Abstract
Synthetic oligodeoxynucleotides (ODNs) containing unmethylated CpG motifs trigger cells that express Toll-like receptor 9 (including human plasmacytoid dendritic cells and B cells) to mount an innate immune response characterized by the production of Th1 and proinflammatory cytokines. When used as vaccine adjuvants, CpG ODNs improve the function of professional antigen-presenting cells and boost the generation of humoral and cellular vaccine-specific immune responses. These effects are optimized by maintaining ODNs and vaccine in close proximity. The adjuvant properties of CpG ODNs are observed when administered either systemically or mucosally, and persist in immunocompromised hosts. Preclinical studies indicate that CpG ODNs improve the activity of vaccines targeting infectious diseases and cancer. Clinical trials demonstrate that CpG ODNs have a good safety profile and increase the immunogenicity of coadministered vaccines.
Keywords: adjuvant, cancer, CpG oligodeoxynucleotide, immunity, infectious disease, Toll-like receptor, vaccine
The host employs a number of active and passive mechanisms to defend against infection. Active protection is mediated by the innate and adaptive immune systems. Innate immunity is induced by exposure to evolutionarily conserved molecular structures termed pathogen-associated molecular patterns (PAMPs) that are expressed by a wide variety of infectious microorganisms [1]. The recognition of PAMPs is mediated by pattern recognition receptors including Toll-like receptors (TLRs), Nod-like receptors and RIG-I-like receptors [2]. TLRs constitute one of the largest and most extensively studied classes of pattern recognition receptors [3]. The innate immune response elicited by TLR activation is primarily characterized by the production of proinflammatory cytokines, chemokines, type I interferons and antimicrobial peptides [1,4].
Innate immunity provides the first line of active defense against infectious pathogens and serves to limit their early proliferation and spread in vivo. Subsequently, the host mounts an adaptive immune response characterized by the expansion of antigen (Ag)-specific T and B cells, resulting in the production of high affinity antibodies (Abs) and the generation of cytotoxic T cells (CTLs) that provide sterilizing immunity and insure long-lasting memory to protect against subsequent infection [5].
Bacterial DNA represents an illustrative example of a PAMP that activates the innate immune system. Unmethylated CG dinucleotides are present at high frequency in prokaryotic DNA but rare in eukaryotic DNA [6,7]. Bacterial DNA released during infection exposes cells that express TLR-9 to these unmethylated CpG motifs (consisting of a central unmethylated CG dinucleotide plus flanking regions), thereby triggering a protective immune response that improves the host’s capacity to eliminate the pathogen [8–11]. The immunostimulatory activity of bacterial DNA is mimicked by synthetic oligodeoxynucleotides (ODNs) expressing unmethylated CpG motifs [12–14]. After internalization by target cells, CpG ODNs reach the late endosomal/lysosomal compartment where they signal by interacting with TLR-9 [9,15]. Confirmation of the importance of TLR-9 was provided by studies of cells transfected to express various TLRs. Only transfection with TLR-9 conferred responsiveness to CpG ODNs or bacterial DNA [9,10]. Consistent with that finding, TLR-9 knockout mice do not respond to stimulation by CpG DNA [9].
B cells and plasmacytoid dendritic cells (pDCs) are the primary cell types in humans that express TLR-9 and respond to CpG stimulation [9,10,16,17]. By comparison, TLR-9 is expressed by, and causes the stimulation of, multiple cells of the myeloid lineage (including monocytes, macrophages and conventional dendritic cells [DCs]) in mice [18–20].
To date, four classes of synthetic CpG ODN have been described, each with distinct structural and biological properties (Table 1) [21–25]. K-type ODNs (also referred to as B type) encode multiple CpG motifs on a phosphorothioate backbone. The use of phosphorothioate nucleotides enhances resistance to nuclease digestion when compared with native phosphodiester nucleotides, resulting in a substantially longer in vivo half life (30–60 min compared with 5–10 min for phosphodiester) [26]. K-type ODNs trigger pDCs to differentiate and produce TNF-α , and B cells to proliferate and secrete IgM (Table 1) [21,22,27].
Table 1.
Comparison of D, K, C and P-type oligodeoxynucleotide.
| ODN type | Representative sequence | Structural characteristics | Immune effects |
|---|---|---|---|
| D- also referred to as A-class |
GGTGCATCGATGCAGGGGGG | Mixed phosphodiester/phosphorothioate backbone Single CpG motif CpG flanking region forms a palindrome Poly G tail at 3′ end |
Induces strong pDC IFN-α secretion APC maturation |
| K- also referred to as B-class |
TCCATGGACGTTCCTGAGCGTT | Phosphorothioate backbone Multiple CpG motifs 5′ motif most stimulatory |
Induces strong B-cell activation pDC maturation Preferentially supports the production of TNF-α and IL-6 |
| C | TCGTCGTTCGAACGACGTTGAT | Phosphorothioate backbone Multiple CpG motifs TCG dimer at 5′ end CpG motif imbedded in a central palindrome |
Induces B-cell and pDC proliferation and differentiation Induces production of IL-6 and IFN-α |
| P | TCGTCGACGATCGGCGCGCGCCG | Phosphorothioate backbone Two palindromes Multiple CpG motifs |
Stimulates pDC and B cells Strong IFN-α secretion |
Bold letters in ODN sequences indicate self-complementary palindromes; CpG motifs are underlined.
APC: Antigen-presenting cell; ODN: Oligodeoxynucleotide; pDC: Plasmacytoid dendritic cell.
D-type ODNs (also referred to as A type) are constructed of a mixed phosphodiester/phosphorothioate backbone, contain a single CpG motif flanked by palindromic sequences and have poly G tails at the 3′ and 5′ ends (a structural motif that facilitates the formation of concatamers) [21]. D-type ODNs trigger pDCs to mature and secrete IFN-α, but have no effect on B cells [22,28]. The distinct activities of K- versus D-type ODNs have been traced to differences in the retention times of CpG/ TLR-9 complexes in the endosomes of pDCs [29,30]. Whereas K-type ODNs are rapidly transported through early endosomes into late endosomes, D-type ODNs are retained for longer periods in the early endosome. Here, D-type ODNs interact with MyD88/IRF-7 complexes, triggering a signaling cascade that supports IFN-α production [29].
C-type ODNs resemble K-type in being composed entirely of phosphorothioate nucleotides, but resemble D-type in containing palindromic CpG motifs. This class of ODNs stimulate B cells to secrete IL-6 and pDCs to produce IFN-α [23,24]. C-type ODNs are present in both early and late endosomes, and thus express properties in common with both K- and D-type ODNs [30]. While additional classes of immunomodulatory ODNs have been described, of particular interest is the recently discovered P-type [25]. P-type ODNs contain two palindromic sequences, enabling them to form higher ordered structures. P-type ODNs activate B cells and pDCs, and induce substantially greater IFN-α production when compared with C-type ODNs [25].
Animal studies and a number of Phase I–III clinical trials support the therapeutic potential of CpG ODNs [31,32]. This article will focus on our current understanding of the mechanism of action of CpG ODNs, and on efforts to harness them as vaccine adjuvants to improve antigen-presenting cell (APC) function and promote the induction of an Ag-specific adaptive immune response.
Mechanisms of action
Intracellular signaling & gene expression
The signaling cascade triggered by the interaction of TLR-9 with CpG ODN culminates in the activation of genes that mediate an inflammatory response. This signaling pathway proceeds through the stimulation of MyD88, IRAK and TRAF-6. Subsequently, the recruitment of various MAP kinases and transcription factors (including NF-κB, AP1 and IRF-7) upregulates the expression of proinflammatory genes [33].
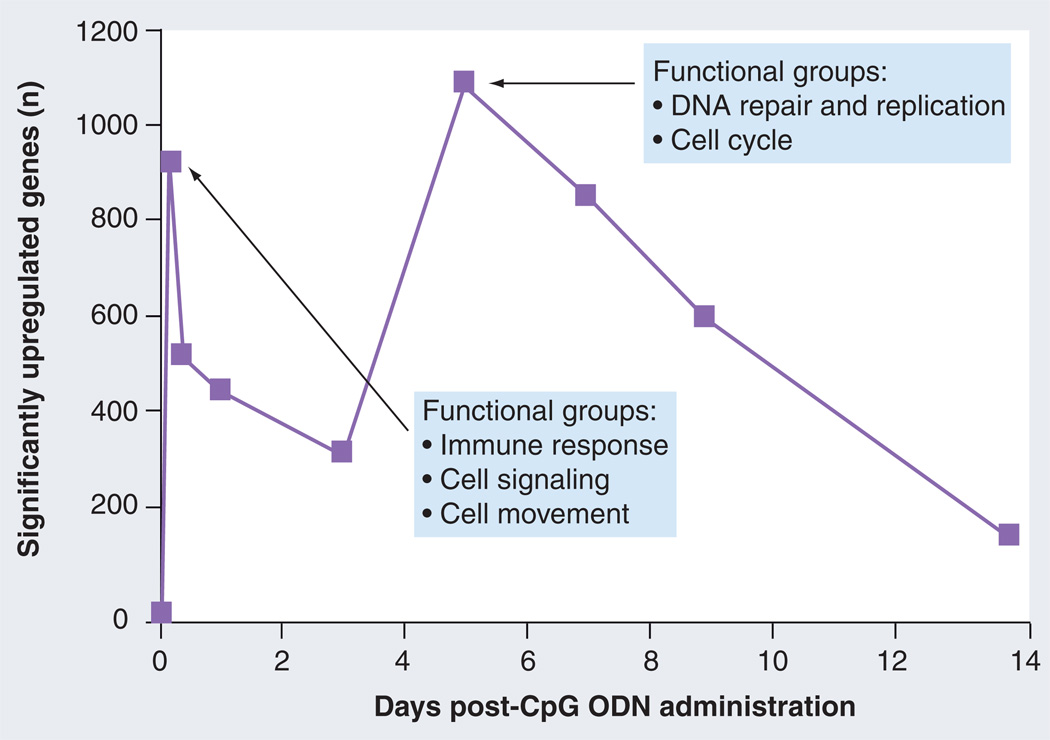
Changes in gene expression can be detected within 30 min of in vivo administration of CpG ODN, and involves the upregulation of TNF, IL-1β and IL-1α [34]. Gene expression peaks at 3 h after CpG ODN treatment, at which time nearly 1000 genes are upregulated (Figure 1) [5]. These genes fall into functional categories (as defined by Ingenuity Pathway Analysis) including immune response (e.g., IL-1α, IL-1β, TLR-9 and TNF), cell signaling (e.g., NFKB1A, MyD88, IRAK-M and A20) and cell movement (e.g., BFL-1, NAP-1, NAK and EGFR)[35]. Gene expression levels then decline over a 3-day period before rising again at day 5. Of interest, the function of the genes activated at 3 h differ from those at 5 days. The second peak of gene activation primarily involves genes in the functional groups cell cycle regulation (e.g., PLK1, WEE1, CHK2 and TOPO2A) and DNA damage response (BRCA1, GADD4A, E2F4 and RPA1) [35].
