Abstract
Pemphigus is a dreaded disease encountered not infrequently in dermatology settings. While scoring systems in various dermatological conditions exist, objective parameters for assessing disease activity and therapeutic responses in pemphigus are not uniform and foolproof. This article presents various scoring systems in pemphigus.
Keywords: Autoimmune bullous disease, index, pemphigus, score
Introduction
Pemphigus, an autoimmune vesicobullous disease characterized by autoantibodies against distinct adhesion molecules of the epidermis, was almost fatal before the advent of corticosteroids in its management.[1] Due to the wide variations in the presentation of the disease, various emerging therapeutic options offered for its treatment and the wide range in responsiveness, this spectrum of diseases has largely been described by various workers in various subjective terms. There was a felt need to devise objective parameters to evaluate the progress of the disease or its response to therapy in order to compare the outcome parameters reported in different studies on a common platform removing all possible inter-observer variability; in short, to lend some method to the madness. An ongoing Cochrane review of clinical trials studying pemphigus revealed an astounding total of 116 outcome measures described in 96 articles over the past 25 years.[2]
The aim of this article is to present on a single platter some salient scoring systems devised on pemphigus.
Scoring Systems
While measurement of body surface area (BSA) involvement using Wallace's rule of nine could, at first glance, provide a simple parameter to assess and grade the severity of pemphigus, it may be too simplistic and is fraught with flaws of subjectivity. Skin area assessments to measure inflammatory skin disease can be difficult, reportedly even for physicians.[3–5]
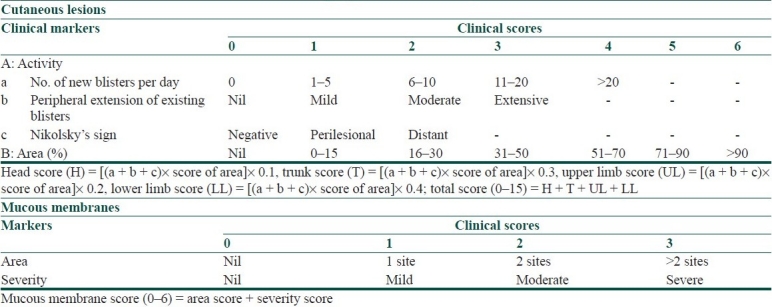
Pemphigus Area and Activity Score [Table 1] is one of the earliest scoring systems devised for pemphigus. While it caters for BSA and number of lesions, severity description is subjective and quantification of lesions does not incorporate size, making it an imprecise tool for assessing disease activity.[6]
Table 1.
Pemphigus area and activity score[6]
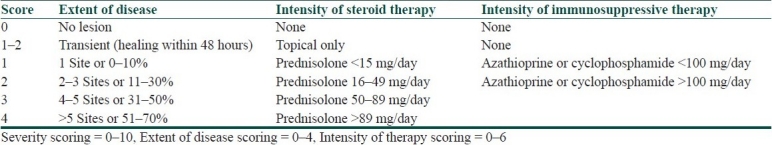
Pemphigus Activity Score [Table 2] introduces intensity of steroid and immunosuppressive therapy along with the extent of disease. But it suffers from a lack of differential clinical involvement of mucosal and cutaneous lesions and an insensitive quantification of lesions.[7]
Table 2.
