Abstract
Castleman’s disease (CD) is a very rare lymphoproliferative disorder whose underlying pathophysiology is not fully understood and for which no standard treatment exists. Because interleukin-1 (IL-1) might promote the production of interleukin-6 (IL-6), a key pathogenic factor for the disease, we hypothesized that blocking the IL-1 receptor would be a useful therapy for CD. We report the case of a 61-year-old woman with CD who had undergone multiple treatments, including cladribine, rituximab, steroids, etanercept, and anti-IL-6 monoclonal antibody, and whose disease was refractory to all of these treatments. She was started on the recombinant IL-1 receptor antagonist, Anakinra, at a subcutaneous dose of 100 mg daily. Within one week, her fatigue and anorexia markedly improved, and her laboratory abnormalities, including anemia, thrombocytosis, leukocytosis, and elevated markers of inflammation, all resolved. Our observation suggests that Anakinra may be an attractive therapeutic approach for refractory multicentric CD.
Introduction
Castleman’s disease (CD) is a very rare lymphoproliferative disorder for which no standard treatment exists. It was originally described by Dr. Benjamin Castleman in 1954, in a patient with a solitary hyperplastic mediastinal lymph node. To date, CD is classified according to histopathologic findings as either hyaline vascular variant, plasma cell variant, or mixed type. It can also be classified into two clinical entities that correlate with the pathology: localized or unicentric CD, and systemic or multicentric CD (MCD). In unicentric CD, a single area is affected, and it is usually the hyaline vascular variant. Patients are often minimally symptomatic and can be cured by surgical excision of the mass. Systemic, or multicentric, CD is clinically more aggressive and generally associated with systemic manifestations such as fever, fatigue, rash, and increased acute-phase reactants. It is typically the plasma cell or mixed variant, and rarely the hyaline vascular variant. MCD requires systemic treatment and the prognosis is often guarded (1–3). Although the mechanisms underlying CD are not fully understood, recent advances in elucidating its biologic basis, particularly the pivotal role of human herpes virus (HHV-8; ref. 4) and interleukin-6 (IL-6; ref. 5), have led to the development of novel investigational therapies, such as antibodies against IL-6 receptor and IL-6 itself, which are showing efficacy in the clinic (6–8). Because interleukin-1 (IL-1) induces the production of IL-6 (9), we hypothesized that blocking the IL-1 receptor may have a salutary impact. We, therefore, administered Anakinra, a recombinant IL-1 receptor antagonist, to a patient with refractory MCD. She has shown a remarkable and ongoing response.
Materials and Methods
A 61-year-old Caucasian woman was referred to MD Anderson Cancer Center for the management of CD in May 2005. Her pertinent medical history began in 2003, with a recurrent, diffuse pruritic rash, low-grade fever, and fatigue. Extensive evaluation on different occasions revealed elevated platelet and white blood cell counts and anemia. She was briefly treated with cyclosporine without improvement. Positron emission tomography-computed tomography (PET-CT) scan done in April 2005, showed 18FDG-avid bilateral inguinal and internal iliac adenopathy and hepatomegaly as well as a soft tissue pulmonary nodule in the left upper lobe with low standardized uptake value. Bone marrow and liver biopsies were negative and bronchoscopy was nondiagnostic. Left axillary lymph node biopsy (reviewed by an MD Anderson Cancer Center hematopathologist) revealed reactive follicular hyperplasia with Castleman-type changes in some germinal centers and marked interfollicular plasmocytosis without cytologic atypia, consistent with the plasma cell variant of CD. No monoclonal B- or plasma-cell population was identified on flow cytometry. Immunostaining for HHV-8 and serology for HIV were negative.
