Abstract
Using three surveys, a comparative assessment of needle performance and patient preference for 27-gauge (G) and 29G needles for glatiramer acetate administration for multiple sclerosis therapy was performed. Eligible patients participated in a specialty pharmacy program and administered glatiramer acetate for ≥1 month. In Survey 1 on the 27G needle, 545 (82.70%) patients reported no needle problems, 106 (16.09%) cited one type (dull, bent, or broken), five (0.76%) cited two types, and three (0.46%) cited all three types. In Survey 2 on the 29G needle, 553 (98.05%) indicated no problems, two (0.35%) cited dull needles, and nine (1.60%) cited bent needles. On the 29G needles versus 27G needles pain comparison, 219 (38.83%) reported the 29G needle was a little better, and 155 (27.48%) reported it was a lot better than the 27G. For injection-site experiences, 515 patients (91.31%) reported no, very slight, or mild reactions with the 29G needle. In Survey 3, over 76% of patients preferred the 29G to the 27G needle and significantly fewer patients reported one or more problems with the 29G needle compared to patients reporting problems with the 27G needle (P < 0.00001). In conclusion, significantly fewer patients reported problems after 30 days of use of the 29G than the 27G needle. Fewer injection-site experiences occurred with the 29G needle and the 29G needle was preferred overall.
Keywords: 29 gauge needle, subcutaneous injection, glatiramer acetate
Introduction
Glatiramer acetate (Copaxone®, 20 mg; Teva Pharmaceuticals, Inc., Kansas City, MO, USA) is approved for the treatment of clinically isolated syndrome (CIS) and relapsing remitting multiple sclerosis (RRMS).1,2 Glatiramer acetate is believed to reduce both the inflammation and neurodegeneration occurring in the pathophysiology of multiple sclerosis by a variety of mechanisms, but it is primarily regarded as a T-cell-directed immunotherapeutic agent.3–5
Disease-modifying drugs (DMDs) for multiple sclerosis (MS) retard progression by reducing the incidence of relapses and magnetic resonance imaging (MRI) activity associated with the underlying pathology of the disease.6 However, all currently available DMDs for MS are delivered by injection. Injection-site experiences or reactions (ISEs) characterized by pain, swelling, redness, or inflammation have been experienced by patients who self-inject glatiramer acetate, interferon beta-1b, or interferon beta-1a.7 Indeed, ISEs are the single most common side effect of glatiramer acetate.7 Although these experiences are typically not serious, they can be uncomfortable and weaken a patient’s commitment to continuing treatment. Furthermore, compliance with, and acceptance of, a DMD with proven effectiveness is influenced by a patient’s perception of the pain caused by drug delivery. Also, pain perception is more enhanced in females than in males, coincident with the increased prevalence of MS in woman over men.8,9
Injection needles are characterized by their gauge (G), which reflects the diameter of the cannula. The higher the gauge, the thinner the needle. With the improvements of the 29G needle geometry, the outer diameter is reduced but the inner diameter of the lumen remains the same so that the same functionality is maintained with respect to flow rate, and speed of injection as well as the force applied on the syringe and drug solution.10 With some needles, an increase in the needle gauge, or thinness, may be associated with an increase in bending or breakage. Thus, improving the design of the needle to reduce injection pain and site experiences without compromising the needle’s performance is an important improvement in the ongoing management of MS using glatiramer acetate. Improvements such as these are likely to enhance patient adherence to the drug administration regimen.
A thinner 29G needle was expected to reduce injection pain, injection site experiences, and be more preferred relative to a 27G needle. Accordingly, three surveys were conducted to determine if the 29G needle was, indeed, an improvement over the previous 27G needle in terms of pain, ISEs and patient preference. The primary objective of the surveys was to provide a comparative assessment of patient-reported gross needle performance such as bending, dullness, or breaking between a 27G and 29G needle after 30 days of use. Another objective was a comparative assessment between the two needles in terms of overall patient preference.
Methods
Patients
Patients with MS who were registered in a specialty pharmacy program and used glatiramer acetate 27G prefilled syringe (PFS) for a minimum of one month prior to study entry. Patient participation in the study was approximately 30 days.
