Abstract
Pulmonary arterial hypertension (PAH) is a progressive disease that causes severe disability and has no cure. Over the past 20 years, a variety of treatment options have evolved for the management of PAH. With an expanded therapeutic armamentarium come more complex decisions regarding treatment options. Agent selection depends upon several factors including efficacy, side effect profile, and cost, as well as convenience of administration. We have undertaken a review of phosphodiesterase-5 (PDE-5) inhibitors in PAH with a focus on efficacy and safety. A literature search was conducted using the Medline and Cochrane Central Register of Controlled Trials databases (1966–February 2010) for relevant randomized clinical studies. Overall, 10 studies met our inclusion criteria. Sildenafil was the most commonly studied agent, followed by tadalafil and vardenafil. Most trials found that the PDE-5 inhibitors significantly improved exercise capacity and lowered pulmonary pressures. However, there were conflicting results regarding these agents’ impact on improving cardiac function and functional class. Overall, these medications were effective and well tolerated with a relatively benign side effect profile. The PDE-5 inhibitors are an important option in treating PAH. While most of the published clinical data involved sildenafil, the other PDE-5 inhibitors show promise as well. Further studies are needed to determine the optimal doses of this therapeutic drug class, as well as its effects as adjunctive therapy with other agents in PAH.
Keywords: sildenafil, tadalafil, vardenafil, pulmonary hypertension
Introduction
Pulmonary arterial hypertension (PAH) is a debilitating chronic disease of the small pulmonary arteries. The prevalence of PAH varies according to its etiology and among specific populations.1 Idiopathic PAH has an annual incidence of one to two cases per million.1 The incidence of PAH in scleroderma patients is dramatically higher.2 Symptoms are nonspecific and often result in long delays in diagnosis.2 Common clinical manifestations include dyspnea, fatigue, weakness, and low exercise capacity.2 As the disease progresses, it leads to right ventricular failure and death.3 The estimated median survival without medical treatment is approximately 2.8 years.3 Fortunately, advances in treatment options have resulted in improved survival.2
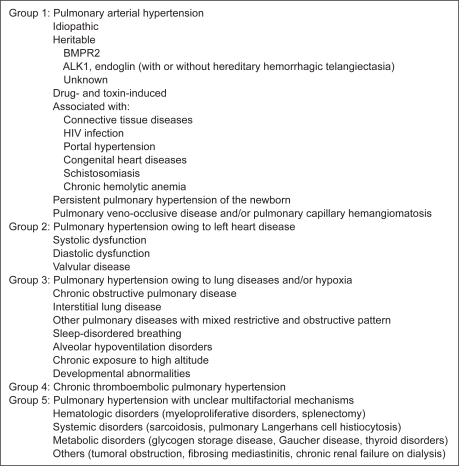
The most widely accepted definition of PAH is a mean pulmonary arterial pressure (MPAP) ≥25 mmHg at rest, normal left-sided filling pressures (pulmonary capillary wedge pressure <15 mmHg), and elevated pulmonary vascular resistance (>3 Wood units).3,4 A classification system has evolved and undergone several revisions.5 The most recently updated Dana Point clinical classification from the Fourth World Symposium on Pulmonary Hypertension is illustrated in Figure 1.6
Figure 1.
Updated clinical classification system on the 2008 World Symposium.6
Abbreviations: ALK1, activin receptor-like kinase type 1; BMPR2, bone morphogenetic protein receptor type 2; HIV, human immunodeficiency virus.
