Introduction
The first hormonal contraceptive was approved for marketing in the United States in 1960. This contraceptive, known then and now as “the pill,” was taken orally and consisted of an estrogen and a progestin designed to be taken by women.
The combined estrogen/progestin oral contraceptive was a breakthrough in contraception for three reasons: because it was highly effective for preventing conception; because, unlike the condom and the diaphragm, the effectiveness of the oral contraceptive does not depend on its being used in conjunction with the act of intercourse; and because, unlike tubal ligation and vasectomy, the effect of the oral contraceptive is reversible. Female hormonal contraceptives administered by injection, transdermally, vaginally, and released from a subdermal implant are now available in the United States and elsewhere. All these contraceptive agents are based on the same general physiologic-biochemical principles as “the pill.” Hormonal contraceptives have been used by at least 500 million women alive today.
Kaiser Permanente (KP) became involved in oral contraceptives in the mid-1960s and has been actively involved in research on hormonal contraceptives since the late 1960s. This review describes the historical background of KP initial research on oral contraceptive safety and the contributions of KP research on hormonal contraception in the subsequent four decades.
Walnut Creek Contraceptive Drug Study
Even before oral contraceptives were marketed, concern about the noncontraceptive health effects of these drugs was acute. Similar concerns about safety have accompanied introduction of other forms of hormonal contraception.
All hormonal contraceptives designed for use by women involve exogenous administration of synthetic estrogen, progestin, or both at doses that have been termed “unphysiologic.” Administration of exogenous estrogen and progestin can alter secretion of hypothalamic, ovarian, and other hormones and thus can theoretically affect multiple organ systems and physiologic processes. As early as the 1930s, exogenous administration of estrogen was known to cause breast malignancy in some rodent species.
Soon after these drugs were first marketed, the US Food and Drug Administration (FDA) began to receive spontaneous reports of venous thromboembolic events and stroke in users of oral contraceptives. Published reports of thromboembolic events1,2 heightened concern about the safety of oral contraceptives.
By the mid-1960s, the need for epidemiologic studies of the noncontraceptive effects of oral contraceptives on women's health had become apparent. The enormous popularity of “the pill” brought recognition that tens of millions of women in the United States and hundreds of millions worldwide would be exposed to exogenous hormones over many years. Thus, any effect of oral contraceptives on cancer or other health conditions had enormous public health implications.
Hormonal contraceptives have been used by at least 500 million women alive today.
In 1966, in response to concern about the safety of “the pill,” Dr James Shannon (then Director of the National Institutes of Health, NIH) transferred $3 million to the National Institute of Child Health and Human Development (NICHD) to study this problem, and a decision was made to commission a large cohort study to evaluate the noncontraceptive health effects of oral contraceptives. Dr Philip Corfman (later to become NICHD's Director of the Center for Population Research) and Dr Daniel Siegel (an NIHCD statistician) investigated several possible sites for such an ambitious study—including the Mayo Clinic, the Health Insurance Plan of New York, and the US Department of Defense—but none appeared to have as much interest or ability as the KP Northern California Region to conduct such a study.
KP was considered a potential research site because personnel at the FDA had worked with Morris Collen, MD—a founder of The Permanente Medical Group (TPMG)—on a project to collect electronic data on prescriptions and on outpatient and inpatient diagnoses to facilitate identification of adverse drug effects. Personnel at NICHD were familiar with the capabilities of KP because they had worked with Jacob Yerushalmy, PhD, (a University of California at Berke-ley statistician affiliated with KP) on the Collaborative Perinatal Project. This was an epidemiologic study that included data collection from more than 50,000 pregnant women and long-term follow-up of outcomes in these women as well as their offspring. The KP Oakland Medical Center was a research site in the project.
In 1967, NICHD officials approached Dr Collen about KP's interest in conducting the epidemiologic cohort study. KP decision makers decided to conduct the study at the KP Walnut Creek Medical Center. The study began in 1968 with Fred Pellegrin, MD, and Irwin Fisch, MD, as the Co-Principal Investigators. Later, Drs Pellegrin and Fisch recruited Savitri Ramcharan, MD—who had trained in epidemiology at the University of California Berkeley School of Public Health under Dr Yerushalmy—to head the study, which was named the Walnut Creek Contraceptive Drug Study (WCCDS).