Study injection system
A PFS consisting of a 1 mL Hypack Physiolis syringe barrel (Becton Dickinson, Franklin Lakes, NJ, USA) fitted with a 29G/5 bevel/0.5 inch needle with a thermoplastic elastometer (TPE) shield (Hypak Physiolis syringe, Becton Dickinson). Patients were advised that they could continue using the autoinject 2 device (Owen Mumford Ltd., Woodstock, UK) or manually inject as they see fit.
Study design
This was a nonrandomized, unblinded, patient reported, comparative assessment of needle performance and preference.
Surveys
Patients with MS who had been using the 27G needle to administer 20 mg of glatiramer acetate daily were interviewed in three separate surveys to determine if injections with the 29G needle were associated with reduced pain, fewer ISEs and were more preferred. Three telephone surveys were administered over 30 days.
First telephone survey
The survey was administered by a specialty pharmacy health care professional (HCP) who obtained verbal informed consent from the patient. The first survey consisted of a needle performance question on the 27G needle. The needle performance question consisted of asking the patient whether they had experienced in the last 30 days a needle issue which may have included the needle feeling dull or the needle bending during injection. Through the usual BioScrip refill procedure, a 30-day supply of the new glatiramer acetate 29G PFS was sent to the patient and a 7–10-day post-drug-shipment call with the patient was scheduled.
Second telephone survey
The second survey was a 7–10-day post-drug-shipment call administered by a HCP. Survey 2 consisted of: 1) Qualifying questions to ensure patients had started using the 29G needle; 2) Questions on whether they had experienced pain with the 29G needle and how they would rate it on a 5-point scale from 0 (no pain at all) to 4 (severe pain) and how would they compared it to pain they had experienced in the past with the 27G needle on a 5-point scale from +2 (a lot better than your usual experience) to −2 (a lot worse than your usual experience); 3) Questions on whether they had experienced ISEs and how they would rate them on a scale from 0 (no site reactions at all) to 4 (severe site reactions) and how they would compared them to the ISEs they had experienced in the past with the 27G needle using a scale from +2 (a lot better than your usual experience) to −2 (a lot worse than your usual experience); and 4) A needle performance question on the 29G needle, asking whether there were needle issues such as it felt dull or possible bending, similar to the question in Survey 1 concerning the 27G needle.
Third telephone survey
The third survey was administered by a HCP approximately 30 days after the 1st survey. Survey 3 consisted of: 1) A needle performance question regarding the 29G needle on whether there were needle issues in the last 30 days, such as being dull or bent; and 2) An overall preference question consisting of asking the patient whether they preferred the new needle to their past experience with the 27G needles.
Statistical analyses
The patient ratings for each of the surveys to the questions on pain, needle performance and needle preference were analyzed using descriptive statistics. Chi-squared analyses were used to determine whether there were differences in the proportion of patients reporting needle problems, and single-sample t-tests were used to compare the ratings means between the 27G and 29G needles for pain, ISEs, and patient preference.
Results
Survey 1
Six hundred seventy-two patients were contacted by the specialty pharmacy for participation in this study. Thirteen patients declined to participate. A total of 659 patients participated in Survey 1, 564 in Survey 2, and 562 in Survey 3. Eighty-five percent of patients who began the study provided complete data on all three survey calls conducted by the specialty pharmacy program.
Demographic characteristics of patients who completed the study are shown in Table 1.
Table 1.
