Abstract
Risk assessment during clinical product development needs to be conducted in a thorough and rigorous manner. However, it is impossible to identify all safety concerns during controlled clinical trials. Once a product is marketed, there is generally a large increase in the number of patients exposed, including those with comorbid conditions and those being treated with concomitant medications. Therefore, postmarketing safety data collection and clinical risk assessment based on observational data are critical for evaluating and characterizing a product’s risk profile and for making informed decisions on risk minimization. Information science promises to deliver effective e-clinical or e-health solutions to realize several core benefits: time savings, high quality, cost reductions, and increased efficiencies with safer and more efficacious medicines. The development and use of standard-based pharmacovigilance system with integration connection to electronic medical records, electronic health records, and clinical data management system holds promise as a tool for enabling early drug safety detections, data mining, results interpretation, assisting in safety decision making, and clinical collaborations among clinical partners or different functional groups. The availability of a publicly accessible global safety database updated on a frequent basis would further enhance detection and communication about safety issues. Due to recent high-profile drug safety problems, the pharmaceutical industry is faced with greater regulatory enforcement and increased accountability demands for the protection and welfare of patients. This changing climate requires biopharmaceutical companies to take a more proactive approach in dealing with drug safety and pharmacovigilance.
Keywords: information technology, pharmacovigilance, safety, standard, risk management, adverse event, adverse drug reaction
Introduction
It is recognized that information technology (IT) has entered and transformed the world of health care and clinical medicine in which the work of doctors and the care of patients proceed with higher quality, efficiency and lower cost. It is also no secret that IT has merged into clinical safety practice and sparks the creation of worldwide pharmacovigilance systems for safety signal detection. The IT transformative force and health IT adoption have fundamentally changed the conduct of clinical research, practice of medicine, and medicinal safety monitoring.
In the wake of recent drug withdrawals, to regain the trust of patients, health care providers and regulators demand that biopharmaceutical or medical device firms show a demonstrated commitment to safety that goes beyond mere compliance. In today’s world, pharmacovigilance pushes new boundaries and it is no longer sufficient to simply report adverse events along with efficacy and quality requirements. Regulators are demanding proactive surveillance programs that include comprehensive risk management plans and signal detection/analysis throughout a clinical product’s lifecycle. Organizations that take the lead in developing a more proactive and long-term approach to manage the safety of their products recognize that success requires a continuous, consistent process from preclinical research onward. This is achieved through developing a good clinical safety practice that shows the company was aware of and acted on every safety issue as it developed for a product or device. In this review, we seek to clarify some of the issues that are central to current discussions about pharmacovigilance, focusing on topics critical to biopharmaceutical or medical device companies with marketed products in human use. This paper is prepared from industry perspectives to present and analyze benefits, advantages, challenges and risks associated with pharmacovigilance based on systematic overview. This article addresses four questions: What exactly is pharmacovigilance? What do we know of its benefits and risks? What challenges are out there preventing its widespread usage? And what does the future hold for pharmacovigilance in worldwide medicine?
It is now generally accepted that part of the process of evaluating drug safety needs to happen in the postmarketing phase though judgment as to whether and how this might happen lies with the regulators. The stronger the national systems of pharmacovigilance and adverse drug reaction (ADR) reporting, the more likely reasonable regulatory decisions will be made for the early release of new drugs with the promise of therapeutic advances. Legislation governing the regulatory process in most countries allows for conditions to be placed on approvals, such as a requirement that there should be detailed pharmacovigilance in the early years after a drug’s release. Careful safety monitoring is not restricted, however, to new drugs or to significant therapeutic advances. It has a critical role to play in the introduction of generic medicines, and in review of the safety profile of older medicines already available as well, where new safety issues may have arisen. While spontaneous reporting remains a cornerstone of pharmacovigilance in the regulatory environment, and is indispensable for signal detection, the need for more active surveillance has also become increasingly clear. Without information on utilization and on the extent of consumption, spontaneous reports are unable to determine the frequency of an ADR attributable to a product, or its safety in relation to a comparator.1 More systematic and robust epidemiological methods that take into account the limitations of spontaneous reporting or postmarketing studies are required to address these key safety questions. They need to be incorporated into postmarketing surveillance programs.
What is pharmacovigilance?
