Abstract
Overexpression of α-synuclein causes familial Parkinson’s disease and abnormal aggregates of the protein are present in sporadic cases of the disease. We have examined the behavioral effects of direct and indirect dopaminergic agonists in transgenic mice expressing human α-synuclein under the Thy-1 promoter (Thy1-aSyn, alpha-synuclein overexpressor), which exhibit progressive impairments in behavioral tests sensitive to nigrostriatal dopamine dysfunction. Male Thy1-aSyn and wild-type mice received vehicle, benserazide/L-DOPA (25 mg/kg, i.p.), high (2 mg/kg, s.c.) and low doses (0.125, 0.25, 0.5 mg/kg, s.c.) of apomorphine, and amphetamine (5 mg/kg, i.p.), beginning at 3 months of age, and were tested on the challenging beam, spontaneous activity, pole test, and gait. L-DOPA had a paradoxical effect and worsened the deficits in Thy1-aSyn mice compared with controls, whereas the high dose of apomorphine only produced few deficits above those already present in Thy1-aSyn. In contrast to wild-type mice, Thy1-aSyn mice did not show amphetamine-induced stereotypies. The results indicate that chronic overexpression of α-synuclein led to abnormal pharmacological responses in mice.
Keywords: sensorimotor function, Parkinson’s disease, amphetamine, apomorphine, L-DOPA
Alterations in synaptic function may be critical in neurodegenerative diseases that are characterized by an abnormal accumulation of the vesicular protein α-synuclein (synucleinopathies; Lotharius and Brundin, 2002). One of the synucleinopathies is Parkinson’s disease (PD), a frequent neurodegenerative disease in which nigrostriatal dopaminergic neurons progressively die, leading to profound motor disability. Point mutations and overexpression of α-synuclein are associated with rare familial forms of PD (Polymeropoulos et al., 1997; Kruger et al., 1998; Singleton et al., 2003), and α-synuclein is a major component of Lewy bodies, the pathological hallmark of sporadic PD (Spillantini et al., 1997; Takeda et al., 1998). The delay in nigrostriatal dopamine (DA) cell loss in human α-synuclein mutations as well as the discovery of pathological α-synuclein accumulation in the brain of some individuals without nigrostriatal DA cell loss (incidental Lewy body disease) suggests that DA cell loss may be preceded by a protracted period of α-synuclein accumulation (Braak et al., 2003).
Transgenic mice expressing human α-synuclein under the Thy-1 promoter (Thy1-aSyn, alpha-synuclein overexpressor) show widespread overexpression and accumulation of α-synuclein in brain neurons, including the nigrostriatal neurons (Rockenstein et al., 2002). These mice do not show loss of nigrostriatal dopaminergic cell bodies or terminals (Rockenstein et al., 2002; Fernagut et al., 2004, 2005) but they exhibit increased mitochondrial pathology in nigrostriatal neurons in response to the neurotoxin MPTP, suggesting that these neurons are altered by the overexpression of α-synuclein (Song et al., 2004). These mice exhibit robust behavioral deficits under challenging motor tests, such as the beam traversal and pole tests (Fleming et al., 2004). Mice with a loss of DA neurons also show impairments on these tests (Hwang et al., 2005) suggesting a role for DA in the behavioral anomalies observed in Thy1-aSyn mice. The effect of broad α-synuclein overexpression on behaviors elicited by DA receptor stimulation, however, is unknown.
In this study we determined whether behavioral responses to direct and indirect DA agonists were altered in Thy1-aSyn mice compared with control mice. L-DOPA, the direct DA agonist apomorphine, and the indirect agonist amphetamine were administered to male Thy1-aSyn and their wild-type littermates, which were then tested on sensorimotor tests that show alterations in Thy1-aSyn mice.
EXPERIMENTAL PROCEDURES
Animal care was conducted in accordance with the United States Public Health Service Guide for the Care and Use of Laboratory Animals, and procedures were approved by the Institutional Animal Care and Use Committee at the University of California Los Angeles (UCLA). Every effort was made to minimize the number of animals used and their suffering. Transgenic mice overexpressing human wild-type α-synuclein under the Thy-1 promoter (Thy1-aSyn) were created previously (Rockenstein et al., 2002), crossed into a mixed C57BL/6-DBA/2 background, and maintained on the mixed C57BL/6-DBA/2 background by breeding mutant females with wild-type males. Genotype of all mice was verified with polymerase chain reaction (PCR) amplification analysis of tail DNA. All animals were group housed with littermates of the same sex with a maximum of four mice per cage. Animals were maintained on a12-h dark/light cycle and all testing was performed during the dark cycle. Water and food were available ad libitum.
In a first set of experiments, drugs and vehicle were administered in a Latin square design to a cohort of 21 male mice from seven litters (Thy1-aSyn, n=10, wild-types, n=11). Mice were repeatedly tested from 3 to 5 months of age. Each drug/vehicle treatment was administered on two test days one week apart in a counterbalanced manner. The peripheral dopa decarboxylase inhibitor benserazide (12.5 mg/kg, i.p.; Sigma, St. Louis, MO, USA) was administered 20 min prior to L-DOPA (25 mg/kg, i.p.) while vehicle-treated animals received injections of 0.9% saline 20 min prior to 0.9% saline. Two weeks after completion of L-DOPA testing, animals were treated with the direct DA agonist apomorphine (Sigma; 2 mg/kg apomorphine dissolved in 0.1% ascorbic saline, s.c.) or 0.1% ascorbic saline. Similar to L-DOPA, apomorphine and vehicle treatments were administered in two sessions one week apart in a counterbalanced manner. The indirect agonist amphetamine (5 mg/kg, i.p.; Sigma) was given in two sessions one week after completion of testing with apomorphine. Following each drug or vehicle administration all mice were successively tested on the challenging beam, spontaneous activity, gait and on the pole test. Behavioral tests were administered in the same order to all animals.
