Abstract
Background
Critical gaps exist in the understanding of cancer symptoms, particularly for cancer-related fatigue (CRF). Existing theories and models do not examine the key role perceived self-efficacy (PSE) plays in a person's ability to manage symptoms.
Objectives
To test the hypothesis that physical functional status (PFS) is predicted through patient characteristics, CRF, other symptoms, and PSE for fatigue self-management in persons with cancer.
Methods
This study is a secondary data analysis from the baseline observation of two randomized control trials. The combined data set includes 298 subjects who were undergoing a course of chemotherapy. Key variables included physiological and contextual patient characteristics, the severity from CRF and other symptoms, PSE, and PFS. Path analysis examined the relationships among the variables in the proposed theoretical model.
Results
Persons with cancer reported CRF as the most prevalent symptom among a mean of 7.4 other concurrent symptoms. The severity from CRF had a direct and indirect effect on PFS, with CRF having a direct adverse impact on PFS (t = -7.02) and an indirect adverse effect as part of the severity from the other symptoms (t = 9.69) which also adversely impacted PFS (t = -2.71). Consistent with the proposed theoretical model, PSE had a positive effect on the PFS (t = 2.87) of persons with cancer while serving as a mediator between CRF severity and PFS.
Discussion
Cancer-related fatigure is prevalent and related to the presence of other symptoms, and PSE for fatigue self-management is an important factor influencing CRF and PFS. A foundation is provided for future intervention studies to increase PSE to achieve optimal PFS in persons with cancer.
In 2008, nearly 1.5 million Americans will learn they have cancer and 600,000 will die from the disease (American Cancer Society, 2008). Consequently, many Americans will live longer with the effects of the disease and its treatment. Regrettably, one of the major effects of cancer and its treatment is the occurrence of multiple symptoms that place persons with cancer at risk for poor outcomes (Miaskowski et al., 2006). Among the multiple concurrent symptoms, fatigue is a highly prevalent and distressing symptom (Gupta, Lis, & Grutsch, 2007). Cancer-related fatigue (CRF) is accompanied by many other severe symptoms that are managed poorly by patients and professionals (Gift, Stommel, Jablonski, & Given, 2003).
Symptoms such as CRF are one of the major determinants of physical functional status (PFS; Doorenbos, Given, Given, & Verbitsky, 2006). Physical functional status (PFS) is the physical activity that people accomplish in the normal course of their lives to meet basic needs, fulfill usual roles, and maintain their health and well-being (Leidy, 1994). Research indicates that symptoms such as CRF have an adverse impact on PFS, making symptom self-management an important component to maximize PFS (Dodd, Miaskowski, & Paul, 2001; Gift, Jablonski, Stommel, & Given, 2004; Given, Given, Azzouz, & Stommel, 2001). Symptom management occurs through self-directed action, with perceived self-efficacy (PSE) being a key factor. In some populations, a positive relationship between a person's PSE and his or her ability to manage symptoms has been shown (Lorig, Ritter, & Plant, 2005). Currently, most CRF management is carried out by patients via self-care strategies (Stone et al., 2003). These strategies often are impacted by the person's level of fatigue and PSE. In order to manage fatigue, it is critical to know what a person thinks of his or her ability to manage fatigue and how it impacts any self-directed action. Perceived self-efficacy forms the basis of any decision to act, the course of action selected, the degree of effort exerted, and the perseverance to continue in the face of adversity (Bandura, 1997). Thus, the ability to exercise control over self-directed action is fundamental to symptom self-management and other actions characteristic of living with a serious chronic illness such as cancer.
Experts agree there are critical gaps in knowledge of CRF management and that research should be focused on CRF interventions to understand the mediating mechanisms of CRF (Ahlberg, Ekman, Gaston-Johansson, & Mock, 2003). Existing models and theories provide insight into factors necessary for the successful management of symptoms but do not address the important role PSE plays in a person's ability to self-manage symptoms (Dodd, Janson, et al., 2001; Lenz, Pugh, Milligan, Gift, & Suppe, 1997). Using Medical Subject Headings (MeSH) terms, key words, and thesaurus terms via PubMed, MEDLINE, CINAHL, and PsycINFO (i.e., fatigue, symptoms, self-efficacy, and cancer), no studies were found examining the role PSE plays in the self-management of fatigue and other associated symptoms to achieve optimal PFS for the cancer population.
The purpose of this study is to test a theoretical model with the hypothesis that PFS is predicted through patient characteristics, CRF, other symptoms, and PSE for fatigue self-management in persons with cancer. Consequently, this study will result in a foundation for future intervention studies to increase PSE to achieve optimal CRF self-management and PFS in persons with cancer.
Theoretical Framework
This study was guided by a synthesis of the Theory of Unpleasant Symptoms (TOUS; Lenz et al., 1997) and Bandura's (1997) Self-Efficacy Theory. The theoretical framework is used to provide insight into how a person's belief (PSE) in ability to self-manage symptoms influences performance of those behaviors. To understand what influences symptom self-management, the factors that affect symptoms must be understood. The TOUS sheds light on this by exposing the multidimensional nature and impact of symptoms. Patient and symptom characteristics and symptom interactions all have an affect on a patient's PFS. Bandura's (1997) Self-Efficacy Theory posits that PSE serves as a mediator between symptoms and PFS. According to Bandura, PSE is a person's perception of ability to implement behaviors to self-manage symptoms such as CRF. Perceived self-efficacy is specific to each behavior or situation, and this study is focused on PSE to manage CRF. Once multiple facets of symptoms such as CRF and the role PSE plays in symptom self-management are understood, symptom self-management strategies can be derived and tailored to enhance PSE and ultimately PFS.