Pharmacovigilance is a branch of pharmacological science encompassing all scientific and data gathering activities relating to the detection, assessment, understanding and prevention of adverse events of medicines and medical devices.1 This includes the use of pharmacoepidemiologic studies. These activities are undertaken with the goal of identifying adverse events and understanding, to the extent possible, their nature, frequency, and potential risk factors. Pharmacovigilance in principle involves the identification and evaluation of safety signals. Safety signal refers to a concern about an excess of adverse events compared to what would be expected to be associated with a product’s use. Signals can arise from postmarketing data and other sources, such as preclinical data and events associated with other products in the same pharmacologic class. It is possible that even a single well documented case report can be viewed as a signal, particularly if the report describes a positive rechallenge or if the event is extremely rare in the absence of drug use. Signals generally indicate the need for further investigation, which may or may not lead to the conclusion that the product caused the event. After a signal is identified, it should be further assessed to determine whether it represents a potential safety risk and whether other action should be taken.2 Pharmacovigilance is particularly concerned with adverse drug reactions, or ADRs, which are defined as: “A response to a drug which is noxious and unintended, and which occurs at doses normally used… for the prophylaxis, diagnosis or therapy of disease, or for the modification of physiological function.”3 Many other issues are also relevant to pharmacovigilance science:1
Substandard medicines
Medication errors
Lack of efficacy reports
Use of medicines for indications that are not approved and for which there is inadequate scientific basis
Case reports of acute and chronic poisoning
Assessment of drug-related mortality
Abuse and misuse of medicines
Adverse interactions of medicines with chemicals, other medicines, and food
The specific aims of pharmacovigilance are to:1
Improve patient care and safety in relation to the use of medicines and all medical and paramedical interventions
Improve public health and safety in relation to the use of medicines
Contribute to the assessment of benefit, harm, effectiveness and risk of medicines, encouraging their safe, rational and more effective (including cost-effective) use
Promote understanding, education and clinical training in pharmacovigilance and its effective communication to the public.
Pharmacovigilance has developed and will continue to develop in response to the special needs and according to the particular strengths of members of the WHO program and beyond. Such active influence needs to be encouraged and fostered; it is a source of vigor and originality that has contributed much to international practice and standards. Pharmacovigilance is gaining traction among doctors and scientists as the number of stories of drug recalls increases in the global mass media. Because clinical trials involve several thousand patients at most, less common side effects and ADRs are often unknown at the time a drug enters the market. Even very severe ADRs, such as liver damage, are often undetected because study populations are small. Postmarketing pharmacovigilance uses tools such as data mining and electronic case report forms to identify the relationships between drugs and ADRs. In brief, an electronic data capture (EDC) system is a computerized system designed for automated support of clinical data collection, reporting, query resolution, randomization, and validation, among other features, in conducting clinical trials. Though EDC technologies offer superior advantages over traditional paper-based systems, collecting, monitoring, coding, reconciling, and analyzing safety data can be challenging.4 To realize the full potential of the information revolution in e-clinical research as compared with traditional paper-based studies, both sponsor and site users will probably have to change the way their offices and days are organized, how they enter and retrieve patient information, how data is entered, the process by which they issue, answer, or close queries, and the ways in which they relate to colleagues and clinical research organizations (CROs) and interact with their patients. Safety scientists will have to find ways to understand and analyze huge amounts of safety information across different studies or systems and coordinate with third party independent committees to enter adjudication results. In other words, effective use of e-technologies depends as much on managing change as it does on information management, and change has never been easy for sponsor e-clinical system implementation and integration.
The capacity of IT staff to realize this transformational vision will also depend on something else: whether the e-systems introduced are designed or configured to capture the protocol required or compliance necessitated information such as unanticipated ADRs (UADRs). It is one thing to digitize the current case report form so that the information sponsors now require is available to them electronically. It is another thing to make certain that all the data needed for pharmacovigilance purposes are collected, coded properly, and data are accessible for functional group review and reconcile with in-house product performance system, organized, apply decision algorithms, and provide the result to management and regulatory agencies when and where they need it. The EDC technology products now being sold are intended to meet the present needs of both sponsor and site users with certain vendor-based differential functions – as would any product be that is aimed at attracting consumers in a well-functioning market. Indeed, our experience indicates that understanding limitations and opportunities offered by EDC vendor, configuring EDC system to meet data capturing needs based on sponsor IT or data management profile, and collaborating with vendor to offer flexible configurations, are key to EDC implementation success.5 Technology innovators and EDC vendors, however, imagine a world in which electronic system meets needs that most sponsor and site users do not think they have. How to meet future needs, how to integrate EDC clinical trial data with eHR, and how to persuade EDC vendors to invest in such innovative systems, is something involving collaborative efforts from many players.
Benefits and risks of pharmacovigilance technologies
The idea that randomized clinical trials can establish product safety and effectiveness is a core principle of the pharmaceutical industry. Neither the clinical trials process nor the approval procedures of the US Food and Drug Administration (FDA) provide a perfect guarantee of safety for all potential consumers under all circumstances. Despite this fact, there are viable pharmacovigilance technology solutions that biopharmaceutical companies can implement to systematically detect, assess, understand, and prevent adverse drug reactions. When built into clinical research and development practices, pharmacovigilance technologies assist biopharmaceutical firms in enhancing patient safety while reducing or even preventing costly safety-related withdrawals. It is recognized that clinical data mining and signal detection associated with pharmacovigilance technology contribute to potential benefits in providing:6
Systematic, automated and practical means of screening large datasets
Better utilization of the large safety databases maintained by the FDA, the World Health Organization (WHO) and other organizations
Improved efficiency by focusing pharmacovigilance efforts on key reporting associations
Positive contributions to public health by identifying potential safety issues more quickly and/or more accurately than traditional pharmacovigilance methods
Better decision support for the pharmaceutical industry and regulators
Potential to clarify the many complex interdependent factors (eg, concomitant drugs and/or diseases) that can play a role in the development of adverse events in a clinical setting
Value by detecting disproportionalities involving multiple drugs or multiple events that would be too difficult to detect by traditional methods.
