Abstract
Adhesion and degranulation-promoting adapter protein (ADAP) is a multi-functional hematopoietic adapter protein that regulates TCR-dependent increases in both integrin function and activation of the NF-κB transcription factor. Activation of integrin function requires both ADAP and the ADAP-associated adapter SKAP55. In contrast, ADAP-mediated regulation of NF-κB involves distinct binding sites in ADAP that promote the inducible association of ADAP, but not SKAP55, with the CARMA1 adapter and the TAK1 kinase. This suggests that the presence or absence of associated SKAP55 defines functionally distinct pools of ADAP. To test this hypothesis, we developed a novel SKAP/ADAP chimeric fusion protein and demonstrated that physical association of ADAP with SKAP55 is both sufficient and necessary for the rescue of integrin function in ADAP-deficient T cells. Similar to wild-type ADAP, the SKAP/ADAP chimera associated with the LFA-1 integrin following TCR stimulation. Although the SKAP/ADAP chimera contains the CARMA1 and TAK1 binding sequences from ADAP, expression of the chimera does not restore NF-κB signaling in ADAP−/− T cells. A single point mutation in the pleckstrin homology (PH) domain of SKAP55 (R131M) blocks the ability of the SKAP/ADAP chimera to restore integrin function and to associate with LFA-1. However, the R131M mutant was now able to restore NF-κB signaling in ADAP-deficient T cells. We conclude that integrin regulation by ADAP involves the recruitment of ADAP to LFA-1 integrin complexes by the PH domain of SKAP55 and this recruitment restricts the ability of ADAP to interact with the NF-κB signalosome and regulate NF-κB activation.
INTRODUCTION
The activation of naïve T cells in secondary lymphoid tissues is initiated by T cell interactions with APCs. Cytoplasmic signals transduced from the TCR promote inside-out signaling that rapidly increases the functional activity of the LFA-1 integrin (CD11a/CD18; αLβ2), leading to firm T:APC conjugation and formation of the immunological synapse (1, 2). T cell stimulation also promotes activation of NF-κB, a key transcription factor in T cell function (3, 4). Distinct signaling mechanisms regulate TCR-dependent integrin and NF-κB activation. Functional activation of LFA-1 integrins on the T cell surface requires upstream phosphorylation of the linker for activation of T cells (LAT)2 and subsequent signaling through the SLP-76 complex (5-7). By contrast, TCR mediated NF-κB activation involves PKCθ-dependent formation of the CARMA1/Bcl-10/MALT1 (CBM) complex, which in turn promotes IKK kinase mediated phosphorylation and degradation of IκBα and liberates NF-κB for translocation to the T cell nucleus (8, 9).
Several adapter proteins downstream of LAT and SLP-76 are important for TCR-dependent integrin activation and T:APC conjugate formation (1, 10, 11). ADAP (adhesion and degranulation promoting adapter protein; also known as Fyb or SLAP-120/130) and SKAP55 (Src kinase-associated phosphoprotein; also known as SKAP1) are hematopoietic specific adapter proteins that have emerged as key mediators of T cell integrin activation. T cells from either ADAP−/− or SKAP55−/− mice show profound defects in TCR-dependent LFA-1 integrin activation (12-15). The constitutive association between ADAP and SKAP55 has been proposed to be critical to the regulation of integrin function by these adapters (16-20). SKAP55 contains a unique N-terminal domain putatively involved in homodimerization (21), a central atypical PH domain, and a C-terminal SH3 domain that mediates constitutive interaction with the central proline-rich domain of ADAP (22, 23). ADAP additionally contains several protein-protein interaction domains and was initially identified by its TCR-dependent interactions with the Src family tyrosine kinase Fyn and SLP-76 (24, 25). While the N-terminus of ADAP has been reported to bind HIP55 (26), no function has been attributed to this domain. By contrast, the C-terminus of ADAP is also important for TCR dependent integrin activation (11, 20), presumably through tyrosine phosphorylation and recruitment of ADAP to the LAT/SLP-76 complex downstream of TCR stimulation.
Beyond its role in promoting TCR-dependent integrin activation, ADAP is also critical for the formation of the NF-κB regulatory CBM complex (27). This function of ADAP traces to TCR-inducible interactions between ADAP and both the CARMA1 and TAK1 proteins (27, 28). Mutation of the CARMA1 binding site within the C-terminus of ADAP does not affect T:APC conjugate formation, and conversely an ADAP construct deficient in SKAP55 binding and integrin function retains the ability to rescue NF-κB activation (20). Consistent with these segregated functions of ADAP towards integrin versus NF-κB activation, we and others have described two pools of ADAP in T lymphocytes. Studies in Jurkat T cells indicate that 70% of ADAP is associated with SKAP55, while essentially all SKAP55 in T cells is associated with ADAP (22). In addition, ADAP, but not SKAP55, is inducibly recruited to the CBM complex for promotion of NF-κB activation following CD3/CD28 stimulation of primary naïve T cells (20, 27). The association of SKAP55 with ADAP is also critical for maintaining SKAP55 protein expression in T cells, as SKAP55 is severely destabilized in the absence of ADAP (29). Thus, ADAP−/− T lymphocytes also have dramatically reduced levels of SKAP55 (30). While ectopic re-expression of ADAP into ADAP−/− cells promotes stabilization and restoration of SKAP55 expression, ADAP constructs defective for SKAP55 binding leave ADAP−/− cells deficient in SKAP55 (20). This finding suggests that the role of ADAP in regulating integrin function is to stabilize SKAP55 (1, 11). However, the forced expression of SKAP55 in the absence of ADAP is unable to restore integrin activation (20), indicating that both SKAP55 and ADAP play distinct functions in this signaling pathway.
The mechanism by which the constitutive association of ADAP with SKAP55 specifically controls TCR-inducible changes in integrin function, but not NF-κB activation, remains unclear. Several key integrin regulatory proteins, including RIAM and RapL, have been reported to bind SKAP55, but these interactions have been shown to be constitutive and not affected by TCR stimulation (18, 31). We hypothesized that SKAP55 may play an active role in controlling ADAP-dependent integrin and NF-κB function. To test this hypothesis, we developed a novel SKAP/ADAP chimeric molecule that retains the CARMA1 and TAK1 binding sites and can substitute for both ADAP and SKAP55 in the absence of endogenous expression of either protein. We show that expression of the SKAP/ADAP chimera in ADAP−/− T cells can efficiently rescue ADAP-dependent T:APC conjugate formation, but not NF-κB activation. The PH domain of SKAP55 is critical for the integrin regulatory function of the SKAP/ADAP complex and mutation of this domain now renders the chimera permissive for NF-κB activation. This indicates that SKAP55 attenuates NF-κB signaling by restricting access of ADAP to the NF-κB signalosome.
MATERIALS AND METHODS
Mice
DO11.10 and DO11.10/ADAP−/− mice on the Balb/c background have been previously described and were crossed to hCAR transgenic mice expressing the human coxsackie adenovirus receptor (13, 20, 27). Mice were housed in specific pathogen-free facilities at the University of Minnesota, and were used between 8 and 12 weeks of age. All experimental protocols involving the use of mice were approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Antibodies and reagents
Anti-Thy1.1 APC, Anti-B220 PerCP-Cy5.5, and PE-conjugated anti-mouse IgG1 were purchased from eBioscience (San Diego, CA). Anti-D011.10 TCR FITC-conjugated KJ1-26 was purchased from Biolegend (San Diego, CA). Rabbit anti-SKAP55 was from Millipore/Upstate (Lake Placid, NY). Mouse anti-IκBα (L35A5; #4814) as well as rabbit anti-phospho-IκBα (14D4; #2859), anti-ERK (#9102), and anti-phospho-ERK (#9101) were from Cell Signaling (Danvers, MA), and mouse anti-Bcl10 (331.3) and rabbit anti-Bcl10 (H-197) was from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-SKAP55 and mouse anti-ADAP C-terminus antibody were from Transduction Labs and sheep anti-ADAP N-terminus polyclonal antiserum was the kind gift of Dr. G. Koretzky and used as previously described for intracellular staining by FACS (20). Anti-rabbit or anti-sheep AlexaFluor 488 (Invitrogen, Carlsbad, CA) was used for secondary detection of intracellular staining. Donkey anti-sheep IR800 and donkey anti-rabbit secondary IR680 antibodies (LI-COR Biosciences, Lincoln, NE) and goat anti-mouse Alexa 680 (Invitrogen) were used for Western blotting in most experiments. Cell Tracker Orange and OVAp (ovalbumin aa323-339) for conjugate assays were from Invitrogen.
