Abstract
Background. Adults with end-stage renal disease who are awaiting kidney transplantation are at risk for low quality of life, high psychological disturbance and relationship distress. Effective psychological interventions to improve functioning in these areas are lacking.
Methods. Sixty-two adults approved for kidney transplantation at one centre in the USA were randomized to quality of life therapy (QOLT), supportive therapy (ST) or standard care (SC) with repeated assessments of quality of life, psychological distress, and social intimacy at pre-intervention (T1), 1 week post-intervention (T2) and 12-week follow-up (T3).
Results. QOLT patients had higher quality of life scores than ST and SC patients at T2 and T3, and higher social intimacy scores compared with SC patients at T3. Both QOLT and ST patients had lower psychological distress scores compared with SC patients at T2, although only QOLT continued to show reductions in psychological distress scores relative to SC patients at T3.
Conclusions. The findings show that it is possible to improve quality of life, psychological functioning and social intimacy with QOLT while patients await kidney transplantation. Study limitations include small sample size, single-centre study and possible patient self-selection biases. Future research will examine whether QOLT effectiveness is affected by treatment modality (face-to-face vs. telephone) and timing (pre- vs. post-transplantation).
Keywords: clinical trial, kidney, psychosocial, quality of life, transplantation
Introduction
Kidney transplantation (KT) is superior to long-term haemodialysis in reducing chronic morbidities, extending life and improving quality of life (QOL) for most adults with chronic kidney failure. However, transplant waiting times are long, and psychological distress and QOL impairment are common [1–8]. Depression has been linked with poor medication adherence and mortality [9–11]. Also, chronic kidney disease and its treatments can contribute to relationship stress and further compromise QOL and psychological functioning [3,12]. The development and evaluation of effective interventions to reduce psychological distress, improve QOL and enhance social intimacy are of clinical and scientific importance to KT patients, their family members and healthcare providers.
Some research supports the benefits of psychological interventions with KT patients. Tsay [13] randomized patients with end-stage renal disease (ESRD) to a cognitive–behavioural coping skills and stress management training programme or standard care (primarily education). Cognitive–behavioural treatment reduced symptoms of stress and depression, and improved QOL, compared with a standard care condition. Chang et al. [14] combined education, vocational rehabilitation and social support enhancement, and found significant QOL improvements in KT recipients. Gross et al. [15] used a mindfulness-based stress reduction programme in KT recipients to reduce depression and anxiety, although no QOL improvements were found.
Quality of life therapy (QOLT) is the only cognitive–behavioural treatment that targets happiness and life satisfaction in multiple life domains (e.g. relationships, enjoyable activities, self-esteem, etc.) with a specific goal of improving overall QOL [16]. This is important because the World Health Organization has emphasized the importance of a patient’s subjective perception of life in the context of his or her value systems, goals, expectations and standards [17]. In QOLT, the patient’s assessment of his or her most important life needs and goals and whether they have been fulfilled is of central importance. In one randomized trial of lung transplant candidates, QOLT was superior to a supportive treatment in achieving QOL, mood and social intimacy benefits, and these clinical benefits were maintained for 3 months [18]. Whether QOLT is effective with KT patients is currently unknown. Therefore, this study examined the effectiveness of QOLT in improving QOL, psychological functioning and social intimacy in adults approved for KT. We hypothesized, based on prior research, that QOLT would be more effective than a supportive therapy (ST) intervention or standard care (SC) services across all outcomes at post-intervention and at 12-week follow-up.
Materials and methods
Participants
Participants were enrolled from January 2007 until February 2009 at Beth Israel Deaconess Medical Center in Boston, MA, USA. Inclusion criteria were: age 18–70 years, chronic kidney disease or ESRD, medically approved for KT, and living within 60 miles of transplant centre. We excluded patients who were already receiving psychological treatment, who had received a prior transplant, who were listed for liver/kidney transplantation, who did not speak English or who had known cognitive impairment (Mini Mental Status Exam score <23). An a priori decision was made to enrol patients who had potential living donors who were at some point in the donor evaluation process because of the possibility that they may not progress to live-donor kidney transplantation.
Study design
Patients were informed about the study via letter and during transplant clinic visits. Written informed consent was obtained, and patients completed a baseline telephone assessment. After the baseline assessment (T1), patients were randomized to QOLT, ST or SC. Post-intervention (T2) and follow-up (T3) assessments were completed 1 and 12 weeks, respectively, after the last treatment session for QOLT and ST patients. SC patients completed the T2 and T3 assessments at 9 and 20 weeks, respectively, following their baseline (T1) assessment. The Institutional Review Board at Beth Israel Deaconess Medical Center approved all study procedures.