Because apomorphine has been shown to have differential effects on activity depending of the dose administered (Cabib and Puglisi-Allegra, 1985), a separate group of male Thy1-aSyn (n=8) and wild-type (n=4) mice from four litters was repeatedly administered varying low doses of apomorphine at 3–4 months of age. Thy1-aSyn and wild-type mice were administered vehicle, 0.125, 0.25, or 0.5 mg/kg apomorphine (s.c.; dissolved in 0.1% ascorbic saline) in a total of four tests sessions, one session a week for four consecutive weeks. Drug order was counterbalanced between animals and all animals received all drug doses and one vehicle injection. Following drug and vehicle administration, all animals were tested on the challenging beam, spontaneous activity, and gait, and all animals were administered the tests in the same order.
Challenging beam traversal
Motor performance was measured with a challenging beam traversal test as previously described (Goldberg et al., 2003; Fleming et al., 2004; Fleming and Chesselet, 2005; Hwang et al., 2005). Briefly, animals were trained to traverse the length of a Plexiglas beam consisting of four sections (25 cm each, 1 m total length) of different width (3.5 cm to 0.5 cm by 1 cm increments) with support ledges attached along each side and leading to the animals’ home-cage. After two days of training a mesh grid (1 cm squares) of corresponding width was placed over the beam surface leaving approximately a 1 cm space between the grid and the beam surface. Animals were then videotaped while traversing the grid-surfaced beam for a total of five trials. Videotapes were viewed and rated in slow motion for errors, number of steps made by each animal, and time to traverse across five trials by an investigator blind to the mouse genotype and drug treatment. Scores were calculated across all five trials and averaged for each mouse.
Spontaneous activity
Spontaneous movements were videotaped for 3 min in a small transparent cylinder (height, 15.5 cm, diameter, 12.7 cm) placed on a piece of glass, with a mirror underneath to permit a clear view of motor movements along the floor and along the walls of the cylinder (Fleming et al., 2004; Fleming and Chesselet, 2005; Hwang et al., 2005). The number of rears, forelimb and hindlimb steps, and time spent grooming were measured. In addition, because the dose of amphetamine used induces stereotypic behaviors in mice and rats (Kuczenski and Segal, 1999), stereotypy was also measured in the cylinder following amphetamine administration. Videotapes were viewed and rated in slow motion by an experimenter blind to the mouse genotype and drug treatment. For stereotypy, time spent licking, head bobbing, turning, and jumping were measured for each mouse.
Pole test
The pole test has been used previously to assess basal ganglia–related movement disorders in mice (Ogawa et al., 1985, 1987; Fernagut et al., 2002a; Matsuura et al., 1997; Fleming et al., 2004; Hwang et al., 2005). Briefly, animals are placed head upwards on top of a vertical wooden pole 50 cm long (1 cm in diameter) with the base in the home cage. After two days of training (five trials for each session), animals received five trials and time to orient downward and time to descend were measured and averaged for each mouse.
Gait analysis
Animals were trained to walk through a narrow alley leading into their home-cage. Once trained, paper was placed along the alley floor and each animal’s forelimbs and hindlimbs were brushed with non-toxic paint (Schallert et al., 1978; Fernagut et al., 2002b; Tillerson et al., 2002; Fleming et al., 2004; Fleming and Chesselet, 2005). Stride length was determined by measuring the distance between paw prints. Stride width was calculated by measuring the distance between hindlimbs. In addition, the range of stride lengths within each animal was measured by subtracting the shortest from the longest stride length (Dunnett, 2003).
Statistics
For L-DOPA, the high dose of apomorphine, and amphetamine, a 2×2 mixed design ANOVA was used to compare genotype (wild-type and Thy1-aSyn mice) and drug (drug and vehicle) for beam traversal, spontaneous activity, the pole test, and gait analysis. Planned comparisons were used to compare the effect of drug in wild-type and Thy1-aSyn mice. For the low doses of apomorphine, challenging beam traversal and gait were analyzed with a 2×4 mixed design ANOVA comparing genotype and dose. Post hoc analysis was performed with Fisher’s LSD. For spontaneous activity, nonparametric tests were used because many animals had activity scores approaching zero, creating significant heterogeneity of variance. A Mann-Whitney U was used to compare wild-type and Thy1-aSyn mice at each dose and a Wilcoxon signed rank test was used to compare apomorphine doses within each genotype. All statistics were calculated with GB-Stat software (Dynamic Microsystems, Inc. Silver Spring, MD, USA, 2000) for Macintosh. The level of significance was set at P<0.05.
RESULTS
L-DOPA
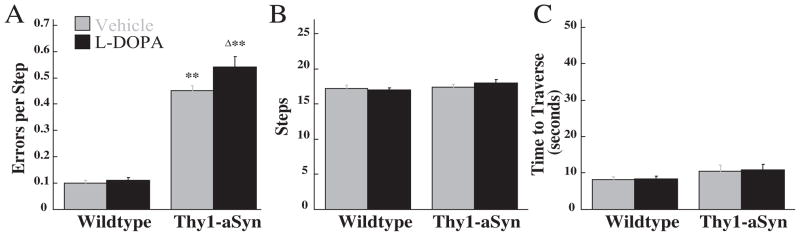
A significant genotype×drug interaction F(1,19)=4.66, P<0.05 was observed for the number of errors per step after L-DOPA administration. As previously reported (Fleming et al., 2004), planned comparisons indicated that Thy1-aSyn mice made more errors per step compared with wild-type mice. This was further increased following L-DOPA administration in Thy1-aSyn but not in wild-type mice. No effect of genotype or drug was found for the number of steps or time to traverse the beam (P>0.05; Fig. 1).
Fig. 1.
L-DOPA (25 mg/kg) effects in Thy1-aSyn (n=10) and wild-type (n=11) mice on the challenging beam. Errors per step (A), steps (B), and time to traverse (C) were measured. ** Indicates P<0.01 compared with similarly treated wild-type mice. Δ Indicates P<0.05 compared with vehicle-treated mice of the same genotype.