In June 2005, after obtaining informed consent, the patient was treated with CNTO-328, an experimental anti-IL-6 antibody. Although her blood counts improved and lymphadenopathy resolved, intermittent fever and rash persisted. She remained on therapy for approximately 3 years, even though her treatment was interrupted for a technical reason from December 2005 through February 2006, at which time her disease flared up. Starting in early 2008, the patient’s symptoms gradually worsened, and included fever, malaise, fatigue, and significant bone and epigastric pain, leading to multiple hospital admissions for pain management. Anti-IL-6 treatment was discontinued in November 2008. She was started on steroids and etanercept, a tumor necrosis factor-blocking agent, for her bone pain (localized mainly in the hip and pelvic areas) without improvement. PET-CT scan showed no adenopapthy or bone lesions. The patient underwent consecutive plateletpheresis for thrombocytosis, with counts greater than 1 × 109 per μL, and then received cladribine 0.14 mg/kg intravenously for 5 days. She completed one cycle without significant improvement. A bone marrow biopsy prior to treatment showed decreased hematopoietic elements and nontrabecular lymphoplasmacytic infiltration. Shortly after cladribine administration, she began to experience exacerbated episodes of fever, chills, rash, fatigue, and confusion, requiring multiple additional hospitalizations. A thorough infectious and rheumatologic work-up was negative and included blood, urine, and sputum cultures as well as histoplasma antigen in urine, cerebrospinal fluid cultures, serology for cytomegalovirus and Epstein Barr virus, rickettsia, Lyme and Ehrlichia diseases, antinuclear antibody, rheumatoid factor, antineutrophil cytoplasmic (c-ANCA) and perinuclear (p-ANCA) antibodies, anti-Sjögren’s syndrome (anti-SSA; anti-Ro; anti-SSB; anti-La), and anti-cyclic citrullinated peptide antibody (anti-CCP). Serum and urine immunoelectrophoresis with immunofixation showed no monoclonal gammopathy. The patient was treated with repeated courses of steroids, and later was started on naprosyn for possible neoplastic fever. She also received a weekly dose of rituximab for 8 weeks, with persistence of disease flare up.
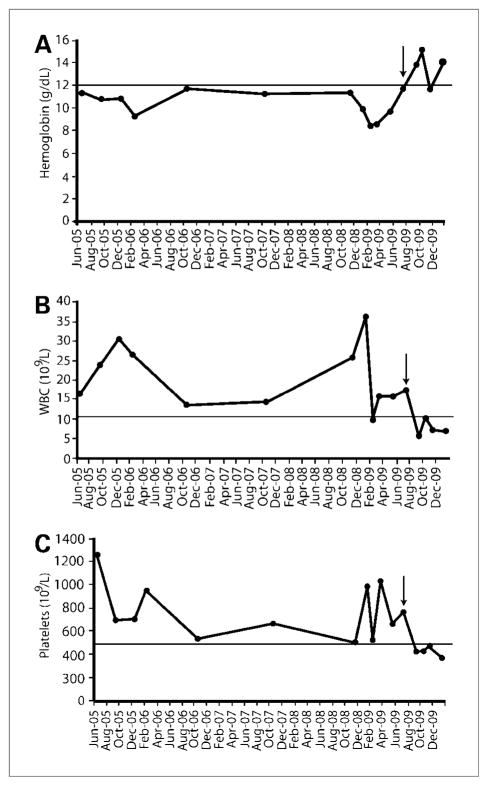
Because IL-1 induces the production and secretion of IL-6, a major driver of CD, we hypothesized that abrogating IL-1 might produce a salutary effect. For that reason, and in view of the patient’s deteriorating condition, Anakinra, a recombinant IL-1 receptor antagonist, was started at a daily subcutaneous dose of 100 mg. Within 1 week, there was a marked improvement in the patient’s energy level and she was no longer wheelchair bound. Her Eastern Cooperative Oncology Group performance status improved from 3 to 1, and complete blood cell count (Fig. 1), erythrocyte sedimentation rate, and C-reactive protein levels normalized.
Figure 1.
Representative blood cell counts prior to and after treatment with Anakinra. Hemoglobin (A), white blood cell count (B), and platelet count (C) improved after initiation of Anakinra. Arrow indicates when treatment with Anakinra was begun. Note: Initial drop in counts in 2005 corresponded with initiation of anti-IL-6 treatment. Also, patient underwent repeat plateletpheresis when platelet counts became elevated to more than 1,000 × 10 9/L.