Cloning and mutagenesis
The FLAG-tagged human SKAP55 cDNA in pEF-BOS was kindly provided by Dr. B. Schraven (Otto-von-Guericke University, Magdeburg, Germany). Subcloning to generate pENTR-FLAG-SKAP55 was previously described (20). To create the SKAP/ADAP chimera, cDNA encoding amino acids 1-299 of human SKAP55 was PCR amplified from FLAG-SKAP55 construct using Pfx DNA polymerase (Invitrogen) and the following primers: EcoRV-Met-SKAP55-sense, 5′-GAGATATCATGGACTACAAAGACGACGA -3′, and SalI-SKAP55-antisense, 5′-GCAGTCGACGTAGTAACTGGCATAGTCTA -3′. The digested product was cloned into pENTR-UP-IT (27) to generate pENTR-SKAP55ΔSH3. Separately, cDNA encoding amino acids 426-819 of murine ADAP was PCR amplified using the following primers: SalI-ADAP-sense 5′-GCAGTCGACCAAGATGGTGTCATGCACTCT –3′, and XmaI-ADAP-stop-antisense, 5′-GACCCGGGCTAGTCATTGTCATAGATGCA -3′. The digested product was cloned in frame into pENTR-SKAP55ΔSH3 to generate pENTR-SKAPΔSH3-ADAP(426-819) which we designate as the SKAP/ADAP chimera. Deletion and point mutant constructs were generated as previously described by site directed mutagenesis (Stratagene) (20) using pENTR-HA-ADAP to generate HA-ADAPΔ1-425 or the SKAP/ADAP chimera to generate the PH domain deletion or R131M mutation (20). To generate the GFP-SKAP55 expression construct, FLAG-SKAP55 was excised from pEF-BOS using Sal I and Pml I and subcloned in frame into the Sal I and Sma I sites of pEGFP-C1 (Clontech, Mountain View, CA) to make pEGFP-FLAG-SKAP55. The Nhe I/Hpa I restriction fragment containing the entire GFP-SKAP55 fusion was then subcloned into the Sma I site of pENTR-UP-IT by blunt ligation. The R131M mutation to GFP-SKAP55 was generated by site-directed mutagenesis as described above. All recombinant DNA sequences were verified using the University of Minnesota Biomedical Genomics Center microsequencing facility.
Adenovirus production
Adenovirus expression plasmids not previously described were generated as described (20, 27) using recombination of pAD-PL-DEST (Invitrogen) with the various pENTR plasmid constructs described above. The adenovirus was produced by transfecting purified Pac I digested adenovirus expression plasmid into AD293 cells (Agilent Technologies, Santa Clara, CA). Viral particles were cultured, purified and titered as previously described (20, 27).
Conjugate assays
Flow cytometry-based conjugate assays were performed as previously described (13, 20). Fresh control hCAR/DO11.10 and hCAR/DO11.10/ADAP−/− lymph node T cells were transduced with adenovirus and incubated at 37C for three days as previously described (20). Separately, fresh wild-type splenocytes were labeled with Cell Tracker Orange and pulsed with OVAp for 30 minutes at 37C. An aliquot of transduced T cells was preexamined to determine the percentage of DO11.10+Thy1.1+ cells and the prewarmed experimental samples were then combined with the Cell Tracker Orange-labeled splenocytes at a ratio of 0.2 × 106 DO11.10+Thy1.1+ cells to 0.8 × 106 splenocytes. The cells were immediately pelleted into a round-bottom plate, incubated for 10 min at 37C, vortexed on a plate mixer for 20 seconds, and fixed with 1% paraformaldehyde. The samples were stained with anti-Thy1.1-APC, anti-B220-PE-Cy5.5, and anti-D011.10 TCR FITC-conjugated KJ1-26 and analyzed by flow cytometry using a FACSCalibur equipped with a high-throughput plate reader. Conjugates were defined as KJ1-26+Thy1.1+ events co-staining with Cell Tracker Orange and B220. Intracellular staining to verify transgene expression by flow cytometry was performed as previously described (20). Based on expression of the construct of interest, Thy1.1 expression levels were used to gate cells containing SKAP55 levels that matched the endogenous level of SKAP55 protein observed in wild-type cells. Flow cytometry data was analyzed using FlowJo software (Tree Star, Ashland, OR).
Western blotting
Western blotting was performed as previously described (20, 28). Briefly, freshly harvested lymphocytes were transduced as described above and after 3 days were washed with PBS containing 0.2% BSA and stimulated with 1 μg anti-CD3 (clone 2C11) and 0.1 μg anti-CD28 per 3 × 106 cells in a 50 μl volume. Samples were lysed at the indicated time point by the addition of 2% NP40 lysis buffer. The lysates were spun to pellet insoluble materials and the supernatant added to 4X NuPAGE sample buffer (Invitrogen) and separated by SDS-PAGE. For whole cell lysates, 1 million cell equivalents were loaded per lane. After western transfer, PVDF membrane was blocked with 0.2% casein for 1 hr, primary antibody was incubated 2 hours at room temperature or overnight at 4C in PBS/0.2% Casein/0.2% Tween-20, washed with PBS containing 0.2% Tween-20, incubated for 1 hr in secondary antibody in PBS/0.2% Casein /0.2% Tween-20, and finally washed with PBS/0.2% Tween-20 and stored in PBS. The membrane was imaged with on Odyssey Infrared Imager (LI-COR Biosciences, Lincoln, NE). Endogenous SKAP55 in β2 integrin immunoprecipitations was detected using mouse anti-SKAP55 diluted in 2.5% nonfat milk in PBS containing 0.2% Tween-20 followed by light-chain specific anti-mouse IgG conjugated to horseradish peroxidase (Jackson Laboratories), developed with SuperSignal West Femto Substrate (Pierce), and imaged on an ImageQuant LAS4000 workstation (GE Healthcare).
Immunoprecipitation
To identify protein complexes by immunoprecipitation, cell lysates were stimulated with anti-CD3/CD28 and lysed as described above at a density of 10 × 106 cells/200 μl. For immunoprecipitation, anti-mouse β2 integrin CD18 (clone M18/4, Biolegend) or mouse anti-Bcl10 was crosslinked to Gamma-bind Sepharose Plus beads (GE Healthcare, Piscataway, NJ) at a ratio of 10 μg to 20 μl settled beads as previously described (20, 28). Beads were added to cleared cell lysates and rotated overnight at 4C, washed with 1% NP40 lysis buffer and samples prepared for SDS-PAGE by resuspending the samples into 30 μl of 1X lysis buffer and bringing the samples to 1X SDS loading buffer and 5% β-mercaptoethanol. For transduction and immunoprecipitation from CD4+ T cell blasts, hCAR+/DO11.10 or hCAR+/DO11.10/ADAP −/− bulk splenocytes were stimulated with PMA (100 ng/ml) and ionomycin (1 μg/μl) for 48h, followed by expansion with recombinant human IL-2 (5 IU/ml). After 9-10 days, T cell blasts were transduced with adenovirus as previously described (20) except that an MOI of 50 was used at a density of 4 × 107 cells/ml. After 48h cells were analyzed for Thy1.1 expression to verify efficient transduction and 80-100% of the T cells routinely expressed Thy1.1. Ten million cells per condition were washed with PBS containing 0.2% BSA and stimulated at a concentration of 1 × 107 cells/300 μl. Anti-CD3 and anti-CD28 mAbs were added at 1μg or 0.1 μg per 50 μl volume, respectively. After 15 mins preincubation on ice, cells were stimulated at 37C for the indicated times and then lysed by slow addition of 2% NP40 lysis buffer (20, 28) and then processed for immunoprecipitation. To detect reconstituted ADAP and SKAP55 in β2 immunoprecipitates, ADAP−/− cells expressing high levels of wild-type ADAP were obtained by magnetic enrichment. Briefly, cells were stained with subsaturating FITC-conjugated anti-Thy1.1 (eBiosciences, 0.04 μg per 5 × 107 cells) and anti-FITC microbeads were then captured on MACS LS columns according to the manufacturer’s instructions (Miltenyi). Thy1.1 expression on the recovered cells was verified by staining with saturating APC-conjugated anti-Thy1.1. These cells contained levels of ADAP and SKAP55 comparable to wild-type cells as assessed by western blotting (data not shown). At least 3 × 107 Thy1.1-high enriched cells were required in order to detect endogenous SKAP55 in β2 immunoprecipitations. Lysates were precleared with gamma-bind sepharose for 1h, and β-mercaptoethanol was also omitted when eluting samples from the IP beads, to minimize the amount of reduced immunoglobulin heavy chain that migrates just above endogenous SKAP55 on the immunoblots.
RESULTS
Physical association of ADAP and SKAP55 is required for efficient T:APC conjugate formation
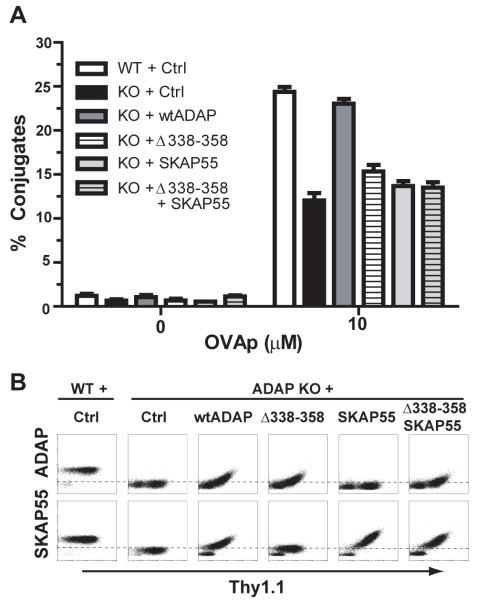
The association between ADAP and SKAP55 is important for TCR-dependent increases in LFA-1 function. Incubation of OVAp-pulsed splenocytes with naïve DO11.10 CD4 T cells (32) promotes the formation of LFA-1 dependent T:APC conjugates in a peptide antigen-dependent manner. We have used this system to show that DO11.10/ADAP−/− T cells show a 30-50% decrease in the efficiency of T:APC conjugate formation (13, 20). We utilized hCAR/D011.10 transgenic mice to allow expression of adenovirus-encoded proteins in naïve antigen-specific primary murine T lymphocytes (33). Our recombinant adenoviruses also encode an IRES-driven Thy1.1 (CD90.1) cell surface reporter for identification of adenovirus-transduced cells. Consistent with previous observations (20), expression of wild-type ADAP in ADAP−/− cells restores conjugate formation to levels similar to wild-type DO11.10 T cells (Fig. 1A). Expression of ADAPΔ338-358 lacking the proline-rich SKAP55 binding site in ADAP, or forced expression of SKAP55 in the absence of ADAP, failed to rescue T:APC conjugation formation (Fig. 1A), consistent with the requirement for ADAP:SKAP55 interaction to promote efficient LFA-1 function.