An effect of L-DOPA, F(1,19)=12.14, P<0.01, was observed on rearing in the cylinder. Although vehicle- treated Thy1-aSyn and wild-type mice did not exhibit significant differences in this behavior, L-DOPA significantly reduced rearing in Thy1-aSyn mice. L-DOPA also affected forelimb stepping: F(1,19)=13.98, P<0.01. Both Thy1-aSyn and wild-type mice made fewer forelimb steps compared with their vehicle scores after L-DOPA administration. Both genotypes, F(1,19)=12.84, P<0.01, and L-DOPA, F(1,19)=8.51, P<0.01 affected hindlimb stepping. Vehicle-treated Thy1-aSyn mice made significantly fewer hindlimb steps compared with wild-type mice. L-DOPA decreased hindlimb stepping in wild-type mice but did not worsen the deficit observed in Thy1-aSyn mice. A main effect of drug was observed on grooming F(1,19)=7.32, P<0.05, which was increased by L-DOPA in wild-type mice (Fig. 2).
Fig. 2.
L-DOPA (25 mg/kg) effects in Thy1-aSyn (n=10) and wild-type (n=11) mice on spontaneous activity. Rears (A), forelimb (B) and hindlimb (C) stepping, and grooming (D) were measured over three minutes. ** Indicates P<0.01 compared with similarly treated wild-type mice. Δ Indicates P<0.05 compared with vehicle-treated mice of the same genotype.
Apomorphine
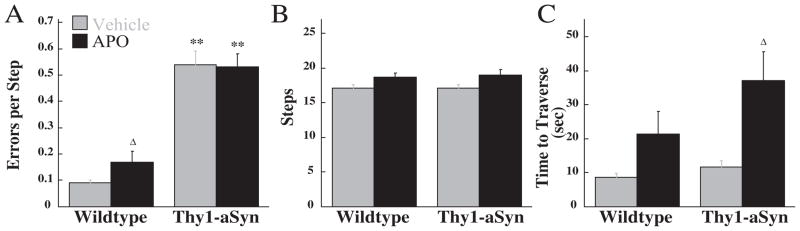
On the challenging beam, the same cohort of mice showed an effect of genotype on errors per step, F(1,19)=101.01, P<0.01, indicating that repeated testing did not affect this deficit. Similar to the previous testing session, Thy1-aSyn mice made more errors per step compared with wild-type mice, however in contrast to L-DOPA, apomorphine increased the number of errors in wild-type mice only. Thus the high dose of apomorphine caused in wild-type an impairment similar to that seen in Thy1-aSyn mice but did not worsen the deficit already present in the transgenics. No effect of genotype or apomorphine was observed on number of steps but an effect of drug was seen on the time to traverse the beam: F(1,19)=10.71, P<0.01. This behavior was significantly increased by apomorphine in Thy1-aSyn mice (Fig. 3).
Fig. 3.
Apomorphine (2 mg/kg) effects in Thy1-aSyn (n=10) and wild-type (n=11) mice on the challenging beam. Errors per step (A), steps (B), and time to traverse (C) were measured. ** Indicates P<0.01 compared with similarly treated wild-type mice. Δ Indicates P<0.05 compared with vehicle-treated mice of the same genotype.
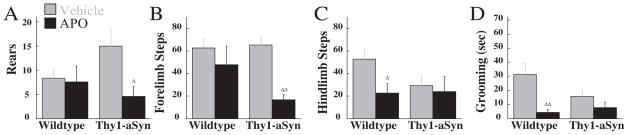
In the cylinder, apomorphine affected rearing, F(1,19)=4.44, P<0.05, significantly decreasing this behavior in Thy1-aSyn compared with vehicle. A similar effect of apomorphine was observed in forelimb stepping, with a significant effect of drug: F(1,19)=13.26, P<0.01. In contrast to previous observations in the same cohort of mice, no main effect of genotype or apomorphine was detected in hind-limb stepping during this second experiment, suggesting that this behavioral deficit may be sensitive to repeated testing. However, planned comparisons indicated that hindlimb stepping was reduced in wild-type mice following apomorphine. An effect of apomorphine, F(1,19)=12.04, P<0.01, was detected on grooming. Apomorphine decreased grooming in wild-type mice (Fig. 4).
Fig. 4.
Apomorphine (2 mg/kg) effects in Thy1-aSyn (n=10) and wild-type (n=11) mice on spontaneous activity. Rears (A), forelimb (B) and hindlimb (C) stepping, and grooming (D) were measured over three minutes. Δ And ΔΔ indicate P<0.05 and 0.01 compared with vehicle-treated mice of the same genotype.
Low dose apomorphine
To further examine the effect of a wider range of apomorphine doses, apomorphine (0.125, 0.25, 0.5 mg/kg) and vehicle were administered using a Latin square design to a separate cohort of male mice (Table 1). On the challenging beam, the effect of genotype on errors per step was confirmed in this cohort: F(1,10)=4.9, P<0.05. Thy1-aSyn mice made significantly more errors per step compared with wild-type mice at all doses. Errors per step were not altered by apomorphine in wild-type mice. In contrast, Thy1-aSyn mice receiving the highest dose of apomorphine (0.5 mg/kg) made more errors compared with their performance at the lower doses and after vehicle. A significant genotype×dose interaction, F(3,30)=3.23, P<0.05, was observed for the number of steps taken to traverse the beam. Thy1-aSyn mice made fewer steps compared with wild-type mice at the 0.125 and 0.25 mg/kg doses of apomorphine. All doses of apomorphine caused wild-type mice to make more steps compared with their vehicle scores but only the 0.125 and 0.5 mg/kg doses significantly increased the number of steps in Thy1-aSyn compared with their vehicle scores. Apomorphine dose affected the time to traverse the beam: F(3,30)=5.47, P<0.01. Although this measure did not significantly differ between Thy1-aSyn and wild-type mice, Thy1-aSyn mice receiving the 0.25 and 0.5 mg/kg doses of apomorphine were significantly slower compared with vehicle and the 0.125 mg/kg dose. Only the 0.5 mg/kg apomorphine dose caused slower traversal times in wild-type mice.
