Abstract
Background:
Linear IgA bullous dermatosis (LAD) of children is relatively frequent in Africa.
Aim:
We undertook this study to evaluate the frequency of this disease among autoimmune bullous diseases (AIBDs) in Tunisian children.
Materials and Methods:
We present a 32-year retrospective study (January 1976 to December 2007). Children with chronic acquired bullous diseases seen at the Charles Nicolle Hospital of Tunis and for who direct immunofluorescence (DIF) of the perilesional skin demonstrated linear IgA immunoglobulin deposits were included in the study population.
Results:
Thirty-one children with LAD were selected representing 65.9% of all AIBDs of children selected in the same period, with a mean age of 5.5 years and a sex ratio (M/F) of 2.4. Most of the children had generalized eruption (28/31), more profuse on the face, pelvic region, buttocks and limbs. Mucosal lesions happened in only four children (12.9%). The mean duration of the disease was 14 months. DIF demonstrated linear IgA deposits along the dermal–epidermal junction in all patients. IgG, IgM, and complement were also seen (20/31). Indirect immunofluorescence was negative in 67% of cases. Eight patients responded to dapsone; however, prednisone had to be added in seven children to control the disease and erythromycin in four others. A long-term remission period was achieved in 76.1% of patients.
Conclusion:
This study confirms that LAD is the most common AIBD in children in Tunisia which frequently occurs in preschool-aged males. Independently of the used drug, a long-term remission is frequently observed.
Keywords: Linear IgA bullous dermatosis, children, direct immunofluorescence, Tunisian children
Introduction
Linear IgA dermatosis (LAD) is a rare acquired autoimmune subepidermal vesiculobullous disease characterized by continuous linear IgA deposits in the basement membrane zone (BMZ), visualized on direct immunofluorescence microscopy. The clinical presentation is heterogeneous and can mimic other blistering diseases such as dermatitis herpetiformis and bullous pemphigoid. Linear IgA dermatosis can affect both adult and children; in the latter the disease is historically referred to as chronic bullous dermatosis of childhood (CBDC). The two forms which differ slightly in their clinical presentations have identical immunopathological and immunogenetic features. The frequency of the disease differs in children and adults and also from a country to another. LAD is the most frequent bullous dermatosis of children in France, Asia, and South Africa. There are very few epidemiologic studies of children with this distinct childhood disorder especially from Tunisia.[1,2] The aim of the present study is to highlight the epidemiological and clinical features and response to treatment in children with LAD attending our tertiary care hospital in Tunis during 32 years.
Materials and Methods
All children under 16 years of age followed up for blistering diseases at the Department of Dermatology of Charles Nicolle Hospital of Tunis over the past 32 years (January 1976 to December 2007) were studied. Only children who showed evidence for linear IgA immunoglobulin deposits in perilesional skin on direct immunofluorescence (DIF) were included in the study group and analyzed with the diagnosis of linear IgA dermatosis. In addition to demographic data, details regarding the age at onset, duration and distribution of lesions, treatment and outcome were précised. Are considered in remission patients who achieve a 3-month period of healing.