Figure 1. Impaired conjugate efficiency in the absence of ADAP and SKAP55 interaction.
(A) Naïve hCAR/DO11.10 (WT) or hCAR/DO11.10/ADAP−/− (KO) CD4 T cells were transduced with control (Ctrl) Thy1.1 adenovirus or the indicated ADAP or SKAP55 expression construct(s). After 3d, the transduced cells were incubated with OVAp-pulsed splenic B cells and analyzed by flow cytometry for efficiency of T:APC conjugate formation as described in Material and Methods. (B) Intracellular staining for ADAP or SKAP55 was performed on the cells transduced in (A), and plotted versus Thy1.1 expression. Conjugates depicted in A were gated on the top one-third of the Thy1.1 gate to ensure that SKAP55 and/or ADAP levels were comparable to endogenous SKAP55 and/or ADAP expression in wild-type control cells. Results for both the conjugate assays and the intracellular staining are representative of at least 3 independent experiments.
ADAP−/− T cells display severely destabilized SKAP55 expression (20, 29), but endogenous SKAP55 expression can be restored following reintroduction of wild-type ADAP (Fig. 1B). In contrast, the ADAPΔ338-358 mutant does not restore endogenous SKAP55. Thus, it remained a formal possibility that simultaneous expression of the SKAP55 binding-deficient ADAPΔ338-358 construct along with SKAP55 could restore T:APC conjugate formation. When we transduced hCAR/D011.10/ADAP−/− T cells with adenoviruses expressing ADAPΔ338-358 and SKAP55, we were able to detect the presence of both proteins in Thy1.1+ cells by flow cytometry (Fig. 1B). However, even when we gated specifically on cells expressing high levels of Thy1.1, we were unable to rescue T:APC conjugate formation with co-expression of ADAPΔ338-358 and SKAP55 (Fig. 1A). This suggests that expression of SKAP55 is not sufficient to restore ADAP-dependent regulation of integrin function when ADAP is unable to associate with SKAP55.
Rescue of T:APC conjugate formation by expression of chimeric SKAP/ADAP protein
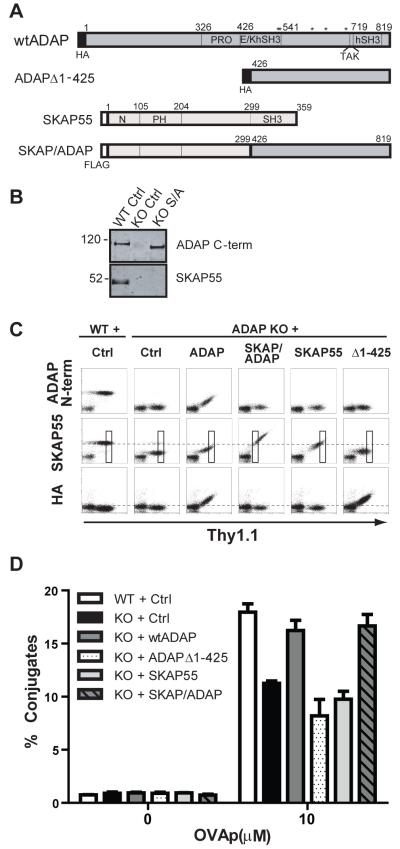
The major interaction site between ADAP and SKAP55 resides in the central proline-rich domain of ADAP and the C-terminal SH3 domain of SKAP55. To decipher the mechanism by which this constitutive protein complex responds to TCR signals, we reasoned that a chimeric construct fusing the two proteins together into a single gene product would regulate T cell conjugation to APCs similarly to the endogenous ADAP:SKAP55 complex. We designed such a construct by removing the binding domains between the two proteins and fusing the N-terminus of SKAP55 to the C-terminus of ADAP. The chimera was constructed with the N-terminal 299 amino acids of SKAP55, deleting the C-terminal SH3 region of SKAP55. This portion of SKAP55 was fused to the C-terminal 393 amino acids of ADAP, deleting the N-terminus and the central proline-rich SKAP55-binding domain of ADAP. This molecule (hereafter designated SKAP/ADAP chimera or S/A) thus lacks the regions normally required for ADAP:SKAP55 interaction but still retains the putative sites for signaling (Fig. 2A).
Figure 2. A SKAP/ADAP chimeric fusion protein restores T:APC conjugate formation in ADAP−/− T cells.
(A) Schematic diagram of key expression constructs used in this study. Amino acid numbering is given for the murine ADAP p130kDa isoform and for human SKAP55. PRO, proline-rich domain; E/K, glutamic acid and lysine-rich domain; hSH3N or hSH3C, N-terminal or C-terminal helical SH3 domain; PH, pleckstrin homology domain. Asterisks indicate the positions of key tyrosine residues (547/549/584/615/687) that have been reported for SLP-76 and Fyn binding to ADAP. The SKAP/ADAP chimera is a 692 aa molecule comprised of the N terminal 299 aa of human SKAP55 fused to the C-terminus of murine ADAP beginning at aa426. (B) Western blot analysis of SKAP55 and ADAP expression in whole cell lysates prepared from naïve wild-type (WT) or ADAP−/− (KO) cells expressing the indicated construct. (C) Naïve hCAR/DO11.10 (WT) or hCAR/DO11.10/ADAP−/− (KO) T cells were transduced for 3d with adenovirus for the indicated construct, fixed, and analyzed by intracellular staining for ADAP (N-terminus), SKAP55, or the HA-Tag. (D) Conjugate assays were performed as in Figure 1. The conjugate efficiency was evaluated from the Thy1.1 gated boxes depicted in panel C, to ensure that the level of the SKAP/ADAP chimera and the control constructs matched that of endogenous SKAP55 observed in wild-type control cells. Results are representative of at least 4 independent experiments for each construct.
We transduced naive hCAR/DO11.10 T cells with the SKAP/ADAP chimera and performed Western blotting with an anti-ADAP antibody that recognizes the C-terminus of ADAP (Fig. 2B, upper blot) and with an anti-FLAG antibody (data not shown) to verify expression of the construct in whole cell lysates. The molecular mass of the SKAP/ADAP chimera is ~110 kDa, slightly smaller than that of endogenous wild-type ADAP (Fig. 2B, upper blot). Expression of the SKAP/ADAP chimera was also verified in naive hCAR/DO11.10/ADAP−/− T lymphocytes by flow cytometry with intracellular staining using anti-SKAP55 and anti-ADAP antibodies (Fig. 2C). In T cells expressing high levels of the Thy1.1 adenovirus marker, we routinely observed levels of expression of the SKAP/ADAP chimera that exceeded levels of endogenous SKAP55 expression in control wild-type T cells, as assessed by intracellular staining with an anti-SKAP55 antibody. The anti-ADAP antibody used for intracellular staining recognizes the N-terminus of ADAP and thus does not recognize either the SKAP/ADAP chimera or the ADAPΔ1-425 control construct lacking the ADAP N-terminus (Fig. 2C top row). Importantly, while ectopic SKAP55 is readily detected in ADAP−/− cells via intracellular staining following transduction of the SKAP/ADAP chimera (Fig. 2C), Western blotting for SKAP55 indicates that this staining is not due to upregulation of any endogenous SKAP55 as indicated by an absence of the ~50 kDa band for endogenous SKAP55 that is observed in wild-type cells (Fig. 2B, lower blot).
We next evaluated T:APC conjugate formation of ADAP−/− cells expressing the SKAP/ADAP chimera. Since the SKAP/ADAP chimera is expressed in abundance compared to endogenous SKAP55 in wild-type cells (Fig. 2C, middle row), we analyzed conjugate formation by specifically gating on cells expressing SKAP55 similar to the levels of endogenous T cells. Thus, ADAP−/− cells expressing the SKAP/ADAP chimera were gated on Thy1.1lo while cells expressing ADAP or SKAP55 required gating on Thy1.1hi to ensure that the cells analyzed contained endogenous levels of SKAP55 (boxes in Fig. 2C). ADAP−/− T cells expressing the SKAP/ADAP chimera showed a rescue of conjugate efficiency at levels similar to wild-type cells or ADAP−/− cells expressing wild-type ADAP (Fig. 2D). By contrast, expression of either SKAP55 alone or the C-terminal portion of ADAP contained in the SKAP/ADAP chimera (ADAPΔ1-425) failed to rescue T:APC conjugate formation in T cells lacking ADAP (Fig. 2D). Together, these results indicate that the SKAP/ADAP chimera contains essential regions of both SKAP55 and ADAP that, when fused, are sufficient for TCR dependent integrin activation in the absence of endogenous ADAP and SKAP55.
