Abstract
Background
Activating autoantibodies to β-adrenergic receptors (AAβ1/2AR) and M2 muscarinic receptors (AAM2R) have been reported in several cardiac diseases and may have pathophysiologic relevance. However, the interactions and relative effects of AAβ1AR, AAβ2AR and AAM2R on contractile function have not been characterized.
Methods
The inotropic effects of IgG from 18 selected patients with cardiomyopathy and/or atrial tachyarrhythmias positive by ELISA for antibodies to β1/2AR were studied using an isolated canine Purkinje fiber contractility assay. M2R-blockade was tested using atropine while selective β1AR and β2AR blockade used CGP-20712A and ICI-118551 respectively.
Results
Fifteen of the 18 anti-β1/2AR ELISA-positive samples demonstrated evidence for negative inotropic muscarinic effects which were blocked using atropine. Atropine failed to uncover a positive inotropic response in 2 of the 18 IgG samples (false positive ELISA for AAβAR). In the remaining 16 AAβAR true-positive subjects, the β1AR-induced increase in contractility (concurrent M2/β2 blockade) was augmented to 140.5 ± 12.2% of baseline compared to 127.4 ± 7.2% of baseline with M2 blockade (atropine) only (p<0.001, n = 16). The β2AR-induced increase in contractility (concurrent M2/β1 blockade) was only 114.5 ± 4.3% of baseline (p<0.001, n = 16). Combined M2 and β1/β2 blockade eliminated any increase in contractility.
Conclusions
The inherently positive inotropic effect of AAβ1AR was negatively modulated by AAM2R and AAβ2AR. These opposing effects of receptor-activating autoantibodies may alter cardiac performance and influence clinical outcome depending on their receptor type and relative contractile activity.
Keywords: Cardiomyopathy, Activating autoantibodies, β-adrenergic receptors, M2 muscarinic receptor, Inotropy
1. Introduction
Activating autoantibodies to β-adrenergic receptors (AAβAR) and M2 muscarinic receptors (AAM2R) are variably present in patients with idiopathic dilated cardiomyopathy (IDCM), ischemic cardiomyopathy, and patients with atrial tachyarrhythmias [1–5]. These antibodies are found in the sera of healthy subjects, but in lower frequency and titers [6]. They primarily target the 2nd extracellular receptor loop and mediate physiologic effects [7,8]. AAβ1AR and AAβ2AR display positive chronotropic and inotropic effects that are blocked wholly or in part by non-selective β-blockers [9–11]. These antibodies are implicated in the pathogenesis of IDCM since endogenous production or passive transfer of AAβ1AR and AAM2R induce cardiomyopathic changes in animal models [12–15]. AAM2R are notable for their negative chronotropic effects in the atria but they also predispose to atrial fibrillation in patients with IDCM [5].
Parasympathetic receptor activation may decrease ventricular βAR-mediated positive inotropic responses as evidenced by the effect of the muscarinic agonist carbachol [16,17]. M2R-mediated ventricular function is considered less important than atrial effects owing to limited ventricular parasympathetic innervation [18]. Since activating autoantibodies are independent of innervation, they may exert a more diffuse and greater impact on ventricular function than via parasym-pathetic nerves. There are no studies on the opposing effects and interactions of AAβAR and AAM2R on contractility in vitro or on the relative effects of antibodies directed against β1AR vs. β2AR. We have examined both functions using specific adrenergic and muscarinic blocking agents with an in vitro canine Purkinje fiber contractility assay.
2. Methods
2.1. Patients
To obtain serum likely to contain high antibody content for study, we chose eighteen sera from a panel of patients with cardiomyopathy and/or atrial tachyarrhythmias with significantly elevated ELISA autoantibodies against βAR. Ten control sera were obtained from healthy donors. This study was approved by the University of Oklahoma Health Sciences Center Institutional Review Board and all subjects provided written informed consent.
2.2. ELISA
Enzyme-linked immunosorbent assay (ELISA) microtiter plates were coated with human β1AR, β2AR or M2R expressed in Sf9 or CHO cell membranes (PerkinElmer, Waltham, WA) [19]. Titers were the highest dilution with an optical density (OD) value of 0.10 at 60 min compared to positive and negative standard sera. Intraassay variation was minimal (±0.1 OD). Titers ≥1:3200 (anti-β1/2AR) and ≥1:1600 (anti-M2R) were considered positive.
2.3. Purification of IgG antibody
The IgG fraction from the patient serum was purified using the NAb Protein A/G Spin Kit (Pierce, Rockford, IL), according to the manufacturer's protocol.