Figure 1. Time course of CpG-mediated gene regulation in vivo.
Mice were injected intraperitoneally with 400 µg of CpG ODN. Gene expression in the spleen was monitored over time by microarray. At least four biological replicates were analyzed independently at each time point in at least two independent experiments. Differentially regulated genes were identified by comparison to untreated mice (n = 6) using a stringency cutoff of p < 0.00001. Ingenuity Pathway Analysis was used to identify those genes differentially regulated during this period based on their function and role in signaling pathways.
ODN: Oligodeoxynucleotide.
Cellular immunology
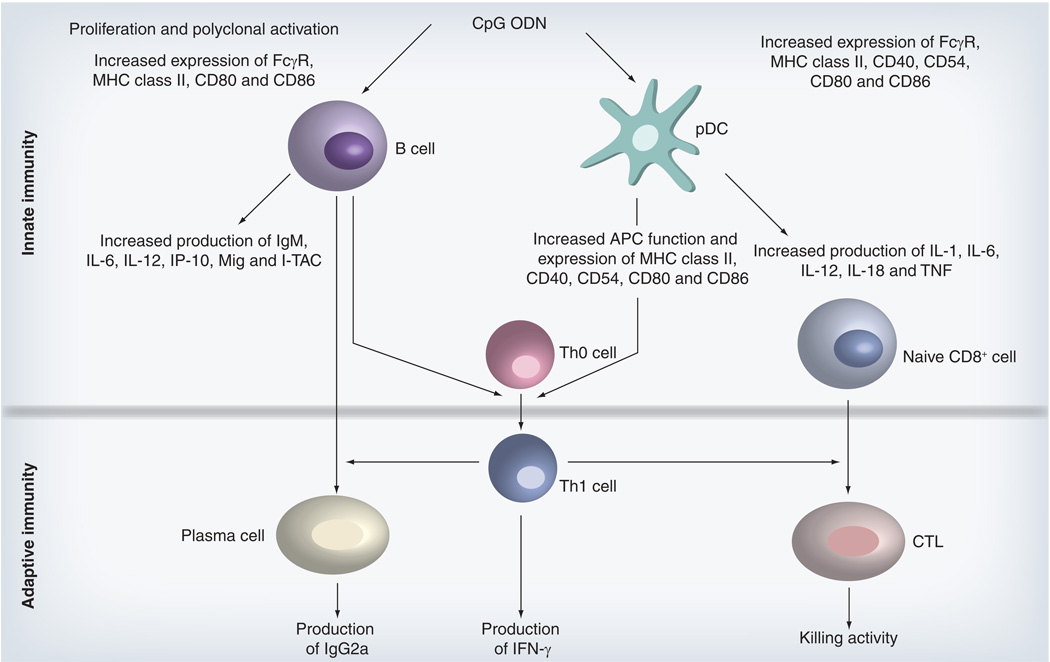
CpG DNA directly activates pDCs and B cells, contributing to the induction of both innate and adaptive immune responses. The cascade of events initiated by CpG DNA indirectly supports the maturation, differentiation and proliferation of natural killer cells, T cells and monocytes/macrophages (Figure 2) [28,36–39]. TLR-9-stimulated B cells produce IL-6, IL-12 and the CXCR3 chemokines IP-10, Mig and I-TAC [40]. They also secrete IgM in a process partially dependent on IL-6 expression [8,41]. B cells activated by CpG DNA upregulate expression of their Fc receptor (FcR) and costimulatory molecules including MHC class II, CD40, CD80 and CD86 [8,42,43]. Subsequently, the CpG-stimulated B cells proliferate and differentiate into plasma cells and memory B cells [44].
Figure 2. Mechanism by which CpG oligodeoxynucleotides facilitate innate and adaptive immune responses.
CpG ODNs directly activate pDCs and B cells. The resultant immune response is characterized by the production of proinflammatory and Th1 cytokines and polyreactive immunoglobulin. This innate immune response forms a foundation on which antigen-specific adaptive immunity is based. By improving the function of professional APCs, CpG ODNs facilitate the generation of humoral and cellular adaptive immune responses against coadministered vaccine antigens.
APC: Antigen-presenting cell; CTL: Cytotoxic T lymphocyte; ODN: Oligodeoxynucleotide; pDC: Plasmacytoid dendritic cell; TNF: Tumor necrosis factor.
Whereas TLR-9 activation alone is sufficient to induce human memory B cells to secrete Ab [45], naive B cells require coactivation through their B-cell Ag receptor [46]. Of note, CpG-activated CD5+ B cells secrete large amounts of IL-10, and this inhibits IL-12 secretion by DCs, which thereby suppresses their Th1 priming function [47].
TLR-9 stimulated pDCs are characterized by their upregulation of CD40, CD54, CD80, CD86 and MHC class II molecules [48–50], increased production of IL-1, IL-6, IL-12, IL-18 and TNF-α [33], resistance to IL-4-induced apoptosis, and differentiation to a mature stage in which Ag processing and presentation are enhanced [51]. pDC also respond to CpG DNA by producing type I IFNs, which can limit the growth of a broad spectrum of viruses and bacteria [52–54]. These multiple effects of CpG ODN suggest they might be of therapeutic utility as vaccine adjuvants.
Early murine experiments involving K-type ODNs established that CpG ODN could improve both the humoral and cell- mediated responses induced by proteins such as ovalbumin, and hen egg lysozyme [55,56]. The adjuvant properties of CpG ODNs were significantly improved when adjuvant and Ag were presented to the immune system in close spatial and temporal proximity. The direct conjugation of CpG ODN to Ag, binding them together with alum, or incorporating them together in lipid emulsions or vesicles generated IgG responses 10–1000-fold greater than induced by Ag alone [42,54,57]. In this context, conjugates containing ODN plus Ag were preferentially taken up by professional APCs, resulting in significantly higher CTL responses than unconjugated mixtures of protein plus ODN [58]. The duration of the immune response was also improved by CpG-based adjuvants. For example, CpG ODNs significantly prolonged the protection induced by Anthrax Vaccine Adsorbed (AVA [Emergent Biosolutions, MI, USA] the licensed human anthrax vaccine) [59]. Mice vaccinated with CpG-adjuvanted AVA produced greater than tenfold more IgG Abs against anthrax protective Ag (PA) than animals vaccinated with AVA alone (p < 0.01) [59]. Moreover, Ab titers persisted in the protective range for more than 1 year, significantly longer than in animals vaccinated with AVA alone (p < 0.001) [59].
Even after IgG anti-PA titers waned, a subset of mice vaccinated with CpG-adjuvanted AVA (but not AVA alone) survived infectious challenge. Studies suggest that the survival of these animals was mediated by the de novo production of protective Abs by high-affinity memory B cells that were stimulated by the Ag present on the challenging pathogen [59]. Similar results were observed in a clinical study involving malaria-naive individuals immunized with a malaria vaccine (consisting of an apical membrane plus merozoite surface protein) [60]. Adding CpG ODN to this vaccine stimulated a significantly larger fraction of B cells to secrete Ab (3.45 vs 0.75%), induced this response more rapidly (day 31 vs day 84), and the response persisted longer (>236 vs 140 days).
CpG-based adjuvants can also impact cellular immune responses. In a recent study, the number and survival of CD8+ T cells was significantly enhanced when CpG ODN was administered 2 days before peptide vaccination [61]. The persistence of the CD8+ T cells in the circulation doubled when compared with vaccine alone (p < 0.001). These effects were not observed with other TLR ligands. A similar prolongation in the number and survival of Ag-specific CD8+ T cells was observed when CpG ODN was added to a peptide-based cancer vaccine [62]. The CpG-adjuvanted vaccine generated fourfold more Ag-specific T cells (p < 0.05), which correlated with an inhibition of tumor growth in both prophylactic and therapeutic vaccination models (p < 0.05).
It is postulated that CpG-induced Th1 cytokine production (including IFN-γ and IL-12) may contribute to improving the CD8+ T-cell response by inducing pDCs to mature [63–65]. Ag presented by immature pDCs can induce Fas and programmed death-1 expression by T cells, culminating in their eventual death [62,61]. By contrast, T cells triggered by CpG-matured pDCs are stimulated but do not express these apoptosis-related proteins [62].