Results
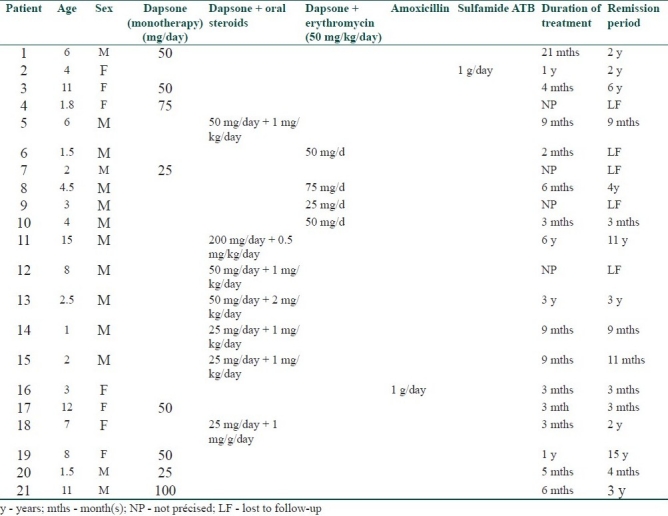
During the study period, 47 children of all ages were treated for a confirmed bullous dermatosis. LAD was by far the most common disease with up to 31 cases (65.9%). The other cases were distributed as follows: eight cases of bullous pemphigoid, five cases of dermatitis herpetiformis, and only three cases of pemphigus. In children with LAD the disease begun at a mean age of 5.5 years (range: 8 months and 16 years). Both sexes were affected but males outnumbered females in a ratio of 2.4:1. Most of the children gave a history of a sudden onset of the bullous eruption (14/31). The other patients noted a period of prodromal itching or transient mild pruritus before specific lesions. Only one of them had an intense pruritus with a burning sensation. Differentiation from an infectious bullous dermatosis was difficult in eight children. Physical examination was misleading and resulted in a delayed diagnosis. In all the children treated for LAD, we noted a significant polymorphism in cutaneous lesions with coexistence of several types of lesions at different stages of the disease. Cutaneous examination showed tense pruritic blisters of variable sizes and in some cases, small clear-filled vesicles were associated (12/31); these arose on normal appearing skin, or at the periphery of an annular erythema. A total of 12 of 31 patients had a cluster of jewels appearance, with lesions in a rosette pattern [Table 1]. Crusts, excoriations, and erosions were also present in some of the children. Seven children had lesions mimicking dermatitis herpetiformis with small-grouped blisters. Nine children had features of bullous pemphigoid with large and tense bullae. The rest of the children had a mixed pattern of both groups. Most of the children had a generalized eruption (28 children, 93%), with an asymmetrical distribution. Two of our patients had localized lesions on their legs. In 70% of cases, the lesions were more profuse on the perineum and particularly on the genital areas and buttocks especially in boys (12 out of 13 cases). Perianal lesions were noticed in only one child. The face (chin and helix) was frequently involved (53% of the cases). Mucous membrane involvement (oral and genital) was noticed in only four boys (12.9%). Histological features showed a subepidermal bulla in all patients with eosinophils and neutrophils and papillary abscesses. In all patients, DIF demonstrated linear IgA deposits along the dermoepidermal junction. A total of 20 out of 31 children showed evidence of IgG, IgM, and complement deposition associated with IgA. Indirect immunofluorescence (IIF) was negative in 67% of the patients [Table 1]. Malabsorption tests were conducted in three patients with no digestive signs, but one patient had partial jejunal atrophy. HLA studies showed a B8, DR3 antigen pattern in two of the three patients studied. In our series, dapsone was used as a first-line therapy in 70% of cases (19 children). The initial dose varied from 25 mg to 100 mg/day, according to the patient's age and weight, the spreading of lesions, and the severity of the disease. Dapsone was used as a monotherapy in eight cases and led to a complete healing of lesions in all of them after a mean duration of 13 days (extremes = 3 and 23 days). Treatment with dapsone was maintained at the same initial dose during a mean duration of 6.5 months (extremes: 3 and 21 months). A remission was obtained in six of them even after stopping dapsone (two patients were lost to follow-up) with a mean remission period of 2.5 years (range: 4 months and 6 years). In 11 patients, because of an initial resistance, dapsone was secondarily associated with either oral corticosteroids (7 patients) (0.5–2 mg/kg/day) or with erythromycin (4 patients). The associated therapy (dapsone–oral steroids) healed the lesions in a mean period of 12 days. Corticosteroids were tapered until a maintaining dose of 5–15 mg/day during a mean period of 20 months (extremes: 3 months and 6 years). Six of these seven patients (one patient was lost to follow-up) had a long-standing remission (off treatment) of a mean period of 3 years (extremes: 9 months and 11 years). Two of the four patients receiving erythromycin associated with dapsone had achieved remission (3 months and 4 years). Two patients had completely healed with antibiotics: amoxicillin only (1 g/day) for one of them and oral sulfonamide only for the other (3 months and 2 years of remission, respectively) [Table 2]. A total of 10 of the 31 diagnosed patients with LAD were lost to follow-up. Independently of the used drug, a total remission was observed in 16 patients (76.1% of cases) with a total remission period, after stopping the treatment, of 10.6 months (extremes 3 months to 6 years). Complications included methemoglobinemia and anemia in three children treated by dapsone, leading to a tapering of the doses with a good response. In one patient, IFD performed in the remission period (4 years after stopping dapsone) was negative, testifying the associated histological cure.
