Abstract
Background:
The incidence of uncomplicated psoriasis is 1–3% in the general population. The involvement of palm and sole is seen in 7–14.5% of cases. There are different topicals and systemic therapies available for treating the case of psoriasis but none is satisfactory for longer duration.
Aim:
The study involved the comparative therapeutic evaluation of the different topical regimens and narrow band ultraviolet B (NB-UVB) therapy in combination with systemic methotrexate.
Materials and Methods:
The study was held in out-patient department of Skin, VD and Leprosy of B.R.D. Medical College, Gorakhpur, from July 2007 to December 2008. The group included 98 new cases of palmoplantar psoriasis. These cases were divided into eight groups according to the eight regimens involved in the study. The severity of psoriasis was assessed by the ESIF (erythema, scaling, induration and fissuring) score.
Results:
The study showed that all the regimens had significant response rates. The combination of NB-UVB with systemic methotrexate had maximum response rate (64.85±4.52%) that was statistically significant (paired “t” at 16d.f. = 33.329, P<0.001) with minimum number of recurrences after stopping the treatment. The combination of halobetasol ointment with systemic methotrexate also had significant response rate (paired “t” at 19d.f. = 13.5183, P<0.001) but had maximum number of cases with recurrence (70%) after stopping the treatment.
Conclusion:
These results suggest that the combination of every regimen with systemic methotrexate resulted in an early and a good improvement in the quality of life of patients suffering from psoriasis. It also shows that NB-UVB in combination with systemic methotrexate is more efficacious and has minimum recurrence rate and side effects in the treatment of palmoplantar psoriasis.
Keywords: Methotrexate, narrow band ultraviolet B, palmoplantar psoriasis, tacrolimus, tazarotene
Introduction
Psoriasis is a common inflammatory disease of the skin and joints. Its cause remains unknown; however, it has been linked to complex interactions between predisposing genes and the environment. The pathophysiology of psoriasis is characterized by epidermal hyperproliferation, enhanced antigen presentation, helper T cell (Th1) and Th17 cytokine production, T cell expansion and angiogenesis.
The therapeutic paradigm for treating psoriasis with different regimens including topical agents, phototherapy and systemic agents continues to evolve. Recent consensus statements suggest that indications for systemic agents or phototherapy are psoriasis affecting greater than or equal to 5% body surface area, psoriasis affecting vulnerable areas (e.g. palmer-plantar), psoriasis with concomitant psoriatic arthritis, pustular, erythrodermic and guttate variants and psoriasis unresponsive to topical medications in which significant physical, social or emotional impairments are involved.[1] Traditional therapies include topical agents (salicylic acid, coal tar, dithranol, steroid, tazarotene, tacrolimus, vitamin D derivatives), phototherapy [PUVA, psoralen-ultraviolet A, narrow band ultraviolet B (NB-UVB) and laser] and systemic agents (methotrexate, acitretin, cyclosporine and biologics).
Aims and objectives
To evaluate the efficacy of various regimens
To assess the various side effects of different regimens
To assess the recurrences after stopping the treatment
To compare the efficacy and acceptability of various regimens.
Materials and Methods
The study was carried out in a series of patients with psoriasis who attended dermatology out-patient department of Nehru Hospital, B.R.D. Medical College, Gorakhpur, from July 2007 to December 2008.
Ninety-eight patients diagnosed clinically as palmoplantar psoriasis were taken for comparative evaluation of eight different regimens, after obtaining informed consent from them. None of the patients were on any systemic or topical antipsoriatic therapy for at least 1 month prior to the study, except for a bland emollient. Women were included only if they were willing to avoid conception during and for 4 months subsequently to the conclusion of the study.
The patients were divided into eight groups randomly for eight different regimens. The total duration of treatment for each regimen was 8 weeks. Patients were examined once weekly for the initial 1 month and subsequently every 2 weeks. Patients were followed for up to 5 months without any treatment.
