Abstract
Background. Despite a higher incidence of end-stage renal disease (stage 5), blacks have been shown to have the same or lower prevalence of chronic kidney disease (CKD stages 3 and 4). Current creatinine-based glomerular filtration rate (GFR)-estimating equations may misclassify young, healthy blacks.
Methods. Among 3501 young adults (mean age 45), we compared the prevalence of CKD in blacks and whites using the Modification of Diet in Renal Disease (MDRD) and the CKD Epidemiology Collaboration (CKD-EPI) equations. In addition, we used measured creatinine excretion rates to determine the actual excretion ratio for CARDIA (race coefficient 12%) and applied this to the CKD-EPI equation. We also studied the prevalence of CKD risk factors among black and white participants near the CKD threshold cut-off (eGFR CKD-EPI 60–80 mL/min/1.73 m2) to estimate the relative likelihood of misclassification in blacks and whites.
Results. Using the MDRD equation, prevalence of CKD stages 4 and 5 was higher for blacks compared with whites (0.6% vs. 0.1%, P-value 0.05). In contrast, prevalence of eGFR <60 mL/min/1.73 m2 was significantly higher for whites (3.6%) compared with blacks (1.9%), due to higher prevalence of stage 3 among whites. Prevalence of CKD was similar for blacks and whites using CKD-EPI equation (1.2%), but was higher among blacks when using the CARDIA-derived race coefficient (1.6% vs.1.2%, P-value = 0.03). Among persons with eGFR by CKD-EPI of 60–80 mL/min/1.73 m2, blacks had higher levels of albuminuria, uric acid, systolic blood pressure and higher diabetes prevalence.
Conclusions. CKD classification among young blacks is very sensitive to the race coefficients. Despite whites having higher rates of CKD stage 3, blacks with eGFRs just above the CKD threshold had higher rates of CKD risk factors. Current equations used to define CKD may systematically miss a high-risk group of blacks at a time in the disease course when interventions are crucial.
Keywords: chronic kidney disease, glomerular filtration rate, race
Introduction
Black Americans have a 2–4-fold incidence of end-stage renal disease (ESRD) compared with whites [1,2]. Paradoxically, national studies have found that chronic kidney disease (CKD) prevalence is lower for blacks compared with whites, except at advanced stages [3]. These observations have led to the hypothesis that blacks may progress faster from CKD to ESRD compared with whites [4].
An additional explanation for these observations may be that prevalence studies are limited by the lack of accurate tools for estimation of glomerular filtration rate (GFR) in non-white populations without known CKD (eGFR ≥60 mL/min/1.73 m2). This inaccuracy could lead to spurious or biased estimates of CKD prevalence. The current standard is to estimate GFR by using the creatinine-based Modification of Diet in Renal Disease (MDRD) equation [5,6], which includes a correction factor for race (a 21% higher eGFR for the same creatinine value for blacks compared with whites) [5]. This equation was developed in a cohort with advanced CKD, so the race correction factor may not apply to a younger population of blacks without CKD. In addition, the MDRD equation is known to be less accurate among subjects with normal or mildly reduced kidney function when tested against gold standard direct GFR measurement methods [7–10]. Recently, a new equation was developed by the CKD Epidemiology Collaboration (CKD-EPI) group that may be more accurate at higher GFR levels. The development datasets for CKD-EPI included a larger proportion of younger participants, and the race correction factor is lower (16%). However, this equation has not yet been validated in young blacks without CKD [11].
The use of creatinine-based GFR-estimating equations with differing race correction coefficients could directly impact the classification of CKD, which is critical for understanding racial/ethnic differences in CKD prevalence. Moreover, classification of CKD is important for accurately capturing those at high risk of adverse events in clinical practice, where GFR is now routinely reported [12]. We designed these analyses to: (i) study the prevalence of CKD in a young, healthy, bi-racial cohort using the MDRD and the newly developed CKD-EPI equations; and (ii) evaluate the impact of the race correction coefficients on CKD classification by race.
