Abstract
Background. Proteinuria is a candidate surrogate end point for randomized controlled trials (RCTs) in chronic kidney disease (CKD). There is a reasonably sound biological basis for this hypothesis, but only preliminary empirical evidence currently exists.
Methods. A systematic review and creation of a patient-level dataset of randomized controlled trials (RCTs) in CKD that reported changes in proteinuria and assessed progression of kidney disease as defined by dialysis, transplantation, death, or changes in GFR or creatinine were performed.
Results. Systematic review. Seventy RCTs met the eligibility criteria; 17 eligible RCTs contained analyses of proteinuria as a predictor of outcomes; 15 RCTs concluded that greater proteinuria was associated with adverse outcomes. A majority were studies of diabetic or hypertensive kidney disease and tested renin–angiotensin system blockade. Definitions of predictor and outcome variables were too variable to conduct a meta-analysis of group data. Database creation. Over 4 years was required to create the patient-level dataset. The final dataset included 34 studies and > 9000 patients with a variety of CKD types and interventions.
Conclusions. There are a relatively small number of RCTs designed to rigorously test therapies for kidney disease progression. Current analyses of change in proteinuria as a predictor of CKD progression are heterogeneous and incomplete, indicating further evaluation in a pooled individual patient-level database is necessary to advance knowledge in this field.
Keywords: chronic kidney disease, proteinuria, randomized clinical trials, surrogate markers, systematic review
Introduction
Many chronic kidney diseases worsen over time by transitions through a sequence of stages, regardless of the specific cause or rate of progression. Recent guidelines and public health campaigns have focused on early detection and treatment of chronic kidney disease based on the rationale that treatments initiated early in the disease course can slow progression of the disease and delay onset of kidney failure.
Kidney failure, defined as the initiation of dialysis or transplantation and doubling of serum creatinine, is an accepted end point for kidney disease progression in clinical trials. However, because many chronic kidney diseases progress slowly, a long duration of follow-up is required, increasing the expense and complexity of trials and leading to a paucity of therapies to slow progression. The use of surrogate end points may accelerate testing of new therapies, particularly in earlier stages of CKD.
Proteinuria is commonly considered as a candidate for a surrogate end point for kidney disease progression. There is a reasonably sound biological basis for this hypothesis [1,2], but to date, there is only preliminary empirical evidence [3–9]. A rigorous evaluation of the surrogacy of proteinuria will avoid erroneous conclusions in instances where the effect of the intervention on proteinuria does not predict the effect on the clinical end point. In May 2008, the National Kidney Foundation (NKF) and the Food and Drug Administration (FDA) co-sponsored a scientific workshop on ‘proteinuria as a surrogate outcome in chronic kidney disease’, with the objectives to (i) evaluate the strengths and limitations of criteria for assessment of proteinuria as a potential surrogate end point in CKD, (ii) explore the strengths and limitations of available data on proteinuria as a potential surrogate end point, focusing on specific clinical circumstances and therapeutic agents, and (iii) delineate what else needs to be done to evaluate proteinuria as a potential surrogate end point [10]. Preceding this conference, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) charged a research group, Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), to undertake a formal evaluation of proteinuria as a surrogate marker [11]. CKD-EPI will accomplish the formal evaluation using a systematic review and a pooled individual patient-level meta-analysis of randomized controlled trials (RCT). The purpose of this manuscript is to describe, first, the results of the systematic review of prior analyses addressing this question as proteinuria as a surrogate marker for kidney disease progression, and second, the formation of the collaborative group and mechanics of development of a pooled individual patient-level dataset to be used in the formal evaluation of proteinuria as a surrogate marker, the results of which will be published separately.
Materials and methods
Literature search
We conducted a comprehensive search of the literature in MEDLINE on 7 April 2005 and updated it on 15 May 2007 to identify articles to address the following research question: ‘Does the short-term effect of treatment on changes in proteinuria predict the long-term effect of treatment on changes in GFR during randomized controlled trials in chronic kidney disease?’ The search strategy retrieved articles using the following key or text words restricted to RCTs: kidney disease, chronic renal insufficiency, chronic kidney disease, renal disease, IgA nephropathy, lupus nephritis, diabetic nephropathy, glomerular disease, polycystic kidney disease, kidney transplant, focal sclerosis, membranous nephropathy, and proteinuria or albuminuria. All abstracts were screened according to the criteria listed in Table 1. Abstracts were excluded in instances where they clearly did not meet one of the criteria. Articles were obtained for the remaining abstracts and screened again against the inclusion criteria. The above search was repeated restricting to meta-analyses, and the resulting studies were searched for additional RCTs. Finally, studies were added from the general knowledge of CKD-EPI investigators and collaborators.
Table 1.
Inclusion criteria for literature search
| 1. Population: CKD (as defined by GFR < 60 mL/min/1.73 m2 or microalbuminuria) |
| 2. Design: randomized controlled trial (RCT) |
| 3. Intervention: any |
| 4. Comparator: any |
| 5. Outcome: dialysis, transplant, death or serum creatinine. At least one person in the study had progression of kidney disease defined by 50% increase in creatinine in at least one patient |
| 6. Sample size: > 100 in non-glomerular disease except IgA nephropathy (n > 25) |
| 7. Measurements: urine protein at baseline and at 6–12 months; GFR or GFR estimate or serum creatinine at baseline and serially in follow-up |
| 8. Duration: at least 1 year after the second measure of proteinuria |
Systematic review of prior analyses
The resulting papers from the literature search were examined to determine if analyses of proteinuria as a predictor of kidney disease progression were performed. To determine if such analyses were reported separately, we conducted an additional MEDLINE search for each study with > 150 participants. Analyses were subdivided into four types depending upon the use of proteinuria as the predictor of outcomes: (i) Baseline—level of proteinuria at baseline, (ii) Intermediate change—change from proteinuria at baseline to an intermediate time point, (iii) Residual—level of proteinuria at an intermediate time point, and (iv) Prentice/Freedman—comparison of treatment effect with and without adjustment for change in proteinuria, where loss of a significant treatment effect after adjustment indicates support for proteinuria as a surrogate marker [12].
Creation of a patient-level pooled database
Obtaining data
Once a study was determined to meet the inclusion criteria (Table 1), the study’s primary author was invited to join CKD-EPI as a collaborator. Invitations were sent initially via electronic mail. Follow-up messages were sent if no response was received. If collaborators expressed potential interest, a subsequent message was sent to explain the specifics of the overall project, study design, and analytical methods as well as publication and ancillary study policies. After agreement to participate was received, a discussion about the required variables and definitions, timing, and method for data transfer occurred.
Pooled dataset development.
Once data were received from a study, variables that were to be included in the pooled dataset were selected, and where necessary, units of numeric variables were converted from SI to traditional units; any character variables, such as sex and race, were changed to numeric variables (e.g. 0 = male and 1 = female) and renamed to be consistent with the pooled dataset. Once a dataset was in acceptable format, the output’s descriptive statistics were gathered, and outliers were manually checked and compared with the original study publication. Inquiries were sent to the collaborators for questions that arose in the data.
