Abstract
Aim:
To assess both serum concentration of metallotionein (MT) and anti-metallothionein (anti-MT) immunoglobulin G (IgG) in autistic children with gastrointestinal (GI) symptoms and controls, and to test the hypothesis that there is an association between the presence of MT, anti-MT IgG, and inflammatory GI disease seen in many children with autistic spectrum disorder (ASD).
Subjects and methods:
ELISAs were used to measure serum MT and anti-MT IgG in 41 autistic children with chronic digestive disease (many with ileo-colonic lymphoid nodular hyperplasia [LNH] and inflammation of the colorectum, small bowel, and/or stomach), and 33 controls (17 age-matched autistic children with no GI disease and 16 age-matched children without autism or GI disease).
Results:
Ten of 41 autistic children with chronic digestive disease had high serum concentration of MT compared to only one of the 33 controls (p < 0.01). Thirteen of the 41 autistic children with chronic digestive disease had anti-MT IgG compared to only four of 33 controls (p < 0.01). Nine of 10 (90%) of autistic children with GI disease with high MT levels had a regressive onset (compared to the expected 25 of 41, or 61%, in this group) (p < 0.05), whereas only nine of 13 of the autistic children with GI disease and anti-MT IgG had a regressive onset (70%) which was not significantly higher than the expected. We didn’t find any correlation between severity of GI disease and MT concentration or anti-MT IgG.
Discussion:
These results suggest a relationship between MT, anti-MT IgG and GI disease seen in many ASD individuals.
Keywords: autism, metallothionein, anti-metallothionein, GI disease
Introduction
Autistic spectrum disorder (ASD) is a neurodevelopmental syndrome with onset prior to age 36 months. Diagnostic criteria consist of impairments in sociality and communication plus repetitive and stereotypic behaviors.1 Traits strongly associated with autism include movement disorders and sensory dysfunctions.2 Although autism may be apparent soon after birth, most autistic children experience at least several months, up to a year or more in some cases, of normal development, followed by regression, defined as loss of function or failure to progress.2–4
The neurotoxicity of mercury (Hg) has long been recognized.5 Primary data come from victims of contaminated fish or grain, from acrodynia induced by Hg in teething powders, and from individual instances of mercury poisoning (HgP), many occurring in occupational settings. More recently, the Food and Drug Administration (FDA) and the American Academy of Pediatrics (AAP) have determined that the typical amount of Hg injected into infants and toddlers via childhood immunizations has exceeded government safety guidelines on an individual6 and cumulative vaccine basis.7 The mercury in vaccines derives from thimerosal (TMS), a preservative which is 49.6% ethylmercury (eHg).7 There may be an association between mercury toxicity and the onset of autism.4,8–10 This association is still unclear, however, since there are several recent reports showing no relationship between mercury exposure and autism.11–13
The metallothioneins (MT) are a family of small proteins containing 61–68 amino acids with an unusually high concentration of cysteine (30%). MT-1, the most functional and active MT in humans, has 21 cysteines. Cysteine contains a sulfhydryl group (SH) that has the ability to react with a number of metals including zinc, mercury, copper and cadmium.
Divalent metals such as copper, zinc, and manganese are toxic to cells in elemental or ionic form. These metals are “enveloped” or “bound” to the small linear MT, which supervises and regulates metal levels in blood, brain and the periphery and therefore plays a major role in heavy metal detoxification of these metals.14–17
Besides detoxification of heavy metals, functions of MT in the body include development of brain neurons, maturation of the GI tract, antioxidation, boosting immune function and delivery of zinc to cells.14,18–20 Evidence also suggests that autistic individuals are prone to developing autoimmune disorders and autism appears to be more common in families with a history of autoimmune disorders.21–24
MT dysfunction may result, then, in many of the issues seen with autistic children, such as the leaky gut syndrome, incomplete breakdown of casein/gluten protein by zinc-dependent enzymes, disrupted ability to combat yeast, reduced production of stomach acid, and impaired stimulation of the pancreas by secretin. It may also lead to inability to clear the body of heavy metals, a dysfunctional immune system, and ultimately to the neurological changes seen in ASD. It would also explain the male sex predominance (4:1) seen in autism, because MT synthesis is enhanced by estrogen and progesterone. In a study of 503 autism-spectrum patients at The Pfeiffer Treatment Center, scientists found abnormal levels of copper and zinc in blood indicating defective functioning of MT proteins.25
MT levels fluctuate in direct response to heavy metal levels such as zinc.26 The fluctuation of MT levels and/or the production of abnormally structured MT in autistic individuals may stimulate an autoimmune response.