The SKAP55 PH domain is required for efficient T:APC conjugate formation
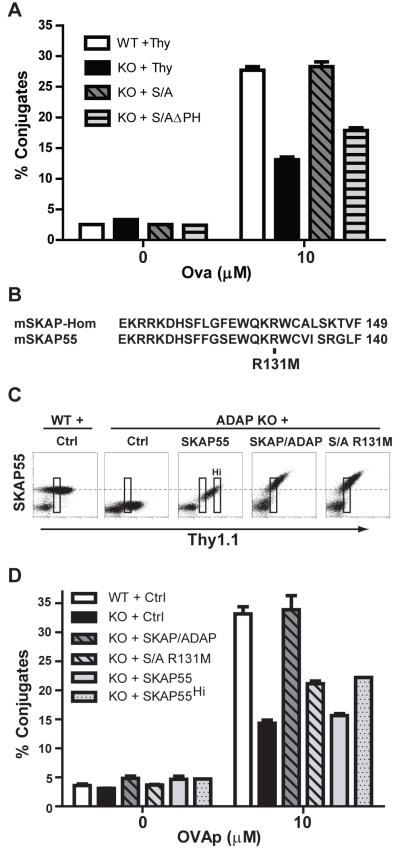
We next used the SKAP/ADAP chimera as a platform to perform mutational and functional analysis to identify critical regions in SKAP55 that drive ADAP-dependent T:APC conjugate formation. In initial deletion mutagenesis studies, we observed that removal of the PH domain (aa 105-204) of SKAP55 severely impaired the ability of the SKAP/ADAP chimera to rescue T:APC conjugate formation in ADAP−/− T cells (Fig. 3A). Analysis of the recently reported crystal structure of the SKAP55 homolog SKAP-HOM revealed a novel intramolecular interaction between the N-terminus and the PH domain of SKAP-HOM (21). This association, which is constitutive under basal conditions, has been proposed to function as a switch such that PI[3,4,5]P3 abundance opens the intramolecular interaction and unmasks a PI[3,4,5]P3 binding site at R140 in the PH domain of SKAP-HOM, ultimately serving to recruit SKAP-HOM to actin-rich membrane ruffles. Alignment of the highly similar PH domains of SKAP-HOM and SKAP55 (21) (see also Fig. 3B) reveals extensive amino acid identity near the critical R140 residue in SKAP-HOM (R131 in SKAP55). To test the functional significance of this residue in the PH domain of SKAP55, we mutated amino acid 131 in the SKAP/ADAP chimera to methionine (R131M). The SKAP/ADAP R131M mutant chimera was expressed equivalently to the intact SKAP/ADAP chimera in hCAR/D011.10/ADAP−/− T cells (Fig. 3C). We present data from a Thy1.1lo gate of ADAP−/− cells expressing the SKAP/ADAP chimera, to identify ADAP−/− cells expressing the chimera at physiological levels similar to that of endogenous SKAP55 found in wild-type cells (boxes in Fig. 3C). Interestingly, when DO11.10/ADAP−/− T cells expressing the R131M mutant were analyzed for T:APC conjugate formation, we consistently observed impaired rescue of conjugate formation, to levels only slightly above that of DO11.10/ADAP−/− T cells expressing the control adenovirus (Fig. 3D, light grey hatched bars). By contrast, the intact SKAP/ADAP chimera again rescued T:APC conjugate formation to levels at or above what we observed in wild-type DO11.10 T cells (Fig. 3D, dark grey hatched bars). As an internal negative control, we also ectopically expressed native SKAP55, which does not rescue T:APC conjugate formation in ADAP−/− T cells. Because wild-type levels of SKAP55 re-expression in the absence of ADAP are only achieved in Thy1.1hi cells, an additional Thy1.1hi gate is also presented (Fig. 3D, grey dotted bars). Together, these experiments reveal a previously unrecognized importance for the PH domain of SKAP55 in controlling ADAP-dependent T:APC conjugate formation.
Figure 3. The R131 amino acid residue in the SKAP55 PH domain is critical for efficient ADAP-dependent T:APC conjugate formation.
(A) Naïve hCAR/DO11.10 (WT) or hCAR/DO11.10/ADAP−/− (KO) T cells were transduced with the indicated constructs and conjugate assays were performed as described in Figures 1-2. The SKAP/ADAPΔPH construct (S/AΔPH) lacks the entire 100 aa PH domain of SKAP55. (B) Amino acid alignment of SKAP-HOM and SKAP55 depicting the lipid binding region of the PH domain. Arginine 140 in SKAP-HOM corresponds to R131 in SKAP55. (C) Expression of SKAP55, SKAP/ADAP, or SKAP/ADAP R131M (S/A R131M) in naïve hCAR/DO11.10/ADAP−/− T cells. Note that the highest levels of SKAP55 expression achieved in the absence of ADAP (Thy1.1hi cells; boxed gate marked “Hi” in middle panel) approach the levels of endogenous SKAP55 found in naïve wild-type T cells, and correspond to the SKAP55 level found in Thy1.1lo cells expressing the SKAP/ADAP chimera or the SKAP/ADAP R131M mutant. (D) Conjugate assays were performed as described in Figures 1-2. T cells were gated on Thy1 lo cells (leftmost gate on each plot in C) to match endogenous levels of SKAP55 found in wild-type cells. For comparison, the conjugate efficiency of Thy1.1hi cells following SKAP55 expression alone in ADAP−/− is also shown (SKAP55Hi). Similar results were obtained in at least 4 independent experiments.
The SKAP55 PH domain is required for recruitment to LFA-1 integrin adhesion complexes
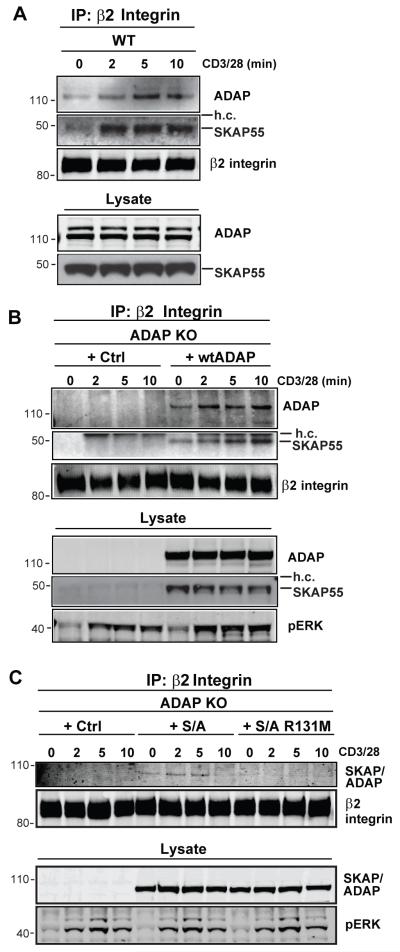
Recent studies have demonstrated that SKAP55 and RapL can be biochemically isolated with LFA-1 integrin adhesion complexes (31, 34, 35). Because SKAP55 is insufficient to control T:APC conjugate formation in the absence of ADAP, we speculated that ADAP would also be recruited to LFA-1 upon TCR stimulation. Indeed, when the LFA-1 β2 integrin subunit CD18 is immunoprecipitated from CD3/28-stimulated wild-type mouse T cells, we observed an accumulation of ADAP in these integrin complexes after 5-10 min of stimulation (Fig. 4A). As expected, endogenous SKAP55 was also detected in the CD18 IP complexes from wild-type cells following TCR stimulation (Fig. 4A). Since rescue of T:APC conjugate formation in ADAP−/− T cells critically depends on the expression of both ADAP and SKAP55, we also verified that endogenous SKAP55 could be detected along with ectopic ADAP in β2 immunoprecipitates from ADAP−/− cells reconstituted with wild-type ADAP. Indeed, when ADAP−/− cells enriched for reconstitution of ADAP to wild-type levels were subjected to CD3/CD28 stimulation, SKAP55 could be detected in β2 immunoprecipitates from these cells. The specificity of this ADAP and SKAP55 immunoblotting is shown by the input lysate from unstimulated cells and by the lack of signal in ADAP−/− T cells under identical stimulation (Fig. 4A,B, and data not shown).
Figure 4. The SKAP55 PH domain is required for TCR-dependent recruitment to β2 integrins.
(A) hCAR/DO11.10 (WT) CD4 T cell blasts were stimulated for the indicated times with anti-CD3/CD28. The cells were lysed and subjected to anti-β2 (CD18) integrin immunoprecipitation. Western blots were probed for ADAP, SKAP55 and for the β2 integrin. Whole cell lysate inputs (lower 2 panels) are shown to demonstrate ADAP and SKAP55 expression. (B,C) hCAR/DO11.10/ADAP−/− (ADAP KO) T cell blasts were transduced with the indicated adenovirus constructs and stimulated and immunoprecipitated for β2 integrin as in (A). Western blots were probed with antibodies against ADAP and SKAP55 to detect ADAP and SKAP55 (B) or the SKAP/ADAP chimera (C), and with antibodies against the β2 integrin to detect the immunoprecipitated CD18 integrin subunit. Whole cell lysate inputs (lower panels) are shown to demonstrate expression of ADAP, SKAP55, and the SKAP/ADAP chimera, and activation was confirmed by blotting for phosphoERK. Similar results were observed in 4 independent experiments.
We next asked whether the SKAP/ADAP chimera or the SKAP/ADAP chimera with the R131M mutation in the SKAP55 PH domain could associate with LFA-1 following T cell activation. ADAP−/− T cells expressing the SKAP/ADAP chimera without or with the R131M PH domain mutation were stimulated with anti-CD3/28 and then cell lysates were subjected to anti-CD18 immunoprecipitation. We detected the recruitment of the SKAP/ADAP chimera to LFA-1 integrin complexes at 2-5 minutes of CD3/CD28 stimulation. By contrast, the R131M domain mutant was not efficiently recruited to LFA-1 at any time point examined (Fig. 4B). Anti-SKAP55 blotting of input cell lysate from unstimulated samples showed equal expression of both chimeric constructs, and antibodies against the β2 integrin subunit showed equal recovery of the integrin chain (Fig. 4B). Taken together with the above adhesion assays, these results suggest that the SKAP55 PH domain is important for both SKAP55 recruitment into TCR-dependent integrin adhesion complexes and for the functional ADAP-dependent activation of LFA-1 integrins.
