Presented at the Global Congress of Gynecologic Endoscopy, the 33rd Annual meeting of the American Association of Gynecologic Laparoscopists, San Francisco, California, November 10–13, 2004, and published in abstract form in J Am Assoc Gynecol Laparosc 2004 Aug;11(3):S13; related publication, Glasser MH. Minilaparotomy myomectomy: a minimally invasive alternative for the large fibroid uterus. J Am Assoc Gynecol Laparosc May/June 2005. In press.
Introduction
In the 1960s or 1970s, gynecology residency training emphasized vaginal hysterectomy as the preferred technique for treating many conditions now managed by less invasive alternatives. This led gynecologists to become very skilled at operating through very small incisions. More recently, as these less invasive procedures are rapidly becoming the standard of care, and women are having fewer babies, vaginal surgery is performed less often. Because our young colleagues are acquiring less experience with this technique, the skill of operating through a very small incision is becoming a lost art. Of the 600,000 hysterectomies done in the United States each year—a number which has remained stable for the past 20 years—65% to 75% are done through large abdominal incisions.1 Rates in the Kaiser Permanente Northern California (KPNC) Region are somewhat better: The rate of abdominal hysterectomy is 68%, the rate of vaginal hysterectomy is 21%, and 11% of these procedures are done laparoscopically.
Although laparoscopic hysterectomy offers a minimally invasive alternative when vaginal hysterectomy is contraindicated or considered too difficult by the surgeon, laparoscopic hysterectomy has many drawbacks. The procedure is very costly because of its requirements for equipment and time in the operating suite and because the procedure has a very steep learning curve. However, when length of hospital stay and postoperative utilization of medical services are taken into account, laparoscopic hysterectomy in the KPNC Region is less expensive than abdominal hysterectomy but substantially more expensive than vaginal hysterectomy. In addition, compared with patients who have the more invasive (ie, abdominal) procedure, our patients who undergo vaginal or laparoscopic hysterectomy have better postoperative quality of life.2
Minilaparotomy is technically less difficult to perform than laparoscopic myomectomy …
Development of Minilaparotomy Techniques
Use of minilaparotomy in surgery for benign gynecologic disease has been well established.3 Laparoscopically assisted myomectomy was first reported by Nezhat et al in 1994.4 In their review of 57 cases, these authors concluded that the use of the minilaparotomy incision is a safe alternative to myomectomy done by laparotomy. Minilaparotomy is technically less difficult to perform than laparoscopic myomectomy, allows better closure of the uterine defect, and may require less time to perform. Most women in the series reported by Nezhat et al4 returned to normal activity within three weeks.
In 2002, we adopted the Pelosi minilaparotomy hysterectomy technique as an effective alternative to laparoscopic hysterectomy and standard open laparotomy hysterectomy. First presented at the Global Congress of Gynecologic Endoscopy in 2002 and described in OBG Management in April 2003,5 the procedure relies on traditional open techniques learned by all Ob/Gyn residents and relies also on use of an inexpensive, soft, sleeve-type self-retaining abdominal retractor. Minilaparotomy is a minimally invasive procedure ideal for gynecologists who are less skilled in vaginal or laparoscopic surgery and who are more comfortable with the (standard) abdominal approach. In addition to combining the surgical principles and techniques of vaginal and laparoscopic surgery, minilaparotomy requires the same postoperative care as less invasive procedures.
Detailed description of the surgical technique would be more appropriate for an obstetrics/gynecology journal; here I describe some of the most important technical principles of minilaparotomy.
Technical Overview of Minilaparotomy
The minilaparotomy procedure begins with provision of patient education and clarifying appropriate expectations. We inform patients that instead of using the laparoscope (for which, incidentally, I have been a zealous advocate for the past 15 years), we will instead make a 4- to 6-cm suprapubic incision which will allow the patient to go home the same day. We freely show patients actual surgical photographs and videotapes (one6 of which contains a postoperative interview with a patient and is available in the KPNC Multimedia Library in Oakland). Our patients—and especially those referred to us from distant KP facilities—are always given the option of spending the night in the hospital.
The 4- to 6-cm cruciate suprapubic incision was first described by Kustner in 1896 and was recently modified by Pelosi.7 The horizontal skin incision and vertical incisions on the deeper layers allow more exposure than the standard Pfannensteil or Maylard horizontal incisions. The skin and fascia are first injected with 0.25% bupivacaine (Marcaine, AstraZeneca Pharmaceuticals, Wilmington DE) with epinephrine even though the procedure is done with the patient under general anesthesia. Use of the atraumatic Mobius retractor (Apple Medical, Marlboro, MA) provides a symmetric round operating field that excellently exposes the underlying pelvic viscera.
Retraction force is distributed equally around the incision, and the rectus muscles are not traumatized by the overstretched metal blades of the commonly-used Balfour or O'Connor-O'Sullivan self-retaining retractors. This situation creates much less postoperative abdominal discomfort for the patient and enables early ambulation. The flexible plastic material of the retractor lines the incision and thus protects it from contamination. In addition, by compressing the layers of the abdominal wall, the retractor provides tamponade of small bleeders. (This reduction of abdominal wall thickness may be helpful, particularly during surgery in obese patients.) Instead of exposing the entire uterus—which, if the myoma is large, may extend to the level of the umbilicus—we need only to expose the vascular pedicles which are being clamped and cut (Figure 1). A good uterine manipulator allows us to deviate and rotate the uterus to expose these pedicles. Regardless of uterine size, the major vasculature to the uterus arises from the pelvic sidewall at the same level (ie, below the pelvic brim). The flexible retractor allows us to move the incision from one side to the other. Because of this flexibility, performing minilaparotomy is like performing a vaginal hysterectomy abdominally.
Figure 1.
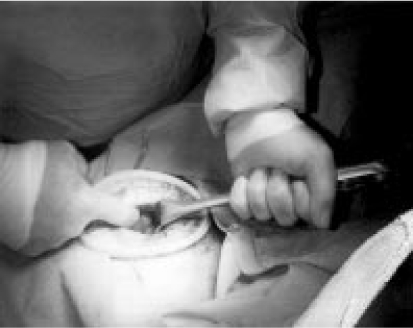
Photograph taken during supracervical hysterectomy shows adnexal pedicle being elevated through the minilaparotomy incision before clamping. The 5-cm incision is held open by a Mobius retractor.
After the pedicles are clamped, cut, and tied, the uterus is amputated from the cervix and is morcellated by using a standard #10 scalpel blade (Figures 2,3). Figure 4 shows an 848-g fibroid which had been removed through a 5-cm minilaparotomy incision. Removal of this large tumor dramatically reduced the patient's abdominal profile (Figures 5,6). The largest uterus removed using this technique weighed 3250 g (more than some infants) and was removed through an 8-cm incision. Another weighed 1780 g (the size of the uterus in the 26th week of pregnancy) and was removed through a 6-cm incision. That patient was discharged from the hospital 16 hours postoperatively after undergoing a 115-minute procedure and returned to work less than two weeks later. We are compiling for possible publication our data from the last two years comparing minilaparotomy supracervical hysterectomy and standard abdominal hysterectomy. Initial results of this comparison are encouraging.
Figure 2.