Table 1.
Epidemioclinical, histological, and immunopathological characteristics of the 31 patients with LAD
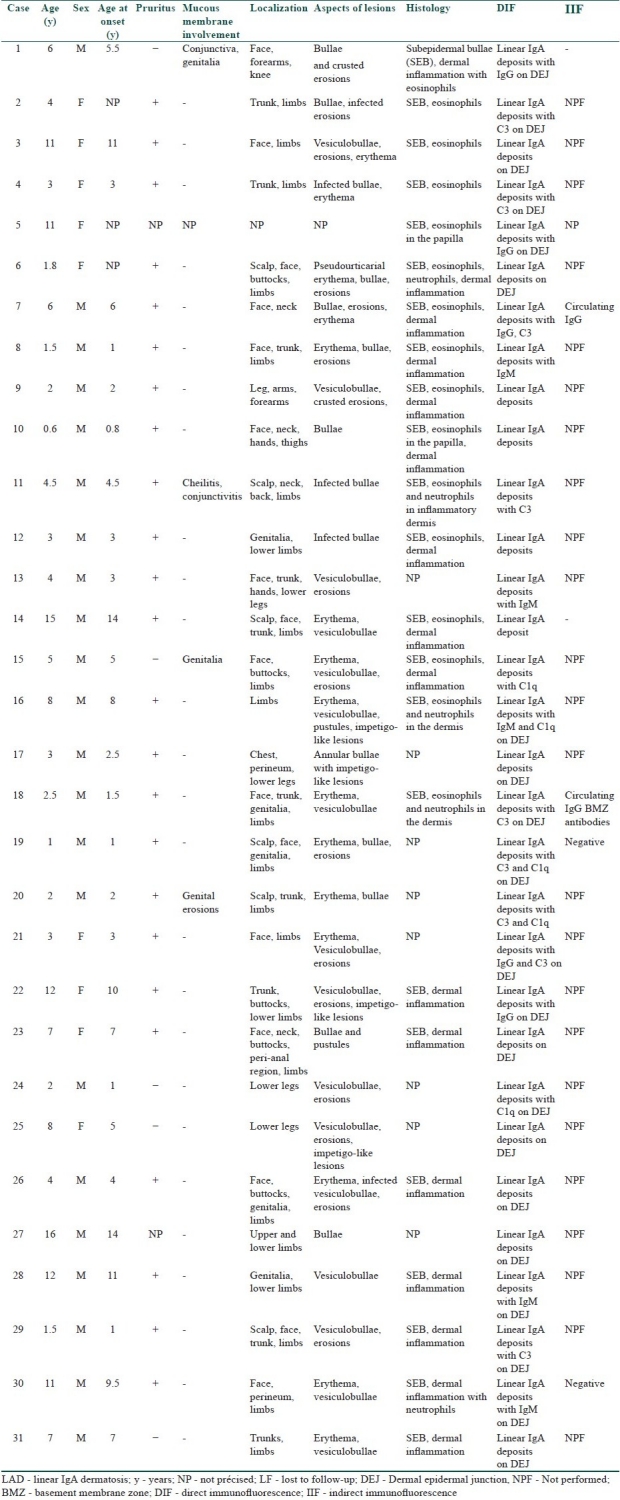
Table 2.
