Abstract
Sulfotransferases (SULTs) are phase II drug-metabolizing enzymes. While the induction of SULTs by hormones and endogenous molecules is relatively well studied, induction by xenobiotics is not well studied. Isoflavones are naturally occurring phytoestrogens, mainly existing in soy food products. They have been described as health-promoting, disease-preventing dietary supplements and as agents with cancer preventive activities. Recently, isoflavones have been reported to interact with nuclear receptors, including those that are known to mediate the induction of drug metabolizing enzymes. In the present investigation, the isoflavone genistein was shown to be a xenobiotic inducer of human SULTs in transformed human liver cells (HepG2) and colon carcinoma cells (Caco-2). Enzymatic activity assay, Western blot, and real-time reverse-transcription polymerase chain reaction (RT-PCR) results demonstrated that genistein significantly induced protein and mRNA expression of human simple phenol sulfotransferase (hSULT1A1) and human dehydroepiandrosterone sulfotransferase (hSULT2A1) in HepG2 and Caco-2 cells. Induction was time- and dose-dependent. Western blot results agreed well with real-time RT-PCR results, suggesting that induction occurred at the gene transcription level. This isoflavone is the first nutritionally related phytoestrogen shown to induce human SULTs in HepG2 and Caco-2 cells.
Keywords: Genistein, Isoflavone, Induction, Human sulfotransferase (SULT)
1. Introduction
Cytosolic sulfotransferases (SULTs) are one of the major families of phase II drug-metabolizing enzymes, which catalyze the sulfation of hydroxyl-containing molecules (Klaassen 1997). The co-substrate (sulfuryl group donor) for all sulfotransferases is 3’-phosphoadenosine-5’-phosphosulfate (PAPS). Sulfation is widely observed in various biological processes. SULT-catalyzed sulfation is important in the regulation of different biological signaling molecules, including hormones, neurotransmitters, bile acids, and peptides. Some SULT isoforms have a broad range of substrate specificities and catalyze the sulfation of many xenobiotics (Duffel 2001). SULTs also play an important role in the detoxification of hydroxyl-containing xenobiotics and bioactivation of procarcinogens. Regulation of SULTs by different endogenous compounds has been relatively well studied (Dunn 2000; Klaassen 1998; Runge-Morris 1998), but information on SULTs induction by xenobiotics is very limited (Runge-Morris 1998).
Soy intake has long been recognized to reduce the incidence of different cancers, cardiovascular disease, postmenopausal syndrome in women, diabetes mellitus, and osteoporosis (Klein 2007; Kousidou 2006; Setchell 1999). Soybeans contain several biologically active components that may contribute individually or synergistically to health benefits. The isoflavone phytoestrogen is particularly abundant in soy. Genistein, a natural isoflavone found in soybean products, has been reported to have both chemopreventive and chemotherapeutic potential against estrogen-responsive diseases, including inhibition of tumor cell growth (Miodini 1999; Mitchell 2000; Setchell 1999; Xiang 2002), lowering of serum cholesterol, and prevention of bone loss in rodents (Ishimi 2002; Kirk 1998; Nakajima 2001; Paik 2003). Genistein is the most potent estrogenic compound in soy and soy products (Chen 2007). Several in vitro and in vivo studies have demonstrated that genistein has anti-cancer effects in prostate, breast, colon, gastric, lung, and pancreatic adenocarcinomas and in lymphoma (Kousidou 2006). The mechanisms underlying genistein’s anti-cancer properties include induction of apoptosis; inhibition of protein-tyrosine kinase; G2/M phase cell cycle arrest; inhibition of topoisomerase II-mediated DNA repair; suppression of telomerase activity; inhibition of epidermal growth factor autophosphorylation, mutagenesis, or conversely antimutagenesis; elaboration of DNA-damaging oxidation or its prevention by genistein’s antioxidant properties; and inhibition of angiogenesis (Klein 2007). Chodon et al. (2007a, b) recently demonstrated that genistein inhibits the growth of HepG2 cells in a dose-dependent manner and induces apoptosis (Chodon 2007a, 2007b). In contrast to positive effects on adult human health, isoflavone consumption during ontogenesis has been associated with some toxic effects, such as disrupted reproductive function in animal models. Thus, how drug-metabolizing enzymes control detoxification and transformation of isoflavones has gained much interest (Ronis 2006).
In recent years, isoflavones have been reported to induce phase I and phase II drug-metabolizing enzymes, such as cytochrome P450s, and to interact with nuclear receptors, such as those that are well known to mediate the induction of drug-metabolizing enzymes (Chen 2007; Li 2007; Mezei 2003; Moon 2006a; Ricketts 2005; Scatena 2004; Shay 2005). Isoflavones are sulfated in vivo by SULTs, even though they are also potent inhibitors of SULTs (Mesia-Vela 2003; Moon 2006a; Waring 2007). To the best of our knowledge, induction of human SULTs by isoflavones has not been reported. Knowledge of drug-metabolizing enzyme induction mechanisms will have significant impact on food safety, cancer prevention, toxicology, drug design and development, drug-drug interaction, drug resistance, and general human health. Studies on the induction of human SULTs by soy isoflavones such as genistein may reveal novel biological functions as well as novel induction mechanisms for human SULTs.
In this report, the induction of human SULT1A1 (hSULT1A1) and human SULT2A1 (hSULT2A1) by genistein at both protein and mRNA levels was investigated in human HepG2 and Caco-2 cells.
2. Materials and methods
2.1. Materials
Genistein, 2-naphthol, [14C] 2-naphthol (4.7 mCi mmol−1), and 3’-phosphoadenosine-5’-phosphosulfate (PAPS) were purchased from Sigma-Aldrich (St. Louis, MO). [1,2,6,7-3H(N)]Dehydroepiandrosterone ([3H]-DHEA, 60 Cimmol−1) was purchased from NEN (Boston, MA). Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) reagents were obtained from Bio-Rad (Hercules, CA). Western blot chemiluminescence reagent kits (Super Signal West Pico Stable Peroxide and Super Signal West Pico Luminol/Enhancer solutions) were purchased from Pierce Chemical (Rockford, IL). Nitrocellulose membranes (Immobilon-P; Millipore Corporation, Bedford, MA) used for Western blots were purchased from Fisher Scientific (Fair Lawn, NJ). Total RNA extraction Kit (TRIZOL reagent) was purchased from Molecular Research Center, Inc. (Cincinnati, OH). SuperScript™II Reverse Transcriptase was from Invitrogen Corporation (Carlsbad, CA). Real-time PCR Kits (qPCR MasterMix Plus for SYBR Green I dNTP) were from EUROGENTEC (San Diego, CA). Antibody against human phenol sulfotransferase (SULT1A1, P-PST) was a generous gift from Dr. N. Falany (University of Alabama at Birmingham). Antibody against human DHEA sulfotransferase (hSULT2A1, DHEA-ST) was purchased from PanVera (Madison, WI). Protein assay reagent was obtained from Bio-Rad. All other reagents and chemicals were of the highest analytical grade available.