2.4. Contractility bioassay
Assays used 5–7 mm segments of free running Purkinje fibers or papillary muscles from canine ventricles in a chamber mounted on an inverted microscope (Olympus, Olympus America Inc., Melville, NY) [19]. The fibers were perfused with normal Tyrode's solution (in mmol/L: NaCl 145, KCl 4.5, CaCl2 1.8, MgCl2 1, NaH2PO4 1, glucose 11, HEPES 10, pH 7.36) at 36±0.1 °C and paced at 2 Hz via extracellular platinum electrodes. Isometric contractions were recorded before, during steady state and following washout using a video edge detector (Model VED-205, Crescent Electronics, Sandy, UT) at 120 Hz. After achieving stable contractile responses over 3–5 min, IgG equivalent to a 1:100 serum dilution was examined for 5 min. With subsequent 5-minute washout periods, IgG plus atropine (100 nmol/L) or nadolol (100 nmol/L) was assayed to determine the effect attributable to the AAβAR or AAM2R components of IgG, respectively. IgG in the presence of atropine (to eliminate the effects of AAM2R) was then tested either with the selective β1 blocker CGP-20712A (300 nmol/L) or the selective β2 blocker ICI-118551 (300 nmol/L). Finally, IgG was tested with dual βAR and M2R blockade. The βAR agonist isoproterenol (ISO, 10 nmol/L) served as a positive control. IgG from healthy donors served as negative controls. Contractility indices were analyzed offline using pClamp 9.2 (Axon Instruments, Foster City, CA). Indices were calculated as the mean of 15 consecutive contraction cycles after a stable baseline or response. This technique was quite stable. The intraassay and interassay coefficients of variation were 4.2% (n = 18) and 8.3% (n = 18), respectively.
2.5. Protein kinase A assays
PKA assays were performed as previously described [19]. Sera (1:100 dilution) with and without atropine (100 nmol/L) were incubated with 1×107 rat cardiac H9c2 cells in T75 culture flasks for 1-h before reactions were stopped by ice-cold PBS. Cells were mechanically dislodged, centrifuged, and homogenized in 0.3 ml of extraction buffer. PKA was measured using a SignaTECT PKA assay system (Promega, Madison, WI). The β-blocker nadolol (1 μmol/L) inhibited antibody-induced PKA activity while ISO (100 nmol/L) was the positive control. Specific activity was expressed as pmol/L/min/μg and results were presented as percentages of basal PKA activity.
2.6. Statistical analysis
Contractility values were normalized to their respective baseline values. Data are expressed as mean±SD. Differences in contractility between intervention and nonintervention were tested by subtracting the normalized values for each subject and using a one-sided t-test on these differences. A p value of 0.05 was used for each test. Differences between means were assessed by a paired or unpaired Student's t-test as appropriate with a Bonferroni correction to adjust for the multiple comparisons. McNemar's test and linear regression analysis were used to compare the results of the Purkinje fiber contractility assay with the PKA assay, and the ELISA titers or the papillary muscle contractility assay, respectively.
3. Results
3.1. ELISA for AAβAR and AAM2R
AAβ1/2AR “ELISA-positive” sera (titer ≥ 1:3200) from 18 high risk cardiac patients (Table 1) provided the antibody “reagents” for this study. AAM2R were elevated by ELISA (≥1:1600) in 10 (56%) of the same 18 patients. Their mean age was 65±6 years, 50% were male, 12 were diabetic (6 with type 1 and 6 with type 2), 13 had some form of cardiomyopathy (7 with systolic and 6 with diastolic dysfunction), and 7 had atrial tachyarrhythmias (6 with atrial fibrillation and 1 with atrial tachycardia).
Table 1.
Clinical profile of the 18 patients.
| Number | Age | Sex | DM | HF | AF | HTN | IHD | EF % |
|---|---|---|---|---|---|---|---|---|
| K104 | 65 | F | 2 | x | x | x | 40 | |
| K105 | 58 | M | 1 | DD | >50 | |||
| K106 | 78 | M | 1 | x | x | x | 30 | |
| K108 | 76 | F | 1 | x | 25 | |||
| K110 | 71 | M | 2 | x | x | x | 40 | |
| K111 | 26 | F | 1 | DD | >50 | |||
| K112 | 70 | M | DD | x | >50 | |||
| K113 | 48 | F | 1 | x | x | 28 | ||
| K114 | 77 | F | 1 | DD | x | x | >50 | |
| K115 | 74 | M | 2 | x | x | x | 40 | |
| K121 | 58 | F | AT | >50 | ||||
| K126 | 63 | M | x | |||||
| K129 | 77 | M | 2 | DD | >50 | |||
| K136 | 64 | F | 2 | x | x | |||
| K137 | 73 | F | 2 | x | x | x | 35 | |
| K141 | 79 | M | DD | x | >50 | |||
| K142 | 73 | F | x | >50 | ||||
| K160 | 56 | M | x | x | >50 |
DM, diabetes mellitus; HF, heart failure; AF, atrial fibrillation; AT, atrial tachycardia; HTN, hypertension; IHD, ischemic heart disease; EF, left ventricular ejection fraction; DD, diastolic dysfunction.