In this study, we tested the hypothesis that levels of MT and anti-MT IgG might be associated with gastrointestinal (GI) disease, particularly inflammation, found in many autistic children.
Materials and methods
ELISA to measure auto-antibodies to metallothionein
Purified metallothionein-1 (Sigma, St. Louis, MO), at a concentration of 500 ng/μl of bicarbonate buffer (pH 9.6), was fixed to wells of a 96 well polystyrene microculture plate (Corning Glass Works Co., Corning, NY) by incubation overnight at 4 °C. Excess metallothionein was dumped from wells and all wells were blocked by washing 3× with 300 microliters of blocking solution (Superblock, Pierce Chemical Co., Rockford, IL). One hundred microliters of primary antibody (Positive control – mouse Mab to metallothionein-1 diluted 1:500 with phosphate buffer solution [PBS]; Negative control – PBS; Experimental serum from autistic and nonautistic individuals diluted 1:500 with PBS) was added to appropriate wells and plate was incubated for two hours at 3 °C. All wells were washed 3× using PBS/tween. One hundred microliters of goat anti-mouse secondary antibody, conjugated with alkaline phosphatase (Bio-Rad Laboratories, Hercules, CA), diluted 1:5000 with PBS to positive control; Goat anti-human (Bio-Rad) diluted 1:5000 with PBS to all other wells, added to appropriate wells and incubated for 45 minutes at 37 degrees C. All wells washed 5× with PBS/tween. One hundred microliters of substrate for alkaline phosphatase added to all wells and plate was incubated at room temperature until significant color change in positive control. Color change measured using ELISA (enzyme-linked immunosorbent assay) Reader (Bio-Rad).
Indirect ELISA to quantitate the concentration of serum metallothionein
Human Metallothionein ELISA Kit, (USCN Life Science and Technology Company, Wuhan, China): Ninety-six well microculture plate strips were coated with polyclonal IgG to human metallothionein. Purified metallothionein-1 (Sigma) at concentrations of 100 μg/ml, 50 ng/ml, 12.5 ng/ml, 6.25 ng/ml, 3.12 ng/ml, and 1.56 ng/ml bicarbonate buffer (pH 9.6), and serum at a dilution of 1:100, were fixed to wells of 96 well polystyrene microculture plate (Corning) by incubation overnight at 4 °C. Excess MT/serum was dumped from wells, and all wells were blocked by washing 3× with 300 mL of blocking solution (Superblock, Pierce). One hundred microliters of primary antibody (mouse Mab to metallothionein-1, diluted 1:500 with PBS) was added to all wells and plate was incubated for 2 hours at 3 °C. All wells were washed three times using PBS/tween. One hundred microliters of secondary antibody, conjugated with alkaline phosphatase (goat anti-mouse [Bio-Rad], diluted 1:5000 with PBS) added to all wells and incubated for 45 minutes at 3 °C. All wells washed five times with PBS/tween. One hundred microliters of substrate for alkaline phosphatase added to all wells and plate was incubated at room temperature until significant color change in positive control. Color change measured using ELISA Reader (Bio-Rad).
Subjects
Autistic children with GI disease
Serum from autistic individuals with GI disease was obtained from the Thoughtful House, Austin, Texas. These patients ranged in age from two to 16 years with a median age of 71 months. Thirty-four (85%) were male. Most patients were referred by their primary care physician for evaluation of ongoing GI symptoms, while some patients were parent-referred.