The SKAP55 PH domain impairs ADAP-dependent NF-κB activation
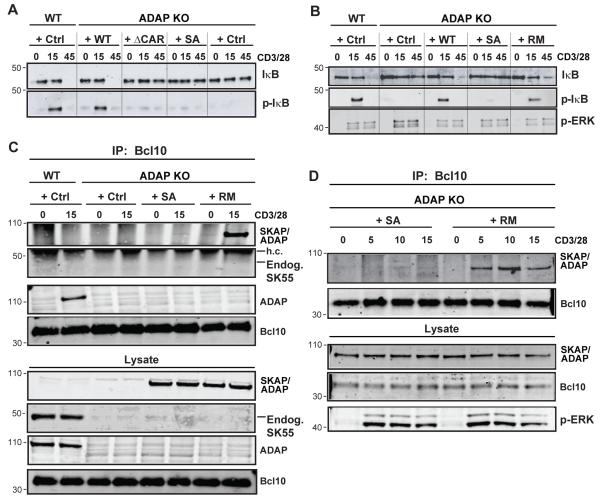
In addition to controlling TCR-dependent integrin-mediated adhesion through interaction with SKAP55, ADAP is also critical for activation of the NF-κB transcription factor following TCR stimulation (27). Activation of NF-κB downstream of the TCR requires assembly of the CBM complex and we have previously shown that the ADAP C-terminus interacts with both CARMA1 and the TAK1 kinase such that ADAP C-terminal mutants deficient for binding either CARMA1 or TAK1 fail to rescue NF-κB activation defects in ADAP−/− T cells (27, 28). Because the SKAP/ADAP chimera contains the entire ADAP C-terminus including both CARMA1 and TAK1 binding domains (see Fig. 2A), we speculated that the SKAP/ADAP chimera would be able to rescue defects in CD3/CD28-mediated IκB degradation and phosphorylation when expressed in ADAP−/− T cells. We stimulated and prepared whole-cell lysates from naïve wild-type or ADAP−/− T cells expressing either control virus, wild-type ADAP, the CARMA1 binding Δ426-541 mutant of ADAP (ADAPΔCAR), or the SKAP/ADAP chimera. As expected, stimulation of wild-type T cells resulted in normal activation of the NF-κB pathway, typified by IκBα phosphorylation that is detected after 15 min of stimulation, followed by loss of total IκBα due to degradation, which is assessed after 45 min (Fig. 5A, Ctrl cells). By comparison, ADAP−/− cells transduced with a control adenovirus expressing Thy1.1 demonstrated defective NF-κB activation in that CD3/28 stimulation failed to induce IκB phosphorylation after 15 min and degradation after 45min. Consistent with previous observations (27, 28), expression of wild-type ADAP, but not ADAPΔCAR, in ADAP−/− cells restored IκBα phosphorylation and degradation. Surprisingly, expression of the SKAP/ADAP chimera failed to rescue the NF-κB pathway downstream of CD3/CD28 stimulation (Fig. 5A and 5B, +S/A lanes). By contrast, expression of the SKAP/ADAP R131M mutant restored CD3/CD28-mediated activation of NF-κB in ADAP−/− cells, as assessed by the rescue of IκB phosphorylation and eventual degradation (Fig. 5B, + RM lanes). To determine if the SKAP/ADAP chimera could interact with the CBM complex, we immunoprecipitated Bcl10 from naive ADAP−/− T cells expressing the SKAP/ADAP chimera or the SKAP/ADAP R131M mutant chimera. While the SKAP/ADAP chimera was not detected in Bcl10 immunoprecipitates following 5 to 15 minutes of anti-CD3/28 stimulation, the SKAP/ADAP R131M mutant was robustly recruited into the Bcl10 complex as early as 5 minutes after stimulation and was sustained until at least 15 minutes (Fig. 5 C,D), consistent with the rescue of NF-κB signaling observed with this construct.
Figure 5. The SKAP55 PH domain inhibits ADAP-dependent NF-κB activation.
(A) Naïve hCAR/DO11.10 (WT) and hCAR/DO11.10/ADAP−/− (ADAP KO) T cells were transduced with control Thy1.1 adenovirus or adenovirus expressing wild-type ADAP (WT), ADAPΔCAR (ΔCAR), or SKAP/ADAP chimera (S/A). After 3d, cells were stimulated with anti-CD3/CD28 for the indicated times (minutes) and lysates prepared. Western blots were probed with antibodies to total IκBα and phospho-IκBα to demonstrate IκBα degradation and phosphorylation, respectively. (B) Western blots from hCAR/DO11.10 and hCAR/DO11.10/ADAP−/− T cells expressing the indicated constructs were performed as in A. RM; SKAP/ADAP R131M mutant. (C) T cells were transduced and stimulated for 15 min as in (A-B) and subjected to immunoprecipitation with mouse anti-Bcl10 antibodies. Immune complexes were analyzed by Western blot with rabbit anti-SKAP55 antibody to detect the appearance of the SKAP/ADAP chimera in the Bcl10 complex. Whole cell lysates are shown in the lower 3 panels and were probed with anti-SKAP55 (to detect the SKAP/ADAP chimera), anti-ADAP, or rabbit anti-Bcl10. (D) T cells from hCAR/DO11.10/ADAP−/− mice were transduced with the indicated constructs as in (C) and stimulated for the indicated times followed by Bcl10 immunoprecipitation and western blotting as described for (C). Similar results were obtained in 3 independent experiments for each panel.
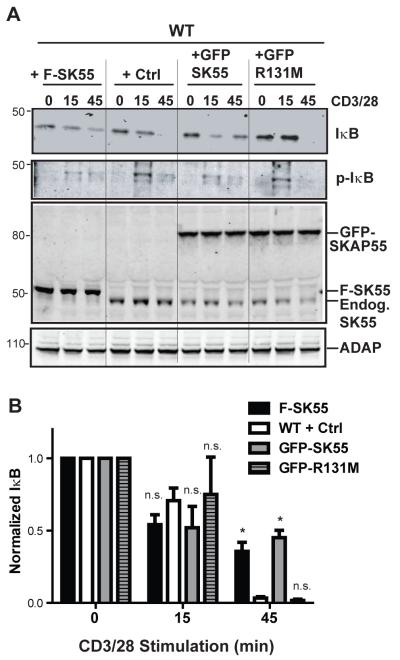
Because only the SKAP/ADAP R131M mutant chimera was able to rescue NF-κB activation or associate with Bcl10 in ADAP−/− T cells (Fig. 5B,C), we next speculated that changes in SKAP55 expression may modulate ADAP-dependent NF-κB activation. To test this hypothesis, we overexpressed a FLAG-tagged form of wild-type SKAP55, a GFP-SKAP55 fusion protein, or a GFP-SKAP55/R131M mutant fusion protein in naïve wild-type T cells. In Western blots, we consistently observed that ectopic expression of SKAP55 in wild-type T cells diminished the expression of endogenous SKAP55, consistent with a finite size for the SKAP55 pool in T cells (Fig. 6A, lower panels showing western blot for total SKAP55). Overexpression of either FLAG-SKAP55 or GFP-SKAP55 reduced the extent of IκBα phosphorylation at 15 minutes and impaired its full degradation at 45 minutes compared to control T cells (Figs. 6A and 6B). In contrast, wild-type T cells overexpressing GFP-SKAP55 containing the R131M mutation exhibited normal IκBα phosphorylation and degradation at 15 and 45 minutes, respectively, that was comparable to wild-type T cells transduced with a control adenovirus (Fig. 6B). This was not due to effects of the R131M mutation on expression of GFP-SKAP55, as the GFP-SKAP55/R131M mutant was expressed at similar levels to the GFP-SKAP55 protein (Fig. 6, top 2 panels respectively). These observations are consistent with a model where arginine 131 in the PH domain of SKAP55 controls the extent of TCR dependent NF-κB activation by defining a pool of ADAP that is restricted from regulating the CBM complex (Fig. 7).
Figure 6. The SKAP55 PH domain suppresses endogenous NF-κB activation.
(A) Naïve wild-type hCAR T cells were transduced with adenoviruses to overexpress SKAP55 (F-SK55), GFP-SKAP55 (GFP SK55), or GFP-SKAP55-R131M (GFP R131M). Upper 2 panels, Western blots were performed as in A and B to detect total IκBα or phosphorylated IκBα (p-IκB). Lower 2 panels, Western blots for SKAP55 and ADAP are depicted showing expression of endogenous SKAP55 (Endog. SK55), FLAG-SKAP55 (F-SKAP55), or GFP-SKAP55 (GFP-SKAP55) and equal expression of ADAP in all samples. Results are representative of 3 independent assays performed. (B) Quantification of total IκBα degradation following CD3/CD28 stimulation as described in A. Results are normalized to IκBα densitometry values from the unstimulated samples, and averaged between 3 independent experiments performed. Asterisk (*) indicates P < 0.05 compared to the wild-type control condition within each time point using Bonferroni’s multiple comparison test of the one-way ANOVA. n.s., P>0.05.