The development of pulmonary hypertension is a complex and incompletely understood process involving both genetic and environmental factors.4 Although significant advances in our understanding of PAH have been achieved over the past 20 years, much remains unknown regarding the pathophysiology.2,7 Pulmonary vascular endothelial dysfunction plays a key role in the development of PAH.2,7 Three identified neurohormonal pathways involved in this process are endothelin (ET), nitric oxide (NO), and prostacyclin I2 (PGI2).7 Pulmonary vasoconstriction may result in an overexpression of endothelin-1 (ET-1), as well as a deficiency in either PGI2 or NO.7 ET-1 is a potent vasoconstrictor that induces cell proliferation in vascular smooth muscle and promotes fibrosis and inflammation.8 ET-1 stimulates ETA receptors located on pulmonary vascular smooth muscle cells as well as ETB receptors located on smooth muscle cells and pulmonary vascular endothelial cells.3,8 The concentration of intracellular calcium increases when ETA receptors are activated, resulting in vasoconstriction and proliferation of vascular smooth muscle cells.7 Stimulation of ETB receptors leads to increased production of PGI2 and NO, which results in vasodilation and plays a role in clearing ET-1.8 NO is a potent vasodilator synthesized from L-arginine by NO synthase in lung vascular endothelium.8 Production of NO increases intracellular cyclic guanosine monophosphate (cGMP), which relaxes vascular smooth muscle.7 The lungs have higher concentrations of phosphodiesterase-5 (PDE-5) and this enzyme is responsible for the degradation of cGMP.7 Prostacyclin is a potent vasodilator with antiproliferative and antiplatelet properties.7 Several enzymes are involved in its biosynthesis. Arachidonic acid is cleaved from phospholipids by cytosolic phospholipase A2 and converted to prostaglandin H2 by the cyclooxygenase enzyme. Subsequently, prostaglandin H2 is converted to PGI2 by prostacyclin synthase. Prostacyclin further binds to endothelial prostacyclin and G protein-coupled receptors which increase production of cyclic adenosine 3′,5′-monophosphate (cAMP). Higher concentrations of cAMP ultimately result in smooth muscle relaxation and vasodilation.7 Thromboxane A2 and PGI2 have opposing effects on pulmonary vascular smooth muscle and platelet aggregation, which are delicately balanced during normal homeostasis. In PAH, a decreased expression of prostacyclin synthase results in a reduction of PGI2 levels, while thromboxane A2 levels increase; the imbalance of these hormones produces pulmonary vasoconstriction.9 It is not clear whether one primary pathway is responsible for the development and progression of PAH or a combination of these neurohormonal abnormalities is involved.7 Ultimately, vasoconstriction in conjunction with vascular remodeling and cell proliferation, lead to advanced vascular changes, such as the plexiform lesion.10
Currently, there are seven treatments approved by the Food and Drug Administration (FDA) available in the US, including the prostacyclins (epoprostenol, treprostinil, iloprost), endothelin receptor antagonists (bosentan, ambrisentan), and PDE-5 inhibitors (tadalafil, sildenafil).4 Despite the many available treatments for PAH, the disease remains incurable and most patients will progress to death from right heart failure.2 In the absence of a cure for PAH, the ideal treatment would be an orally administered agent with few side effects and requiring minimal monitoring. It would rapidly and durably improve pulmonary blood flow, prevent right heart failure, and improve survival as well as quality of life. Lastly, the medication would be inexpensive and readily available. Of the currently approved therapies, none meet all these criteria.
Despite 15 years of clinical studies, epoprostenol remains the only agent to improve survival in prospective, randomized controlled trials.11,12 However, the widespread use of the prostacyclins may be limited by cost, risk of infections from indwelling catheters, inconvenient intravenous or subcutaneous administration, and systemic side effects including hypotension, headache, diarrhea, and leg pain. Inhaled iloprost requires six to nine administrations daily because the duration of action only lasts for about 1–2 hours.13 Also, patients have been found to be subtherapeutic during the evening hours while sleeping.13 Endothelin receptor antagonists are an attractive option. Increasing reports have shown these agents to be safe and effective.14 Although ease of administration may be considered more alluring over the prostacyclins, adverse effects (eg, hepatotoxicity, teratogenicity, edema) and drug interactions may limit their use as well.
The PDE-5 inhibitors are an attractive option among the currently available therapies in the management of PAH. Over the years, several small, nonrandomized studies as well as randomized clinical trials have been published evaluating the use of sildenafil in PAH.15–34 More recently, a few clinical trials have also investigated tadalafil, supporting its FDA approval for the treatment of PAH.35,36 Compared with the prostacyclins and endothelin receptor antagonists, the PDE-5 inhibitors offer clear advantages as monotherapy in PAH. In contrast with the prostacyclins, most patients have few side effects, once or thrice daily dosing is convenient, and routine laboratory monitoring is not required. Lastly, these agents are much less expensive than the alternatives. The purpose of this review was to evaluate the safety and efficacy of the PDE-5 inhibitors in the management of PAH.
Literature review
A systematic search was conducted using the MEDLINE database and the Cochrane Central Register of Controlled Trials for articles published between 1966 and February 2010. Searches were limited to English, human, and clinical trial using the terms sildenafil, tadalafil, vardenafil, phosphodiesterase inhibitor, and pulmonary hypertension.