Research Hypotheses Related to the Theoretical Model
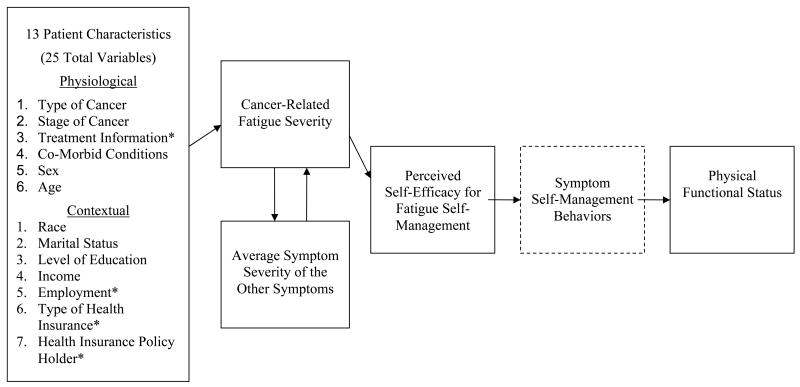
The hypothesized theoretical model for this study is depicted in Figure 1. Current literature provides limited evidence of the etiology of CRF in the cancer population (National Comprehensive Cancer Network, 2007). While patient characteristics likely influence other symptoms from cancer and its treatment, this study is focused on identifying patient characteristics that affect CRF. Physiological and contextual characteristics are hypothesized to influence CRF (Hypothesis 1). During the course of their illness and treatment trajectory, persons with cancer present with CRF and other concurrent symptoms. The concurrence of symptoms are likely to catalyze each other, worsening the level of symptom severity (Lenz et al., 1997). Consequently, a reciprocal relationship is hypothesized to occur between CRF severity and average symptom severity (excluding CRF; Hypothesis 2). A contributing factor to the achievement of CRF self-management to attain maximum PFS may be a person's PSE. Investigators have substantiated that PSE plays a central role in producing positive outcomes in symptom self-management and functional ability in persons living with chronic conditions (Lorig et al., 2005; Motl, Snook, McAuley, & Gliottoni, 2006). While it is believed that the effect of PSE on functional ability is through symptom self-management behaviors, assessing symptom self-management behaviors was beyond the scope of this study and thus appears in the model as an untested assumption for the study. Few studies have been conducted relative to PSE and symptom management in the cancer population, and those that have indicate that increased PSE has a positive impact on the lives of persons with cancer (Eller et al., 2006; Lev et al., 2001). Moreover, although limited research exists describing the relationship between fatigue and PFS in persons with cancer, research documents that increased CRF contributes to decreased PFS (Given, Given, Azzouz, Kozachik, & Stommel, 2001). Therefore, PSE for fatigue self-management is hypothesized to mediate the relationship between CRF and PFS with PSE having a positive effect on PFS (Hypothesis 3).
Figure 1.
- Asterisk (*) means there are more than one variable in certain patient characteristic categories. See details of each patient characteristic in the Measures Section of the manuscript.
- Dashed line means the relationship between perceived self-efficacy for fatigue self-management and symptom self-management behaviors is an untested assumption of the theoretical framework and assessing symptom self-management behaviors was beyond the scope of this study.
Methods
Design and Sample
Secondary analysis was conducted using data from two National Cancer Institute sponsored randomized controlled trials (RCTs) of persons with cancer (Barbara Given,1998-2007, The Family Home Care for Cancer: A Community-based Model for Symptom Management, R01 CA-079280 and Charles Given, 2003-2007, The Automated Telephone Information and Monitoring of Symptoms, R01 CA-30724). In this secondary analysis, a descriptive design was employed, using data from baseline measures in the RCTs collected prior to any intervention. The sample included persons with breast (n = 105), lung (n = 63), colon (n = 44), and other sites of cancer diagnoses (n = 86). Study participants were at least 21 years old and undergoing a course of chemotherapy with at least two cycles remaining at time of enrollment for a new or recurrent diagnosis of breast, colorectal, or lung cancer; other solid tumors; and non-Hodgkin's lymphoma, and may have been receiving concurrent radiation therapy. Persons had to be intact cognitively, English-speaking, able to conduct telephone interviews, and not receiving hospice care. Specific inclusion criteria for the Family Home Care for Cancer: A Community-based Model for Symptom Management included reaching a symptom severity threshold of 2 out of 10 (10 = most severe) for both pain and fatigue or 3 out of 10 on either pain or fatigue. Specific inclusion criteria for the Automated Telephone Monitoring for Symptom Management required a symptom severity threshold of 2 out of 10 for at least 1 of 16 possible symptoms related to cancer and its treatment. This secondary analysis was approved by the Michigan State University Institutional Review Board for the protection of human subjects.
Measures
Measures for key variables are presented and organized according to the hypothesized theoretical model and include: physiological and contextual patient characteristics; symptoms; PSE for fatigue self-management; and PFS (Figure 1).
Physiological and contextual patient characteristics
Most patient characteristics were collected in the original study through a demographic questionnaire and medical records. A total of 13 patient characteristics (6 physiological and 7 contextual), some composed of more than one variable, were included in the hypothesized theoretical model.
Physiological patient characteristics consisted of type and stage of cancer, treatment information, comorbid conditions, sex, and age. Given that persons with lung cancer report more symptoms than persons with other cancer diagnoses (Doorenbos, Given, Given, & Verbitsky, 2006), type of cancer was coded into two groups: lung cancer and other cancer diagnoses. For stage of cancer, within a diverse cancer population, data indicate that advanced cancer is more likely to be related to both pain and fatigue when compared with pain alone (Given, Given, Azzouz, Kozachik, et al., 2001). Stage of cancer was classified according to the tumor-node-metastasis staging system of the American Joint Committee on Cancer. For small cell lung cancer, a two-staged system was used: limited or extensive. In order to compare those with limited disease to those with advanced disease, the researchers combined and coded stages 0-II and limited stage into the early stage, and stages III-IV and extensive stage as late stage. Various types of cancer treatment have been shown to be related to fatigue and other symptoms (Cooley, Short, & Moriarty, 2003). For this study, treatment information included radiation therapy and surgery. Radiation therapy was coded into two groups (receiving and not receiving). Surgery consisted of two variables: surgery prior and surgery during. Surgery prior means surgery occurred anytime prior to chemotherapy and may or may not be related to this episode of cancer. This variable was dummy-coded into four groups with no surgery serving as the reference group (yes had surgery; don't know if had surgery; this response choice was not selected; no surgery). Surgery during means that surgery occurred during chemotherapy between the time of consent and the last interview. This variable was dummy-coded into three groups with no surgery serving as the reference group (yes had surgery; this response choice was not selected; no surgery). The variables requiring dummy coding, surgery prior and surgery during, were included in regression and path models after dummy coding. Comorbid conditions were assessed using a modified version of the Comorbidity Questionnaire (Katz, Chang, Sangha, Fossel, & Bates, 1996), which is used to inquire about the presence of 15 chronic health conditions; content validity and test-retest reliability have been established. For this study, this variable consisted of a sum of the total number of health problems. Sex and age were included in the hypothesized theoretical model since women, as compared to men, have been found to be more likely to report CRF, and women 60 years of age or greater with metastatic cancer reported fatigue to be more disruptive than men (Given, Given, Azzouz, Kozachik, et al., 2001).