Measurements
The assessment protocol included QOL, psychological distress and social intimacy questionnaires selected because of their strong psychometric properties and utility with chronically ill patients.
Quality of Life Inventory (QOLI) [19]. The QOLI comprises 32 statements reflecting 16 life domains that are rated by the patient for their relative importance and current satisfaction level. These domains include: health; self-esteem; goals-and-values; money; work; play; learning; creativity; helping; love relationship; relationships with children, relatives and friends; home; neighbourhood; and community. Combining these different domains, a standardized overall T score was calculated for this study. Higher score indicates higher QOL.
SF-36 Health Survey (v2) [20]. The SF-36 is a widely used generic health status measurement with eight QOL domains: physical functioning, role functioning–physical, role functioning–emotional, vitality, pain, general health, social functioning and mental health. These domains are included in two composite scores—Physical Component Summary (PCS) and Mental Component Summary (MCS)—which we used in our analyses. Higher scores reflect better functioning.
Profile of Mood States-Short Form (POMS) [21]. The POMS lists adjectives that patients use to indicate how they felt in the past week. Responses yield a total mood disturbance score, with higher scores indicating more mood disturbance.
Hopkins Symptom Checklist-25 (HSCL) [22]. Using the HSCL, patients indicated the degree to which 25 anxiety/depression symptoms bothered them in the past month. A total score was obtained, with a higher score indicative of more distress.
Number of unhealthy mental health days in the past month [23]. Patients were asked to estimate how many unhealthy mental health days (e.g. stress, depression and anxiety) they had in the past month.
Miller Social Intimacy Scale (MSIS) [24]. The MSIS is a 17-item instrument that asks about intimacy frequency and intensity. Only those patients who had a spouse or partner completed the MSIS (QOLT n = 15, ST n = 13 and SC n = 11). Responses yield a total intimacy score, with a higher score indicative of greater intimacy.
Patients and therapists in the two active treatments (QOLT and ST) completed a 12-item treatment process and satisfaction rating scale, which assessed comfort level, attentiveness, rapport, perceived treatment helpfulness and convenience.
Specification of treatment conditions
We sought to evaluate the effectiveness of QOLT in comparison with an active treatment alternative and with a no-treatment control group. ST was chosen as the active treatment alternative because all transplant centres employ social workers who provide educational and supportive interventions—thus, we viewed this as the intervention most likely to be used in the setting of transplantation. However, as standard of care, most transplant centres do not provide structured psychological treatment services to their wait-listed patients. The use of this no-treatment control group allowed us to examine whether QOLT was superior to no treatment, something that was not done in the one prior QOLT study using adult lung transplant candidates [18].
Both active treatments were intended to occur once weekly, face-to-face, for ∼50 min per session, with participants receiving eight individual treatment sessions over a 2-month time period. A ‘full dose’ of treatment was defined as ≥6 sessions. Participants who missed sessions (e.g. illness, inclement weather and no-show) had make-up sessions scheduled as close in time as possible to the originally scheduled session.
QOLT was developed as an integrative and comprehensive approach to improve QOL [16,25]. In the current study, patients completed the QOLI, which provided specific information about current QOL levels across multiple life domains. The therapist then worked with the patient to identify specific areas of life contributing to the patient’s overall QOL. The causes of dissatisfaction in specific life domains were identified, and specific strategies were developed to facilitate change in the patient’s subjective circumstances of the domain (e.g. problem solve to improve the situation), attitudes or perceptions of the domain, standards of fulfilment for the domain, and/or the relative importance placed on the domain for overall happiness. One common example was the desire of patients to improve their satisfaction with the quality of their relationships with family members or friends. QOLT guided patients through the process of assessing the relationship, understanding their thoughts and feelings about the relationship, articulating how they want the relationship to change or be different, and setting goals for the relationship. Throughout this process, the therapist helped the patient to identify and build upon key relationship skills, to practise effective communication strategies, and to evaluate whether these change efforts have been successful.
ST was designed to mirror the supportive services that many transplant centres commonly offer to patients. The goal of ST was to provide a structured approach to deliver emotional and educational support to patients in coping with the demands of waiting for KT. Additional treatment objectives were to promote a supportive therapist–patient relationship, enhance the patient’s strengths, coping skills and capacity to use environmental supports, and reduce the patient’s subjective distress. Session topics included understanding the transplant process, understanding medications and their effects, coping with illness and transplantation, identifying and dealing with emotions, dealing with issues of death and dying, communicating with others, and navigating the healthcare system, among others. Therapists listened actively to their concerns and worries, displayed a genuine interest in their life activities and well-being as well as a non-judgmental acceptance of their current state, provided encouragement and reinforcement, and promoted the use of other support systems [26].