Adopting good pharmacovigilance practice in clinical safety monitoring and analysis and having an aptitude to utilize the advantages pharmacovigilance technology solutions provide are key to unlock the power of pharmacovigilance and maximize clinical safety returns in an evolving drug safety environment. One needs to realize that pharmacovigilance is a tool and should be applied into clinical context to achieve its intended functions. One critical component of good pharmacovigilance practice is centered on acquiring complete quality data from reported source on adverse events. Spontaneous case reports of adverse events submitted to the sponsor and FDA, and reports from other sources, such as the medical literature or clinical studies, may generate signals of adverse effects of drugs. The quality of the reports is critical for appropriate evaluation of the relationship between the product and adverse events.2 Table 1 summarizes necessary good case report data elements, what to be included for reporting medication errors for recommended good case reports, key features that may suggest a causal relationship between the use of a product and the adverse event, and critical analysis points that characterize and identify risk factors.
Table 1.
Good case report characteristics and summarized descriptive analysis points of a case series
| Clinical data elements | Medication error | Causal relationship features | Critical analysis – case series |
|---|---|---|---|
| AE description or disease experience, including time to onset of signs or symptoms | Products involved | Occurrence of the adverse event in the expected time | The clinical and laboratory manifestations and course of the event |
| Suspected and concomitant MEDS details (dose, lot number, schedule, dates, duration) | Sequence of events leading up to the error | Absence of symptoms related to the event prior to exposure | Demographic characteristics of patients with events (age, gender, race) |
| Documentation of AE diagnosis, including methods used to make the diagnosis | Work environment in which the error occurred | Evidence of positive dechallenge or positive rechallenge | Exposure duration |
| Clinical course of the event and patient outcomes (eg, hospitalization or death) | Types of personnel involved with the error, type(s) of error, and contributing factors | Consistency of the event with the established pharmacological/toxicological effects of the product | Time from initiation of product exposure to the adverse event; |
| Relevant therapeutic measures and laboratory data at baseline, during therapy, and subsequent to therapy | Patient outcomes may not be available at initial reporting. F/U reports can convey important information about the course of the event and serious outcomes, such as hospitalization or death | Consistency of the event with the known effects of other products in the class | Doses used in cases, including labeled doses, greater than labeled doses, and overdoses; |
| Information about response to dechallenge and rechallenge | All appropriate information outlined in NCC MERP | Other supporting evidence from preclinical, clinical, and/or pharmacoepidemiologic studies | Use of concomitant medications |
| Any other relevant information | Absence of alternative explanations for the event | Presence of co-morbid conditions Route of administration Lot numbers, if available, for products used in patients with AE Changes in event reporting rate over calendar time or product life cycle |
Abbreviations: AE, adverse event; MEDS, medications; NCC MERP, National Coordinating Council for Medication Error Reporting and Prevention; F/U, Follow-up.
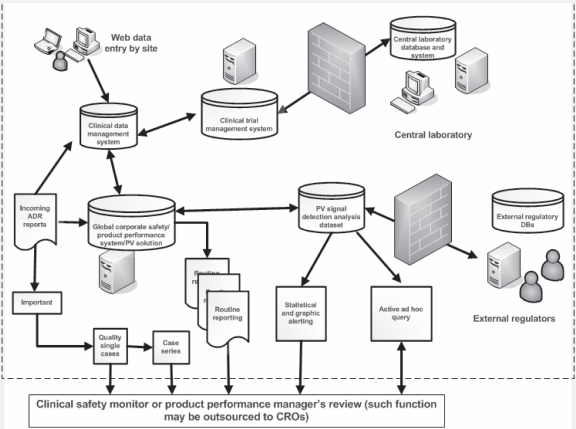
Good pharmacovigilance processes (GPVP) focus on the enhancement of the reports that are most likely to be important. In parallel to any regulatory reporting (submission) triage, these reports need to have both their potential maximized and the value measured. That measurement must be both as an individual and in a case-series. The goal of GPVP is to clearly and accurately identify rare, serious, unusual or unexpected adverse drug reactions such as UADEs as soon as possible after product market launch. Therefore, GPVP in the 21st century is best practices-driven with IT systems-enabling support. Modern internet and communication technologies have allowed these best practices to be joined in a supportable process. They support the ‘clinical art’ of pharmacovigilance by providing service-oriented architecture, powerful data-mining and query tools, and work environments for case-series management and product preparation. Pharmacovigilance technology systems can be effective in detecting what may be otherwise invisible to the human eye, increasing productivity and are less likely to miss important public health information hidden in “haystacks” of data.7 It is demonstrated that full product safety assessment can be conducted by such technologies in an efficient and effective manner (Figure 1).