Pregnant, lactating, hypertensive, diabetic, active pulmonary tuberculosis patients and patients having contraindication to any one of regimens were not included in the study.
These cases were divided into eight groups randomly:
Group I (11 patients): Regimen I (salicylic acid 6% ointment daily + methotrexate 7.5 mg PO weekly)
Group II (4 patients): Regimen II (topical tacrolimus 0.1% + methotrexate 7.5 mg PO weekly)
Group III (17 patients): Regimen III (crude coal tar ointment + methotrexate 7.5 mg PO weekly)
Group IV (9 patients): Regimen IV (topical tazarotene 0.1% + methotrexate 7.5 mg PO weekly)
Group V (20 patients): Regimen V (halobetasol propionate 0.05% ointment + methotrexate 7.5 mg PO weekly)
Group VI (12 patients): Regimen VI (NB-UVB phototherapy)
Group VII (17 patients): Regimen VII (NB-UVB phototherapy + methotrexate 7.5 mg PO weekly)
Group VIII (8 patients): Regimen VIII (bland emollient + methotrexate 7.5 mg PO weekly)
The initial dose of NB-UVB was 280 mJ/cm2, which was delivered by the whole body NB-UVB chamber. NB-UVB therapy was given on every alternate day, i.e. 3 times in a week up to 8 weeks (24 settings) and 20 mJ/cm2 increment in NB-UVB dose was done in every setting. Thus, the total cumulative dose of NB-UVB after 8 weeks was 12.24 J/cm2.
The lesions were assessed for the degree of erythema, scaling, induration and fissuring (ESIF) and were scored on a severity scale of 0–3 (0 clear, 1 mild, 2 moderate and 3 severe). The most severe condition was given 12 points. The percentage of overall improvement was calculated by deducting the sum of the clinical scores after therapy from the sum of the pretreatment scores and dividing it by pretreatment clinical scores categorized as follows:
Minimal improvement up to 25%
Moderate improvement 26–50%
Marked improvement 51–75%
Total/near total clearance >75%.
The patients were considered to have good improvement when clinical improvement was more than 50% and any improvement less than that was considered as poor response.
Evaluation of response to treatment, laboratory investigations and side effects, if any, were recorded at regular intervals.
At the end of study, the data were tabulated and statistical analysis was done to know the level of significance.
Results and Discussion
Psoriasis usually has a chronic course with periods of remission and exacerbation. Treatment depends upon age, sex, occupation, personality, general health, type extent, and duration. It should never be more unpleasant, intolerable or dangerous than the disease.
Regimen I (salicylic acid 6% ointment daily + methotrexate 7.5 mg PO weekly)
Salicylic acid is commonly used in psoriasis as a 2-10% ointment applied twice daily over thick lesions. It is keratoplastic when used in less than 2% concentration, whereas above 3% it is keratolytic. It is best avoided for unstable psoriasis and exfoliative psoriasis. Systemic absorption of salicylic acid can result in salicylism and even death.