Data concerning treatment and outcome in the 21 children out of 31 with LAD followed up
Discussion
LAD is an autoimmune disease characterized immunopathologically by the linear deposition of IgA at the basement membrane zone. This antibody deposition leads to complement activation and to a cascade of immunoreactions resulting in the loss of adhesion at the dermal–epidermal junction with a major consequence that is blister formation.[3]
LAD is not a very common disease in adults. Its prevalence has been estimated in Europe as 0.5 per 1,000,000 adults.[4] The prevalence in children has rarely been reported but its incidence is reported to be high in South Africa, North Africa, and Asia.[2,5–7] Taking into account the previous reported series, our series accounting 31 cases of LAD in 32 years is to our knowledge among the highest frequencies ever reported.[6,7] The reason of the difference between the frequencies of two series needs to be determined. We have also confirmed that LAD is the most common autoimmune bullous disease (AIBD) in children in Tunisia with up to 66% of all cases of AIBD registered during 32 years.
LAD has a bimodal age at onset; it occurs in children and in adults. The disease in children begins usually in the preschool age, with a mean age at onset of 4–5 years.[7,8] In our series, the mean age at onset was in agreement with the literature finding. The disease affects both sexes, but some studies reported a slight female preponderance[9] while other studies, as our study, report boys being increasingly affected compared to girls.[6,7]
The disease has been associated most commonly with infections and drugs. Drug-induced LAD is more likely to occur in the older population with a sufficient evidence for vancomycin.[10,11] Drug-induced LAD is exceptionally described in children.[12] In a series of 25 cases of childhood LAD reported by Wojnarowska et al., 38% of them had either preceding infection or ingestion of drugs. Drugs that have been associated with childhood LAD include antibiotics and nonsteroidal inflammatory agents, but the exact agents are not known.[13] In our series, no causes have been implicated with the onset of the disease.
Clinically there are no major differences between the adult and the childhood form of LAD. Only the distribution of the lesions differs. In fact, lesions in children are typically localized in the lower abdomen and anogenital areas with a frequent involvement of the perineum. The other sites include face, especially the perioral area. Lesions may be symmetrically or asymmetrically distributed.[9]
Mucosal involvement is more frequent in adults than in children particularly in Europe with oral, ocular, genital, and digestive manifestations.[13,14] In African and Asian series, children presenting with LAD rarely suffer from mucosal involvement.[6,7,15] This is confirmed in our study where only 12.9% of children had evidence of oral or genital lesions. But, even if no signs of mucosal affection are reported by the patient, an ocular examination is necessary and may include subconjunctival fibrosis, symblepharon formation, and cicatricial entropion.
The association of LAD with a gluten-sensitive enteropathy is less frequently observed than with dermatitis herpetiformis (DH) and remains controversial, due to the absence of clinical signs and the negativity of complementary examinations.[3] None of our patients had a digestive history but one had jejunal atrophy on systematic digestive endoscopy.
LAD in children has been reported in association with HLA B8, DR3, Cw7 more frequently than in adults. In addition, this is more likely to happen, if these genes are present in a homozygote state.[3] Moreover, the TNF2 gene is thought to play a role in the duration of the disease and is associated with a worse prognosis.[14] In our series, two of the three children investigated for HLA haplotype, and had B8, DR3 haplotype.