Materials and methods
Participants
Study design details of Coronary Artery Risk Development in Young Adults (CARDIA) have been previously published [13]. Briefly, a cohort of healthy young adults from the community, age 18–30 at the time of enrolment, balanced by race (black and white) and sex was recruited by telephone or door-to-door between 1985 and 1986 from four participating sites: Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA. In order to be enrolled in CARDIA, participants had to be free of any chronic disease. Follow-up examinations occurred at Year 2, 5, 7, 10, 15 and 20. We restricted the present analysis to the 3549 participants who completed the Year 20 examination. We excluded those with no serum creatinine measure at Year 20, for a total sample size of 3501. All study protocols were approved by the appropriate institutional review boards.
Kidney function measures
Serum creatinine was measured by nephelometry and calibrated to National Institute of Standards and Technology (NIST) standards as recommended by the National Kidney Disease Education Program (NKDEP) Laboratory Working Group [14]. We estimated GFR for blacks and whites separately using both the MDRD equation [5] and the newly developed CKD-EPI equation using Year 20 serum creatinine values [11].
Variables of interest
Sociodemographic factors were assessed by questionnaire at all study visits. Race was determined by participant self-report at baseline. We used socioeconomic data from Year 20 rather than baseline as a more accurate reflection of a person's socioeconomic status at the time of kidney function assessment. As a marker of socioeconomic status, we used income (categorized as <$25 000, $25 000–<$50 000 and ≥$50 000) and level of highest educational attainment (categorized as less than high school, high school graduate, some college, completed college and more than college). Systolic and diastolic blood pressure were defined as average of the second and third of three measurements taken at 1-min intervals after a 5-min rest. A diagnosis of hypertension was recorded if the participant ever had a systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, or use of anti-hypertensive medications. Height and weight were used to calculate body mass index [weight (kilogram) divided by height squared (square metre)]. Diabetes was defined by fasting glucose ≥126 mg/dL or use of diabetes medications. History of cardiovascular disease (CVD) was defined by an adjudicated CVD CARDIA end point [myocardial infarction, congestive heart failure (CHF) or stroke] before the Year 20 examination. LDL cholesterol (LDL-c) was derived using the Friedewald formula from fasting samples. Serum uric acid was calibrated to NIST standards and expressed in milligram per decilitre. Urinary albumin and creatinine from Year 20 were reported as albumin-to-creatinine ratio in milligram per gram.
Statistical analyses
We first estimated GFR by each of the equations, MDRD and CKD-EPI for all participants, and classified them by CKD stage, according to National Kidney Foundation criteria [6]. Using each equation separately, we estimated the prevalence of stage 3 (eGFR 30–59 mL/min/1.73 m2) and stages 4 and 5 (eGFR <30 mL/min/1.73 m2) for blacks and whites separately. We also estimated the overall prevalence of CKD, defined as eGFR <60 mL/min/1.73 m2 at Year 20, overall and by race. We then estimated the prevalence rate ratio of CKD for blacks compared with whites, using each equation.
In a second analysis, we estimated the overall prevalence of CKD and each CKD stage at Year 20 using the CKD-EPI equation with a CARDIA-derived race coefficient of 1.12 (i.e. 12% increase in eGFR for blacks for same value of creatinine). This coefficient was derived using three separate 24-h urine collections from a subsample of 443 black and 392 white CARDIA participants. Creatinine excretion rates for each individual were expressed in milligram/24 h. Mean creatinine excretion by race was estimated. The ratio of creatinine excretion was estimated at 12% milligram/24 h higher for blacks [15]. We then estimated the prevalence rate ratio of CKD for blacks compared with whites, using the CKD-EPI equation with CARDIA-derived race coefficient.
We hypothesized that the magnitude of the race coefficient (i.e. 1.21 vs. 1.16 in MDRD vs. CKD-EPI) would strongly influence the prevalence of stage 3 CKD among blacks and would change the estimates of racial differences. We further hypothesized that the current estimating equations may systematically misclassify blacks as being free of CKD. If this were true, the misclassification would occur in those with eGFR 60–80 mL/min/1.73 m2, and blacks would have more advanced CKD than whites when captured by the equations. Therefore, we compared characteristics of black vs. whites with CKD by gender using chi-square or t-test where appropriate. Furthermore, we examined the characteristics of those with eGFR 60–80 mL/min/1.73 m2 reasoning that blacks in this group near the CKD threshold would have greater evidence of kidney disease and kidney disease risk factors than whites in this range. We compared the prevalence of the major CKD risk factors among blacks and white subjects with eGFR 60–80 mL/min/1.73 m2 (systolic blood pressure, diabetes, lipids and BMI) in addition to possible markers of CKD presence including albuminuria and serum uric acid levels using chi-square, Fisher's exact or Wilcoxon tests where appropriate. We presented the mean values of the albumin-to-creatinine ratio and log-transformed the ratio for comparisons to achieve normality.