There is a little evidence concerning how contextual characteristics affect CRF in the general cancer population (Montazeri, Gillis, & McEwen, 1998). The burden associated with cancer requires great amounts of support through contextual characteristics to sustain the patient and their family. Consequently, contextual patient characteristics included race, marital status, level of education, income, employment, type of health insurance, and health insurance policy holder. For the purpose of this analysis, race was coded into two groups (majority and minority); marital status coded into married and not married; employment data included whether a person was retired, was receiving disability, was on a temporary leave, and whether they had to quit employment; type of health insurance held was coded in three groups (private, Medicare, or Medicaid); and health insurance policy holder status was coded in three groups (patient, spouse, or no policy).
Symptoms
The Brief Fatigue Inventory (BFI) is a 9-item measure focused on the assessment of CRF severity. Substantial evidence supports the psychometrics of the BFI in the cancer population (Mendoza et al., 1999). For this study, two items (patient's current and worst severity of CRF within the past 7 days) from the BFI were used to calculate a CRF severity score (0-10, 10 = most severe). Current and worst fatigue severity were used since these observations were distributed identically, as indicated by the high degree of correlation between these two items by a Cronbach's alpha of 0.85. The CRF severity score was calculated by summing each subject's response for both CRF severity items and dividing by two for a composite score on an 11-point scale.
The Symptom Experience Inventory is a self-report measure of 16 symptoms related to cancer and its treatment (Given et al., 2002). On an 11-point scale (0-10, 10 = most severe), patients were asked to rate the current severity of each of the symptoms. For this study, evaluation of internal consistency reliability resulted in a Cronbach's α of 0.72. The average symptom severity was calculated by summing each subject's severity scores for each symptom reported and dividing by the number of symptoms reported for a composite score on an 11-point scale. The average symptom severity score did not include the symptom of fatigue. Current fatigue was compared with the current severity of the other symptoms.
Perceived self-efficacy for fatigue self-management
In a review of the literature, no existing tool was found to measure PSE for fatigue self-management in persons with cancer. A 6-item subscale adapted from the Lorig Arthritis Self-Efficacy Scale (ASE) was developed to measure PSE for fatigue self-management. Lorig created the ASE to measure a person's PSE to cope with the symptoms of arthritis. The ASE has a 3-factor solution, accounting for 61% of the variance in PSE. Internal coefficient alphas for each of the subscales (n = 143) have been reported as 0.76 for pain management, 0.89 for physical functioning, and 0.87 for coping with other symptoms (Lorig, Chastain, Ung, Shoor, & Holman, 1989).
The Coping with Other Symptoms Lorig subscale, which has been successfully adapted in the past for patient populations including those with cancer pain and human immunodeficiency virus (Keefe et al., 2003; Shively, Smith, Bormann, & Gifford, 2002), was modified to focus on fatigue by replacing other identified symptoms with fatigue to create the PSE for Fatigue Self-Management Scale (PSEFSMS). The PSEFSMS is a self-report measure containing 6 items related to PSE for fatigue self-management. On an 11-point scale (0-10, 10 = very certain) respondents rated their certainty in performing fatigue-managing behaviors. Content validity for the PSEFSMS was evaluated by a panel of 13 content experts. These experts were selected using the selection criteria established by Grant and Davis (1997) and have experience in areas such as cancer-related fatigue, clinical oncology, self-efficacy theory, research methods and statistics, or a combination of these. The panel of experts was provided with conceptual definitions, the measurement model, and a description of the population and setting in which the PSEFSMS would be used (Grant & Davis, 1997). The expert panel members were asked to identify items that were not stated clearly, and to comment on the representativeness of each item and the comprehensiveness of the total instrument (Grant & Davis, 1997). For this study, reliability of the instrument was established in the form of internal consistency reliability which resulted in a Cronbach's α of 0.92.
Physical functional status
The outcome, PFS, was measured using the 10-item physical functioning subscale of the Medical Outcomes Study 36-Item Short Form Health Survey (Ware, Snow, Kosinski, & Gandek, 1993). The items capture the extent of PFS using a 3-point Likert-type scale, are summed, and then are transformed to a 0-100 scale, with higher scores indicating greater PFS. This measure has been used extensively in populations with chronic illness and has provided evidence of psychometric soundness. In this study, the internal consistency reliability value is a Cronbach's α of 0.91.
Data Analysis
An EM algorithm (maximum likelihood; von Eye & Schuster, 1998) was used for missing value analysis and imputation using SYSAT version 11.0 statistical software package (SYSAT, Point Richmond, CA). For this study, 2.9% of the data incurred missing values requiring estimation and imputation. Except for 7 items (5.7-30% missing values), most items had complete data or a minimal rate of missingness (<1-2% missing values). All missing data were evaluated and determined to be missing completely at random via Little's Missing Completely at Random test statistic. Descriptive statistics and Cronbach's α of the main study variables are presented in Table 1. Correlations among the main study variables are presented in Table 2. The components of the hypothesized theoretical model were examined using an exogenous-endogenous nonrecursive path model to test the hypothesis that PFS for persons with cancer is predicted through patient characteristics, CRF severity, other symptoms, and PSE for fatigue self-management. This approach was selected because multiple hypothesized paths of influence are examined simultaneously and global indices of the fit can be provided between the data and the hypothesized theoretical model (Raykov & Marcoulides, 2006). This incorporated a sequence of predictions tested through a path model using the LISREL Version 8.72 statistical software package.
Table 1. Descriptive Statistics for the Main Study Variables.
| Measure | n | M (SD) | Skewness (Kurtosis) | Potential Range | Internal Consistency Cronbach's alpha |
|---|---|---|---|---|---|
| Age | 298 | 57.10 (11.88) | -0.411 (-0.459) | ≥ 21 | --- |
| Comorbid Conditions | 298 | 2.02 (1.59) | 6.97 (5.91) | 0-15 | --- |
| Cancer-Related Fatigue Severity | 298 | 5.84 (2.23) | -0.759 (-2.90) | 0-10 | .85 |
| Total Symptom Severity | 296 | 4.64 (1.58) | 0.387 (1.11) | 0-10 | .72 |
| Perceived Self-Efficacy for Fatigue Self-Management | 298 | 6.43 (2.25) | -1.33 (-2.87) | 0-10 | .92 |
| Physical Functional Status | 298 | 58.10 (27.10) | -1.94 (-3.63) | 0-100 | .91 |
Table 2. Bivariate Correlations of the Main Study Variables.
