Abstract
Background:
The metabolic complications and pathologic changes that occur in diabetes mellitus (DM) influence the occurrence of various dermatoses.
Aim:
To study the impact of control of diabetes on the pattern of cutaneous disorders.
Materials and Methods:
A cross-sectional descriptive study of patients attending diabetic clinic in a tertiary care hospital. A total of 500 consecutive patients were studied. Detailed history, clinical examination and relevant investigations were done to diagnose diabetic complications and cutaneous disorders. Dermatoses with or without known pathogenesis were correlated with age, gender, fasting plasma glucose (FPG), duration of diabetes, and complications of DM. Statistical analysis was carried out using Student “t” test and Chi-square test with 5% confidence interval (P value 0.05).
Results:
Majority of patients had well-controlled (FPG<130 mg/ml, 60%) type 2 DM (98.8%). No statistically significant difference (P>0.05) between the patients with or without DM specific cutaneous disorders was noticed with reference to age and gender distribution, duration of DM and FPG. Signs of insulin resistance, acrochordon (26.2%), and acanthosis nigricans (5%) were common, followed by fungal (13.8%) and bacterial (6.8%) infections. Eruptive xanthoma (0.6%), diabetic foot (0.2%), diabetic bulla (0.4%), diabetic dermopathy (0.2%), generalized granuloma annulare (0.2%), and insulin reactions (6.2%) and lipodystrophy (14%) were also seen.
Conclusion:
Well-controlled diabetes decreases the prevalence of DM-specific cutaneous disorders associated with chronic hyperglycemia. It is necessary to have a dermatologist in the diabetic clinic for early detection of potentially grave or predisposing conditions.
Keywords: Cutaneous manifestations, diabetic clinic, diabetes mellitus
Introduction
Diabetes mellitus (DM) is a metabolic disorder affecting various organ systems including skin. Chronic hyperglycemia resulting in production of advanced glycosylated end products (AGE) is responsible for most of the complications of diabetes.[1] One of the main goals of diabetic clinic is good glycemic control. As most of the DM-specific cutaneous disorders are related to the duration of diabetes and chronic hyperglycemia, a study of cutaneous disorders in patients attending diabetic clinic was undertaken to know the impact of control of diabetes on the pattern of these disorders.
Materials and Methods
A cross-sectional descriptive study of 500 consecutive patients attending diabetic clinic was conducted in a tertiary care hospital. Patients who had not registered in diabetic clinic, either attending the dermatology clinic directly or referred from wards, were excluded from the study to ensure the uniformity in diabetes care received in diabetic clinic. Pregnant women with gestational diabetes were also excluded. A detailed history regarding age, gender, duration of DM, type of diabetes, and treatment taken for diabetes was taken. Any associated systemic diseases were also recorded. All the patients were examined thoroughly and screened for any cutaneous disorders. Complete hemogram, urine analysis, fasting and postprandial plasma sugar tests were done in all the patients. Serum creatinine, serum lipid profile, and fundus examination were also done to detect diabetic complications. Relevant microbiologic and histopathologic investigations were done whenever necessary to diagnose the cutaneous disorder. The dermatoses were divided into specific and nonspecific disorders. The former was further divided into four categories: dermatoses with known pathogenesis, dermatoses without known pathogenesis, dermatoses that occur with increased frequency, and dermatoses due to diabetic treatment. The first two categories were correlated with gender, fasting plasma glucose (FPG), duration of diabetes, and complications of diabetes like retinopathy, nephropathy, and peripheral neuropathy.
Statistical analysis was carried out using Student “t” test and Chi-square test with 5% confidence interval (P value 0.05).
Results
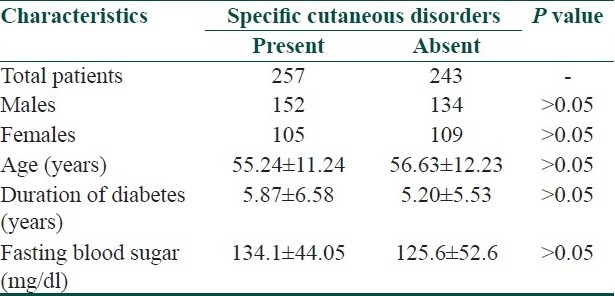
Type 2 DM was present in 98.8% (494/500) of patients. Males and females constituted 57.2% (286/500) and 42.8% (214/500) of cases with mean age of 58.2±11.96 and 53.3±10.78 years, respectively. The mean duration of DM was 5.5±5.8 years. In 82% of patients, the duration of diabetes was <10 years. The mean FPG was 129.97±48.65 mg/dl. In 60% of patients, FPG was <130 mg/dl and it was 130-140 mg/dl in 12% of patients. General characteristics of diabetic patients with or without specific cutaneous disorders are compared in Table 1. Cutaneous disorders specific to diabetes were present in 257 (51.1%) patients. Systemic diseases such as hypertension (1.8%), ischemic heart disease (1%), and stroke (0.4%) were present in 16 (3.2%) patients. Retinopathy was present in 14 (2.8%), nephropathy in 11 (2.2%), and peripheral neuropathy in 268 (53.6%) patients.