CpG ODN as a vaccine adjuvant
Results from preclinical studies
More than 600 preclinical studies examining the immunogenicity of CpG-adjuvanted vaccines have been published. Although there are differences in the cellular targets and nature of the response induced by CpG ODNs in rodents versus primates, murine studies provide valuable insights into the mechanism of action of these agents. CpG ODNs boosted the humoral immune response induced by vaccines against a large number of pathogens, including anthrax, leishmania, influenza virus, measles virus, lymphocytic choriomeningitis virus, orthopox viruses, hepatitis B surface Ag and tetanus toxoid, with Ag-specific Ab titers improving and rising by up to three orders of magnitude [37,56,66–72]. There have been fewer studies of vaccines designed to induce T-cell responses, but these also identify a benefit to CpG ODN adjuvants. For example, in the murine model of leishmania infection, inclusion of CpG ODN with a vaccine consisting of a low dose of metacyclic promastigotes accelerated the generation of CD4+ IFN-γ-secreting cells and increased the number of IFN-γ-producing CD4+ and CD8+ T cells by two- to threefold [73]. This pathogen-specific IFN-γ response had a significant effect on disease, reducing the parasite load by >1000-fold while reducing the extent and duration of disease (p < 0.001).
While vaccines are typically administered prophylactically to reduce host susceptibility to infection, there are situations in which pathogen-specific immunity is needed after exposure (e.g., following release of biothreat pathogens). In such cases, accelerating the induction of protective immunity is critical. Several studies indicate that CpG ODNs speed up the development of vaccine-induced responses. For example, mice vaccinated with CpG-adjuvanted AVA developed protective immunity threefold faster than those immunized with AVA alone [74], with significant protection being observed within 5 versus 15 days (p < 0.05). The combination of CpG ODN with AVA accelerated the serum IgG anti-PA response, yielding serum anti-PA titers that were tenfold higher and significantly more protective by day 10 (p < 0.05) [74].
As pathogens can gain access to the host through the respiratory, gastrointestinal, vaginal and/or rectal mucosa, the ability of CpG ODNs to boost the immune response elicited by mucosal vaccines was also examined. Animals immunized intravaginally with a herpes simplex virus (HSV)-2 vaccine combined with CpG ODN rapidly developed strong mucosal and systemic Th1 immune responses that protected against lethal HSV-2 infection [75]. They also generated MHC-restricted, Ag-specific CTLs, replicating the effects of parenterally injected CpG ODN plus Ag. Administering CpG ODN with recombinant HSV-1 glyco-protein B intranasally induced significant glycoprotein B-specific IgA levels and anti-HSV CTLs in the genital tract and protected mice from genital HSV challenge [76]. Newborn mice orally immunized with sonicated salmonella proteins (SSPs) plus CpG ODN developed both mucosal and systemic immune responses that were more protective than those induced by vaccination with SSP alone [77]. Combining SSP with CpG ODN induced sixfold higher pathogen-specific IgG (p < 0.05) and markedly (but not significantly) increased IgA serum Ab levels [77]. In addition, mice vaccinated with SSP plus CpG ODN showed significantly enhanced survival following enteric challenge with live Salmonella enteritidis whereas conventional vaccination was nonprotective [77]. Ocular application of an HSV-1 vaccine combined with CpG ODN to rabbits generated Ag-specific virus neutralizing IgA and IgG in both tears and serum. Whereas the conventional HSV-1 vaccine failed to induce Ag-specific T-cell immunity, immunization with the CpG-adjuvanted vaccine generated local and systemic Ag-specific T-cell responses polarized towards Th1 immunity [78].
The ability of CpG ODNs to induce a Th1-biased immune milieu and support CD8+ T-cell responses suggests they might be promising adjuvants for cancer vaccines [63–65]. In preclinical animal studies, CpG ODNs enhanced the production of cytotoxic CD8+ T cells targeting tumor Ags [31,79]. This effect was observed when ODNs were conjugated to, or simply coadministered with, tumor Ag [80,81]. In a preclincal study of colon cancer, a vaccine targeting cell surface-associated mucin (MUC1) combined with CpG ODN and GM-CSF elicited a robust anti-tumor response [82]. Prophylactic vaccination with CpG ODN + MUC1 peptide completely prevented tumor growth (p < 0.001) versus peptide alone.
The adjuvant-like properties of CpG ODN observed in immune competent mice triggered interest in their potential to improve the response in aged animals that develop defects in cell-mediated and humoral immunity [83,84]. Vaccines for the elderly must compensate for these alterations in immune function. Several reports indicate that adding CpG ODN significantly boosts vaccine immunogenicity in geriatric mice and/or restores IgG and CD4+ T-cell priming to levels found in young adults [85–87]. Similarly, newborns are less capable of developing robust immune responses. For example, newborns respond poorly when immunized with hepatitis B surface Ag, attenuated measles virus, or tetanus toxoid [68,88]. Several studies indicate that CpG ODNs enhance the Ab and CTL responses of young animals when coadministered with Ag [77,88]. Interpretation of these findings is complicated, however, because the newborn animals were immunized repeatedly, obscuring the effect of a single early dose of CpG ODN on subsequent immune responsiveness.
Immunodeficiency is a hallmark of HIV-induced AIDS. HIV infection reduces the number and functional activity of CD4+ T cells, resulting in suboptimal responses following vaccination [89–91]. However, retrovirus infection has less effect on the innate than adaptive immune systems [92]. Thus, peripheral blood mononuclear cells from HIV-infected subjects (and simian immunodeficiency virus [SIV]-infected macaques) can respond to CpG stimulation despite declines in Ag-specific immunity [92]. The ability of CpG ODNs to boost the immune response of SIV-infected macaques to a hepatitis B vaccine was investigated. Unlike healthy macaques, SIV-infected animals were generally unable to mount a protective Ab response when repeatedly vaccinated with Engerix B® (GlaxoSmithKline, London, UK), the licenced hepatitis B virus vaccine (HIV patients show a similar loss of vaccine responsiveness) [89,90]. By comparison, the addition of CpG ODN to the vaccine boosted Ab titers to protective levels in all animals with viral loads <107 copies/ml [92]. Although the Ab levels achieved were significantly lower than those of similarly immunized uninfected animals, these findings indicate that inclusion of CpG ODN can boost the immunogenicity of vaccines in immunocompromised as well as normal hosts.
Supporting preclinical studies in mice, the adjuvant effect of CpG ODNs has been observed in several large animal models. Immunizing calves with a vaccine prepared by formalin-inactivation of bovine respiratory syncytial virus combined with CpG ODN enhanced the Ag-specific Ab response and reduced the amount of bovine respiratory syncytial virus in the lungs of matched animals immunized with vaccine alone [93]. Oral vaccination of newborn piglets with live-attenuated pseudorabies virus plus CpG ODN yielded up to 18-fold higher serum IgG titers and tenfold higher mucosal IgA levels when compared with animals immunized with pseudorabies virus alone [94]. Similarly, CpG ODN improved the activity of a vaccine targeting Newcastle disease in chickens, increasing the Ag-specific IgG response by threefold when compared with the conventional vaccine and protecting chickens from challenge with an otherwise lethal dose of Newcastle disease virus [95].
It is important to demonstrate that CpG ODN designed for human use are effective as adjuvants in primates. The TLR-9 molecule expressed by humans and mice differs by 24% at the amino acid level [9], and the cells that express TLR-9 vary between these species [18–20]. Moreover, the motif (consisting of a CpG dinucleotide plus flanking regions) that optimally stimulates immune cells differs between mice and humans [8,24,28,96]. Fortunately, nonhuman primates recognize and respond to all classes of CpG ODNs that stimulate human cells [97–99]. Several studies confirm that CpG ODNs act as vaccine adjuvants in nonhuman primates [92,98–101]. For example, K-type CpG ODNs boosted AVA-specific Ab titers by sixfold in studies of rhesus macaques [102]. In that system, D-type ODNs were somewhat less effective, inducing only a threefold increase in anti-PA titers [102]. These enhanced Ab responses developed more rapidly, were of high avidity and provided significantly greater protection against anthrax infection than those observed after vaccination with AVA alone, reproducing the effects observed in mice [71,74,102]. Similarly K-type ODNs significantly increased the Ag-specific serum IgG response of Aotus monkeys immunized with a peptide-based malaria vaccine [99]. These effects were sequence specific, as control ODNs had no effect on vaccine immunogenicity [99].
Results from clinical studies
Human clinical trials have focused on the use of K-type ODNs as adjuvants for vaccines targeting infectious diseases and cancer [103]. Table 2 provides a partial list of relevant clinical trials. In studies of Energix B, subjects immunized with the CpG adjuvanted vaccine developed 13-fold higher primary and 45-fold higher secondary serum IgG Ab responses than those immunized with vaccine alone. The adjuvanted vaccine also induced a more rapid Ab response [104,105]. In another Phase I study, subjects mounted significantly stronger Ab responses to the poorly immunogenic apical membrane antigen (AMA) 1 malaria vaccine candidate when it was codelivered with CpG ODN. The coadministration of CpG ODN with AMA1 increased the mean anti-AMA1 Ab titer by 5.5-fold when compared with AMA1 alone [106]. This enhanced Ab response was persistent: at 236 days after vaccination, those immunized with AMA1 plus CpG ODN maintained serum Ab titers 4.6-fold higher than those vaccinated with just AMA1 [106]. However, CpG ODNs did not overcome the lack of immunogenicity of AMA1 when administered to semi-immune adults with a history of multiple previous Plasmodium infections and circulating AMA1-specific Abs [107].
Table 2.