The overall search strategy was to identify clinical studies evaluating the use of oral PDE-5 inhibitors (sildenafil, tadalafil, and vardenafil) in PAH. Articles meeting the following criteria were included in this review: study design was a randomized controlled trial; subjects included had pulmonary arterial hypertension; oral sildenafil, tadalafil, or vardenafil was one of the study treatments; and subjects included the adult patient population ≥18 years. Studies were excluded if the study population was exclusively done in pediatrics or neonates, a PAH subgroup was not included, or the route of administration of the PDE-5 inhibitor was not oral.
Two authors (MSB, LMW) independently searched the titles and abstracts of potentially eligible studies identified. Articles with indeterminate abstracts or potentially meeting our criteria were obtained for full manuscript review and evaluated for inclusion criteria. The references of all identified articles were also manually searched to identify additional studies. The literature search yielded 10 randomized studies (Table 1). The majority of studies involved sildenafil with a few using tadalafil. Only a single, small, randomized study evaluated vardenafil compared with the other two PDE-5 inhibitors in PAH.34
Table 1.
Randomized clinical studies of the phosphodiesterase-5 inhibitors in pulmonary arterial hypertension
| Author | n | PAH definition | PAH class/type | Study regimen(s) | Control regimen(s) | Concomitant medications |
Results |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise capacity | MPAP/SPAP | CI/CO | Functional class | QOL | |||||||
| Galiè (SUPER study)27 | 278 | MPAP ≥ 25 and PCWP ≤ 15 | WHO Class I–IV | Sildenafil (3 study doses): 20, 40, or 80 mg PO TID × 12 weeks. Long-term study phase: all patients titrated to 80 mg PO TID. | Placebo | Conventional therapy allowed (specific drugs N/A) | ↑ | ↓ | ↑ | ↓ | N/A |
| Galiè (PHIRST study)35 | 405 | MPAP ≥ 25, PVR ≥ 3, PCWP ≤15 | WHO Class I–IV | Tadalafil 2.5 mg, 10 mg, 20 mg, 40 mg or placebo PO daily × 16 weeks. | Placebo | Bosentan: maximal use 125 mg twice daily for minimum of 12 weeks at time of screening | ↑ | ↓ | ↑ (CI) 40 mg group only | ↔ | ↑ 40 mg group only |
| Simmonneau28 | 267 | N/A | WHO Class I–IV) | IV epoprostenol + sildenafil 20 mg PO TID × 4 weeks, increased to 40 mg PO TID × 4 weeks, 80 mg PO TID × 4 weeks. | Placebo + epoprostenol | None | ↑ | ↓ | ↑ (CO) | N/A | N/A |
| Bharani36 | 11 | SPAP > 35 | WHO Class II–III | Tadalafil 20 mg once daily or placebo × 4 weeks, followed by 2-week drug-free interval, then crossed over to other regimen. | Placebo | Diuretics, oral anticoagulants, digoxin | ↑ | ↓ (SPAP) | N/A | ↔ | N/A |
| Bharani29 | 9 | SPAP ≥ 35 | NYHA II–IV | 25 mg PO BID × 2 weeks; with 2-week washout, then crossover × 2 weeks. | Placebo | Digoxin, diuretics, oral anticoagulants, nifedipine | ↑ | ↓ (SPAP) | N/A | ↔ | N/A |
| Sastry30 | 22 | MPAP ≥ 30 | NYHA II and III | Weight-based dosing: <26 kg = 25 mg PO TID; 26–50 kg = 50 mg PO TID; >50 kg = 100 mg PO TID × 6 weeks. Crossover between sildenafil and placebo, then by another 6 weeks. | Placebo | Digoxin, diuretics, oral anticoagulants | ↑ | ↔ (SPAP) | ↑ | N/A | ↑ |
| Wilkins (SERAPH study)31 | 26 | MPAP ≥ 25 | NYHA III only | 50 mg PO BID × 4 weeks; 50 mg PO TID × 12 wks. (all sildenafil patients were transitioned to open-label bosentan in fifth month) | Bosentan: 62.5 mg PO BID × 4 weeks; 125 mg PO BID × 12 weeks. Open-label bosentan in fifth month. | Digoxin, diuretics, oral anticoagulants, calcium channel blockers | ↑ | N/A | ↔ | N/A | |
| Singh32 | 20 | N/A | NYHA II–IV | Adults: 25 mg × 1; repeated 6 hours. If tolerated, 100 mg PO TID × 6 weeks; 2-week wash-out then crossover × 6 weeks. (Children very detailed regimen) | Placebo | N/A | ↑ | ↓ | N/A | ↓ | N/A |