SC patients received usual care services at our centre. This included clinic visits, every 3–6 months, with the transplant nephrologist, surgeon and/or nurse coordinator. A brief visit with the transplant social worker or transplant pharmacist might also be scheduled. Patients in both QOLT and ST also received SC, which allowed us to evaluate the benefits of these two active treatments beyond the usual clinical services rendered by the transplant programme.
Therapists were Master’s or PhD level social workers and psychologists with at least 2-year experience in transplantation who delivered only one of the interventions. All non-study transplant personnel were blinded to the participants’ group assignment, as were research assistants responsible for data collection, scoring, coding and entry.
Statistical analysis
This study was designed to have a statistical power of 80% to detect a difference of at least one-half standard deviation change in the QOLI T score in the three groups. A 15% dropout rate was assumed in calculating sample size. All subjects randomized (n = 62) were included in the analyses according to the original intent-to-treat design. Exploratory analyses were conducted to identify outliers, missing data and data distributional characteristics. We used maximum-likelihood estimates to impute missing data [27]. The Shapiro–Wilk’s test was used to examine distributional characteristics. Age and HSCL scores were not normally distributed, and log transformations were performed. For analyses predicting outcome measures (QOL, psychological distress and social intimacy) based on group status, analysis of covariance was used with the respective baseline (T1) value as a covariate. Effect sizes were computed using Cohen’s d. Total summary scores (versus individual scaled scores) were used to reduce the risk of Type I error. A Reliable Change Index (RCI) [28] was used to evaluate clinically significant change at both post-intervention (T2) and 12-week follow-up (T3). Chi-square analyses were conducted to examine whether rates of reliable change differed across groups. PASW Statistics 17.0 was used for all statistical analyses.
Results
Recruitment, retention and sample characteristics
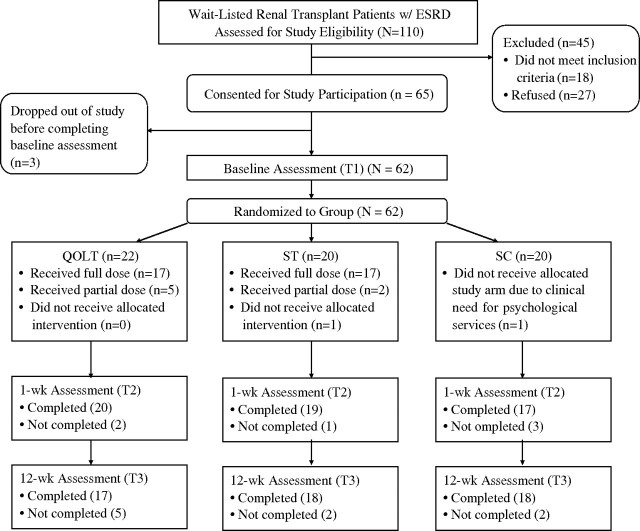
Of the 110 patients approached about the study, 18 did not fulfil study criteria, and 27 refused to participate (Figure 1). Of the 65 patients who consented, 62 completed the baseline assessment and were randomized. Baseline sociodemographic and medical characteristics were similar across the three groups (Table 1). However, fewer QOLT patients had ≥12 years of education compared with ST and SC patients (χ2 = 22.1, P = 0.005).
Fig. 1.
CONSORT diagram showing patient progression through the study.
Table 1.
Sample characteristics by group assignment
| Treatment condition |
|||
|---|---|---|---|
| QOLT | ST | SC | |
| (n = 22) | (n = 20) | (n = 20) | |
| Age, year | 53.2 (11.1) | 48.6 (11.9) | 52.7 (12.7) |
| Sex, female | 10 (46%) | 12 (60%) | 11 (55%) |
| Race, non-white | 8 (36%) | 6 (30%) | 8 (40%) |
| Marital status, married | 8 (36%) | 7 (35%) | 9 (45%) |
| Education, ≥12 yearsa | 18 (82%) | 19 (95%) | 20 (100%) |
| Employed | 4 (18%) | 6 (30%) | 9 (45%) |
| Time since KT approval, months | 10.9 (12.3) | 9.4 (7.7) | 14.0 (13.3) |
| Primary disease | |||
| Diabetes | 7 (32%) | 8 (40%) | 7 (35%) |
| Hypertension | 6 (27%) | 3 (15%) | 7 (35%) |
| Polycystic kidneys | 2 (9%) | 3 (15%) | 0 (0%) |
| Other | 7 (32%) | 6 (30%) | 6 (30%) |
| Dialysis | |||
| None | 5 (23%) | 4 (20%) | 5 (25%) |
| Haemodialysis | 12 (55%) | 12 (60%) | 12 (60%) |
| Peritoneal dialysis | 5 (23%) | 4 (20%) | 3 (15%) |
| Dialysis exposure, monthsb | 18.4 (±14.5) | 18.9 (±17.1) | 25.9 (±21.6) |
| Body mass index | 30.6 (±4.7) | 28.6 (±6.8) | 31.2 (±5.6) |
Data are presented as means (SD) for continuous variables or n (%) for categorical variables.