2.2. Cell culture and drug treatment
Both HepG2 and Caco-2 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). The cells were cultured and maintained according to the manufacturer’s instructions. Briefly, HepG2 cells were grown and maintained in Dulbecco’s Modified Eagles’s Medium Nutrient Mixture F-12 Ham (Sigma) supplemented with 10% fetal bovine serum (FBS). Caco-2 cells were grown and maintained in Dulbecco’s Modified Eagles’s Medium (Sigma) supplemented with 20% FBS. The cultures were incubated at 37°C in a humidified incubator containing 5% CO2, 95% ambient air.
HepG2 and Caco-2 cells were used for genistein treatment. On day 0, cells were seeded in 100 × 20 mm dishes at a density of 1.0 × 107 cells per dish. After 24 h, various concentrations of genistein dissolved in sterile DMSO were added to the culture medium (0.2, 1, 5, or 25 µM final concentration in the medium). Medium was replaced every 2 days and replenished with freshly dissolved genistein. On days 2, 4, and 7, cells were collected after genistein treatment. Total RNA was extracted from cells with TRIZOL reagent and subjected to real-time RT-PCR. Cell cytosols were prepared for enzymatic activity assays and Western blot analyses.
2.3. Cytosol preparation
Both HepG2 and Caco-2 cells were harvested from their culture dishes using 0.25% trypsin-EDTA solution (Sigma), washed with phosphate-buffered saline, and then homogenized in 1 ml of cell lysis buffer (3 mM 2-mercaptoethanol; 0.1 mM phenylmethylsulfonyl fluoride; 20 mM Tris, pH 7.4; 160 mM NaCl; 0.03% Tween-20; and 0.1 mM EDTA). The homogenate was then centrifuged at 12,000 × g for 30 min, and the supernatant was used in the enzymatic assays and Western blot studies (below).
2.4. Enzyme assays
SULT activity in the cytosol was determined using a radioactive assay method previously described (Maiti 2003a). Briefly, 50 µg of protein from HepG2 cytosol and 200 µg of protein from Caco-2 cytosol were used as the enzyme source for each assay. For radioactive 2-naphthol sulfation activity, [14C] 2-naphthol (4.7 mCi/mmol; 0.1 mM final concentration) was the substrate. To determine DHEA sulfation activity, [3H]-DHEA (diluted to 0.4 Ci/mmol; 2 µM final concentration) was the substrate. For all assays, 20 µM PAPS was used. All enzymatic assays were performed in a total reaction volume of 250 µl. After 30 min of incubation at 37°C in a shaking water bath, the reaction was stopped by adding 250 µl stop buffer (0.25 M Tris, pH 8.7). Extraction was performed twice by adding 0.5 ml of water-saturated chloroform. After the final extraction, 100 µl of the aqueous phase was used for scintillation counting. Assays were run in duplicate. All data represented the average of the results from three experiments.
2.5. Western blot analysis
Both anti-hSULT1A1 and anti-hSULT2A1 antibodies were used for Western blot analyses of human SULT induction in HepG2 and Caco-2 cells. The specific Western blot procedure has been described previously (Chen 2003a; Maiti 2003a, 2003b).
2.6. Quantitative real-time RT-PCR
Total RNA was prepared from genistein-treated HepG2 and Caco-2 cells using TRIZOL reagent according to the manufacturer’s protocol. RNA samples were incubated with RQ1 DNase at 37°C for 30 min and then inactivated at 65°C for 10 min. Superscriptase II (Invitrogen) reverse transcriptase with 100 ng of total RNA was used to synthesize the first strand cDNA, and 1 µl of reverse-transcribed product served as the template in polymerase chain reactions. Real-time PCR was performed using qPCR MasterMix Plus for SYBR Green I dNTP Kit (EUROGENTEC) following the manufacturer’s instructions. Primers were designed with Primer Express software (Applied Biosystems, Foster City, CA) as follows: ACTBF321: 5’-AGAAAATCTGGCACCACACC-3’; ACTBR462: 5’-GGGGTGTTGAAGGTCTCAAA-3’, GI, L5016088; hSULT1A1F596: 5’-CATGGTCGGAAGTGTCCT-3’; hSULT1A1R712: 5’-TTCGGGTTCTCCTTCATGTC-3’, GI, L32189358; hSULT2A1F163: 5’-TGAGTTCGTGATAAGGGATGAA-3’; hSULT2A1R294: 5’-CAGATGGGCAGATTGGAT-3’, GI, L29540544. Real-time PCR was performed using an ABI PRISM 7500 Fast System (Applied Biosystems). Initially, regular PCR product DNA was purified with GENECLEAN Turbo (Qbiogene, Carlsbad, CA) for constructing standard curves (102–108 copies gene). A standard curve was plotted with the threshold cycle (CT) vs. the logarithmic value of the gene copy number. The gene copy number of unknown samples was generated directly from the standard curve by Sequence Detector (ver. 1.7) software (Applied Biosystems). At least two duplications were run for each experiment and each experiment was repeated at least three times. All gene copy numbers were normalized to human β-actin mRNA.
2.7. Statistical analysis
Student’s t-tests were performed to evaluate statistical significance with the difference between means of control and genistein-treated HepG2 and Caco-2 cells. Data presented in the figures are means ± S.E.M. (standard error) of the data collected separately from at least three independent experiments.