All analyses were performed using STATA version 9 (StataCorp, TX, USA). Statistical significance was determined at P < 0.05.
Results
Participant characteristics
Of the 3501 CARDIA participants included in these analyses, 47% self-identified as black. Mean age of the cohort at Year 20 visit was 45 years, 11% had diabetes and 21% had hypertension. Mean eGFR by MDRD was 90 ± 22 mL/min/1.73 m2, and mean eGFR CKD-EPI was 97 ± 18 mL/min/1.73 m2. Mean albumin-to-creatinine ratio was 13 ± 60 mg/g.
Compared with white, blacks had higher levels of albuminuria, body mass index and systolic blood pressure, and were more likely to be hypertensive and diabetic (Table 1).
Table 1.
Characteristics of 3501 CARDIA participants at Year 20 examination by race
| Characteristic | Black (n = 1651) | White (n = 1898) | P-value |
|---|---|---|---|
| Mean (SD) or n (%) | |||
| Age | 45 ± 4 | 46 ± 3 | <0.001 |
| Female | 991 (61%) | 989 (53%) | <0.001 |
| Income | <0.001 | ||
| <$25 000 | 392 (24%) | 148 (8%) | |
| $25 000–$49 999 | 411 (25%) | 263 (14%) | |
| $50 000+ | 788 (48%) | 1447 (77%) | |
| Education | <0.001 | ||
| Less than high school graduate | 108 (7%) | 40 (2%) | |
| High school graduate | 449 (28%) | 242 (13%) | |
| Some college | 569 (35%) | 376 (20%) | |
| College | 322 (20%) | 581 (31%) | |
| More than college | 167 (10%) | 628 (34%) | |
| Body mass index (kg/m2) | 31 ± 8 | 28 ± 7 | <0.001 |
| LDL cholesterol (mg/dL) | 110 ± 34 | 110 ± 31 | 0.89 |
| Systolic blood pressure (mmHg) | 121 ± 16 | 113 ± 13 | <0.001 |
| Fasting glucose (mg/dL) | 100 ± 31 | 96 ± 22 | 0.0001 |
| Serum creatinine (mg/dL) | 0.94 ± 0.55 | 0.87 ± 0.24 | <0.001 |
| MDRD estimated GFR | 97 ± 24 | 84 ± 19 | <0.001 |
| CKD-EPI estimated GFR | 102 ± 20 | 93 ± 14 | <0.001 |
| Albumin/creatinine ratio (mg/g) | 16.5 ± 63.6 | 10.5 ± 56.2 | 0.003 |
| Hx of cardiovascular eventsa | 19 (1%) | 13 (1%) | 0.14 |
| Diabetes | 231 (14%) | 161 (9%) | <0.001 |
| Hypertension | 486 (30%) | 234 (12%) | <0.001 |
| Smoker | 651 (40%) | 763 (41%) | 0.69 |
History of cardiovascular events includes heart failure, myocardial infarction or stroke during study period.
Prevalence of CKD in blacks and whites by creatinine-based eGFR-estimating equations
The overall prevalence of eGFR <30 mL/min/1.73 m2 (CKD stages 4 and 5) in CARDIA was 0.3%, and this estimate did not vary significantly when using the CKD-EPI or MDRD equation. Blacks had a higher prevalence of eGFR <30 mL/min/1.73 m2 compared with whites using either equation (0.6% vs. 0.1%, P-value 0.05 for MDRD; and 0.6% vs. 0.05%, P-value 0.05 with CKD-EPI).