Figure 1.
The good pharmacovigilance processes and workflow in a typical sponsor biopharmaceutical firm supported by information technology. Modern internet and communication technologies have enabled improved clinical safety monitoring in a significant way despite anticipated ongoing challenges.
Abbreviations: ADR, adverse drug reaction; DB, database; PV, pharmacovigilance; CRO, clinical research organization.
Spontaneous reporting is the core data-generating system of international pharmacovigilance, relying on health care professionals (and in some places consumers) to identify and report any suspected ADR to their national pharmacovigilance center or to the manufacturer.8 Spontaneous reports are almost always submitted voluntarily. One of this system’s major weaknesses is under-reporting, a major potential risk area in data mining inherent to postmarketing safety databases that no signal detection method is likely to overcome. There are published examples of known safety issues that are not retrospectively identified by data-mining methods using pre-defined thresholds.6 Another problem is that overworked medical personnel do not always see reporting as a priority. If the symptoms are not serious, they may not notice them at all. Even if the symptoms are serious, they may not be recognized as the effect of a particular drug. Even so, spontaneous reports are a crucial element in the worldwide enterprise of pharmacovigilance and form the core of the WHO database, which includes around 4.6 million reports (as of January 2009),9 growing annually by about 250,000.10 There are concerns that, in some situations, data mining may generate more signals than can be followed up effectively with available resources. In this case, focus might be directed to signals with the greatest public health impact and seriousness. There is also concern about the lack of systematic, objective validation of the methods, a problem that also exists for traditional pharmacovigilance methods. Unfortunately, efforts to validate data-mining methods (and traditional methods) are complicated by the absence of a gold standard for identifying true drug toxicities, although various imperfect reference standards may be used to obtain useful insights on the performance of any method. It is not practical to evaluate data-mining methods or traditional methods using performance criteria generally accepted for screening and diagnostic tests.6 The final risk factor worth noting is that pharmacovigilance systems may generate errant signals that turn out to be false alarms. This can occur because other factors, not adequately adjusted for, confound the apparent relationship. Physicians generally select treatments on the basis of the subtleties of a patient’s clinical status, as well as their own practice preferences. As automated algorithms search for associations between medication use and adverse events in large observational data sets, rigorous analytic techniques will be necessary to ensure that confounding does not produce spurious associations that could generate safety signals warning of nonexistent hazards. Or, equally problematic, inadequate analysis could conceal a true risk signal that might have been evident with more careful adjustment. Simplistic data mining yielding inadequately adjusted drug–event associations could thus be counterproductive even for first-step signal-generation analysis. Fortunately, more sophisticated approaches are available to mitigate these risk-assessment risks. Partially automated processes based on epidemiologic principles can be used to derive relevant covariate information from large, comprehensive data sets and use them for advanced multivariable adjustment procedures.11
Challenges of pharmacovigilance
There are well-known inherent issues in systematically analyzing and interpreting voluntarily submitted data involving multiple drugs, medical conditions, and events per report, without the benefit of a research protocol, randomization, and a control group of persons taking the placebo. Other difficulties include chronic under-reporting, occasional publicity-driven and litigation-driven episodes of over-reporting and misreporting, incomplete and missing information, and inconsistencies and changes over time in reporting and naming/coding practices.7 In addition, there is considerable uncertainty regarding the quality and completeness of the information contained in each data field, including dosage, formulation type, timing of exposure, and length of exposure and follow-up and in estimating the corresponding product exposure and background rate of adverse events. The extraction of useful information from this database presents multiple challenges, including managing, storing, querying, and analyzing such a large amount of data, and resolving event and drug dictionary problems and data miscoding. There is a need for analytical methods that are capable of systematically screening this database to identify potential serious adverse events of concern in such a noisy background that properly balance the concerns for excessive signaling and accounting for background noise.