| Ghofrani33 | 30 | MPAP > 40 | NYHA III–IV | Sildenafil 12.5 mg; sildenafil 12.5 mg + iloprost 2.8 μg; sildenafil 50 mg; sildenafil 50 mg + iloprost 2.8 μg. Iloprost administered 1 hour after sildenafil dose. | iNO administered, iloprost administered after hemodynamic values returned to baseline. | None | N/A | ↓(dose-depend) | ↑(increase x 5% 12.5 mg and 13.2% 50 mg) | N/A | N/A |
| Ghofrani34 | 60 | N/A | NYHA II–IV | Sildenafil 50 mg × 1; vardenafil 10 mg × 1; vardenafil 20 mg × 1; tadalafil 20 mg × 1; tadalafil 40 mg × 1; or tadalafil 60 mg × 1 | iNO 20–40 ppm | N/A | N/A | ↓ (all treatments) | N/A | N/A | N/A |
Abbreviations: BID, twice daily; CI, cardiac index; CO, cardiac output; iNO, inhaled nitric oxide; IV, intravenous; LV, left ventricle; MPAP, mean pulmonary arterial hypertension; N/A, not reported; NYHA, New York Heart Association; PAH, pulmonary arterial hypertension; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; QOL, quality of life; SPAP, systolic pulmonary arterial hypertension; TID, three times daily; WHO, World Health Organization; ↑, increased effect/variable; ↓, decreased effect/variable; ↔, no effect.
Pharmacology
Eleven PDE isoenzymes have been identified, all of which have distinct substrates and tissue distribution.37 The PDE-5 isoenzyme is expressed in the lungs, platelets, vascular smooth muscle, and myocardium.37 This enzyme is responsible for degrading cGMP through hydrolysis. Decreased cGMP concentrations lead to increased intracellular calcium, which produces smooth muscle vasoconstriction.37 Therefore, PDE-5 inhibitors oppose the vasoconstrictive effects within the pulmonary smooth muscle by increasing the intracellular cGMP concentrations, as well as allowing for enhanced NO-mediated vasodilatory action.13,38,39
Sildenafil, tadalafil, and vardenafil are all selective PDE-5 inhibitors. The selectivity of inhibition for each of these agents varies due to differences in their affinity for the PDE enzyme.38,40,41 Their relative selectivity for each PDE enzyme is measured in terms of drug concentration inhibiting 50% of the enzymatic reaction (IC50). The IC50 of sildenafil, tadalafil, and vardenafil for PDE-5 are approximately 3.5 nM, 6. nM, and 0.1 nM, respectively. The PDE-6 family is localized in the retina. Sildenafil has selectivity at PDE-6 in roughly 10-fold higher concentrations than PDE-5, which may explain its impact on vision. Vardenafil has up to 25-fold selectivity at PDE-6 compared with PDE-5, which could also contribute to ocular adverse effects. Tadalafil does not have enhanced selectivity for PDE-6. However, tadalafil has selectivity at PDE-11 at 5-fold higher concentrations than at PDE-5.38,40,41 PDE-11 is present in skeletal muscle, prostate, kidney, liver, pituitary glands, salivary glands, and testes.37 The clinical significance of the effect of tadalafil on PDE-11 is unknown.41–43,46
The PDE-5 inhibitors also vary in their pharmacokinetic profiles, as illustrated in Table 2. Differences among these agents can be seen in their bioavailability, half-life, and primary method of elimination. Although these drugs share a common mechanism for the primary metabolism of the parent drug, dissimilarities exist regarding their metabolites. The major metabolite of sildenafil is N-desmethylsildenafil, which is half as potent as the parent drug and excreted 80% in feces. Tadalafil’s metabolite is considerably less potent than the parent drug and is excreted in stool (60%) and urine (36%). Renal impairment increases the half-life of both tadalafil and its metabolite.47
Table 2.
| Drug | Bioavailability (%) | Time to peak onset (hours) | Metabolism (metabolite activity) | Half-life (hours) | Elimination | Protein binding (%) |
|---|---|---|---|---|---|---|
| Sildenafil | 41 | 0.5–2 | Hepatic (active) | 4 | Fecal (80%) Renal (13%) |
96 |
| Tadalafil | N/A | 2–8 | Hepatic (inactive) | 15 | Fecal (61%) Renal (36%) |
94 |
| Vardenafil | 15 | 0.5–2 | Hepatic (active) | 4–5 | Fecal (91%–95%) Renal (2%–6%) |
95 |
Abbreviation: N/A, not available.