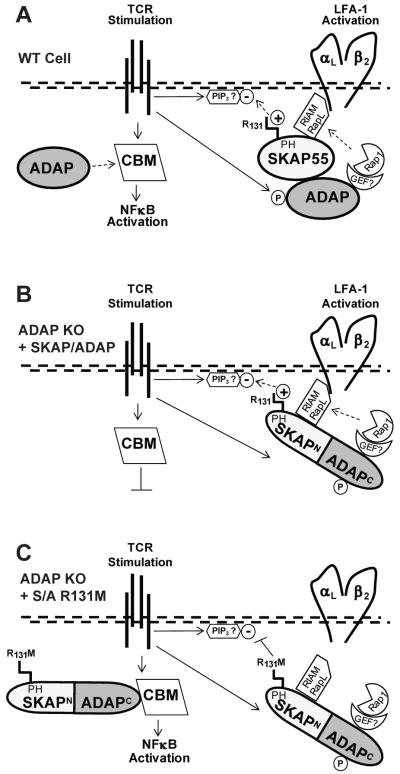
Figure 7. Model for ADAP and SKAP55 control of LFA-1 integrin activation and NF-κB activation.
(A) In wild-type T cells, TCR stimulation promotes ADAP phosphorylation (P) and activation of the constitutively associated ADAP:SKAP55 adapter module. Positively charged arginine 131 (R131) in the SKAP55 pleckstrin homology (PH) domain recruits and restricts the complex to negatively charged phosphoinositides (such as PIP3) in the plasma membrane. Downstream integrin activation components including Rap1 and a putative Rap1-GEF are provided through ADAP and the Rap1 effectors RIAM and/or RapL through SKAP55 are recruited to LFA-1 for promotion of integrin activation. Only free excess ADAP is available for concurrent assembly of the CARMA-1/Bcl10/Malt1 (CBM) complex, which initiates IκB phosphorylation/degradation and release of NFκB to the nucleus. (B) The SKAP/ADAP chimera is exclusively recruited to the integrin pathway. As there is no excess ADAP that is not associated with SKAP55 in ADAP−/− T cells expressing the chimera, no free ADAP is available for NF-κB activation. (C) In ADAP−/− cells expressing the R131M mutation in the SKAP/ADAP chimera, SKAP55 PH domain-mediated interaction of the chimera with membrane lipids is attenuated. This allows the chimera (through the ADAP C-terminus) to engage the CBM complex and promote NF-κB activation.
DISCUSSION
This study investigated the mechanism by which distinct biochemical pools of ADAP control integrin activation and NF-κB signaling following TCR stimulation of primary murine T lymphocytes. We tested the hypothesis that the physical association of ADAP with SKAP55 not only regulates integrin activation following TCR stimulation but also attenuates NF-κB activation by controlling the ability of ADAP to interact with the CBM complex. We have identified an arginine motif (R131) in the PH domain of SKAP55 that is required for the recruitment of the ADAP:SKAP55 complex to LFA-1 integrins and subsequent ADAP-dependent T:APC conjugate formation, and restrains efficient NF-κB activation (Fig. 7A).
Although the constitutive association of ADAP and SKAP55 is critical for efficient TCR-mediated activation of integrins (18, 20), the precise mechanism by which this scaffolding complex triggers changes in integrin function remains unclear. SKAP55 is obligately associated with ADAP(29, 30), while studies with both Jurkat T cells and ADAP−/− T cells have shown that there is a biochemical and functional pool of ADAP in T cells that is not associated with SKAP55 and regulates NF-κB signaling (22, 27, 28). In this study, we utilized the fact that ADAP−/− T cells lack expression of both endogenous ADAP and SKAP55 in order to define the mechanism by which the ADAP:SKAP55 complex specifically regulates integrin activation but not NF-κB activation.
We developed a SKAP/ADAP chimeric molecule consisting of the N-terminus of SKAP55 fused in frame to the C-terminus of ADAP. This chimera was efficiently expressed and did not induce upregulation of endogenous SKAP55, allowing us to analyze T cells where all of the ADAP was constitutively associated with SKAP55. Importantly, the chimera efficiently rescued conjugate formation defects in ADAP−/− T cells, demonstrating that these minimal domains of SKAP55 and ADAP are sufficient for integrin activation in a system where neither protein is endogenously expressed. It has been speculated by several investigators that SKAP55 contributes key integrin regulatory information to the ADAP:SKAP55 complex (18, 20, 21, 31). The SKAP/ADAP chimera allowed us to define a novel function for the PH domain of SKAP55 in regulating conjugate formation by controlling the localization of the ADAP:SKAP complex to integrins (Fig. 7B). We focused on the SKAP55 PH domain because of recent crystallographic studies of the SKAP55 homolog SKAP-HOM (SKAP55R; SKAP2) (21). This work demonstrated that mutation of R140 in the PH domain of SKAP-HOM to methionine abrogated the binding of the SKAP-HOM PH domain to PI[3,4,5]P3 and the recruitment of SKAP-HOM to actin-rich membrane ruffles (21). These findings are consistent with the known role of other PH domains in recruiting proteins to sites of phosphoinositide synthesis (36) and are particularly intriguing given the role of PI3K in inside-out integrin activation (36, 37). Interestingly, the PH domain in SKAP-HOM is atypical in that it folds into a novel intramolecular interaction with another domain within SKAP-HOM that controls SKAP-HOM dimerization, resulting in constitutive dimers of SKAP-HOM that contain masked PI[3,4,5]P3 binding sites (21). High concentrations of PI[3,4,5]P3 release the PH domain from this intramolecular association, allowing for SKAP-HOM recruitment to membranes rich in PI[3,4,5]P3. Although this has yet to be demonstrated, the high sequence identity and homology between the PH domains of SKAP-HOM and SKAP55 suggest that SKAP55 may also undergo similar PI[3,4,5]P3 dependent conformational switching and membrane recruitment via its PH domain (21, 31).
Consistent with this model, we found that removal of either the entire PH domain or mutation of the conserved arginine at position 131 in the SKAP/ADAP chimera to methionine severely impaired the rescue of T:APC conjugate formation in ADAP−/− T cells, likely tracing to the impaired accumulation of the SKAP/ADAP chimera with LFA-1 adhesion complexes observed following CD3/28 simulation (Fig. 7C). Our results provide direct structural and functional demonstration of an integrin-regulatory domain within SKAP55 that is distinct from the ADAP-binding SH3 domain. In addition, our findings establish in primary T lymphocytes a model where the SKAP55 PH domain senses activated T cell membranes and thereby targets the ADAP:SKAP55 complex to LFA-1 integrins.
It is currently not known how PI[3,4,5]P3 dependent conformational shifts in the SKAP55 PH domain promote T:APC conjugate formation via association with LFA-1. One likely possibility is that there exist high concentrations of TCR-dependent signaling lipids in membranes that are also rich in integrin heterodimers, bringing the SKAP55:ADAP complex into close proximity with these integrins. Current models of integrin activation propose that recruitment of the small GTPase Rap1 and its effectors RIAM and RapL are critical final steps required for integrin activation (38, 39). A role for the SKAP55 PH domain in recruiting the SKAP:ADAP complex to integrins is consistent with this model, as both RIAM and RapL have been identified as binding partners for SKAP55 (19, 31). While no RIAM binding site within SKAP55 has yet been defined, RapL appears to bind the N-terminus of SKAP55 (31). It is unclear whether RapL binding would affect SKAP55 constitutive homodimerization through this domain, but one scenario is that the opened PH domain exposes part of the N-terminal dimerization domain to trigger RapL binding, further recruiting the ADAP:SKAP complex to PI[3,4,5]P3 rich membranes where RapL can recognize the αL integrin chain of LFA-1 (34). It is also not known why the ADAP/SKAP55 complex does not fully account for all TCR-dependent LFA-1 activation in T cells, as evidenced by the observation that ADAP−/− T cells show only a partial reduction in T:APC conjugate formation. Since ADAP appears to be most critical in situations of weak or limiting antigen stimulation (13), this suggests the existence of ADAP-independent modes of integrin activation downstream of the TCR. One possibility is that since Rap1 activation is normal in the absence of ADAP (18), this pool of activated Rap1 may positively regulate integrin activation even in the context of the defective Rap1 plasma membrane targeting that has been reported in ADAP−/− cells (18).
In addition to the targeting information provided by SKAP55, the requirement for the ADAP C-terminus on the SKAP/ADAP chimera in our present work reinforces previous findings pointing to the importance of the ADAP C-terminus towards LFA-1 integrin activation (18, 20). Since ADAP inducibly associates with the SH2 domain of SLP-76 (40-42), it remains likely that phosphorylation of the ADAP C-terminus following TCR simulation couples ADAP to the LAT/SLP-76 complex. In contrast, other studies have placed ADAP within LFA-1 outside-in signaling pathways following TCR and LFA-1 costimulation (43, 44), suggesting that additional levels of ADAP regulation may be operative in activated T cells. In addition, a number of previously unrecognized phosphorylated tyrosine residues in the ADAP C-terminus as well as novel ADAP interacting proteins have recently been reported, including cytoskeletal regulatory proteins such as Dock2 and Nck (45-47). These results are consistent with a proposed role for ADAP in controlling TCR-dependent cytoskeletal rearrangements (48, 49). Future studies using well controlled systems such as ADAP−/− cells expressing C-terminal tyrosine or actin-binding mutants of the SKAP/ADAP chimera could help clarify these signaling networks. In addition, the atypical helical SH3 domains within the ADAP C-terminus have been reported to bind negatively charged phospholipids and potentially alter adhesion to immobilized integrin ligands (50, 51). Future studies will need to address whether lipid- or phosphoinositide- binding cooperativity exists involving the PH domain of SKAP55 and the helical SH3 domains of ADAP.