Pemphigus activity score[7]
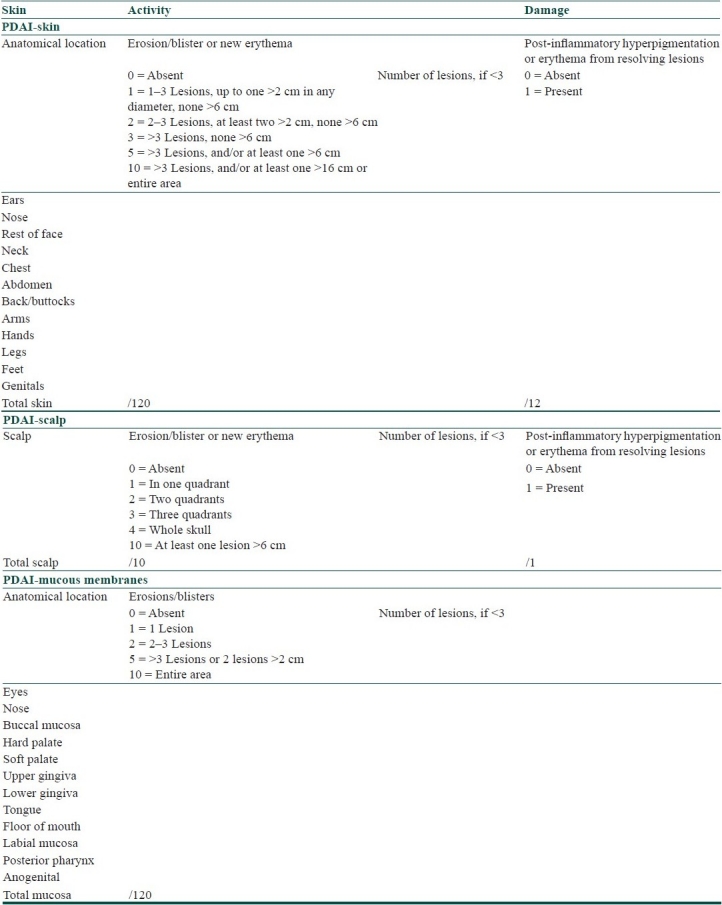
Pemphigus Disease Area Index [Table 3] integrates cutaneous with mucosal disease in well-defined anatomical locations, assesses number and sizes of lesions and also scores post-inflammatory hyperpigmentation of resolving lesions.[8]
Table 3.
Pemphigus disease activity index[8]
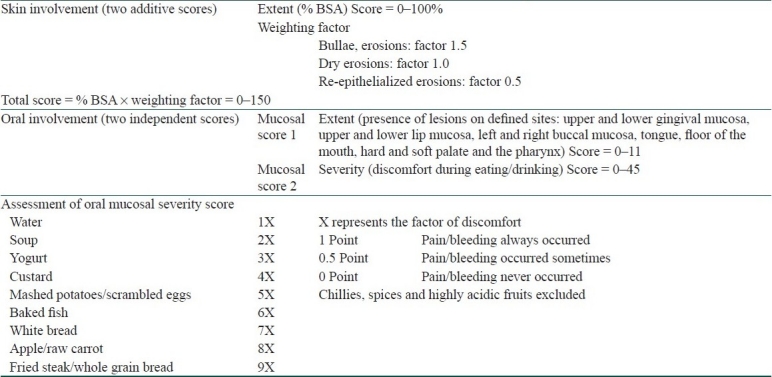
Autoimmune Bullous Skin Disorder Intensity Score [Table 4] is a quality- and quantity-based score for cutaneous and oral mucosal lesions. This system claims to monitor the clinical status of individual patients over time vis-à-vis inter-patient differentiation assessed by other systems. In addition, this system can be used for assessing autoimmune diseases other than pemphigus alone, and thus is more versatile.[9]
Table 4.