Another challenge will be determining rules to trigger an alert, when to consider a signal likely enough to be real to warrant follow-up, and when a signal needs to be elevated to represent a potential safety risk.12 If data mining analysis was performed on data for millions of people taking thousands of drugs, statistic significance could emerge as data on a drug–event relationship accumulate, even after adjustment for repeated testing. Such P value-driven thresholds could result from the size of the population and the strength of the supposed association. Taking account of multiple covariates such as severity of adverse events, whether a safe alternative treatment is available, or how much benefit the drug provides will likely cut down the list to prioritize focused follow-up. Sundström and Hallberg applied Bayesian confidence propagation neural network (BCPNN) methodology to calculate the information component (IC) value for drug-event combinations for drugs belonging to the anatomic therapeutic chemical (ATC) classes of the cardiovascular system, musculoskeletal system, and nervous system (number of reports = 51,270) where only the suspected drug was considered, and also where both concomitant and suspected drugs were considered using data from the Swedish Drug Information System and reported that the proportion of “type C” reactions signaled when considering both concomitant and suspected drugs as compared with suspected drugs only.13 Conversely, taking action prematurely on the basis of inadequate data could result in unnecessary confusion and harmful discontinuations of useful treatments. We cannot know now what inputs will be optimal for each decision analysis. But stating such inputs transparently up front will help to clarify the decision-making process of regulators who will have to act on these signals. It will also facilitate the communication of decisions, by enabling regulators to frame recommendations or actions in terms of prestated assumptions about acceptable risks for a given product. If such tools are applied well, the system will be able to provide early notice of adverse drug effects that have previously taken years to discover. It seems that there is a fine balance of judgment on public warnings on possible hazards. Caution needs to be exercised to issue public announcement on unreal hazards. An excessively high threshold for warnings would keep real risks hidden too long, but an excessively low threshold could undermine public trust in clinical products, the surveillance system, and the entire medical world. Proper implementation of the pharmacovigilance technology solution will require expertise in intelligibly communicating information about risks in relation to benefits to clinicians and patients alike.
Challenge area also lies in clinical process re-engineering to ensure modern pharmacovigilance technology systems are configured, tailored, and implemented in the context of addressing safety process improvements and organizational needs to support daily clinical safety operations. In the past four decades from the thalidomide tragedy to the recent drug recalls, companies have used pharmacovigilance methods to identify rare, easily identified safety problems. During the same four decades, we have seen the growth of a fragmented clinical or healt hcare system that lacks a unifying infrastructure. As a result, this system operates primarily in reaction to rather than in anticipation of major pharmaceutical safety events. As drug consumption has increased and the public has grown to expect greater drug safety, the traditional reactive approach has proven largely incapable of addressing both shifts in public expectations and regulatory and media scrutiny. This reality has revealed improvement areas involved in patient safety operations: organizational alignment, operations management, data management, and risk management. Table 2 summarizes key functional activities and recommended best practices under the specified four areas to enable realization of the capability of pharmacovigilance systems in an adaptive operations framework.
Table 2.
Proactive pharmacovigilance best practices and key processes or activities in the areas of organizational alignment, operations management, data management, and risk management
| Organizational alignment | Operations management | Data management | Risk management |
|---|---|---|---|
| Align operational activities across different functional groups and departments | Implement process-driven standard operating procedures, work instructions, and training materials | Design science-driven, site workflow-focused, and standard-based case report forms for post-marketing studies | Develop an objective, data-driven, team-oriented approach to risk monitoring and evaluation |
| Implement well-defined decision-making models, escalation processes, and communication channels | Designate a pharmacovigilance operating model and business process owner (Debatably, this may be under “Risk management”) | Implement data mining techniques to bolster safety analytics, reporting, and investigation | Determine the pharmacovigilance workload and sufficiently resource the required effort |
| Incorporate continuous improvement activities and standardized risk communication plans (Need buy-in from “Risk management”) | Ensure that appropriate process and organizational checks and balances are in place to limit bias and manage regulatory risk | Develop standard edit check specifications for AEs and adjudication process forms | Implement workflow management technology to ensure appropriate transparency and accessibility of safety information |
| Retain key pharmacovigilance personnel with cross-disciplinary expertise and skill sets – This will involve all others as well | Create dashboard to summarize timely awareness of safety risks across the portfolio and timely execution of safety risk minimization activities | Develop standard based metrics reports and data management reports | Select a vendor that best matches the pharmacovigilance operating model, business process and vendor/system selection criteria (Need buy-in from DM) |
| Examine corporate IT platform and have vision for a long term pharmacovigilance strategy | Manage and provide oversight to CROs recruited for portion or all of a clinical study | Lead integration efforts in building interoperability among CDMS, CTMS, Safety System, coding application | Develop risk management action plans based on pre-established risk scoring mitigation processes |
| Re-organize functional groups as needed | May be the owner of coding application and data migration Provide trainings to other functional groups |
Define and provide alert or signal threshold – This will involve others as well |
Abbreviations: AE, adverse event; CRO, clinical research organization; DM, data management; CDMS, clinical data management system; CTMS, clinical trial management system.