Baseline was defined as the date closest to randomization where not otherwise specified. The diagnosis of aetiology of kidney disease and diabetes was as assigned based on the individual patient-level data or explicit study inclusion criteria. The specific type and collection method for urine protein were recorded. Hypertension was defined by diagnosis provided, taking antihypertensive medication or baseline blood pressure > 140/90 mmHg. If only the haematocrit was provided, then the haemoglobin was estimated by dividing haematocrit by 3. Information on assignment to treatment or control arm, and specific interventions given in each arm was included, including both treatments included in a factorial design. In some studies, data on non-study medications were available, but only information on angiotensin-converting enzyme inhibitors or renin–angiotensin receptor blockers were retained in the study datasets.
Follow-up variables included in the datasets consisted of urine protein, serum creatinine and blood pressure. Among the included studies, urine protein was measured in a 24-h collection or a spot sample, as total protein or albumin, and given as a total value, a concentration, or as a protein to creatinine ratio.
Outcomes included doubling of serum creatinine, ESRD (dialysis or transplant) or death. Doubling of serum creatinine was either provided in the dataset or calculated from the follow-up creatinine. Administrative censoring data or the last follow-up dates were included.
Data pooling.
After data cleaning, studies were combined into pooled datasets suitable for analysis. Five pooled datasets were created: baseline, follow-up visits (urine protein, serum creatinine and blood pressure) and outcomes. The baseline dataset included baseline demographic information, clinical information and laboratory values. The follow-up datasets included multiple observations per patient and included serial follow-up measurements of blood pressure, urine protein, and serum creatinine with time from randomization specified for each measurement. The outcome dataset included information on administrative censoring date, doubling of serum creatinine, ESRD, and death and dates for each event.
Results
Literature search
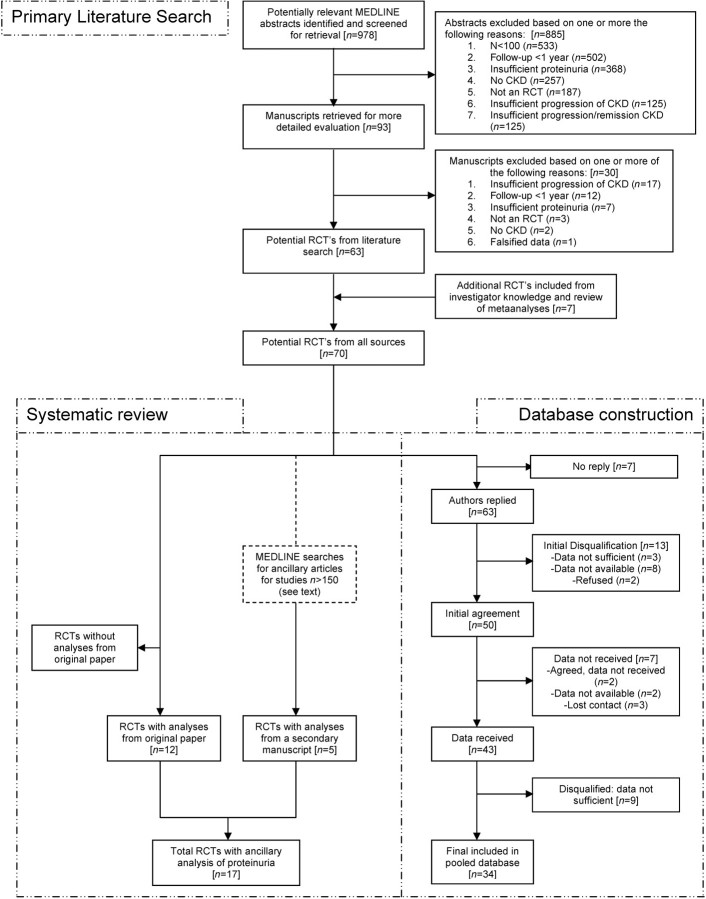
Based on review of abstracts, a total of 93 RCTs met the inclusion criteria (Figure 1, upper half). One study is now known to have been falsified, and was removed from further consideration [13]. Manuscripts were reviewed, and additional RCTs were included based on investigators’ knowledge. At the end of the selection process, 70 RCTs met the inclusion criteria (Table 2, first column). Of the 23 205 participants, 9811 (42%) were included in trials of diabetic kidney disease, 6737 (23%) in non-diabetic kidney disease and 3257 (14%) in transplant kidney disease. Of the 70 studies, angiotensin-converting enzymes (ACE) inhibitors or angiotensin receptor blockers (ARB) (n = 23) and immunosuppressives (n = 27) were the most common interventions. In addition, low protein diets (n = 5), other antihypertensives (n = 4), varying blood pressure goals (n = 2), and new investigational therapies (n = 3) are also represented.
Fig. 1.
Steps involved in the primary literature search, systemic review and database construction.
Table 2.
Number of patients and studies by disease for systematic review and creation of patient-level pooled database
| Disease | Requested Subjects (studies) | Initial agreement Subjects (studies) | Data received Subjects (studies) | Final Subjects (studies) |
|---|---|---|---|---|
| Diabetic kidney disease | ||||
| Type 1 | 409 (1) | 409 (1) | 409 (1) | 409 (1) |
| Type 2 | 9402 (6) | 9300 (5) | 8610 (4) | 3620 (3) |
| Subtotal | 9811 (7) | 9709 (6) | 9019 (5) | 4029 (4) |
| Non-diabetic kidney disease | ||||
| IgA | 807 (12) | 593 (9) | 593 (9) | 387 (6) |
| Lupus | 481 (7) | 315 (4) | 315 (4) | 228 (3) |
| Membranous | 581 (9) | 514 (8) | 391 (6) | 334 (6) |
| FSGS | 106 (2) | 49 (1) | 49 (1) | 11 (1) |
| MPGN | 59 (1) | 0 | 0 | 0 |
| Hypertension | 1094 (1) | 1094 (1) | 1094 (1) | 1094 (1) |
| Amyloid | 183 (1) | 183 (1) | 0 | 0 |
| ADPKD | 89 (1) | 89 (1) | 89 (1) | 0 |
| Various | 6737 (23) | 3678 (16) | 3492 (15) | 3030 (13) |
| Subtotal | 10 137 (57) | 6518 (41) | 6023 (37) | 5084 (30) |
| Transplant | ||||
| Transplant | 3257 (6) | 814 (3) | 581 (1) | 0 |
| Subtotal | 3257 (6) | 814 (3) | 581 (1) | 0 |
| Total | 23 205 (70) | 17 038 (50) | 15 623 (43) | 9113 (34) |
The number of authors initially approached is indicated in the ‘Requested’ column. Authors who responded that were interested in participating in the collaboration are indicated in the ‘Initial agreement’ column. We only received data on a subset of these studies, indicated in the ‘Data received’ column, and all of the data received was not eligible for inclusion; thus, the final dataset is indicated in the ‘Final’ column. In all columns, ‘Subjects’ refers to the number of participants, and ‘studies’ refers to the number of studies.
Systematic review of prior analyses
Figure 1 (lower left) describes the additional steps required for the systematic review. Of 70 eligible RCTs, 12 contained analyses of proteinuria as a predictor of CKD progression in the original manuscript. In a second MEDLINE search for relevant analyses that were published separately, we identified 1510 abstracts, of which 25 manuscripts were retrieved for further review. From these, five additional RCTs were included in which an analysis of proteinuria was reported in the ancillary paper but not in the original manuscript (Table 3).
Table 3.