3.2. Contractile activity assays
IgG from 10 of 18 (56%) patients produced a positive contractile response (109.8 to 119.6% of buffer control, p<0.05) (Fig. 1). The remaining 8 IgG samples produced a negative contractile response (82.0 to 87.5% of buffer control, p<0.05). Seven of the 10 samples with a positive IgG contractility had a further increase in contractility in the presence of the muscarinic blocker atropine (p<0.05 vs. IgG alone) while 3 did not. By contrast, all 8 samples with an initially negative contractility rose with atropine. In 2 of these 8, atropine abolished the negative inotropic response but failed to produce a positive inotropic response and are considered false positive by ELISA for AAβAR. Thus, a total of 15 (7 and 8 from the two subgroups) of the 18 IgG samples demonstrated evidence for negative inotropic muscarinic effects. These included the 10 subjects who were ELISA-positive for anti-M2R and 5 not detected by ELISA. The response to atropine did not differ between AAM2R ELISA-positive and AAM2R ELISA-negative samples. Atropine alone failed to produce a significant change in Purkinje fiber contractility.
Fig. 1.
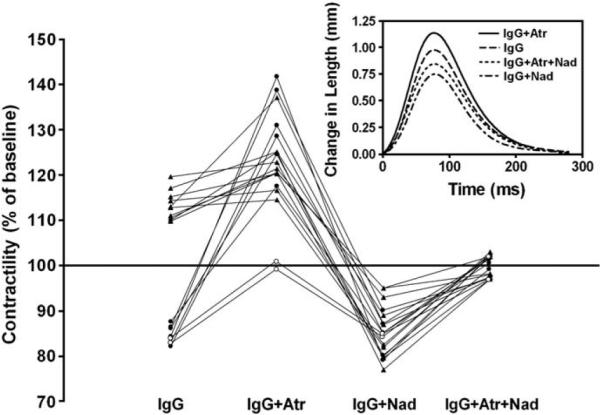
The effect of IgG from the 18 patients on the contractility of isolated Purkinje fibers. Data are expressed as percent of basal buffer control (horizontal line) and represent the mean of 2–6 assays for each IgG. IgG alone from 10 patients (closed triangles) demonstrated a positive inotropic effect, whereas IgG from the other 8 patients (open and closed circles) had a negative inotropic effect (p<0.05 vs. basal buffer control). Two (open circles) of these 8 subjects with a negative IgG effect had a positive response to atropine (Atr) but returned only to baseline. Overall, IgG plus atropine increased contractility in 15 of 18 (p<0.05 vs. IgG alone). IgG in the presence of nadolol (Nad) decreased contractility in all 18 patients (p<0.05 vs. basal buffer control). Combined blockade with atropine and nadolol eliminated any change in contractility in all 18. IgG effects from a representative patient on the contractility of isolated Purkinje fibers in the presence of atropine, nadolol and together are shown in the inset.
IgG from all 18 patients in the presence of the non-selective β-blocker nadolol (100 nmol/L) was associated with a decreased Purkinje fiber contractility (Fig. 1). This decrease ranged from 75.0 to 95.0% of buffer control (p<0.05). These data include the 2 subjects that had false positive anti-βAR autoantibodies. Their decrease in contractility is due to unopposed M2R activity. Nadolol alone had no effect on baseline Purkinje contractility. Dual blockade with atropine (100 nmol/L) and nadolol (100 nmol/L) returned all values to baseline. The mean effects of IgG from the 18 patients in the presence of M2R and βAR blockade are shown in Fig. 2. IgG in the presence of atropine (100 nmol/L) increased Purkinje fiber contractility to 123.5±11.5% of baseline (p<0.001 vs. IgG, n=18), whereas IgG in the presence of nadolol decreased contractility to 84.8±6.2% of baseline (p<0.001 vs. IgG, n=18). ELISA titers for AAβ1/2AR and AAM2R did not correlate with their respective functional effects, as assessed by the Purkinje fiber contractility assay (r2=0.004, p=0.82 and r2=0.071, p=0.28, for AAβ1/2AR and AAM2R, respectively). IgG from 10 healthy controls had no significant effect either in the absence or presence of atropine, nadolol and their combination. ISO (10 nmol/L) increased the contractility to 148.4±3.4% of basal buffer control (p<0.001), and this positive inotropic effect was completely blocked with nadolol (100 nmol/L).
Fig. 2.