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 1.00 | ||||||||
| 2. Comorbid Conditions | .41** | 1.00 | |||||||
| 3. Sex | -.12* | -.06 | 1.00 | ||||||
| 4. Cancer Stage | -.10 | .01 | .22** | 1.00 | |||||
| 5. Surgery anytime prior to chemotherapy | .01 | -.08 | .10 | .16* | 1.00 | ||||
| 6. CRF Severity | -.08 | .14* | .15** | .15* | .04 | 1.00 | |||
| 7. Total Symptom Severity | -.03 | .09 | .00 | .02 | -.12* | .51** | 1.00 | ||
| 8. PSE for Fatigue Self-Management | .06 | -.06 | -.13* | -.09 | .07 | -.39** | -.14* | 1.00 | |
| 9. Physical Functional Status | -.16** | -.38** | -.11 | -.02 | -.02 | -.50** | -.36** | .32** | 1.00 |
p < .05 (2-tailed),
p < .01 (2-tailed)
CRF = cancer-related fatigue; PSE = perceived self-efficacy
Analyses were conducted using the Satorra-Bentler Robust Maximum Likelihood Method of parameter estimation to adjust model chi-square for nonnormally distributed variables. Fisher's skewness and kurtosis statistic for the main study variables are reported in Table 1. Since multiple models were tested and decisions about the parameters were made based on earlier statistical results, the chi-square statistic may not approximate the chi-square distribution properly and the standard errors estimated for the confidence intervals can be underestimated. Therefore, the weight placed on the chi-square statistic was small, and several other model fitting measures were used to attain a parsimonious final solution (Raykov & Marcoulides, 2006). The Satorra-Bentler Scaled chi-square reflects the degree of discrepancy between the observed covariance matrix derived from the data and that predicted by the model. A well-fitting model is one where the resulting chi-square value is small and not significant. The Root-Mean Square Error of Approximation (RMSEA) takes into account model complexity as reflected in the degrees of freedom. A RMSEA value ≤ 0.05 indicates close fit with a value of 0 (indicating exact fit). The 90% confidence intervals (CI) for population parameters estimated by the RMSEA reflects the degree of uncertainty associated with RMSEA as a point estimate at the 90% level of statistical confidence. If the lower bound of a 90% CI is ≤ 0.05, and the interval is not excessively wide, the model has close fit in the population. The Comparative Fit Index (CFI) was used indicating the proportional reduction in the chi-square (or the fit function) achieved by a particular model compared to a null model in which the variables are specified to be uncorrelated. A CFI value above 0.90 indicates good model fit. The Goodness-of-Fit Index (GFI) is the percent of observed covariances explained by the covariances implied by the model. A GFI = 1.0 indicates perfect model fit and a GFI > 0.90 indicates a good fit. Last, the Akaike Information Criteria (AIC) is a parsimony adjusted index used to select among competing models favoring simpler models; a lower AIC reflects the better fitting model.
In the final parsimonious model, the chi-square difference statistic was used to evaluate the mediating role of PSE for fatigue self-management and the average severity of the other symptoms in the relationship between CRF severity and PFS (Raykov & Marcoulides, 2006). The chi-square difference statistic can be used to determine whether two nested models differ in their model fit. Models are nested if one model contains all parameters of the other plus at least one more. Chi-square difference is computed as the chi-square value for the larger unconstrained model minus the chi-square value of the smaller nested model and the difference, also a chi-square, is evaluated with degrees of freedom (df) equal to the difference between the degrees of freedom in the two models. The null hypothesis is that there is no difference in fit. If the chi-square difference is not significant, then the null hypothesis is retained and the two models have comparable fit to the data. Following the suggestions of Baron and Kenny (1986) and Kenny (1998), the model that included both the direct and indirect paths from CRF severity to PFS was compared with the model where the direct path from CRF severity to PFS was constrained to 0. Sample size for power to conduct this analysis supports reasonably stable results. This analysis meets the criteria set by Kline (2005), who states a desirable goal to have is 10 to 20 times as many cases as to the number of free parameters.
Finally, the estimated odds ratio and 95% confidence interval were calculated to clarify the degree to which the number of comorbid conditions and being a woman as compared to a man influenced level of CRF severity. Comorbid conditions were dichotomized at two, which reflects the mean and median number of comorbid conditions reported by persons with cancer in this study (i.e., < 2 comorbid conditions; ≥ 2 or more comorbid conditions). Similarly, the median score for CRF severity was 6 and CRF severity was divided into two groups (i.e., CRF score < 7; CRF score ≥ 7). This cut-point was determined since research has found patients rating their worst fatigue and worst pain at a 7 or greater, and by using this range (7-10) to demarcate severe fatigue, 38% of the sample would be defined as severe fatigue, similar to other symptom studies (Mendoza et al., 1999; Serlin, Mendoza, Nakamura, Edwards, & Cleeland, 1995).
Results
Patient Characteristics
The analysis was completed on 298 persons with cancer. Physiological and contextual patient characteristics are shown in Table 3. Participants ranged in age from 25 to 90 years with a mean of 57 years; 70% were men; 87% were Caucasian; and 69% were married. The majority of participants had completed some college education and had an annual combined income of $50,000 to $74,999. The median number of comorbid conditions per participant was two, with hypertension (45%), emotional problems (28%), and other major health problems (21%) accounting for the majority of comorbid conditions. Most were diagnosed with late-stage cancer (74%), and had a surgery prior to receiving chemotherapy (62%).