The first participants in the WCCDS were recruited in late 1968. From 1968 through early 1972, a total of 16,638 women aged 18 to 54 years were recruited into the follow-up study of oral contraception. An additional 1800 women who were pregnant or recently postpar-tum were recruited to a special cross-sectional study. Active follow-up of women in the WCCDS continued through 1978. From its start until its last publication, the study included analyses conducted by a number of visiting researchers, including Susan Harlap, MD (an Israeli scientist then on sabbatical), Valerie Beral, PhD (a United Kingdom scientist then on sabbatical), and Diana Petitti, MD, MPH (then an Epidemic Intelligence Service (EIS) officer with the US Centers for Disease Control and Prevention [CDC]). Dr Petitti continued to work with data from the study well into the 1980s. Supplemented by data from record linkage, from chart review, and from reexamining subjects, data from the study were used in studies published as late as 1993.
The WCCDS helped to establish the reputation of KP in epidemiologic research, demonstrated KP's ability to recruit subjects for large studies …
Table 13–43 separately lists WCCDS publications that address issues of contraception3–13 and that address other topics related to women's health.14–18 The total number of these publications is large. Equally important are other contributions of the WCCDS to research in the KP Northern California Region specifically and in KP more generally. The WCCDS helped to establish the reputation of KP in epidemiologic research, demonstrated KP's ability to recruit subjects for large studies, and helped develop the infrastructure for conducting federally funded research at KP.
Table 1.
Kaiser Permanente studies of hormonal contraception
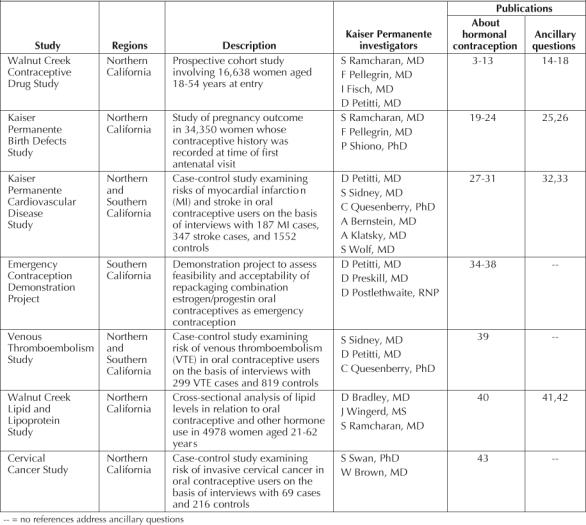
The Kaiser Permanente Birth Defects Study
In the early 1970s, studies from other countries raised concern about the possibility that use of hormonal contraceptives might affect a fetus in either of two circumstances: 1) when the fetus was conceived during use of oral contraceptives (which failed to prevent pregnancy) or 2) as a carry over from past exposure to hormones. Success of the WCCDS led the WCCDS team to receive an award from NICHD to conduct a study evaluating the effects of hormone exposure during early gestation on birth defects. At the first antenatal visit, the study collected information from more than 35,000 women receiving prenatal care at the KP Oakland, Hayward, Richmond, and Walnut Creek Medical Centers in Northern California. Pregnancy outcomes were ascertained by chart review.
Publications from this study—the KP Birth Defects Study—are listed in Table 1.19–26 As with the WCCDS, data from the Birth Defects Study were used to answer not only questions about the effect of contraceptives on birth defects but also many other questions about pregnancy outcome. In addition to its substantive contribution to knowledge about birth defects, the study further demonstrated the research capabilities of KP, enhanced the reputation of KP in the community, and contributed to the development of an infrastructure for conducting research in KP Northern California.
Vascular Disease Case-Control Studies
Epidemiologic studies conducted in the 1970s and 1980s established the increased risk of venous thromboembolism, ischemic stroke, and myocardial infarction from use of combined estrogen/progestin oral contraceptives. Shortly after reports first appeared describing vascular disease in oral contraceptive users, doses of estrogen in combined estrogen/progestin oral contraceptives were lowered in an attempt to reduce the vascular risks of oral contraceptive use. Attempts were also made to limit oral contraceptive use to women who were not at high risk for vascular disease (because of smoking or hypertension, for example). By the middle of the 1980s, confidence was high that changes in estrogen dose and in selection by clinicians of women for oral contraceptive use had successfully reduced the vascular risks of oral contraceptive use; however, empirical data to prove this point were limited.