Photograph shows 12-cm anterior myoma morcellated through a 4-cm minilaparotomy incision.
Figure 3.
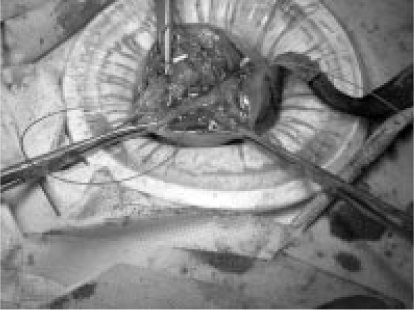
Photograph taken during minilaparotomy myomectomy shows deep suturing of uterine defect.
Figure 4.

Photograph shows 848-g fibroid removed through a 5-cm minilaparotomy incision. (Balance shown by permission of the manufacturer, Acculab, Edgewood, New York.)
Figure 5.

Photograph shows preoperative abdominal profile of patient from whom 848-g fibroid was later removed.
Figure 6.

Photograph shows postoperative abdominal profile of same patient, who left the hospital four hours after surgery.
The small incision is not the only factor that enables the patient to be discharged early from the hospital. During the early postoperative phase, much of the incisional discomfort is eliminated by liberal use of long-acting local anesthetic before making the skin incision and before closing. This reduction of incisional pain allows early ambulation and more rapid return of bowel function. We use a large (8-mg) intraoperative dose of dexamethasone (Decadron, Merck, Whitehouse Station NJ) intravenously and 60 mg of ketorolac (Toradol, Roche, Nutley NJ) intramuscularly to minimize emesis and inflammation.
… reduction of incisional pain allows early ambulation and more rapid return of bowel function.
The Foley catheter is removed in the operating suite, and the patient is fed as soon as she wants food or drink. The intravenous line is removed as soon as the patient can tolerate oral administration of fluid. Encouragement and help from the nursing staff is the key to both early ambulation and early discharge from the hospital. Keeping these patients in the ambulatory surgery unit is advantageous because of the skill of the nurses in this area. Nothing is more disheartening to a physician than finding the patient—who was scheduled for discharge that morning—semicomatose in bed with the siderails up, oxygen being administered, and an intravenous line running. Unfortunately, this care path is common after standard abdominal hysterectomy, and many of our inpatient nurses are accustomed to it. We are therefore now developing an intensive education program in which nurses from the ambulatory surgery department educate our medical-surgical nurses about early discharge.
Minilaparotomy is now our standard technique for performing abdominal myomectomy. We have performed nearly 150 of these procedures at the KP San Rafael Medical Center since 1995, and a report on our first 139 cases (Table 1) is in press.8 In that paper, I conclude that:
Table 1.
Characteristics of minilaparotomy myomectomy performed in 139 patients a at the KP San Rafael Medical Center from January 1995 through December 2003