CpG oligodeoxynucleotides as vaccine adjuvants.
| Treatment | Disease | Study phase | Outcome | Ref. |
|---|---|---|---|---|
| Infectious diseases | ||||
| CpG 7909 + Engerix B® | Hepatitis B | Phase I/II | Seroprotection achieved faster and anti-HBs Ab titers 45-fold higher after secondary immunization vs Engerix B alone |
[86,104,105] |
| 100% seroconversion after 6 weeks (vs 63% in controls) in HIV-infected adults, seroprotection maintained 5 years |
[108,142] | |||
| CpG 7909 + AMA1 | Malaria | Phase I | 5.5-fold increase in anti-AMA1 Ab titer, enhanced Ab after 236 days post-immunization and decreasing the Ag dose by a quarter compared to AMA1 alone |
[106] |
| No enhancement in the acquisition of memory B cells in semi-immune adults |
[107] | |||
| CpG 7909 + PCV-7/ PPV-23 |
Pneumonia | Phase I/II | Twofold increase in Ag-specific IgG levels at 3, 4 and 10 months in HIV-infected adults |
[109] |
| Cancer | ||||
| CpG 7909 + Melan A MART 1 |
Melanoma | Phase I | Tenfold increase in Melan A-specific CD8+ T cells (>3%) | [110] |
| CpG 7909 + NY-ESO-1 | Melanoma, breast cancer, sarcoma, ovarian cancer |
Phase I | Increased CD8+ T-cell response and prolonged survival | [111] |
| CpG 1018 + GM-CSF + Htert peptide |
Sarcoma, glioblastoma |
Phase I | CD8+ T-cell response in only one subject | [112] |
| CpG 7909 + low-dose radiotherapy |
Lymphoma | Phase I/II | Four of 15 patients with complete or partial clinical response, induction of tumor reactive memory CD8+ T cells |
[114] |
Ab: Antibody; Ag: Antigen; AMA1: Apical membrane antigen 1; Engerix B: Licensed hepatitis B vaccine; GM-CSF: Granulocyte-macrophage colony-stimulating factor; hTERT: Human telomerase reverse transcriptase; MART: Melanoma antigen recognized by T cells; NY-ESO-1: New York-esophageal cancer-1; PCV-7: 7-valent pneumococcal conjugate vaccine; PPV-23: 23-valent pneumococcal polysaccharide vaccine.
CpG ODNs were evaluated as vaccine adjuvants in immunocompromised HIV-infected subjects. When coadministered with Engerix B, CpG ODNs significantly improved the Ab and the T-cell proliferative response of adults who failed to respond to Engerix B alone [108]. All patients receiving the CpG-adjuvanted vaccine maintained titers in the seroprotective range for >1 year [108]. When administered with a pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine (PPV-23), CpG ODNs also improved the Ag-specific IgG response of HIV-infected adults for nearly 1 year (p < 0.001) [109].
The use of CpG ODNs in combination with cancer vaccines was the focus of several clinical studies. The induction of tumor-reactive CD8+ T cells is believed to play a central role in tumor eradication. Patients with melanoma who received melanoma Ag A plus CpG ODN in incomplete Freund’s adjuvant generated a stronger and more rapid CD8+ T-cell response. In that study, four immunizations with the adjuvanted vaccine resulted in >3% of circulating CD8+ T cells being Melan-A-specific. This was an order of magnitude higher than in patients treated with vaccine alone [110]. Similar effects were achieved in studies targeting tumors expressing the New York-esophageal cancer (NY-ESO)-1 protein. Vaccination with recombinant NY-ESO-1 protein plus CpG ODN and montanide significantly boosted the tumor-specific Ab response within 6 weeks, while cross-primed NY-ESO-1-specific CD8+ T cells were detected in a subset of patients by 12 weeks [111].
By contrast, when CpG ODN was coadministered with granulocyte-macrophage colony-stimulating factor and a peptide corresponding to the immunodominant epitope from the tumor Ag human telomerase reverse transcriptase, no beneficial effect on the CTL response of patients with sarcoma or glioblastoma was detected [112]. In this context, there is little evidence that CpG-adjuvanted tumor vaccines lead to clinical improvement. In a Phase I trial involving 14 patients with different types of cancer, Ag-specific CD8+ T-cell responses were induced in nine patients following combined vaccination with NY-ESO-1, CpG ODN and montanide [113]. Six of those nine patients lived an average of 39 months, far longer than their predicted survival of only 4 months. Whether vaccine-induced immunity was responsible for this improved clinical outcome could not be determined in that uncontrolled study. Another trial examined the effect of in situ tumor vaccination in patients with low-grade B-cell lymphoma. A total of 15 patients received an intratumoral injection of CpG ODN plus low-dose irradiation. One of these patients had a complete clinical response and three had partial responses [114]. Up to a threefold induction of tumor- reactive memory CD8+ T cells was detected in three patients who mounted clinical responses. In summary, clinical studies suggest that CpG ODN can boost both humoral and cell-mediated responses to vaccines targeting infectious diseases and cancer, an effect also observed in immunocompromised individuals.
Safety
The safety profile of CpG ODN was investigated in rodents, nonhuman primates and humans. Safety issues included the possibility that CpG ODN might increase host susceptibility to autoimmune disease or predispose to toxic shock. The immune stimulation elicited by CpG motifs can reduce the apoptotic death of stimulated lymphocytes, induce polyclonal B-cell activation and increase the production of auto-Abs and proinflammatory cytokines [115–117]. All of these effects could increase the risk of autoimmune disease [118–121]. To establish the magnitude of this safety concern, in vivo experiments were conducted in which mice were repeatedly injected with immunostimulatory doses of CpG DNA. Although the number of IgG anti-DNA secreting B cells rose by two- to threefold and serum IgG anti-DNA antibody titers rose by up to 60%, the magnitude of these effects was insufficient to induce or worsen systemic autoimmunity [122–124].
The situation was somewhat more complex for organ-specific autoimmune diseases, which are typically promoted by the type of Th1 response preferentially elicited by CpG DNA. In an IL-12-dependent model of experimental allergic encephalomyelitis (that mimics multiple sclerosis), animals treated with CpG DNA and then challenged with auto-Ag developed autoreactive Th1 effector cells that caused disease, whereas mice injected with auto-Ag alone remained disease free [125,126]. In a molecular mimicry model, CpG DNA coadministered with Chlamydia-derived Ag promoted the induction of autoimmune myocarditis [127]. CpG ODN also increased the susceptibility of mice to interventions that can induce arthritis [128]. These findings indicate that CpG motifs can promote the development of deleterious autoimmune reactions under certain circumstances. This concern was heightened when a clinical trial using CpG ODN as an adjuvant for the hepatitis B vaccine was halted when one subject developed Wegener’s granulomatosis, an autoimmune disease characterized by inflammation of the vasculature [129]. As there are multiple reports of vasculitis developing after administration of the conventional hepatitis vaccine [130,131], the implication and causality of this single event is difficult to evaluate.
Toxic shock can be induced by exposing the host repeatedly to agents that induce TNF-α, such as lipopolysaccharide and d-galactosamine [132]. In this context, toxic shock was observed in murine studies involving the administration of CpG ODN in conjunction with sublethal doses of lipopolysaccharide or to animals pre-sensitized with d-galactosamine [13,133,134]. There are no reports of toxic shock when CpG ODN is administered at concentrations typically present in vaccines [135,136].
Evidence from clinical trials indicates that CpG ODNs are reasonably safe when administered as vaccine adjuvants. This includes reports showing that conventional and CpG-adjuvanted vaccines have similar safety profiles [137,138]. Yet several studies noted an elevation in the frequency and/or severity of local adverse events (injection site reactions such as pain, swelling, induration, pruritus and erythema) and systemic symptoms (including flu-like symptoms) by CpG-adjuvanted vaccines. Most of the adverse events reported in clinical studies were mild-to-moderate, appeared within 24 h of dosing and persisted for only a few days. In one double-blind placebo-controlled study involving HIV-infected adults, the injection of PPV-23 led to a significantly higher incidence of influenza-like symptoms when combined with CpG ODN when compared with vaccine alone (37 of 41 vs 2 of 41 participants; p < 0.001) [109]. A total of 75.6% of the subjects vaccinated with CpG ODN plus PPV-23 developed adverse events graded as moderate–severe in intensity, and one subject was admitted to the hospital with fever and malaise for 5 days. By contrast, influenza-like symptoms were uncommon among those who received PPV-23 alone. It is possible that the increased reactogenicity found with the adjuvanted vaccine reflected synergy between CpG ODNs and the bacterial cell wall PAMPs present in the PPV-23 vaccine [139].
Expert commentary & five-year view
CpG ODNs activate the innate immune system by binding to their cognate TLR-9 receptors within the cell. The resultant innate immune response is characterized by the production of Th1 and proinflammatory cytokines and chemokines, and supports the induction of an adaptive immune response in the presence of foreign Ag. CpG ODNs also improve the Ag-presenting function of DCs, monocytes and macrophages, induces the proliferation of B cells and indirectly stimulate the immunoprotective activity of natural killer cells while recruiting T cells to the site of CpG ODN administration. These diverse effects support the ability of CpG ODNs to act as vaccine adjuvants, inducing faster, stronger, higher affinity and longer-lasting humoral and cellular immune responses. The adjuvant properties of CpG ODNs are observed after either systemic or mucosal vaccine administration.
An important benefit of CpG ODNs is their ability to boost immunity in groups with reduced immune function, such as the elderly, newborns and the immunosuppressed. Difficulty inducing strong pathogen-specific responses in those populations represents a stumbling block in efforts to achieve ‘herd immunity’. Preclinical and clinical studies demonstrate that CpG ODNs can boost immunity in subjects with weak adaptive immune systems. Future efforts should identify the populations and conditions that best benefit from the addition of CpG ODNs as vaccine adjuvants.
Progress toward developing therapeutically useful CpG ODNs will also include efforts to identify additional classes of stimulatory ODNs and to improve the activity of those already identified. Such efforts will establish how the new classes of ODNs (including the recently described P-type) regulate discrete elements of the immune system.
