Abstract
Accumulation of α-synuclein in neurons of the central and peripheral nervous system is a hallmark of sporadic Parkinson’s disease (PD) and mutations that increase α-synuclein levels cause familial PD. Transgenic mice overexpressing α-synuclein under the Thy1 promoter (Thy1-aSyn) have high levels of α-synuclein expression throughout the brain but no loss of nigrostriatal dopamine neurons up to 8 months, suggesting that they may be useful to model pre-clinical stages of PD. Olfactory dysfunction often precedes the onset of the cardinal motor symptoms of PD by several years and includes deficits in odor detection, discrimination and identification. In the present study, we measured olfactory function in 3- and 9-month-old male Thy1-aSyn mice with a buried pellet test based on latency to find an exposed or hidden odorant, a block test based on exposure to self and non-self odors, and a habituation/dishabituation test based on exposure to non-social odors. In a separate group of mice, α-synuclein immunoreactivity was assessed in the olfactory bulb. Compared with wildtype littermates, Thy1-aSyn mice could still detect and habituate to odors but showed olfactory impairments in aspects of all three testing paradigms. Thy1-aSyn mice also displayed proteinase K-resistant α-synuclein inclusions throughout the olfactory bulb. These data indicate that overexpression of α-synuclein is sufficient to cause olfactory deficits in mice similar to that observed in patients with PD. Furthermore, the buried pellet and block tests provided sufficient power for the detection of a 50% drug effect, indicating their usefulness for testing novel neuroprotective therapies.
Keywords: behavior, movement, odor, olfaction, Parkinson’s disease
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder primarily characterized by motor impairments due to a loss of nigrostriatal dopaminergic neurons. Mutations that cause familial forms of PD point to new pathophysiological mechanisms that can be targeted for neuroprotection but a major issue remains the detection of PD at an early stage, when neuroprotective therapies may be most effective. Among other non-motor symptoms, a variable degree of olfactory impairment, including deficits in odor identification, detection and discrimination, is found in 70–95% of patients with PD (Ward et al., 1983; Doty et al., 1988, 1992; Tissingh et al., 2001). Importantly, the deficits can precede the cardinal motor symptoms of PD by up to 4 years and relatives of patients with PD with olfactory deficits are at greater risk of developing the disease, suggesting that these olfactory symptoms may signal early stages of PD (Montgomery et al., 1999; Berendse et al., 2001; Ponsen et al., 2004; Langston, 2006; Ross et al., 2008). This underscores the need to understand the mechanism of the deficits and their potential value in assessing neuroprotective therapies. However, this research has been hindered by the lack of an animal model of PD-related olfactory deficits.
Abnormal accumulation of α-synuclein is a pathological hallmark of sporadic PD and its potential role in the development of the disease is strongly supported by genetic evidence that mutations in α-synuclein or multiplication of the α-synuclein gene lead to familial PD (Chesselet, 2007). Mice overexpressing wildtype (WT) human α-synuclein under the Thy1 promoter (Thy1-aSyn) have high levels of α-synuclein and proteinase K-resistant inclusions containing α-synuclein in many brain regions, including the substantia nigra and locus coeruleus (Rockenstein et al., 2002; Fernagut et al., 2007). At 2–8 months of age, these mice develop mild sensorimotor impairments but these are not L-dopa responsive and are not related to nigrostriatal cell loss, suggesting that at this age the mice may model a pre-parkinsonism (i.e. ‘pre-manifest’) phase of PD (Fleming et al., 2004, 2006; Fernagut et al., 2007). Indeed, we have preliminary evidence that they exhibit digestive and autonomic dysfunction reminiscent of that observed in early or pre-manifest PD (Pfeiffer, 2003; Fleming et al., 2007; Goldstein et al., 2007; Wang et al., 2008).
In the present study, we assessed olfactory function in male Thy1-aSyn and WT littermates using multiple olfactory tests designed to detect graded differences in olfactory abilities. Heterozygous male transgenic mice and WT littermates were assessed in: (i) a buried food test compared with surface food exploration; (ii) a modified block test (Spinetta et al., 2005, 2006; Tillerson et al., 2006) to evaluate sensitivity to social smells, an ethologically essential ability in mice; and (iii) an olfactory habituation/dishabituation test employing novel non-social scents. The olfactory bulb was examined for the presence of α-synuclein aggregates. A power analysis was performed on each olfactory test in order to identify which tests may be optimal for future pre-clinical drug testing.
Materials and methods
Animal care was conducted in accordance with the United States Public Health Service Guide for the Care and Use of Laboratory Animals, and procedures were approved by the Institutional Animal Care and Use Committee at the University of California Los Angeles. Thy1-aSyn mice were created previously and crossed into a hybrid C57BL/6-DBA/2 background (Rockenstein et al., 2002). Animals were maintained on the hybrid C57BL/6-DBA/2 background by mating N5 females hemizygous for the transgene with male WT mice on the hybrid background obtained from Charles River Laboratories, Inc. (Wilmington, MA, USA) (Fleming et al., 2004, 2006). Male and female mice from the same litters were never bred together. Litter sizes ranged from four to 11 mice per litter. The genotype of all Thy1-aSyn and WT mice was verified with polymerase chain reaction amplification analysis of tail DNA. Animals were maintained on a reverse light/dark cycle with lights off at 10:00 h and all testing was performed between 12:00 and 16:00 h during the dark cycle under low light. Food and water were available ad libitum except during testing.
Only male mice were used in the study. Hemizygous Thy1-aSyn and WT littermate mice from a total of 18 litters were tested on three different olfactory tests including the buried pellet, block and habituation/dishabituation tests. At 3–4 months of age, Thy1-aSyn (n = 35) and WT (n = 36) mice (18 litters) were tested on the block test and a subgroup of the same mice (from 12 litters) was tested on the habituation/dishabituation test (Thy1-aSyn, n = 22; WT, n = 24). A subset of Thy1-aSyn (n = 7) and WT (n = 16) mice (from five litters) were tested on the buried pellet test at 5–6 months of age. Another subset of Thy1-aSyn (n = 10) and WT (n = 7) mice (from seven litters) was aged to 9 months and then tested again on the block and habituation/dishabituation tests. At 11 months of age the remaining mice from the 9 month cohort (Thy1-aSyn, n = 5; WT, n = 7) were tested on the 24 h block test.
Buried pellet test
The buried pellet test was performed as previously described (Crawley, 2000; Nathan et al., 2004). Individually housed mice were food restricted and maintained at ~90% body weight for 2 days prior to and during testing. Food-restricted mice were given 3–4 g of mouse chow per animal per day depending on weight. Weights were monitored each day during food restriction. Buried pellet testing was performed for 5 days and the surface pellet control test was performed for one trial 1 day after the buried pellet. For the buried pellet test, a clean mouse cage (15 × 25 × 13 cm) was filled with 3 cm of clean bedding. One piece of sweetened cereal (~250 mg; Cap’n Crunch®; Quaker, Chicago, IL, USA) was buried along the perimeter of the cage approximately 0.5 cm below the bedding so that it was not visible. A mouse was then placed in the centre of the cage and the latency to dig up and begin to eat the cereal was measured using a stopwatch (accurate to 0.01 s). After the trial, each animal was returned to its homecage. If an animal did not locate the food pellet within 5 min then it was removed, returned to its homecage and given a score of 5 min. The bedding was changed between mice for all testing periods and the cereal was buried in a different location on each test day. The surface pellet test was set up in a similar way except that the piece of cereal was placed on top of the bedding.