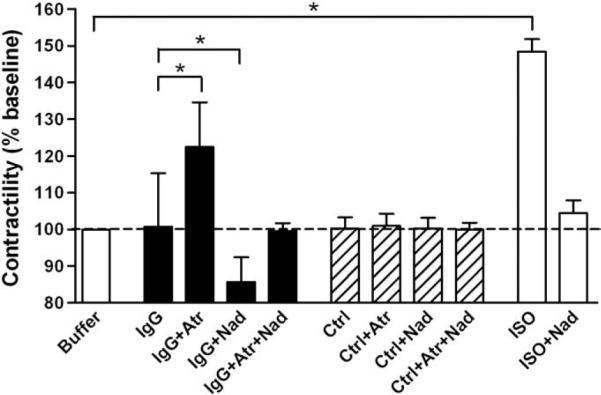
Mean values of the effect of IgG from the 18 patients on Purkinje fiber contractility. Data are expressed as percent of basal buffer control (dashed line) (mean±SD). IgG in the presence of atropine (Atr) increased the contractile response of isolated canine Purkinje fibers to 123.5±11.5% of baseline (*p<0.001 vs. IgG, n=18). IgG in the presence of the non-selective β-blocker, nadolol (Nad) decreased the contractile response to 84.8±6.2% of baseline (*p<0.001 vs. IgG, n=18). Combined blockade with atropine and nadolol eliminated any change in contractility. Normal control IgG did not demonstrate any significant contractile response, either alone or in the presence of atropine, nadolol or their combination (n=10). The positive control, isoproterenol (ISO) significantly increased Purkinje fiber contractility to 148.4 ±3.4% of basal buffer control (*p<0.001 vs. basal buffer control, n=18), which was effectively blocked by nadolol.
In our cohort, the presence of AAβ1AR was always accompanied by AAβ2AR. To dissect their relative impact on contractility, we examined the effect of IgG in the presence of atropine (to eliminate the effects of AAM2R) either with the selective β1 blocker CGP-20712A (300 nmol/L) or the selective β2 blocker ICI-118551 (300 nmol/L) (Fig. 3). IgG from the 16 patients showing a positive AAβAR inotropic effect in the presence of muscarinic blockade was examined. IgG plus atropine increased the contractility of the Purkinje fibers to 127.3±7.2% of baseline (p<0.001). When the selective β2AR blocker ICI-118551 was added the contractility further increased to 140.5±12.2% of baseline (p<0.001 vs. IgG plus atropine) indicating that the co-existence of AAβ2AR partially suppressed the effect of the AAβ1AR. Contractility was increased when AAβ1AR was blocked with CGP-20712A; but only to 114.5±4.3% of baseline (p<0.001 vs. IgG plus atropine and vs. IgG plus atropine plus ICI-118551), indicating that the net AAβ2AR inotropic effects mediated by Gs and Gi were weakly positive compared to the AAβ1AR. Combined blockade with atropine, CGP-20712A and ICI-118551 eliminated any positive inotropic effect of IgG.
Fig. 3.
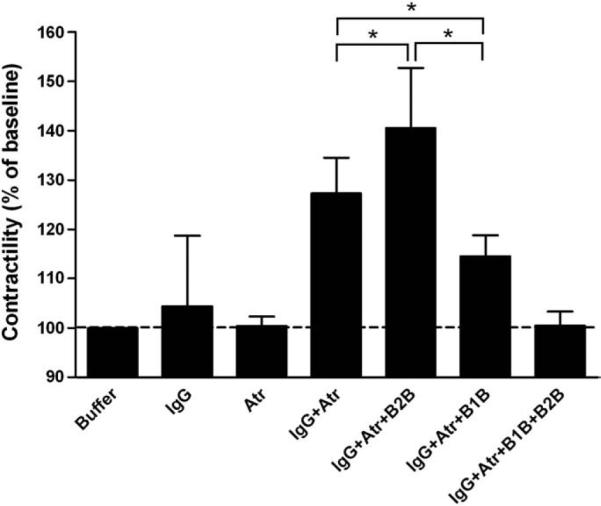
The effect of selective β1 blockade (B1B, CGP-20712A) and β2 blockade (B2B, ICI-118551) on Purkinje fiber contractility in the presence of atropine (Atr). Data are expressed as percent of basal buffer control (dashed line) (mean±SD). IgG with combined M2/β2 blockade resulted in increased contractility compared to M2 blockade alone (*p<0.001 vs. IgG plus atropine, n=16). IgG with combined M2/β1 blockade had a less pronounced, yet significant positive inotropic effect (*p<0.001 vs. IgG plus atropine and vs. IgG plus atropine plus B2B, n=16). Combined M2, β1 and β2 blockade eliminated any positive inotropic effect of IgG (NS vs. atropine, n=16).
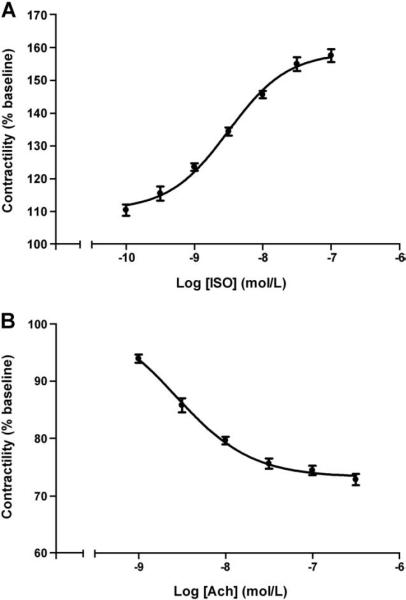
We quantified the effect of IgG from each patient by testing increasing concentrations of ISO and acetylcholine (Ach) in half log increments from 1×10−10 to 1×10−7 and 1×10−9 to 3.2×10−7 mol/L, respectively. ISO produced a dose-dependent increase in contractility with an EC50 value of 3.6±0.5×10−9 mol/L (mean±SEM, n=4) (Fig. 4A). Likewise, Ach produced a dose-dependent decrease in contractility of the Purkinje fibers, with an EC50 value of 2.7±0.4×10−9 mol/L (mean±SEM, n=4) (Fig. 4B).