Clinical studies have been conducted to evaluate the safety and activity of CpG ODNs in humans. Available results suggest that these agents are reasonably safe and improve the response to prophylactic pathogen-specific vaccines. Such studies also provide valuable insight into the optimal dose, duration and site(s) of delivery of CpG ODN plus vaccine. As discussed earlier, greater insight into the mechanisms of action of CpG ODNs was recently obtained, including knowledge of their signaling pathways and broad effect on gene expression. Understanding these effects is likely to improve our ability to design better vaccines.
Some vaccination strategies involve the use of multiple Ags or Ags that are difficult/expensive to prepare. In those cases, the dose sparing effects of CpG ODNs may be of value. The first clinical trial examining this effect is underway [201]. Another important avenue of research will examine the use of CpG ODN combined with other adjuvants (including ligands that target other TLRs). Such combinations could provide a powerful tool for enhancing vaccine immunogenicity.
The utility of CpG ODNs as adjuvants for tumor vaccines is unclear. CpG ODNs have been shown to induce strong cellular responses following vaccination, manifested by increased numbers of CD8+ T cells. Yet the promising results observed in animal studies have yet to be reproduced in the clinic. One explanation for this discrepancy is that TLR-9 is expressed by murine but not human conventional DCs [140]. Since conventional DCs play a critical role in the cross-presentation of tumor Ag to CD8+ T cells [141], it is possible that CpG ODNs will be less successful at inducing anti-tumor immunity in humans versus mice. Many of the favorable outcomes found in murine models were obtained by prophylactic vaccination using specific tumor Ags. Due to the diversity of Ags expressed by human tumors, this may not be a suitable approach for most patients. In this context, it remains an open question whether the adaptive immune system can be harnessed to resolve most solid tumors, especially since this system failed to detect or destroy the original cancer cells.
A promising alternative is to prevent tumor recurrence/metastases by combining CpG vaccination with surgery. In murine models, a vaccine prepared by coupling CpG ODNs to apoptotic tumor cells effectively prevented metastatic spread after removal of the primary cancer. Based on the encouraging results from preclinical and clinical studies showing that CpG ODNs can be safe and effective when used as adjuvants for vaccines targeting infectious diseases, their use as vaccines for the treatment of cancer deserve continued support.
Key issues.
CpG oligodeoxynucleotides (ODNs) directly activate human B cells and plasmacytoid dendritic cells via TLR-9. CpG ODN indirectly support the maturation and proliferation of natural killer cells, T cells and monocytes/macrophages.
The resulting immune response is characterized by the production of Th1-type and proinflammatory cytokines, chemokines and polyreactive IgM.
The immunogenicity of conventional protein antigens and peptide-based vaccines is enhanced by CpG ODN. The adjuvant effect of CpG ODNs is mediated through improved function of professional antigen-presenting cells and the resultant generation of humoral and cellular vaccine-specific immune responses.
CpG ODNs increase the magnitude and accelerate the development of vaccine-induced responses. They also improve the induction of memory, thereby extending the duration of humoral and cellular immunity.
CpG ODNs boost immunity in groups with reduced immune function, such as the elderly and immunosuppressed. They are effective when administered either systemically or mucosally.
Preclinical and clinical trials demonstrate CpG ODNs can boost the immunogenicity of vaccines targeting infectious diseases and cancer.
Clinical trials indicate that CpG ODNs are reasonably safe when administered as vaccine adjuvants.
Footnotes
Financial & competing interests disclosure
Dennis M Klinman and members of his laboratory hold or have applied for patents concerning the activity of CpG ODN, including their use as vaccine adjuvants. The rights to all such patents have been transferred to the US government. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009;22(2):240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 5.Paul WE. Fundamental Immunology. New York, NY, USA: Raven Press; 1993. [Google Scholar]
- 6.Razin A, Friedman J. DNA methylation and its possible biological roles. Prog. Nucleic Acid Res. Mol. Biol. 1981;25:33–52. doi: 10.1016/s0079-6603(08)60482-1. [DOI] [PubMed] [Google Scholar]
- 7.Cardon LR, Burge C, Clayton DA, Karlin S. Pervasive CpG suppression in animal mitochondrial genomes. Proc. Natl Acad. Sci. USA. 1994;91:3799–3803. doi: 10.1073/pnas.91.9.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krieg AM, Yi A, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–548. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 9. Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123.•• The first paper to show that the response to CpG DNA is mediated via TLR-9
- 10.Takeshita F, Leifer CA, Gursel I, et al. Cutting Edge: role of toll-like receptor 9 in CpG DNA-induced activation of human cells. J. Immunol. 2001;167(7):3555–3558. doi: 10.4049/jimmunol.167.7.3555. [DOI] [PubMed] [Google Scholar]
- 11.Bauer S, Kirschning CJ, Hacker H, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl Acad. Sci. USA. 2001;98(16):9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner H. Bacterial CpG-DNA activates immune cells to signal “infectious danger”. Adv. Immunol. 1999;73:329–368. doi: 10.1016/s0065-2776(08)60790-7. [DOI] [PubMed] [Google Scholar]
- 13.Sparwasser T, Meithke T, Lipford G, et al. Bacterial DNA causes septic shock. Nature. 1997;386:336–338. doi: 10.1038/386336a0. [DOI] [PubMed] [Google Scholar]
- 14.Sparwasser T, Miethke T, Lipford G, et al. Macrophages sense pathogens via DNA motifs: induction of tumor necrosis factor-a-mediated shock. Eur. J. Immunol. 1997;27:1671–1679. doi: 10.1002/eji.1830270712. [DOI] [PubMed] [Google Scholar]
- 15.Ishii KJ, Takeshita F, Gursel I, et al. Potential role of phosphatidylinositol 3 kinase, rather than DNA-dependent protein kinase, in CpG DNA-induced immune activation. J. Exp. Med. 2002;196(2):269–274. doi: 10.1084/jem.20020773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gursel M, Verthelyi D, Gursel I, Ishii KJ, Klinman DM. Differential and competitive activation of human immune cells by distinct classes of CpG oligodeoxynucleotides. J. Leukoc. Biol. 2002;71:813–820. [PubMed] [Google Scholar]
- 17.Hornung V, Rothenfusser S, Britsch S, et al. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 2002;168(9):4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 18.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 2001;194(6):863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krug A, Towarowski A, Britsch S, et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur. J. Immunol. 2001;31(10):3026–3037. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Bauer M, Redecke V, Ellwart JW, et al. Bacterial CpG DNA triggers activation and maturation of human CD11c(−), CD123(+) dendritic cells. J. Immunol. 2001;166(8):5000–5007. doi: 10.4049/jimmunol.166.8.5000. [DOI] [PubMed] [Google Scholar]
- 21.Verthelyi D, Ishii KJ, Gursel M, Takeshita F, Klinman DM. Human peripheral blood cells differentially recognize and respond to two distinct CpG motifs. J. Immunol. 2001;166:2372–2377. doi: 10.4049/jimmunol.166.4.2372. [DOI] [PubMed] [Google Scholar]
- 22.Krug A, Rothenfusser S, Hornung V, et al. Identification of CpG oligonucleotide sequences with high induction of IFNα/β in plasmacytoid dendritic cells. Eur. J. Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 23.Hartmann G, Battiany J, Poeck H, et al. Rational design of new CpG oligonucleotides that combine B cell activation with high IFN-a induction in plasmacytoid dendritic cells. Eur. J. Immunol. 2003;33(6):1633–1641. doi: 10.1002/eji.200323813. [DOI] [PubMed] [Google Scholar]
- 24.Marshall JD, Fearon K, Abbate C, et al. Identification of a novel CpG DNA class and motif that optimally stimulate B cell and plasmacytoid dendritic cell functions. J. Leukoc. Biol. 2003;73(6):781–792. doi: 10.1189/jlb.1202630. [DOI] [PubMed] [Google Scholar]
- 25.Samulowitz U, Weber M, Weeratna R, et al. A novel class of immune-stimulatory CpG oligodeoxynucleotides unifies high potency in type I interferon induction with preferred structural properties. Oligonucleotides. 2010;20(2):93–101. doi: 10.1089/oli.2009.0210. [DOI] [PubMed] [Google Scholar]
- 26.Mutwiri GK, Nichani AK, Babiuk S, Babiuk LA. Strategies for enhancing the immunostimulatory effects of CpG oligodeoxynucleotides. J. Control. Release. 2004;97(1):1–17. doi: 10.1016/j.jconrel.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 27.Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J. Immunol. 2000;164(1):944–952. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- 28.Ballas ZK, Rasmussen WL, Krieg AM. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J. Immunol. 1996;157:1840–1847. [PubMed] [Google Scholar]
- 29.Honda K, Ohba Y, Yanai H, et al. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434(7036):1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 30.Guiducci C, Ott G, Chan JH, et al. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J. Exp. Med. 2006;203(8):1999–2008. doi: 10.1084/jem.20060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006;5(6):471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 32.Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv. Drug Deliv. Rev. 2009;61(3):195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Kumagai Y, Takeuchi O, Akira S. TLR9 as a key receptor for the recognition of DNA. Adv. Drug Deliv. Rev. 2008;60(7):795–804. doi: 10.1016/j.addr.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Klaschik S, Tross D, Klinman DM. Inductive and suppressive networks regulate TLR9-dependent gene expression in vivo. J. Leukoc. Biol. 2009;85(5):788–795. doi: 10.1189/jlb.1008671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klaschik S, Tross D, Shirota H, Klinman DM. Short- and long-term changes in gene expression mediated by the activation of TLR9. Mol. Immunol. 2010;47(6):1317–1324. doi: 10.1016/j.molimm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klinman DM, Yi A, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete IL-6, IL-12 and IFNg. Proc. Natl Acad. Sci. USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879.•• First work to establish that unmethylated CpG motifs elicited a complex immunomodulatory cascade that included the production of Th1-type and proinflammatory cytokines.