Block test
The block test was performed as previously described (Spinetta et al., 2005, 2006; Tillerson et al., 2006) with some modifications. Individually housed animals were exposed to five wood blocks (A–E; 2.5 cm3) placed inside each animal’s cage for 7 days. During this time the cage bedding was not changed so that the blocks took on the odor of that animal. On the day of the test, animals were transferred into a separate testing area and the blocks were removed along with some of the cage bedding, both of which were placed in a plastic bag labeled with the animal’s identification. This allowed the blocks to maintain the scent of that animal. Water bottles, food and metal cage grids were removed and just the filtered cage lid remained on the cage. Animals were habituated to the room for 1 h. For Trial 1, blocks A–D, originally from the animal’s own cage, were placed at the same time in the middle of the cage as close to the mouse as possible. The blocks were placed approximately 1–2 cm apart. The mouse was then videotaped for 30 s. All mice received a total of six exposures to blocks A–D with an inter-trial interval of ~5 min. On the seventh trial, block D was removed and replaced with block E from another mouse’s bedding. Blocks A–C and E were then placed into the cage and the animal was videotaped for 30 s as in Trials 1–6. The order of the blocks varied between mice. The experimenter wore gloves throughout the entire procedure and changed them for each trial for each mouse. A rater who was blind to genotype measured the time spent sniffing each block, with sniffing defined as nasal contact with that block. Time to approach the novel block (E) during Trial 7 was also measured. In addition, total activity was measured as the number of movements made along the length and width of the homecage during each trial. The rater scored the time spent sniffing and time to approach from the videotape using a stopwatch (accurate to 0.01 s).
In an additional experiment to directly assess olfactory detection, the block test was performed in a group of Thy1-aSyn (n = 5) and WT (n = 7) mice with blocks exposed to the homecage for only 24 h. This resulted in a less intense odor compared with blocks that had been in the cage for 7 days, thus increasing the difficulty of detecting the novel block.
Habituation to the block procedure and approach habituation were also measured in the 24 h block test (Thy1-aSyn, n = 5; WT, n = 7). Total time spent sniffing the blocks and latency to approach any block in trials 1–6 (prior to exposure to the novel block E) was measured. trials 1 and 2, 3 and 4, and 5 and 6 were pooled and then averaged to produce a mean sniffing or approach latency for each mouse.
Habituation/dishabituation test
The habituation/dishabituation test was performed as previously described (Bielsky et al., 2004). In this test, individually housed animals were habituated to a stimulus by placing a small empty plastic tissue cartridge (Fisher Scientific, Pittsburgh, PA, USA) in each animal’s cage for several days. Similar to the block test, on the day of the test the stimulus was removed from the cage and animals were transferred into a separate testing area where water bottles, food and metal cage grids were removed and just the filtered cage lid remained on each cage. Animals were habituated to the room for 1 h. A cotton ball injected with 5–40 μL of odorant was placed inside a new plastic cartridge; 5 μL was used for the extracts of pine and cinnamon (All Natural Botanicals, Pinellas Park, FL, USA), as well as anise and coconut (McCormick & Co., Inc., Hunt Valley, MD, USA), whereas 40 μL was used for the less intense juice concentrates of orange, lemon and lime (Minute Maid®; The Coca-Cola Company, Atlanta, GA, USA). The scented cartridge was then placed in the cage and the mouse was videotaped for 30 s. After 30 s the cartridge was removed and the trial for the next mouse was initiated in a similar manner with a separate cartridge. Each mouse received six exposures to the same scented cartridge with an inter-trial interval of ~5 min. On the seventh trial, a new cartridge holding a cotton ball scented with a different scent was placed in the cage. The experimenter wore gloves throughout the entire procedure and changed them for each trial for each mouse. Time spent sniffing the cartridge was measured, with sniffing defined as nasal contact with the cartridge. Time to approach the cartridge and total activity were also measured. Similar to the block test, the rater scored time spent sniffing and time to approach from the videotape using a stopwatch (accurate to 0.01 s). Different pairs of scents were tested, which included banana/lemon, coconut/anise and pine/cinnamon with the first scent being the habituated scent (e.g. banana) and the second being the novel scent (e.g. lemon). To test whether the mice had more difficulty in discriminating between similar odors they were also exposed to a series of citrus odors including lemon/lime, lemon/orange and lime/orange.
α-synuclein immunohistochemistry
To conserve animals, the mice used for immunohistochemistry were part of a previous behavioral experiment (Fleming et al., 2006). Male mice (Thy1-aSyn, n = 15; WT, n = 8; ~5 months of age) were anesthetized with pentobarbital (100 mg/kg, i.p.) and intracardially perfused with 0.1 M phosphate-buffered saline (PBS) followed by ice-cold 4% paraformaldehyde. Brains were quickly removed, post-fixed for 2 h in 4% paraformaldehyde, cryoprotected in 30% sucrose in 0.1 M PBS, frozen on powdered dry ice and stored at −80°C. Free-floating coronal sections (40 μm) were collected for analysis. Sections were washed in 0.1 M PBS and then in 0.1 M PBS containing 10 μg/mL of proteinase K (Invitrogen, Carlsbad, CA, USA) at room temperature (23°C) for 10 min and then washed with 0.1 M PBS. An alternative set of sections did not receive proteinase K treatment and was just washed in 0.1 M PBS. All sections were incubated for 1 h in a blocking solution containing 0.1 M PBS and mouse-on-mouse blocking solution (Vector Laboratories, Burlingame, CA, USA). Sections were then incubated overnight with the primary antibody for α-synuclein (4 mL/mL mouse anti-α-synuclein; BD Biosciences, San Jose, CA, USA), which recognizes both mouse and human α-synuclein (van der Putten et al., 2000), at 4°C in the presence of 2% normal goat serum. Sections were washed in 0.1 M PBS followed by a 2 h incubation with a biotinylated secondary antibody, goat anti-mouse IgG F (ab)2, at room temperature in the presence of 2% normal goat serum. Sections were then rinsed in 0.1 M PBS and subsequently incubated in avidin-biotin complex (ABC, Vector Laboratories) for 45 min. Sections were rinsed again in 0.1 M PBS followed by an incubation in 0.05 M Tris-buffered saline containing 3-3′diam-inobenzidine (Sigma) and 0.3% H2O2 (Sigma) to reveal staining. Sections were rinsed with 0.1 M PBS, mounted on gelatin-coated slides, dehydrated, cleared with xylene and mounted with Eukit mounting medium (Calibrated Instruments, Hawthorne, NY, USA).