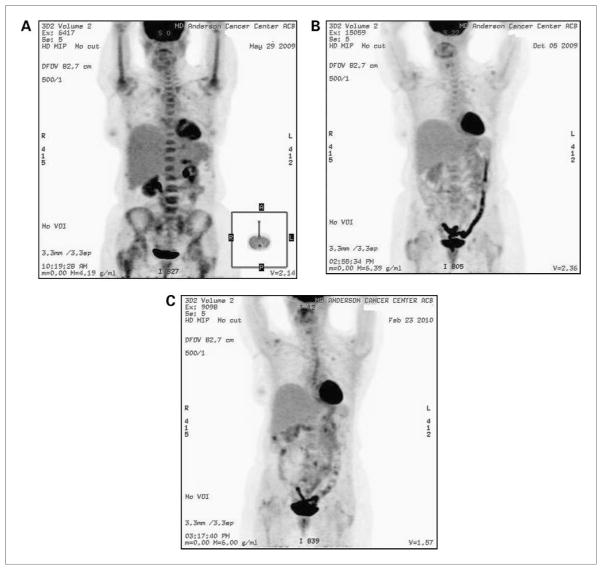
Repeat PET-CT scans 3 and 7 months later showed no evidence of metabolically active lymphadenopathy and decreased FDG uptake throughout bone marrow (Fig. 2). After 7 months of treatment with Anakinra, the patient remained in remission.
Figure 2.
Whole-body 18FDG PET scan done immediately before (A), and 3 months (B), and 7 months (C) after treatment with Anakinra. Increased FDG uptake throughout the bone marrow is markedly less prominent after 3 and 7 months of treatment with Anakinra.
Results and Discussion
MCD is an uncommon lymphoproliferative disorder for which the best therapeutic approach is not yet established. Cytotoxic chemotherapy and steroids were used with variable efficacy (10). New studies have shed light on the molecular basis of the disease, providing hope for the development of a rational approach in managing the disease. However, due to its heterogeneity, rarity, and lack of definitive trials, most of the available data on CD treatment are based on small case series and case reports.
We report the case of a 61-year-old woman with MCD that was refractory to multiple therapies, including cladribine, rituximab, steroids, etanercept, and anti-IL-6 monoclonal antibody. She was then started on the recombinant IL-1 receptor antagonist Anakinra at a dose of 100 mg subcutaneously daily. Within 1 week, her fatigue, anorexia, and bone pain markedly improved, and her laboratory abnormalities, including anemia, thrombocytosis, leukocytosis, and elevated markers of inflammation, all resolved.
Galeotti and colleagues (11) described a case of a 13-year-old boy with MCD who failed to respond to chemotherapy and rituximab treatment, but had a marked response to Anakinra. Of interest, Gherardi and colleagues (12) showed elevated serum levels of IL-1β and IL-6 in five patients with POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes), four of whom had concomitant MCD. Abundant IL-1β mRNA-producing cells were present in the interfollicular spaces in two patients, suggesting that lymph nodes may be a source of aberrant Il-1β production.
There are two distinct IL-1 receptors, IL-1RI and IL-1RII (9). IL-1, by binding to IL-1RI, activates the nuclear factor-κB pathway, which triggers gene transcription of proteins involved in the inflammatory process, including IL-6. In contrast, IL-1RII is unable to transduce intracellular signals. IL-1 receptor antagonist (IL-1RA) is a naturally occurring molecule that binds to IL1-1RI and blocks its biologic effects. Anakinra is the recombinant form of human IL-1RA. It is an injectable drug, with an acceptable safety profile, and is U.S. Food and Drug Administration (FDA) approved in the United States for treatment of rheumatoid arthritis (13). During the last few years, evidence of its efficacy in other inflammatory conditions has accumulated, including Still’s disease, and systemic-onset juvenile rheumatoid arthritis, both of which are IL-6-driven disorders (14).
Our results indicate that the blockade of IL-1 signaling might be an attractive therapeutic strategy for MCD. Furthermore, therapeutic targeting of the IL-1 receptor has a strong biologic rationale. We, therefore, propose that Anakinra warrants further evaluation in the management of this disorder.
Acknowledgments
The authors thank Joann Aaron for her assistance with figures and editing.
Grant Support
RR024148 from the National Center for Research Resources, a component of the NIH Roadmap for Medical Research (http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp).