Table 1.
General characteristics of diabetic patients with or without specific cutaneous disorders
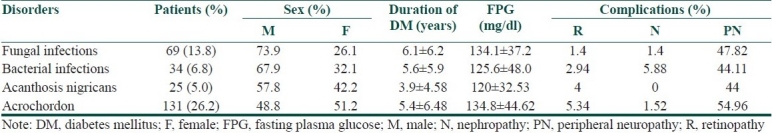
Among specific dermatoses with known pathogenesis, infections and signs of insulin resistance were common [Table 2]. Among the fungal infections, tinea cruris was common (37.68%), followed by tinea corporis (18.84%), pityriasis versicolor (15.94%), candidal intertrigo (13.04%), tinea unguium (11.59%), candidal balanoposthitis (8.69%), candidal vulvovaginitis (4.34%), tinea pedis (1.4%), and tinea barbae (1.4%). Among the bacterial infections, furuncles were present in 70.58%, folliculitis in 17.64%, and ecthyma gangrenosum, erysipelas, carbuncle, and cellulitis each in 2.94% of patients.
Table 2.
Dermatoses with known pathogenesis
Among the specific dermatoses without known pathogenesis, bullous diabeticorum was present in two (0.4%) patients, and generalized granuloma annulare and diabetic dermopathy in one (0.2%) patient each.
Among dermatoses that occur with increased frequency, vitiligo was present in eight (1.6%), lichen planus in nine (1.8%) and psoriasis in three (0.6%) patients.
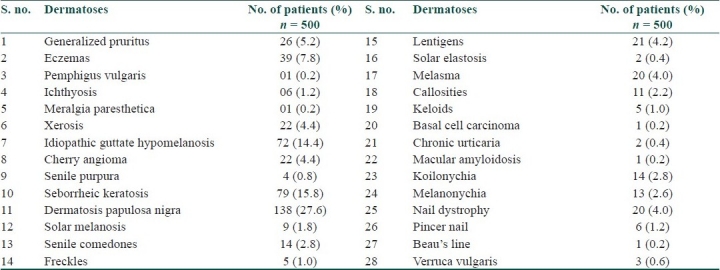
Sixty-four (12.8%) patients were on insulin therapy. Local insulin reaction such as erythema and induration was seen in four (6.2%) patients, and nine (14.0%) patients showed insulin lipodystrophy. Apart from specific cutaneous disorders, various other dermatoses were also present [Table 3].
Table 3.
Nonspecific cutaneous disorders
Discussion
Various cutaneous disorders are related to diabetic complications such as microangiopathy (diabetic dermopathy), glycosylation of proteins (diabetic thick skin), neuropathy (diabetic foot), immunologic dysfunction (infections), insulin resistance (acanthosis nigricans), and abnormal lipid metabolism (xanthoma).[1] Fasting hyperglycemia is a main contributor to the overall diurnal hyperglycemia in poorly controlled diabetes.[2] The results (FPG<130 mg/dl) of the present study showed well-controlled diabetes in the majority of patients attending diabetic clinic. Also, the duration of DM was less than 10 years in the majority of patients. No statistically significant difference (P>0.05) between the patients with or without DM-specific cutaneous disorders was noticed in the present study with particular reference to age and gender distribution, duration of DM, and FPG. This is mainly because the majority of specific skin disorders in the present study were acanthosis nigricans, acrochordon, and uncomplicated infections which are not related to chronic hyperglycemia (discussed in detail below). There was no statistically significant difference between the occurrence of peripheral neuritis and various DM-specific cutaneous disorders. Other diabetic complications were not compared because of less number of patients. Low prevalence of systemic complications in the present study may be due to shorter duration of DM.
Acanthosis nigricans and acrochordon, manifestations of insulin resistance which may be present before the expression of DM, were the predominant dermatoses with known pathogenesis. Increased levels of insulin act on insulin like growth factor (IGF) receptors, resulting in development of acanthosis nigricans.[1] Association between multiple acrochordons and DM has been reported.[3] Acrochordon has been regarded as a sign of impaired glucose tolerance, DM, and increased cardiovascular (atherogenic lipid profile) risk.[4] Infections were the next commonest group of dermatoses in the present study. Cutaneous infections are seen more frequently in type 2 DM.[5] The association between DM and increased susceptibility to infection in general is not supported by strong evidence. However, streptococcal, pseudomonas, and candidal infections are known to occur with increased frequency in DM.[6] There are no conclusive evidences that diabetic patients are susceptible to dermatophytoses[7] or staphylococcal skin infections.[8] However, the patients especially with poorly controlled diabetes are at increased risk of severe and recurrent infections, and complications.[9] As in nondiabetics, rate of exposure to the organisms seems to determine the occurrence of infections in diabetic patients. The predominance of infection in males in the present study may be due to increased likelihood of exposure to the infectious organisms and humid climatic and working conditions.