Statistics
The buried pellet test was analysed using nonparametric statistics as the latency scores showed heterogeneity of variance. The latency to locate the pellet was averaged across days 3–5 and then compared between WT and Thy1-aSyn mice using a Mann–Whitney U-test. Similarly, for the surface pellet part of the test, latency to find the pellet was analysed using a Mann–Whitney U-test to compare WT and Thy1-aSyn mice. For the block test, analyses were performed on data from Trial 7 (when the novel scented block was introduced into the cage). Nonparametric analyses were used to account for heterogeneity of variance. The Mann–Whitney U-test was used to compare time spent sniffing the novel block and time to approach the blocks for Thy1-aSyn and WT mice. Time spent sniffing the novel block compared with the familiar blocks was analysed using the Wilcoxon Signed Rank test. In addition, both time spent sniffing the blocks and time to approach any block during Trials 1–6 (prior to the presentation of the novel block) were analysed using a mixed design 2 × 6 ANOVA followed by Fisher’s LSD post-hoc. To assess both habituation and dishabituation in the habituation/dishabituation test, parametric analyses were performed on Trials 1, 6 and 7, similar to previous studies (Macknin et al., 2004). A 2 × 3 mixed design ANOVA was used to compare genotype (WT vs. Thy1-aSyn) and trial [Trials 1 (novel), 6 (habituated) and 7 (novel)] on time spent sniffing. Post-hoc analysis was performed using Fisher’s LSD. Time to approach for WT and Thy1-aSyn mice was analysed using Student’s t-tests. Total activity for both the block and habituation/dishabituation tests was analysed using Student’s t-test. All statistics were calculated with GB-Stat software (2000; Dynamic Microsystems, Inc., Silver Spring, MD, USA) for Macintosh. The level of significance was set at P < 0.05.
Results
Buried pellet test
In the buried pellet test, we compared the latency to locate a pellet of food buried beneath the bedding or placed on its surface. Thy1-aSyn mice took significantly longer to locate the buried pellet compared with WT mice (Mann–Whitney U-test, P < 0.05; Fig. 1). However, Thy1-aSyn and WT mice showed similar interest, mobility and attention to the stimulus.
Fig. 1.
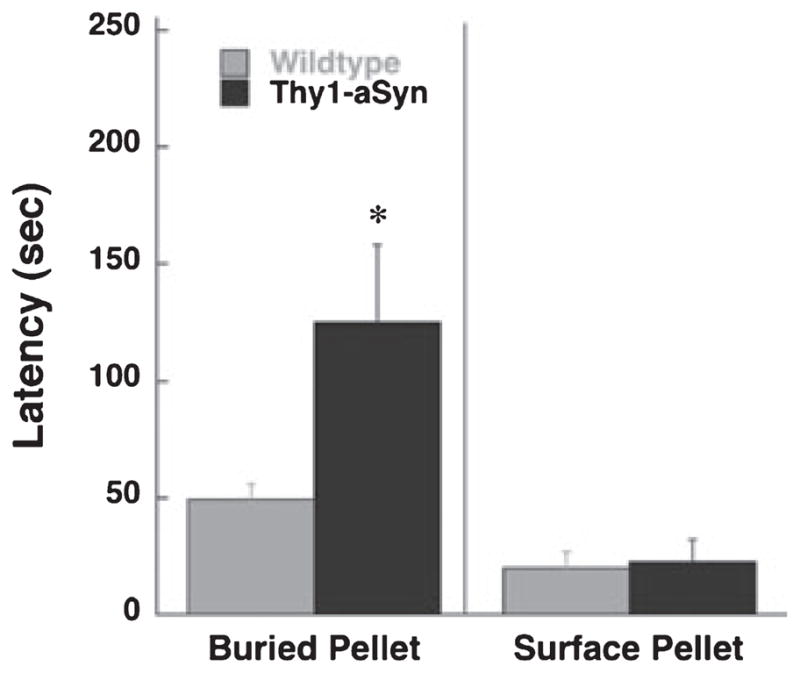
Latency to uncover the pellet in 5–6-month-old Thy1-aSyn (n = 7) and WT (n = 16) mice. *P < 0.05 compared with WT (Mann–Whitney U-test).
Block test
Mice were tested for olfaction detection and discrimination using a novel block test (adapted from Spinetta et al., 2005, 2006; Tillerson et al., 2006). At this age, both WT and Thy1-aSyn mice were able to discriminate the novel block and spent more time sniffing block E (the novel block) compared with the other blocks in Trial 7 (Wilcoxon Signed Rank test, P < 0.01). Thy1-aSyn mice, however, spent significantly less time sniffing this novel block (block E) compared with WT mice (Mann–Whitney U-test, P < 0.01; Fig. 2A), suggesting a partial impairment in olfaction. There was no significant difference in the time to approach block E between Thy1-aSyn and WT mice (P > 0.05; Table 1) and total activity was not significantly different between Thy1-aSyn and WT mice (total activity: t = −0.27, P > 0.05; Table 1), indicating that the deficit was not due to decreased interest or locomotion.
Fig. 2.
(A) Time spent sniffing in the block test in 3–4-month-old Thy1-aSyn (n = 35) and WT (n = 36) mice. (B) Time spent sniffing in the block test in Thy1-aSyn (n = 10) and WT (n = 7) mice at 9 months. Blocks were kept in the homecage for 7 days prior to testing at 3–4 and 9 months. (C) Time spent sniffing in the block test in Thy1-aSyn (n = 5) and WT (n = 7) mice at 11 months. Blocks were kept in the cage for 24 h prior to testing. *, **P < 0.05 and 0.01, respectively, compared with WT. ΔΔP < 0.05 and 0.01, respectively, compared with blocks A–C (Mann–Whitney U-test and Wilcoxon Signed Rank test).
Table 1.
Time to approach and total activity in the block test in Thy1-aSyn and WT mice
| Experiment | Time to approach novel block (s)
|
Total activity (line crosses)
|
||
|---|---|---|---|---|
| WT (n) | Thy1-aSyn (n) | WT (n) | Thy1-aSyn (n) | |
| 3–4 months | 8.03 ± 1.07 (36) | 12.52 ± 1.76 (35) | 23.28 ± 2.32 (36) | 24.17 ± 2.37 (35) |
| 9 months | 7.19 ± 4.06 (7) | 11.86 ± 4.12 (10) | 18.86 ± 3.81 (7) | 21.40 ± 2.16 (10) |
A subset of the same mice was allowed to age and tested again at 9 months of age. At this greater age, the Thy1-aSyn mice again showed a decrease in sniffing without change in approach or activity behavior, similar to what was observed at 3–4 months (Fig. 2B and Table 1).
In the previous experiment, the blocks were heavily scented by prolonged (7 days) exposure to bedding, thus making it difficult to detect mild deficits in detection. To address this issue, an additional experiment was performed in older (11-month-old) mice using blocks that were left in the homecage for only 24 h to minimize the intensity of the odor. Even at this age WT mice were able to discriminate between the novel and familiar blocks and spent significantly more time sniffing the novel block compared with the familiar homecage blocks (Wilcoxon Signed Rank test, P < 0.01 for blocks A–C compared with block E). Similar to the previous data, Thy1-aSyn mice sniffed the novel block significantly less than WT mice (Mann–Whitney – test, P < 0.05; Fig. 2C). However, Thy1-aSyn mice did not sniff the novel block more than the other blocks carrying the odor of their own homecage (Wilcoxon Signed Rank test, P > 0.05), indicating that the transgenics had difficulty in discriminating the novel block from the familiar blocks. Time to approach (Thy1-aSyn, 9.98 ± 5.46 s; WT, 6.93 ± 3.92 s) and activity levels (Thy1-aSyn, 24.40 ± 1.86, n = 5; WT, 33.43 ± 7.88 line crosses, n = 7) were not significantly different between Thy1-aSyn and WT mice (total activity: t = −1.12, P > 0.05), indicating that the differences were not due to impairments in motor behavior even at this greater age.