Fig. 4.
The dose effect of isoproterenol (ISO) and acetylcholine (Ach) on the contractility of Purkinje fibers. Data are expressed as percent of basal buffer control (mean ± SEM). A. ISO-induced increase in contractility (n=4). B. Ach-induced decrease in contractility (n=4).
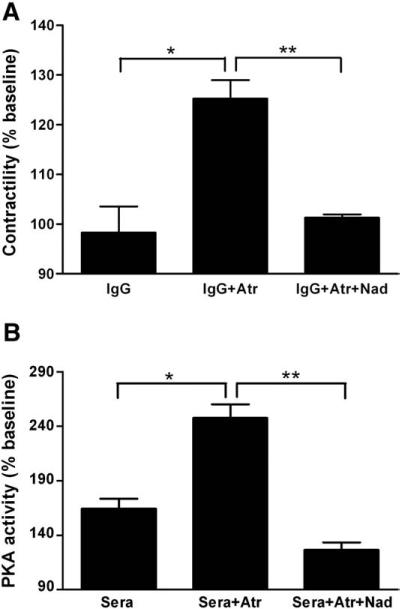
We validated our assay by comparing the effects of 8 IgG samples on Purkinje fiber contractility with their serum effects on PKA activity in H9c2 cardiomyocytes (Fig. 5). The qualitative effects of IgG and their parent serum samples on Purkinje fiber contractility and PKA activity, respectively, in the presence of the atropine, as well as atropine plus nadolol, were similar. The number of discordant pairs was not significantly different between the two assays (p = 0.480 and 1.000 for comparison of the results of the two assays in the presence of atropine and atropine plus nadolol, respectively), indicating similar qualitative βAR and M2 muscarinic IgG effects in the two assays. When small papillary muscles were available in the experimental canine heart (n = 6), the effects of IgG were tested on Purkinje fiber contractility as well as on small papillary muscle contractility (Fig. 6). There was a strong positive correlation of the contractile response of Purkinje fibers and papillary muscles to IgG (r2 = 0.832, p = 0.011), indicating a similar effect of IgG on the two tissues.
Fig. 5.
Similar qualitative effects of 8 selected IgG samples on Purkinje fiber contractility and their parent sera on PKA activity, in the presence of atropine (Atr) and combined blockade with atropine and nadolol (Nad). Data are expressed as percent of baseline (mean ± SD). A. Purkinje fiber contractility. B. PKA activity in H9c2 cells. *p<0.01, **p<0.001.
Fig. 6.
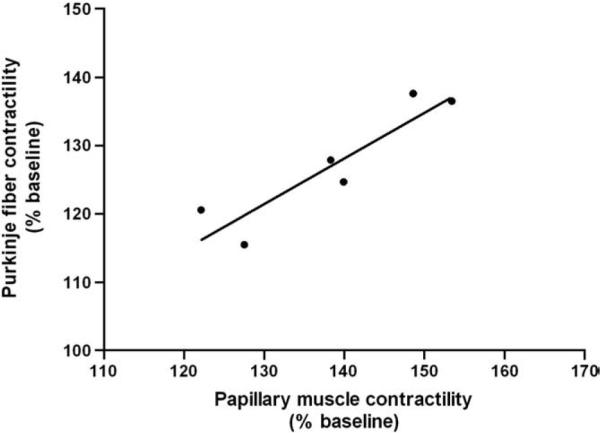
Linear regression of IgG effects from 6 patients on Purkinje fiber and papillary muscle contractility. There is a highly significant and positive relationship (r2=0.832, p=0.011) of these two different assays of autoantibody activation.