χ2 = 22.1, P = 0.005 (QOLT < ST, SC).
Calculated only for those receiving haemodialysis or peritoneal dialysis.
Figure 1 illustrates the patients who received the assigned condition (full or partial) and who completed each assessment. In the QOLT group, intervention was terminated prematurely by two patients (4 and 5 sessions, respectively), and was terminated administratively for one patient who received KT after three sessions. In the ST group, intervention was terminated prematurely by two patients (2 and 3 sessions, respectively). In the SC group, one patient was withdrawn administratively because urgent psychiatric treatment was warranted. Across all groups, reasons for not completing assessments included time constraints (n = 3), multiple failures to reach by telephone (n = 1), KT (n = 2) and death (n = 1).
The mean number of QOLT and ST sessions did not differ significantly (7.1 vs. 6.9, respectively, t = 0.32, P = 0.75), and total intervention exposure was comparable (381.9 min vs. 351.3 min, respectively, t = 0.94, P = 0.35) and not significantly correlated with outcomes. There were no differences (all Ps > 0.05) between QOLT and ST patients on treatment process ratings, with both patients and therapists reporting high levels of comfort, rapport, supportiveness and overall helpfulness.
Intervention effectiveness
Means and standard deviations are broken down by group and time in Table 2. There were no baseline (T1) group differences across the seven measures (all Ps > 0.17).
Table 2.
Means and standard deviations for dependent measures at baseline (T1), 1-week post-intervention (T2) and 12-week follow-up (T3)
| QOLT |
ST |
SC |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 |
| QOLI | 37.8 (12.0) | 46.7 (14.9)a | 45.8 (13.1)a | 38.1 (13.1) | 37.0 (12.3)b | 38.3 (11.0)b | 38.3 (15.6) | 36.9 (15.8)b | 36.3 (16.8)b |
| SF-36 PCS | 36.5 (7.5) | 36.4 (6.6) | 36.5 (6.2) | 39.1 (7.4) | 36.6 (9.3) | 39.9 (8.6) | 36.9 (9.8) | 35.2 (9.5) | 36.0 (10.4) |
| SF-36 MCS | 40.7 (13.7) | 46.2 (11.3) | 46.1 (9.6)a | 41.3 (15.3) | 40.5 (15.7) | 40.1 (13.6)b | 42.4 (10.5) | 42.8 (12.0) | 39.4 (15.3)b |
| POMS | 30.9 (15.9) | 23.8 (18.1)a | 20.7 (16.1)a | 28.5 (22.8) | 23.0 (22.7)a | 23.1 (20.4) | 24.5 (14.9) | 28.7 (19.4)b | 26.8 (18.4)b |
| HSCL | 49.0 (11.4) | 35.5 (13.7)a | 38.6 (8.3)a | 45.6 (13.8) | 33.7 (15.6)a | 37.4 (10.8)a | 42.0 (11.5) | 41.6 (13.2)b | 43.0 (14.1)b |
| Mentally unhealthy days | 11.8 (9.5) | 9.5 (7.5)a | 7.4 (6.9)a | 10.8 (10.6) | 9.4 (6.8)a | 9.6 (7.6)a | 11.2 (7.3) | 13.7 (9.3)b | 14.4 (10.0)b |
| MSIS | 60.4 (12.4) | 65.7 (11.2) | 65.5 (11.4)a | 62.7 (14.0) | 63.6 (11.8) | 64.4 (13.6) | 63.3 (9.2) | 62.5 (14.8) | 58.7 (15.5)b |
Different superscripts between columns representing the same time point represent statistically significant differences between the treatment conditions (P < 0.05).
QOLI, Quality of Life Inventory total T score; SF-36 PCS, SF-36 Health Survey Physical Component Summary; SF-36 MCS, SF-36 Health Survey Mental Component Summary; POMS, Profile of Mood States total mood disturbance score; HSCL, Hopkins Symptom Checklist total score; MSIS, Miller Social Intimacy Scale total score.