Table 1.
Apomorphine (low doses) effects in Thy1-aSyn and wild-type mice
| Thy1-aSyn mice | Vehicle | 0.125 | 0.25 | 0.5 |
|---|---|---|---|---|
| Beam | ||||
| Err/step | 0.38±0.08** | 0.33±0.10** | 0.38±0.11** | 0.53±0.14**Δ |
| Steps | 16.2±0.59 | 17.2±0.77*Δ | 17.0±0.68* | 18.6±0.36ΔΔ |
| Time | 22.1±7.04 | 26.6±4.43 | 46.0±9.85ΔΔ | 51.3±10.65ΔΔ |
| Activity | ||||
| Rears | 7.5±2.49 | 1.0±0.53Δ | 0.63±0.32Δ | 0.75±0.62Δ |
| FL steps | 57.5±17.8 | 25.9±3.22** | 17.4±8.25Δ | 13.0±4.48*Δ |
| HL steps | 24.0±11.4 | 5.3±1.53** | 5.3±3.40 | 2.1±1.01* |
| Groom | 23.5±7.86 | 22.9±8.50* | 6.4±3.12Δ | 11.3±4.55* |
| Wild-type mice | Vehicle | 0.125 | 0.25 | 0.5 |
| Beam | ||||
| Err/step | 0.10±0.02 | 0.08±0.01 | 0.09±0.02 | 0.13±0.03 |
| Steps | 15.4±0.65 | 18.6±0.36ΔΔ | 18.4±0.34ΔΔ | 19.6±0.53ΔΔ |
| Time | 7.9±0.86 | 21.9±2.90 | 25.4±1.59 | 32.6±6.02Δ |
| Activity | ||||
| Rears | 13.3±5.12 | 6.5±3.59 | 1.5±0.87 | 0.75±0.75 |
| FL steps | 70.5±7.96 | 61.5±13.73 | 26.5±3.80 | 39.0±7.67 |
| HL steps | 45.8±14.2 | 41.3±9.02 | 13.0±6.62 | 16.0±9.15 |
| Groom | 40.8±2.36 | 53.3±13.35 | 52.0±24.96 | 30.5±2.10 |
Challenging beam (errors per step, steps, time to traverse) and activity scores (rears, forelimb steps, hindlimb steps, time spent grooming) in Thy1-aSyn (n=8) and wild-type (n=4) mice following vehicle, 0.125, 0.25, 0.5 mg/kg of apomorphine.
indicate P<0.05 and P<0.01, respectively, compared to similarly treated wild-type mice.
indicate P<0.05 and P<0.01, respectively, compared to vehicle-treated mice of the same genotype.
Regarding spontaneous activity (Table 1), Thy1-aSyn mice did not significantly differ from wild-type mice in rearing. Thy1-aSyn mice reared less compared with their vehicle scores after all apomorphine doses, whereas rearing was unaffected by apomorphine in wild-type mice. Thy1-aSyn mice made fewer forelimb steps compared with wild-type mice at the 0.125 and 0.5 mg/kg doses. In addition, Thy1-aSyn mice made fewer forelimb steps compared with their vehicle scores following the 0.25 and 0.5 mg/kg apomorphine doses. Forelimb stepping was unaltered by apomorphine in wild-type mice. For hindlimb stepping, Thy1-aSyn mice made fewer hindlimb steps compared with wild-type mice at the 0.125 and 0.5 mg/kg doses however, hindlimb stepping was not affected by apomorphine in Thy1-aSyn or wild-type mice. For grooming, Thy1-aSyn mice groomed less than wild-type mice at the 0.125 and 0.5 mg/kg doses. Apomorphine had no effect in wild-type mice, but did decrease grooming in Thy1-aSyn mice at the 0.25 mg/kg dose. Apomorphine did not induce any significant effects on stride length, width or stride difference in either Thy1-aSyn or wild-type mice (P>0.05).
Amphetamine
Amphetamine was administered to the first cohort of mice after the tests with L-DOPA and high dose of apomorphine were completed. On the challenging beam, the effect of genotype on errors per step observed in previous testing sessions was confirmed: F(1,19)=35.96, P<0.01, and again, Thy1-aSyn made more errors per step compared with wild-type mice. Amphetamine administration did not alter this behavior in either wild-type or Thy1-aSyn mice. No effect of genotype or amphetamine was detected on the number of steps to traverse the beam. Vehicle-treated Thy1-aSyn and wild-type mice did not differ however, planned comparisons indicated that following amphetamine wild-type but not Thy1-aSyn mice made significantly fewer steps (P<0.05) compared with their vehicle scores. No effect of genotype or drug was found for the time to traverse: vehicle-treated wild-type and Thy1-aSyn mice did not differ, and only wild-type mice were significantly faster following amphetamine than when they received vehicle (P<0.05; Fig. 5).
Fig. 5.
Amphetamine (5 mg/kg) effects in Thy1-aSyn (n=10) and wild-type (n=11) mice on the challenging beam. Errors per step (A), steps (B), and time to traverse (C) were measured. * And ** indicate P<0.05 and 0.01 compared with similarly treated wild-type mice. Δ Indicates P<0.05 compared with vehicle-treated mice of the same genotype.