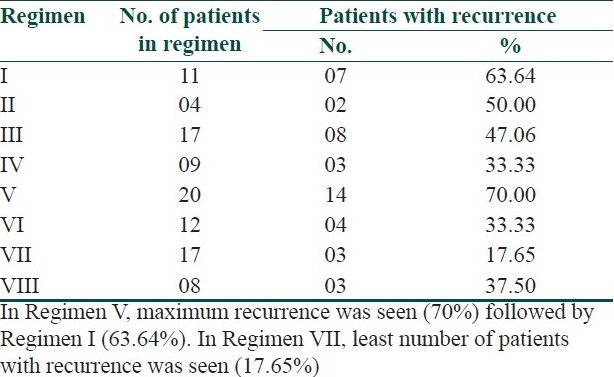
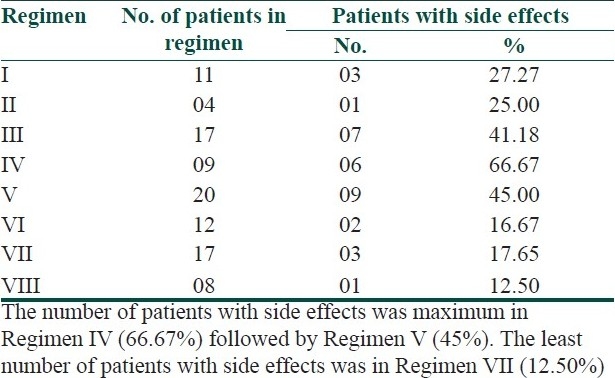
In the present study, 90.91% (10 out of 11) patients with palmer lesions and 80% (8 out of 10) patients with plantar lesions showed marked improvement with Regimen I [Table 1]. The P value was <0.001 which was most highly significant. In this regimen, 63.64% (7 out of 11) patients developed the recurrence after stopping the treatment and 27.27% (3 out of 11) patients had side effects in the form of irritation and burning [Table 2].
Table 1.
Comparative study of different regimens in the treatment of palmoplantar psoriasis
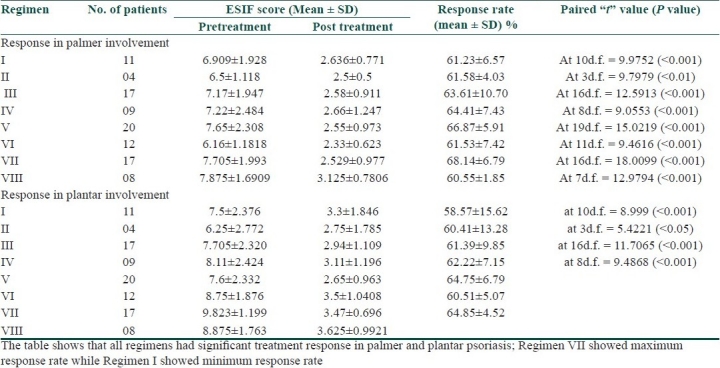
Table 2.
Recurrences seen in different regimens after stopping the treatment
Regimen II (topical tacrolimus 0.1% + methotrexate 7.5 mg PO weekly)
The calcineurin inhibitors, tacrolimus and pimacrolimus, have both been assessed in the treatment of psoriasis. Both are approved for the topical treatment of psoriasis and, unlike corticosteroids, do not cause skin atrophy. Unfortunately, their efficacy in the treatment of psoriasis is limited unless they are used under occlusion or on the thinner skin of the face, intertriginous areas or genitals.[2,3]
In our study, tacrolimus 0.1% ointment was combined with oral methotrexate 7.5 mg weekly to treat the patients of palmoplantar psoriasis. In the regimen, all patients with palmer involvement showed marked improvement with average response rate 61.58±4.03% (P<0.01), while 75% (3 out of 4) patients with plantar involvement had marked improvement with average response rate 60.14±13.28% (P<0.05). In this regimen, 50% (2 out of 4) patients developed the recurrence after stopping the treatment, while one patient (25%) developed side effects in the form of irritation and burning [Table 3]. The number of patients in this regimen was less at the end of 8 weeks trial due to cost effect, and thus, this regimen is not affordable by most of the patients.
Table 3.