3. Results
2.1. Genistein induction of hSULT1A1 in HepG2 cells
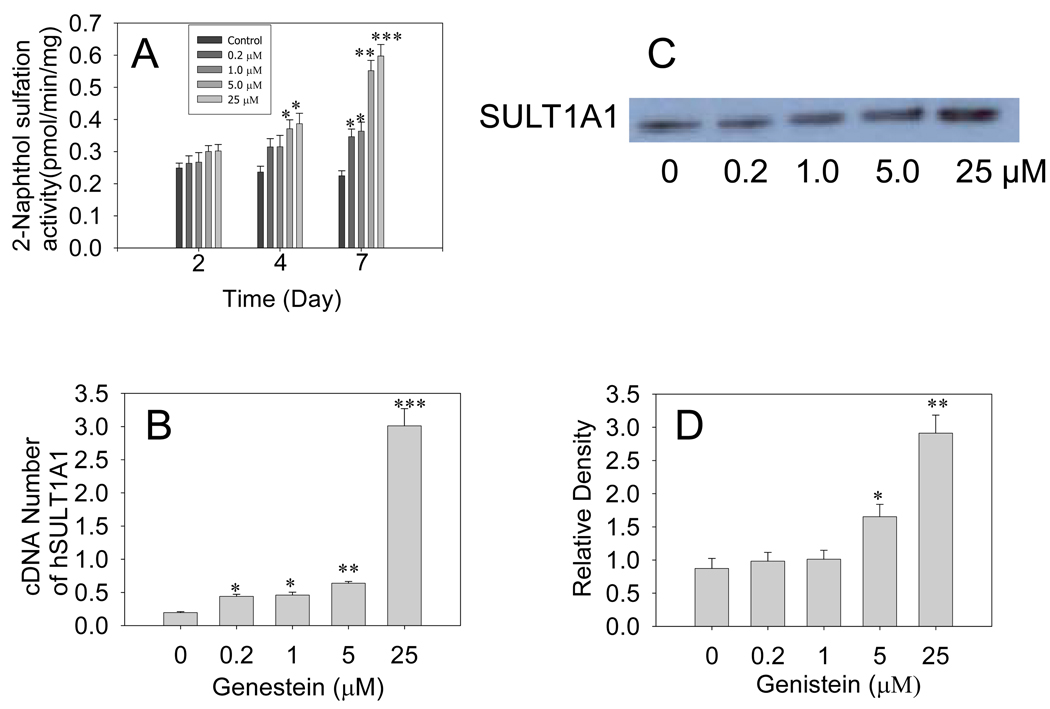
Genistein treatment induced both hSULT1A1 protein and mRNA in HepG2 cells. Induction was time- and dose-dependent. Enzymatic assays demonstrated that treating HepG2 cells with different doses of genistein for 7 days significantly increased 2-naphthol sulfation-specific activity (Fig 1A). Treatment of HepG2 cells for 7 days with 25 µM genistein increased 2-naphthol sulfation activity up to 1.8-fold (P<0.001). Western blot densitometry data showed that hSULT1A1 protein levels increased significantly in response to 7 days of genistein treatment at concentrations of 5 and 25 µM (P<0.05 and P<0.01, respectively; Fig. 1C, 1D). Treatment with 25 µM genistein induced hSULT1A1 protein levels up to 2.5-fold (P<0.01). Real-time RT-PCR also showed that 25 µM genistein significantly induced hSULT1A1 mRNA levels up to 10-fold (P<0.001) (Fig. 1B). These Western blot and real-time RT-PCR results are in good agreement with the enzymatic activity data. These results suggest that genistein induced hSULT1A1 at the gene transcription level.
Fig. 1. Genistein induction of hSULT1A1 in HepG2 cells.
(A) Enzymatic assay showing the genistein time- and dose-dependent induction of hSULT1A1 (P-PST) enzymatic activity in HepG2 cells. The 2-naphthol sulfation activity of cell cytosol was determined using the radioactive enzyme assay method. (B) Real-time RT-PCR showing the dose-dependent induction of hSULT1A1 mRNA expression in HepG2 cells treated with different doses of genistein for 7 days. (C) Representative Western blot of HepG2 cell cytosol. (D) Densitometry analysis showing human sulfotransferase hSULT1A1 protein expression in HepG2 cells treated with different doses of genistein for 7 days. Each experiment was repeated at least three times; data presented are means ± S.E.M. of data collected separately from at least three independent experiments (*P<0.05, **P<0.01, ***P<0.001).
2.2. Genistein induction of hSULT2A1 in HepG2 cells
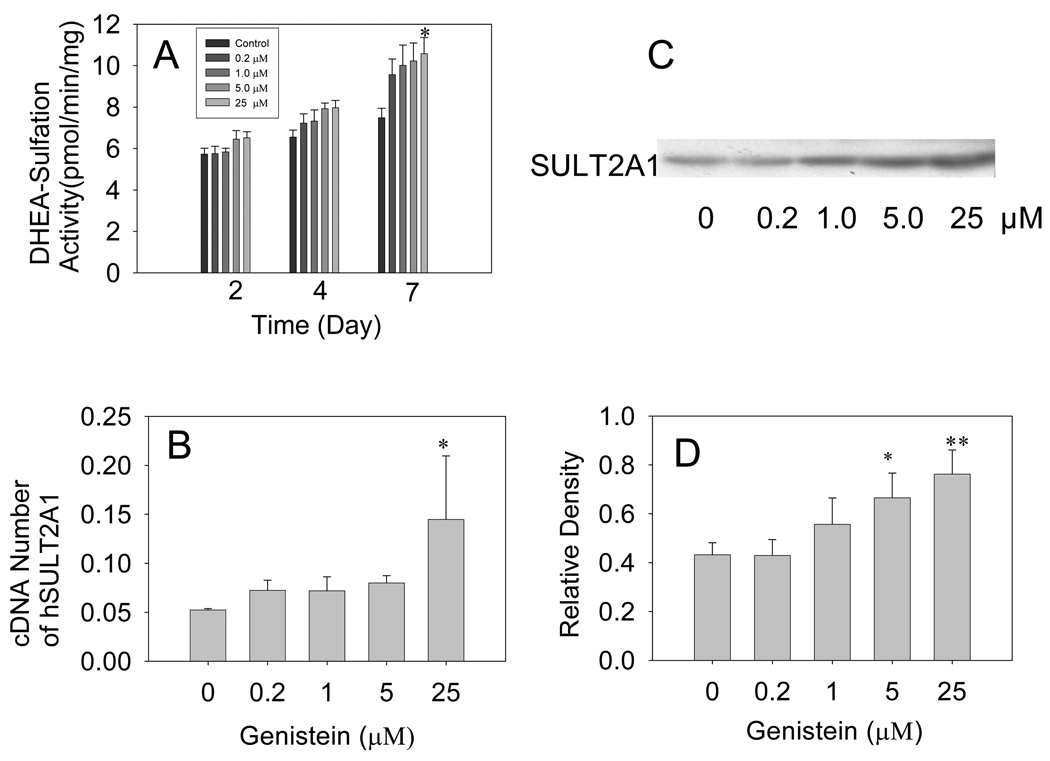
Figure 2 shows that genistein treatment induced hSULT2A1 in HepG2 cells in a time- and dose-dependent manner. Enzymatic assays demonstrated that treating HepG2 cells with different doses of genistein for either 2 days or 4 days failed to significantly change hSULT2A1 activity; however, treating the cells with 25 µM genistein for 7 days increased DHEA sulfation activity by about 57.5% (P<0.05) (Fig. 2A). Western blot data showed that hSULT2A1 protein expression increased by about 54% and 81%, respectively, in response to treatment with 5 µM and 25 µM genistein for 7 days (P<0.05, Fig 2C, 2D). Real-time RT-PCR data also demonstrated 25 µM genistein significantly increased hSULT2A1 mRNA levels up to 2.7-fold (P<0.01) (Fig. 2B). These data suggest that the regulation of hSULT2A1 gene expression might occur at the gene transcription level.