All patients had a diagnosis of autism, ASD, pervasive developmental disorder (PDD), or Asperger’s syndrome. The developmental diagnosis was established by either single or multiple members of the following specialties: pediatric neurologists, developmental pediatricians, pediatric psychiatrists, or psychologists. A clear history relating to the onset of developmental disorder was obtained from the parents of all 41 patients. Of these, the large majority (61%) was reported to have had a regressive onset. Regression was determined as a normal development until at least the age of 12 months followed by inexplicable loss of previously achieved developmental milestones between 12 and 24 months and accompanied by the appearance of typical autistic behaviors or normal development until at least the age of 12 months followed by a developmental plateau in which milestones are not noticeably lost but the rate of development suffers marked deceleration, accompanied by the appearance of typical autistic behaviors. Nonregression individuals had definite onset of autistic behaviors prior to the age of 12 months.
Scoring of severity of GI disease
All the children in our study (41) had chronic GI symptoms and all were investigated by ileo-colonoscopy. Macroscopic and histological features of the upper and lower GI tract were scored. A point system was developed to assess the severity of GI disease (particularly inflammation). Patients were scored according to mild (1 point), moderate (2 points), and marked (3 points) disease in each area (upper and lower GI) and for endoscopic assessment (macroscopic) and histological assessment of each area. Therefore, the maximum score for GI disease was 12 (3 points each for upper scope, upper histology, lower scope, and lower histology). A point system was also developed for severity of lymphoid nodular hyperplasia (LNH). Patients were scored according to mild (1 point), moderate (2 points), and marked (3 points) LNH in each area (upper and lower GI) for a maximum of 6 points. And finally, a point system was also developed for severity of erythema. Patients were scored according to mild (1 point), moderate (2 points), and marked (3 points) erythema in each area (upper and lower GI) for a maximum of 6 points. The rationale for this scoring was to provide an objective and unbiased way to compare clinical and pathological findings and immune dysfunction.
Controls
Two control groups (total n = 33) were studied, including 17 age- (mean 68 months), gender- (80% male), and diagnosis- (61% regressive onset) matched autistic children with no GI disease and 16 age- (mean 71 months) and gender- (75% male) matched children without autism or GI disease. Serum and medical history were obtained from the Autism Genetic Resource Exchange [AGRE].*
Statistics
Inferential statistics were derived from t-test and odds ratios with 95% confidence intervals.
Results
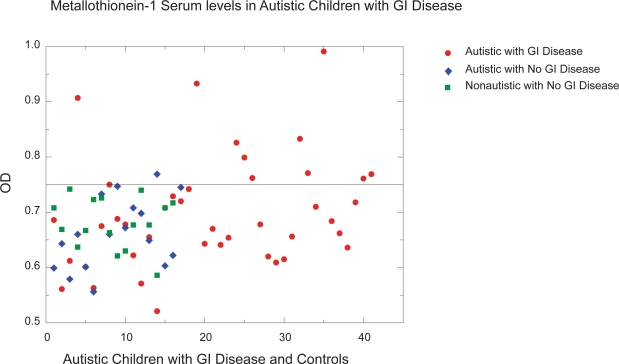
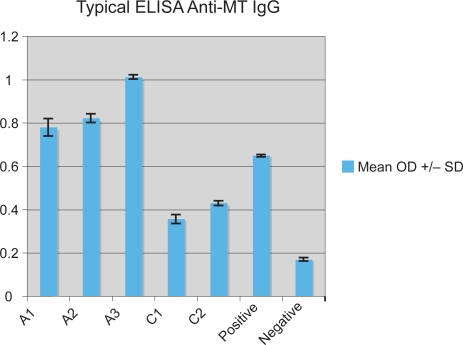
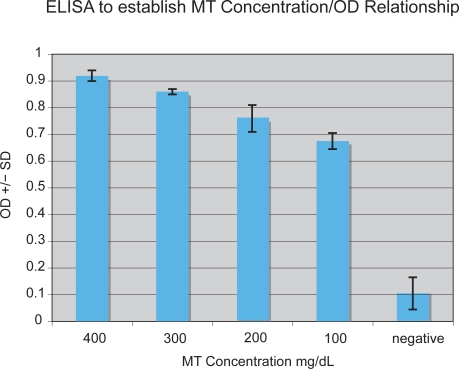
We used the ELISA described above to measure serum MT and anti-MT IgG levels in 41 autistic children with chronic digestive disease and 33 age/gender-matched controls (as described above). All assays were performed in triplicate. Any variation in OD greater than 0.05 was not included in this data. A typical assay measuring anti-MT IgG is shown on Figure 1. An ELISA was performed to establish MT concentration/OD relationship. Normal MT serum concentration is approximately 80–200 mg/dL serum. Above 200 mg/dL (0.75 OD) was considered high (above normal) (Figure 2).