Number of patients with side effects seen in different regimens
Regimen III (crude coal tar ointment + methotrexate 7.5 mg PO weekly)
Crude coal tar which contains 48% hydrocarbons, 42% carbon and 10% water, is composed of about 10,000 compounds. Goeckerman used a combination of tar with ultraviolet radiation in the treatment of psoriasis.[4] Prasad et al. did a retrospective analysis in which 35 patients admitted with psoriasis were analyzed and 16 patients received methotrexate in a weekly oral schedule (15 mg/week). After 4 weeks of therapy, there was total clearance in 52.6% of the patients with combination therapy, whereas 12.5% of the patients with conventional therapy achieved this.[5]
In the present study, coal tar ointment was given in combination with methotrexate 7.5 mg PO weekly to 17 patients of palmoplantar psoriasis. In this series, 11.76% (2 out of 17) patients had near total clearing and 70.58% (12 out of 17) patients had marked improvement in palmer involvement. In plantar lesion, 5.88% (1 out of 17) patients had near total clearing and 76.47% (13 out of 17) patients had marked improvement. The average response rate was 63.61±10.7% (P<0.001) for palms and 61.39±9.85% (P<0.001) for soles. Eight patients (47.06%) showed recurrence of disease after stopping the treatment. Seven patients (41.18%) developed side effects in the form of irritation, burning, staining of clothes and contact dermatitis due to two times application in a day.
Regimen IV (topical tazarotene 0.1% + methotrexate 7.5 mg PO weekly)
Tazarotene is the only retinoid approved for the topical treatment of plaque psoriasis. Tazarotene restores normal epidermal proliferation and differentiation and reduces epidermal inflammation.[6] It is neither sensitizing nor phototoxic, but dose-related skin irritation is common and often necessitates combination with topical steroid, applied at the same or different time of the day, to decrease irritation and improve overall efficacy.[7,8]
In our study, tazarotene 0.1% gel was combined with oral methotrexate 7.5 mg weekly. The regimen showed marked improvement in 88.88% (8 out of 9) patients with both palmer and plantar lesions. The average response rate was 64.41±7.43% (P<0.001) for palms and 62.22±7.15% (P<0.001) for soles. Three patients (33.33%) showed recurrence of disease after stopping the treatment. Six patients (66.67%) developed side effects in the form of irritation, burning, erythema and exfoliation of skin which was assessed by recent change and by ruling out the other precipitating factors.
Regimen V (halobetasol propionate 0.05% ointment + methotrexate 7.5 mg PO weekly)
Corticosteroids remain among the first-line agents in the topical treatment of psoriasis in all age groups. They have anti-inflammatory and antiproliferative properties, and when applied to psoriatic patients, they reduce erythema, scaling and pruritus.[9] They range in potency from very weak class VII agents to super potent class I agents as ranked by the Stoughton-Cornell vasoconstriction classification.[10] A double blind, parallel group, multicenter trial revealed that 0.05% halobetasol propionate ointment is more effective in the treatment of patients with chronic localized psoriasis compared to 0.05% clobetasole propionate ointment (86% versus 70%).[11]
In the present study, halobetasol propionate 0.05% ointment was combined with oral methotrexate 7.5 mg weekly. In this series, both palmer and plantar involvement showed marked improvement in 90% (18 out of 20) patients. The average response rate was 66.87±5.91% (P<0.001) for palms and 64.75±6.79% (P<0.001) for soles. In this regimen, 70% (14 out of 20) patients developed recurrence of lesion after stopping the treatment. Nine patients (45%) developed side effects in the form of atrophy of skin, pustular psoriasis, acneiform eruption and Cushingoid facies during treatment due to ultrapotent nature and long duration of application of the topical steroid.
Regimen VI (NB-UVB phototherapy)
Natural sunlight has been used for centuries in the treatment of psoriasis. The most effective wavelength of UVR for psoriasis is in the range of 311–313 nm (NB-UVB).[12] Treatment with NB-UVB rapidly depletes infiltrating T cells from psoriatic plaques and result in faster clearance, less erythema and longer remission.[13] Topical therapies, such as calcipotriene, tazarotene and anthralin, combined with NB-UVB will enhance the efficacy of both modalities and decrease the overall exposure to UV radiation.[14–16] The combination of acitretin with NB-UVB (Re-NB-UVB) appears to have synergistic effects and may decrease the time to clearance and the overall exposure to both modalities.[17,18] The acute dose-dependent side effects of NB-UVB are erythema, burning, pigmentation and rare transient lesional blistering.[19,20]
In the present study, NB-UVB was tried on 12 patients with palmoplantar psoriasis. The regimen achieved marked improvement in 91.66% of patients with both palmer and plantar lesions. The average response rate was 61.53±7.42% (P<0.001) for palms and 60.51±5.07% (P<0.001) for soles. Four patients out of 12 (33.33%) came with recurrence of lesions after stopping the treatment. Two patients (16.67%) had side effects in the form of erythema and stinging sensation on their exposed body part (face and fore arms) due to whole body NB-UVB chamber.