Fig. 2. Genistein induction of hSULT2A1 in HepG2 cells.
(A) Enzymatic assay showing genistein time- and dose-dependent induction of hSULT2A1 (DHEA-ST) enzymatic activity in HepG2 cells. The DHEA sulfation activity of cell cytosol was determined using the radioactive enzyme assay method. (B) Real-time RT-PCR showing the dose-dependent induction of hSULT2A1 mRNA expression in HepG2 cells treated with different doses of genistein for 7 days. (C) Representative Western blot of HepG2 cell cytosol. (D) Densitometry analysis showing dose-dependent induction of human sulfotransferase hSULT2A1 protein expression in HepG2 cells treated with different doses of genistein for 7 days. Each experiment was repeated at least three times and data presented are means ± S.E.M. of the data collected separately from at least three independent experiments (*P<0.05, **P<0.01, ***P<0.001).
2.3. Genistein induction of hSULT1A1 in Caco-2 cells
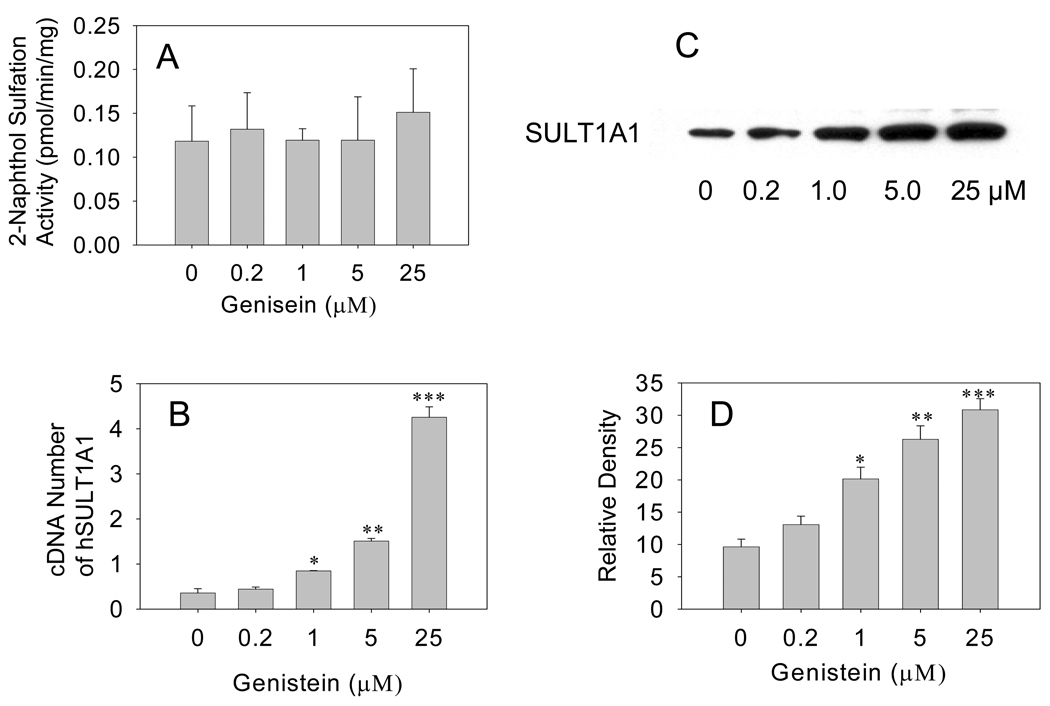
In Caco-2 cells, genistein treatment for 7 days failed to significantly increase 2-naphthol sulfation activity (Fig. 3A). Western blot analysis, however, demonstrated that genistein significantly increased hSULT1A1 protein levels in Caco-2 cells in a dose-dependent manner. Western blot densitometry data showed that hSULT1A1 protein levels increased by 1.2-, 1.8- and 2.3-fold, respectively, in response to genistein treatment for 7 days at concentrations of 1, 5, and 25 µM (P<0.05, 0.01, and 0.001, respectively; Fig. 3C, 3D). Real-time RT-PCR data showed that hSULT1A1 mRNA expression was induced by 1.3-, 3.4-, and 11.3-fold, respectively, in response to the treatment with 1, 5, and 25 µM genistein for 7 days (P<0.05, 0.01, and 0.001, respectively; Fig. 3B). The Western blot results agreed with the real-time RT-PCR data, suggesting that the gene regulation of hSULT1A1 by genistein in Caco-2 cells occurred at the gene transcription level.
Fig. 3. Genistein induction of hSULT1A1 in Caco-2 cells.
(A) Enzymatic assay showing dose-dependent induction of hSULT1A1 (P-PST) enzymatic activity in Caco-2 cells treated with different doses of genistein for 7 days. The 2-naphthol sulfation activity of cell cytosol was determined using the radioactive enzyme assay method. (B) Real-time RT-PCR showing the dose-dependent induction of human sulfotransferase hSULT1A1 mRNA expression in Caco-2 cells treated with different doses of genistein for 7 days. (C) Representative Western blot of Caco-2 cell cytosol. (D) Densitometry analysis showing the dose-dependent induction of hSULT1A1 protein expression in Caco-2 cells treated with different doses of genistein for 7 days. Each experiment was repeated at least three times and data presented are means ± S.E.M. of the data collected separately from at least three independent experiments (*P<0.05, **P<0.01, ***P<0.001).