Systematic review of previous analyses relating proteinuria to kidney disease progression
| Author, year, reference (study name) | Number | Population | Treatment | Protein modality | Control | Observation |
|||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Intermediate change | Residual | Prentice/Freedman analysis | ||||||
| Dember 2007 [29] | 183 | Non-diabetic (glomerular disease) | Eprodisate | 24-h P | Placebo | ○ | |||
| Hou 2006 [26] | 422 | Non-diabetic (various) | Benazapril | 24-h P | Placebo | ● | |||
| Hogg 2006 [14] | 96 | Non-diabetic (glomerular disease) | Fish oil/prednisone | Spot A | Placebo | ● | |||
| Ruggenenti 2005 [15] (REIN-2) | 338 | Non-diabetic (various) | Intensive BP | 24-h P | Conventional BP | ● | |||
| Lea 2005 [5] (AASK) | 1094 | Non-diabetic (hypertensive) | Ramipril or metoprolol/intensive BPa | 24-h P | Amlodipine/conventional BP | ● | ● | ||
| Bolton 2004 [27] | 690 | Diabetic | Pimagedine | Placebo | ● | ||||
| Atkins 2005 [16] Hunsicker 2004 [8] (IDNT) | 1715 | Diabetic | Irbesartan or amlodipine | 24-h P | Placebo | ● | ● | ● | |
| Praga 2003 [17] | 44 | Non-diabetic (glomerular disease) | Enalapril | 24-h P | Conventional BP except ACEI | ○ | ● | ||
| De Zeeuw 2004 [3] | 1513 | Diabetic | Losartan | Spot A | Placebo | ● | ● | ● | ● |
| Shahinfar 2002 [18] (RENAAL) | 1513 | ||||||||
| Pozzi 1999 [28] | 86 | Non-diabetic (glomerular disease) | Methylprednisolone | 24-h P | Supportive therapy | ● | ● | ||
| van Essen 1997 [19] | 103 | Non-diabetic (various) | Enalapril/atenolol | 24-h P | β-blocker | ● | |||
| Ruggenenti 2003 [20] | 273 | Non-diabetic (various) | Ramipril | 24-h P | Placebo; conventional BP | ● | ● | ● | ● |
| GISEN 1997 [21] (REIN) | 166 | ||||||||
| Ihle 1996 [22] | 70 | Non-diabetic (various) | Enalapril | 24-h P | Placebo | ● | |||
| Breyer 1996 [23] (CSG) | 409 | Diabetic | Captopril | 24-h P | Placebo | ● | |||
| Hunsicker 1997 [4] | 585 | Non-diabetic (various) | Low protein diet/intensive BPa | 24-h P | Usual protein diet/conventional BP | ● | ● | ||
| Peterson 1995 [24] (MDRD Study A) | 255 | ● | ● | ||||||
| Hunsicker 1997 [4] | 585 | Non-diabetic (various) | Very low protein diet/intensive BPa | 24-h P | Low protein diet/conventional BP | ● | ● | ||
| Peterson, 1995 [24] (MDRD Study B) | 255 | ● | ● | ||||||
| D’Amico 1994 [25] | 128 | Non-diabetic (various) | Low protein diet | 24-h P | Controlled protein diet | ● | |||
Filled circles, significant; unfilled circles, not significant; 24-h P, 24-h measurement of total urinary protein excretion over 24 h; 24-h A, urinary albumin excretion over 24 h; spot P, urine albumin to creatinine ratio from a single urine specimen.
Factorial study design.
Baseline. A total of 13 individual RCTs [3–5,8,14–25] examined the association of baseline proteinuria to kidney disease outcomes, with proteinuria quantified as total urine protein, log-transformed urine protein, protein to creatinine ratio, and albumin to creatinine ratio. Analyses were heterogeneous and included both linear regression of GFR slope and time-dependent outcomes. Twelve of the 13 analyses found a statistically significant relationship between baseline proteinuria and outcome [3–5,8,14–16,18–25]. The exception was one study testing enalapril in 44 patients with IgA nephropathy [17].
Intermediate change. Ten studies examined intermediate change in proteinuria [3–5,8,16–18,20,21,24,26–28]. Here too, the measures of proteinuria varied (i.e. 24-h urine protein and albumin to creatinine ratio) as did time intervals (e.g. absolute change over 4 months, quartile decrease in proteinuria over 6 months, or percent decrease). Analyses included both linear regression of GFR slope and time-dependent outcomes. All published analyses of intermediate change in proteinuria found that greater reduction in proteinuria was associated with a favourable effect on the outcome measure.
Residual. Two studies examined residual proteinuria [3,18,20,21]. One study examined 24-h total urine protein at 3 months [20,21], while the other examined spot albumin to creatinine ratio at 6 months. Both analyses found that greater reduction in proteinuria was associated with a favourable effect on the outcome measure.
Prentice–Freedman. Five studies employed the Prentice–Freedman type of analysis [3,8,16,18,20,21,28,29], examining treatment effect with and without adjustment for proteinuria. Time intervals varied in that two studies adjusted for baseline proteinuria [8,29], while one study each adjusted for 6-month change [8,28], time-dependent change [18], and 6-month residual proteinuria [3]. Of these five studies, four found that the main treatment effect lost statistical significance when adjusted for its measure of proteinuria [3,8,16,18,20,21,28]. The exception was a study testing eprodisate in 183 patients with AA amyloidosis with a median baseline proteinuria of 3.1 g/day and a composite time-dependent outcome followed up for 24 months, where the treatment effect was maintained after adjustment for baseline urine protein [29].
Creation of a patient-level pooled database
Figure 1 (lower right) describes additional steps for creation of the patient-level pooled database.
Process of data transfer
The majority of authors were willing to collaborate, although in some instances, the process of obtaining agreement required several iterations. Altogether, the time from initial contact to agreement to participate ranged from immediately to 6 months, and multiple contacts were often needed with some ranging up to 16.
Data transfer required even more intense follow-up with time from agreement to data transfer ranged from 9 to 108 weeks, and number of contacts ranged from 3 to 31, with a mean of 11. Of the 70 studies contacted, we did not receive a reply from seven authors [28,30–35], 13 studies were disqualified after initial discussion with the authors [25,36–47], the data was never received for 7 studies despite initial agreement [27,29,42,48–51] and 9 studies were disqualified after data were received for insufficient data [14,52–59], leaving 34 studies included in the dataset (Table 2, second, third and fourth columns) [15,17,19,22,26,42,43,60–84]. Overall, more than 3 years have passed since the first author contact to the transfer of the last dataset. The final dataset included studies published from 1989 to 2007, multi-centre (n = 27) and single centre (n = 7), with a range of participants from 11 to 1715, per each study (Table 4).
Table 4.