Last but not least, standard-based systems integration will present challenges. In sponsor corporate environment, pharmacovigilance technology system needs to establish interoperable channels with other numerous systems: Clinical data management system (CDMS), clinical trial management system, product performance system, clinical coding application, and potential CRO systems. It seems that standardization on signal definitions, common medical domains, clinical data elements, case report forms, adverse events, and medication coding are critical to ensure quality signal analysis. Standardization is also key to ensure success of pooled data analysis among all subjects in the pharmacovigilance databases used. Standardization is challenging because we do not have a standard framework yet to allow full system integration. Though industry seems to agree that XML is the default file format for interchange and messaging, there are many implementation details to be defined and agreed to enable, for instance, a sponsor postmarketing study to talk directly with a hospital electronic health care system. It is due to this same systems interoperability challenge that current sponsor clinical studies need to collect clinical data in a separate EDC or via paper-based case report forms though convergence is expected to continue until electronic medical or electronic health records become more pervasive within the broader health care system. At that point, the ideal solution would be to extract patient data directly from the electronic medical records as opposed to collecting the data in a separate data collection instrument or enable bidirectional channels between electronic medical records and CDMS. Collaboration has begun in several initiatives between Clinical Data Interchange Standards Consortium (CDISC), HL7, National Cancer Institute (NCI), and FDA to encourage adoption of its global standards for clinical research, which should continue to be harmonized with health care standards, to provide a means for interoperability among health care and research systems such that clinical research can support informed health care decisions and improve patient safety.14,15
The future of pharmacovigilance technology
The challenges to manage drug safety efficiently and to adhere to regulatory requirements create the strong impression that widespread adoption of pharmacovigilance is inevitable. As an instrument of reform, pharmacovigilance has attributes that ensure its attractiveness to many groups in a politically and economically divided health care system that is struggling with seemingly insurmountable problems of cost and quality and postmarketing clinical studies as well.
Regulatory bodies such as FDA and European Medicines Agency (EMEA) are intensifying safety regulations, therefore boosting the adoption rates of pharmacovigilance systems by biopharmaceutical firms.16 However, the apparent certainty of pharmacovigilance adoption needs to be constantly reexamined due to considerations of a number of challenging issues. One is whether the current standardization initiatives in reaching interoperability between differential clinical or e-health systems among several standard consortiums such as CDISC, HL7, NCI, and FDA will have any effect on pharmacovigilance. If so, to what extent such implementation level standard may bring changes and affect ongoing pharmacovigilance monitoring activities? On the technical architecture perspective, will modern pharmacovigilance technology system offer multi-tier web based application framework so that even a new clinical standard definition causes minimum modification behind the scene? This certainly presents a challenge call to pharmacovigilance technology vendors to partner with pharmaceutical firms and health care providers to offer flexible, configurable, scalable, and interoperable pharmacovigilance technology solutions to meet the future pharmacovigilance needs in:1 a) increasing globalization; b) web-based sales and information; c) broader safety concerns linked to the patterns of drug use within society; d) collaborative working approach among biopharmaceutical firms, health providers, regulatory agencies, insurance payers, CROs, standards consortiums, and central laboratories.
A second debatable question is whether, if the apparent automation of technical edit checks of pharmacovigilance offers systematic assurance, their definition, range, threshold determination, or data-mining statistical methodology associated with alert or signal triggering requires some level of standardizations to enable consolidated efforts, comparability, and interoperability. If so, achieving this goal requires multiple stakeholders’ contribution and collaboration, among which clinical safety science and statistical modeling matter experts will play ongoing critical roles in ensuring deliverability and objectivity. The primary purpose of these technical autochecks within GPVP are to send alerts or signals, based on pre-defined and configurable thresholds or ranges,13,17 to the reviewers e-mail box for assessment as to whether it is a true signal. It is vital that the clinical safety monitors be assured that any data or sets of data that may have a causal link to one of their drugs be detected as an alert for further evaluation by the clinical risk assessor.
A third unanswered question is how, exactly, the modern pharmacovigilance revolution will recruit the majority of small to mid-sized companies, pharmacies, health care providers, and academic communities who still use labor-intensive traditional pharmacovigilance tools and prefer not to change due to various reasons, concerns, or skeptics on pharmacovigilance. As yet, no clear strategy has developed to assist these entities with the costs of installing, configuring, and integrating, and maintaining pharmacovigilance systems or for convincing them that they can effectively function within the new practice regimes that the new pharmacovigilance may offer and support with better improved return on investment as compared to traditional pharmacovigilance. Additionally, convincing top pharmaceutical companies with well established systems and processes to switch to modern pharmacovigilance systems can be both challenging and exciting. One needs to possess at least the following assets to succeed: demonstration of system functionality, understanding business requirement, commitment to customer service, enhancing configurability, assisting with data migration and system knowledge transfer, offer consultation in preparation of new standard operating procedures or modification of existing ones, and prove cost-saving advantages in the long-term. The most difficult seems to be aligning existing processes to fit in the new system. Often times, biopharmaceutical firms would want the system to have more configurable features.