2.4. Genistein induction of hSULT2A1 in Caco-2 cells
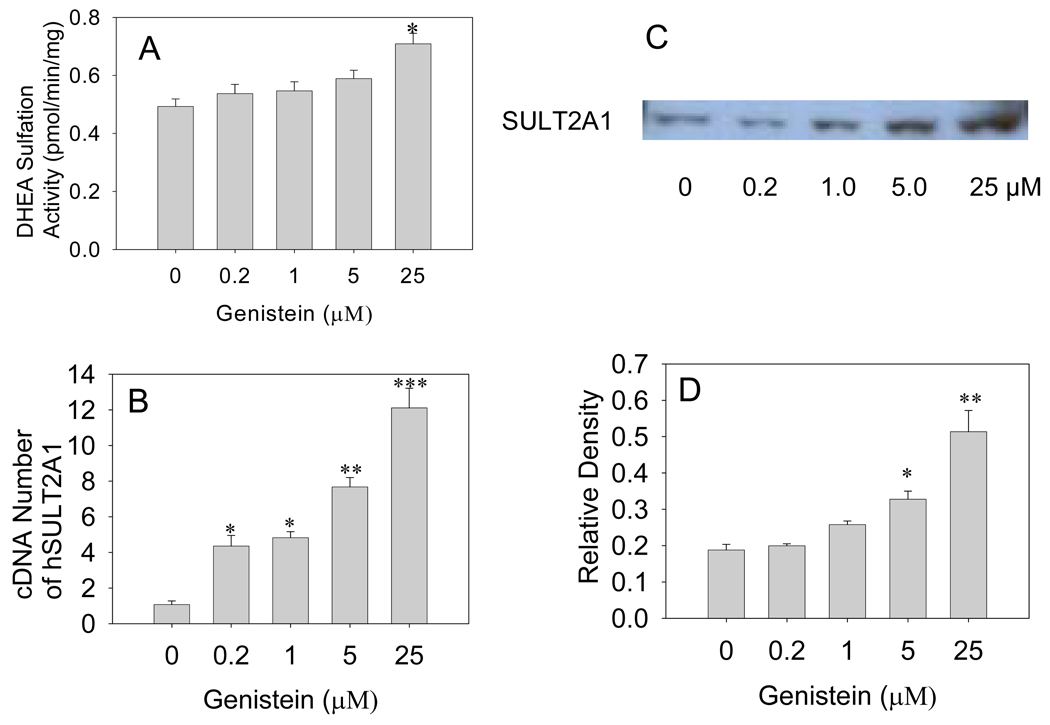
Enzymatic activity assays demonstrated that treating Caco-2 cells with 25 µM genistein for 7 days induced DHEA sulfation activity by about 62% (P<0.05, Fig 4A). Western blot densitometry data showed that hSULT2A1 protein level increased by 42.2%, 84%, and 170%, respectively, in response to treatment with 1, 5, and 25 µM genistein for 7 days (P<0.05 , 0.05, and 0.01, respectively; Fig. 4C). Real-time RT-PCR results indicated that hSULT2A1 mRNA levels increased 2.6-, 3.2-, 5.7-, and 9.4-fold, respectively, in response to treatment with 0.2, 1, 5, and 25 µM genistein for 7 days (P<0.05, 0.05, 0.01, and 0.001, respectively; Fig. 4B). Obviously, in Caco-2 cells genistein induced hSULT2A1 mRNA expression more significantly than protein expression.
Fig. 4. Genistein induction of hSULT2A1 in Caco-2 cells.
(A) Enzymatic assay showing dose-dependent induction of human sulfotransferase hSULT2A1 (DHEA-ST) enzymatic activity in Caco-2 cells treated with different doses of genistein for 7 days. The DHEA sulfation activity of cell cytosol was determined using the radioactive enzyme assay method. (B) Real-time RT-PCR result showing the dose-dependent induction of hSULT2A1 mRNA expression in Caco-2 cells treated with different doses of genistein for 7 days. (C) Representative Western blot of Caco-2 cell cytosol. (D) Densitometry analysis showing dose-dependent induction of hSULT2A1 protein expression in Caco-2 cells treated with different doses of genistein for 7 days. Each experiment was repeated at least three times and data presented are means ± S.E.M. of the data collected separately from at least three independent experiments (*P<0.05, **P<0.01, ***P<0.001).
4. Discussion
Regulation of SULT expression by endogenous molecules (i.e., hormones and bile acids) has been documented to some degree (Dunn 2000; Klaassen 1998; Runge-Morris 1998). The biochemical consequences of this regulation importantly affect various physiological processes. However, only a few studies have examined the induction of SULTs by drugs or xenobiotics in either animal tissues or cultured human cells (Maiti 2003a, 2003b; Runge-Morris 1998). The induction of drug-metabolizing enzymes by genistein is basically unknown. While screening different soybean phytoestrogens for their ability to induce SULTs, we observed that genistein could regulate human SULTs expression in HepG2 and Caco-2 cell lines.
Phytoestrogen isoflavones exist naturally as biologically inactive glycosylated glucosides. Like estrogen, isoflavones can be sulfur conjugated. They are deconjugated by large intestinal microflora to produce the readily absorbed free aglycones genistein and daidzein, which are thought to be the bioactive components of soy (Andlauer 2000; Setchell 2002; Sfakianos 1997). Isoflavone glucuronidation was previously demonstrated to occur primarily during first-pass metabolism in the gut of both the rat and human (Andlauer 2000; Setchell 2002; Sfakianos 1997). However, Ronis et al. (2006) recently reported that in humans sulfation of genistein and daidzein occurred to a large extent throughout the GI tract, suggesting a high level of first-pass sulfation. Thus, these sulfate metabolites may contribute to the bioactive pool of isoflavones following soy consumption.
Our previous experimental data suggested that hSULT1A1, hSULT1E1, and hSULT2A1, three members of human SULT families, all exist in the human GI tract, but with different distributions (Chen 2003b; Ronis 2006). Moreover, genistein sulfation in the human GI tract at physiologically relevant concentrations appears to correlate well with sulfation of the phenolic SULT1A1 family marker substrate, 2-napthol (Chen 2003b; Ronis 2006). In 2004, Nokano et al. (2004) also demonstrated that SULT1A1, one of the four major SULTs occurring in the human liver, plays the most important role among the four SULTs in the hepatic cytosolic 7-preferential sulfation of genistein and daidzein, and that SULT1E1 plays an important role in disulfation of these two phytoestrogens(Nakano 2004). They also determined the regioselectivity of sulfation in human hepatic cytosol (Nakano 2004).