In 1988, NICHD issued a request for proposals for case-control studies of the risk of stroke and myocardial infarction in users of low-estrogen-dose oral contraceptives. KP was successful in its bid for a contract to conduct this study. The study was a milestone for KP insofar as data collection for the research spanned both the KP Northern and Southern California Regions.
The study of stroke and myocardial infarction was followed by an identically designed study that assessed the risk of venous thromboembolic disease in users of low-estrogen-dose oral contraceptives. For the study, data were collected in both the KP Northern and Southern California Regions. These data were the subject of publications addressing the primary question at the outset of the research27–31,39 as well as ancillary questions32,33 about vascular disease epidemiology in women of reproductive age (Table 1). The studies were important for establishing the success of interregional collaborative research.
Emergency Contraception Demonstration Project
As early as 1975, researchers and clinicians recognized that a high dose of combination estrogen/progestin oral contraceptives could prevent pregnancy if taken shortly after an unprotected act of intercourse. (A hormonal contraceptive drug taken this way was initially called “the morning-after pill” and was later renamed “emergency contraception.”) This practice constituted off-label use of combined estrogen/progestin oral contraceptives and was not widespread.
… contributions take many forms, including case reports, new methodology for contraceptive research, and reviews and summaries of the relevant medical literature.
Beginning in the mid-1990s, several women's advocacy groups began to promote emergency contraception and to educate the public and physicians about it. Emergency contraception was difficult to promote, in part because it required physicians to provide individualized instruction on how to break up a package of combined oral contraceptives. Moreover, combined estrogen/progestin oral contraceptives exist in many different formulations containing different amounts of estrogen and progestin. Thus, the number of pills to be taken differs for different formulations of combined estrogen/progestin oral contraceptives.
In 1996, KP was approached by the Pacific Women's Health Institute (a not-for-profit women's health research institute based in Los Angeles) about a possible joint project designed to demonstrate the feasibility and acceptability of promoting hormonal emergency contraception in a community setting. External funds were secured to conduct such a project in San Diego County, but partner organizations in San Diego withdrew from the project because of concern about legal liability of promoting off-label use of a drug. The project went forward through KP in San Diego.
The project was highly successful and was the KP Southern California Region's nominee for the Vohs Award for Quality37 as well as the basis for several publications (Table 1).34–36,38 The success of the project at KP was influential in the decision of other organizations (for example, Planned Parenthood and various community clinics) to get involved in promoting emergency hormonal contraception.
Other Studies and Contributions
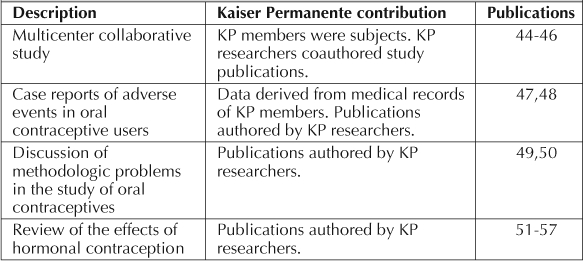
Table 1 lists other research studies on hormonal contraception conducted solely at KP.40–42 KP researchers and KP data made additional contributions to knowledge about hormonal contraception (Table 2).44–57 These contributions take many forms, including case reports, new methodology for contraceptive research, and reviews and summaries of the relevant medical literature.
Table 2.
Other publications on hormonal contraception involving Kaiser Permanente members, data, and researchers
Summary and Conclusion
The availability of hormonal contraceptives spawned changes in the social relations between men and women and enabled revolutionary changes in the roles of women in society. The contribution of hormonal contraception to improving the status of women worldwide is difficult to overestimate. Studies that have established the magnitude of risks and benefits of hormonal contraception have been instrumental for developing policies regarding hormonal contraception and for providing information that helps individuals and couples to make informed choices about childbearing.