Table 3. Physiological and Contextual Patient Characteristics (n = 298).
| Characteristics | n | % |
|---|---|---|
| Gender | ||
| Men | 89 | 30 |
| Women | 209 | 70 |
| Race | ||
| Caucasian | 259 | 87 |
| Other | 39 | 13 |
| Marital Status | ||
| Married | 205 | 69 |
| Not married | 93 | 31 |
| Education Completed | ||
| Less than high school | 30 | 10 |
| High school | 73 | 24 |
| Some college/Technical training | 86 | 30 |
| College | 60 | 20 |
| Graduate/Professional | 49 | 16 |
| Annual Household Income | ||
| < $24,999 | 43 | 15 |
| $25,000-$49,999 | 77 | 26 |
| $50,000-$74,999 | 84 | 28 |
| $75,000-$99,999 | 34 | 11 |
| > $100,000 | 60 | 20 |
| Comorbid Conditions | ||
| Hypertension | 134 | 45 |
| Emotional problems | 82 | 28 |
| Other major health problems | 61 | 21 |
| Other cancer | 58 | 20 |
| Heart problem | 52 | 17 |
| Loss of urine beyond control | 48 | 16 |
| Diabetes | 41 | 14 |
| Cataract surgery | 30 | 10 |
| Arthritis | 29 | 10 |
| Emphysema | 28 | 9 |
| Wear a hearing aid | 15 | 5 |
| Surgical replacement of joint | 11 | 4 |
| Stroke | 6 | 2 |
| Angina | 7 | 2 |
| Fractured hip | 1 | -- |
| Stage of cancer | ||
| Early | 78 | 26 |
| Late | 220 | 74 |
Symptoms
Data indicate a high number of concurrent symptoms in persons with cancer with a mean of 7.4 symptoms (SD = 2.60). Fatigue was the most prevalent symptom occurring 100% of the time in the 7 days prior to the baseline interview followed by insomnia (77%), lack of appetite (63%), weakness (60%), dry mouth (60%), pain (55%), and nausea (53%). Out of 16 symptoms, alopecia was the most severe (M = 5.77; SD = 3.24) followed by insomnia (M = 5.40; SD = 2.63), vomiting (M = 5.29; SD = 2.97), constipation (M = 5.26; SD = 2.81), and fatigue (M = 5.24; SD = 2.33; Table 4). Considering the top five most prevalent symptoms for all persons with cancer, fatigue was the most severe. The average symptom severity score for persons with cancer was 4.64 (SD = 1.58) which was correlated positively with CRF severity (r = .51; p < .001).
Table 4. Most Frequent and Most Severe Symptoms (n = 298).
| Symptom | Frequency | Severity | ||
|---|---|---|---|---|
| Rank | n (%) | Rank | M (SD) | |
| Fatigue | 1 | 298 (100) | 5 | 5.24 (2.33) |
| Insomnia | 2 | 229 (77) | 2 | 5.54 (2.63) |
| Lack of Appetite | 3 | 187 (63) | 7 | 5.04 (2.48) |
| Weakness | 4 | 180 (60) | 8 | 4.84 (2.42) |
| Dry Mouth | 5 | 178 (60) | 9 | 4.80 (2.60) |
| Pain | 6 | 164 (55) | 10 | 4.62 (2.27) |
| Nausea | 7 | 159 (53) | 12 | 4.54 (2.64) |
| Difficulty Remembering | 8 | 133 (45) | 16 | 3.71 (2.44) |
| Numbness/Tingling | 9 | 124 (41) | 13 | 4.44 (2.55) |
| Constipation | 10 | 118 (40) | 4 | 5.26 (2.81) |
| Dyspnea | 11 | 115 (39) | 11 | 4.59 (2.14) |
| Cough | 12 | 105 (35) | 15 | 3.89 (2.36) |
| Alopecia | 13 | 100 (34) | 1 | 5.77 (3.24) |
| Diarrhea | 14 | 93 (31) | 6 | 5.10 (2.56) |
| Vomiting | 15 | 45 (15) | 3 | 5.29 (2.97) |
| Fever | 16 | 30 (10) | 14 | 4.07 (2.32) |
Testing the Fit of the Hypothesized Theoretical Model
Patient characteristics included in the hypothesized theoretical model were refined by combining bivariate and multiple regression analyses (inclusion criterion set at p < .20) with an evaluation of each characteristic's merit based on past research and theory. These criteria were chosen to retain as many patient characteristics as possible since they could be significant in the final analyses of theory testing. As a result, 2 (i.e., race and health insurance policy holder) of the 13 patient characteristics to the prediction of CRF severity were eliminated. Similarly, two other patient characteristics (i.e., employment data and treatment information) to the prediction of CRF severity had some but not all variables eliminated (i.e., was on a temporary leave; radiation therapy; surgery prior groups don't know if I had surgery and response choice was not selected; surgery during group this response choice was not selected). Next, the exogenous-endogenous model was tested to examine the overall fit. While the solution converged, the fit of the model was not acceptable (X2 = 160.18; p < .01; df = 50; RMSEA = .089; lower bound 90% CI = 0.074; upper bound 90% CI = 0.10; CFI = 0.92; GFI = 0.95; AIC = 482). The model was improved by removing nonsignificant paths one at a time and by including paths that had not been taken into account in the first solution. Both removing and including paths were based on evaluation of parameter estimates, modification indexes, goodness-of-fit tests, and theoretical considerations.
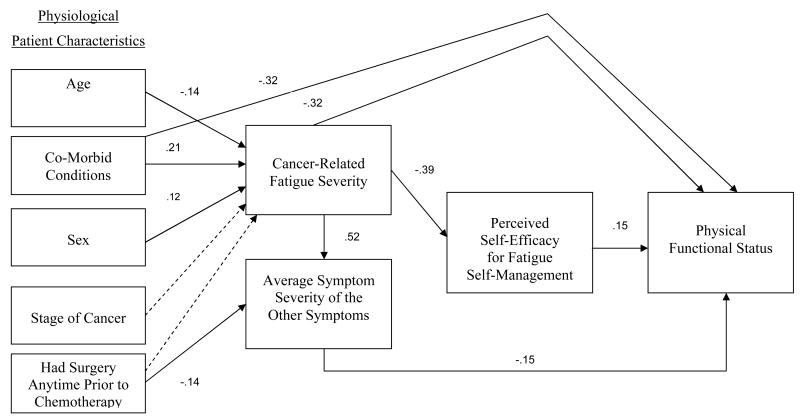
The direct and indirect paths in the final parsimonious theoretical model are shown in Figure 2, with the fitting measures indicating a good fit, improved over the original model (X2 = 17.76; p = .22; df = 14; RMSEA = .03; lower bound 90% CI = 0.00; upper bound 90% CI = .068; CFI = .99; GFI = 0.99; AIC = 79). The direct paths demonstrate the following for persons with cancer: younger age (t = -2.18), greater number of comorbid conditions (t = 3.36), and being female (t = 2.11) predicts greater CRF severity. Having surgery anytime prior to chemotherapy (t = -2.85) predicts greater average severity of the other symptoms. Contrary to expectations, the relationship between CRF severity and average symptom severity was not reciprocal. Instead, greater CRF severity predicts greater average symptom severity (t = 9.69). The effect of CRF severity on the average symptom severity was found to be significant (t = 2.07), but the reverse effect was not significant (t = 1.16). Correspondingly, the model fitting measures indicated that when eliminating the effect of the average symptom severity on CRF severity while retaining the effect of CRF severity on the average symptom severity, the model fit was improved. The direct paths also demonstrate that greater CRF severity predicts lower PSE for fatigue self-management (t = -7.02). Greater PSE for fatigue self-management predicts greater PFS (t = 2.87). Last, more comorbid conditions (t = -7.47), greater CRF severity (t = -5.30) and greater average severity of the other symptoms (t = -2.71) predicted lower PFS.