SULTs are important in regulating the levels and activities of neurotransmitters and hormones as well as in eliminating xenobiotics (Falany 1997). The isoflavones genistein and equol have shown to be potent mixed inhibitors of hepatic estrogen SULT, with inhibitory constant values of 500 and 400 nM, respectively (Harris 2004). But SULT induction by genistein has not been reported.
Our results demonstrate that a 7-day treatment of genistein can significantly induce hSULT1A1 and hSULT2A1 enzymatic activities in human HepG2 cells, as well as increase protein and mRNA expression levels. Induction of hSULT1A1 enzymatic activity and gene expression in HepG2 cells is much higher than that of hSULT2A1. Of human SULTs, hSULT1A1 plays the most important role in the hepatic cytosolic sulfation of isoflavones. The Western blot data of Ronis et al. (2006) demonstrated that hSULT2A1 apoprotein is expressed mainly in the segments of human small intestine and to a lesser extent in human liver. There was a good correlation between hSULT2A1 apoprotein expression and its activities in different human GI tract segments (r2=0.68) (Ronis 2006). This suggests that hSULT2A1 enzyme may play an important role in the sulfation of genistein in the small intestine but not in the liver in humans.
Caco-2 is a human colon carcinoma cell line that is widely used as a human intestine model for testing orally administered drugs. Our data demonstrated that 7-day genistein treatments significantly induce hSULT1A1 and hSULT2A1 mRNA and protein expression in Caco-2 cells. But genistein significantly induced only hSULT2A1 enzyme activity, not SULT1A1 enzyme activity in Caco-2 cells. These results are consistent with the conclusions of Ronis et al.(2006), who observed that genistein sulfation activity was essentially the same in both human liver and small intestine ., indicating that hSULT2A1 enzyme plays an important role in the sulfation of genistein in different segments of the small intestine.
In humans, genistein plasma or serum levels derived from ingesting soy foods range from 1 µM to about 5 µM (Allred 2004; Maubach 2003; Safford 2003; Wiseman 2004). Since most genistein in serum may become bound to serum proteins, the actual concentration of biologically active genistein in serum is more likely to be higher. To obtain relevant data from in vitro models, Klein and King (2007) recommended using a genistein concentration of 5 µM as the upper limit for in vitro studies (Klein 2007). This concentration represents the maximal physiological serum level (free form) of genistein achievable by diet or dietary supplementation. The lower concentration limit for in vitro studies is 10–20 µM (Klein 2007). In the present study, we treated HepG2 and Caco-2 cells with 0, 0.2, 1, 5, and 25 µM genistein. These genistein concentrations reflect the range of genistein serum levels resulting from the absorption of soy isoflavones humans ingest in their daily diet.
According to our Western blot and real-time RT-PCR results, genistein significantly induced hSULT1A1 and hSULT2A1 gene and protein expression in HepG2 and Caco-2 cells in a dose- and time-dependent manner. Induction of hSULT1A1 and hSULT2A1 enzymatic activities, however, was not as high as that of hSULT1A1 and hSULT2A1 gene expression. Recent published reports on genistein inhibition of SULT1A1and SULT2A1 enzymatic activities may explain why genistein induction of hSULT1A1 and hSULT2A1 enzymatic activities was less than that of hSULT1A1 and hSULT2A1 mRNA and protein expression. In 2003, Mesia-Vela and Kauffman (2003) reported that dietary flavonoids are potent inhibitors of SULTs (Mesia-Vela 2003). Both SULT1A1 and SULT2A1, in rat liver cytosol were inhibited by flavonoids. Interestingly, inhibition of SULT2A1 was at least 100 times less than that of SULT1A1, leading to the conclusion that SULT1A1, not SULT2A1, is highly sensitive to inhibition by dietary flavonoids including genistein (Mesia-Vela 2003). Genistein also competitively inhibits human liver phenol SULT (IC50 of about 0.1 µM) (Eaton 1996; Walle 1995) and human platelet phenol SULT, at Ki values ranging from 0.1 to 0.3 µM (Ghazali 1999). The potent inhibitory effects of genistein on SULT enzymatic activity explains why we did not detect high-level increases of hSULT1A1 and hSULT2A1 enzymatic activities after genistein treatment, even though mRNA and protein expression was significant induced. Lewis et al. (1998) found that the potent non-competitive inhibition of SULT1A1 by dietary flavonoids may be potentially beneficial for human health, since SULTs that metabolize estrogen also catalyze the bioactivation of N-hydroxy-2 amino-1-methyl-6-phenylimidazo-[4,5-b] pyridine, a mutagen and procarcinogen in cooked food, and its subsequent binding to DNA (Lewis 1998).
Isoflavone sulfation, which is one of the main pathways for conjugation, occurs to a large extent through out the GI tract of both of rat and human (Moon 2006b; Ronis 2006). In rats, genistein exhibits high clearance and volume of distribution (Moon 2006b; Ronis 2006). In humans, SULTs play an important role in isoflavone sulfation in the liver and small intestine. In the current study, we demonstrated for the first time that genistein significantly induces hSULT1A1 and hSULT2A1 in human HepG2 and Caco-2 cells. Enzymatic activity assay, Western blot, and real-time RT-PCR results demonstrated that genistein significantly induced hSULT1A1 and hSULT2A1 protein and mRNA expression in human HepG2 and Caco-2 cells. Induction of hSULT1A1 and hSULT2A1 in HepG2 and Caco-2 cells was time- and dose-dependent. Enzymatic activity assay and Western blot results were in good agreement with real-time RT-PCR results, suggesting that induction occurs at the gene transcription level. The isoflavone genistein is the first nutritionally related phytoestrogen to show human SULT induction activity in HepG2 and Caco-2 cells. The molecular mechanisms underlying the genistein-mediated induction of hSULT1A1 and hSULT2A1 will be investigated in the near future.
Acknowledgments
This work was supported in part by NIH grant GM078606 (G.C.); American Cancer Society grant RSG-07-028-01-CNE (G.C.); USDA grant 2006-35200-17137 (G.C.); Oklahoma Center for the Advancement of Science and Technology (OCAST) grant HR05-015 (G.C.); and Oklahoma State University CVHS Research Advisory Committee (RAC) grant (G.C. and Y.C.).