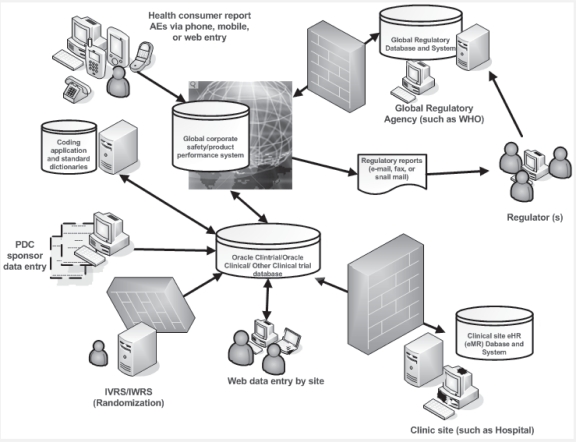
Perhaps the biggest uncertainty concerning pharmacovigilance is whether it will accomplish dramatic, transformational improvement in accurately and reliably detecting clinical safety signals among the millions of haystack of voluntarily reported data. It seems reasonable that traditional pharmacovigilance approaches are still necessitated for confirming a potential signal from an autofired alert, determining a potential safety risk or any action to be taken from a signal in pharmacovigilance. It would be premature to assume that modern pharmacovigilance technology will offer such critical decision-making capability. Even if it does, a thorough manual confirmation would be required at the detailed clinical data levels. Sponsor management is already grappling with the fact that implementing pharmacovigilance corporate wide will require changing, quite dramatically, the work of many different functional groups including but not limited to: IT, clinical data management, safety, product performance, operations, CROs if applicable, clinical sites in order to create an operational safety framework and foster the gear switch to support the implementation of new technology. In the face of this challenge, the will to improve and prosper will be primary, the technology and innovation secondary, and patience and collaboration critical. Creating standards-based and interoperable clinical pharmacovigilance systems in which corporate management and safety staff can find the quality improvement in signal detection and cost reduction essential to accomplishing corporate financial and professional goals will be necessary to widespread adoption of modern pharmacovigilance and to assessing its transformative potential (Figure 2).
Figure 2.
The interoperable clinical research and pharmacovigilance network (ICRPN). Data standard allows communications between electronic health record or medical record and clinical trial data, enabling clinicians and patients to share clinical information across institutional and geographic boundaries with industry sponsors. ICRPNs facilitate this information exchange by bringing together the groups that must participate in it to make the exchange effective. ICRPNs may also provide ongoing governance of the process of data sharing. Data exchange occurs through the information-exchange networks that provide the technical means of exchanging data between the records and databases maintained by clinicians, health care institutions, individual consumers, industry sponsors, and regulators. A systematic contextual approach must be employed to understand and address evolving pharmacovigilance issues.
Abbreviations: PDC, paper based data collection; IVRS, interactive voice response system; IWRS, interactive web response system; AE, adverse event; WHO, world health organization; eHR, electronic health record; eMR, electronic medical record.
The modern pharmacovigilance system will have the potential to identify and quantify adverse-event signals with unprecedented power and performance.18–20 Such data-mining capability coupled with improving standards will provide great benefits to optimize medications’ safety and benefit–risk relationships. Setting up the system to function and ensuring its interoperability with multiple other systems such as clinical data management system, coding applications, clinical trial management system, or product performance system will be a daunting task yet achievable, but making sure the alerts or signals it generates are epidemiologically rigorous and clinically valuable will be of paramount criticality. Ultimately, knowing what data mining numbers mean for practice, confirming potential signal or safety risk via further case report or case-series, and communicating that meaning effectively and promptly will present the biggest challenges of all.11 Collectively, modern pharmacovigilance system is a tool like all other IT ventures, and one still likely to be driven by humans.
Conclusion
The assessment of spontaneous reports is most effective when it is conducted within the defined and rigorous good pharmacovigilance process (GPVP) framework, a functional structure for both public health, health care delivery and corporate risk management strategy. These practices are designed to efficiently and effectively detect and alert the drug safety professional to new and potentially important information on drug adverse reactions. Data mining of adverse event databases is a tool to help with the challenging task of systematically detecting signals among the over 300,000 MedWatch or other similar reports submitted annually to the FDA or similar agencies and is most effectively utilized with full awareness of the limitations and circumstances of voluntary reporting, coding, database characteristics, or quality. Data-mining signals by themselves are not indicators of problems, but indicators of possible problems. Data mining is not intended to replace traditional pharmacovigilance techniques, but to engender improvement and add efficiency. Signals are generated for a relatively small proportion of all distinct drug–event pairs in the database. These signals capture a high proportion of the total number of drug–event pairs reported, greatly facilitating more focused follow-up and prioritized risk assessment.21 Such practices and the overall GPVP are supported by modern internet-based systems with powerful analytical engines, workflow, security, and audit trails to allow validated systems support for proactive drug safety signaling efforts. Future pharmacovigilance technology will have more standardization and interoperability capabilities. It reasons to state that pharmacovigilance has the potential to meet the challenges of the increasing range and potency of medicines (including vaccines); however, there are issues, concerns, challenges and risks involved in implementing and adopting modern pharmacovigilance solutions.
Footnotes
Disclosure
The author reports no conflicts of interest in this work. Moreover, opinions or views expressed through this article represent individual perspectives only.