At each age tested, WT scores for time spent sniffing block E (3–4 months, 10.02 ± 0.90 s; 9 months, 10.08 ± 2.21 s; 11 months, 16.24 ± 3.28 s) were similar to WT mice tested in pilot studies (12.57 ± 1.94 s). The majority of scores were similar to the mean of that group and there were no outliers, indicating the reproducibility of the behavioral measure.
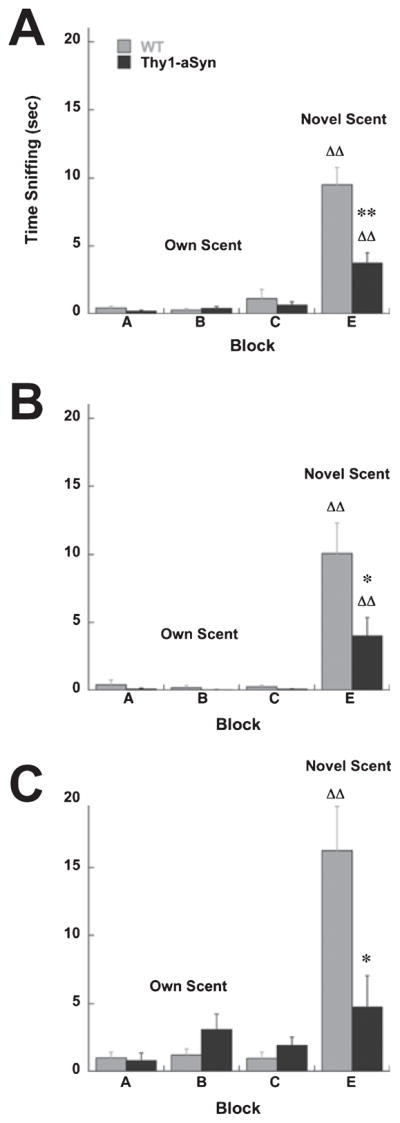
Habituation to the block procedure and approach habituation were also measured in the 24 h block test. Total time spent sniffing the blocks and latency to approach any block in Trials 1–6 (prior to exposure to the novel block E) was measured. There was no difference in time spent sniffing the blocks or in time to approach any block in Trials 1–6 between Thy1-aSyn and WT mice, indicating a similar interest in the blocks and sustained attention during repeated trials (P > 0.05; Fig. 3).
Fig. 3.
(A) Time spent sniffing all blocks during Trials 1–6 (prior to the presentation of the novel block E) in the 24 h block test in Thy1-aSyn (n = 5) and WT (n = 7) mice. (B) Time to approach any block during Trials 1–6 in the 24 h block test in Thy1-aSyn (n = 5) and WT (n = 7) mice.
Habituation/dishabituation test
It is conceivable that the decrease in sniffing observed in the block test could be due to an abnormal social response to the scent of a different mouse. To address this question, 3–4-month-old Thy1-aSyn and WT mice previously tested on the block test were also tested in a habituation/dishabituation test that uses non-social scents. A mixed design ANOVA was used to compare Trials 1, 6 and 7 (novel, habituated and novel) for each pair of scents. Data for banana/lemon, coconut/anise and pine/cinnamon are presented in Table 2. For the banana/lemon scent pair there was a main effect of trial (F2,92 = 32.34, P < 0.01), indicating that both Thy1-aSyn and WT mice habituated to the banana scent and were then able to discriminate the lemon scent as different. Post-hoc analysis showed significant differences in sniffing between Trials 1 (novel) and 6 (habituated), and Trials 7 (novel) and 6 for both Thy1-aSyn and WT mice (P < 0.01). Similarly, for the coconut/anise and pine/cinnamon scent pairs, there was a main effect of trial (coconut/anise, F2,90 = 35.25, P < 0.01; pine/cinnamon, F2,90 = 50.59, P < 0.01) and post-hoc analysis indicated that there were significant differences in sniffing in both scent pairs between Trials 1 and 6, and Trials 6 and 7 (P < 0.01) for both Thy1-aSyn and WT mice. In general, both Thy1-aSyn and WT mice were able to habituate to a novel odor and then discriminate between a habituated odor and a novel odor at 3–4 months of age in these strongly different pairs of scents. However, Thy1-aSyn mice spent less time sniffing the coconut scent in Trial 1 compared with WT mice (P < 0.05).
Table 2.
Time spent sniffing the scented cartridge in the habituation/dishabituation test in Thy1-aSyn (3–4 months, n = 22; 9 months, n = 10) and WT (3–4 months, n = 24; 9 months, n = 7) mice
| Scents | Time spent sniffing (s)
|
|||||
|---|---|---|---|---|---|---|
| Trial 1
|
Trial 6
|
Trial 7
|
||||
| WT | Thy1-aSyn | WT | Thy1-aSyn | WT | Thy1-aSyn | |
| 3–4 months | ||||||
| Banana/lemon | 4.82 ± 0.81 | 5.37 ± 1.01 | 0.78 ± 0.19††,ΔΔ | 0.53 ± 0.20††,ΔΔ | 4.44 ± 0.78 | 3.62 ± 0.74 |
| Coconut/anise | 5.20 ± 0.75 | 3.57 ± 0.83* | 1.01 ± 0.32††,ΔΔ | 0.54 ± 0.18††,ΔΔ | 3.81 ± 0.77 | 3.88 ± 0.72 |
| Pine/cinnamon | 4.83 ± 0.68 | 3.63 ± 0.76 | 0.55 ± 0.17††,ΔΔ | 0.59 ± 0.31††,ΔΔ | 5.49 ± 0.78 | 4.55 ± 0.86 |
| 9 months | ||||||
| Banana/lemon | 4.58 ± 1.06 | 3.74 ± 0.96 | 0.20 ± 0.10††,ΔΔ | 0.10 ± 0.07††,ΔΔ | 5.03 ± 1.59 | 3.32 ± 0.92 |
| Coconut/anise | 4.73 ± 1.43 | 2.37 ± 0.89* | 0.15 ± 0.10††,Δ | 0.06 ± 0.04† | 2.72 ± 1.12 | 1.88 ± 0.74 |
| Pine/cinnamon | 4.08 ± 1.34 | 2.82 ± 0.86 | 0.56 ± 0.25††,ΔΔ | 0.15 ± 0.09††,ΔΔ | 3.42 ± 1.12 | 2.70 ± 0.96 |
P < 0.05 and
P < 0.01, compared with Trial 1 within the same genotype;
P < 0.05 and
P < 0.01, compared with Trial 7 within the same genotype;
P < 0.05, compared with WT within the same trial.