Figure 1.
A typical ELISA measuring anti-MT IgG of autistic children with GI disease (A) compared to control (C) children with no GI disease. Positive control is anti-MT IgG Mab and purified MT. Negative control is PBS replacing anti-MT IgG.
Abbreviations: anti-MT, anti-metallothionein; ELISA, enzyme-linked immunosorbent assay; GI, gastrointestinal; IgG, immunoglobulin G; MT, metallothionein; PBS, phosphate buffer solution.
Figure 2.
ELISA to establish MT concentration/OD relationship. Normal MT serum concentration is approximately 80–200 mg/dL serum. Above 200 mg/dL (0.75 OD) was considered high (above normal).
Abbreviations: ELISA, enzyme-linked immunosorbent assay; MT, metallothionein; OD, oxidation ditch.
Ten of 41 autistic children with chronic digestive disease had high serum concentration of metallothionein compared to only one of the 33 controls (Figure 3) (p < 0.01).
Figure 3.
Ten of 41 autistic children with chronic digestive disease had high serum concentration of metallothionein compared to only one of the 33 controls (Figure 1) (p < 0.01).
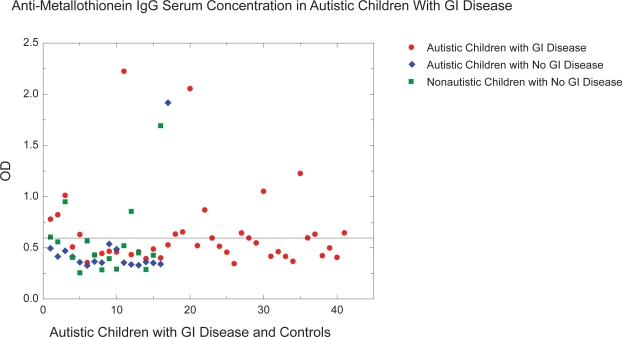
Thirteen of the 41 autistic children with chronic digestive disease had anti-MT IgG compared to only four of 33 controls (Figure 4) (p < 0.01).
Figure 4.
Thirteen of the 41 autistic children with chronic digestive disease had high levels (OD ≥ 0.63 based on greater than mean ± SD of normal controls) of anti-MT IgG compared to only 4 of 33 controls.
Abbreviations: anti-MT, anti-metallothionein; IgG, immunoglobulin G; MT, metallothionein; OD, oxidation ditch.
Nine of 10 of autistic children with GI disease with high MT levels had a regressive onset (compared to the expected 25 of 41, or 61%, in this group) (p < 0.05), whereas, only nine of 13 of the autistic children with GI disease and anti-MT IgG had a regressive onset (70%) which was not significantly higher than expected (Table 1).
Table 1.
Nine of 10 of autistic children with GI disease with high MT levels had a regressive onset (compared to the expected 25 of 41, or 61%, in this group) (p < 0.05), whereas only nine of 13 of the autistic children with GI disease and anti-MT IgG had a regressive onset (70%) which was not significantly higher than the expected. Only four of 10 autistic children with GI disease with high MT levels also had severe GI disease (P > 0.1). However, although only six of 14 with high anti-MT IgG had severe GI disease, four of five of those autistic children with severe GI erythema, and four of six with severe LNH had these autoantibodies.