Immunofluorescence studies are very important for the diagnosis of LAD. DIF of perilesional and normal skin shows typically a linear deposition of IgA on the basement membrane.[9] Complement deposition is frequent (C3) and IgG or IgM can also be present at the same time as IgA antibodies. In some cases, the DIF can be negative and the dermatosis can be considered as an infectious disease leading to a considerable delay in the treatment. Repeated laboratory studies must be considered in cases with obvious clinical presentation of LAD and negative DIF results. Circulating IgA BMZ antibodies have been reported in up to 80% of patients.[6] But IIF seems to be more likely positive in children than in adult patients with LAD (72% versus 20%).[16] The mechanism of the loss of self-tolerance to target antigens is unknown. Many studies have focused on the targeted dermal–epidermal junction antigens during LAD, and different proteins have been identified in the lamina lucida, sublamina densa, or both locations simultaneously. The 97 kDa and the 120 kDa proteins extracted from human epidermis are the best characterized antigens binding IgA antibodies from sera of patients with LAD and are localized in the lamina lucida. These proteins may represent a cleaved portion of the extracellular domain of bullous pemphigoid antigen (180 kDa), which can exist in vivo, or could also be alternative splicing products of the same antigen. More recently, patients with LAD have been reported to show evidence of IgA antibodies reacting with both bullous pemphigoid antigens (180 kDa and 230 kDa). This may be explained by a specific immune response against pemphigoid antigens.[3,17]
A minority of patients can present with antibodies directed against different antigens located on both lamina lucida and lamina densa. The 280 kDa antigen, the collagen VII (250 kDa) antigen, and many more can be identified in those patients and can mislead the diagnosis of bullous pemphigoid (BP) or epidermolysis bullosa acquisita. More confusing are the patients presenting with both IgA and IgG antibodies targeting the same antigens. Patients with linear IgA antibodies are continued to be grouped into a single category of disease, LAD, no matter what kind of antigen their antibodies are targeting. Because of the molecular heterogeneity encountered in such a disease, and in sight of our current knowledge, patients should be classified according to the antigen targeted, thus we may have BP with IgA deposits, epidermolysis bullosa acquisita with IgA deposit, and the IgA/IgG linear dermatosis.[17]
Our series, so do several other reported series, confirm the efficiency of dapsone in controlling LAD.[6,7,18] However, a combination with systemic steroids is sometimes required to control the disease (as seven patients of our series). Other useful medications including colchicine, niacinamide, antibiotics, and a gluten-free diet were reported.[3,18,19] Local care of lesions must be conducted; ruptured lesions, scratching, and infected lesions should be treated with sterile dressings and must be checked twice a day. A particular hygiene must be granted to the perineal lesions in children. LAD of childhood has usually a benign and self-limiting effect, and a long-standing remission can be achieved either with dapsone or a combination therapy.[6,7] Seventy-six percent of patients (16 over 21 followed patients: 76.1%) had a remission period of 10.6 months after stopping the treatment. The disease tends to wax and wane in severity all over the years until it disappears spontaneously.[13] Cutaneous lesions heal without scarring. As demonstrated by one of our cases, IgA deposits on DIF disappear completely in children after remission, while they remain positive in adults even after healing.[3]
In conclusion, our study was in agreement with the occidental literature for some clinical features but differs in the frequency of the disease, the male predominance, the rare mucosal involvement, and the shorter mean duration. These differences were also reported by other authors from African and Asian countries.[5,6] Molecular and genetic studies may be helpful to prιcis the existence of specificities of this disease in African countries.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Reid C, Wojnarowska F, Psthupityia G, Aboobaker J, Bhogal B, Black MM. Chronic bullous disease of childhood around the world. Br J Dermatol. 1988;119:33–41. [Google Scholar]