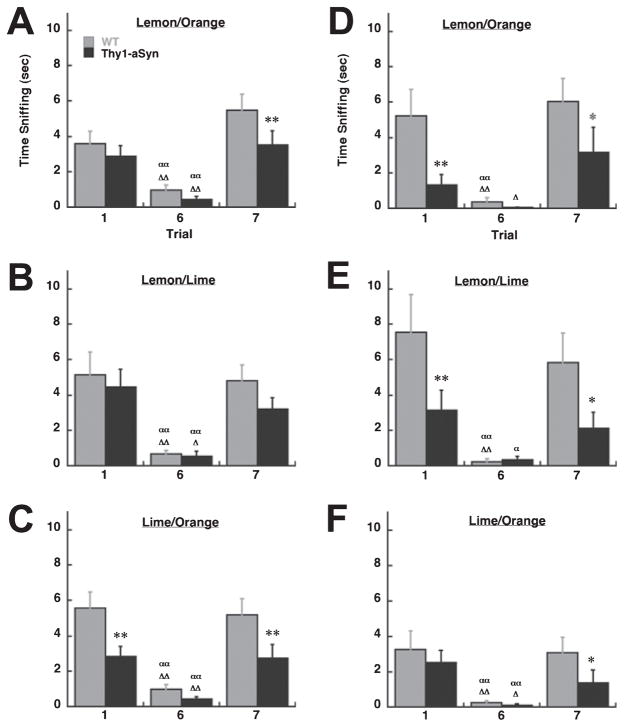
To test whether the mice had more difficulty in discriminating between similar odors they were also exposed to a series of citrus odors at 3–4 months (Fig. 4A–C). For both the lemon/orange and lemon/lime scent combinations, there was a main effect of trial (lemon/orange, F2,92 = 33.12, P < 0.01; lemon/lime, F2,92 = 26.18, P < 0.01). Post-hoc analysis indicated significant differences between Trials 1 and 6, and Trials 6 and 7 (P < 0.01) for both Thy1-aSyn and WT mice in both scent pairs. However, for the lemon/orange scent pair Thy1-aSyn mice spent less time sniffing the novel scent during Trial 7 (P < 0.01). In the lime/orange combination there was a main effect of genotype (F1,45 = 7.04, P < 0.05) and a main effect of trial (F2,92 = 25.98, P < 0.01). Although post-hoc analysis indicated significant differences between Trials 1 and 6, and Trials 6 and 7 (P < 0.01) for both Thy1-aSyn and WT mice, Thy1-aSyn mice also spent significantly less time sniffing the novel scents in Trials 1 and 7 compared with WT mice (P < 0.01; Fig. 4C).
Fig. 4.
(A–C) Time spent sniffing in the habituation/dishabituation test in 3–4-month-old Thy1-aSyn (n = 22) and WT (n = 24) mice. (D–F) Time spent sniffing in the habituation/dishabituation test in 9-month-old Thy1-aSyn (n = 10) and WT (n = 7) mice. *, **P < 0.05 and 0.01 compared with WT within the same trial; α,ααP < 0.05 and 0.01, respectively, compared with Trial 1 within the same genotype; Δ, ΔΔP < 0.05 and 0.01, respectively, compared with Trial 7 within the same genotype (2 × 3 ANOVA followed by Fisher’s LSD).
A subset of the 3–4-month-old mice that were allowed to age was again tested in the habituation/dishabituation test at 9 months of age. Data for the banana/lemon, coconut/anise and pine/cinnamon scent pairs are shown in Table 2. Similar to testing at 3–4 months there was a main effect of trial for the banana/lemon, coconut/anise and pine/cinnamon scent pairs (banana/lemon, F2,34 = 19.48, P < 0.01; coconut/anise, F2,34 = 11.52, P < 0.01; pine/cinnamon, F2,34 = 14.13, P < 0.01), indicating that both Thy1-aSyn and WT mice were able to habituate and discriminate the novel odor. Again, post-hoc analysis showed significant differences in time spent sniffing between Trials 1 (novel) and 6 (habituated), and Trials 7 (novel) and 6 for both Thy1-aSyn and WT mice for the banana/lemon and pine/cinnamon scent pairs (P < 0.01). However, in the coconut/anise pair, Thy1-aSyn mice spent less time sniffing the coconut scent in Trial 1 compared with WT mice (P < 0.05) and sniffing in Trial 1 did not differ significantly from that in Trial 6 (P > 0.05). These results are similar to testing at 3–4 months, except that Thy1-aSyn mice began to show subtle differences in discriminating between the coconut and anise scents.
Data from the citrus scents at 9 months are presented in Fig. 4D–F. For the lemon/orange scent pair, there was a main effect of genotype (F1,16 = 6.09, P < 0.05) and trial (F2,34 = 11.97, P < 0.01). Post-hoc analysis indicated significant differences between Trials 1 and 6 for WT mice (P < 0.01) but not for Thy1-aSyn mice (P > 0.05). There were significant differences in sniffing between Trials 6 and 7 for both Thy1-aSyn (P < 0.05) and WT (P < 0.01) mice. Although Thy1-aSyn mice were able to discriminate between the habituated and novel scents, they did spend significantly less time sniffing the scented cartridge in Trials 1 (P < 0.01) and 7 (P < 0.05) compared with WT mice. For the lemon/lime combination, there was also a main effect of genotype (F1,16 = 5.31, P < 0.05) and trial (F2,34 = 12.70, P < 0.01). Post-hoc analysis indicated significant differences between Trials 1 and 6 for both Thy1-aSyn (P < 0.05) and WT (P < 0.01) mice. However, the difference in sniffing between Trials 6 and 7 was significant for WT (P < 0.01) but not for Thy1-aSyn (P > 0.05) mice, indicating that the transgenics had difficulty in discriminating between the lemon and lime scents. Thy1-aSyn mice also spent less time sniffing the scented cartridge in Trials 1 (P < 0.01) and 7 (P < 0.05) compared with WT mice. For the lime/orange combination there was a main effect of trial (F2,34 = 17.56, P < 0.01). Post-hoc analysis indicated significant differences between Trials 1 and 6, and Trials 6 and 7 (P < 0.05) for both Thy1-aSyn and WT mice; however, Thy1-aSyn mice spent less time sniffing the novel scent in Trial 7 compared with WT mice (P < 0.05). In general, at 9 months of age Thy1-aSyn mice showed impairments in discriminating between citrus odors. At all ages tested WT and Thy1-aSyn mice did not differ in the time to approach the novel scent or in total activity during testing for each scent pair (P > 0.05, Table 3).
Table 3.
Time to approach and total activity in the habituation/dishabituation test in Thy1-aSyn (3–4 months, n = 22; 9 months, n = 10) and WT (3–4 months, n = 24; 9 months, n = 7) mice
| Scents | Time to approach (s)
|
Total activity (line crosses)
|
||
|---|---|---|---|---|
| WT | Thy1-aSyn | WT | Thy1-aSyn | |
| 3–4 months | ||||
| Banana/lemon | 12.09 ± 1.86 | 13.32 ± 2.16 | 10.29 ± 1.34 | 15.18 ± 2.33 |
| Coconut/anise | 13.25 ± 2.43 | 16.01 ± 2.57 | 8.96 ± 1.12 | 11.81 ± 1.42 |
| Pine/cinnamon | 8.06 ± 2.08 | 9.61 ± 2.17 | 8.42 ± 1.40 | 10.33 ± 1.18 |
| Lemon/lime | 9.33 ± 2.15 | 15.17 ± 2.38 | 6.63 ± 1.31 | 10.95 ± 2.29 |
| Lemon/orange | 11.92 ± 2.40 | 13.04 ± 2.53 | 8.42 ± 1.42 | 11.36 ± 1.71 |
| Lime/orange | 9.94 ± 2.36 | 13.68 ± 2.48 | 8.42 ± 1.42 | 11.50 ± 3.38 |
| 9 months | ||||
| Banana/lemon | 6.84 ± 2.22 | 7.89 ± 3.13 | 15.18 ± 2.33 | 16.11 ± 2.34 |
| Coconut/anise | 13.60 ± 5.19 | 13.73 ± 3.83 | 11.81 ± 1.42 | 14.11 ± 2.41 |
| Pine/cinnamon | 13.07 ± 5.01 | 13.47 ± 3.91 | 15.50 ± 3.40 | 19.00 ± 2.37 |
| Lemon/lime | 10.86 ± 5.01 | 14.68 ± 3.94 | 13.87 ± 3.53 | 14.22 ± 4.07 |
| Lemon/orange | 8.08 ± 3.88 | 13.97 ± 3.63 | 14.25 ± 3.73 | 12.78 ± 2.34 |
| Lime/orange | 6.90 ± 3.99 | 14.74 ± 4.35 | 14.62 ± 3.09 | 12.89 ± 3.35 |
Power analysis
In the present study each olfaction test had sufficient power to detect differences between Thy1-aSyn and WT mice. The block and buried pellet tests had 95% and 90% power, respectively, whereas the habituation/dishabituation test had 50–80% power. In order to identify which tests may be optimal for screening in potential pre-clinical studies, additional power analyses were performed to determine how many mice would be required to detect a 20% or 50% improvement with 80% power and an alpha level of 0.05. Based on the present data in Thy1-aSyn mice, only 16 subjects are needed in the buried pellet test to detect an improvement of 50%, whereas in the block test 31 subjects are needed. The habituation/dishabituation test required the largest number of animals with 52 mice required to detect a 50% improvement. This indicates that the buried pellet test is practical to detect 50% improvement. However, none of the tests could be expected to detect a 20% improvement as much larger numbers of mice would be required (95, 114 and 326, respectively).