For almost four decades, KP researchers have made sustained contributions to the advancement of knowledge on hormonal contraception. Data collected in studies of hormonal contraception have been used to address a variety of other important questions about women's health. Participation in research on hormonal contraception has made important contributions to the research infrastructure of the KP Northern and Southern California Regions and at KP nationally. Research on hormonal contraception has enhanced the research reputation of KP locally, nationally, and internationally.
Acknowledgments
We wish to acknowledge the many people, both inside and outside Kaiser Permanente, who contributed to the research described here, including those who played a role in formulating scientific questions, authoring and coauthoring publications, collecting and analyzing data, and participating in the research as subjects. Special thanks to Philip A Corfman, MD, who filled in many of the details on the history of the Walnut Creek Contraceptive Drug Study.
References
- Jordan WM. Pulmonary embolism. Lancet. 1961;2:1146–7. [Google Scholar]
- Boyce J, Fawcett JW, Nolan DC. Coronary thrombosis and Conovide. Lancet. 1963 Jan 12;1:111. doi: 10.1016/s0140-6736(63)91117-6. [DOI] [PubMed] [Google Scholar]
- Beral V, Ramcharan S, Faris R. Malignant melanoma and oral contraceptive use among women in California. Br J Cancer. 1977 Dec;36(6):804–9. doi: 10.1038/bjc.1977.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitti DB, Wingerd J, Pellegrin F, Ramcharan S. Oral contraceptives, smoking, and other factors in relation to risk of venous thromboembolic disease. Am J Epidemiol. 1978 Dec;108(6):480–5. doi: 10.1093/oxfordjournals.aje.a112646. [DOI] [PubMed] [Google Scholar]
- Petitti DB, Wingerd J. Use of oral contraceptives, cigarette smoking, and risk of subarachnoid haemorrhage. Lancet. 1978 Jul 29;2(8083):234–5. doi: 10.1016/s0140-6736(78)91745-2. [DOI] [PubMed] [Google Scholar]
- Ramcharan S, Sponzilli EE, Wingerd JC. Serum protein fractions. Effects of oral contraceptives and pregnancy. Obstet Gynecol. 1976 Aug;48(2):211–5. [PubMed] [Google Scholar]
- Swan SH. Inflammatory disease associated with oral contraceptive use. Lancet. 1981 Oct 10;2(8250):809. doi: 10.1016/s0140-6736(81)90218-x. [DOI] [PubMed] [Google Scholar]
- Sponzilli EE, Ramcharan S, Wingerd J. Rheumatoid factor (antigammaglobulin) in women: effects of oral contraceptives use of its prevalence. Arthritis Rheum. 1976 May–Jun;19(3):602–6. doi: 10.1002/art.1780190312. [DOI] [PubMed] [Google Scholar]
- Wingerd J, Duffy TJ, Creek W. Oral contraceptive use and other factors in the standard glucose tolerance test. Diabetes. 1977 Nov;26(11):1024–33. doi: 10.2337/diab.26.11.1024. [DOI] [PubMed] [Google Scholar]
- Wingerd J, Sponzilli EE. Concentrations of serum protein fractions in white women: effects of age, weight, smoking, tonsillectomy, and other factors. Clin Chem. 1977 Jul;23(7):1310–7. [PubMed] [Google Scholar]
- Ramcharan S, editor. The Walnut Creek Contraceptive Drug Study: a prospective study of the side effects of oral contraceptives. Volume I: Findings in oral contraceptive users and nonusers at entry to the study. Bethesda (MD): US Department of Health, Education, and Welfare, Center for Population Research; 1974. editor. (DHEW Publication No. (NIH) 74–562, Center for Population Research Monograph) [Google Scholar]