- 2.Denguezli M, Ben Nejma B, Nouira R, Korbi S, Bardi R, Ayed K, et al. IgA linear bullous dermatosis in children.A series of 12 Tunisian patients. Ann Dermatol Venereol. 1994;121:888–92. [PubMed] [Google Scholar]
- 3.Guide S V, Marinkovich M P. Linear Ig A bullous dermatosis. Clin Dermatol. 2001;19:719–27. doi: 10.1016/s0738-081x(00)00185-1. [DOI] [PubMed] [Google Scholar]
- 4.Zillikens D, Wever S, Roth A, Weidenthaler-Barth B, Hashimoto T, Brocker EB. Incidence of autoimmune subepidermal blistering dermatoses in a region of central Germany. Arch Dermatol. 1995;131:957–8. doi: 10.1001/archderm.131.8.957. [DOI] [PubMed] [Google Scholar]
- 5.Aboobaker J, Wojnarowska FT, Bhogal B, Black MM. Chronic bullous dermatitis of childhood-clinical and immunological features seen in African patients. Clin Exp Dermatol. 1991;16:160–4. doi: 10.1111/j.1365-2230.1991.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 6.Kanwar AJ, Sandhu K, Handa S. Chronic bullous dermatosis of childhood in north India. Pediatr Dermatol. 2004;21:610–2. doi: 10.1111/j.0736-8046.2004.21521.x. [DOI] [PubMed] [Google Scholar]
- 7.Nanda A, Dvorak R, Al-Sabah H, Alsaleh QA. Linear IgA bullous disease of childhood: an experience from Kuwait. Pediatr Dermatol. 2006;23:443–7. doi: 10.1111/j.1525-1470.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- 8.Hruza LL, Mallory SB, Fitzgibbons J, Mallory GB., Jr Linear IgA bullous dermatosis in a neonate. Pediatr Dermatol. 1993;10:171–6. doi: 10.1111/j.1525-1470.1993.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 9.Brenner S, Mashiah J. Autoimmune Blistering diseases in children: signposts in the process of evaluation. Clin dermatol. 2000;18:711–24. doi: 10.1016/s0738-081x(00)00154-1. [DOI] [PubMed] [Google Scholar]
- 10.Kuechle MK, Stegemeir E, Maynard B, Gibson LE, Leiferman KM, Peters MS. Drug-induced linear Ig A bullous dermatosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994;30:187–92. doi: 10.1016/s0190-9622(94)70015-x. [DOI] [PubMed] [Google Scholar]
- 11.Nousari HC, Kimyai-Asadi A, Caeiro JP, Anhalt GJ. Clinical, demographic, and immunohistologic features of vancomycin-induced linear IgA bullous disease of the skin. Report of 2 cases and review of the literature. Medicine (Baltimore) 1999;78:1–8. doi: 10.1097/00005792-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Ho JC, Ng PL, Tan SH, Giam YC. Childhood linear IgA bullous disease triggered by amoxicillin-clavulanic acid. Pediatr Dermatol. 2007;24:40–3. doi: 10.1111/j.1525-1470.2007.00438.x. [DOI] [PubMed] [Google Scholar]
- 13.Wojnarowska F, Marsden RA, Bhogal B, Black MM. Chronic bullous disease of childhood, childhood cicatricial pemphigoid, and linear IgA disease of adults. A comparative study demonstrating clinical and immunopathologic overlap. J Am Acad Dermatol. 1988;19:792–805. doi: 10.1016/s0190-9622(88)70236-4. [DOI] [PubMed] [Google Scholar]
- 14.Collier PM, Wojnarowska F, Welsh K, McGuire W, Black MM. Adult linear Ig A disease and chronic bullous disease of childhood: the association with human lymphocyte antigens cw7,B8, DR3 and tumor necrosis factor influences disease expression. Br J Dermatol. 1999;141:867–75. doi: 10.1046/j.1365-2133.1999.03110.x. [DOI] [PubMed] [Google Scholar]
- 15.Mahe A, Flageul B, Bobin P. Bullous IgA linear dermatosis of children in Mali. Ann Dermatol Venereol. 1996;123:544–8. [PubMed] [Google Scholar]
- 16.Bhogal B, Wojnarowska F, Marsden RA, Das A, Black MM, McKee PH. Linear Ig A bullous dermatosis of adults and children: an immunoelectron microscopic study. Br J Dermatol. 1987;117:289–96. doi: 10.1111/j.1365-2133.1987.tb04134.x. [DOI] [PubMed] [Google Scholar]
- 17.Ghohestani RF, Nicolas JF, Kanitakis J, Claudy A. Linear Ig A bullous dermatosis with Ig A antibodies exclusively directed against the 180- or 230–kDa epidermal antigens. J Invest Dermatol. 1997;108:854–8. doi: 10.1111/1523-1747.ep12292581. [DOI] [PubMed] [Google Scholar]
- 18.Egan CA, Zone JJ. Linear Ig A bullous dermatosis. Int J Dermatol. 1999;38:818–27. doi: 10.1046/j.1365-4362.1999.00813.x. [DOI] [PubMed] [Google Scholar]
- 19.Alajlan A, Al-Khawajah M, Al-Sheikh O, Al-Saif F, Al-Rasheed S, Al-Hoqail I, et al. Treatment of linear IgA bullous dermatosis of childhood with flucloxacillin. J Am Acad Dermatol. 2006;54:652–6. doi: 10.1016/j.jaad.2005.11.1102. [DOI] [PubMed] [Google Scholar]