- 37.Sun S, Zhang X, Tough DF, Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. J. Exp. Med. 1998;188:2335–2342. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stacey KJ, Sweet MJ, Hume DA. Macrophages ingest and are activated by bacterial DNA. J. Immunol. 1996;157:2116–2120. [PubMed] [Google Scholar]
- 39.Roman M, Martin-Orozco E, Goodman JS, et al. Immunostimulatory DNA sequences function as T helper-1 promoting adjuvants. Nat. Med. 1997;3:849–854. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 40.Kato A, Ogasawara T, Homma T, et al. CpG oligodeoxynucleotides directly induce CXCR3 chemokines in human B cells. Biochem. Biophys. Res. Commun. 2004;320(4):1139–1147. doi: 10.1016/j.bbrc.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 41.Yi A, Klinman DM, Martin TL, Matson S, Krieg AM. Rapid immune activation by CpG motifs in bacterial DNA. J. Immunol. 1996;157:5394–5402. [PubMed] [Google Scholar]
- 42.Davis HL, Weeranta R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J. Immunol. 1998;160:870–876. [PubMed] [Google Scholar]
- 43.Kobayashi H, Horner AA, Takabayashi K, et al. Immunostimulatory DNA pre-priming: a novel approach for prolonged Th1-biased immunity. Cell. Immunol. 1999;198:69–75. doi: 10.1006/cimm.1999.1572. [DOI] [PubMed] [Google Scholar]
- 44.Jung J, Yi AK, Zhang X, Choe J, Li L, Choi YS. Distinct response of human B cell subpopulations in recognition of an innate immune signal, CpG DNA. J. Immunol. 2002;169(5):2368–2373. doi: 10.4049/jimmunol.169.5.2368. [DOI] [PubMed] [Google Scholar]
- 45.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298(5601) doi: 10.1126/science.1076071. 2199-2120. [DOI] [PubMed] [Google Scholar]
- 46.Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur. J. Immunol. 2006;36(4):810–816. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- 47.Sun CM, Deriaud E, Leclerc C, Lo-Man R. Upon TLR9 signaling, CD5+ B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity. 2005;22(4):467–477. doi: 10.1016/j.immuni.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Hartmann G, Weiner GJ, Krieg AM. CpG DNA: a potential signal for growth, activation and maturation of human dendritic cells. Proc. Natl Acad. Sci. USA. 1999;96:9305–9310. doi: 10.1073/pnas.96.16.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 2001;31(11):3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 50.Kerkmann M, Rothenfusser S, Hornung V, et al. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J. Immunol. 2003;170(9):4465–4474. doi: 10.4049/jimmunol.170.9.4465. [DOI] [PubMed] [Google Scholar]
- 51. Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842.•• This work was the first to identify unmethylated CpG motifs as crucial mediators of immune activation. Studies showed that B cells responded to CpG motifs by proliferating and secreting immunoglobulin.
- 52.Ishii KJ, Ito S, Conover J, et al. CpG-activated Thy1.2+ dendritic cells protect against lethal Listeria monocytogenes infection. Eur. J. Immunol. 2003;35:2397–2405. doi: 10.1002/eji.200425795. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto T, Yamamoto S, Katoaka T, Tokunaga T. Lipofection of synthetic oligodeoxyribonucleotide having a palindromic sequence of AACGTT to murine splenocytes enhances IFN production and natural killer activity. Microbiol. Immunol. 1994;38:831–836. doi: 10.1111/j.1348-0421.1994.tb01867.x. [DOI] [PubMed] [Google Scholar]
- 54.Klinman DM, Barnhart KM, Conover J. CpG motifs as immune adjuvants. Vaccine. 1999;17:19–25. doi: 10.1016/s0264-410x(98)00151-0. [DOI] [PubMed] [Google Scholar]
- 55.Lipford GB, Bauer M, Blank C, Reiter R, Wagner H, Heeg K. CpG-containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen: a new class of vaccine adjuvants. Eur. J. Immunol. 1997;27:2340–2344. doi: 10.1002/eji.1830270931. [DOI] [PubMed] [Google Scholar]
- 56.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper (Th1) immunity. J. Exp. Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gursel I, Gursel M, Ishii KJ, Klinman DM. Sterically stabilized cationic liposomes improve the uptake and immunostimulatory activity of CpG oligonucleotides. J. Immunol. 2001;167(6):3324–3328. doi: 10.4049/jimmunol.167.6.3324. [DOI] [PubMed] [Google Scholar]
- 58.Klinman DM. Adjuvant activity of CpG oligodeoxynucleotides. Int. Rev. Immunol. 2006;25(3–4):135–154. doi: 10.1080/08830180600743057. [DOI] [PubMed] [Google Scholar]
- 59. Tross D, Klinman DM. Effect of CpG oligonucleotides on vaccine-induced B cell memory. J. Immunol. 2008;181(8):5785–5790. doi: 10.4049/jimmunol.181.8.5785.• This report demonstrates that CpG oligodeoxynucleotides (ODNs) as vaccine adjuvants generate a large pool of high-affinity memory B cells
- 60.Crompton PD, Traore B, Kayentao K, et al. Sickle cell trait is associated with a delayed onset of malaria: implications for time-to-event analysis in clinical studies of malaria. J. Infect. Dis. 2008;198(9):1265–1275. doi: 10.1086/592224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Overstreet MG, Freyberger H, Cockburn IA, Chen YC, Tse SW, Zavala F. CpG-enhanced CD8+ T-cell responses to peptide immunization are severely inhibited by B cells. Eur. J. Immunol. 2010;40(1):124–133. doi: 10.1002/eji.200939493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muraoka D, Kato T, Wang L, et al. Peptide vaccine induces enhanced tumor growth associated with apoptosis induction in CD8+ T cells. J. Immunol. 2010;185(6):3768–3776. doi: 10.4049/jimmunol.0903649. [DOI] [PubMed] [Google Scholar]
- 63.Stern BV, Boehm BO, Tary-Lehmann M. Vaccination with tumor peptide in CpG adjuvant protects via IFN-γ-dependent CD4 cell immunity. J. Immunol. 2002;168(12):6099–6105. doi: 10.4049/jimmunol.168.12.6099. [DOI] [PubMed] [Google Scholar]
- 64.Sandler AD, Chihara H, Kobayashi G, et al. CpG oligonucleotides enhance the tumor antigen-specific immune response of a granulocyte macrophage colony-stimulating factor-based vaccine strategy in neuroblastoma. Cancer Res. 2003;63(2):394–399. [PubMed] [Google Scholar]
- 65.Wille-Reece U, Flynn BJ, Lore K, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J. Exp. Med. 2006;203(5):1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCluskie MJ, Davis HL. CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J. Immunol. 1998;161:4463–4466. [PubMed] [Google Scholar]
- 67.von Hunolstein C, Mariotti S, Teloni R, et al. The adjuvant effect of synthetic oligodeoxynucleotide containing CpG motif converts the anti-Haemophilus influenzae type b glycoconjugates into efficient anti-polysaccharide and anti-carrier polyvalent vaccines. Vaccine. 2001;19(23–24):3058–3066. doi: 10.1016/s0264-410x(01)00048-2. [DOI] [PubMed] [Google Scholar]
- 68.Kovarik J, Bozzotti P, Love-Homan L, et al. CpG oligonucleotides can cirmcuvent the TH2 polorization of neonatal responses to vaccines but fail to fully redirect TH2 responses established by neonatal priming. J. Immunol. 1999;162:1611–1617. [PubMed] [Google Scholar]
- 69.Eastcott JW, Holmberg CJ, Dewhirst FE, Esch TR, Smith DJ, Taubman MA. Oligonucleotide containing CpG motifs enhances immune response to mucosally or systemically administered tetanus toxoid. Vaccine. 2001;19(13–14):1636–1642. doi: 10.1016/s0264-410x(00)00422-9. [DOI] [PubMed] [Google Scholar]
- 70.Al Mariri A, Tibor A, Mertens P, et al. Protection of BALB/c mice against Brucella abortus 544 challenge by vaccination with bacterioferritin or P39 recombinant proteins with CpG oligodeoxynucleotides as adjuvant. Infect. Immun. 2001;69(8):4816–4822. doi: 10.1128/IAI.69.8.4816-4822.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xie H, Gursel I, Ivins BE, et al. CpG oligodeoxynucleotides adsorbed onto polylactide-co-glycolide microparticles improve the immunogenicity and protective activity of the licensed anthrax vaccine. Infect. Immun. 2005;73(2):828–833. doi: 10.1128/IAI.73.2.828-833.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fogg CN, Americo JL, Lustig S, et al. Adjuvant-enhanced antibody responses to recombinant proteins correlates with protection of mice and monkeys to orthopoxvirus challenges. Vaccine. 2007;25(15):2787–2799. doi: 10.1016/j.vaccine.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mendez S, Tabbara K, Belkaid Y, et al. Coinjection with CpG-containing immunostimulatory oligodeoxynucleotides reduces the pathogenicity of a live vaccine against cutaneous Leishmaniasis but maintains its potency and durability. Infect. Immun. 2003;71(9):5121–5129. doi: 10.1128/IAI.71.9.5121-5129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klinman DM, Currie D, Lee G, Grippe V, Merkel T. Systemic but not mucosal immunity induced by AVA prevents inhalational anthrax. Microbes Infect. 2007;9(12–13):1478–1483. doi: 10.1016/j.micinf.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tengvall S, Lundqvist A, Eisenberg RJ, Cohen GH, Harandi AM. Mucosal administration of CpG oligodeoxynucleotide elicits strong CC and CXC chemokine responses in the vagina and serves as a potent Th1-tilting adjuvant for recombinant gD2 protein vaccination against genital herpes. J. Virol. 2006;80(11):5283–5291. doi: 10.1128/JVI.02013-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gallichan WS, Woolstencroft RN, Guarasci T, McCluskie MJ, Davis HL, Rosenthal KL. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol. 2001;166(5):3451–3457. doi: 10.4049/jimmunol.166.5.3451. [DOI] [PubMed] [Google Scholar]