Regimen VII (NB-UVB phototherapy + methotrexate 7.5 mg PO weekly)
Methotrexate has been used for psoriasis since the 1950s and remains the most widely prescribed drug for severe psoriasis worldwide. It is a folic acid analogue that reversibly inhibits dihydrofolate reductase, resulting in interference with DNA synthesis and effects on T cells.[21] Methotrexate is usually prescribed orally in a once weekly single dose or three divided dose schedule over 24 hours, after a 2.5–5 mg test dose, in dosages ranging from 7.5 to 22.5 mg per week, pending clinical response. Oral folic acid 1–5 mg daily is added to prevent stomatitis and macrocytic anemia and to decrease gastrointestinal symptoms such as nausea, vomiting and anorexia; however, it may reduce the efficacy of methotrexate.[22] Methotrexate can be used in a sequential regimen with UVB (broad band or narrow band) in an effort to decrease total overall dose and potential toxicity.
Methotrexate has the potential for severe side effects, necessitating careful selection and monitoring of patients. Methotrexate, because of its teratogenicity, is absolutely contraindicated during pregnancy, and pregnancy should be avoided for at least 3 months after discontinuing treatment.[23] Methotrexate, on long-term use in psoriatic patients causes liver fibrosis and cirrhosis. Current guidelines recommend a liver biopsy after a cumulative methotrexate dose of 1.5 g and then after every subsequent 1.5 g.[24] Recently, Chalmers and colleagues reported that the measurement of type III procollagen aminopeptide in serum can be used to detect liver fibrosis or cirrhosis and reduce the need for liver biopsy.[25]
In a recent clinical trial, only oral methotrexate was given to 25 patients with moderate to severe psoriasis. An interim analysis showed that 15 patients completed 24 weeks of therapy with a mean methotrexate dose of 19 mg weekly. The mean improvement at 24 weeks was approximately 50% and 10 of 15 patients (67%) achieved 50% response.[26]
There was also a clinical trial that showed effectiveness of methotrexate in combination with NB-UVB phototherapy in which 24 patients with plaque type psoriasis participated; 19 patients completed the trial. Ten out of 11 patients who received methotrexate + NB-UVB achieved clearance of psoriasis lesions compared with 5 of 13 patients treated with NBUVB alone. The median time to clear was 4 weeks in the methotrexate group, which was significantly sooner than the UVB-only group (P<0.0001). More than half of the patients in the UVB-only group still had lesions at the end of the 24 weeks study.[27]
In the present study, oral methotrexate 7.5 mg weekly was combined with NB-UVB therapy to treat the 17 patients of palmoplantar psoriasis. In this regimen, all patients showed marked improvement in both palmer and plantar lesions. The average response rate was 68.14±6.79% (P<0.001) for palms and 64.85±4.52% (P<0.001) for soles. Minimum number of patients with recurrence (three patients) was seen in this regimen. Three patients (17.65%) had developed side effects like erythema and irritation on their exposed body part.