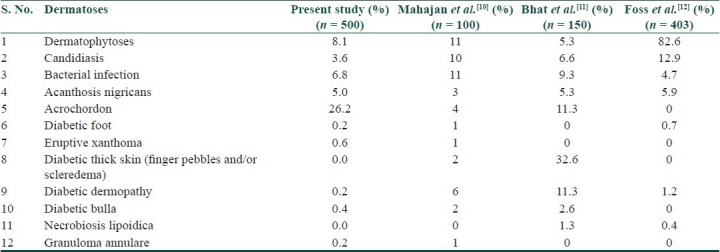
The absence of diabetic thick skin and severe infections, and the lower incidence of specific dermatoses with or without known pathogenesis in the present study when compared to other studies[10–12] [Table 4] is mainly due to difference in the diabetic population (type 1 vs. type 2), well-controlled diabetes, and shorter duration of DM in the majority of patients, and also the source from where patients were drawn, i.e., diabetic clinic. In spite of about half of the patients having peripheral neuritis, only one patient had diabetic foot. This underlines the significance of health education regarding foot care in diabetic patients.
Table 4.
Comparison of specific cutaneous disorders with or without known pathogenesis, with other studies
Certain dermatoses with underlying autoimmune pathogenesis like vitiligo are known to occur in DM as a part of polyglandular autoimmune syndrome.[13] Oral lichen planus has been suggested to occur with increased frequency in DM. However, such an association was not noticed in several other studies,[5] including the present one. An association between psoriasis and increased cardiovascular risk and metabolic syndrome has been reported. While treating psoriasis patients, screening for metabolic syndrome and cardiovascular risk factors is advised.[14]
Insulin reactions may be local (erythema or urticarial pruritic nodules) or systemic (generalized urticaria, rarely anaphylaxis). These reactions occur usually within the first month of therapy. The reactions are due to impurities in insulin preparations such as bovine or porcine proteins, additional peptides, preservatives, and additives.[15] In the present study, no systemic reactions were encountered. Use of highly purified and recombinant insulin reduces the prevalence of insulin reactions.[16] Similarly, lipodystrophy can be prevented by highly purified and recombinant insulin[17] and injection site rotation.[5] Lower prevalence of insulin reactions in the present study may be due to the use of human insulin.
The occurrence of nonspecific cutaneous disorders also has pathogenetic, prognostic, and therapeutic importance in diabetic patients. Autonomic neuropathy or AGE of stratum corneum proteins has been attributed to the pathogenesis of ichthyosis, xerosis, and pruritus.[18] The loss of cutaneous barrier in nonspecific disorders predisposes already susceptible diabetic patients to chronic and recurrent infections. While treating the dermatoses with underlying DM, three issues need to be kept in mind. First one is the duration and modality of therapy. Usually, a prolonged systemic therapy is required because of chronicity and severity of dermatoses. Patients with onychomycosis should be treated aggressively with oral antifungals to avoid devastating secondary bacterial infections.[19] The second point is the interactions between the drugs used to treat dermatoses and oral hypoglycemic agents. Due care should be taken to avoid any major drug interactions. Lastly, the treatment of steroid responsive dermatoses has to be kept in mind. Corticosteroids are known to affect the glycemic control adversely. Therefore, blood glucose levels should be monitored carefully, and accordingly, the dosage schedules of antidiabetic treatment need to be adjusted. Accelerated aging of skin has been reported in patients with both types of DM, especially type 1.[12] However, in the present study, such skin lesions (seborrheic keratosis, senile comedones, senile purpura, solar elastosis, idiopathic guttate hypomelanosis) are mainly due to chronologic and photo aging.