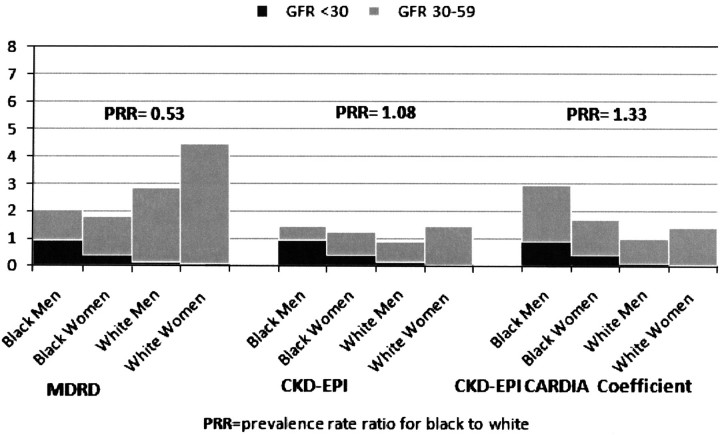
In contrast, when we considered the combined CKD stages 3–5, the overall prevalence of eGFR <60 mL/min/1.73 m2 using the MDRD equation was higher for whites compared with blacks. Blacks had an overall CKD prevalence of 1.9%, compared with 3.7% for whites (P-value = 0.001), with a prevalence rate ratio of 0.53 for blacks compared with whites. The observed contrast in the race differences was due to a higher prevalence of CKD stage 3 among whites compared with blacks (Figure 1).
Fig. 1.
Prevalence of CKD (GFR <60 mL/min/1.73 m2) by race and gender using MDRD, CKD-EPI equation and CKD-EPI equation with CARDIA-derived coefficient.
With the CKD-EPI equation, the overall prevalence of eGFR <60 mL/min/1.73 m2 was reduced across the entire CARDIA cohort, and this was due to a lower prevalence of CKD stage 3. This effect was more pronounced among whites. In contrast to the findings with the MDRD equation, the overall prevalence of eGFR <60 mL/min/1.73 m2 did not differ significantly by race using CKD-EPI equation (prevalence 1.2% for whites vs. 1.3% for blacks, P-value = 0.76) (Figure 1).
In analyses using the CKD-EPI equation with the CARDIA-derived race coefficient (12%), the overall prevalence of CKD was 1.4% (n = 48). Similar to the results above, blacks had a higher prevalence of CKD stage 4 and 5. However, when including stage 3 in our estimates, the overall prevalence of CKD was higher for blacks compared with whites. The prevalence of CKD was 1.6% for blacks, compared with 1.2% for whites, for a prevalence rate ratio of 1.33 (P-value = 0.03) (Figure 1).
The prevalence of CKD varied within each ethnic group using different equations, but the magnitude of this difference was higher for whites. The overall prevalence of CKD varied by ∼50% (1.9% by MDRD compared with 1.3% by CKD-EPI) among blacks and over 3-fold among whites (3.7% by MDRD compared with 1.2% by CKD-EPI).
Characteristics of participants with CKD (CKD-EPI eGFR <60 mL/min/1.73 m2)
Among those with CKD, black men had much lower eGFR and higher prevalence of albuminuria. Mean CKD-EPI eGFR for black men was 26 ± 22 mL/min/1.73 m2, compared with 49 ± 19 mL/min/1.73 m2 among white men (P-value 0.04). Mean albumin-to-creatinine ratio was 147 ± 131 mg/g among black men and 11 ± 12 mg/g for white men (P-value 0.02).
Similar patterns of a more severe disease were also seen in black women. Among women with eGFR <60 mL/min/1.73 m2, mean eGFR for black women was 38 ± 19 mL/min/1.73 m2, compared with 52 ± 10 mL/min/1.73 m2 for white women (P-value 0.003). Mean albumin-to-creatinine ratio was similarly higher among black compared with white women, though not statistically significant (167 ± 535 mg/g for black women and 113 ± 195 mg/g for white women, P-value 0.73).
Characteristics of participants with CKD-EPI eGFR 60–80 mL/min/1.73 m2
Because we found that the prevalence of CKD stage 3 was surprisingly lower among young blacks compared with young whites and that this was due to the race coefficient in estimating equations, we hypothesized that some blacks may be misclassified as being free of CKD. Because this misclassification would most likely occur among those with eGFR 60–80 mL/min/1.73 m2, we compared the characteristics of black and white CARDIA participants with eGFR by CKD-EPI 60–80 mL/min/1.73 m2. Among men with eGFR by CKD-EPI of 60–80 mL/min/1.73 m2, black men had a 2.5-fold higher prevalence of albuminuria and higher serum uric acid levels compared with white men. In addition, blacks had 80% higher prevalence of hypertension and higher BMI. Among women with eGFR 60–80 mL/min/1.73 m2, black women had a 4-fold higher prevalence of albuminuria, 10-fold prevalence of hypertension, and substantially higher BMI, fasting glucose and LDL levels compared with white women (Table 2).