Regimen VIII (bland emollient + methotrexate 7.5 mg PO weekly)
Liberal application of bland emollients is often effective for mild psoriasis and always plays an adjunctive role, particularly in winter when low humidity and lack of sunlight initiate predictable flares. Emollients are antipruritic, mildly desquamative, relatively inexpensive and well tolerated by almost all patients.[9]
In the present study, emollient cream was applied on the lesion of palmoplantar psoriasis in combination with oral methotrexate 7.5 mg weekly. This regimen was tried on eight patients. In this regimen, all the patients had marked improvement. The average response rate was 60.55±1.85% (P<0.001) for palms and 59.74±4.20 (P<0.001) for soles. In three patients (37.5%), recurrence had been recorded after stopping the treatment. One patient (12.5%) had developed side effects in the form of irritation.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Pariser DM, Bagel J, Gelfand JM, Korman NJ, Ritchlin CT, Strober BE, et al. National Psoriasis Foundation Clinical consensus on disease severity. Arch dermatol. 2007;143:239–42. doi: 10.1001/archderm.143.2.239. [DOI] [PubMed] [Google Scholar]
- 2.Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359–62. doi: 10.1111/1523-1747.ep12520022. [DOI] [PubMed] [Google Scholar]
- 3.Stern RS. Genital tumors among men with psoriasis exposed to radiation.The photochemotherapy follow-up study. N Engel J Med. 1990;322:1093–7. doi: 10.1056/NEJM199004193221601. [DOI] [PubMed] [Google Scholar]
- 4.Goeckerman WH. Treatment of psoriasis. Arch Dermatol Syphilol. 1931;24:446–50. [Google Scholar]
- 5.Prasad P, Felix J. Topical coal tar alone and in combination oral methotrexate in management of psoriasis. Indian J Dermatol Venereol Leprol. 1997;63:26–8. [PubMed] [Google Scholar]
- 6.Esgleyes-Ribot T, Chandraratna RA, Lew-Kaya DA, Sefton J, Duvic M. Response of psoriasis to a new topical retinoid, AGN 190168. J Am Acad Dermatol Apr. 1994;30:581–90. doi: 10.1016/s0190-9622(94)70066-4. [DOI] [PubMed] [Google Scholar]
- 7.Marks R. Early clinical development of tazarotene.Br. J Dermatol. 1996;135:26–31. doi: 10.1111/j.1365-2133.1996.tb15663.x. [DOI] [PubMed] [Google Scholar]
- 8.Lebwohl MG, Breneman DL, Goffe BS, Grossman JR, Ling MR, Milbauer J, et al. Tazarotene 0.1% gel plus corticosteroid cream in the treatment of plaque psoriasis. J Am Acad Dermatol Apr. 1994;30:581–90. doi: 10.1016/s0190-9622(98)70008-8. [DOI] [PubMed] [Google Scholar]
- 9.Leman J, Burden D. Psoriasis in children: A guide to its diagnosis and management. Paediater Drugs. 2001;3:673–80. doi: 10.2165/00128072-200103090-00005. [DOI] [PubMed] [Google Scholar]
- 10.Cornell RC, Stroughton RB. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch Dermatol. 1985;121:63–7. [PubMed] [Google Scholar]
- 11.Goldberg B, Hartdegen R, Presbury D, Smith EH, Yawalkar S. A double blind multicenter comparision of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate in patients with chronic localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145–8. doi: 10.1016/0190-9622(91)70313-q. [DOI] [PubMed] [Google Scholar]
- 12.Coven TR, Burack LH, Gilleaudeau R, Keogh M, Ozawa M, Krueger JG. NB-UVB produces superior clinical and histopathological resolution of moderate to severe psoriasis in patients compared with BB-UVB. Arch Dermatol. 1997;133:1514–22. [PubMed] [Google Scholar]