The main goals of diabetic care are good glycemic control, physical exercise, medical nutritional therapy, diabetes self-management education, foot care, and early detection of nephropathy, retinopathy, and metabolic alterations.[20] A good glycemic control definitely reduces the incidence and severity of cutaneous disorders with or without known pathogenesis, as observed in the present study. However, several nonspecific cutaneous disorders that occur in diabetic patients can increase the likelihood of exposure to infectious organisms and contact allergens, resulting in chronic and recurrent infections and eczemas, respectively. These further worsen the diabetes control of the patients. Long-term effects of DM on the microcirculation and on dermal collagen eventually result in skin disorders in almost all the diabetic patients. Thus, dermatologists play an important role in reducing the dermatologic morbidity, improvement of quality of life, and management strategy of diabetic patients. It is necessary to have a dermatologist in the diabetic clinic along with other specialists for early detection of potentially grave or predisposing conditions and to provide a comprehensive diabetic care to the patients. Further prospective studies are required to ascertain the role of dermatologists in diabetic clinic.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Kalus AA, Chien AJ, Olerud JE. Diabetes mellitus and other endocrinal disorders. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Lefell DJ, editors. Fitzpatrick's Dermatology in General Medicine. 7th ed. Newyork: McGraw Hill Medical; 2008. pp. 1461–84. [Google Scholar]
- 2.Monnier L, Lapinski H, Collette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetes patients. Diabetes Care. 2003;26:881–5. doi: 10.2337/diacare.26.3.881. [DOI] [PubMed] [Google Scholar]
- 3.Kahana M, Grossman E, Feinstein A, Ronnen M, Cohen M, Millet MS. Skin tags: A cutaneous marker for diabetes mellitus. Acta Derm Venereol. 1987;67:175–7. [PubMed] [Google Scholar]
- 4.Crook MA. Skin tags and atherogenic lipid profile. J Clin Pathol. 2000;53:873–4. doi: 10.1136/jcp.53.11.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferringer T, Miller F. Cutaneous manifestations of diabetes mellitus. Dermatol Clin. 2002;20:483–92. doi: 10.1016/s0733-8635(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 6.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341:1906–12. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 7.Lugo-Somolinos A, Sanchez JL. Prevalence of dermatophytosis in patients with diabetes. J Am Acad Dermatol. 1992;26:408–10. doi: 10.1016/0190-9622(92)70063-l. [DOI] [PubMed] [Google Scholar]
- 8.Breen JD, Karchmer AW. Staphylococcus aureus infection in diabetic patients. Infec Dis Clin North Am. 1995;9:11–24. [PubMed] [Google Scholar]
- 9.Jorizzo JL. Diabetes mellitus. In: Callen JP, Jorizzo JL, editors. Dermatological signs of internal disease. 3rd ed. Philadelphia: Saunders; 2003. pp. 169–74. [Google Scholar]
- 10.Mahajan S, Koranne RV, Sharma SK. Cutaneous manifestation of diabetes mellitus. Indian J Dermatol Venereol Leprol. 2003;69:105–08. [PubMed] [Google Scholar]
- 11.Bhat Y, Gupta V, Kudyar RP. Cutaneous manifestations of diabetes mellitus. Int J Diab Dev Ctries. 2006;26:152–5. [Google Scholar]
- 12.Foss NT, Polon DP, Takada MH, Fross-Freitas MC, Foss MC. Skin lesions in diabetic patients. Rev Saude Publica. 2005;39:1–5. doi: 10.1590/s0034-89102005000400024. [DOI] [PubMed] [Google Scholar]
- 13.Dittmar M, Kahaly GJ. Polyglandular autoimmune syndromes: Immunogenetics and long-term follow up. J Clin Endocrinol Metab. 2003;88:2983–92. doi: 10.1210/jc.2002-021845. [DOI] [PubMed] [Google Scholar]
- 14.Wakee M, Thio HB, Prens EP, Sijbrands EJ, Neumann HA. Unfavorable cardiovascular risk profiles in untreated and treated psoriasis patients. Atherosclerosis. 2007;190:1–9. doi: 10.1016/j.atherosclerosis.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Huntley AC. The cutaneous manifestations of diabetes mellitus. J Am Acad Dermatol. 1982;7:427–55. doi: 10.1016/s0190-9622(82)80248-x. [DOI] [PubMed] [Google Scholar]
- 16.Paron NG, Lambert PW. Cutaneous manifestations of diabetes mellitus. Prim Care. 2000;27:371–83. doi: 10.1016/s0095-4543(05)70201-3. [DOI] [PubMed] [Google Scholar]
- 17.Sibbald RG, Landolt SJ, Toth D. Skin and diabetes. Endocrinol Metab Clin North Am. 1996;25:463–72. doi: 10.1016/s0889-8529(05)70334-0. [DOI] [PubMed] [Google Scholar]
- 18.Yosipovitch G, Hodak E, Vardi P, Shraga I, Karp M, Sprecher E, et al. The prevalence of cutaneous manifestations in IDDM patients and their association with diabetes risk factors and microvascular complications. Diabetes Care. 1998;21:506–9. doi: 10.2337/diacare.21.4.506. [DOI] [PubMed] [Google Scholar]
- 19.Gupta AK, Humke S. The prevalence and management of onychomycosis in diabetic patients. Eur J Dermatol. 2000;10:379–84. [PubMed] [Google Scholar]
- 20.American Diabetes Association. Standards of medical care in diabetes-2007. Diabetes Care. 2007;30:S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]