4. Discussion
In our study AAM2R and acetylcholine separately produced negative inotropic effects in canine Purkinje fibers. Our data with activating autoantibodies are similar to reports of acetylcholine-induced negative inotropic effects on ventricular muscle in the presence of βAR stimulation (accentuated antagonism) [20,21]. This likely is mediated via M2R coupling to Gi, inhibiting adenylate cyclase and attenuating the βAR-mediated increase in cAMP; although alternative effects through increased K channel conductance and activation of an outward K current (IK,Ach) exist in some species [22,23]. Herein, we are the first to demonstrate that circulating AAM2R, via activation of M2R, compromised the AAβAR-mediated increase in contractility in isolated Purkinje fibers. This AAM2R effect on the contractile response of the ventricular myocardium, with or without the presence of an adrenergic agonist, is capable of altering βAR pathophysiology in vivo and influencing cardiac performance. This “humoral” non-desensitizing exposure to the M2R agonist may be more pervasive than the effects caused by parasympathetic nerve activity. We have demonstrated evidence of AAM2R-mediated physiologic effects in some patients without elevated anti-M2R titers by ELISA. While anti-β1/2AR autoantibodies were used to choose subjects for activity study, it is well established that ELISA alone will not detect all such antibodies [7,24]. Additionally, they will not predict functionality of the antibodies so detected. We found no significant correlation between the ELISA titers and their degree of activity.
Selective β2AR blockade enhanced the IgG-mediated contractility. Unlike β1AR, β2AR couples to both Gs and Gi proteins [25]. Our data suggest that the β1-Gs-mediated effect was partially attenuated by the β2-Gi effect. This is the first report of such an interaction of AAβ1AR and AAβ2AR on myocardial contractility. This interaction may be important in light of the potential role of β2-Gi signaling in the failing heart. β2AR activation via Gi in failing canine cardiac cells blunts β1AR stimulation of ICa,L [26]. AAβ2AR acting via Gi may further attenuate the β1AR contractile effects in the failing heart.
The main purpose of this study was to characterize the interaction between these autoantibodies and not necessarily correlate these changes with any specific etiology. Hence, sera were obtained from a heterogeneous pool of cardiac-related patients based only on their demonstrating high antibody titers. A potential limitation of our study is that Purkinje fibers are a “non-physiologic” model of contractility since they do not contribute to ventricular contractility in vivo. Purkinje fibers were used because they are more readily available than small papillary muscles in the canine heart. To validate this methodology, we compared data derived from the Purkinje model with two supplementary methods (PKA activity assay and papillary muscle contractility). These data were consonant with the Purkinje data and support the validity of our method as a surrogate marker for the effects of IgG on contractility in vivo.
4.1. Implications
These data are the first to document the opposing effects of activating autoantibodies from human sources on ventricular function. The detrimental effects of long-term activation of the β-adrenergic system from stress [27], pheochromocytoma [28], intrinsic molecular activation of the βAR [27] or from circulating activating autoantibodies [29] have been previously recognized. The present data support the concept that circulating activating autoantibodies to the muscarinic M2R may exert an inhibitory effect on ventricular contractility and thereby play a role in cardiomyopathic pathophysiology. M2R activation exerts a negative impact on cAMP-mediated PKA activation and would exert a similarly negative effect on diastolic relaxation often observed in cardiomyopathies. Although not measured in this study, the co-presence of activating autoantibodies to both β1/2AR and M2R has relevance to the genesis of atrial tachyarrhythmias [30].
This study is the first to examine the relative effects of autoantibody activation of specific β1 and β2ARs. The relative changes in density of these two receptors have been recognized as having significance to the failing heart [31,32]; but there is controversy as to their relative importance in either genesis or amelioration of the increasing desensitization of the heart to adrenergic stimulation. The relative importance of β1AR vs. β2AR stimulation as deleterious factors in the progression of heart failure is unclear [33]. Our study demonstrates that β1AR and β2AR activation by antibodies differ quantitatively and studies of their role in disease states should be mindful of their differential activities. The relative specificity of autoantibodies to the differing 2nd extracellular loop sequences for β1AR and β2AR provides an opportunity to examine their relative importance in contractility. The present study confirms and extends these concepts to demonstrate that activation of the β2AR by the circulating autoantibody exerts a partially inhibitory effect on β1AR activation even while it possesses intrinsic activation potential when β1AR are blocked. These data suggest that production of monoclonal activating autoantibodies to each receptor subtype will provide powerful tools for dissecting the interactions of cardiac receptors with increasing specificity compared to presently available pharmacologic tools.
4.2. From theory to practice
These data support an inhibitory effect of AAM2R on AAβAR-induced contractility in Purkinje fibers. Inasmuch as these data mimic those observed in ventricular papillary tissues, there is reason to believe that their co-presence similarly may negatively influence ventricular contractility and performance in patients harboring these autoantibo-dies. Since β-blockers are routinely used in many of these clinical conditions, the absence of specific M2R blockade may place the patients in a disadvantageous situation because of the unopposed muscarinic effect. In cases in which an unexplained or inverse response to β-blockers is encountered, one might suspect the presence of opposing muscarinic autoantibodies. Our data support somewhat different effects of β1and β2AR activation on contractility. This raises the question as to whether cardioselective β1AR blockade or non-selective β1/2AR blockade might be indicated for a given patient depending on the patient's “mix” of activating autoantibodies. Since the several studies using these agents have not screened their subjects for activating autoantibodies, this is a question that will require further study in a prospective manner before any objective decision can be made.
Acknowledgements
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology [34].