Post-intervention (T2). There was a significant group effect on post-intervention (T2) QOLI (F = 10.6, P < 0.001, ηp2 = 0.27), POMS (F = 4.3, P = 0.02, ηp2 = 0.13) and HSCL (F = 4.5, P = 0.02, ηp2 = 0.14) scores, and number of mentally unhealthy days (F = 4.3, P = 0.02, ηp2 = 0.13). The QOLT group had higher QOLI scores than the ST (Cohen’s d = 0.71 and d = 0.47, respectively) and SC (d = 0.64 and d = 0.65, respectively) groups. Relative to the SC group, both the QOLT and ST groups had lower POMS (d = 0.26 and d = 0.27, respectively), lower HSCL scores (d = 0.46 and d = 0.55, respectively), and fewer mentally unhealthy days (d = 0.50 and d = 0.53, respectively). There was no group effect at post-intervention (T2) for SF-36 PCS (P = 0.36), SF-36 MCS (P = 0.11) or MSIS (P = 0.13) scores.
Twelve-week follow-up (T3). There was a significant group effect on follow-up (T3) QOLI (F = 9.1, P < 0.001, ηp2 = 0.24), SF-36 MCS (F = 4.4, P = 0.02, ηp2 = 0.13), POMS (F = 4.0, P = 0.02, ηp2 = 0.12) and HSCL (F = 4.5, P = 0.02, ηp2 = 0.13) scores, number of mentally unhealthy days (F = 7.5, P = 0.001, ηp2 = 0.21), and MSIS scores (F = 3.4, P = 0.05, ηp2 = 0.16). The QOLT group had higher QOLI and SF-36 MCS scores than both the ST (d = 0.62 and d = 0.51, respectively) and SC (d = 0.63 and d = 0.52, respectively) groups. The QOLT group had lower POMS (d = 0.35) and HSCL (d = 0.38) scores, fewer mentally unhealthy days (d = 0.81) and higher MSIS scores (d = 0.37) compared with the SC group. The ST group also had lower HSCL scores (d = 0.55) and fewer mentally unhealthy days than the SC group (d = 0.54). There was no group effect on follow-up (T3) SF-36 PCS scores (P = 0.47).
Clinically significant change (Table 3)
Table 3.
Number (%) of patients in each intervention group with clinical improvement, clinical decline or no clinical change at post-intervention (T2) based on the calculated RCI
| QOLT |
ST |
SC |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | Improved | Declined | No change | Improved | Declined | No change | Improved | Declined | No change |
| QOLI | 14 (64%)a | 2 (9%) | 6 (27%) | 0 (0%)b | 4 (20%) | 16 (80%) | 2 (10%)b | 2 (10%) | 16 (80%) |
| SF-36 PCS | 1 (5%) | 2 (9%) | 19 (86%) | 5 (25%) | 5 (25%) | 10 (50%) | 2 (10%) | 2 (10%) | 16 (80%) |
| SF-36 MCS | 8 (36%)a | 1 (5%) | 13 (59%) | 3 (15%)b | 5 (25%) | 12 (60%) | 2 (10%)b | 3 (15%) | 15 (75%) |
| POMS | 10 (46%)a | 2 (9%) | 10 (46%) | 6 (30%)a | 1 (5%) | 13 (65%) | 2 (10%)b | 3 (15%) | 15 (75%) |
| HSCL | 11 (50%)a | 2 (9%) | 9 (41%) | 9 (45%)a | 1 (5%) | 10 (50%) | 1 (5%)b | 1 (5%) | 18 (90%) |
| HRQoL mentally unhealthy days | 6 (27%)a | 0 (0%) | 16 (73%) | 4 (20%) | 3 (15%) | 13 (65%) | 2 (10%)b | 6 (30%) | 12 (60%) |
| MSIS | 6 (40%)a | 0 (0%) | 9 (60%) | 3 (23%) | 2 (15%) | 8 (62%) | 1 (9%)b | 2 (18%) | 8 (73%) |
Different superscripts between columns labelled ‘Improved’ represent statistically significant differences between the treatment conditions (P < 0.05).
QOLI, Quality of Life Inventory total T score; SF-36 PCS, SF-36 Health Survey Physical Component Summary; SF-36 MCS, SF-36 Health Survey Mental Component Summary; POMS, Profile of Mood States total mood disturbance score; HSCL, Hopkins Symptom Checklist total score; MSIS, Miller Social Intimacy Scale total score.
Using RCI criteria [28], there were different rates of clinical change for QOLI T (χ2 = 26.0, P < 0.001), POMS (χ2 = 6.4, P = 0.04) and HSCL (χ2 = 9.6, P = 0.01) scores. Follow-up analyses (all Ps < 0.05) showed that the QOLT group displayed more clinical improvement than the SC group on all measures, while the ST group displayed more improvement than the SC group on psychological distress measures (POMS and HSCL) only. The QOLT group was more likely than the ST group to show clinical improvement on the QOLI, but these groups did not differ from each other on the POMS or HSCL.