- 77.Huang CF, Wang CC, Wu TC, Wu KG, Lee CC, Peng HJ. Neonatal sublingual vaccination with Salmonella proteins and adjuvant cholera toxin or CpG oligodeoxynucleotides induces mucosal and systemic immunity in mice. J. Pediatr. Gastroenterol. Nutr. 2008;46(3):262–271. doi: 10.1097/MPG.0b013e318156050d. [DOI] [PubMed] [Google Scholar]
- 78.Nesburn AB, Ramos TV, Zhu X, Asgarzadeh H, Nguyen V, BenMohamed L. Local and systemic B cell and Th1 responses induced following ocular mucosal delivery of multiple epitopes of herpes simplex virus type 1 glycoprotein D together with cytosine-phosphate-guanine adjuvant. Vaccine. 2005;23(7):873–883. doi: 10.1016/j.vaccine.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 79.Rothenfusser S, Hornung V, Ayyoub M, et al. CpG-A and CpG-B oligonucleotides differentially enhance human peptide-specific primary and memory CD8+ T-cell responses in vitro. Blood. 2004;103(6):2162–2169. doi: 10.1182/blood-2003-04-1091. [DOI] [PubMed] [Google Scholar]
- 80.Tighe H, Takabayashi K, Schwartz D, et al. Conjugation of immunostimulatory DNA to the short ragweed allergen amb a 1 enhances its immunogenicity and reduces its allergenicity. J. Allergy Clin. Immunol. 2000;106:124–134. doi: 10.1067/mai.2000.107927. [DOI] [PubMed] [Google Scholar]
- 81.Fourcade J, Kudela P, Andrade Filho PA, et al. Immunization with analog peptide in combination with CpG and montanide expands tumor antigen-specific CD8+ T cells in melanoma patients. J. Immunother. 2008;31(8):781–791. doi: 10.1097/CJI.0b013e318183af0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mukherjee P, Pathangey LB, Bradley JB, et al. MUC1-specific immune therapy generates a strong anti-tumor response in a MUC1-tolerant colon cancer model. Vaccine. 2007;25(9):1607–1618. doi: 10.1016/j.vaccine.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller RA. The cell bilogy of aging: immunologic models. J. Gerontol. 1989;44:B4–0. doi: 10.1093/geronj/44.1.b4. [DOI] [PubMed] [Google Scholar]
- 84.Thoman ML, Weigle WO. The cellular and subcellular bases of immunosenescence. Adv. Immunol. 1989;46:221–261. doi: 10.1016/s0065-2776(08)60655-0. [DOI] [PubMed] [Google Scholar]
- 85.Qin W, Jiang J, Chen Q, et al. CpG ODN enhances immunization effects of hepatitis B vaccine in aged mice. Cell. Mol. Immunol. 2004;1(2):148–152. [PubMed] [Google Scholar]
- 86.Siegrist CA, Pihlgren M, Tougne C, et al. Co-administration of CpG oligonucleotides enhances the late affinity maturation process of human anti-hepatitis B vaccine response. Vaccine. 2004;23(5):615–622. doi: 10.1016/j.vaccine.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 87.Sen G, Chen Q, Snapper CM. Immunization of aged mice with a pneumococcal conjugate vaccine combined with an unmethylated CpG-containing oligodeoxynucleotide restores defective immunoglobulin G antipolysaccharide responses and specific CD4+-T-cell priming to young adult levels. Infect. Immun. 2006;74(4):2177–2186. doi: 10.1128/IAI.74.4.2177-2186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brazolot Millan CL, Weeratna R, Krieg AM, Siegrist CA, Davis HL. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc. Natl Acad. Sci. USA. 1998;95:15553–15558. doi: 10.1073/pnas.95.26.15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Diamant EP, Schechter C, Hodes DS, Peters VB. Immunogenicity of hepatitis B vaccine in human immunodeficiency virus-infected children. Pediatr. Infect. Dis. J. 1993;12(10):877–878. doi: 10.1097/00006454-199310000-00014. [DOI] [PubMed] [Google Scholar]
- 90.Wong EK, Bodsworth NJ, Slade MA, Mulhall BP, Donovan B. Response to hepatitis B vaccination in a primary care setting: influence of HIV infection, CD4+ lymphocyte count and vaccination schedule. Int. J. STD AIDS. 1996;7(7):490–494. doi: 10.1258/0956462961918563. [DOI] [PubMed] [Google Scholar]
- 91.Pacanowski J, Kahi S, Baillet M, et al. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood. 2001;98(10):3016–3021. doi: 10.1182/blood.v98.10.3016. [DOI] [PubMed] [Google Scholar]
- 92.Verthelyi D, Gursel M, Kenney RT, et al. CpG oligodeoxynucleotides protect normal and SIV-infected macaques from Leishmania infection. J. Immunol. 2003;170(9):4717–4723. doi: 10.4049/jimmunol.170.9.4717. [DOI] [PubMed] [Google Scholar]
- 93.Mapletoft JW, Oumouna M, Townsend HG, Gomis S, Babiuk LA, van Drunen Littel-van den Hurk Formulation with CpG oligodeoxynucleotides increases cellular immunity and protection induced by vaccination of calves with formalin-inactivated bovine respiratory syncytial virus. Virology. 2006;353(2):316–323. doi: 10.1016/j.virol.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 94.Linghua Z, Xingshan T, Fengzhen Z. In vivo oral administration effects of various oligodeoxynucleotides containing synthetic immunostimulatory motifs in the immune response to pseudorabies attenuated virus vaccine in newborn piglets. Vaccine. 2008;26(2):224–233. doi: 10.1016/j.vaccine.2007.10.058. [DOI] [PubMed] [Google Scholar]
- 95.Linghua Z, Xingshan T, Fengzhen Z. Vaccination with Newcastle disease vaccine and CpG oligodeoxynucleotides induces specific immunity and protection against Newcastle disease virus in SPF chicken. Vet. Immunol. Immunopathol. 2007;115(3–4):216–222. doi: 10.1016/j.vetimm.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 96.Halpern MD, Kurlander RJ, Pisetsky DS. Bacterial DNA induces murine interferon-g production by stimulation of IL-12 and tumor necrosis factor-a. Cell. Immunol. 1996;167:72–78. doi: 10.1006/cimm.1996.0009. [DOI] [PubMed] [Google Scholar]
- 97.Verthelyi D, Kenney RT, Seder RA, Gam AA, Friedag B, Klinman DM. CpG oligodeoxynucleotides as vaccine adjuvants in primates. J. Immunol. 2002;168(4):1659–1663. doi: 10.4049/jimmunol.168.4.1659. [DOI] [PubMed] [Google Scholar]
- 98.Davis HL, Suparto II, Weeratna RR, et al. CpG DNA overcomes hyporesponsiveness to hepatitis B vaccine in orangutans. Vaccine. 2000;18(4–5):1920–1924. doi: 10.1016/s0264-410x(99)00443-0. [DOI] [PubMed] [Google Scholar]
- 99.Jones TR, Obaldia N, Gramzinski RA, et al. Synthetic oligodeoxynucleotides containing CpG motifs enhance immunogenic vaccine in Aotus monkeys. Vaccine. 1999;17(23–24):3065–3071. doi: 10.1016/s0264-410x(99)00145-0. [DOI] [PubMed] [Google Scholar]
- 100.Hartmann G, Weeratna RD, Ballas ZK, et al. Delineation of a CpG phosphorothioate oligodeoxinucleotide for activating primate immune responses in vitro and in vivo. J. Immunol. 2000;164:1617–1624. doi: 10.4049/jimmunol.164.3.1617. [DOI] [PubMed] [Google Scholar]
- 101.Verthelyi D, Wang VW, Lifson JD, Klinman DM. CpG oligodeoxynucleotides improve the response to hepatitis B immunization in healthy and SIV-infected rhesus macaques. AIDS. 2004;18(7):1003–1008. doi: 10.1097/00002030-200404300-00007. [DOI] [PubMed] [Google Scholar]
- 102.Klinman DM, Xie H, Little SF, Currie D, Ivins BE. CpG oligonucleotides improve the protective immune response induced by the anthrax vaccination of rhesus macaques. Vaccine. 2004;22(21–22):2881–2886. doi: 10.1016/j.vaccine.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 103.Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine. 2010 doi: 10.1016/j.vaccine.2010.08.002. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cooper CL, Davis HL, Morris ML, et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind Phase I/II study. J. Clin. Immunol. 2004;24(6):693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 105.Halperin SA, Van Nest G, Smith B, Abtahi S, Whiley H, Eiden JJ. A Phase I study of the safety and immunogenicity of recombinant hepatitis B surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide adjuvant. Vaccine. 2003;21(19–20):2461–2467. doi: 10.1016/s0264-410x(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 106.Mullen GE, Ellis RD, Miura K, et al. Phase 1 trial of AMA1-C1/Alhydrogel plus CPG 7909: an asexual blood-stage vaccine for Plasmodium falciparum malaria. PLoS ONE. 2008;3(8):e2940. doi: 10.1371/journal.pone.0002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Traore B, Kone Y, Doumbo S, et al. The TLR9 agonist CpG fails to enhance the acquisition of Plasmodium falciparum-specific memory B cells in semi-immune adults in Mali. Vaccine. 2009;27(52):7299–7303. doi: 10.1016/j.vaccine.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cooper CL, Davis HL, Angel JB, et al. CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. AIDS. 2005;19(14):1473–1479. doi: 10.1097/01.aids.0000183514.37513.d2.• This clinical trial (together with [109]) demonstrates a potential adjuvant role for CpG ODNs in vaccine hyporesponsive populations (HIV-infected adults)