In the cylinder, neither genotype nor drug significantly altered rearing. Both genotype F(1,19)=5.59, P<0.05 and drug F(1,19)=13.26, P<0.01 affected forelimb stepping. Although not different between vehicle-treated Thy1-aSyn and wild-type mice, forelimb stepping increased in both Thy1-aSyn and wild-type mice after amphetamine but to a lesser extent in the transgenics. An effect of amphetamine, F(1,19)=13.72 (P<0.01) was found on hindlimb stepping. Similar to the previous test session in, hindlimb stepping did not differ between Thy1-aSyn and wild-type mice but it increased in both types of mice after amphetamine. Both genotype F(1,19)=6.74, P<0.05 and amphetamine, F(1,19)=18.05, P<0.01 affected grooming. Vehicle-treated Thy1-aSyn mice groomed less than wild-type mice and following amphetamine, grooming was decreased in both Thy1-aSyn and wild-type mice (Fig. 6).
Fig. 6.
Amphetamine (5 mg/kg) effects in Thy1-aSyn (n=10) and wild-type (n=11) mice on spontaneous activity. Rears (A), forelimb (B) and hindlimb (C) stepping, and grooming (D) were measured over three minutes. * Indicates P<0.05 compared with similarly treated wild-type mice. Δ And ΔΔ indicate P<0.05 and 0.01 compared with vehicle-treated mice of the same genotype.
Because increase in stereotypies is a major effect of amphetamine, stereotyped behaviors were also measured in the cylinder. A significant genotype×amphetamine interaction, F(1,19)=15.99, P<0.01, on stereotyped behavior was observed. Vehicle-treated Thy1-aSyn mice showed less stereotyped behaviors compared with wild-type mice (P<0.05). Following amphetamine, wild-type mice showed a robust increase in stereotypic behaviors compared with their vehicle scores (P<0.01). In marked contrast, amphetamine did not alter stereotyped behavior in Thy1-aSyn mice (Fig. 7).
Fig. 7.
Amphetamine-induced stereotypies in Thy1-aSyn (n=10) and wild-type (n=11) mice. In the cylinder stereotyped behaviors such as licking, head bobbing, turning and jumping were measured in the cylinder following amphetamine (5 mg/kg). * And ** indicate P<0.05 and P<0.01, respectively compared with similarly treated wild-type mice. ΔΔ Indicates P<0.01 compared with vehicle-treated wild-type mice.
Pole test and gait analysis
In the pole test, although vehicle-treated Thy1-aSyn mice had consistently longer t-turn and t-down time than wild-type mice (Fleming et al., 2004), the dopaminergic agents had little or no effect in this test. Only apomorphine (2 mg/kg) caused an increase in t-turn and t-down and this occurred in both wild-type and Thy1-aSyn mice (Table 2). Only modest effects of genotype or drug were observed on the different gait parameters. Vehicle-treated Thy1-aSyn and wild-type mice differed in stride width in the apomorphine and amphetamine experiments. Amphetamine significantly increased stride width in Thy1-aSyn mice only (Table 2).
Table 2.
The effect of L-DOPA, apomorphine, and amphetamine in Thy1-aSyn and wild-type mice on the pole test and gait analysis
| Drug treatment | Pole
|
Gait
|
|||
|---|---|---|---|---|---|
| T-turn | T-down | Length | Width | Difference | |
| Vehicle | |||||
| WT | 1.56±0.25 | 4.31±0.47 | 7.52±0.27 | 4.13±0.11 | 0.77±0.16 |
| Thy1-aSyn | 3.92±0.56* | 7.60±0.78** | 6.87±0.35 | 3.81±0.18 | 1.15±0.21 |
| L-DOPA | |||||
| WT | 1.60±0.35 | 4.87±0.60 | 7.30±0.37 | 3.90±0.14 | 1.23±0.23 |
| Thy1-aSyn | 3.42±0.53 | 7.52±0.72* | 6.46±0.38 | 3.74±0.19 | 1.88±0.41 |
| Vehicle | |||||
| WT | 1.62±0.20 | 4.84±0.32 | 7.02±0.27 | 3.99±0.08 | 1.09±0.18 |
| Thy1-aSyn | 4.62±0.7** | 8.32±0.78* | 6.21±0.41 | 3.49±0.16* | 1.86±0.31 |
| Vehicle | |||||
| WT | 4.09±0.79Δ | 8.33±1.18Δ | 6.88±0.27 | 3.78±0.13 | 1.22±0.19 |
| Thy1-aSyn | 8.16±1.86 | 14.82±2.95Δ | 6.75±0.29 | 3.50±0.15 | 1.95±0.43 |
| APO | |||||
| WT | 1.85±0.44 | 4.07±0.59 | 7.74±0.28 | 4.21±0.18 | 0.89±0.21 |
| Thy1-aSyn | 3.52±0.51* | 6.24±0.59* | 7.31±0.40 | 3.47±0.20* | 1.47±0.28 |
| AMP | |||||
| WT | 1.25±0.09 | 3.15±0.20 | 8.34±0.26 | 4.60±0.21 | 1.22±0.19 |
| Thy1-aSyn | 5.34±1.06** | 7.68±1.06** | 8.03±0.25 | 4.53±0.30Δ | 1.41±0.21 |
Performance on the pole test (T-turn, T-down) and gait (stride length, stride width, maximum stride difference) in Thy1-aSyn (n=10) and wild-type (WT; n=11) mice following vehicle/L-DOPA, vehicle/apomorphine (APO), and vehicle/amphetamine (AMP).
indicate P<0.05 and P<0.01, respectively, compared to similarly treated wild-type mice.
Indicates P<0.05 compared to vehicle-treated mice of the same genotype.
DISCUSSION
Mice overexpressing wild-type α-synuclein were used in this study to assess the effect of excess α-synuclein on the DA system in the absence of DA cell death, a situation that may precede the onset of PD in familial (Singleton et al., 2003) and sporadic cases of the disease (Braak et al., 2003). The results confirm the presence of behavioral anomalies in Thy1-aSyn mice, and reveal that their behavioral response to DA agonists differs from wild-type, the most striking difference observed being a resistance to amphetamine-induced stereotypies.