References
- Klaassen C, Boles JW. Sulfation and sulfotransferases 5: the importance of 3'-phosphoadenosine 5'-phosphosulfate (PAPS) in the regulation of sulfation. Faseb J. 1997;11:404–418. doi: 10.1096/fasebj.11.6.9194521. [DOI] [PubMed] [Google Scholar]
- Duffel M, Marshal AD, McPhie P, Sharma V, Jakoby WB. Enzymatic aspects of the phenol (aryl) sulfotransferases. Drug Metab Rev. 2001;33:369–395. doi: 10.1081/dmr-120001394. [DOI] [PubMed] [Google Scholar]
- Dunn RT, Klaassen CD. Thyroid hormone modulation of rat sulfotransferase mRNA expression. Xenobiotica. 2000;30:345–357. doi: 10.1080/004982500237550. [DOI] [PubMed] [Google Scholar]
- Klaassen C, Liu L, Dunn RT., II Regulation of sulfotransferase mRNA expression in male and female rats of various ages. Chem. Biol. Interact. 1998;109:299–313. doi: 10.1016/s0009-2797(97)00141-5. [DOI] [PubMed] [Google Scholar]
- Runge-Morris M. Regulation of sulfotransferase gene expression by glucocorticoid hormones and xenobiotics in primary rat hepatocyte culture. Chem. Biol. Interact. 1998;109:315–527. doi: 10.1016/s0009-2797(97)00142-7. [DOI] [PubMed] [Google Scholar]
- Klein CB, King AA. Genistein genotoxicity: Critical considerations of in vitro exposure dose. Toxicol Appl Pharmacol. 2007;224:1–11. doi: 10.1016/j.taap.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Kousidou O, Tzanakakis GN, et al. Effects of the natural isoflavonoid genistein on growth, signaling pathways and gene expression of matrix macromolecules by breast cancer cells. Mini Rev Med Chem. 2006;6:331–337. doi: 10.2174/138955706776073420. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:758–767. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- Miodini P, Fioravanti L, Di Fronzo G, Cappelletti V. The two phyto-oestrogens genistein and quercetin exert different effects on oestrogen receptor function. Br J Cancer. 1999;80:1150–1155. doi: 10.1038/sj.bjc.6690479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Duthie SJ, et al. Effects of phytoestrogens on growth and DNA integrity in human prostate tumor cell lines: PC-3 and LNCaP. Nutr Cancer. 2000;38:223–228. doi: 10.1207/S15327914NC382_12. [DOI] [PubMed] [Google Scholar]
- Xiang H, Schevzov G, et al. A comparative study of growth-inhibitory effects of isoflavones and their metabolites on human breast and prostate cancer cell lines. Nutr Cancer. 2002;42:224–232. doi: 10.1207/S15327914NC422_12. [DOI] [PubMed] [Google Scholar]
- Ishimi Y, Yoshida M, Wakimoto S, Wu J, Chiba H, Wang X, Takeda K, Miyaura C. Genistein, a soybean isoflavone, affects bone marrow lymphopoiesis and prevents bone loss in castrated male mice. Bone. 2002;31:180–185. doi: 10.1016/s8756-3282(02)00780-9. [DOI] [PubMed] [Google Scholar]
- Kirk E, Sutherland P, Wang SA, Chait A, LeBoeuf RC. Dietary isoflavones reduce plasma cholesterol and atherosclerosis in C57BL/6 mice but not LDL receptor-deficient mice. J Nutr. 1998;128:954–959. doi: 10.1093/jn/128.6.954. [DOI] [PubMed] [Google Scholar]
- Nakajima D, Kim CS, Oh TW, Yang CY, Naka T, Igawa S, Ohta F. Suppressive effects of genistein dosage and resistance exercise on bone loss in ovariectomized rats. J Physiol Anthropol Appl Human Sci. 2001;20:285–291. doi: 10.2114/jpa.20.285. [DOI] [PubMed] [Google Scholar]
- Paik M, Lee HO, Chung HS, Yang SO, Kim JH, Om AS. Genistein may prevent cadmium-induced bone loss in ovariectomized rats. J Med Food. 2003;6:337–343. doi: 10.1089/109662003772519895. [DOI] [PubMed] [Google Scholar]
- Chen Y-C, Nagpal ML, Stocco DM, Lin T. Effects of genistein, resveratrol, and quercetin on steroidogenesis and proliferation of MA-10 mouse Leydig tumor cells. Journal of Endocrinology. 2007;192:527–537. doi: 10.1677/JOE-06-0087. [DOI] [PubMed] [Google Scholar]
- Chodon D, Banu SM, et al. Inhibition of cell proliferation and induction of apoptosis by genistein in experimental hepatocellular carcinoma. Mol Cell Biochem. 2007a;297:73–80. doi: 10.1007/s11010-006-9324-2. [DOI] [PubMed] [Google Scholar]
- Chodon D, Ramamurty N, et al. Preliminary studies on induction of apoptosis by genistein on HepG2 cell line. Toxicol In Vitro. 2007b;21:887–891. doi: 10.1016/j.tiv.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Ronis M, Little JM, Barone GW, Chen G, Radominska-Pandya A, Badger TM. Sulfation of the isoflavones genistein and daidzein in human and rat liver and gastrointestinal tract. J Med Food. 2006;9:348–355. doi: 10.1089/jmf.2006.9.348. [DOI] [PubMed] [Google Scholar]
- Li Y, Mezei O, Shay NF. Human and murine hepatic sterol-12-alpha-hydroxylase and other xenobiotic metabolism mRNA are upregulated by soy isoflavones. J Nutr. 2007;137:1705–1712. doi: 10.1093/jn/137.7.1705. [DOI] [PubMed] [Google Scholar]
- Mezei O, et al. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J Nutr. 2003;133:1238–1243. doi: 10.1093/jn/133.5.1238. [DOI] [PubMed] [Google Scholar]
- Moon YJ, Wang X, et al. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006a;20:187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Ricketts ML, et al. Molecular mechanisms of action of the soy isoflavones includes activation of promiscuous nuclear receptors. A review. J Nutr Biochem. 2005;16:321–330. doi: 10.1016/j.jnutbio.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Scatena R, et al. Mitochondrial dysfunction by synthetic ligands of peroxisome proliferator activated receptors (PPARs) IUBMB Life. 2004;56:477–482. doi: 10.1080/15216540400008416. [DOI] [PubMed] [Google Scholar]