ADAP has recently emerged as a key regulator of TCR dependent NF-κB activation (27). This pathway depends on C-terminal amino acid motifs in ADAP that are distinct from the central SKAP55 binding domain in ADAP. Surpisingly, the SKAP/ADAP chimera did not rescue NF-κB activation in ADAP−/− cells despite containing the sites in ADAP critical for binding to CARMA1 and TAK1. However, the SKAP/ADAP R131M mutant, which is unable to efficiently rescue integrin function when expressed in ADAP−/− T cells, is able to restore NF-κB activation, suggesting that the intact SKAP55 PH domain plays a previously unappreciated role in excluding bound ADAP from interacting with the CBM complex even in TCR stimulated cells. Since SKAP55 is limiting in T cells, increases in SKAP55 expression might reduce the amount of ADAP able to interact with the CBM signalosome and promote NF-κB activation. Our finding that over-expression of wild-type SKAP55 in wild-type T cells impairs IκB phosphorylation and degradation is consistent with this model. In contrast, over-expression of the SKAP55/R131M mutant did not affect CD3/CD28-mediated activation of NF-κB. Overall, our results are consistent with the hypothesis that the fraction of ADAP that associates with SKAP55 is recruited by the PH domain of SKAP55 to integrins and thus impairs ADAP from interacting with the CBM signalosome and regulating the NF-κB pathway (Fig. 7).
In summary, our results demonstrate a critical function for the SKAP55 PH domain not only in controlling integrin function via recruitment of ADAP/SKAP complexes to integrins, but also in controlling the ability of ADAP to interact with the CBM signalosome and regulate NF-κB (Fig. 7). The ability to modulate NF-κB activation by changing levels of SKAP55 expression in T cells suggests the intriguing possibility that changes in the expression patterns of SKAP55 and/or ADAP during the course of T cell activation may be a mechanism by which T cells modulate this critical transcription factor signaling pathway.
ACKNOWLEDGMENTS
We thank Dr. E. Peterson for providing helpful discussions and reagents, Dr. G. Koretzky and B. Schraven for reagents, and T. Lee, M. Schwartz, D. Loughran, H. Nguyen and L. Yang for mouse genotyping and colony maintenance.
Footnotes
This work was supported by National Institutes of Health Grant RO1 AI038474 to Y.S. Y.S. is also supported in part by the Harry Kay Chair in Biomedical Research at the University of Minnesota.
- ADAP
- adhesion and degranulation promoting adapter protein
- Bcl-10
- B cell lymphoma 10
- CARMA1
- caspase recruitment domain membrane-associated guanylate kinase protein 1
- CBM
- CARMA-1-BCL10-MALT1
- GEF
- guanine nucleotide exchange factor
- HA
- hemagglutinin
- h.c.
- IgG heavy chain
- hCAR
- human coxsackie adenovirus receptor
- LAT
- linker for activated T cells
- PI[3,4,5]P3
- phosphatidylinositol 3,4,5 trisphosphate
- PH
- pleckstrin homology
- PKC
- protein kinase C
- Rap1
- Ras proximate 1
- RapL
- regulator of cell adhesion and polarization enriched in lymphoid tissues
- RIAM
- Rap1-GTP-interacting adapter molecule
- S/A
- SKAP/ADAP chimera
- SH2
- src homology 2
- SH3
- src homology 3
- SKAP55
- src kinase associated phosphoprotein of 55 kDa
- SKAP-HOM
- SKAP55 homolog
- SLP-76
- SH2-domain containing leukocyte phosphoprotein of 76 kda
- TAK1
- TGF-β-activated kinase 1
REFERENCES
- 1.Burbach BJ, Medeiros RB, Mueller KL, Shimizu Y. T cell receptor signaling to integrins. Immunol. Rev. 2007;218:65–81. doi: 10.1111/j.1600-065X.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 2.Dustin ML, Tseng SY, Varma R, Campi G. T cell-dendritic cell immunological synapses. Curr. Opin. Immunol. 2006;18:512–516. doi: 10.1016/j.coi.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Kane LP, Lin J, Weiss A. It’s all Rel-ative: NF-κB and CD28 costimulation of T-cell activation. Trends Immunol. 2002;23:413–420. doi: 10.1016/s1471-4906(02)02264-0. [DOI] [PubMed] [Google Scholar]
- 4.Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 5.Koretzky GA, Abtahian F, Silverman MA. SLP76 and SLP65: complex regulation of signalling in lymphocytes and beyond. Nat. Rev. Immunol. 2006;6:67–78. doi: 10.1038/nri1750. [DOI] [PubMed] [Google Scholar]
- 6.Baker RG, Hsu CJ, Lee D, Jordan MS, Maltzman JS, Hammer DA, Baumgart T, Koretzky GA. The adapter protein SLP-76 mediates “outside-in” integrin signaling and function in T cells. Mol. Cell Biol. 2009;29:5578–5589. doi: 10.1128/MCB.00283-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goda S, Quale AC, Woods ML, Felthauser A, Shimizu Y. Control of TCR-mediated activation of β1 integrins by the ZAP-70 interdomain B region and the LAT adapter protein. J. Immunol. 2004;172:5379–5387. doi: 10.4049/jimmunol.172.9.5379. [DOI] [PubMed] [Google Scholar]
- 8.Thome M. CARMA1, BCL-10 and MALT1 in lymphocyte development and activation. Nat. Rev. Immunol. 2004;4:348–359. doi: 10.1038/nri1352. [DOI] [PubMed] [Google Scholar]
- 9.Perkins ND. Integrating cell-signalling pathways with NF-κB and IKK function. Nat. Rev. Mol. Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 10.Simeoni L, Kliche S, Lindquist J, Schraven B. Adaptors and linkers in T and B cells. Curr. Opin. Immunol. 2004;16:304–313. doi: 10.1016/j.coi.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Rudd CE. SKAP-55, SKAP-55-related and ADAP adaptors modulate integrin-mediated immune-cell adhesion. Trends Cell Biol. 2008;18:486–493. doi: 10.1016/j.tcb.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Liu H, Lu Y, Lovatt M, Wei B, Rudd CE. Functional defects of SKAP-55-deficient T cells identify a regulatory role for the adaptor in LFA-1 adhesion. Mol. Cell Biol. 2007;27:6863–6875. doi: 10.1128/MCB.00556-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller KL, Thomas MS, Burbach BJ, Peterson EJ, Shimizu Y. Adhesion and degranulation promoting adapter protein (ADAP) positively regulates T cell sensitivity to antigen and T cell survival. J. Immunol. 2007;179:3559–3569. doi: 10.4049/jimmunol.179.6.3559. [DOI] [PubMed] [Google Scholar]
- 14.Peterson EJ, Woods ML, Dmowski SA, Derimanov G, Jordan MS, Wu JN, Myung PS, Liu Q-H, Pribila JT, Freedman BD, Shimizu Y, Koretzky GA. Coupling of the TCR to integrin activation by SLAP-130/Fyb. Science. 2001;293:2263–2265. doi: 10.1126/science.1063486. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths EK, Krawczyk C, Kong YY, Raab M, Hyduk SJ, Bouchard D, Chan VS, Kozieradzki I, Oliveira-dos-Santos AJ, Wakeham A, Ohashi PS, Cybulsky MI, Rudd CE, Penninger JM. Positive regulation of T cell activation and integrin adhesion by the adapter Fyb/Slap. Science. 2001;293:2260–2263. doi: 10.1126/science.1063397. [DOI] [PubMed] [Google Scholar]
- 16.Hogg N, Laschinger M, Giles K, McDowall A. T-cell integrins: more than just sticking points. J. Cell Sci. 2003;116:4695–4705. doi: 10.1242/jcs.00876. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Moon EY, Azouz A, Wu X, Smith A, Schneider H, Hogg N, Rudd CE. SKAP-55 regulates integrin adhesion and formation of T cell-APC conjugates. Nat. Immunol. 2003;4:366–374. doi: 10.1038/ni913. [DOI] [PubMed] [Google Scholar]
- 18.Kliche S, Breitling D, Togni M, Pusch R, Heuer K, Wang X, Freund C, Kasirer-Friede A, Menasche G, Koretzky GA, Schraven B. The ADAP/SKAP55 signaling module regulates T-cell receptor-mediated integrin activation through plasma membrane targeting of Rap1. Mol. Cell. Biol. 2006;26:7130–7144. doi: 10.1128/MCB.00331-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menasche G, Kliche S, Chen EJ, Stradal TE, Schraven B, Koretzky G. RIAM links the ADAP/SKAP-55 signaling module to Rap1, facilitating T-cell-receptor-mediated integrin activation. Mol. Cell Biol. 2007;27:4070–4081. doi: 10.1128/MCB.02011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burbach BJ, Srivastava R, Medeiros RB, O’Gorman WE, Peterson EJ, Shimizu Y. Distinct regulation of integrin-dependent T cell conjugate formation and NF-κB activation by the adapter protein ADAP. J. Immunol. 2008;181:4840–4851. doi: 10.4049/jimmunol.181.7.4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swanson KD, Tang Y, Ceccarelli DF, Poy F, Sliwa JP, Neel BG, Eck MJ. The Skap-hom dimerization and PH domains comprise a 3′-phosphoinositide-gated molecular switch. Mol. Cell. 2008;32:564–575. doi: 10.1016/j.molcel.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marie-Cardine A, Hendricks-Taylor LR, Boerth NJ, Zhao H, Schraven B, Koretzky GA. Molecular interaction between the Fyn-associated protein SKAP55 and the SLP-76-associated phosphoprotein SLAP-130. J. Biol. Chem. 1998;273:25789–25795. doi: 10.1074/jbc.273.40.25789. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Kang H, Raab M, Da Silva AJ, Kraeft SK, Rudd CE. FYB (FYN binding protein) serves as a binding partner for lymphoid protein and FYN kinase substrate SKAP55 and a SKAP55-related protein in T cells. Proc. Natl. Acad. Sci. USA. 1998;95:8779–8784. doi: 10.1073/pnas.95.15.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musci MA, Hendricks-Taylor LR, Motto DG, Paskind M, Kamens J, Turck CW, Koretzky GA. Molecular cloning of SLAP-130, an SLP-76-associated substrate of the T cell antigen receptor-stimulated protein tyrosine kinases. J. Biol. Chem. 1997;272:11674–11677. doi: 10.1074/jbc.272.18.11674. [DOI] [PubMed] [Google Scholar]