Table 2.
Characteristics of CARDIA participants with eGFR 60–80 mL/min/1.73 m2 by CKD-EPI
| Characteristic | Black men | White men | P-value | Black women | White women | P-value |
|---|---|---|---|---|---|---|
| (n = 86) | (n = 161) | (n = 104) | (n = 223) | |||
| Albumin/creatinine ratio (mg/g)b | 38 ± 200 | 13 ± 55 | 0.004a | 21 ± 60 | 7 ± 11 | 0.006a |
| Serum uric acid (mg/dL) | 7.34 ± 1.53 | 6.88 ± 1.29 | 0.02 | 5.86 ± 1.61 | 4.98 ± 0.99 | <0.001 |
| Systolic blood pressure (mmHg) | 124 ± 16 | 117 ± 13 | 0.001 | 123 ± 20 | 108 ± 11 | <0.001 |
| Hypertension % (n) | 35 (30) | 19 (31) | 0.006 | 38 (40) | 4 (10) | <0.001 |
| Fasting glucose (mg/dL) | 104 ± 25 | 101 ± 25 | 0.41 | 105 ± 50 | 91 ± 8 | 0.005 |
| Diabetes % (n) | 7 (6) | 5 (8) | 0.52 | 17 (18) | 6 (14) | 0.001 |
| Body mass index | 31 ± 6 | 28 ± 4 | <0.001 | 32 ± 7 | 26 ± 6 | <0.001 |
| LDL cholesterol (mg/dL) | 113 ± 32 | 115 ± 30 | 0.69 | 112 ± 35 | 104 ± 30 | 0.03 |
Represents mean albumin/creatinine ratio, and P-value is for log-transformed comparisons.
Corrected for age and sex.
t-test for continuous variables, Wilcoxon test for median, chi-square test or Fisher exact test for categorical variables.
Discussion
We found that racial differences in prevalence estimates of CKD are very sensitive to the equation used to estimate GFR in a young, bi-racial cohort. When using the MDRD equation, whites had higher overall prevalence of CKD compared with blacks, due to a higher prevalence of stage 3 CKD among whites. In contrast, when using the CKD-EPI equation, not only was the overall prevalence of CKD reduced but we observed no race differences in CKD estimates. Moreover, when using the CARDIA-derived coefficient for the CKD-EPI equation, blacks had higher prevalence of CKD. Interestingly, despite having a higher prevalence of CKD stage 3 compared with blacks based on the validated equations, whites had a strikingly lower prevalence of risk factors for CKD. Rather than attributing this constellation of findings to an ‘epidemiological paradox’, we believe that an alternative explanation is that the current race coefficient for blacks may lead to a systematic misclassification of CKD among young blacks.
Our study shows that applying different race coefficients (1.21 in MDRD vs. 1.16 in CKD-EPI) in a young, healthy population significantly changes the association between race and CKD. This is of substantial importance epidemiologically as we attempt to understand why reports show that blacks have a lower prevalence of CKD [3] but higher ESRD incidence compared with whites [1]. Blacks have been shown to progress faster from CKD to ESRD [4], but this faster progression may not fully account for the higher ESRD incidence rates.
Our findings also show that blacks who are classified as having CKD (eGFR <60 mL/min/1.73 m2) have much lower eGFR and a worse CKD risk factor profile compared with whites in CARDIA. The creatinine threshold required to reach an eGFR of 60 mL/min/1.73 m2 is substantially higher for blacks (1.7 mg/dL for a black man age 45 compared with 1.4 mg/dL for a white man age 45). In young adults, it is unclear whether these creatinine differences are solely a correction for muscle mass or rather represent different severities of kidney disease.