Characteristics of studies included in the pooled dataset
| Disease | Study/author/reference | Year | Site | Number | Intervention in treatment arm | Intervention in control arm | Protein modality | Baseline protein (mean) |
|---|---|---|---|---|---|---|---|---|
| Type 1 DM | CSG [61] | 1993 | MC | 409 | ACEI | Placebo | 24-h P | 2.8 |
| Type 2 DM | IDNT [60] | 2001 | MC | 1715 | ARB | CCB, placebo | 24-h P, 24-h A | 2.9 |
| RENAAL [63] | 2001 | MC | 1513 | ARB | Placebo | Spot A | 1.25 g/g | |
| ABCDa [62] | 2000 | MC | 392 | Intensive BP | Moderate BP | 24-h A | 171 μg/min | |
| IgA | Donadio [64] | 1999 | MC | 97 | Fish oil | Placebo | 24-h P | 2.9 |
| Donadio [65] | 2001 | MC | 73 | High-dose fish oil | Low-dose fish oil | 24-h P | 1.7 | |
| Praga [17] | 2003 | SC | 44 | ACEI | Standard medical therapy | 24-h P | 1.9 | |
| Maes [66] | 1999 | SC | 34 | ACEI; MMF | Placebo | 24-h P | 1.7 | |
| Appel [67] | 2003 | SC | 30 | MMF | Placebo | 24-h P | 2.7 | |
| HKVIN [68] | 2006 | MC | 109 | ARB | Placebo | 24-h P | 2.1 | |
| Lupus | Lupus CSG [69] | 1992 | MC | 83 | Prednisone CYC | Prednisone CYC + plasmapheresis | 24-h P | 5.5 |
| Chan [70] | 2005 | MC | 62 | MMF prednisolone | CTX-AZA prednisolone | 24-h P | 5.3 | |
| Euro lupus [71] | 2002 | MC | 83 | High-dose CYC | Low-dose CYC | 24-h P | 3 | |
| Membranous | Ponticelli AJKD [72] | 2006 | MC | 31 | Steroids chlorambucil or CYC | Steroids | 24-h P | 6.1 |
| Ponticelli JASN [43] | 1997 | MC | 91 | Steroids + Chlorambucil | Steroids + cyclophosphamide | 24-h P | 7.4 | |
| Ponticelli NEJM [74] | 1992 | MC | 76 | Steroids + chlorambucil | Steroids alone | 24-h P | 7.3 | |
| Ponticelli NEJM [73] | 1989 | MC | 77 | Steroids + chlorambucil | Supportive therapy | 24-h P | 5.8 | |
| Cattranb [42] | 2001 | MC | 11 | Cyclosporine | Placebo | 24-h P | 9.3 | |
| Spain, Praga [76] | 2007 | SC | 48 | Tacrolimus | Placebo | 24-h P | 7.8 | |
| FSGS | Cattranc [75] | 1999 | MC | 11 | Cyclosporine | Placebo | 24-h P | 7.8 |
| Hypertension | AASK [77] | 2002 | MC | 1094 | ACEI intensive BP | CCB BB conventional BP | 24-h P | 0.5 |
| Various | Hannedouche [78] | 1994 | MC | 99 | ACEI | BB | 24-h P | 2.2 |
| Ihle [22] | 1996 | SC | 70 | ACEI | Placebo | 24-h P | 2.2 | |
| Brenner [79] | 1993 | MC | 112 | ACEI | Placebo | 24-h P | 2.3 | |
| Toto [79] | 1993 | MC | 124 | ACEI | Placebo | 24-h P | 0.7 | |
| Kamper [80] | 1992 | SC | 69 | ACEI | Standard medical therapy | 24-h A | 0.02 | |
| Maschio [81] | 1996 | MC | 583 | ACEI | Placebo | 24-h P | 1.8 | |
| Zuchelli [82] | 1992 | MC | 121 | ACEI | CCB | 24-h P | 1.6 | |
| Hou [26] | 2006 | SC | 224 | ACEI | Placebo | 24-h P | 1.6 | |
| REIN [83] | 1999 | MC | 351 | ACEI | Placebo | 24-h P | 3.6 | |
| REIN 2 [15] | 2005 | MC | 333 | ACEI (conventional) | ACEI + CCB (intensive) | 24-h P | 2.9 | |
| van Essen [19] | 1997 | MC | 103 | ACEI | BB | 24-h P | 3.3 | |
| MDRD-A [84] | 1997 | MC | 585 | Intensive BP, low protein | Conventional BP, usual protein | 24-h P | 0.9 | |
| MDRD-B [84] | 1997 | MC | 255 | Intensive BP, very low protein | Conventional BP, low protein | 24-h P | 1.4 |
Units for urine protein are g/24 h.
This is a subset of the 470 study participants with microalbuminuria.
This is a subset of the 51 study participants.
This is a subset of the 54 study participants.
MC, multiple centre; SC, single centre; CCB, calcium channel blocker; CTX-AZA, cyclophosphamide–azathioprine; BB, beta-blocker; 24-h P, 24-h measurement of total urinary protein excretion over 24 h; 24-h A, urinary albumin excretion over 24 h; Spot A, urine albumin to creatinine ratio from a single urine specimen.
Database description
Table 5 shows the variables that were provided according to number of participants and number of studies. Most studies did not provide all the requested variables.
Table 5.
Available data for inclusion in the pooled dataset
| Variable | Studies | Patients |
|---|---|---|
| Study population | 34 | 9113 |
| Baseline variables | ||
| Age | 34 | 9051 |
| Sex | 34 | 9111 |
| Race | 33 | 9087 |
| Kidneydiseasediagnosis | 34 | 9113 |
| Duration of kidney disease | 15 | 3329 |
| Diabetes status | 25 | 8525 |
| HTN statusa | 32 | 8678 |
| Systolic BPa | 30 | 8742 |
| Diastolic BPa | 30 | 8742 |
| Height | 25 | 7609 |
| Weight | 29 | 8591 |
| BMIa | 25 | 7597 |
| Medicationsa | 26 | 8629 |
| Treatment assignmenta | 34 | 9113 |
| BUN | 13 | 6604 |
| Serum creatinine | 34 | 9008 |
| GFR (measured)a | 9 | 2340 |
| Creatinine clearance (measured) | 14 | 3229 |
| Urine protein measurementsa | 34 | 9112 |
| Haemoglobina | 16 | 6346 |
| Albumin | 19 | 7150 |
| Glucose | 34 | 9112 |
| Calcium | 12 | 5790 |
| Phosphate | 12 | 6784 |
| Potassium | 7 | 3173 |
| PTH | 1 | 224 |
| CRP | 2 | 258 |
| Cholesterol | 17 | 6889 |
| HDL | 10 | 5271 |
| LDL | 11 | 4810 |
| Triglycerides | 16 | 6376 |
| Follow-up | ||
| Systolic blood pressure | 30 | 8467 |
| Diastolic blood pressure | 30 | 8472 |
| Serum creatinine | 34 | 8970 |
| Urine protein | 34 | 8577 |
| Measured GFR | 9 | 1832 |
| Measured creatinine clearance | 15 | 3842 |
Bold indicates variables included in the dataset.
Variables provided by the study authors needed to be defined, calculated from existing data or selected from multiple potential choices.
Three studies were multifactorial in design with all three studies including a blood pressure arm as well as a second intervention (drug class of diet) [62,77,84]. A total of 16 studies used placebo in the control arms [22,26,42,60,61,63,64,66–68,75,76,79,81,83], whereas 18 studies used a different intervention [15,17,19,43,62,65,69–74,77,78,80,82,84] (Table 4).
Urine protein was measured using 24-h measurement of total urine protein in 30 studies [15,17,19,22,26,42,43,61,64–79,81–84], and 24-h measurement of total urine albumin was used in two studies [62,80] (Table 4). One study [60] assayed both urine protein and urine albumin in a 24-h urine collection, while another used spot urine albumin as the primary measurement, but a subset of participants collected a 24-h urine collection, and total protein was measured [63].