- 25.Raab M, Kang H, Da Silva A, Zhu XC, Rudd CE. FYN-T-FYB-SLP-76 interactions define a T-cell receptor ζ/CD3-mediated tyrosine phosphorylation pathway that up-regulates interleukin 2 transcription in T-cells. J. Biol. Chem. 1999;274:21170–21179. doi: 10.1074/jbc.274.30.21170. [DOI] [PubMed] [Google Scholar]
- 26.Yuan M, Mogemark L, Fällman M. Fyn binding protein, Fyb, interacts with mammalian actin binding protein, mAbp1. FEBS Lett. 2005;579:2339–2347. doi: 10.1016/j.febslet.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 27.Medeiros RB, Burbach BJ, Mueller KL, Srivastava R, Moon JJ, Highfill S, Peterson EJ, Shimizu Y. Regulation of NF-κB activation in T cells via association of the adapter proteins ADAP and CARMA1. Science. 2007;316:754–758. doi: 10.1126/science.1137895. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava R, Burbach BJ, Shimizu Y. NF-κB activation in T cells requires discrete control of IκB kinase α/β (IKKα/β) phosphorylation and IKKγ ubiquitination by the ADAP adapter protein. J. Biol. Chem. 2010;285:11100–11105. doi: 10.1074/jbc.M109.068999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, Norton DD, Precht P, Martindale JL, Burkhardt JK, Wange RL. Deficiency of ADAP/Fyb/SLAP-130 destabilizes SKAP55 in Jurkat T cells. J. Biol. Chem. 2005;280:23576–23583. doi: 10.1074/jbc.M413201200. [DOI] [PubMed] [Google Scholar]
- 30.Togni M, Swanson KD, Reimann S, Kliche S, Pearce AC, Simeoni L, Reinhold D, Wienands J, Neel BG, Schraven B, Gerber A. Regulation of in vitro and in vivo immune functions by the cytosolic adaptor protein SKAP-HOM. Mol. Cell. Biol. 2005;25:8052–8063. doi: 10.1128/MCB.25.18.8052-8063.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raab M, Wang H, Lu Y, Smith X, Wu Z, Strebhardt K, Ladbury JE, Rudd CE. T cell receptor “inside-out” pathway via signaling module SKAP1-RapL regulates T cell motility and interactions in lymph nodes. Immunity. 2010;32:541–556. doi: 10.1016/j.immuni.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 33.Hurez V, Dzialo-Hatton R, Oliver J, Matthews RJ, Weaver CT. Efficient adenovirus-mediated gene transfer into primary T cells and thymocytes in a new coxsackie/adenovirus receptor transgenic model. BMC. Immunol. 2002;3:4. doi: 10.1186/1471-2172-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katagiri K, Maeda A, Shimonaka M, Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat. Immunol. 2003;4:741–748. doi: 10.1038/ni950. [DOI] [PubMed] [Google Scholar]
- 35.Kinashi T, Katagiri K. Regulation of lymphocyte adhesion and migration by the small GTPase Rap1 and its effector molecule, RAPL. Immunol. Lett. 2004;93:1–5. doi: 10.1016/j.imlet.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Lemmon MA. Pleckstrin homology domains: not just for phosphoinositides. Biochem. Soc. Trans. 2004;32:707–711. doi: 10.1042/BST0320707. [DOI] [PubMed] [Google Scholar]
- 37.Woods ML, Kivens WJ, Adelsman MA, Qiu Y, August A, Shimizu Y. A novel function for the Tec family tyrosine kinase Itk in activation of β1 integrins by the T cell receptor. EMBO J. 2001;20:1232–1244. doi: 10.1093/emboj/20.6.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menasche G, Kliche S, Bezman N, Schraven B. Regulation of T-cell antigen receptor-mediated inside-out signaling by cytosolic adapter proteins and Rap1 effector molecules. Immunol. Rev. 2007;218:82–91. doi: 10.1111/j.1600-065X.2007.00543.x. [DOI] [PubMed] [Google Scholar]
- 39.Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, Puzon-McLaughlin W, Lafuente EM, Boussiotis VA, Shattil SJ, Ginsberg MH. Reconstructing and deconstructing agonist-induced activation of integrin αIIbβ3. Curr. Biol. 2006;16:1796–1806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 40.Geng L, Raab M, Rudd CE. Cutting edge: SLP-76 cooperativity with FYB/FYN-T in the up-regulation of TCR-driven IL-2 transcription requires SLP-76 binding to FYB at Tyr595 and Tyr651. J. Immunol. 1999;163:5753–5757. [PubMed] [Google Scholar]
- 41.Wang HY, McCann FE, Gordan JD, Wu X, Raab M, Malik TH, Davis DM, Rudd CE. ADAP-SLP-76 binding differentially regulates supramolecular activation cluster (SMAC) formation relative to T cell-APC conjugation. J. Exp. Med. 2004;200:1063–1074. doi: 10.1084/jem.20040780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boerth NJ, Judd BA, Koretzky GA. Functional association between SLAP-130 and SLP-76 in Jurkat T cells. J. Biol. Chem. 2000;275:5143–5152. doi: 10.1074/jbc.275.7.5143. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Wei B, Bismuth G, Rudd CE. SLP-76-ADAP adaptor module regulates LFA-1 mediated costimulation and T cell motility. Proc. Natl. Acad. Sci. USA. 2009;106:12436–12441. doi: 10.1073/pnas.0900510106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki JI, Yamasaki S, Wu J, Koretzky GA, Saito T. Actin cloud induced by LFA-1-mediated outside-in signals lowers the threshold for T cell activation. Blood. 2006;109:168–175. doi: 10.1182/blood-2005-12-020164. [DOI] [PubMed] [Google Scholar]
- 45.Lehmann R, Meyer J, Schuemann M, Krause E, Freund C. A novel S3S-TAP-tag for the isolation of T-cell interaction partners of adhesion and degranulation promoting adaptor protein. Proteomics. 2009;9:5288–5295. doi: 10.1002/pmic.200900294. [DOI] [PubMed] [Google Scholar]
- 46.Mayya V, Lundgren DH, Hwang SI, Rezaul K, Wu L, Eng JK, Rodionov V, Han DK. Quantitative phosphoproteomic analysis of T cell receptor signaling reveals system-wide modulation of protein-protein interactions. Sci. Signal. 2009;2:ra46. doi: 10.1126/scisignal.2000007. [DOI] [PubMed] [Google Scholar]
- 47.Sylvester M, Kliche S, Lange S, Geithner S, Klemm C, Schlosser A, Grossmann A, Stelzl U, Schraven B, Krause E, Freund C. Adhesion and degranulation promoting adapter protein (ADAP) is a central hub for phosphotyrosine-mediated interactions in T cells. PLoS ONE. 2010;5:e11708. doi: 10.1371/journal.pone.0011708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coppolino MG, Krause M, Hagendorff P, Monner DA, Trimble W, Grinstein S, Wehland J, Sechi AS. Evidence for a molecular complex consisting of Fyb/SLAP, SLP-76, Nck, VASP and WASP that links the actin cytoskeleton to Fcγ receptor signalling during phagocytosis. J. Cell Sci. 2001;114:4307–4318. doi: 10.1242/jcs.114.23.4307. [DOI] [PubMed] [Google Scholar]
- 49.Krause M, Sechi AS, Konradt M, Monner D, Gertler FB, Wehland J. Fyn-binding protein (Fyb)/SLP-76-associated protein (SLAP), Ena/vasodilator-stimulated phosphoprotein (VASP) proteins and the Arp2/3 complex link T cell receptor (TCR) signaling to the actin cytoskeleton. J. Cell Biol. 2000;149:181–194. doi: 10.1083/jcb.149.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heuer K, Sylvester M, Kliche S, Pusch R, Thiemke K, Schraven B, Freund C. Lipid-binding hSH3 domains in immune cell adapter proteins. J. Mol. Biol. 2006;361:94–104. doi: 10.1016/j.jmb.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Heuer K, Arbuzova A, Strauss H, Kofler M, Freund C. The helically extended SH3 domain of the T cell adaptor protein ADAP is a novel lipid interaction domain. J. Mol. Biol. 2005;348:1025–1035. doi: 10.1016/j.jmb.2005.02.069. [DOI] [PubMed] [Google Scholar]