Footnotes
Note: H. El-Osta contributed to collection of data (patient’s chart and review of the literature), manuscript writing, and final approval of the manuscript. R. Kurzrock contributed to provision of study material, collection of data (patient’s chart and review of the literature), manuscript writing, editing, and final approval of the manuscript. F. Janku contributed to writing the article and final approval of the manuscript.
Disclosure of Potential Conflicts of Interest
R. Kurzrock: Commercial research support and honoraria, AMGEN. No other potential conflicts of interest were disclosed.
References
- 1.Dham A, Peterson B. Castleman disease. Curr Opin Hematol. 2007;14:354–9. doi: 10.1097/MOH.0b013e328186ffab. [DOI] [PubMed] [Google Scholar]
- 2.Casper C. The aetiology and management of Castleman disease at 50 years: translating pathophysiology to patient care. Br J Haematol. 2005;129:3–17. doi: 10.1111/j.1365-2141.2004.05311.x. [DOI] [PubMed] [Google Scholar]
- 3.Larroche C, Cacoub P, Godeau P. La maladie de Castleman. Rev Med Interne. 1996;17:1003–13. doi: 10.1016/s0248-8663(97)80844-2. [article in French] [DOI] [PubMed] [Google Scholar]
- 4.Casper C, Nichols WG, Huang ML, Corey L, Wald A. Remission of HHV-8 and HIV-associated multicentric Castleman disease with ganciclovir treatment. Blood. 2004;103:1632–4. doi: 10.1182/blood-2003-05-1721. [DOI] [PubMed] [Google Scholar]
- 5.Katsume A, Saito H, Yamada Y, et al. Anti-interleukin 6 (IL-6) receptor antibody suppresses Castleman’s disease symptoms emerged in IL-6 transgenic mice. Cytokine. 2002;20:304–11. doi: 10.1006/cyto.2002.2012. [DOI] [PubMed] [Google Scholar]
- 6.Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106:2627–32. doi: 10.1182/blood-2004-12-4602. [DOI] [PubMed] [Google Scholar]
- 7.Van Rhee F, Fayad L, Voorhees P, et al. CNT0 328, a monoclonal antibody to interleukin-6, is active as a single agent in Castleman’s disease: Preliminary results of a phase 1 study. Blood. 2008;112:1008. [Google Scholar]
- 8.Ahmed B, Tschen J, Cohen P, et al. Cutaneous castleman’s disease responds to anti-interleukin-6 treatment. Mol Cancer Ther. 2007;6:2386–90. doi: 10.1158/1535-7163.MCT-07-0256. [DOI] [PubMed] [Google Scholar]
- 9.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 10.Bowne WB, Lewis JJ, Filippa DA, et al. The management of unicentric and multicentric Castleman’s disease. A report of 16 cases and a review of the literature. Cancer. 1999;85:706–17. doi: 10.1002/(sici)1097-0142(19990201)85:3<706::aid-cncr21>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Galeotti C, Tran TA, Franchi-Abella S, Fabre M, Pariente D, Koné-Paut I. IL-1RA agonist (Anakinra) in the treatment of multifocal Castleman disease: case report. J Pediatr Hematol Oncol. 2008;30:920–4. doi: 10.1097/MPH.0b013e31818ab31f. [DOI] [PubMed] [Google Scholar]
- 12.Gherardi RK, Belec L, Fromont G, et al. Elevated levels of interleukin-1 beta (IL-1 beta) and IL-6 in serum and increased production of IL-1 beta mRNA in lymph nodes of patients with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes (POEMS) syndrome. Blood. 1994;83:2587–93. [PubMed] [Google Scholar]
- 13.Kalliolias GD, Liossis SN. The future of the IL-1 receptor antagonist Anakinra: from rheumatoid arthritis to adult-onset Still’s disease and systemic-onset juvenile idiopathic arthritis. Expert Opin Investig Drugs. 2008;17:349–59. doi: 10.1517/13543784.17.3.349. [DOI] [PubMed] [Google Scholar]
- 14.Woo P. Anakinra treatment for systemic juvenile idiopathic arthritis and adult onset Still disease. Ann Rheum Dis. 2008;67:281–2. doi: 10.1136/ard.2007.082859. [DOI] [PubMed] [Google Scholar]