- 13.Jain VK, Aggarwal K, Jain K, Bansal A. NB-UVB phototherapy in childhood psoriasis. Int J Dermatol. 2007;46:320–2. doi: 10.1111/j.1365-4632.2007.03148.x. [DOI] [PubMed] [Google Scholar]
- 14.Woo WK, Mc Kenna KE. Combination TL01 UVB phototherapy and topical calcipotriol for psoriasis: A prospective randomized placebo controlled clinical trial. Br J Dermatol. 2003;1491:46–50. doi: 10.1046/j.1365-2133.2003.05380.x. [DOI] [PubMed] [Google Scholar]
- 15.Behrens S, Grundmann-Kollmann M, Schiener R, Peter RU, Kerscher M. Combination phototherapy of psoriasis with NB-UVB irradiation and topical tazarotene gel. J Am Acad Dermatol. 2000;42:493–5. doi: 10.1016/s0190-9622(00)90225-1. [DOI] [PubMed] [Google Scholar]
- 16.Carrozza P, Häusermann P, Nestle FO, Burg G, Böni R. Clinical efficacy of NB-UVB(311nm) combined with dithranol in psoriasis.An open pilot study. Dermatology. 2000;200:35–9. doi: 10.1159/000018312. [DOI] [PubMed] [Google Scholar]
- 17.Lebwohl M, Ali S. Treatment of psoriasis. Part 2. Systemic Therapies. J Am Acad Dermatol. 2001;45:649–61. doi: 10.1067/mjd.2001.117047. [DOI] [PubMed] [Google Scholar]
- 18.Spuls PI, Rozenblit M, Lebwohl M. Retrospective study of the efficacy of NB-UVB and acitretin. J Dermatology Treat. 2003;14:7–20. doi: 10.1080/jdt.14.s2.17.20. [DOI] [PubMed] [Google Scholar]
- 19.Calzavara-Pinton PG, Zane C, Candiago E, Facchetti F. Blisters on psoriatic lesions treated with TL01 lamps. Dermatology. 2000;200:115–9. doi: 10.1159/000018342. [DOI] [PubMed] [Google Scholar]
- 20.George SA, Ferguson J. Lesional blistering following NB-UVB (TL01) phototherapy for psoriasis: A report of four cases. Br J Dermatol. 1992;127:445–6. doi: 10.1111/j.1365-2133.1992.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 21.Cronstein BN. The mechanism of action of methotrexate. Rheum Dis Clin North Am. 1997;23:739–55. doi: 10.1016/s0889-857x(05)70358-6. [DOI] [PubMed] [Google Scholar]
- 22.Salim A, Tan E, Ilchyshyn A, Berth-Jones J. Folic acid supplementation during treatment of psoriasis with methotrexate: A randomized, double blind, placebo controlled trial. Br J Dermatol. 2006;154:169–74. doi: 10.1111/j.1365-2133.2006.07289.x. [DOI] [PubMed] [Google Scholar]
- 23.Bawle EV, Conard JV, Wiess L. Adult and two children with fetal methotrexate syndrome. Teratology. 1998;57:51–5. doi: 10.1002/(SICI)1096-9926(199802)57:2<51::AID-TERA2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 24.Roenigk HH, Jr, Auerbach R, Maibach H, Weinstein G, Lebwohl M. Methotrexate in psoriasis: Consensus conference. J Am Acad Dermatol. 1998;38:478–85. doi: 10.1016/s0190-9622(98)70508-0. [DOI] [PubMed] [Google Scholar]
- 25.Chalmers RJ, Kirby B, Smith A, Burrows P, Little R, Horan M, et al. Replacement of routine liver biopsy by procollagen III aminopeptide for monitoring patients with psoriasis receiving long term mrthotrexate: A multicenter audit and health economic analysis. Br J Dermatol. 2005;152:444–50. doi: 10.1111/j.1365-2133.2005.06422.x. [DOI] [PubMed] [Google Scholar]
- 26.Callis KP, Chadha A, Vaishnaw A, Krueger GG. Reduction of CD45RO+ effector T lymphocytes is not observed in the treatment of psoriasis with methotrexate. J Invest Dermatol. 2002 Abstract 220. [Google Scholar]
- 27.Kaur I, Dogra S, De D, Kanwar AJ. Systemic methotrexate treatment in childhood psoriasis; further experience in 24 children from india. Pediatr Dermatol. 2008;25:184–8. doi: 10.1111/j.1525-1470.2008.00629.x. [DOI] [PubMed] [Google Scholar]