Most interestingly, despite a much lower prevalence of risk factors for CKD, whites had a higher prevalence of CKD stage 3. If the current classification is accurate, then we would expect whites near the CKD cut-off to have higher severity of risk factors for CKD. However, we found that among those with eGFR 60–80 mL/min/1.73 m2, blacks had much higher prevalence of CKD-associated abnormalities and risk factors, particularly albuminuria, a critical marker of kidney damage. Although our study cannot determine the exact coefficient appropriate for young blacks, it is likely that the current most clinically available 21% higher eGFR for the same serum creatinine (i.e. MDRD equation) may misclassify young blacks as having no CKD. In addition, since the prevalence of CKD varied even further among whites than blacks using MDRD vs. CKD-EPI equations, it is possible that whites may be mislabelled as having CKD. These equations have not been validated in young adults with no established CKD. Importantly, the prevalence of stages 4 and 5 did not vary among blacks using either MDRD or CKD-EPI equation, where equations were developed and thus are thought to be most accurate [5,11]. These findings are of major importance because the current use of race coefficients in estimating equations may systematically misclassify young blacks as low CKD risk, and only capture them at advanced stages of disease, or mislabel whites as having CKD. Unfortunately, until a population-based study measures GFR in healthy, young adults, we cannot discern the extent of misclassification in whites and blacks.
Our study is the first to explore the impact of the current creatinine-based equations on CKD prevalence estimates by race and gender. We used a large, well-characterized young cohort with calibrated serum creatinine measures. We used both the accepted standard and the newly improved estimating equations for these analyses in addition to a CARDIA-derived coefficient. However, our study is limited by its cross-sectional nature, so it cannot assess the progression of CKD. We may also be limited by the overall low prevalence of CKD in this cohort. In addition, we did not have gold standard GFR measures to establish true CKD status. Unfortunately, no community-based study of blacks without CKD has measured GFR to date. Although we show that blacks near the CKD threshold have higher albuminuria and higher serum uric acid levels, we do not have information on other important metabolic parameters associated with CKD.
In summary, we found that CKD classification among blacks is very sensitive to the race coefficients of the current GFR-estimating equations, particularly at stage 3. Although whites had a higher prevalence of CKD stage 3 compared with blacks, their CKD risk factor profile was strikingly better than that of blacks. Our findings suggest that the current equations may underestimate CKD in blacks, particularly at stage 3, where interventions are most crucial. Future studies to develop GFR-estimating equations should include young, healthy, non-white populations and should consider alternative filtration markers that may be less biased by race.
Acknowledgments
C.A.P. is funded by grant 1K23DK082793-01 from NIDDK.
Conflict of interest statement. None declared.
References
- 1.US Renal Data System . Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD, USA: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007. USRDS 2007 Annual Data Report. [Google Scholar]
- 2.Klag MJ, Whelton PK, Randall BL, et al. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA. 1997;277:1293–1298. [PubMed] [Google Scholar]
- 3.Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 4.Hsu CY, Lin F, Vittinghoff E, et al. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14:2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 7.Lin J, Knight EL, Hogan ML, et al. A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol. 2003;14:2573–2580. doi: 10.1097/01.asn.0000088721.98173.4b. [DOI] [PubMed] [Google Scholar]
- 8.Perkins BA, Nelson RG, Ostrander BE, et al. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-��year follow-up study. J Am Soc Nephrol. 2005;16:1404–1412. doi: 10.1681/ASN.2004100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poggio ED, Wang X, Greene T, et al. Performance of the modification of diet in renal disease and Cockcroft–Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol. 2005;16:459–466. doi: 10.1681/ASN.2004060447. [DOI] [PubMed] [Google Scholar]
- 10.Stevens LA, Manzi J, Levey AS, et al. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis. 2007;50:21–35. doi: 10.1053/j.ajkd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.den Hartog JR, Reese PP, Cizman B, et al. The costs and benefits of automatic estimated glomerular filtration rate reporting. Clin J Am Soc Nephrol. 2009;4:419–427. doi: 10.2215/CJN.04080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 14.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs DR, Jr, Murtaugh MA, Steffes M, et al. Gender- and race-specific determination of albumin excretion rate using albumin-to-creatinine ratio in single, untimed urine specimens: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2002;155:1114–1119. doi: 10.1093/aje/155.12.1114. [DOI] [PubMed] [Google Scholar]