α-synuclein immunohistochemistry
In order to detect insoluble forms of α-synuclein, sections from the olfactory bulb from Thy1-aSyn and WT mice were pre-treated with proteinase K. Thy1-aSyn mice displayed proteinase K-resistant α-synuclein-immunoreactive inclusions throughout the olfactory bulb compared with WT mice. The inclusions were visible in the external portion of the olfactory nucleus, accessory olfactory bulb and glomerular layer. The α-synuclein-immunoreactive inclusions ranged from < 1 to 5 μm in diameter. No staining was observed in corresponding sections from WT mice treated with proteinase K (Fig. 5).
Fig. 5.
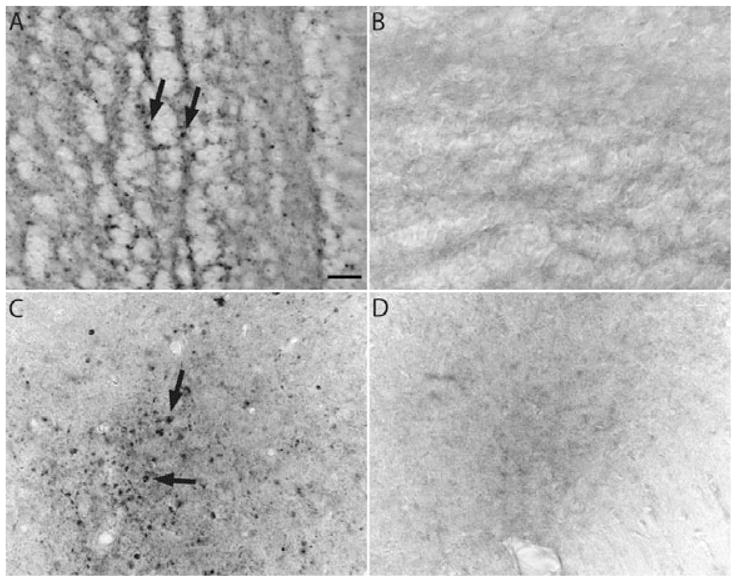
Proteinase K-resistant α-synuclein inclusions in the olfactory bulb of Thy1-aSyn and WT mice at 5 months of age. Thy1-aSyn mice have α-synuclein inclusions throughout the olfactory bulb including the external portion of the olfactory nucleus (A) and mitral cell layer (C). WT mice show no abnormal α-synuclein inclusions (B and D). Scale bar, 20 μm. Arrows indicate proteinase K-resistant inclusions.
Discussion
Mice overexpressing human WT α-synuclein showed olfactory impairments in aspects of multiple olfactory tests that are sensitive to graded differences in olfactory abilities. Although transgenics detected and discriminated odors, they showed significantly lower scores than WT mice in aspects of all three tests, suggesting that overexpression of α-synuclein is sufficient to cause partial but significant olfactory deficits in mice. Importantly, the new behavioral endpoints identified in this study had sufficient power for testing therapies that may protect against α-synuclein-induced neuronal dysfunction.
The olfactory deficits that we have detected in the Thy1-aSyn mice occurred in the absence of obvious motor deficits, as measured by locomotor activity, time to approach the odorant and habituation to the procedures. This is consistent with our previous observations that, at the ages examined in this study, Thy1-aSyn mice only show sensorimotor deficits in tests that strongly challenge motor function (Fleming et al., 2004). Furthermore, these motor deficits were not reversed by L-dopa (Fleming et al., 2006), indicating that they are not equivalent to parkinsonism but rather indicate subtle and progressive neuronal dysfunction, possibly related to other anomalies detected in these mice, such as decreased noradrenaline in the cortex (N.T. Maidment, UCLA, Los Angeles, unpublished observation; Rommelfanger & Weinshenker, 2007), synaptic alterations in striatum (Wu et al., 2005) or dysfunction of dopamine neurons (Maidment et al., 2006). The observation that olfactory deficits in Thy1-aSyn mice occur before dopaminergic cell loss and overt signs of parkinsonism is compatible with observations in humans that olfactory deficits often occur in pre-manifest stages of PD (Berendse et al., 2001; Ponsen et al., 2004; Hentschel et al., 2005; Haehner et al., 2007; Ross et al., 2007, 2008). Recent data suggest that olfaction deficits can precede the clinical manifestations of PD by up to 4 years (Ross et al., 2008).
Multiple complementary tests were used to assess olfactory function in the present study. The buried pellet test revealed the mouse’s ability to detect and find palatable food, the block test evaluated the mouse’s ability to detect social scents, which is an ethologically important behavior in mice, whereas, in the habituation/dishabituation test, mice were exposed to multiple non-social scent combinations. A main finding of the study is that deficits were found across all three testing paradigms, thus further suggesting that they are not due to deficits in social cues or interest in the stimulus. The deficits, however, were usually seen as a decreased scoring compared with WT mice, whereas the ability to smell a novel stimulus and even to discriminate a novel odor were preserved, at least in the young mice. This is similar to observations in patients that olfactory deficits are of variable magnitude and also variably affect different aspects of olfaction, such as odor identification, detection and discrimination (Ward et al., 1983; Doty et al., 1988, 1992; Tissingh et al., 2001; Huisman et al., 2004). It is possible that, in the original block test (7 days exposure to bedding) and some of the pairs in the habituation/dishabituation tests, young mice were able to discriminate between the novel and familiar odors because the odors were intense or markedly different. Deficits in detection began to emerge when older mice were exposed to blocks that were placed in the homecage for only 24 h, resulting in a milder odor. Indeed, WT sniffing scores for the novel block tended to be higher than in the 7 day experiment and previous data in rats show that sniffing bout duration increases as the concentration of an odorant decreases (Youngentob et al., 1987). Although 11-month-old WT mice still showed a clear ability to discriminate a novel odor, Thy1-aSyn mice not only sniffed the novel block less but also had difficulty in discriminating between the novel and familiar blocks. Similarly, deficits in detection emerged in older mice in the habituation/dishabituation test when pairs of similar scents were used. Thy1-aSyn mice were, for the most part, able to detect a novel odor from a habituated odor at 3–4 months of age. However, at 9 months of age Thy1-aSyn mice showed greater difficulty in detecting scents that were similar (lemon, orange and lime) compared with WT mice.