Drug effects in wild-type mice
The dopaminergic precursor L-DOPA is the main treatment for PD and reverses motor deficits in rodents that have a loss of dopaminergic neurons (Lindner et al., 1996; Fleming et al., 2005; Hwang et al., 2005). In contrast, in normal mice L-DOPA would increase stimulation of dopaminergic receptors already stimulated by normal levels of DA, although increases in synaptic DA are blunted by uptake into dopaminergic terminals (Abercrombie et al., 1990). Wild-type mice in this study showed an increase in grooming behavior in response to L-DOPA, which likely accounts for the decrease in the other behaviors in the cylinder. A similar increase in grooming is observed after stimulation of specific D1 DA receptor agonists in rodents (Molloy and Waddington, 1984), suggesting that the effect of L-DOPA in wild-type mice may primarily reflect an increased stimulation of D1 dopaminergic receptors.
Apomorphine, which directly stimulates both D1 and D2 dopaminergic receptors, elicits differential behavioral effects depending on the dose administered and the strain of mouse (Sansone et al., 1981; Vetulani et al., 1982; Cabib and Puglisi-Allegra, 1985; Zhuang et al., 2001). In the present study, mice were maintained on a mixed C57BL/6×DBA/2 background and wild-type mice displayed decreases in hindlimb stepping and grooming similar to what would be expected in the DBA/2 strain (Cabib and Puglisi-Allegra, 1985). With the high dose of apomorphine behavioral deficits were also observed on the challenging beam and in the pole test in wild-type mice, further indicating that in the presence of an intact DA system, overstimulation of DA receptors by apomorphine leads to impaired motor performance in these mice.
In contrast to apomorphine, amphetamine not only releases DA, but also serotonin and noradrenaline, and inhibits DA reuptake and metabolism. The resulting effect is an increase in extracellular DA, noradrenaline, and serotonin (Heikkila et al., 1975; Seiden et al., 1993). As previously observed in rodents, amphetamine induced a general increase in motor activity (Randrup and Munkvad, 1975; Russell and Pihl, 1978), manifested here as a decrease in the time to traverse and number of steps on the challenging beam and increased forelimb and hindlimb movements in the cylinder. In addition, amphetamine induced robust stereotyped behaviors in wild-type mice. In summary, wild-type mice in this study responded to dopaminergic agents in a way compatible with their known effects.
Behavioral deficits in Thy1-aSyn mice
Thy1-aSyn mice, tested between 3 and 5 months of age, showed behavioral deficits in tests of sensorimotor function similar to those we have previously described at the same age (Fleming et al., 2004). As also observed in parkin-deficient mice (Goldberg et al., 2003), the most robust phenotype was an increase in the number of errors per step on the challenging beam. Thy1-aSyn mice also showed a marked deficit in the time to orient and descend from a pole (Fleming et al., 2004), similar to mice lacking nigrostriatal DA neurons (Pitx3 −/− mice; Hwang et al., 2005). These data confirm the robustness of these behavioral deficits and indicate that they can be observed in Thy1-aSyn mice despite frequent repeated testing. However, repeated testing (as well as prior drug exposure) may account for the lack of a consistent decrease in hindlimb stepping and grooming in the cylinder and in the time to traverse the beam (Voikar et al., 2004), all deficits that have been previously observed in Thy1-aSyn mice (Fleming et al., 2004).
Drug effects in Thy1-aSyn mice
L-DOPA therapy is the gold standard treatment for patients with PD and reverses motor impairments, similar to those observed in Thy1-aSyn mice, in mice with a loss of nigro-striatal DA neurons (Hwang et al., 2005; Fleming et al., 2005; Lindner et al., 1996). In contrast to wild-type mice, L-DOPA worsened motor performance on the challenging beam and decreased spontaneous activity in Thy1-aSyn mice. Previous studies indicated that overexpression of A30P mutant α-synuclein leads to reduced capacity of DA storage pool in nigrostriatal terminals and to paradoxical responses to L-DOPA (Yavich et al., 2004). Although the relevance of this observation to our behavioral finding remains to be established, these authors predicted that, behaviorally, mice with altered levels of α-synuclein would perform poorly in tests that required prolonged bursting of DA neurons and that L-DOPA would further worsen their performance on these tests, as we have observed (Yavich et al., 2004). Altered behavioral responses in Thy1-aSyn mice could also be due to abnormal postsynaptic responses because electrophysiological studies on medium spiny neurons in the striatum show paradoxical responses to both the DA D2 receptor agonist quinpirole and the D2 antagonist sulpiride in Thy1-aSyn mice (Wu et al., 2005).
Although amphetamine increased locomotor activity and decreased grooming in both wild-type and Thy1-aSyn mice, transgenic mice were insensitive to both amphetamine-induced stereotypies and decreased motor activity on the beam. The altered response to amphetamine in this study is unlikely presynaptic in origin because Thy1-aSyn mice have normal DA release in response to amphetamine (Maidment et al., 2006). Interestingly, amphetamine has no effect on the frequency of spontaneous EPSCs in striatal slices of Thy1-aSyn mice (Wu et al., 2005), suggesting a postsynaptic dysfunction.
CONCLUSIONS
Although less extensively documented than in our studies of Thy1-aSyn mice, abnormal behavioral responses to DA agonists have been observed in other lines of mice with PD-related mutations. These include mice overexpressing α-synuclein under the tyrosine hydroxylase promoter (Richfield et al., 2002; Thiruchelvam et al., 2004), mice lacking parkin (Itier et al., 2003), DJ-1 knockout mice (Kim et al., 2005) and mice lacking Gpr37, a substrate for parkin (Marazziti et al., 2004). Similarly, increased basal extracellular DA and increased DA content in the striatum have been observed in the Thy1-aSyn mice used in this study (Maidment et al., 2006) as well as in parkin knockout mice (Goldberg et al., 2003) and DJ-1 knockout mice (Chen et al., 2005), indicating a provocative common feature of mice expressing diverse PD-related mutations. Together with electrophysiological evidence for abnormal responses to dopaminergic agents in the striatum (Wu et al., 2005), the data indicate that α-synuclein overexpression, as well as other PD-causing mutations, induces profound perturbations of the nigrostriatal DA system even in the absence of DA neuronal death. Whether these anomalies lead to the abnormal behavioral responses to dopaminergic agonists reported here remains to be determined.