| LNH | Eryth | Total GI | MT Mean OD | Anti-MT Mean OD | |
|---|---|---|---|---|---|
| A | 3 | 6 | 9 | 0.686 | 0.78 |
| R-PDD | 1 | 0 | 3 | 0.561 | 0.823 |
| RA | 1 | 0 | 3 | 0.612 | 1.012 |
| RA | 2 | 2 | 6 | 0.907 | 0.508 |
| RA | 4 | 3 | 8 | 0.601 | 0.63 |
| RA | 2 | 0 | 8 | 0.563 | 0.356 |
| R-UD | 4 | 2 | 8 | 0.675 | 0.43 |
| RA | 3 | 1 | 7 | 0.75 | 0.445 |
| A | 3 | 1 | 6 | 0.688 | 0.466 |
| RA | 1 | 0 | 7 | 0.678 | 0.459 |
| PDD/NOS | 2 | 2 | 6 | 0.622 | 2.225 |
| A | 1 | 2 | 6 | 0.571 | 0.433 |
| A | 3 | 5 | 10 | 0.655 | 0.461 |
| RA | 0 | 2 | 5 | 0.521 | 0.394 |
| A | 2 | 1 | 5 | 0.708 | 0.488 |
| RA | 3 | 1 | NA | 0.729 | 0.401 |
| A | NA | NA | NA | 0.72 | 0.528 |
| R-PDD | 5 | 0 | NA | 0.742 | 0.634 |
| A | 4 | 4 | 11 | 0.933 | 0.655 |
| R-PDD | 3 | 1 | 8 | 0.643 | 2.055 |
| RA | 3 | 2 | 5 | 0.67 | 0.521 |
| RA | 3 | 0 | 7 | 0.641 | 0.87 |
| A | 3 | 2 | 8 | 0.654 | 0.596 |
| RA | 2 | 0 | 4 | 0.826 | 0.515 |
| RA | 3 | 0 | 4 | 0.799 | 0.457 |
| R-ASP | 2 | 1 | 6 | 0.762 | 0.345 |
| A | 3 | 4 | 6 | 0.678 | 0.645 |
| PDD | 2 | 1 | 4 | 0.62 | 0.596 |
| R-PDD/NOS | 2 | 1 | 4 | 0.609 | 0.548 |
| RA | 3 | 0 | 6 | 0.615 | 1.052 |
| A-dev. plateau | 2 | 0 | 4 | 0.656 | 0.417 |
| A | NA | NA | NA | 0.833 | 0.462 |
| RA | 3 | 2 | 6 | 0.771 | 0.416 |
| A | 0 | 0 | NA | 0.71 | 0.368 |
| RA | 3 | 0 | 5 | 0.991 | 1.227 |
| RA | 2 | 2 | 7 | 0.684 | 0.597 |
| A | NA | NA | NA | 0.662 | 0.633 |
| RA | 2 | 0 | 3 | 0.636 | 0.423 |
| A | 4 | 0 | 7 | 0.718 | 0.499 |
| RA | 3 | 2 | 7 | 0.761 | 0.406 |
| RA | 6 | 0 | 10 | 0.769 | 0.646 |
Notes: Diagnosis: A, autistic early onset; RA, regressive onset; ASP, Asperger’s syndrome patients; PDD, pervasive developmental disorder.
Abbreviations: anti-MT, anti-metallothionein; ELISA, enzyme-linked immunosorbent assay; GI, gastrointestinal; IgG, immunoglobulin G; LNH, lymphoid nodular hyperplasia; MT, metallothionein.
We didn’t find any correlation between severity of GI disease and MT concentration. Only four of 10 autistic children with GI disease with high metallothionein levels also had severe GI disease (Table 1) (P > 0.1). However, although only six of 14 with high anti-MT IgG had severe GI disease, four of five of those autistic children with severe GI erythema, and four of six with severe LNH had these auto-antibodies (Table 1) (p < 0.05).
Discussion
Autoimmune reactions after exposure to heavy metals such as mercury have been causally implicated in autism. As MT is the primary metal-detoxifying protein in the body, we conducted a study of serum MT and antibodies to MT in 41 autistic children with severe GI disease, and 33 age/gender-matched controls.
Metallothioneins (MT), a family of small proteins containing 61–68 amino acids with an unusually high concentration of cysteine, function as intracellular distributors and mediators of heavy metals, including copper and zinc, and heavy metal detoxification.
Data indicates that copper to zinc ratio is abnormally high in individuals with autism.25 This might be explained by an altered function due to an altered conformation of the protein.