Patient demographic characteristics
| Characteristic | Mean | SD | Median | Range |
|---|---|---|---|---|
| Age (years) | 48.07 | 10.77 | 48 | 18–85 |
| Age at diagnosis (years) | 38.54 | 10.13 | 39 | 12–68 |
| MS disease duration (years) | 9.53 | 8.21 | 7 | 0–46 |
| Length of time on GA (months) | 45.16 | 38.46 | 40 | 0–340 |
| Gender | ||||
| F: 432 (76.9%) | ||||
| M: 130 (23.1%) | ||||
| Injection method | ||||
| Autoinjector: 355 (63.1%) | ||||
| Manual: 165 (29.4%) | ||||
| Both: 42 (7.5%) |
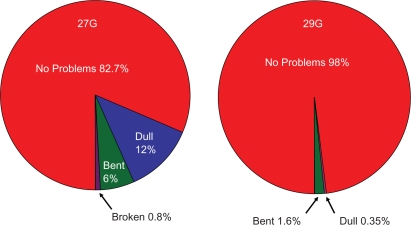
The majority of the survey participants were female (76.9%), whose average age was 48 years. Patients surveyed had MS for approximately 9.5 years, and were taking glatiramer acetate for an average of 45 months. With regard to 27G needle performance, of the 659 patients responding to the needle performance question, 545 (82.7%) indicated no problems, 106 (16.1%) reported one type of problem, five (0.8%) reported two types of problems, and three (0.5%) reported three types of problems (Figure 1).
Figure 1.
Needle performance data on 27G and 29G needles.
Survey 2
Survey 2 was administered to 564 patients after they had been using the new 29G needles from four to 30 days (mean = 21.5, standard deviation [SD] = 5.8). The method used to self-inject for these 564 patients were as follows: 356 (63.1%) used the autoinject device, 166 (29.3%) used manual injections, and 42 (7.4%) used both methods (Table 1). Concerning needle performance, 553 (98%) patients surveyed indicated no problems with 29G needle, two (0.35%) complained of needle dullness, and nine (1.6%) reported needles bending. There were no reports of needle breakage (Figure 1). The subjective pain ratings assigned by the 564 patients are shown in Table 2. The median pain rating for the 29G needle was 1.0, with a mean of 1.09 and SD of 0.81. ISEs assigned by patients (n = 564) are presented in Table 3. The median ISE rating was 2, the mean was 1.35, and the SD was 0.94.
Table 2.
Pain ratings assigned to an injection with a 29G needle (564 respondents)
| Pain rating | Number of patients | Percent |
|---|---|---|
| 0 – No pain at all | 130 | 23.05 |
| 1 – very slight pain, hardly noticeable | 283 | 50.18 |
| 2 – mild pain | 121 | 21.45 |
| 3 – moderate pain | 30 | 5.32 |
| 4 – severe pain | 0 | 0 |
Table 3.
The ISE ratings assigned to 29G needle (564 respondents)
| ISE rating | Number of patients | Percent |
|---|---|---|
| 0 – no site reaction at all | 136 | 24.11 |
| 1 – very slight reaction, barely noticeable | 144 | 25.53 |
| 2 – mild reaction | 235 | 41.67 |
| 3 – moderate reaction | 48 | 8.51 |
| 4 – severe reaction | 1 | 0.18 |
Pain with 29G needles compared to 27G needles
Table 4 shows the patient (n = 564) perceived pain comparison ratings between the 29G needle relative to past experiences with a 27G needle using a scale from +2 (a lot better) to −2 (a lot worse). The median pain comparison rating was 1.0, with a mean of 0.89 and SD of 0.86 (P < 0.001).
Table 4.
Pain and ISE comparison ratings assigned to an injection with a 29G needle in comparison to their experience with 27G needle (564 respondents)
| Comparison rating | Pain comparisons Number of patients (%) | ISE comparisons Number of patients (%) |
|---|---|---|
| +2 – a lot better | 155 (27.48) | 94 (16.67) |
| +1 – a little better | 219 (38.83) | 183 (32.45) |
| 0 – no difference | 168 (29.79) | 263 (46.63) |
| –1 – a little worse | 20 (3.55) | 21 (3.72) |
| –2 – a lot worse | 2 (0.35) | 3 (0.53) |
ISEs with 29G needles compared to the 27G
Table 4 shows the ISE comparison ratings that were assigned by patients (n = 564) to the 29G needle relative to past experience with the 27G needle using a subjective rating scale from +2 (a lot better) to −2 (a lot worse). Fewer ISEs with the 29G needle were reported by 49.2% of patients compared with the 27G needle used previously. The median ISEs comparison rating was 0, the mean was 0.6, and the SD was 0.8 (P < 0.001).