The study was supported by the American Heart Association, Presbyterian Health Foundation (X.Y.), Heart Rhythm Institute, OUHSC and Veterans Administration Research Foundation (D.C.K, E.P.). Private grants from Will and Helen Webster, Britani T. and Paul E. Bowman, Jr., and Stan and Gayle Ward were gratefully received.
Footnotes
Presented in part at the Annual High Blood Pressure Research Conference of the American Heart Association, Sep 26–29, 2007, Tucson, AZ.
References
- [1].Magnusson Y, Marullo S, Hoyer S, et al. Mapping of a functional autoimmune epitope on the beta 1-adrenergic receptor in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1990;86:1658–63. doi: 10.1172/JCI114888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fu LX, Magnusson Y, Bergh CH, et al. Localization of a functional autoimmune epitope on the muscarinic acetylcholine receptor-2 in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1993;91:1964–8. doi: 10.1172/JCI116416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Del Corsso C, de Carvalho AC, Martino HF, Varanda WA. Sera from patients with idiopathic dilated cardiomyopathy decrease ICa in cardiomyocytes isolated from rabbits. Am J Physiol Heart Circ Physiol. 2004;287:H1928–36. doi: 10.1152/ajpheart.00044.2004. [DOI] [PubMed] [Google Scholar]
- [4].Zhang L, Hu D, Li J, Wu Y, Liu X, Yang X. Autoantibodies against the myocardial beta1-adrenergic and M2-muscarinic receptors in patients with congestive heart failure. Chin Med J (Engl) 2002;115:1127–31. [PubMed] [Google Scholar]
- [5].Baba A, Yoshikawa T, Fukuda Y, et al. Autoantibodies against M2-muscarinic acetylcholine receptors: new upstream targets in atrial fibrillation in patients with dilated cardiomyopathy. Eur Heart J. 2004;25:1108–15. doi: 10.1016/j.ehj.2004.05.012. [DOI] [PubMed] [Google Scholar]
- [6].Liu HR, Zhao RR, Zhi JM, Wu BW, Fu ML. Screening of serum autoantibodies to cardiac beta1-adrenoceptors and M2-muscarinic acetylcholine receptors in 408 healthy subjects of varying ages. Autoimmunity. 1999;29:43–51. doi: 10.3109/08916939908995971. [DOI] [PubMed] [Google Scholar]
- [7].Nikolaev VO, Boivin V, Stork S, et al. A novel fluorescence method for the rapid detection of functional beta1-adrenergic receptor autoantibodies in heart failure. J Am Coll Cardiol. 2007;50:423–31. doi: 10.1016/j.jacc.2007.03.051. [DOI] [PubMed] [Google Scholar]
- [8].Wallukat G, Wollenberger A, Morwinski R, Pitschner HF. Anti-beta 1-adrenoceptor autoantibodies with chronotropic activity from the serum of patients with dilated cardiomyopathy: mapping of epitopes in the first and second extracellular loops. J Mol Cell Cardiol. 1995;27:397–406. doi: 10.1016/s0022-2828(08)80036-3. [DOI] [PubMed] [Google Scholar]
- [9].Christ T, Wettwer E, Dobrev D, et al. Autoantibodies against the beta1 adrenoceptor from patients with dilated cardiomyopathy prolong action potential duration and enhance contractility in isolated cardiomyocytes. J Mol Cell Cardiol. 2001;33:1515–25. doi: 10.1006/jmcc.2001.1414. [DOI] [PubMed] [Google Scholar]
- [10].Chiale PA, Ferrari I, Mahler E, et al. Differential profile and biochemical effects of antiautonomic membrane receptor antibodies in ventricular arrhythmias and sinus node dysfunction. Circulation. 2001;103:1765–71. doi: 10.1161/01.cir.103.13.1765. [DOI] [PubMed] [Google Scholar]
- [11].Mijares A, Lebesgue D, Argibay J, Hoebeke J. Anti-peptide antibodies sensitive to the `active' state of the beta2-adrenergic receptor. FEBS Lett. 1996;399:188–91. doi: 10.1016/s0014-5793(96)01321-x. [DOI] [PubMed] [Google Scholar]
- [12].Fu ML, Schulze W, Wallukat G, Hjalmarson A, Hoebeke J. A synthetic peptide corresponding to the second extracellular loop of the human M2 acetylcholine receptor induces pharmacological and morphological changes in cardiomyocytes by active immunization after 6 months in rabbits. Clin Immunol Immunopathol. 1996;78:203–7. doi: 10.1006/clin.1996.0030. [DOI] [PubMed] [Google Scholar]
- [13].Jahns R, Boivin V, Hein L, et al. Direct evidence for a beta 1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest. 2004;113:1419–29. doi: 10.1172/JCI20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Matsui S, Fu M, Hayase M, et al. Transfer of immune components from rabbit autoimmune cardiomyopathy into severe combined immunodeficiency (SCID) mice induces cardiomyopathic changes. Autoimmunity. 2006;39:121–8. doi: 10.1080/08916930500314855. [DOI] [PubMed] [Google Scholar]