- 109.Sogaard OS, Lohse N, Harboe ZB, et al. Improving the immunogenicity of pneumococcal conjugate vaccine in HIV-infected adults with a Toll-like receptor 9 agonist adjuvant: a randomized, controlled trial. Clin. Infect. Dis. 2010;51(1):42–50. doi: 10.1086/653112. [DOI] [PubMed] [Google Scholar]
- 110.Speiser DE, Lienard D, Rufer N, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J. Clin. Invest. 2005;115(3):739–746. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Valmori D, Souleimanian NE, Tosello V, et al. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc. Natl Acad. Sci. USA. 2007;104(21):8947–8952. doi: 10.1073/pnas.0703395104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haining WN, Davies J, Kanzler H, et al. CpG oligodeoxynucleotides alter lymphocyte and dendritic cell trafficking in humans. Clin. Cancer Res. 2008;14(17):5626–5634. doi: 10.1158/1078-0432.CCR-08-0526. [DOI] [PubMed] [Google Scholar]
- 113.Karbach J, Gnjatic S, Bender A, et al. Tumor-reactive CD8+ T-cell responses after vaccination with NY-ESO-1 peptide, CpG 7909 and Montanide ISA-51: association with survival. Int. J. Cancer. 2010;126(4):909–918. doi: 10.1002/ijc.24850. [DOI] [PubMed] [Google Scholar]
- 114. Brody JD, Ai WZ, Czerwinski DK, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a Phase I/II study. J. Clin. Oncol. 2010;28(28):4324–4332. doi: 10.1200/JCO.2010.28.9793.• The authors show thatin situ tumor vaccination with CpG ODNs induces a systemic anti-tumoral clinical response, which is a promising approach for CpG ODNs as cancer adjuvants
- 115.Gilkeson GS, Riuz P, Howell D, Lefkowith JB, Pisetsky DS. Induction of immune-mediated glomerulonephritis in normal mice immunized with bacterial DNA. Clin. Immunol. Immunopathol. 1993;68:283–292. doi: 10.1006/clin.1993.1129. [DOI] [PubMed] [Google Scholar]
- 116.Gilkeson GS, Pippen AM, Pisetsky DS. Induction of cross-reactive anti-dsDNA antibodies in preautoimmune NZB/NZW mice by immunization with bacterial DNA. J. Clin. Invest. 1995;95:1398–1402. doi: 10.1172/JCI117793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Steinberg AD, Krieg AM, Gourley MF, Klinman DM. Theoretical and experimental approaches to generalized autoimmunity. Immunol. Rev. 1990;118:129–163. doi: 10.1111/j.1600-065x.1990.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 118.Klinman DM. Polyclonal B cell activation in lupus-prone mice precedes and predicts the development of autoimmune disease. J. Clin. Invest. 1990;86:1249–1254. doi: 10.1172/JCI114831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Linker-Israeli M, Deans R, Wallace D, Prehn J, Ozeri-Chen T, Klinenberg J. Elevated levels ofendogenous IL-6 in systemic lupus erythematosus. J. Immunol. 1991;147:117–123. [PubMed] [Google Scholar]
- 120.Krieg AM. CpG DNA : a pathogenic factor in systemic lupus erythematosus? J. Clin. Immunol. 1995;15(6):284–292. doi: 10.1007/BF01541318. [DOI] [PubMed] [Google Scholar]
- 121.Yi A-K, Hornbeck P, Lafrenz DE, Krieg AM. CpG DNA rescue of murine B lymphoma cells from anti-IgM induced growth arrest and programmed cell death is associated with increased expression of c-myc and bcl-xl. J. Immunol. 1996;157:4918–4925. [PubMed] [Google Scholar]
- 122.Mor G, Singla M, Steinberg AD, Hoffman SL, Okuda K, Klinman DM. Do DNA vaccines induce autoimmune disease? Hum. Gene Ther. 1997;8:293–300. doi: 10.1089/hum.1997.8.3-293. [DOI] [PubMed] [Google Scholar]
- 123.Katsumi A, Emi N, Abe A, Hasegawa Y, Ito M, Saito H. Humoral and cellular immunity to an encoded protein induced by direct DNA injection. Hum. Gene Ther. 1994;5:1335–1339. doi: 10.1089/hum.1994.5.11-1335. [DOI] [PubMed] [Google Scholar]
- 124.Gilkeson GS, Conover JS, Halpern M, Pisetsky DS, Feagin A, Klinman DM. Effects of bacterial DNA on cytokine production by (NZB/NZW)F1 mice. J. Immunol. 1998;161:3890–3895. [PubMed] [Google Scholar]
- 125.Segal BM, Klinman DM, Shevach EM. Microbial products induce autoimmune disease by an IL-12 dependent process. J. Immunol. 1997;158:5087–5091. [PubMed] [Google Scholar]
- 126.Segal BM, Chang JT, Shevach EM. CpG oligonucleotides are potent adjuvants for the activation of autoreactive encephalotogenic T cells in vivo. J. Immunol. 2000;164:5683–5688. doi: 10.4049/jimmunol.164.11.5683. [DOI] [PubMed] [Google Scholar]
- 127.Bachmaier K, Meu N, Maza LM, Pal S, Nessel A, Penninger JM. Chlamydia infections and heart disease linked through antigenic mimicry. Science. 1999;283:1335–1339. doi: 10.1126/science.283.5406.1335. [DOI] [PubMed] [Google Scholar]
- 128.Zeuner RA, Verthelyi D, Gursel M, Ishii KJ, Klinman DM. Influence of stimulatory and suppressive DNA motifs on host susceptibility to inflammatory arthritis. Arthritis Rheum. 2003;48(6):1701–1707. doi: 10.1002/art.11035. [DOI] [PubMed] [Google Scholar]
- 129.DeFrancesco L. Dynavax trial halted. Nat. Biotechnol. 2008;26(5):484. doi: 10.1038/nbt0508-484a. [DOI] [PubMed] [Google Scholar]
- 130.Le HC, Cohen P, Bousser MG, Letellier P, Guillevin L. Suspected hepatitis B vaccination related vasculitis. J. Rheumatol. 1999;26(1):191–194. [PubMed] [Google Scholar]
- 131.Allen MB, Cockwell P, Page RL. Pulmonary and cutaneous vasculitis following hepatitis B vaccination. Thorax. 1993;48(5):580–581. doi: 10.1136/thx.48.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Opal SM. Endotoxins and other sepsis triggers. Contrib. Nephrol. 2010;167:14–24. doi: 10.1159/000315915. [DOI] [PubMed] [Google Scholar]
- 133.Cowdery JS, Chace JH, Yi AK, Krieg AM. Bacterial DNA induces NK cells to produce IFNγ in vivo and increases the toxicity of lipopolysaccharides. J. Immunol. 1996;156:4570–4575. [PubMed] [Google Scholar]
- 134.Hartmann G, Krug A, Waller K, Endres S. Oligodeoxynucleotides enhance lipopolysaccharide-stimulated synthesis of TNF: dependence on phosphorothioate modification and reversal by heparin. Mol. Med. 1996;2:429–438. [PMC free article] [PubMed] [Google Scholar]
- 135.Klinman DM, Conover J, Coban C. Repeated administration of synthetic oligodeoxynucleotides expressing CpG motifs provides long-term protection against bacterial infection. Infect. Immun. 1999;67:5658–5663. doi: 10.1128/iai.67.11.5658-5663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Krieg AM, Efler SM, Wittpoth M, Al Adhami MJ, Davis HL. Induction of systemic Th1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J. Immunother. 2004;27(6):460–471. doi: 10.1097/00002371-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 137.Sagara I, Ellis RD, Dicko A, et al. A randomized and controlled Phase 1 study of the safety and immunogenicity of the AMA1-C1/Alhydrogel + CPG 7909 vaccine for Plasmodium falciparum malaria in semi-immune Malian adults. Vaccine. 2009;27(52):7292–7298. doi: 10.1016/j.vaccine.2009.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cooper CL, Davis HL, Morris ML, et al. Safety and immunogenicity of CPG 7909 injection as an adjuvant to Fluarix influenza vaccine. Vaccine. 2004;22(23–24):3136–3143. doi: 10.1016/j.vaccine.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 139.Sen G, Khan AQ, Chen Q, Snapper CM. In vivo humoral immune responses to isolated pneumococcal polysaccharides are dependent on the presence of associated TLR ligands. J. Immunol. 2005;175(5):3084–3091. doi: 10.4049/jimmunol.175.5.3084. [DOI] [PubMed] [Google Scholar]
- 140.Barchet W, Wimmenauer V, Schlee M, Hartmann G. Accessing the therapeutic potential of immunostimulatory nucleic acids. Curr. Opin. Immunol. 2008;20(4):389–395. doi: 10.1016/j.coi.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 141.Ullrich E, Menard C, Flament C, et al. Dendritic cells and innate defense against tumor cells. Cytokine Growth Factor Rev. 2008;19(1):79–92. doi: 10.1016/j.cytogfr.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 142.Cooper CL, Angel JB, Seguin I, Davis HL, Cameron DW. CPG 7909 adjuvant plus hepatitis B virus vaccination in HIV-infected adults achieves long-term seroprotection for up to 5 years. Clin. Infect. Dis. 2008;46(8):1310–1314. doi: 10.1086/533467. [DOI] [PubMed] [Google Scholar]
Website
- 201.Phase I study of the safety and immunogenicity of BSAM-2/ alhydrogel(registered trademark)+CPG 7909, an asexual blood stage vaccine for Plasmodium falciparum malaria in adults in the US and Mali. http://clinicaltrials.gov/ct2/show/NCT00889616.