Survey 3
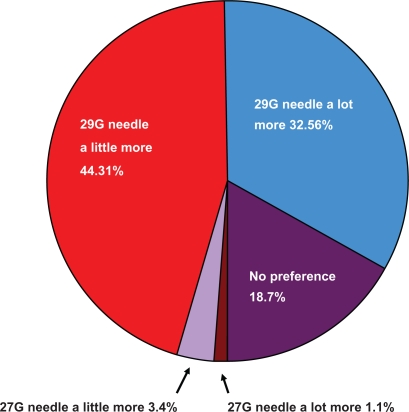
Five hundred sixty-two patients were administered the third survey. All had continued to use the 29G needle since the last survey call. Concerning performance of the 29G needle, 558 (99.3%) patients indicated no problems, and four (0.7%) patients reported a problem with needle bending. There were no reports of difficulties with either dull needles or needles breaking. Concerning overall preference, over 76% of patients preferred the 29G needle to the 27G needle (Figure 2).
Figure 2.
Overall preference for 29G needle relative to the 27G needle.
Discussion
Based on three telephone surveys, significantly fewer MS patients reported problems in the preceding 30 days using a thinner 29G needle to administer glatiramer acetate than the 27G needle used previously for drug delivery. In fact, two-thirds of the patients had less injection pain with the 29G relative to the 27G needle. Almost half of the patients experienced fewer ISEs with the 29G needle and another 46% experienced no difference in ISEs between the 29G and 27G needles. When interpreting the results of the patient surveys, it should be noted that the surveys were unblinded and the results were based on subjective questionnaires. Patients were asked to rate their pain and ISEs using the 29G needle and compare it to their remembered experience with the 27G needle. Additionally, while patients had at least 30 days of experience with injections with the 27G needle prior to entry into the study, there is no comparator arm so it is possible that the decreasing pain and ISEs with the 29G needle may have been a function of time rather than the gauge of needle. Nevertheless, the consistency of the results from the three surveys suggest that the 29G needle is a significant improvement over the 27G needle used previously for glatiramer acetate administration. Since almost three quarters of patients surveyed preferred the 29G needle to the 27G needle, this needle improvement may result in improved patient adherence in glatiramer acetate administration.
Additional studies evaluating differences between the 27G and 29G needles were recently reported by Jaber and colleagues.11 In this report, the results of two clinical trials with healthy volunteers and five surveys with MS patients comparing satisfaction with 29G/5-bevel needle with a TPE shield relative to the 27G/3-bevel needle with standard rubber shield for injection of interferon beta-1a were reviewed. The findings of this report indicate that the pain scores for the 29G/5-bevel needle with TPE shield decreased by 40% and skin penetration improved by 69% compared with the 27G/3-bevel needle with standard rubber shield. Moreover, 63% of MS patients surveyed thought that injections were less painful with the 29G/5-bevel needle than the 27G/3-bevel needle.11
The US Food and Drug Administration recently approved glatiramer acetate administration for patients who are in the early stages of their disease as well as patients diagnosed with RRMS. Early glatiramer acetate use has been shown to be an effective intervention, reducing MS relapse rates significantly.12 Indeed, patients are recommended to start taking glatiramer acetate once they have experienced their first clinical episode and have MRI features that are consistent with MS, even if the symptoms presented are relatively mild.13 Overall the side effects of glatiramer acetate are relatively limited and mild. However, the occurrence of ISEs can act as a deterrent for early and continued glatiramer acetate therapy. To facilitate good injection routines, patients starting daily subcutaneous self-injections of glatiramer acetate receive training on injection-site preparation and injection technique.14
Despite attention to good injection practices, patients who inject glatiramer acetate using the 27G needles sometimes experience ISEs. Improving the design of existing needles to reduce injection-associated pain and ISEs without compromising the needle’s functional integrity is important in the long-term management of MS. The results presented here indicate that the MS patients perceive that the 29G needle is a significant improvement over the 27G needle used previously for glatiramer acetate administration.