- Ramcharan S, editor. The Walnut Creek Contraceptive Drug Study: a prospective study of the side effects of oral contraceptives. Volume II: Additional findings in oral contraceptive users and nonusers. Bethesda (MD): US Department of Health, Education, and Welfare, Center for Population Research; 1976. editor. (DHEW Publication No. (NIH) 76–563, Center for Population Research Monograph) [Google Scholar]
- Ramcharan S, Pellegrin FA, Ray R, Hsu J-P. The Walnut Creek Contraceptive Drug Study: a prospective study of the side effects of oral contraceptives. Volume III: An interim report: a comparison of disease occurrence leading to hospitalization or death in users and nonusers of oral contraceptives. Bethesda (MD): US Department of Health, Education, and Welfare, Center for Population Research; 1981. (NIH Publication No. 81–564, Center for Population Research Monograph) [PubMed] [Google Scholar]
- Petitti DB, Sidney S. Obesity and cholecystectomy among women: implications for prevention. Am J Prev Med. 1988 Nov–Dec;4(6):327–30. [PubMed] [Google Scholar]
- Hahn RC, Petitti DB. Minnesota Multiphasic Personality Inventoryrated depression and the incidence of breast cancer. Cancer. 1988 Feb;61(4):845–8. doi: 10.1002/1097-0142(19880215)61:4<845::aid-cncr2820610434>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Petitti DB, Perlman JA, Sidney S. Noncontraceptive estrogens and mortality: long-term follow-up of women in the Walnut Creek Study. Obstet Gynecol. 1987 Sep;70(3 Pt 1):289–93. [PubMed] [Google Scholar]
- Petitti DB, Sidney S, Perlman JA. Increased risk of cholecystectomy in users of supplemental estrogen. Gastroenterology. 1988 Jan;94(1):91–5. doi: 10.1016/0016-5085(88)90614-2. [DOI] [PubMed] [Google Scholar]
- Hall V, Petitti DB, Ettinger B, Quesenberry C. Does estrogen replacement therapy prevent height loss? Menopause. 1994;1(1):3–9. [Google Scholar]
- Harlap S, Shiono P, Pellegrin F, et al. Chromosome abnormalities in oral contraceptive breakthrough pregnancies. Lancet. 1979 Jun 23;1(8130):1342–3. doi: 10.1016/s0140-6736(79)91969-x. [DOI] [PubMed] [Google Scholar]
- Harlap S, Shiono PH, Ramcharan S. Spontaneous foetal losses in women using different contraceptives around the time of conception. Int J Epidemiol. 1980 Mar;9(1):49–56. doi: 10.1093/ije/9.1.49. [DOI] [PubMed] [Google Scholar]
- Harlap S, Shiono PH, Ramcharan S, et al. Chromosomal abnormalities in the Kaiser Permanente Birth Defects Study, with special reference to contraceptive use around the time of conception. Teratology. 1985 Jun;31(3):381–7. doi: 10.1002/tera.1420310309. [DOI] [PubMed] [Google Scholar]
- Harlap S, Shiono PH, Ramcharan S. Congenital abnormalities in the offspring of women who used oral and other contraceptives around the time of conception. Int J Fertil. 1985;30(2):39–47. [PubMed] [Google Scholar]
- Shiono PH, Harlap S, Ramcharan S, Berendes H, Gupta S, Pellegrin F. Use of contraceptives prior to and after conception and exposure to other fetal hazards. Contraception. 1979 Aug;20(2):105–20. doi: 10.1016/0010-7824(79)90083-0. [DOI] [PubMed] [Google Scholar]
- Shiono PH, Harlap S, Ramcharan S. Sex of offspring of women using oral contraceptives, rhythm, and other methods of birth control around the time of conception. Fertil Steril. 1982 Mar;37(3):367–72. doi: 10.1016/s0015-0282(16)46097-8. [DOI] [PubMed] [Google Scholar]
- Harlap S, Shiono PH, Ramcharan S, Berendes H, Pellegrin F. A prospective study of spontaneous fetal losses after induced abortions. N Engl J Med. 1979 Sep 27;301(13):677–81. doi: 10.1056/NEJM197909273011301. [DOI] [PubMed] [Google Scholar]
- Harlap S, Shiono PH. Alcohol, smoking, and incidence of spontaneous abortions in the first and second trimester. Lancet. 1980 Jul 26;2(8187):173–6. doi: 10.1016/s0140-6736(80)90061-6. [DOI] [PubMed] [Google Scholar]