Measurements of MT levels, as well as zinc, have been used to demonstrate zinc deficiency. MT increases rapidly after zinc supplementation and decreases if the diet is deficient in zinc.26
Studies indicate that many children with autism have an immune abnormality of some type, including myeloperoxidase deficiency, severe combined immunodeficiency, and IgA deficiencies. Twenty percent of autistics have IgG subclass deficiencies, and deficiencies in complement C4b. Concentrations of IL-12 and interferon gamma are much higher in autistic children than in normal children.27–31 Autistic individuals are also susceptible to autoimmune disorders.32,33
It has previously been reported that anti-MT antibody is present in the circulation of healthy persons, including both normal and autistic children.34,35
Singh and Hanson reported in 200636 that serum levels of MT did not significantly differ between normal and autistic children. Furthermore, autistic children harbored normal levels of anti-MT, without any significant difference between normal and autistic children. They suggested that, because autistic children have a normal profile of MT and anti-MT, the mercury-induced autoimmunity to MT may not be implicated in the pathogenesis of autism.
In a previous study,37 we found that a significant number of autistic family members (parents and children) had high levels of anti-MT and serum MT, but the levels of MT and anti-MT did not correlate with autism (parents and nonautistic children were just as likely to have high levels). In that study, however, the only demographic group, which seemed to have higher levels of anti-MT IgG than the controls (although not significant), was the group with GI disease.
In this study, we looked more carefully at autistic children with GI disease and found that they had a significantly higher concentration of serum MT and anti-MT antibodies when compared to age/gender-matched controls of autistic children without GI disease and nonautistic children without GI disease. This suggests an association between the GI disease seen in many autistic children and serum levels of MT and anti-MT IgG. It is possible that the presence of anti-MT IgG in this subgroup could be associated with the fact that autistic children are more susceptible to immune dysfunction, including autoimmune disease. However, it is still unclear whether high levels of MT or the presence of anti-MT IgG is associated with a cause of GI disease or the result of other immune dysfunction found in autistic children.
Footnotes
The Autism Genetic Resource Exchange (AGRE) is the first collaborative gene bank for the study of autism spectrum disorders and one of the world’s largest shared resources for the study of autism and related disorders, with a collection of over 900 well-characterized multiplex and simplex families made available to the greater scientific community. Founded by Cure Autism Now (CAN) in 1997, AGRE is currently funded by the National Institute of Mental Health (NIMH) and Autism Speaks (AS), which merged with CAN in 2006.
Disclosure
We gratefully acknowledge the resources provided by the Autism Genetic Resource Exchange (AGRE) Consortium and the participating AGRE families. The Autism Genetic Resource Exchange is a program of Cure Autism Now and is supported, in part, by grant MH64547 from the National Institute of Mental Health to Daniel H. Geschwind (PI) and The Thoughtful House Center for Children, Austin, Texas.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington D.C.: American Psychiatric Association; 1994. [Google Scholar]
- 2.Gillberg C, Coleman M. The Biology of the Autistic Syndromes. 2nd ed. London: Mac Keith Press; 1992. [Google Scholar]
- 3.Filipek P, Accardo P, Baranek G, et al. The screening and diagnosis of autistic spectrum disorders. J Autism Dev Disord. 1999;29:439–484. doi: 10.1023/a:1021943802493. [DOI] [PubMed] [Google Scholar]