Autoimmune bullous skin disorder intensity score[9]
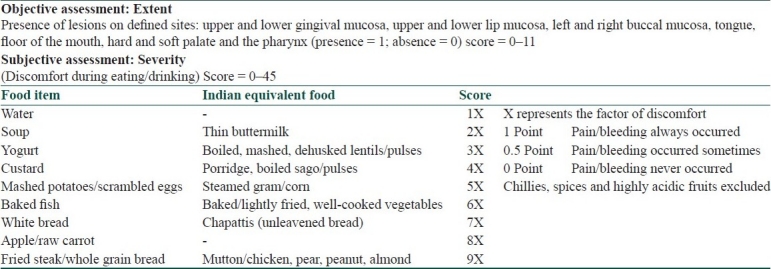
As mucosal lesions in pemphigus often predate cutaneous lesions, are more recalcitrant and recurrent, associated with severe morbidity (halitosis, dysphagia), often improve with reducing depth and not necessarily by reducing count or size, defy BSA assessment and behave differently from cutaneous lesions, an independent scoring system was devised for oral pemphigus [Table 5]. The system is based on a modification of the objectively validated dysphagia grading system by Dakkak and Bennett. It is reportedly useful for other diseases, viz., benign mucosal pemphigoid, herpetic gingivostomatitis and Stevens Johnson syndrome, and eliminates subjective inter-observer variability.[10] The versatility of this system is revealed by the fact that ABSIS system has also borrowed from this scoring system.
Table 5.
Saraswat's oral pemphigus scoring[10]
Numerous other scoring systems [Tables 6–9] have also been described in literature at different times to score disease activity of pemphigus.[11–14] International Pemphigus Committee recently came out with a consensus statement on definitions of disease, end points, and therapeutic response for pemphigus which, for the first time, defines common terms and end points of the disease in order to accurately measure and assess disease extent, activity, severity, and therapeutic response at agreed-on time points.[8]
Table 6.
Pemphigus vulgaris lesion severity ccore[11]
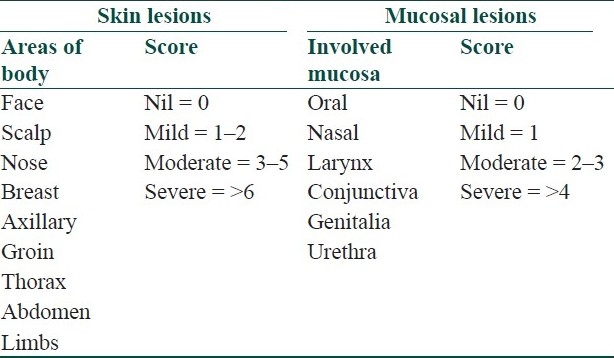
Table 9.
Harman's pemphigus grading[14]
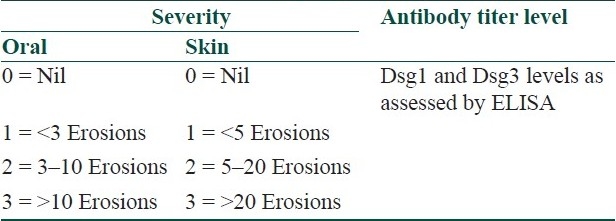
Table 7.
Kumar's scoring system[12]

Table 8.
Mahajan's scoring system[13]

Thusfar, the overwhelming fact revealed is the singular lack of uniformity in comparing disease activity and therapeutic responses. Validating scoring systems and pitting them against each other could be used as a means to compare relative efficacies of various systems. One such study recently compared PDAI with ABSIS and found a better intra-class correlation coefficient for PDAI in reliability of assessing skin activity and in intra-rater test-retest reliability for consistency and reproducibility of scoring, when compared with ABSIS. The study concluded that PDAI was a reliable, quick, and easy-to-use method to capture the extent of skin and mucosal lesions in mild-to-moderate pemphigus, as compared to ABSIS.[15]
Conclusion
Pemphigus, as a group of diseases, is constantly evolving with advances in molecular biology and expanding therapeutic options. While in the past, introduction of steroids remarkably reduced the mortality rate of this dreaded disease, modern times paradoxically incriminate immunosuppressive therapies as one of the major causes of morbidity and mortality in this condition. Hence, the need of an ideal scoring system to assess this disease against contemporary parameters cannot be overstated. Despite a wide array of scoring systems given by various workers, the search for the “perfect one” is still on.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Bystryn JC, Rudolph JL. Pemphigus. Lancet. 2005;366:61–73. doi: 10.1016/S0140-6736(05)66829-8. [DOI] [PubMed] [Google Scholar]