Figure 2.
Final Parsimonious Theoretical Model:
Satorra-Bentler Scaled Chi-Square 17.76; p = .22; df = 14; RMSEA = .03; RMSEA lower bound 90% CI = 0.00; RMSEA upper bound 90% CI = 0.068; CFI = .99; GFI = 0.99; and AIC 79.
• Solid line means a significant direct path (t > -/+ 2.0).
• Dashed line means a nonsignificant direct path (t < 2.0).
• The numerical values represent standardized path coefficients.
• Not depicted in the Figure 2, standardized significant indirect path coefficient from CRF severity to PFS of -0.14.
Moreover, two indirect paths from CRF severity to PFS were identified (t = -3.61), including PSE for fatigue self-management as originally hypothesized in the model, and the average severity of the other symptoms which was not hypothesized a priori. The significance of the mediating variables was evaluated using the chi-square difference test. The difference between the constrained model of total mediation and the unconstrained model of partial mediation was significant (Δχ² = 27.81; Delta df = 1; p < .001). Therefore, the model of partial mediation was retained. Last, two nonsignificant paths were retained, the effects of stage of cancer (t = 1.95) and having surgery anytime prior to chemotherapy (t = 1.60) on CRF severity because the model was significantly worse without these two paths.
Discussion
The results of hypotheses testing in this study supports the credibility of the theoretical framework derived through a synthesis of the TOUS and Bandura's Self-Efficacy Theory and extends the CRF literature by identifying mechanisms associated with CRF severity which ultimately impact the PFS of persons with cancer. The data validated the fundamental hypotheses of the initial model and also provided greater depth, resulting in a final theoretical model that is more complex than the original hypothesized model. Specific patient characteristics that relate to CRF severity were identified and the directional relationship between CRF severity was reoriented, demonstrating that CRF influences the average severity from the other concurrent symptoms rather than the other way around. This is the first known study to demonstrate that PSE for fatigue self-management mediates the relationship between CRF and PFS.
Hypothesis 1: Patient Characteristics Influence Cancer-Related Fatigue
Path analysis revealed that having a greater number of comorbid conditions, being a woman, and being of younger age predicted greater CRF severity. Persons with two or more comorbid conditions were nearly twice as likely to have a higher level of CRF severity (95% CI: 1.07 - 2.74) than those with fewer co-morbid conditions. Women were twice as likely to experience CRF severity at levels of 7 to 10 as were men (95% CI: 1.29 - 3.65). Knowing that these patient characteristics lead to greater CRF allows the clinician to anticipate greater CRF and offer preventative measures. For instance, with this understanding clinicians could develop an assessment process to screen those who are at risk for CRF so an individualized plan of care could be delivered to preempt the development of higher levels of CRF and other symptoms that decrease PFS. Further research is needed, however, to understand why these patient characteristics influence greater CRF severity. Bandura (1997) informs us that human behavior is multideterminant based on the interplay of personal and environmental influences which comprise a person's characteristics. In these instances we know that certain characteristics influence greater CRF, but this could be the outcome of differing behaviors based on differing personal and environmental influences.
Hypothesis 2: Cancer-Related Fatigue and the Other Unpleasant Symptoms are Reciprocally Related
In the final model, it was revealed that the relationship between CRF and the other unpleasant symptoms was not reciprocal with only CRF affecting the other unpleasant symptoms. Parallel to the TOUS, symptoms interact and create a catalyzing effect having a resultant effect on critical patient outcomes. Knowledge gaps exist in multisymptom relationships and symptom clusters. Not all symptoms are equal in importance and there is a need to identify priority symptoms that may dominate and trigger the experience of multisymptom relationships to optimize symptom management (Gift, 2007). Knowing that CRF affects other symptoms and the reciprocal does not occur allows nurse researchers to focus research and practice on CRF, knowing that what benefits can be gained by treating CRF will have cascading positive affects on other co-occurring symptoms. It is also relevant that researchers have found that cancer patients who experienced fatigue reported an average of 4.4 other symptoms (Given, Given, Azzouz, Kozachik, et al., 2001) and patients in this study reported a mean of 7.4 symptoms including fatigue. This study identifies CRF as a priority symptom associated with greater levels of average symptom severity of the other unpleasant symptoms leading to lower PFS.
Hypothesis 3: Perceived Self-Efficacy for Fatigue Self-Management Mediates Between Cancer-Related Fatigue Severity and Physical Functional Status
The severity from CRF indirectly and directly impacts PFS in persons with cancer. The indirect path is a unique finding showing that PSE for fatigue self-management mediates CRF severity and PFS in persons with cancer as greater CRF severity predicted lower PSE for fatigue self-management, and greater PSE for fatigue self-management predicted greater PFS. Bandura (1997) has identified PSE as a powerful mediator of health promoting behaviors (i.e., symptom self-management) which lead to successful outcome attainment (i.e., PFS). According to both Bandura (1997) and Baron and Kenny (1986), mediators are defined as an internal property of a person that transforms the predictor variable to explain how or why outcomes occur. It would be presumed that that influence is mediated through the fatigue self-management behaviors of the individual, information that was not available for this secondary data analysis. This obvious next step in this research is needed to set the stage for later research examining what interventions can be used to enhance PSE, stimulate effective self-management behaviors, and enhance PFS. What this study does show is that PSE for fatigue self-management is an important part of symptoms experienced by the patient with cancer and should be included in the treatment plan. The nurse in partnership with the patient might implement any one or a combination of PSE for fatigue self-management interventions, including direct mastery experiences, vicarious experiences, use of social and verbal persuasion, and interpreting inferences from physiological and psychological states indicative of personal strengths and vulnerabilities to reach goals (Bandura, 1997). Through the use of PSE-enhancing fatigue self-management interventions, nurses can equip the patient to manage CRF and optimizing PFS. As a result, the findings from this analysis provides a platform for further research to develop efficacy enhancing interventions to optimize symptom control and PFS by addressing CRF severity and PSE for fatigue self-management.