- [15].Matsui S, Fu ML, Hayase M, et al. Active immunization of combined beta1-adrenoceptor and M2-muscarinic receptor peptides induces cardiac hypertrophy in rabbits. J Card Fail. 1999;5:246–54. doi: 10.1016/s1071-9164(99)90009-x. [DOI] [PubMed] [Google Scholar]
- [16].Skomedal T, Fu ML, Hjalmarson A, Hoebeke J, Schiander IG, Osnes JB. Anti-M2 muscarinic receptor antibodies inhibit beta-adrenoceptor-mediated inotropic response in rat myocardium. Eur J Pharmacol. 1997;333:169–75. doi: 10.1016/s0014-2999(97)01127-8. [DOI] [PubMed] [Google Scholar]
- [17].Koglin J, Bohm M, von Scheidt W, Stablein A, Erdmann E. Antiadrenergic effect of carbachol but not of adenosine on contractility in the intact human ventricle in vivo. J Am Coll Cardiol. 1994;23:678–83. doi: 10.1016/0735-1097(94)90754-4. [DOI] [PubMed] [Google Scholar]
- [18].Higgins CB, Vatner SF, Braunwald E. Parasympathetic control of the heart. Pharmacol Rev. 1973;25:119–55. [PubMed] [Google Scholar]
- [19].Kem DC, Yu X, Patterson E, et al. Autoimmune hypertensive syndrome. Hypertension. 2007;50:829–34. doi: 10.1161/HYPERTENSIONAHA.107.096750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bailey JC, Watanabe AM, Besch HR, Jr, Lathrop DA. Acetylcholine antagonism of the electrophysiological effects of isoproterenol on canine cardiac Purkinje fibers. Circ Res. 1979;44:378–83. doi: 10.1161/01.res.44.3.378. [DOI] [PubMed] [Google Scholar]
- [21].Du XY, Schoemaker RG, Bos E, Saxena PR. Characterization of the positive and negative inotropic effects of acetylcholine in the human myocardium. Eur J Pharmacol. 1995;284:119–27. doi: 10.1016/0014-2999(95)00384-w. [DOI] [PubMed] [Google Scholar]
- [22].Dhein S, van Koppen CJ, Brodde OE. Muscarinic receptors in the mammalian heart. Pharmacol Res. 2001;44:161–82. doi: 10.1006/phrs.2001.0835. [DOI] [PubMed] [Google Scholar]
- [23].Harvey RD, Belevych AE. Muscarinic regulation of cardiac ion channels. Br J Pharmacol. 2003;139:1074–84. doi: 10.1038/sj.bjp.0705338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Caforio AL, Daliento L, Angelini A, et al. Autoimmune myocarditis and dilated cardiomyopathy: focus on cardiac autoantibodies. Lupus. 2005;14:652–5. doi: 10.1191/0961203305lu2193oa. [DOI] [PubMed] [Google Scholar]
- [25].Zhu W, Zeng X, Zheng M, Xiao RP. The enigma of beta2-adrenergic receptor Gi signaling in the heart: the good, the bad, and the ugly. Circ Res. 2005;97:507–9. doi: 10.1161/01.RES.0000184615.56822.bd. [DOI] [PubMed] [Google Scholar]
- [26].He JQ, Balijepalli RC, Haworth RA, Kamp TJ. Crosstalk of beta-adrenergic receptor subtypes through Gi blunts beta-adrenergic stimulation of L-type Ca2+ channels in canine heart failure. Circ Res. 2005;97:566–73. doi: 10.1161/01.RES.0000181160.31851.05. [DOI] [PubMed] [Google Scholar]
- [27].Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- [28].Sardesai SH, Mourant AJ, Sivathandon Y, Farrow R, Gibbons DO. Phaeochromocytoma and catecholamine induced cardiomyopathy presenting as heart failure. Br Heart J. 1990;63:234–7. doi: 10.1136/hrt.63.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jahns R, Boivin V, Siegmund C, Inselmann G, Lohse MJ, Boege F. Autoantibodies activating human beta1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation. 1999;99:649–54. doi: 10.1161/01.cir.99.5.649. [DOI] [PubMed] [Google Scholar]
- [30].Scherlag BJ, Patterson E, Po SS. The neural basis of atrial fibrillation. J Electrocardiol. 2006;39:S180–3. doi: 10.1016/j.jelectrocard.2006.05.021. [DOI] [PubMed] [Google Scholar]
- [31].Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation. 1993;87:454–63. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- [32].Bristow MR. beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–69. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- [33].Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- [34].Coats AJ. Ethical authorship and publishing. Int J Cardiol. 2009;131:149–50. doi: 10.1016/j.ijcard.2008.11.048. [DOI] [PubMed] [Google Scholar]