For most studies, hypertension status was defined as provided in the dataset, except in one study [71] where hypertensive status was defined as systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mmHg. In 11 studies, baseline sitting measurements of blood pressure were used [19,22,26,61,77–83]. BMI was calculated in all studies except for two that had already provided the calculated value. Haemoglobin was estimated by dividing haematocrit by 3 in 10 studies [19,22,26,77–83].
Some of the original study datasets were organized into one observation per visit with multiple observations per patient [4,5,19,22,24,42,60–65,69,75,78–82,85], while other study datasets were organized into one observation per visit, with multiple observations per patient [15,17,26,43,66–68,70–74,76]. The later form datasets were converted into one observation per visit.
Determination of the last follow-up time differed among studies. Of the 34 studies included, the study end date was determined using an administrative censoring date (n = 4) [60,63,77,84], the last follow-up date or end date (n = 9) [43,64,65,67–69,72–74], or the last available serum creatinine value or outcome in comparison with randomization date (n = 3) [17,26,42,62,66,75]. Four studies [15,61,70,76] did not have either an administrative censoring date or the last follow-up date.
Discussion
In principle, the rationale for the use of surrogate end points is that they may facilitate the conduct of clinical trials and identification of novel therapies. Surrogates can often be measured earlier, more easily or frequently and with higher precision, are less subject to competing risks, and are less affected by other treatment modalities, all of which allow for reduced sample size requirements, less expensive trials and faster decision making. Surrogate end points can be used in a moderately low-risk manner in earlier phases of development of new interventions, as exploratory subgroup analyses, extension of established findings to related patient populations with less severe disease, or with greater risk in extension of established findings to related interventions and in the establishment of the benefit of new interventions. Many in the nephrology community have suggested proteinuria as changes in proteinuria occur earlier than changes in GFR [10]. However, while proteinuria is indeed easy to measure, complete 24-h urine collections are difficult to obtain, and levels may be biased by exercise and treatments. The goal of the CKD-EPI Collaboration is to determine if early reduction in proteinuria can be used as a surrogate marker for hard clinical end points, and as we previously suggested, the creation of a joint dataset of multiple RCT databases is the ideal statistical approach of evaluating change in proteinuria as surrogate [11]. In this publication, we summarize the literature search, evaluate the current literature on the topic, and describe the process by which this joint dataset was created. Analyses underway will describe the results of our evaluation to empirically test proteinuria as a surrogate marker.
Literature search
The treatment of kidney disease is a fundamental problem in nephrology, yet our review of the literature identified only a relatively small number of RCTs designed to rigorously test therapies for kidney disease progression. The majority of studies were of small sample size, and despite the chronic nature of the disease, most studies had less than 1-year follow-up. We were able to identify studies of a variety of CKD aetiologies and therapies; however, some areas are less well represented, and the majority of treatments involve blockade of the renin–angiotensin system. In part, the limitation reflects the lack of studies in the field, but it also reflects the limitations of variables collected among the available studies. For instance, we were able to find studies of transplant patients, but none measured proteinuria or had data available that could be shared with the collaboration. It is likely that in part, the lack of available studies has been due to little enthusiasm by industry for investigations into novel therapeutics for kidney disease because of the need for long and expensive trials.
Systematic review of prior analyses
In our systematic review of the published literature, the majority of analyses found that increasing proteinuria at any time point is associated with kidney disease progression, although we recognize the possibility of publication bias. Despite the apparent homogeneity of the results, these individual associations are not sufficient to determine proteinuria as a surrogate marker. First, these analyses were absent in a majority of studies. Second, as mentioned above, the vast majority of analyses are of patients with diabetic or hypertensive kidney disease, in trials of renin–angiotensin system blockade. Third, there was much heterogeneity in analytic approaches used, with variable definitions for predictor and outcome variables, making it difficult to compare results from individual studies. Until there is consensus on the definition of an early change in proteinuria, it will not be possible to combine the results of clinical trials. Finally, the few studies where proteinuria did not predict CKD progression or fulfil the Prentice–Freedman approach serve as an important reminder that proteinuria as a surrogate marker candidate may depend on its context or pathophysiology [10]. For instance, in amyloidosis, proteinuria may be due to overflow of light chains and not correlated with the degree of glomerular kidney damage [29]. It is thus evident that the pooled dataset will improve generalizability and may assist in explaining the heterogeneity of the current knowledge by allowing for greater flexibility in defining predictors and outcomes, although we will still be limited by the measures collected in the original trials.
Patient-level pooled database
In general, we were impressed that most investigators were very willing to share data and to work with us to clean the dataset, even in the absence of monetary compensation. However, each study defined variables differently and collected different information, which contributed to the time-consuming and labour-intensive nature of this work. The pooled dataset therefore does not contain complete information on all potentially relevant participant characteristics, which would assist in exploring the causes and the likely study heterogeneity we will observe, ultimately restricting our analyses of these datasets. In the future, we would advocate for patterning of academia, industry, the NIDDK, and the NKF to encourage collaborations for development of similar data structures and for data sharing after the completion of the trials. Process and rules about governance of the data would need to be established but are doable. Lack of establishment of such structures could delay recognition of surrogates.
In conclusion, the lack of available treatments for kidney disease progression is a critical problem. Validation of reduction of proteinuria as a surrogate marker will undoubtedly facilitate and accelerate the conduct of studies and the availability of treatments. However, few studies have been used to test treatments for kidney disease, and of those, rigorous analyses of the role of proteinuria are limited. We will address this question in a joint analysis of a pooled dataset in anticipation that positive results will facilitate conduct of studies to bring new therapies more rapidly to the aid of people with chronic kidney disease.
Acknowledgments
This study is supported by grants UO1 DK 053869, UO1 DK 067651 and UO1 DK 35073 from National Institutes of Health. African American Study of Kidney Disease and Hypertension (AASK): Tom Greene, PhD. Appropriate Blood Pressure Control in Diabetes (ABCD): Robert W. Schrier, MD, Raymond O. Estacio, MD. Department of Medicine, University of Toronto, Canada: Daniel C. Cattran, MD, FRCPC. Euro-Lupus Nephritis Trial (ELNT): Frèdèric A. Houssiau, MD, PhD. Hong Kong Study Using Valsartan in IgA Nephropathy (HKVIN): Philip Kam-Tao Li, MD, FRCP, FACP. Department of Medicine, University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong: Tak-Mao Chan, MD. Irbesartan in Diabetic Nephropathy Trial (IDNT): Ed Lewis, MD; Lawrence G. Hunsicker, MD. Division of Clinical Nephrology, New York Presbyterian Hospital, Columbia University, New York, NY, USA: Gerald B. Appel, MD; Gershon Frisch, MD. Mayo Clinic: James Donadio, MD; Fernando Fervenza, MD. Department of Nephrology and Internal Medicine, Heilig Hart Hospital, Roeselare Belgium: Bart Maes, MD, PhD. Nephrology Department, Hospital 12 de Octubre, Madrid, Spain: Manuel Praga, MD. Captopril in Diabetic Nephropathy Study (CSG): Roger A. Rodby, MD; Richard D. Rohde, BS. Lupus Nephritis Collaborative Study (LNCS): Edmund Lewis, MD; John M. Lachin, ScD. Modification of Diet in Renal Disease (MDRD) Study: Gerald Beck, PhD. Department of Nephrology, IRCCS Istituto Humanitas, Milan, Italy: Claudio Ponticelli, MD; Patrizia Passerini, MD; Gabriella Moroni, MD; Giuseppe Montogrino, MD. Grupo Español de estudio de la Nefropatía Membranosa: Manuel Praga, MD. Ramipril Efficacy In Nephropathy (REIN and REIN 2): Giuseppe Remuzzi, MD, FRCP; Piero Ruggenenti, MD. Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL): Dick De Zeeuw, MD, PhD; Barry M. Brenner, MD; William Keane, MD. Renal Division, Brigham and Women's Hospital, Boston, MA, USA: Barry M. Brenner, MD. University of Texas Southwestern Medical Center, Dallas, TX, USA: Robert Toto, MD. Department of Nephrology, University Hospital, Strasbourg, France: Thierry P. Hannedouche, MD. North American IgA Nephropathy Study: Fan Hou, MD, PhD. Department of Nephrology, The Royal Melbourne Hospital, Melbourne, VIC, Australia: Benno U. Ihle MBBS, FRACP, FJFICM; Priscilla S. Kincaid-Smith MD, DSc. University of Copenhagen, Copenhagen, Denmark: Anne-Lise Kamper, MD. Division of Nephrology, University Hospital, Verona, Italy: Guiseppe Maschio, MD. Department of Medicine, University Hospital, Groningen, the Netherlands: GG. van Essen, MD. Department of Nephrology, Malpighi-Bologna, Italy: Pietro Zucchelli, MD.