- Petitti DB, Sidney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stroke in users of low-dose oral contraceptives. N Engl J Med. 1996 Jul 4;335(1):8–15. doi: 10.1056/NEJM199607043350102. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Petitti DB, Siscovick DS, et al. Stroke and use of low-dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke. 1998 Nov;29(11):2277–84. doi: 10.1161/01.str.29.11.2277. [DOI] [PubMed] [Google Scholar]
- Sidney S, Petitti DB, Quesenberry CP, Jr, Klatsky AL, Ziel HK, Wolf S. Myocardial infarction in users of low-dose oral contraceptives. Obstet Gynecol. 1996 Dec;88(6):939–44. doi: 10.1016/s0029-7844(96)00351-1. [DOI] [PubMed] [Google Scholar]
- Sidney S, Siscovick DS, Petitti DB, et al. Myocardial infarction and use of low-dose oral contraceptives: a pooled analysis of 2 US studies. Circulation. 1998 Sep 15;98(11):1058–63. doi: 10.1161/01.cir.98.11.1058. [DOI] [PubMed] [Google Scholar]
- Petitti DB, Siscovick DS, Sidney S, et al. Norplant implants and cardiovascular disease. Contraception. 1998 May;57(5):361–2. doi: 10.1016/s0010-7824(98)00036-5. [DOI] [PubMed] [Google Scholar]
- Petitti DB, Sidney S, Quesenberry CP, Jr, Bernstein A. Incidence of stroke and myocardial infarction in women of reproductive age. Stroke. 1997 Feb;28(2):280–3. doi: 10.1161/01.str.28.2.280. [DOI] [PubMed] [Google Scholar]
- Petitti DB, Sidney S, Quesenberry C, Bernstein A. Stroke and cocaine or amphetamine use. Epidemiology. 1998 Nov;9(6):596–600. [PubMed] [Google Scholar]
- Beckman LJ, Harvey SM, Sherman CA, Petitti DB. Changes in providers' views and practices about emergency contraception with education. Obstet Gynecol. 2001 Jun;97(6):942–6. doi: 10.1016/s0029-7844(01)01365-5. [DOI] [PubMed] [Google Scholar]
- Harvey SM, Beckman LJ, Sherman C, Petitti D. Women's experience and satisfaction with emergency contraception. Fam Plann Perspect. 1999 Sep–Oct;31(5):237–40. 260. [PubMed] [Google Scholar]
- Petitti DB, Harvey SM, Preskill D, et al. Emergency contraception: preliminary report of a demonstration and evaluation project. J Am Med Womens Assoc. 1998;53(5 Suppl 2):251–4. [PubMed] [Google Scholar]
- Petitti DB, Preskill D, Heller K, et al. Emergency contraception research and demonstration project. Perm J. 2000 Spring;4(2):57–65. [Google Scholar]
- Sherman C, Harvey SM, Beckman LJ, Petitti DB. Emergency contraception: knowledge and attitudes of health care providers in a health maintenance organization. Women's Health Issues. 2001 Sep–Oct;11(5):448–57. doi: 10.1016/s1049-3867(01)00087-1. Erratum in: Women's Health Issues 2001 Nov–Dec;11(6):503. [DOI] [PubMed] [Google Scholar]
- Sidney S, Petitti DB, Soff GA, Cundiff DL, Tolan KK, Quesenberry CP., Jr. Venous thromboembolic disease in users of low-estrogen combined estrogen-progestin oral contraceptives. Contraception. 2004 Jul;70(1):3–10. doi: 10.1016/j.contraception.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bradley DD, Wingerd J, Petitti DB, Krauss RM, Ramcharan S. Serum high-density-lipoprotein cholesterol in women using oral contraceptives, estrogens and progestins. N Engl J Med. 1978 Jul 6;299(1):17–20. doi: 10.1056/NEJM197807062990104. [DOI] [PubMed] [Google Scholar]
- Krauss RM, Perlman JA, Ray R, Petitti D. Effects of estrogen dose and smoking on lipid and lipoprotein levels in postmenopausal women. Am J Obstet Gynecol. 1988 Jun;158(6 Pt 2):1606–11. doi: 10.1016/0002-9378(88)90198-6. [DOI] [PubMed] [Google Scholar]