Further Findings
It was also demonstrated through path analysis that patient characteristics (i.e., surgery anytime prior to chemotherapy and comorbid conditions) affected two endogenous variables (i.e., average severity of the other symptoms and PFS) not accounted for in the hypothesized model. Average symptom severity was heightened from the direct influence of undergoing surgery anytime prior to chemotherapy, emphasizing the importance of examining surgical experiences as they influence the total symptom experience and more research is needed to better understand this phenomenon. Second, greater comorbid conditions predicted lower PFS, which is consistent with research that showed in varied cancer populations that patients with greater comorbid conditions scored lower in PFS (Given, Given, Azzouz, Kozachik, et al., 2001). Further research is needed to examine which comorbid conditions have the greatest influence on PFS.
Limitations
This was a secondary analysis, obtaining data from two RCTs, both of which only included persons with cancer undergoing chemotherapy with at least two cycles remaining at the time of enrollment; one also required the presence of either high pain or fatigue while the other required the presence of one symptom. Thus, the findings of this study can be generalized only to cancer patients undergoing chemotherapy and having fatigue or other symptoms, not the general cancer population. Another limitation of this study is the cross-sectional design which precludes determination of temporal effects among the variables examined. However, the measurement within this design was bounded by all persons diagnosed with cancer undergoing chemotherapy with at least two cycles remaining at the time of enrollment. The exact date of onset of chemotherapy as well as the exact date of a surgical procedure was not included in this current requested dataset as a part of a de-identification protocol. The variables surgery prior and surgery during chemotherapy refer to a wide variety of procedures that may or may not be related to cancer treatment and the timing of surgery in relation to the baseline interview is not made specific in the data set used for this analysis. The nature of this analysis was in part exploratory and needs replication through a prospective design.
Conclusion
The theoretical framework presented was developed with the goal of elucidating which patient characteristics impact CRF, how CRF relates to other symptoms, and whether PSE for fatigue self-management mediates the relationship between CRF and PFS. Age, comorbid conditions, and sex were identified as important risk factors in the CRF experience. Additionally, CRF was found to increase the average severity of the other symptoms directly, which affected PFS negatively. Moreover, PSE for fatigue self-management was found to be a mediator that influenced the impact of CRF on PFS positively. Knowledge generated from this study provides the foundation to identify persons at risk for developing CRF, provides data indicating the importance of the treatment of CRF and its effect on the severity of the other symptoms, and it provides the foundation for the need to develop interventions to increase PSE to achieve optimal symptom self-management and PFS in persons with cancer.
Acknowledgments
Supported by (a) National Institutes of Health, National Institute of Nursing Research, Individual Ruth L. Kirschstein National Research Service Award, Grant Number 1F31 NR009621-01A1; Project Title: Fatigue, Self-Efficacy, and Functional Status in Persons with Lung Cancer, Principal Investigator Amy Hoffman; (b) Mary Margaret Walther Cancer Research Fellowship; Behavioral Cooperative Oncology Group. Walther Cancer Institute, Indianapolis, Indiana; (c) Blue Cross Blue Shield of Michigan Foundation, Grant Number 1044.SAP; Project Title: Fatigue, Self-Efficacy, and Functional Status in Persons with Lung Cancer, Principal Investigator Amy Hoffman; (d) Sigma Theta Tau International Honor Society of Nursing, Kappa Epsilon Chapter-At-Large, MI; and (e) John F. Dunkel Scholarship Award, College of Nursing, Michigan State University, 2003 & 2004.
The following data source was used: The Family Home Care for Cancer: A Community-based Model for Symptom Management” (FHCC) project (R01 CA-079280) sponsored by Barbara A. Given, Ph.D., R.N., FAAN, Principal Investigator, and “The Automated Telephone Monitoring for Symptom Management” (ATSM) project (R01 CA-30724) sponsored by Charles W. Given, Ph.D., Principal Investigator.
Contributor Information
Amy J. Hoffman, Kirkhof College of Nursing, Grand Valley State University, Grand Rapids, Michigan.
Alexander von Eye, Psychology Department, Michigan State University, East Lansing, Michigan.
Audrey G. Gift, College of Nursing, Michigan State University, East Lansing, Michigan.
Barbara A. Given, College of Nursing, Michigan State University, East Lansing, Michigan.
Charles W. Given, Department of Family Practice, College of Human Medicine, Michigan State University, East Lansing, Michigan.
Marilyn Rothert, College of Nursing, Michigan State University, East Lansing, Michigan.
References
- Ahlberg K, Ekman T, Gaston-Johansson F, Mock V. Assessment and management of cancer-related fatigue in adults. Lancet. 2003;362(9384):640–650. doi: 10.1016/S0140-6736(03)14186-4. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer facts and figures 2008. 2008 Retrieved February 2, 2008, from http://www.cancer.org/docroot/STT/content/STT_1x_Cancer_Facts_and_Figures_2008.asp http://davidakenny.net/cm/mediate.htm#WIM.