Acknowledgments
We thank Ehud Gruen and Gowry Fernando for their assistance with the mouse colony. Funded by Morris K. Udall Parkinson’s Disease Research Center of Excellence at UCLA (P50NS38367) and NIH/NIEHS (U54ES12078) to M.-F.C. and M.S.L., AG18440 and AG022074 to E.M., and Training Program in Neural Repair T32 NS07449-05 NIH/National Institute of Neurological Disorders & Stroke (SMF/Trainee) and the Chen Family.
Abbreviations
- DA
dopamine
- PD
Parkinson’s disease
- Thy1-aSyn
alpha-synuclein overexpressor
References
- Abercrombie ED, Bonatz AE, Zigmond MJ. Effects of L-DOPA on extracellular dopamine in striatum of normal and 6-hydroxydopamine-treated rats. Brain Res. 1990;525:36–44. doi: 10.1016/0006-8993(90)91318-b. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Chen L, Cagniard B, Matthews T, Jones S, Koh HC, Ding Y, Carvey PM, Ling Z, Kang UJ, Zhuang X. Age-dependent motor deficits and dopaminergic dysfunction in DJ-1 null mice. J Biol Chem. 2005;280(22):21418–21426. doi: 10.1074/jbc.M413955200. [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. Different effects of apomorphine on climbing behavior and locomotor activity in three strains of mice. Pharmacol Biochem Behav. 1985;23:555–557. doi: 10.1016/0091-3057(85)90418-6. [DOI] [PubMed] [Google Scholar]
- Dunnett SB. Assessment of motor impairments in transgenic mice. In: Crawley JN, editor. Mouse behavioral phenotyping. Washington, DC: Society for Neuroscience; 2003. pp. 1–12. [Google Scholar]
- Fernagut P-O, Diguet E, Jaber M, Bioulac B, Tison F. Dopamine transporter knock-out mice are hypersensitive to 3-nitropropionic acid-induced striatal damage. Eur J Neurosci. 2002a;15:2053–2056. doi: 10.1046/j.1460-9568.2002.02047.x. [DOI] [PubMed] [Google Scholar]
- Fernagut P-O, Diguet E, Labattu B, Tison F. A simple method to measure stride length as an index of nigrostriatal dysfunction in mice. J Neurosci Methods. 2002b;113:123–130. doi: 10.1016/s0165-0270(01)00485-x. [DOI] [PubMed] [Google Scholar]
- Fernagut P-O, Hutson CB, Fleming SM, Masliah E, Levine MS, Chesselet M-F. Program No. 559.2. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2004. Behavioral deficits in mice overexpressing alpha-synuclein under the PDGF promoter occur without loss of tyrosine hydroxylase immunoreactivity and is not affected by the pesticide paraquat. [Google Scholar]
- Fernagut PO, Hutson CB, Fleming SM, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Abtract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2005. Nigrostriatal dysfunction in transgenic mice overexpressing α-synuclein. Program No. 85.13. [Google Scholar]
- Fleming SM, Chesselet M-F. Phenotypical characterization of genetic mouse models of Parkinson’s disease. In: LeDoux M, editor. Animal models of movement disorders. San Diego: Academic; 2005. pp. 183–192. [Google Scholar]
- Fleming SM, Delville Y, Schallert T. An intermittent, controlled-rate, slow progressive degeneration model of Parkinson’s disease: validation with Sinemet and methylphenidate. Behav Brain Res. 2005;156(2):201–213. doi: 10.1016/j.bbr.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut P-O, Masliah E, Levine MS, Chesselet M-F. Early and progressive motor abnormalities in mice overexpressing wildtype human alpha-synuclein. J Neurosci. 2004;24(42):9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, Gajendiran M, Roth BL, Chesselet M-F, Maidment NT, Levine MS, Shen J. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Orlansky H, Mytilineou C, Cohen G. Amphetamine: evaluation of d- and l-isomers as releasing agents and uptake inhibitors for 3H-dopamine and 3H-norepinephrine in slices of rat neostriatum and cerebral cortex. J Pharmacol Exp Ther. 1975;194:47–56. [PubMed] [Google Scholar]
- Hwang D-Y, Fleming SM, Ardayfio P, Moran-Gates T, Kim H, Tarazi FI, Chesselet M-F, Kim K-S. 3,4-Dihydroxyphenylalanine reverses the motor deficits in Pitx3-deficient aphakia mice: Behavioral characterization of a novel genetic model of Parkinson’s disease. J Neurosci. 2005;25(8):2132–2137. doi: 10.1523/JNEUROSCI.3718-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier JM, Ibanez P, Mena MA, Abbas N, Cohen-Salmon C, Bohme GA, Laville M, Pratt J, Corti O, Pradier L, Ret G, Joubert C, Periquet M, Araujo F, Negroni J, Casarejos MJ, Canals S, Solano R, Serrano A, Gallego E, Sanchez M, Denefle P, Benavides J, Tremp G, Rooney TA, Brice A, Garcia de Yebenes J. Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum Mol Genet. 2003;12:2277–2291. doi: 10.1093/hmg/ddg239. [DOI] [PubMed] [Google Scholar]
- Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, Westaway D, Lozano AM, Anisman H, Park DS, Mak TW. Hypersensitivity of DJ-1 deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropy-rindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A. 2005;102(14):5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Sensitization of amphetamine-induced stereotyped behaviors during the acute response. J Pharmacol Exp Ther. 1999;288:699–709. [PubMed] [Google Scholar]