Discussion
While KT offers the possibility of improvements in physical health and activity [28,29], the lengthy waiting time combined with the emotional and physical demands of managing chronic kidney disease can contribute to low QOL, heightened stress, feelings of uncertainty and anxiety, and strained relationships [2,3,8,12,30–32]. Identifying effective clinical interventions to improve QOL and psychological functioning in this population is important. The current study found that QOLT had superior QOL outcomes relative to both ST and SC, and superior social intimacy outcomes relative to SC. However, QOLT and ST were comparable in reducing psychological distress.
The current findings are consistent with those of a QOLT clinical trial in which 58 wait-listed lung transplant candidates were randomized to receive telephone-based QOLT or ST [18]. Baseline levels of QOL and mood disturbance were very similar in the two studies, and QOLT was superior to ST in improving QOL but not in attenuating mood disturbance. Interestingly, the degree of QOL change in the QOLT group was nearly identical in both studies. However, there were some differences between the studies. The improvement in mood disturbance for the ST patients was maintained at the 12-week follow-up in the current study, but not in the lung transplant study. Also, QOLT and ST patients in the current study did not differ in their social intimacy levels. QOLT patients did have higher social intimacy scores than SC patients at 12-week follow-up, but this was due to a decline in social intimacy in the SC group. In contrast, Rodrigue et al. [18] reported impressive gains in social intimacy for the QOLT group, relative to ST patients, 1 month after treatment, although these effects were not maintained at 12-week follow-up. One explanation for why social intimacy effects were not seen in the current study is that baseline levels were already quite high in both QOLT and ST groups. Collectively, these two studies raise interesting questions about the QOLT effectiveness across transplant populations and treatment modality. Future research should examine the differential effectiveness of telephone versus face-to-face treatment delivery methods in a single trial, and whether QOLT with other transplant patients (e.g. liver and heart) can produce clinical benefits similar to those observed for lung and kidney transplant patients.
Consistent with previous findings [18], we found that ST was equally effective as QOLT at reducing symptoms of overall mood disturbance and mentally unhealthy days. However, the reduction in psychological distress for ST patients did not lead to a corresponding increase in QOL or social intimacy, as was observed for QOLT patients. In contrast to ST, QOLT provided patients with specific strategies and homework assignments to achieve both symptom reduction and life enhancement while waiting for KT. QOLT encouraged patients to look beyond their physical limitations and to optimize satisfaction in other life domains that are important to them (self-esteem, goals and values, relationships, etc.). Interestingly, QOLT patients showed no changes in their physical QOL (e.g. on the SF-36 PCS), yet were able to achieve higher life satisfaction and less psychological distress.
We recognize that many transplant programmes may not have the same level of mental health services that are available in our centre. However, screening for low QOL and high mood disturbances in wait-listed patients requires very little time and effort, both for patients and staff. High-risk patients could then be offered or referred for treatment. While QOLT may not be easily accessible, ST services are readily available in many transplant centres or dialysis units, and they provide important psychological benefit for patients. However, their limitations in producing more sweeping QOL changes should be noted.
Prior research has found a heightened strain in the patient–caregiver relationship for dialysis and KT patients [3,12]. Excessive worry and anxiety, role changes necessitated by the patient’s physical health, financial strain, alterations in sexual activity and communication patterns, and other responsibilities (e.g. work, childcare and care of elderly parent) can contribute to caregiving burden and psychological distress among spouses or partners. Daneker et al. [3] demonstrated that a high caregiver strain in the context of ESRD contributes to negative relationship changes, which further exacerbate mood disturbances in patients. In the current study, QOLT patients reported a modest increase in social intimacy, while SC patients reported a declining social intimacy over the course of 3 months. Although QOLT may hold some psychological and relationship benefits for spouse caregivers even when they are not an active part of the treatment process [33], the impact of spouse participation in QOLT is unknown and should be evaluated in future trials.
Recent studies provide preliminary evidence for offering psychological interventions to dialysis and KT patients. Lii et al. [34] found that a group-based cognitive–behavioural treatment, relative to a control group, was more effective at decreasing depression symptoms, increasing self-care behaviours and improving QOL in patients receiving haemodialysis. Tsay and her colleagues [13,35] reported similar findings in two other randomized clinical trials evaluating psychological interventions in ESRD patients. Gross et al. [15] showed that a group mindfulness-based stress reduction programme can produce clinically meaningful change in anxiety, depression and sleep quality for solid-organ transplant recipients (including KT), although QOL benefits could not be demonstrated. The findings from the current study add to this promising and growing literature. One advantage of the current study is that QOLT was compared to both an inactive (standard care) control group and an active condition that provided social support, therapeutic attention, and a positive expectancy.