As sniffing and olfaction are involved in almost every type of motivated mouse behavior (mice rely on olfaction to investigate and identify other mice, predators, food and mates), we took care to rule out motivation or attention impairments as a contributing factor to the increased time to find the buried pellet or to the decreased sniffing observed in the block and habituation/dishabituation tests. It is clear in the buried pellet test that motor impairments or differences in eating and motivation were not a contributing factor in our findings because Thy1-aSyn mice were able to find and eat the pellet as quickly as WT mice when the pellet was placed on top of the bedding. In the block and habituation/dishabituation tests the tendency for Thy1-aSyn mice to take longer to approach and the reduced sniffing of the novel stimuli could be interpreted as an attention or motivation deficit. However, this is unlikely because Thy1-aSyn mice habituate to the procedure at the same rate as WT mice and Thy1-aSyn mice also sniff the blocks during the early trials as much as WT mice. This indicates that their interest and attention for novelty are similar to WT mice. We did find a higher latency for the Thy1-aSyn mice to approach the novel block. This may still reflect an impairment in olfaction because the only difference between the previous and novel stimuli is the odor. Therefore, if the mouse does not detect the scent of the novel block then it would not be motivated to approach and investigate it. We also considered the possibility that the trial latency of 30 s was too short, thus decreasing the time available for Thy1-aSyn mice to sniff the novel stimuli if the time taken to approach was increased. This is also unlikely because, upon repeated analysis of the tapes, we found that none of the Thy1-aSyn mice were sniffing the novel stimulus for the last 5 s of the trial. In contrast, some WT mice continued to sniff the novel stimulus even after the trial ended. Furthermore, qualitative analysis of the tapes revealed that Thy1-aSyn mice showed a similar pattern of approach to the blocks in Trials 1–6 (habituation trials) compared with WT mice, again indicating similar interest in the blocks upon initial presentation. Preliminary observations from our group also indicate that Thy1-aSyn mice do not show increased anxiety or a difference in activity levels compared with WT mice in the elevated plus maze, light/dark box test or novelty-suppressed feeding, situations that can also be influenced by motivation and attention factors (Mulligan, Fleming and Chesselet, unpublished observations). Taken together, these data strongly support a partial impairment in olfactory function in Thy1-aSyn mice.
The mechanisms of olfactory deficits in PD remain unclear. Lewy bodies are present in olfactory regions, such as the olfactory bulb and tract (Daniel & Hawkes, 1992; Pearce et al., 1995), and recent studies suggest that α-synuclein accumulation in olfactory regions may even precede pathology in other areas of the brain such as the substantia nigra, the primary locus of neuronal cell loss in PD (Braak et al., 2003). Here we show proteinase K-resistant α-synuclein inclusions throughout the olfactory bulb in Thy1-aSyn mice, suggesting that α-synuclein pathology may interfere with olfactory function in these mice. In addition to α-synuclein pathology, there are other factors that may contribute to the deficit. For example, Huisman et al. (2004) found a 100% increase in dopaminergic neurons in the olfactory bulb in post-mortem tissue from patients with PD. Olfactory impairments have also been observed in mice with alterations in the dopamine system, including dopamine transporter knockout and dopamine D2 receptor knockout mice (Tillerson et al., 2006). Deficits in olfactory neurogenesis and the migration of newly born cells from the subventricular zone to the olfactory bulb, processes that are important for olfactory function (Lledo et al., 2004, 2006), may also play a role. Post-mortem studies of PD brains found fewer precursor cells in the olfactory bulb of patients with PD compared with controls (Hoglinger et al., 2004) and dopamine-depleted mice show reduced subependymal zone proliferation (Hoglinger et al., 2004). Furthermore, impairment in neurogenesis has been shown in mice that overexpress α-synuclein under the platelet-derived growth factor β promoter and in mice expressing the A53T mutation (Winner et al., 2004, 2008). Determining whether impairment in neurogenesis causes or is a consequence of olfactory deficits (Reynolds & Weiss, 1992; Lledo et al., 2004, 2006) will require further investigation. Our data indicate that overexpression of α-synuclein alone, without nigral dopamine cell loss, is sufficient to cause olfactory deficits in mice and identify Thy1-aSyn mice as a valuable tool in which to further study the mechanisms involved in olfactory dysfunction in PD.
The present results provide novel end-points for testing therapies that may interfere with α-synuclein-induced pathology. As olfactory deficits do not respond to current symptomatic treatment (L-dopa), Thy1-aSyn mice will be useful to discriminate between disease-modifying and symptomatic treatment of synucleinopathies like PD. Previous studies have shown olfactory impairments in two different lines of mice associated with Alzheimer’s disease where olfactory dysfunction is, as in PD, an early symptom (Macknin et al., 2004; Nathan et al., 2004). Our data now show that this symptom can also be modeled and used for drug development in a mouse model of synucleinopathy.
Acknowledgments
We thank Maureen Hagan, Ehud Gruen and Gowry Fernando for their help with the mouse colony and Dr Timothy Schallert for the block test procedures. Funded by the Morris K. Udall Parkinson’s Disease Research Center of Excellence at UCLA (P50NS38367), American Parkinson Disease Association and the Chen family.