Conflict of interest statement. None declared.
References
- 1.Burton C, Harris KP. The role of proteinuria in the progression of chronic renal failure. Am J Kidney Dis. 1996;27:765–775. doi: 10.1016/s0272-6386(96)90512-0. [DOI] [PubMed] [Google Scholar]
- 2.Remuzzi G, Chiurchiu C, Ruggenenti P. Proteinuria predicting outcome in renal disease: nondiabetic nephropathies (REIN) Kidney Int Suppl. 2004;92:S90–S96. doi: 10.1111/j.1523-1755.2004.09221.x. [DOI] [PubMed] [Google Scholar]
- 3.de Zeeuw D, Remuzzi G, Parving H-H, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int. 2004;65:2309–2320. doi: 10.1111/j.1523-1755.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 4.Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997;51:1908–1919. doi: 10.1038/ki.1997.260. [DOI] [PubMed] [Google Scholar]
- 5.Lea J, Greene T, Hebert L, et al. The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease: results of the African American study of kidney disease and hypertension. Arch Intern Med. 2005;165:947–953. doi: 10.1001/archinte.165.8.947. [DOI] [PubMed] [Google Scholar]
- 6.Rossing K, Christensen PK, Hovind P, et al. Progression of nephropathy in type 2 diabetic patients. Kidney Int. 2004;66:1596–1605. doi: 10.1111/j.1523-1755.2004.00925.x. [DOI] [PubMed] [Google Scholar]
- 7.Ruggenenti P, Perna A, Mosconi L, et al. Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN) Kidney Int. 1998;53:1209–1216. doi: 10.1046/j.1523-1755.1998.00874.x. [DOI] [PubMed] [Google Scholar]
- 8.Hunsicker LG, Atkins RC, Lewis JB, et al. Impact of irbesartan, blood pressure control, and proteinuria on renal outcomes in the Irbesartan Diabetic Nephropathy Trial. Kidney Int Suppl. 2004;92:S99–S101. doi: 10.1111/j.1523-1755.2004.09223.x. [DOI] [PubMed] [Google Scholar]
- 9.Jafar TH, Stark PC, Schmid CH, et al. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int. 2001;60:1131–1140. doi: 10.1046/j.1523-1755.2001.0600031131.x. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Cattran D, Friedman A, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009;54:205–226. doi: 10.1053/j.ajkd.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 11.Stevens LA, Greene T, Levey AS. Surrogate end points for clinical trials of kidney disease progression. Clin J Am Soc Nephrol. 2006;1:874–884. doi: 10.2215/CJN.00600206. [DOI] [PubMed] [Google Scholar]
- 12.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8:431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 13.Nakao N, Yoshimura A, Morita H, et al. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet. 2003;361:117–124. doi: 10.1016/S0140-6736(03)12229-5. [DOI] [PubMed] [Google Scholar]
- 14.Hogg RJ, Lee J, Nardelli N, et al. Clinical trial to evaluate omega-3 fatty acids and alternate day prednisone in patients with IgA nephropathy: report from the Southwest Pediatric Nephrology Study Group. Clin J Am Soc Nephrol. 2006;1:467–474. doi: 10.2215/CJN.01020905. [DOI] [PubMed] [Google Scholar]
- 15.Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939–946. doi: 10.1016/S0140-6736(05)71082-5. [DOI] [PubMed] [Google Scholar]
- 16.Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis. 2005;45:281–287. doi: 10.1053/j.ajkd.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Praga M, Gutierrez E, Gonzalez E, et al. Treatment of IgA nephropathy with ACE inhibitors: a randomized and controlled trial. J Am Soc Nephrol. 2003;14:1578–1583. doi: 10.1097/01.asn.0000068460.37369.dc. [DOI] [PubMed] [Google Scholar]
- 18.Shahinfar S, Dickson TZ, Ahmed T, et al. Losartan in patients with type 2 diabetes and proteinuria: observations from the RENAAL Study. Kidney Int Suppl. 2002;62:S64–S67. doi: 10.1046/j.1523-1755.62.s82.13.x. [DOI] [PubMed] [Google Scholar]
- 19.van Essen GG, Apperloo AJ, Rensma PL, et al. Are angiotensin converting enzyme inhibitors superior to beta blockers in retarding progressive renal function decline? Kidney Int Suppl. 1997;63:S58–S62. [PubMed] [Google Scholar]
- 20.Ruggenenti P, Perna A, Remuzzi G. Retarding progression of chronic renal disease: the neglected issue of residual proteinuria. Kidney Int. 2003;63:2254–2261. doi: 10.1046/j.1523-1755.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 21.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997;349:1857–1863. [PubMed] [Google Scholar]
- 22.Ihle BU, Whitworth JA, Shahinfar S, et al. Angiotensin-converting enzyme inhibition in nondiabetic progressive renal insufficiency: a controlled double-blind trial. Am J Kidney Dis. 1996;27:489–495. doi: 10.1016/s0272-6386(96)90158-4. [DOI] [PubMed] [Google Scholar]
- 23.Breyer JA, Bain RP, Evans JK, et al. Predictors of the progression of renal insufficiency in patients with insulin-dependent diabetes and overt diabetic nephropathy. The Collaborative Study Group. Kidney Int. 1996;50:1651–1658. doi: 10.1038/ki.1996.481. [DOI] [PubMed] [Google Scholar]
- 24.Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123:754–762. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]
- 25.D'Amico G, Gentile MG, Fellin G, et al. Effect of dietary protein restriction on the progression of renal failure: a prospective randomized trial. Nephrol Dial Transplant. 1994;9:1590–1594. [PubMed] [Google Scholar]
- 26.Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354:131–140. doi: 10.1056/NEJMoa053107. [DOI] [PubMed] [Google Scholar]
- 27.Bolton WK, Cattran DC, Williams ME, et al. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol. 2004;24:32–40. doi: 10.1159/000075627. [DOI] [PubMed] [Google Scholar]
- 28.Pozzi C, Bolasco PG, Fogazzi GB, et al. Corticosteroids in IgA nephropathy: a randomised controlled trial. Lancet. 1999;353:883–887. doi: 10.1016/s0140-6736(98)03563-6. [DOI] [PubMed] [Google Scholar]
- 29.Dember LM, Hawkins PN, Hazenberg BP, et al. Eprodisate for the treatment of renal disease in AA amyloidosis. N Engl J Med. 2007;356:2349–2360. doi: 10.1056/NEJMoa065644. [DOI] [PubMed] [Google Scholar]
- 30.Cinotti GA, Zucchelli PC. Effect of lisinopril on the progression of renal insufficiency in mild proteinuric non-diabetic nephropathies. Nephrol Dial Transplant. 2001;16:961–966. doi: 10.1093/ndt/16.5.961. [DOI] [PubMed] [Google Scholar]