Interpretation of these findings should be considered in the context of a few methodological limitations. Statistical power was adequate to detect meaningful clinical change, but our small sample did not allow us to identify predictors of treatment response, the patients most likely to benefit from which treatment approach, and specific mechanisms of change. For instance, it is possible that patients receiving dialysis have a differential response to treatment than those not yet requiring dialysis. We found that there were no statistically significant differences between dialysis and non-dialysis patients on the outcome measures (data not reported), but <20% of our sample was not yet on dialysis, and meaningful comparisons cannot be made. Another limitation is that our SC group was not an attention control group, thus introducing the possibility that QOLT and ST benefits were due to non-specific factors. Also, we cannot rule out biases due to subject self-selection and conducting the study at one transplant centre. Patients who agree to take part in this type of study may be more amenable and less recalcitrant to psychological intervention, so they may be more likely to report therapeutic gains than those who choose not to participate. Also, our transplant centre employs three full-time mental health professionals, which may influence patient expectations about proactively attending to mental health needs.
Acknowledgments
This research was supported by a grant (DK077322) from the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK). The authors are grateful to the patients who participated in this study. Also, this research would have not been possible without the many individuals who facilitated patient recruitment, treatment delivery, assessments and data management: Timothy Antonellis, Noelle Dimitri, Ogo Egbuna, Jonathan Florman, Jean Francis, Robert Guenther, Kathleen MacNaughton, Richard McCartney, Colleen Morse, Matthew Paek, Amanda Reed and Hongying Tang.
Conflict of interest statement. None declared.
References
- 1.Son YJ, Choi KS, Park YR, et al. Depression, symptoms and the quality of life in patients on hemodialysis for end-stage renal disease. Am J Nephrol. 2009;29:36–42. doi: 10.1159/000150599. [DOI] [PubMed] [Google Scholar]
- 2.Kimmel PL, Cukor D, Cohen SD, et al. Depression in end-stage renal disease patients: a critical review. Adv Chron Kidney Dis. 2007;14:328–334. doi: 10.1053/j.ackd.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Daneker B, Kimmel PL, Ranich T, et al. Depression and marital dissatisfaction in patients with end-stage renal disease and in their spouses. Am J Kidney Dis. 2001;38:839–846. doi: 10.1053/ajkd.2001.27704. [DOI] [PubMed] [Google Scholar]
- 4.Tomasz W, Piotr S. A trial of objective comparison of quality of life between chronic renal failure patients treated with hemodialysis and renal transplantation. Ann Transplant. 2003;8:47–53. [PubMed] [Google Scholar]
- 5.Deligiannis A. Report from the Second International Congress on Quality of Life in End-stage Renal Disease, Thessaloniki, Greece, 8–9 March 2002. Nephrol Dial Transplant. 2002;17:1888–1889. doi: 10.1093/ndt/17.11.1888. [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol. 2009;4:1057–1064. doi: 10.2215/CJN.00430109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perlman RL, Finkelstein FO, Liu L, et al. Quality of life in chronic kidney disease (CKD): a cross-sectional analysis in the Renal Research Institute-CKD study. Am J Kidney Dis. 2005;45:658–666. doi: 10.1053/j.ajkd.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Cukor D, Coplan J, Brown C, et al. Anxiety disorders in adults treated by hemodialysis: a single-center study. Am J Kidney Dis. 2008;52:128–136. doi: 10.1053/j.ajkd.2008.02.300. [DOI] [PubMed] [Google Scholar]
- 9.Cukor D, Rosenthal DS, Jindal RM, et al. Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients. Kidney Int. 2009;75:1223–1229. doi: 10.1038/ki.2009.51. [DOI] [PubMed] [Google Scholar]
- 10.Lopes AA, Bragg J, Young E, et al. Dialysis Outcomes and Practice Patterns Study (DOPPS): depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney Int. 2002;62:199–207. doi: 10.1046/j.1523-1755.2002.00411.x. [DOI] [PubMed] [Google Scholar]
- 11.Kimmel PL, Peterson RA, Weihs KL, et al. Multiple measurements of depression predict mortality in a longitudinal study of chronic hemodialysis patients. Kidney Int. 2000;57:2093–2098. doi: 10.1046/j.1523-1755.2000.00059.x. [DOI] [PubMed] [Google Scholar]
- 12.Frazier PA, Davis-Ali SH, Dahl KE. Stressors, social support, and adjustment in kidney transplant patients and their spouses. Soc Work Health Care. 1995;21:93–108. doi: 10.1300/J010v21n02_07. [DOI] [PubMed] [Google Scholar]