Abbreviations
- PBS
phosphate-buffered saline
- PD
Parkinson’s disease
- Thy1-aSyn
transgenic mice overexpressing wildtype human α-synuclein under the Thy1 promoter
- WT
wildtype
References
- Berendse HW, Booij J, Francot CM, Bergmans PL, Hijman R, Stoof JC, Wolters EC. Subclinical dopaminergic dysfunction in asymptomatic Parkinson’s disease patients’ relatives with a decreased sense of smell. Ann Neurol. 2001;50:34–41. doi: 10.1002/ana.1049. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Chesselet MF. In vivo alpha-synuclein overexpression in rodents: a useful model of Parkinson’s disease? Exp Neurol. 2007;209:22–27. doi: 10.1016/j.expneurol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. What’s Wrong with My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. Wiley-Liss; NY: 2000. [Google Scholar]
- Daniel SE, Hawkes CH. Preliminary diagnosis of Parkinson’s disease by olfactory bulb pathology. Lancet. 1992;340:186. doi: 10.1016/0140-6736(92)93275-r. [DOI] [PubMed] [Google Scholar]
- Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38:1237–1244. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- Doty RL, Stern MB, Pfeiffer C, Gollomp SM, Hurtig HI. Bilateral olfactory dysfunction in early stage treated and untreated idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1992;55:138–142. doi: 10.1136/jnnp.55.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernagut PO, Hutson CB, Fleming SM, Tetreaut N, Salcedo J, Masliah E, Chesselet MF. Behavioral and histopathological consequences of paraquat intoxication in mice: effects of alpha-synuclein overexpression. Synapse. 2007;61:991–1001. doi: 10.1002/syn.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci. 2004;24:9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Hutson CB, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Behavioral effects of dopaminergic agonists in transgenic mice overexpressing human wildtype alpha-synuclein. Neuroscience. 2006;142:1245–1253. doi: 10.1016/j.neuroscience.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Jordan MC, Masliah E, Chesselet M-F, Roos KP. Program No. 50.9. 2007 Neuroscience Meeting Planner. Society for Neuroscience; San Diego, CA: 2007. Alterations in Baroreceptor Function in Transgenic Mice Overexpressing Human Wildtype Alpha Synuclein. [Google Scholar]
- Goldstein DS, Sharabi Y, Karp BI, Bentho O, Saleem A, Pacak K, Eisenhofer G. Cardiac sympathetic denervation preceding motor signs in Parkinson disease. Clin Auton Res. 2007;17:118–121. doi: 10.1007/s10286-007-0396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehner A, Hummel T, Hummel C, Sommer U, Junghanns S, Reichmann H. Olfactory loss may be a first sign of idiopathic Parkinson’s disease. Mov Disord. 2007;22:839–842. doi: 10.1002/mds.21413. [DOI] [PubMed] [Google Scholar]
- Hentschel K, Furtado S, Markopoulou K, Doty RL, Uitti RJ, Wszolek ZK. Differences in olfactory dysfunction between sporadic PD and familial Parkinson’s disease kindreds by UPSIT subgroup analysis. Mov Disord. 2005;20(Suppl 10):S55. [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Huisman E, Uylings HB, Hoogland PV. A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson’s disease. Mov Disord. 2004;19:687–692. doi: 10.1002/mds.10713. [DOI] [PubMed] [Google Scholar]
- Langston JW. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59:591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Saghatelyan A, Lemasson M. Inhibitory interneurons in the olfactory bulb: from development to function. Neuroscientist. 2004;10:292–303. doi: 10.1177/1073858404263460. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Macknin JB, Higuchi M, Lee VM, Trojanowski JQ, Doty RL. Olfactory dysfunction occurs in transgenic mice overexpressing human tau protein. Brain Res. 2004;1000:174–178. doi: 10.1016/j.brainres.2004.01.047. [DOI] [PubMed] [Google Scholar]
- Maidment NT, Lam HA, Ackerson LC, Rockenstein E, Masliah E. Program No. 378.4. 2006 Neuroscience Meeting Planner. Society for Neuroscience; Atlanta, GA: 2006. Dysregulation of Dopamine Transmission in Mice Over-Expressing Wildt-Type Human Alpha-Synuclein. [Google Scholar]
- Montgomery EB, Jr, Baker KB, Lyons K, Koller WC. Abnormal performance on the PD test battery by asymptomatic first-degree relatives. Neurology. 1999;52:757–762. doi: 10.1212/wnl.52.4.757. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Yost J, Litherland MT, Struble RG, Switzer PV. Olfactory function in apoE knockout mice. Behav Brain Res. 2004;150:1–7. doi: 10.1016/S0166-4328(03)00219-5. [DOI] [PubMed] [Google Scholar]
- Pearce RK, Hawkes CH, Daniel SE. The anterior olfactory nucleus in Parkinson’s disease. Mov Disord. 1995;10:283–287. doi: 10.1002/mds.870100309. [DOI] [PubMed] [Google Scholar]
- Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2003;2:107–116. doi: 10.1016/s1474-4422(03)00307-7. [DOI] [PubMed] [Google Scholar]
- Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters ECh, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann Neurol. 2004;56:173–181. doi: 10.1002/ana.20160. [DOI] [PubMed] [Google Scholar]
- van der Putten H, Wiederhold KH, Probst A, Barbieri S, Mistl C, Danner S, Kauffmann S, Hofele K, Spooren WP, Ruegg MA, Lin S, Caroni P, Sommer B, Tolnay M, Bilbe G. Neuropathology in mice expressing human alpha-synuclein. J Neurosci. 2000;20:6021–6029. doi: 10.1523/JNEUROSCI.20-16-06021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68:568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Weinshenker D. Norepinephrine: the redheaded stepchild of Parkinson’s disease. Biochem Pharmacol. 2007;74:177–190. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Ross GW, Petrovitch H, Abbott RD, Tanner CM, Popper J, Masaki K, Launer L, White LR. Association of olfactory dysfunction with risk for future Parkinson’s disease. Mov Disord. 2007;21:2062–2067. doi: 10.1002/ana.21291. [DOI] [PubMed] [Google Scholar]
- Ross GW, Petrovitch H, Abbott RD, Tanner CM, Popper J, Masaki K, Launer L, White LR. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol. 2008;63:167–173. doi: 10.1002/ana.21291. [DOI] [PubMed] [Google Scholar]
- Spinetta MJ, Woodlee MT, Hester NW, Heymann JC, Feinberg LM, Rajagopalan KN, Lichtenwalner R, Parent J, Schallert T. Program No. 999.8. 2005 Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2005. A Simple and Sensitive Odor Recognition Test for Rats and Mice Detects Retrograde Amnesia Caused by Ethanol or Other Drugs That Interfere with Memory Consolidation. [Google Scholar]
- Spinetta M, Woodlee M, Feinberg L, Heymann J, O’Connell W, Parent J, Lichtenwalner R, Schallert T. Program No. 753.1. 2006 Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2006. Alcohol and Retrograde Amnesia: Can Rats Have Blackouts and Does Caffeine Help? [Google Scholar]
- Tillerson JL, Caudle WM, Parent JM, Gong C, Schallert T, Miller GW. Olfactory discrimination deficits in mice lacking the dopamine transporter or the D2 dopamine receptor. Behav Brain Res. 2006;172:97–105. doi: 10.1016/j.bbr.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Tissingh G, Berendse HW, Bergmans P, DeWaard R, Drukarch B, Stoof JC, Wolters EC. Loss of olfaction in de novo and treated Parkinson’s disease: possible implications for early diagnosis. Mov Disord. 2001;16:41–46. doi: 10.1002/1531-8257(200101)16:1<41::aid-mds1017>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Wang L, Fleming SM, Chesselet MF, Taché Y. Abnormal colonic motility in mice overexpressing human wild-type α-synuclein. Neuroreport. 2008;19:873–876. doi: 10.1097/WNR.0b013e3282ffda5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward CD, Hess WA, Calne DB. Olfactory impairment in Parkinson’s disease. Neurology. 1983;33:943–946. doi: 10.1212/wnl.33.7.943. [DOI] [PubMed] [Google Scholar]
- Winner B, Lie DC, Rockenstein E, Aigner R, Aigner L, Masliah E, Kuhn HG, Winkler J. Human wild-type alpha-synuclein impairs neurogenesis. J Neuropathol Exp Neurol. 2004;63:1155–1166. doi: 10.1093/jnen/63.11.1155. [DOI] [PubMed] [Google Scholar]
- Winner B, Rockenstein E, Lie DC, Aigner R, Mante M, Bogdahn U, Couillard-Depres S, Masliah E, Winkler J. Mutant alpha-synuclein exacerbates age-related decrease of neurogenesis. Neurobiol Aging. 2008;29:913–925. doi: 10.1016/j.neurobiolaging.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Cepeda C, Masliah E, Levine MS. Program No. 85.12. AbstractViewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2005. Abnormal Glutamate and Dopamine Receptor Function in the Striatum of α-Synuclein-Overexpressing Mice. [Google Scholar]
- Youngentob SL, Mozell MM, Sheehe PR, Hornung DE. A quantitative analysis of sniffing strategies in rats performing odor detection tests. Physiol Behav. 1987;41:59–69. doi: 10.1016/0031-9384(87)90131-4. [DOI] [PubMed] [Google Scholar]