References
- 1.WHO Collaborating Centre for International Drug Monitoring . The Importance of Pharmacovigilance – Safety Monitoring of Medicinal Products. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- 2.FDA Clinical Medical . Guidance for Industry – Good Pharmacovigilance Practices and Pharmacoepidemiologic Assessment. Rockville, MD: US Food and Drug Administration; 2005. [Google Scholar]
- 3.Aronson JK, Ferner RE. Clarification of terminology in drug safety. Drug Saf. 2005;28:851–870. doi: 10.2165/00002018-200528100-00003. [DOI] [PubMed] [Google Scholar]
- 4.Bobo R, Notte J. Safety reporting in clinical trials. Next Generation Pharmaceutical. Available from: http://www.ngpharma.com/pastissue/article.asp?art=26377&issue=159. Accessed August 22, 2009.
- 5.Spink C. Pharmaceutical Clinical Development: Realising the full rewards of Electronic Data Capture (EDC) IBM Global Services. Mar, 2002. Available from: http://www-935.ibm.com/services/uk/igs/pdf/esrpharmaceutical-clinincal-development-realising-the-full-rewards-of-edc.pdf. Accessed October 6, 2009.
- 6.Almenoff J, Tonning JM, Gould AL, et al. Perspectives on the use of data mining in pharmacovigilance. Drug Saf. 2005;28:981–1007. doi: 10.2165/00002018-200528110-00002. [DOI] [PubMed] [Google Scholar]
- 7.Nelson RC, Palsulich B, Gogolak V. Good pharmacovigilance practices: Technology enabled. Drug Saf. 2002;25:407–414. doi: 10.2165/00002018-200225060-00004. [DOI] [PubMed] [Google Scholar]
- 8.Lindquist M. VigiBase. The WHO Global ICSR Database System: Basic facts. Drug Inf J. 2008;42:409–419. [Google Scholar]
- 9.World Health Organization WHO Programme for International Drug Monitoring. Available from: http://www.who-umc.org/DynPage.aspx?id=13140&mn=1514. Accessed on August 22, 2009.
- 10.Mann RD, Andrews EB, editors. Pharmacovigilance. Chichester, NH: John Wiley & Sons Ltd; 2002. [Google Scholar]
- 11.Avorn J, Schneeweiss S. Managing drug-risk information: What to do with all those new numbers. N Engl J Med. 2009;361:647–649. doi: 10.1056/NEJMp0905466. [DOI] [PubMed] [Google Scholar]
- 12.Szarfman A, Machado SG, O’Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf. 2002;25:381–392. doi: 10.2165/00002018-200225060-00001. [DOI] [PubMed] [Google Scholar]
- 13.Sundström A, Hallberg P. Data mining in pharmacovigilance – detecting the unexpected: the role of index of suspicion of the reporter. Drug Saf. 2009;32:419–427. doi: 10.2165/00002018-200932050-00005. [DOI] [PubMed] [Google Scholar]
- 14.Clinical Data Interchange Standards Consortium (CDISC); CDASH Core and Domain Teams . Austin, TX: Clinical Data Interchange Standards Consortium; Oct, 2008. Clinical Data Acquisition Standards Harmonization (CDASH) [Google Scholar]
- 15.Buetow K. Building a 21st Century Biomedical System: the cancer biomedical informatics grid (caBIG®) Philadelphia, PA: Annual Meeting of DIA Data Conference; Mar 9–11, 2009. [Google Scholar]
- 16.Hochberg AM, Hauben M. Time-to-signal comparison for drug safety data-mining algorithms vs traditional signaling criteria. Clin Pharmacol Ther. 2009;85:600–606. doi: 10.1038/clpt.2009.26. [DOI] [PubMed] [Google Scholar]
- 17.Hauben M, Aronson JK. Defining “signal” and its subtypes in pharma-covigilance based on a systematic review of previous definitions. Drug Saf. 2009;32:99–110. doi: 10.2165/00002018-200932020-00003. [DOI] [PubMed] [Google Scholar]
- 18.Klein DF, O’Brien CP. Improving detection of adverse effects of marketed drugs. JAMA. 2007;298:333–334. doi: 10.1001/jama.298.3.333. [DOI] [PubMed] [Google Scholar]
- 19.Alemenoff JS, Pattishall EN, Gibbs TG, DuMouchel W, Evans SJ, Yuen N. Novel statistical tools for monitoring the safety of marketed drugs. Clin Pharmacol Ther. 2007;82:157–166. doi: 10.1038/sj.clpt.6100258. [DOI] [PubMed] [Google Scholar]
- 20.Hauben M, Aronson JK. Gold standards in pharmacovigilance: the use of definitive anecdotal reports of adverse drug reactions as pure gold and high grade ore. Drug Saf. 2007;30:645–655. doi: 10.2165/00002018-200730080-00001. [DOI] [PubMed] [Google Scholar]
- 21.US Food and Drug Administration The Sentinel Initiative: A National Strategy for Monitoring Medical Product Safety. May, 2008. Available from: http://www.fda.gov/Safety/FDAsSentinelInitiative/ucm089474.htm. Accessed October 6, 2009.