- 4.Bailey A, Phillips W, Rutter M. Autism: towards an integration of clinical, genetic, neuro-psychological, and neurobiological perspectives. J Child Psychol Psychiatry. 1996;37:89–126. doi: 10.1111/j.1469-7610.1996.tb01381.x. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki T, Takemoto TI, Kashiwazaki H, Miyama T. Metabolic fate of ethylmercury salts in man and animal. In: Miller MW, Clarkson TW, editors. Mercury, Mercurials, and Mercaptans. Vol. 12. Springfield, IL: Charles C Thomas; 1973. pp. 209–233. [Google Scholar]
- 6.Halsey NA. Perspective on the use of thimerosal-containing vaccines; Presentation at the National Vaccine Advisory Committee Workshop on Thimerosal and Vaccines, Institute of Vaccine Safety; Aug 11–12, 1999; Cited on Dec 2, 2008. Available from: http://www.vaccinesafety.edu/. [Google Scholar]
- 7.Egan WM. Thimerosal in Vaccines; Presentation to the FDA; Sept 14, 1999. [Google Scholar]
- 8.Davis LE, Kornfeld M, Mooney HS, et al. Methyl mercury poisoning: long term clinical, radiological, toxicological, and pathological studies of an affected family. Ann Neurol. 1994;35:680–688. doi: 10.1002/ana.410350608. [DOI] [PubMed] [Google Scholar]
- 9.Bernard S, Enayati A, Roger H, Binstock T, Redwood T, McGinnis W. Autism: A Unique Type of Mercury Poisoning; ARC Research Report; April, 2000; Cited on Dec 2, 2008. Available from: http://www.vaccinationnews.com/DailyNews/July2001/AutismUniqueMercPoison.htm. [Google Scholar]
- 10.Schubert J, Riley E, Tyler S. Combined effects in toxicology, a rapid systemic testing procedure: cadmium, mercury and lead. J Toxicol Environ Health. 1978;4:763–776. doi: 10.1080/15287397809529698. [DOI] [PubMed] [Google Scholar]
- 11.Pichichero ME, Gentile A, Giglio N, et al. Mercury levels in newborns and infants after receipt of thimerosal-containing vaccines. Pediatrics. 2008;121:e208–14. doi: 10.1542/peds.2006-3363. [DOI] [PubMed] [Google Scholar]
- 12.Fombonne E. Thimerosal disappears but autism remains. Arch Gen Psychiatry. 2008;65:15–16. doi: 10.1001/archgenpsychiatry.2007.2. [DOI] [PubMed] [Google Scholar]
- 13.Schechter R, Grether JK. Continuing increases in autism reported to California’s developmental services system: mercury in retrograde. Arch Gen Psychiatry. 2008;65:19–24. doi: 10.1001/archgenpsychiatry.2007.1. [DOI] [PubMed] [Google Scholar]
- 14.Goering PL, Klaassen CD. Metals Toxicology. Academic Press; San Diego: 1995. Hepatotoxicity of metals; pp. 339–361. [Google Scholar]
- 15.Klaassen CD, Habeebu SSM. Role of metallothionein in cadmium-induced hepatotoxicity. In: Lee SK, Surh Y-J, editors. Trends in Biopharmaceutical and Toxicological Sciences. Seoul National University Press; Seoul: 1998. pp. 1–17. [Google Scholar]
- 16.Takeda A, Kodama Y, Okada S. Metallothionein induction in rat brain after intrastriatal injection of zinc and cadmium salts. J Health Sci. 1999;45:20–23. [Google Scholar]
- 17.Koizumi S, Suzuki K, Ogra Y, Gong P, Otuska F. Roles of zinc fingers and other regions of the transcription factor human MTF-1 in zinc-regulated DNA binding. J Cell Physiol. 2000;185:464–472. doi: 10.1002/1097-4652(200012)185:3<464::AID-JCP18>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Aschner M, Cherian MG, Klaassen CD, Palmiter RD, Erickson JC, Bush AI. Metallothioneins in brain – the role in physiology and pathology. Toxicol Appl Pharmacol. 1997;142:229–242. doi: 10.1006/taap.1996.8054. [DOI] [PubMed] [Google Scholar]
- 19.Goering PL, Klaassen CD. Hepatotoxicology of copper, iron, and cadmium. Comprehensive Toxicology. 1997;9:389–406. [Google Scholar]
- 20.Cherian MG, Chan HM. Biological functions of metallothionein – a review. In: Suzuki KT, Imura N, Kimura M, editors. Metallothionein III. Basel: Birkhauser Verlag; 1993. pp. 87–109. [Google Scholar]