- 31.Heering P, Braun N, Mullejans R, et al. Cyclosporine A and chlorambucil in the treatment of idiopathic focal segmental glomerulosclerosis. Am J Kidney Dis. 2004;43:10–18. doi: 10.1053/j.ajkd.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 32.Pelletier RP, Akin B, Ferguson RM. Prospective, randomized trial of steroid withdrawal in kidney recipients treated with mycophenolate mofetil and cyclosporine. Clin Transplant. 2006;20:10–18. doi: 10.1111/j.1399-0012.2005.00430.x. [DOI] [PubMed] [Google Scholar]
- 33.Katafuchi R, Ikeda K, Mizumasa T, et al. Controlled, prospective trial of steroid treatment in IgA nephropathy: a limitation of low-dose prednisolone therapy. Am J Kidney Dis. 2003;41:972–983. doi: 10.1016/s0272-6386(03)00194-x. [DOI] [PubMed] [Google Scholar]
- 34.Endo K, Miyashita Y, Sasaki H, et al. Probucol delays progression of diabetic nephropathy. Diabetes Res Clin Pract. 2006;71:156–163. doi: 10.1016/j.diabres.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Stefoni S, Mosconi G, La Manna G, et al. Low-dosage ibopamine treatment in progressive renal failure: a long-term multicentre trial. Am J Nephrol. 1996;16:489–499. doi: 10.1159/000169049. [DOI] [PubMed] [Google Scholar]
- 36.Grootscholten C, Ligtenberg G, Hagen EC, et al. Azathioprine/methylprednisolone versus cyclophosphamide in proliferative lupus nephritis. A randomized controlled trial. Kidney Int. 2006;70:732–742. doi: 10.1038/sj.ki.5001630. [DOI] [PubMed] [Google Scholar]
- 37.Ballardie FW, Roberts IS. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol. 2002;13:142–148. doi: 10.1681/ASN.V131142. [DOI] [PubMed] [Google Scholar]
- 38.Marin R, Ruilope LM, Aljama P, et al. A random comparison of fosinopril and nifedipine GITS in patients with primary renal disease. J Hypertens. 2001;19:1871–1876. doi: 10.1097/00004872-200110000-00023. [DOI] [PubMed] [Google Scholar]
- 39.Rosman JB, ter Wee PM, Meijer S, et al. Prospective randomised trial of early dietary protein restriction in chronic renal failure. Lancet. 1984;2:1291–1296. doi: 10.1016/s0140-6736(84)90818-3. [DOI] [PubMed] [Google Scholar]
- 40.Fellstrom B, Holdaas H, Jardine AG, et al. Risk factors for reaching renal endpoints in the Assessment of Lescol in Renal Transplantation (ALERT) trial. Transplantation. 2005;79:205–212. doi: 10.1097/01.tp.0000147338.34323.12. [DOI] [PubMed] [Google Scholar]
- 41.Mycophenolate mofetil for the treatment of a first acute renal allograft rejection: three-year follow-up. The Mycophenolate Mofetil Acute Renal Rejection Study Group. Transplantation. 2001;71:1091–1097. doi: 10.1097/00007890-200104270-00014. [DOI] [PubMed] [Google Scholar]
- 42.Cattran DC, Appel GB, Hebert LA, et al. Cyclosporine in patients with steroid-resistant membranous nephropathy: a randomized trial. Kidney Int. 2001;59:1484–1490. doi: 10.1046/j.1523-1755.2001.0590041484.x. [DOI] [PubMed] [Google Scholar]
- 43.Ponticelli C, Altieri P, Scolari F, et al. A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol. 1998;9:444–450. doi: 10.1681/ASN.V93444. [DOI] [PubMed] [Google Scholar]
- 44.Mann JF, Gerstein HC, Yi QL, et al. Development of renal disease in people at high cardiovascular risk: results of the HOPE randomized study. J Am Soc Nephrol. 2003;14:641–647. doi: 10.1097/01.asn.0000051594.21922.99. [DOI] [PubMed] [Google Scholar]
- 45.Herlitz H, Harris K, Risler T, et al. The effects of an ACE inhibitor and a calcium antagonist on the progression of renal disease: the Nephros Study. Nephrol Dial Transplant. 2001;16:2158–2165. doi: 10.1093/ndt/16.11.2158. [DOI] [PubMed] [Google Scholar]
- 46.Dinant HJ, Decker JL, Klippel JH, et al. Alternative modes of cyclophosphamide and azathioprine therapy in lupus nephritis. Ann Intern Med. 1982;96:728–736. doi: 10.7326/0003-4819-96-6-728. [DOI] [PubMed] [Google Scholar]
- 47.Decker JL, Klippel JH, Plotz PH, et al. Cyclophosphamide or azathioprine in lupus glomerulonephritis. A controlled trial: results at 28 months. Ann Intern Med. 1975;83:606–615. doi: 10.7326/0003-4819-83-5-606. [DOI] [PubMed] [Google Scholar]
- 48.Bakker RC, Hollander AA, Mallat MJ, et al. Conversion from cyclosporine to azathioprine at three months reduces the incidence of chronic allograft nephropathy. Kidney Int. 2003;64:1027–1034. doi: 10.1046/j.1523-1755.2003.00175.x. [DOI] [PubMed] [Google Scholar]
- 49.Teplan V, Schuck O, Knotek A, et al. Enhanced metabolic effect of erythropoietin and keto acids in CRF patients on low-protein diet: Czech multicenter study. Am J Kidney Dis. 2003;41:S26–S30. doi: 10.1053/ajkd.2003.50079. [DOI] [PubMed] [Google Scholar]
- 50.Teplan V, Schuck O, Knotek A, et al. Effects of low-protein diet supplemented with ketoacids and erythropoietin in chronic renal failure: a long-term metabolic study. Ann Transplant. 2001;6:47–53. [PubMed] [Google Scholar]
- 51.Cattran DC, Greenwood C, Ritchie S, et al. A controlled trial of cyclosporine in patients with progressive membranous nephropathy. Canadian Glomerulonephritis Study Group. Kidney Int. 1995;47:1130–1135. doi: 10.1038/ki.1995.161. [DOI] [PubMed] [Google Scholar]
- 52.Marre M, Lievre M, Chatellier G, et al. Effects of low dose ramipril on cardiovascular and renal outcomes in patients with type 2 diabetes and raised excretion of urinary albumin: randomised, double blind, placebo controlled trial (the DIABHYCAR study) BMJ. 2004;328:495–499. doi: 10.1136/bmj.37970.629537.0D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X, Qiu Q, Tang L, et al. Effects of co-administration of urokinase and benazepril on severe IgA nephropathy. Nephrol Dial Transplant. 2004;19:852–857. doi: 10.1093/ndt/gfh069. [DOI] [PubMed] [Google Scholar]
- 54.Bannister KM, Weaver A, Clarkson AR, et al. Effect of angiotensin-converting enzyme and calcium channel inhibition on progression of IgA nephropathy. Contrib Nephrol. 1995;111:184–192. doi: 10.1159/000423895. [DOI] [PubMed] [Google Scholar]