- 2.Martin L, Murrell DF. Measuring the immeasurable: A systematic review of outcome measures in pemphigus. Paper Australas J Dermatol. 2006;47:A32–3. [Google Scholar]
- 3.Tilin-Grosse S, Rees J. Assessment of area of involvement in skin disease: A study using schematic figure outlines. Br J Dermatol. 1993;128:69–74. doi: 10.1111/j.1365-2133.1993.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 4.Charman CR, Venn AJ, Williams HC. Measurement of body surface area involvement in atopic eczema: An impossible task? Br J Dermatol. 1999;25:406–11. doi: 10.1046/j.1365-2133.1999.02617.x. [DOI] [PubMed] [Google Scholar]
- 5.Charman C, Williams H. Outcome measures of disease severity in atopic eczema. Arch Dermatol. 2000;136:763–9. doi: 10.1001/archderm.136.6.763. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal M, Walia R, Kochhar AM, Chander R. Pemphigus Area and Activity Score (PAAS): A novel clinical scoring method for monitoring of pemphigus vulgaris patients. Int J Dermatol. 1998;37:158–60. [PubMed] [Google Scholar]
- 7.Herbst A, Bystryn JC. Patterns of remission in pemphigus vulgaris. J Am Acad Dermatol. 2000;42:422–7. doi: 10.1016/s0190-9622(00)90213-5. [DOI] [PubMed] [Google Scholar]
- 8.Murrell DF, Dick S, Ahmed AR, Amagai M, Barnadas MA, Borradori L, et al. Consensus statement on definitions of disease, end points, and therapeutic response for pemphigus. J Am Acad Dermatol. 2008;58:1043–6. doi: 10.1016/j.jaad.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfütze M, Niedermeier A, Hertl M, Eming R. Introducing a novel Autoimmune Bullous Skin Disorder Intensity Score (ABSIS) in pemphigus. Eur J Dermatol. 2007;17:4–11. doi: 10.1684/ejd.2007.0090. [DOI] [PubMed] [Google Scholar]
- 10.Saraswat A, Kumar B. A new grading system for oral pemphigus. Int J Dermatol. 2003;42:413–4. doi: 10.1046/j.1365-4362.2003.01811.x. [DOI] [PubMed] [Google Scholar]
- 11.Esmaili N, Chams-davatchi C, Valikhani M, Farshidfar F, Parvaneh N, Tamizifar B. Treatment of pemphigus vulgaris with mycophenolate mofetil as a steroid-sparing agent. Eur J Dermatol. 2008;18:159–64. doi: 10.1684/ejd.2008.0354. [DOI] [PubMed] [Google Scholar]
- 12.Kumar B, Arora S, Kumaran MS, Jain R, Dogra S. Study of desmoglein 1 and 3 antibody levels in relation to disease severity in Indian patients with pemphigus. Indian J Dermatol Venereol Leprol. 2006;72:203–6. doi: 10.4103/0378-6323.25780. [DOI] [PubMed] [Google Scholar]
- 13.Mahajan VK, Sharma NL, Sharma RC, Garg G. Twelve-year clinico-therapeutic experience in pemphigus: A retrospective study of 54 cases. Int J Dermatol. 2005;44:821–7. doi: 10.1111/j.1365-4632.2005.02218.x. [DOI] [PubMed] [Google Scholar]
- 14.Harman KE, Seed PT, Gratian MJ, Bhogal BS, Challacombe SJ, Black MM. The severity of cutaneous and oral pemphigus is related to desmoglein 1 and 3 antibody levels. Br J Dermatol. 2001;144:775–80. doi: 10.1046/j.1365-2133.2001.04132.x. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbach M, Murell DF, Bystryn JC, Dulay S, Dick S, Facharzadeh S, et al. Reliability and Convergent Validity of Two Outcome Instruments for Pemphigus. J Invest Dermatol. 2009;129:2404–10. doi: 10.1038/jid.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]