- 13.Daneker B, Kimmel PL, Ranich T, Peterson RA. Depression and marital dissatisfaction in patients with end-stage renal disease and in their spouses. Am J Kidney Dis. 2001;38:839–846. doi: 10.1053/ajkd.2001.27704. [DOI] [PubMed] [Google Scholar]
- 14.Tsay SL. Self-efficacy training for patients with end-stage renal disease. J Adv Nurs. 2003;43:370–375. doi: 10.1046/j.1365-2648.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- 15.Chang CF, Winsett RP, Gaber AO, et al. Cost-effectiveness of post-transplantation quality of life intervention among kidney recipients. Clin Transpl. 2004;18:407–414. doi: 10.1111/j.1399-0012.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 16.Gross CR, Kreitzer MJ, Russas V, et al. Mindfulness meditation to reduce symptoms after organ transplant: a pilot study. Adv Mind Body Med. 2004;20:20–29. [PubMed] [Google Scholar]
- 17.Frisch MB. Quality of life therapy: applying a life satisfaction approach to positive psychology and cognitive therapy. Hoboken, NJ, USA: John Wiley & Sons; 2006. [Google Scholar]
- 18.Harper A, Power M. Development of the World Health Organization WHOQOL-BREF Quality of Life Assessment. Psychol Med. 1998;28:551–558. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigue JR, Baz MA, Widows MR, et al. A randomized evaluation of quality-of-life therapy with patients awaiting lung transplantation. Am J Transplant. 2005;5:2425–2432. doi: 10.1111/j.1600-6143.2005.01038.x. [DOI] [PubMed] [Google Scholar]
- 20.Frisch MB. Quality of Life Inventory (QOLI) Minneapolis, MN, USA: National Computer Systems; 1994. [Google Scholar]
- 21.Ware JE, Kosinski M. Improvements in the content and scoring of the SF-36 Health Survey. Version 2. www.sf-36.org. [Google Scholar]
- 22.McNair D, Lorr M, Droppelman L. Manual for the Profile of Mood States. San Diego, CA, USA: Educational and Industrial Testing Service; 1981. [Google Scholar]
- 23.Derogatis LR, Lipman RS, Rickels K, et al. The Hopkins symptom checklist (HSCL)—a self-report symptom inventory. Behav Sci. 1974;19:1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- 24.Andresen EM, Fitch CA, McLendon PM, et al. Reliability and validity of disability questions for US Census 2000. Am J Public Health. 2000;90:1297–1299. doi: 10.2105/ajph.90.8.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller RS, Lefcourt HM. Miller Social Intimacy Scale. In: Corcoran K, Fischer J, editors. Measures For Clinical Practice: A Sourcebook. 3rd edn. NY, USA: Free Press; 2000. pp. 469–471. [Google Scholar]
- 26.Frisch MB. Quality of life therapy and assessment in health care. Clin Psychol Sci Pract. 1998;5:19–40. [Google Scholar]
- 27.Novalis PN, Rojcewicz SJ, Peele R. Clinical Manual of Supportive Psychotherapy. Washington DC, USA: American Psychiatric Press; 1993. [Google Scholar]
- 28.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Meth. 2002;7:147–177. [PubMed] [Google Scholar]
- 29.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 30.Neipp M, Karavul B, Jackobs S, et al. Quality of life in adult transplant recipients more than 15 years after kidney transplantation. Transplantation. 2006;81:1640–1644. doi: 10.1097/01.tp.0000226070.74443.fb. [DOI] [PubMed] [Google Scholar]
- 31.Tomasz W, Piotr S. A trial of objective comparison of quality of life between chronic renal failure patients treated with hemodialysis and renal transplantation. Ann Transplant. 2003;8:47–53. [PubMed] [Google Scholar]
- 32.Mujais SK, Story K, Brouillette J, et al. Health-related quality of life in CKD patients: correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4:1293–1301. doi: 10.2215/CJN.05541008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berthoux F, Bartiromo M. How to improve quality of life in patients with chronic kidney disease: a personal view. J Nephrol. 2008;21:S7–S8. [PubMed] [Google Scholar]
- 34.Ritz E. Can we ameliorate quality of life in chronic kidney disease? The nephrologist’s point of view. J Nephrol. 2008;21:S9–S11. [PubMed] [Google Scholar]
- 35.Rodrigue JR, Widows MR, Baz MA. Caregivers of lung transplant candidates: do they benefit when the patient is receiving psychological services? Prog Transplant. 2006;16:336–342. doi: 10.1177/152692480601600409. [DOI] [PubMed] [Google Scholar]
- 36.Lii YC, Tsay SL, Wang TJ. Group intervention to improve quality of life in haemodialysis patients. J Clin Nurs. 2007;16:268–275. doi: 10.1111/j.1365-2702.2007.01963.x. [DOI] [PubMed] [Google Scholar]
- 37.Tsay SL, Lee YC. Effects of an adaptation training programme for patients with end-stage renal disease. J Adv Nurs. 2005;50:39–46. doi: 10.1111/j.1365-2648.2004.03347.x. [DOI] [PubMed] [Google Scholar]