- Shay NF. Regulation of Gene Transcription By Botanicals: Novel Regulation Mechanisms. Annu. Rev. Nutr. 2005;25:297–315. doi: 10.1146/annurev.nutr.25.050304.092639. [DOI] [PubMed] [Google Scholar]
- Mesia-Vela S, Kauffman FC. Inhibition of rat liver sulfotransferases SULT1A1 and SULT2A1 and glucuronosyltransferase by dietary flavonoids. XENOBIOTICA. 2003;33:1211–1220. doi: 10.1080/00498250310001615762. [DOI] [PubMed] [Google Scholar]
- Waring R, Ayers S, Gescher AJ, Glatt HR, Meinl W, Jarratt P, Kirk CJ, Pettitt T, Rea D, Harris RM. Phytoestrogens and xenoestrogens: The contribution of diet and environment to endocrine disruption. J Steroid Biochem Mol Biol. 2007 doi: 10.1016/j.jsbmb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Maiti S, Chen G. Methotrexate is a novel inducer of rat liver and intestinal sulfotransferases. Arch Biochem Biophys. 2003a;418:161–168. doi: 10.1016/j.abb.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Chen G, Chen X. Arginine residues in the active site of human phenol sulfotransferase (SULT1A1) J Biol Chem. 2003a;278:36358–36364. doi: 10.1074/jbc.M306045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti S, Chen G. Tamoxifen induction of aryl sulfotransferase and hydroxysteroid sulfotransferase in male and female rat liver and intestine. Drug Metab Dispos. 2003b;31:637–644. doi: 10.1124/dmd.31.5.637. [DOI] [PubMed] [Google Scholar]
- Andlauer W, Kolb J, Stehle P, Furst P. Absorption and metabolism of genistein in isolated rat small intestine. J Nutr. 2000;130:843–846. doi: 10.1093/jn/130.4.843. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr Biochem. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- Sfakianos J, Coward L, Kirk M, Barnes S. Intestinal uptake and biliary excretion of the isoflavone genistein in rats. J Nutr. 1997;127:1260–1268. doi: 10.1093/jn/127.7.1260. [DOI] [PubMed] [Google Scholar]
- Chen G, Zhang D, Jing N, Yin S, Falany CN, Radominska-Pandya A. Human gastrointestinal sulfotransferases: identification and distribution. Toxicol. Appl. Pharmacol. 2003b;187:186–197. doi: 10.1016/s0041-008x(02)00073-x. [DOI] [PubMed] [Google Scholar]
- Nakano H, Ogura Kenichiro, Takahashi Eriko, Harada Tomokazu, et al. Regioselective Monosulfation and Disulfation of the Phytoestrogens Daidzein and Genistein by Human Liver Sulfotransferases. Drug Metab. Pharmacokin. 2004;19:216–226. doi: 10.2133/dmpk.19.216. [DOI] [PubMed] [Google Scholar]
- Falany C. Sulfation and sulfotransferases. Introduction: changing view of sulfation and the cytosolic sulfotransferases. Faseb J. 1997;11:1–2. doi: 10.1096/fasebj.11.1.9034159. [DOI] [PubMed] [Google Scholar]
- Harris RM, Wood DM, et al. Phytoestrogens are potent inhibitors of estrogen sulfation: implications for breast cancer risk and treatment. J Clin Endocrinol Metab. 2004;89:1779–1787. doi: 10.1210/jc.2003-031631. [DOI] [PubMed] [Google Scholar]
- Allred CD, Allred KF, Ju YH, Goeppinger TS, Doerge DR, Helferich WG. Soy processing influences growth of estrogen-dependent breast cancer tumors. Carcinogenesis. 2004;25:1649–1657. doi: 10.1093/carcin/bgh178. [DOI] [PubMed] [Google Scholar]
- Maubach J, Bracke ME, Heyerick A, Depypere HT, Serreyn RF, Mareel MM, De Keukeleire D. Quantitatiion of soy-derived phytoestrogens in human breast tissue and biological fluids by high-performance liquid chromatography. J. Chromatogr., B, Biomed Sci. Appl. 2003;784:137–144. doi: 10.1016/s1570-0232(02)00789-4. [DOI] [PubMed] [Google Scholar]
- Safford B, Dickens A, Halleron N, Briggs D, Carthew P, Baker V. A model to estimate the oestrogen receptor mediated effects from exposure to soy isoflavones in food. Regul. Toxicol. Pharmacol. 2003;38:196–209. doi: 10.1016/s0273-2300(03)00091-6. [DOI] [PubMed] [Google Scholar]
- Wiseman H, Casey K, Bowey EA, Duffy R, Davies M, Rowland IR, Lloyd AS, Murray A, Thompson R, Clarke DB. Influence of 10 wk soy consumption on plasma concentrations and excretion of isoflavonoids and on gut microflora in healthy adults. Am. J. Clin. Nutr. 2004;80:692–699. doi: 10.1093/ajcn/80.3.692. [DOI] [PubMed] [Google Scholar]
- Eaton EA, Walle UK, et al. Flavonoids, potent inhibitors of the human P-form phenolsulfotransferase. Potential role in drug metabolism and chemoprevention. Drug Metab Dispos. 1996;24:232–237. [PubMed] [Google Scholar]
- Walle T, Eaton EA, Walle UK. Quercetin, a potent and specific inhibitor of the human P-form phenosulfotransferase. Biochem Pharmacol. 1995;50:731–734. doi: 10.1016/0006-2952(95)00190-b. [DOI] [PubMed] [Google Scholar]
- Ghazali R, Waring RH. The effects of flavonoids on human phenolsulphotransferases: potential in drug metabolism and chemoprevention. Life Sci. 1999;65:1625–1632. doi: 10.1016/s0024-3205(99)00423-3. [DOI] [PubMed] [Google Scholar]
- Lewis AJ, Walle UK, King RS, Kadlubar FF, Falany CN, Walle T. Bioactivation of the cooked food mutagen N-hydroxy-2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by estrogen sulfotransferase in cultured human mammary epithelial cells. Carcinogenesis. 1998;19:2049–2053. doi: 10.1093/carcin/19.11.2049. [DOI] [PubMed] [Google Scholar]
- Moon YJ, Sagawa K, Frederick K, Zhang S, Morris ME. Pharmacokinetics and bioavailability of the isoflavone biochanin A in rats. The AAPS J. 2006b;8:E433–E442. doi: 10.1208/aapsj080351. [DOI] [PMC free article] [PubMed] [Google Scholar]