- Bandura A. Self-efficacy: The exercise of control. New York, NY: W.H. Freeman and Company; 1997. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social-psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Cooley ME, Short TH, Moriarty HJ. Symptom prevalence, distress, and change over time in adults receiving treatment for lung cancer. Psycho-Oncology. 2003;12(7):694–708. doi: 10.1002/pon.694. [DOI] [PubMed] [Google Scholar]
- Dodd M, Janson S, Facione N, Faucett J, Froelicher ES, Humphreys J, et al. Advancing the science of symptom management. Journal of Advanced Nursing. 2001;33(5):668–676. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncology Nursing Forum. 2001;28(3):465–470. [PubMed] [Google Scholar]
- Doorenbos A, Given B, Given C, Verbitsky N. Physical functioning: Effect of behavioral intervention for symptoms among individuals with cancer. Nursing Research. 2006;55(3):161–171. doi: 10.1097/00006199-200605000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorenbos AZ, Given CW, Given B, Verbitsky N. Symptom experience in the last year of life among individuals with cancer. Journal of Pain and Symptom Management. 2006;32(5):403–412. doi: 10.1016/j.jpainsymman.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller LS, Lev EL, Gejerman G, Colella J, Esposito M, Lanteri V, et al. Prospective study of quality of life of patients receiving treatment for prostate cancer. Nursing Research. 2006;55(2 Suppl):S28–S36. [PubMed] [Google Scholar]
- Gift AG. Symptom clusters related to specific cancers. Seminars In Oncology Nursing. 2007;23(2):136–141. doi: 10.1016/j.soncn.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Gift AG, Jablonski A, Stommel M, Given CW. Symptom clusters in elderly patients with lung cancer. Oncology Nursing Forum. 2004;31(2):203–212. doi: 10.1188/04.ONF.202-212. [DOI] [PubMed] [Google Scholar]
- Gift AG, Stommel M, Jablonski A, Given C. A cluster of symptoms over time in patients with lung cancer. Nursing Research. 2003;52(6):393–400. doi: 10.1097/00006199-200311000-00007. [DOI] [PubMed] [Google Scholar]
- Given CW, Given B, Azzouz F, Kozachik S, Stommel M. Predictors of pain and fatigue in the year following diagnosis among elderly cancer patients. Journal of Pain and Symptom Management. 2001;21(6):456–466. doi: 10.1016/s0885-3924(01)00284-6. [DOI] [PubMed] [Google Scholar]
- Given B, Given C, Azzouz F, Stommel M. Physical functioning of elderly cancer patients prior to diagnosis and following initial treatment. Nursing Research. 2001;50(4):222–232. doi: 10.1097/00006199-200107000-00006. [DOI] [PubMed] [Google Scholar]
- Given B, Given CW, McCorkle R, Kozachik S, Cimprich B, Rahbar MH, et al. Pain and fatigue management: Results of a nursing randomized clinical trial. Oncology Nursing Forum. 2002;29(6):949–956. doi: 10.1188/02.ONF.949-956. [DOI] [PubMed] [Google Scholar]
- Grant JS, Davis LL. Selection and use of content experts for instrument development. Research in Nursing & Health. 1997;20(3):269–274. doi: 10.1002/(sici)1098-240x(199706)20:3<269::aid-nur9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Gupta D, Lis CG, Grutsch JF. The relationship between cancer-related fatigue and patient satisfaction with quality of life in cancer. Journal of Pain and Symptom Management. 2007;34(1):40–47. doi: 10.1016/j.jpainsymman.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Medical Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Ahles TA, Porter LS, Sutton LM, McBride CM, Pope MS, et al. The self-efficacy of family caregivers for helping cancer patients manage pain at end-of-life. Pain. 2003;103(1-2):157–162. doi: 10.1016/s0304-3959(02)00448-7. [DOI] [PubMed] [Google Scholar]
- Kenny D. Multiple factor model. 1998 Retrieved May 3, 2008, from http://davidakenny.net/cm/mfactor.htm.
- Kline R. Principles and practice of structural equation modeling. 2nd. New York: The Guilford Press; 2005. [Google Scholar]
- Leidy N. Functional status and the forward progress of merry-go-rounds: Toward a coherent analytical framework. Nursing Research. 1994;43(4):196–202. [PubMed] [Google Scholar]
- Lenz ER, Pugh LC, Milligan RA, Gift A, Suppe F. The middle-range theory of unpleasant symptoms: An update. ANS: Advances in Nursing Science. 1997;19(3):14–27. doi: 10.1097/00012272-199703000-00003. [DOI] [PubMed] [Google Scholar]
- Lev EL, Daley KM, Conner NE, Reith M, Fernandez C, Owen SV. An intervention to increase quality of life and self-care self-efficacy and decrease symptoms in breast cancer patients. Scholarly Inquiry for Nursing Practice. 2001;15(3):277–294. [PubMed] [Google Scholar]
- Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy In people with arthritis. Arthritis and Rheumatism. 1989;32(1):37–44. doi: 10.1002/anr.1780320107. [DOI] [PubMed] [Google Scholar]
- Lorig K, Ritter PL, Plant K. A disease-specific self-help program compared with a generalized chronic disease self-help program for arthritis patients. Arthritis and Rheumatism. 2005;53(6):950–957. doi: 10.1002/art.21604. [DOI] [PubMed] [Google Scholar]
- Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Cooper BA, Paul SM, Dodd M, Lee K, Aouizerat BE, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: A cluster analysis. Oncology Nursing Forum. 2006;33(5):E79–E89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- Montazeri A, Gillis CR, McEwen J. Quality of life in patients with lung cancer: A review of literature from 1970 to 1995. Chest. 1998;113(2):467–481. doi: 10.1378/chest.113.2.467. [DOI] [PubMed] [Google Scholar]
- Motl RW, Snook EM, McAuley E, Gliottoni RC. Symptoms, self-efficacy, and physical activity among individuals with multiple sclerosis. Research in Nursing & Health. 2006;29(6):597–606. doi: 10.1002/nur.20161. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Cancer-related fatigue version 2.2007. 2007 Retrieved July 30, 2007, from http://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf.
- Raykov T, Marcoulides G. A first course in structural equation modeling. 2nd. Mahwah, NJ: Lawrence Erlbaum Associates; 2006. [Google Scholar]
- Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- Shively M, Smith TL, Bormann J, Gifford AL. Evaluating self-efficacy for HIV disease management skills. AIDS and Behavior. 2002;6(4):371–379. [Google Scholar]
- Stone P, Ream E, Richardson A, Thomas H, Andrews P, Campbell P, et al. Cancer-related fatigue--a difference of opinion? Results of a multicentre survey of healthcare professionals, patients and caregivers. European Journal of Cancer Care. 2003;12(1):20–27. doi: 10.1046/j.1365-2354.2003.00329.x. [DOI] [PubMed] [Google Scholar]
- von Eye A, Schuster C. Regression analysis for social sciences. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and interpretation guide. Boston, MA: Nimrod Press; 1993. [Google Scholar]