- Lindner MD, Plone MA, Francis JM, Emerich DF. Validation of a rodent model of Parkinson’s Disease: evidence of a therapeutic window for oral Sinemet. Brain Res Bull. 1996;39:367–372. doi: 10.1016/0361-9230(96)00027-5. [DOI] [PubMed] [Google Scholar]
- Lotharius J, Brundin P. Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3:932–942. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- Maidment NT, Lam HA, Ackerson LC, Rockenstein E, Masliah E. Dysregulation of dopamine transmission in mice over-expressing wild-type human alpha-synuclein. Soc Neurosci Abstr 2006 [Google Scholar]
- Marazziti D, Golini E, Mandillo S, Magrelli A, Witke W, Matteoni R, Tocchini-Valentini GP. Altered dopamine signaling and MPTP resistance in mice lacking the Parkinson’s disease-associated GPR37/parkin-associated endothelin-like receptor. Proc Natl Acad Sci U S A. 2004;101:10189–10194. doi: 10.1073/pnas.0403661101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K, Kabuto H, Makino H, Ogawa N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods. 1997;73:45–48. doi: 10.1016/s0165-0270(96)02211-x. [DOI] [PubMed] [Google Scholar]
- Molloy AG, Waddington JL. Dopaminergic behaviour stereospecific promoted by the D1 agonist R-SK & F 38393 and selectively blocked by the D1 antagonist SCH 23390. Psychopharmacology (Berl) 1984;82:409–410. doi: 10.1007/BF00427697. [DOI] [PubMed] [Google Scholar]
- Ogawa N, Hirose Y, Ohara S, Ono T, Watanabe Y. A simple quantitative bradykinesia test in MPTP-treated mice. Res Commun Chem Pathol Pharmacol. 1985;50:435–441. [PubMed] [Google Scholar]
- Ogawa N, Mizukawa K, Hirose Y, Kajita S, Ohara S, Watanabe Y. MPTP-induced parkinsonian model in mice: biochemistry, pharmacology and behavior. Eur Neurol. 1987;26 (Suppl 1):16–23. doi: 10.1159/000116351. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Randrup A, Munkvad I. Stereotyped behavior. Pharmacol Ther [B] 1975;1:757–768. doi: 10.1016/0306-039x(75)90027-6. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Thiruchelvam MJ, Cory-Slechta DA, Wuertzer C, Gainetdinov RR, Caron MG, Di Monte DA, Federoff HJ. Behavioral and neurochemical effects of wild-type and mutated human alpha-synuclein in transgenic mice. Exp Neurol. 2002;175:35–48. doi: 10.1006/exnr.2002.7882. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68:568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- Russell RL, Pihl RO. The effect of dose, novelty, and exploration on amphetamine-produced stereotyped behavior. Psychopharmacology (Berl) 1978;60:93–100. doi: 10.1007/BF00429185. [DOI] [PubMed] [Google Scholar]
- Sansone M, Ammassari-Teule M, Renzi P, Oliverio A. Different effects of apomorphine on locomotor activity in C57BL/6 and DBA/2 mice. Pharmacol Biochem Behav. 1981;14:741–743. doi: 10.1016/0091-3057(81)90141-6. [DOI] [PubMed] [Google Scholar]
- Schallert T, Whishaw IQ, Ramirez VD, Teitelbaum P. Compulsive, abnormal walking caused by anticholinergics in akinetic, 6-hy-droxydopamine-treated rats. Science. 1978;199:1461–1463. doi: 10.1126/science.564552. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639–677. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Song DD, Shults CW, Sisk A, Rockenstein E, Masliah E. Enhanced substantia nigra mitochondrial pathology in human alpha-synuclein transgenic mice after treatment with MPTP. Exp Neurol. 2004;186:158–172. doi: 10.1016/S0014-4886(03)00342-X. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Takeda A, Hashimoto M, Mallory M, Sundsumo M, Hansen L, Sisk A, Masliah E. Abnormal distribution of the non-Abeta component of Alzheimer’s disease amyloid precursor/alpha-synuclein in Lewy body disease as revealed by proteinase K and formic acid pretreatment. Lab Invest. 1998;78:1169–1177. [PubMed] [Google Scholar]
- Thiruchelvam MJ, Powers JM, Cory-Slechta DA, Richfield EK. Risk factors for dopaminergic neuron loss in human alpha-synuclein transgenic mice. Eur J Neurosci. 2004;19:845–854. doi: 10.1111/j.0953-816x.2004.03139.x. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Caudle WM, Reveron ME, Miller GW. Detection of behavioral impairments correlated to neurochemical deficits in mice treated with moderate doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Exp Neurol. 2002;178:80–90. doi: 10.1006/exnr.2002.8021. [DOI] [PubMed] [Google Scholar]
- Vetulani J, Sansone M, Oliverio A. Analysis of the difference in the behavioral effects of apomorphine in C57BL/6 and DBA/2 mice. Pharmacol Biochem Behav. 1982;17:967–971. doi: 10.1016/0091-3057(82)90481-6. [DOI] [PubMed] [Google Scholar]
- Voikar V, Vasar E, Rauvala H. Behavioral alterations induced by repeated testing in C57BL/6J and 129S2/Sv mice: implications for phenotyping screens. Genes Brain Behav. 2004;3:27–38. doi: 10.1046/j.1601-183x.2003.0044.x. [DOI] [PubMed] [Google Scholar]
- Wu N, Cepeda C, Masliah E, Levine MS. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2005. Abnormal glutamate and dopamine receptor function in the striatum of α-synuclein-overexpressing mice. Program No. 85.12. [Google Scholar]
- Yavich L, Tanila H, Vepsäläinen S, Jäkälä P. Role of α-synuclein in presynaptic dopamine recruitment. J Neurosci. 2004;24(49):11165–11170. doi: 10.1523/JNEUROSCI.2559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]