- 55.Moroni G, Doria A, Mosca M, et al. A randomized pilot trial comparing cyclosporine and azathioprine for maintenance therapy in diffuse lupus nephritis over four years. Clin J Am Soc Nephrol. 2006;1:925–932. doi: 10.2215/CJN.02271205. [DOI] [PubMed] [Google Scholar]
- 56.Alexopoulos E, Stangou M, Pantzaki A, et al. Treatment of severe IgA nephropathy with omega-3 fatty acids: the effect of a “very low dose” regimen. Ren Fail. 2004;26:453–459. doi: 10.1081/jdi-200026763. [DOI] [PubMed] [Google Scholar]
- 57.Himmelmann A, Hansson L, Hansson BG, et al. ACE inhibition preserves renal function better than beta-blockade in the treatment of essential hypertension. Blood Press. 1995;4:85–90. doi: 10.3109/08037059509077575. [DOI] [PubMed] [Google Scholar]
- 58.van Dijk M, Breuning M, Duiser R, et al. No effect of enalapril on progression in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2003;18:2314–2320. doi: 10.1093/ndt/gfg417. [DOI] [PubMed] [Google Scholar]
- 59.Montagnino G, Kramer BK, Arias M. Efficacy and safety of tacrolimus compared with cyclosporine microemulsion in kidney transplantation: twelve-month follow-up. Transplant Proc. 2002;34:1635–1637. doi: 10.1016/s0041-1345(02)02960-3. [DOI] [PubMed] [Google Scholar]
- 60.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 61.Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 62.Estacio RO, Jeffers BW, Gifford N, et al. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diab Care. 2000;23:B54–B64. [PubMed] [Google Scholar]
- 63.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 64.Donadio JV, Jr., Grande JP, Bergstralh EJ, et al. The long-term outcome of patients with IgA nephropathy treated with fish oil in a controlled trial. Mayo Nephrology Collaborative Group. J Am Soc Nephrol. 1999;10:1772–1777. doi: 10.1681/ASN.V1081772. [DOI] [PubMed] [Google Scholar]
- 65.Donadio JV, Jr., Larson TS, Bergstralh EJ, et al. A randomized trial of high-dose compared with low-dose omega-3 fatty acids in severe IgA nephropathy. J Am Soc Nephrol. 2001;12:791–799. doi: 10.1681/ASN.V124791. [DOI] [PubMed] [Google Scholar]
- 66.Maes BD, Oyen R, Claes K, et al. Mycophenolate mofetil in IgA nephropathy: results of a 3-year prospective placebo-controlled randomized study. Kidney Int. 2004;65:1842–1849. doi: 10.1111/j.1523-1755.2004.00588.x. [DOI] [PubMed] [Google Scholar]
- 67.Frisch G, Lin J, Rosenstock J, et al. Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: a double-blind randomized controlled trial. Nephrol Dial Transplant. 2005;20:2139–2145. doi: 10.1093/ndt/gfh974. [DOI] [PubMed] [Google Scholar]
- 68.Li PK, Leung CB, Chow KM, et al. Hong Kong study using valsartan in IgA nephropathy (HKVIN): a double-blind, randomized, placebo-controlled study. Am J Kidney Dis. 2006;47:751–760. doi: 10.1053/j.ajkd.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 69.Lewis EJ, Hunsicker LG, Lan SP, et al. A controlled trial of plasmapheresis therapy in severe lupus nephritis. The Lupus Nephritis Collaborative Study Group. N Engl J Med. 1992;326:1373–1379. doi: 10.1056/NEJM199205213262101. [DOI] [PubMed] [Google Scholar]
- 70.Chan TM, Tse KC, Tang CS, et al. Long-term study of mycophenolate mofetil as continuous induction and maintenance treatment for diffuse proliferative lupus nephritis. J Am Soc Nephrol. 2005;16:1076–1084. doi: 10.1681/ASN.2004080686. [DOI] [PubMed] [Google Scholar]
- 71.Houssiau FA, Vasconcelos C, D'Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46:2121–2131. doi: 10.1002/art.10461. [DOI] [PubMed] [Google Scholar]
- 72.Ponticelli C, Passerini P, Salvadori M, et al. A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis. 2006;47:233–240. doi: 10.1053/j.ajkd.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 73.Ponticelli C, Zucchelli P, Passerini P, et al. A randomized trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med. 1989;320:8–13. doi: 10.1056/NEJM198901053200102. [DOI] [PubMed] [Google Scholar]
- 74.Ponticelli C, Zucchelli P, Passerini P, et al. Methylprednisolone plus chlorambucil as compared with methylprednisolone alone for the treatment of idiopathic membranous nephropathy. The Italian Idiopathic Membranous Nephropathy Treatment Study Group. N Engl J Med. 1992;327:599–603. doi: 10.1056/NEJM199208273270904. [DOI] [PubMed] [Google Scholar]
- 75.Cattran DC, Appel GB, Hebert LA, et al. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney Int. 1999;56:2220–2226. doi: 10.1046/j.1523-1755.1999.00778.x. [DOI] [PubMed] [Google Scholar]
- 76.Praga M, Barrio V, Juarez GF, et al. Tacrolimus monotherapy in membranous nephropathy: a randomized controlled trial. Kidney Int. 2007;71:924–930. doi: 10.1038/sj.ki.5002215. [DOI] [PubMed] [Google Scholar]
- 77.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 78.Hannedouche T, Landais P, Goldfarb B, et al. Randomised controlled trial of enalapril and beta blockers in non-diabetic chronic renal failure. BMJ. 1994;309:833–837. doi: 10.1136/bmj.309.6958.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 80.Kamper AL, Strandgaard S, Leyssac PP. Effect of enalapril on the progression of chronic renal failure. A randomized controlled trial. Am J Hypertens. 1992;5:423–430. doi: 10.1093/ajh/5.7.423. [DOI] [PubMed] [Google Scholar]
- 81.Maschio G, Alberti D, Janin G, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med. 1996;334:939–945. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- 82.Zucchelli P, Zuccala A, Borghi M, et al. Long-term comparison between captopril and nifedipine in the progression of renal insufficiency. Kidney Int. 1992;42:452–458. doi: 10.1038/ki.1992.309. [DOI] [PubMed] [Google Scholar]
- 83.Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999;354:359–364. doi: 10.1016/S0140-6736(98)10363-X. [DOI] [PubMed] [Google Scholar]
- 84.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 85.Ruggenenti P, Perna A, Mosconi L, et al. A randomized placebo controlled trial of the angiotensin-converting-enzyme inhibitor ramipiril on the decline of the glomerular filtration rate and end stage renal failure in proteinuric, non-diabetic chronic renal disease. Lancet. 1997;349:1857–1863. [Google Scholar]