- 21.van Gent T, Heijnen CJ, Treffers PDA. Autism and the immune system. J Child Psychol Psychiatry. 1997;38:337–349. doi: 10.1111/j.1469-7610.1997.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 22.Hu H, Möller G, Abedi-Valugerdi M. Mechanism of mercury-induced autoimmunity: both T helper 1- and T helper 2-type responses are involved. Immunology. 1999;96:348–357. doi: 10.1046/j.1365-2567.1999.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh VK. Plasma increase of interleukin-12 and interferon-gamma: pathological significance in autism. J Neuroimmunol. 1996;66:143–145. doi: 10.1016/0165-5728(96)00014-8. [DOI] [PubMed] [Google Scholar]
- 24.Singh VK. Serological association of measles virus and human herpesvirus-6 with brain autoantibodies in autism. Clin Immunol Immunopathol. 1998;89:105–108. doi: 10.1006/clin.1998.4588. [DOI] [PubMed] [Google Scholar]
- 25.Walsh W, Usman A. Metal metabolism and autism; Presented at the American Psychiatric Association Annual Meeting; May 10, 2001; New Orleans, LA. [Google Scholar]
- 26.Grider A, Bailey LB, Cousins RJ. Erythrocyte metallothionein as an index of zinc status in humans. Proc Nat Acad Sci U S A. 1990;87:1259–1262. doi: 10.1073/pnas.87.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warren R, Odell J, Warren W, et al. Immunoglobulin A deficiency in a subset of autistic subjects. J Autism Develop Dis. 1997;27:187–192. doi: 10.1023/a:1025895925178. [DOI] [PubMed] [Google Scholar]
- 28.Warren R, Margaretten N, Pace N, Foster A. Immune abnormalities in patients with autism. J Autism Develop Dis. 1986;16:189–197. doi: 10.1007/BF01531729. [DOI] [PubMed] [Google Scholar]
- 29.Warren R, Singh V, Cole P, et al. Possible association of the extended MHC haplotype B44-SC30-DR4 with autism. Immunogenetics. 1992;36:203–207. doi: 10.1007/BF00215048. [DOI] [PubMed] [Google Scholar]
- 30.Warren R, Yonk J, Burger R, Odell J, Warren W. DR positive T cells in autism: association with decreased plasma levels of the complement C4B protein. Neuropsychobiology. 1995;31:53–57. doi: 10.1159/000119172. [DOI] [PubMed] [Google Scholar]
- 31.Gupta S, Aggarwal Heads C. Dysregulated immune system in children with autism. Beneficial effects of intravenous immune globulin on autistic characteristics. Autism Develop Dis. 1996;26:439–452. doi: 10.1007/BF02172828. [DOI] [PubMed] [Google Scholar]
- 32.Bagenstose LM, Salgame P, Monestier M. Murine mercury-induced autoimmunity: a model of chemically related autoimmunity in humans. Immunol Res. 1999;20:67–78. doi: 10.1007/BF02786508. [DOI] [PubMed] [Google Scholar]
- 33.Comi AM, Zimmerman AW, Frye VH, Law PA, Peeden JN. Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. J Child Neurol. 1999;14:388–394. doi: 10.1177/088307389901400608. [DOI] [PubMed] [Google Scholar]
- 34.Singh VK, Hanson J. Assessment of metallothionein and antibodies to metallothionein in normal and autistic children having exposure to vaccine-derived thimerosal. Pediatr Allergy Immunol. 2006;17:291–296. doi: 10.1111/j.1399-3038.2005.00348.x. [DOI] [PubMed] [Google Scholar]
- 35.Jin GB, Nakayama H, Shmyhlo M, et al. High positive frequency of antibodies to metallothionein and heat shock protein 70 in sera of patients with metal allergy. Clin Exp Immunol. 2003;131:275–279. doi: 10.1046/j.1365-2249.2003.02074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh VK, Hanson J. Assessment of metallothionein and antibodies to metallothionein in normal and autistic children having exposure to vaccine-derived thimerosal. Pediatr Allergy Immunol. 2006;17:291–296. doi: 10.1111/j.1399-3038.2005.00348.x. [DOI] [PubMed] [Google Scholar]
- 37.Russo AJ. Anti-metallothionein IgG and levels of metallothionein in autistic families. Swiss Med Weekly. 2008;138:70–77. doi: 10.4414/smw.2008.12014. [DOI] [PubMed] [Google Scholar]