“[m]inilap[arotomy] myomectomy with or without laparoscopic assistance is a minimally invasive alternative to standard open myomectomy and laparoscopic myomectomy. This procedure achieves a uterine repair equal to that of open myomectomy, affords the ability to palpate the uterus and has the additional advantages of same day discharge and rapid return to normal activity. There is no need for expensive electronic morcellators with very expensive disposable blades. The cost of the Mobius retractor is about the same as some disposable trocars. Very large myomas which would not be managed laparoscopically by the vast majority of practicing gynecologists can be removed using this procedure. The procedure is far easier to teach than laparoscopic myomectomy because of the high degree of technical skill required for the latter. We certainly should not abandon laparoscopic myo-mectomy for selected cases, but I feel strongly that this procedure should be adopted as a minimally invasive alternative for those who don't feel comfortable with the laparoscopic approach.”8
Minilaparotomy is contraindicated in cases where severe adhesions might exist (eg, endometriosis, previous myomectomy, previous pelvic inflammatory disease, bowel disease, or malignancy). In those cases, open laparoscopy is strongly recommended to assess severity of the condition and to determine whether minilaparotomy is feasible. If clinically significant pathology inappropriate for minilaparotomy is detected at this assessment, the surgeon should perform standard laparotomy.
A logical question might be, “What size of incision constitutes minilaparotomy?” The size of the incision is not important: If the operation was originally planned to be done through an 8-in Pfannensteil or midline incision, then an 8-cm cruciate incision certainly constitutes a minilaparotomy. If the same meticulous surgical technique is used to avoid tissue trauma, bowel handling, and packing, then the procedure allows the patient to feel better sooner and enables the same early-discharge care path.
… minilaparotomy is not an appropriate substitute for standard vaginal hysterectomy, which remains the most cost-effective procedure with the least disability …
Conclusion
Minilaparotomy is substantially more cost-effective than prolonged laparoscopic supracervical or laparoscopically assisted vaginal hysterectomy. I again emphasize that minilaparotomy is not an appropriate substitute for standard vaginal hysterectomy, which remains the most cost-effective procedure with the least disability if the early-discharge algorithm is followed. At the KP San Rafael Medical Center, 50% of patients who undergo vaginal hysterectomy go home the same day, and 98% are discharged within 23 hours–a dramatic change from the old “rule” I learned in residency, ie, that patients who undergo abdominal hysterectomy stay in the hospital for at least five to seven days postoperatively and that patients who undergo vaginal hysterectomy stay in the hospital for three days.
More and more of our KPNC facilities are beginning to adopt minilaparotomy hysterectomy because it is far easier to teach than vaginal or laparoscopic hysterectomy and produces excellent results. The procedure may also be useful in urologic practice (eg, for pelvic node dissection) and in some general surgery procedures. Nonetheless, despite the utility of having this minimally invasive approach in our surgical repertoire, we must continue to use more conservative alternatives to hysterectomy, such as “watchful waiting,” medical therapy, and endometrial ablation.
References
- Farquhar CM, Steiner CA. Hysterectomy rates in the United States, 1990– 1997. Obstet Gynecol. 2002 Feb;99(2):229–34. doi: 10.1016/s0029-7844(01)01723-9. [DOI] [PubMed] [Google Scholar]
- Van Den Eeden SK, Glasser MH, Mathias SD, Colwell HH, Pasta DJ, Kunz K. Quality of life, health care utilization, and costs among women undergoing hysterectomy in a managed-care setting. Am J Obstet Gynecol. 1998 Jan;178(1 Part 1):91–100. doi: 10.1016/s0002-9378(98)70633-7. [DOI] [PubMed] [Google Scholar]
- Benedetti-Panici P, Maneschi F, Cutillo G, Scambia G, Congiu M, Mancuso S. Surgery by minilaparotomy in benign gynecologic disease. Obstet Gynecol. 1996 Mar;87(3):456–9. doi: 10.1016/0029-7844(95)00441-6. [DOI] [PubMed] [Google Scholar]
- Nezhat C, Nezhat F, Bess O, Nezhat CH, Mashiach R. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil Menopausal Stud. 1994 Jan–Feb;39(1):39–44. [PubMed] [Google Scholar]
- Pelosi MA, 2nd, Pelosi MA., 3rd. Pelosi minilaparotomy hysterectomy: effective alternative to laparoscopy and laparotomy. OBG Management [serial on the Internet] 2003 Apr;15(4):16–33. [cited 2004 Nov 29] Available from: www.obgmanagement.com/obg_contents.asp?which_issue=4/1/03. [Google Scholar]
- Minilap supracervical hysterectomy for the large fibroid uterus [videotape] [Oakland (CA)]: Kaiser Permanente Multimedia Communications; 2004. [Google Scholar]
- Pelosi MA, 3rd, Pelosi MA. The suprapubic cruciate incision for laparoscopic-assisted microceliotomy. JSLS. 1997 Jul–Sep;1(3):269–72. [PMC free article] [PubMed] [Google Scholar]
- Glasser MH. Minilaparotomy myomectomy: a minimally invasive alternative for the large fibroid uterus. J Am Assoc Gynecol Laparosc. 2005 May/June doi: 10.1016/j.jmig.2005.03.009. In press. [DOI] [PubMed] [Google Scholar]