- Wingerd J, Petitti DB. HDL–cholesterol and diabetes. Lancet. 1978 Sep 23;2:676. [PubMed] [Google Scholar]
- Swan SH, Brown WL. Oral contraceptive use, sexual activity, and cervical carcinoma. Am J Obstet Gynecol. 1981 Jan;139(1):52–7. doi: 10.1016/0002-9378(81)90411-7. [DOI] [PubMed] [Google Scholar]
- Cobb KL, Kelsey JL, Sidney S, Ettinger B, Lewis CE. Oral contraceptives and bone mineral density in white and black women in CARDIA. Coronary Risk Development in Young Adults. Osteoporos Int. 2002 Nov;13(11):893–900. doi: 10.1007/s001980200123. [DOI] [PubMed] [Google Scholar]
- Kim C, Siscovick DS, Sidney S, Lewis CE, Kiefe CI, Koepsell TD, CARDIA Study Oral contraceptive use and association with glucose, insulin, and diabetes in young adult women: the CARDIA Study. Coronary Artery Risk Development in Young Adults. Diabetes Care. 2002 Jun;25(6):1027–32. doi: 10.2337/diacare.25.6.1027. [DOI] [PubMed] [Google Scholar]
- London RS, Chapdelaine A, Upmalis D, Olson W, Smith J. Comparative contraceptive efficacy and mechanism of action of the norgestimate-containing triphasic oral contraceptive. Acta Obstet Gynecol Scand Suppl. 1992;156:9–14. doi: 10.3109/00016349209156509. [DOI] [PubMed] [Google Scholar]
- Petitti DB, Klatsky AL. Malignant hypertension in women aged 15 to 44 years and its relation to cigarette smoking and oral contraceptives. Am J Cardiol. 1983 Aug;52(3):297–8. doi: 10.1016/0002-9149(83)90126-1. [DOI] [PubMed] [Google Scholar]
- Wolf SM, Wagner JH, Jr, Davidson S. Oral contraceptives and neurological illness. Bull Los Angl Neuro Soc. 1967;32:141–9. [Google Scholar]
- Petitti DB. Epidemiologic assessment of the risks of oral contraception. J Reprod Med. 1986 Sep;31(9 Suppl):887–91. [PubMed] [Google Scholar]
- Swan SH, Petitti DB. A review of problems of bias and confounding in epidemiologic studies of cervical neoplasia and oral contraceptive use. Am J Epidemiol. 1982 Jan;115(1):10–8. doi: 10.1093/oxfordjournals.aje.a113264. [DOI] [PubMed] [Google Scholar]
- London RS. The new era in oral contraception: pills containing gestodene, norgestimate, and desogestrel. Obstet Gynecol Surv. 1992 Nov;47(11):777–82. doi: 10.1097/00006254-199211000-00014. [DOI] [PubMed] [Google Scholar]
- Petitti DB, Porterfield D. Worldwide variations in the lifetime probability of reproductive cancer in women: implications of best-case, worst-case, and likely-case assumptions about the effect of oral contraceptive use. Contraception. 1992 Feb;45(2):93–104. doi: 10.1016/0010-7824(92)90043-s. [DOI] [PubMed] [Google Scholar]
- Petitti DB. Oral contraceptives and hepatocellular carcinoma. Br Med J (Clin Res Ed) 1986 Jul 19;293(6540):204. doi: 10.1136/bmj.293.6540.204-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitti DB. Safety of birth control pills. In: Samuels SE, Smith MD, editors. The Pill: from prescription to over the counter. Menlo Park (CA): The Henry J Kaiser Family Foundation; 1994. pp. 77–116. p. [Google Scholar]
- Petitti DB, Sidney S, Quesenberry CP. Oral contraceptive use and myocardial infarction. Contraception. 1998 Mar;57(3):143–55. doi: 10.1016/s0010-7824(98)00016-x. [DOI] [PubMed] [Google Scholar]
- Petitti DB. Vascular risks of combined estrogen-progestin oral contraceptives. In: Sciarra JJ, editor. Gynecology and obstetrics. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 1–23. editor. p. [Google Scholar]
- Petitti DB. Clinical practice. Combination estrogen-progestin oral contraceptives. N Engl J Med. 2003 Oct 9;349(15):1443–50. doi: 10.1056/NEJMcp030751. Erratum in: N Engl J Med 2004 Jan 1;350(1):92. [DOI] [PubMed] [Google Scholar]