Conclusions
In this study, MS patients using the 29G needle for daily administration of glatiramer acetate reported significantly fewer problems relative to the 27G needle used previously. The parameters examined were pain, patient satisfaction, and needle performance issues such as dullness, bending or breaking. The increased satisfaction associated with the 29G needle is likely to increase compliance and thus enhance the positive effects of this DMD in curtailing MS progression.
Acknowledgments
The authors thank Pippa Loupe, PhD (Teva Neuroscience, Kansas City, MO) and Dervla Mellerick, PhD (Science Word Doctor, LLC, Ann Arbor, MI) for their assistance in the preparation of this manuscript. All patient satisfaction surveys were funded by Teva Neuroscience, Kansas City MO. SG and JC participated in the design, coordination and analysis of the research presented in this manuscript. SG and JC read and approved the final manuscript.
Footnotes
Disclosure
SC and JC are employed by Medical Affairs, Teva Neuroscience Kansas City MO and hold stock in Teva Neuroscience as well as other companies. The research presented in this manuscript was funded by Teva Neuroscience, Kansas City, MO.
References
- 1.Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45(7):1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 2.Teva Neuroscience Copaxone prescribing information. 2009. Available from: http://www.copaxone.com/. Accessed November 3, 2009.
- 3.Arnon R, Aharoni R. Neurogenesis and neuroprotection in the CNS: Fundamental elements in the effect of glatiramer acetate on treatment of autoimmune neurological disorders. Mol Neurobiol. 2007;36(3):245–253. doi: 10.1007/s12035-007-8002-z. [DOI] [PubMed] [Google Scholar]
- 4.Arnon R, Sela M. Immunomodulation by the copolymer glatiramer acetate. J Mol Recognit. 2003;16(6):412–421. doi: 10.1002/jmr.628. [DOI] [PubMed] [Google Scholar]
- 5.Weber MS, Prodhomme T, Yossef S, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13(8):935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- 6.Brown MG, Kirby S, Skedgel C, et al. How effective are disease-modifying drugs in delaying progression in relapsing-onset MS? Neurology. 2007;69(15):1498–1507. doi: 10.1212/01.wnl.0000271884.11129.f3. [DOI] [PubMed] [Google Scholar]
- 7.Galetta SL, Markowitz C. US FDA-approved disease-modifying treatments for multiple sclerosis: review of adverse event profiles. CNS Drugs. 2005;19(3):239–252. doi: 10.2165/00023210-200519030-00005. [DOI] [PubMed] [Google Scholar]
- 8.Coyle PK, Christie S, Fodor P, et al. Multiple sclerosis gender issues: clinical practices of women neurologists. Mult Scl. 2004;10(5):582–588. doi: 10.1191/1352458504ms1083oa. [DOI] [PubMed] [Google Scholar]
- 9.Avasarala JR, O’Donovan CA, Roach SE, Camacho F, Feldman SR. Analysis of NAMCS data for multiple sclerosis, 1998–2004. BMC Med. 2007;5:6. doi: 10.1186/1741-7015-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang S. 2006. Report dated March 1st, 2006 to Katz R. Beltzville MD: FDA, CDER Regulatory Affairs. Horsham, PA: Teva Neuroscience;
- 11.Jaber A, Bozzato GB, Vedrine L, Prais WA, Berube J, Laurent PE. A novel needle for subcutaneous injection of interferon beta-1a: effect on pain in volunteers and satisfaction in patients with multiple sclerosis. BMC Neurol. 2008;8:38. doi: 10.1186/1471-2377-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comi G, Martinelli V, Rodegher M, et al. for the PreCISe study group Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:1503–1511. doi: 10.1016/S0140-6736(09)61259-9. [DOI] [PubMed] [Google Scholar]
- 13.Teva Neuroscience Copaxone. Available from: http://www.nationalmssociety.org/about-multiplesclerosis/treatments/medications/glatiramer-acetate. Accessed September 9, 2009.
- 14.Teva Neuroscience Your Copaxone Injection Guide. Available from: http://www.copaxone.com/pdf/Injecting_Guide.pdf. Accessed November 3, 2009.