Large randomized clinical studies
The SUPER (Sildenafil Use in Pulmonary Arterial Hypertension) study was a multicenter, randomized, double-blind, placebo-controlled study investigating the short- and long-term outcomes of sildenafil in pulmonary hypertension.27 The short-term phase assigned subjects to either placebo or one of three sildenafil dosing regimens (20, 40, or 80 mg three times daily). Those randomized to the 20 mg and 40 mg groups were initiated on these regimens and continued throughout the 12-week study period without any dose escalation. However, sildenafil was started at 40 mg orally three times daily for the first seven days after enrollment before titrating the dose up to 80 mg three times daily for those in this treatment arm. Subjects not originally assigned to the 80 mg dose group and eligible for the long-term phase of the study were titrated to 80 mg three times daily over a six-week period. Dummy dose escalation was performed in the 12-week and long-term phases to maintain blinding. Sildenafil was associated with a significant improvement in exercise capacity in all three groups over placebo (P < 0.001). Sildenafil significantly increased the six-minute walk distance (6MWD) at week 12 from baseline with all three incremental doses (45 m, 46 m, and 50 m, respectively; P < 0.001). The placebo, 20 mg, 40 mg, and 80 mg sildenafil groups had similar baseline MPAP measurements (56 ± 16, 54 ± 13, 49 ± 13, and 52 ± 16 mmHg, respectively). Furthermore, hemodynamics significantly improved with sildenafil from baseline, while no differences were found with placebo. Mean pulmonary artery pressure decreased by 2.1 (P = 0.04), 2.6 (P = 0.01), and 4.7 mmHg (P < 0.001) for the 20 mg, 40 mg and 80 mg groups, respectively. While all three sildenafil groups significantly increased cardiac index compared with placebo, the greatest increase noted was 0.37 L/mi-n/m2 (P < 0.001) in the 80 mg group. However, differences between active treatment groups did not reach statistical significance. The percentage of subjects observed to improve their World Health Organization (WHO) functional status by at least one class was 28% (P = 0.003), 36% (P < 0.001), and 42% (P < 0.001) in the 20 mg, 40 mg, and 80 mg groups, respectively. Those completing the one-year follow-up study (n = 222) with sildenafil 80 mg three times daily monotherapy showed a mean change in 6MWD of 51 m, which was comparable with those results shown after 12 weeks of therapy. However, a remaining question this study was unable to answer was the optimal dosing for long-term therapy. In fact, the extension phase supports the use of higher doses of sildenafil for the maintenance of efficacy in walk distance and functional class.
The second large randomized, placebo-controlled study with sildenafil was PACES (Pulmonary Arterial Hypertension Combination Study of Epoprostenol and Sildenafil).28 This trial investigated the long-term effects of concomitant sildenafil with intravenous epoprostenol in PAH. This study was unique in that PAH subjects had to have been on intravenous epoprostenol for at least three months prior to randomization without any dose changes within the previous four weeks. Epoprostenol dosing was considered optimized prior to enrollment in the study. The epoprostenol dose varied from 3–181 ng/kg/min; the median dose in the placebo and sildenafil arm was 28 and 29 ng/kg/min, respectively. Following randomization, subjects in the sildenafil arm were administered 20 mg three times daily for four weeks, increased to 40 mg three times daily for another four weeks, then titrated to 80 mg three times daily for an additional eight weeks. Overall, the addition of sildenafil to epoprostenol significantly increased the mean change from the baseline 6MWD over placebo at week 16 (29.8 m, 95% confidence interval [CI] 18.5–41.2; versus 1.0 m, 95% CI −10.9–12.9, respectively, P < 0.001). Subgroup analysis found those subjects whose baseline 6MWD was <325 m did not benefit significantly from adjunct sildenafil compared with placebo. However, sildenafil significantly improved the 6MWD over placebo if the baseline distance was ≥325 m. Also, sildenafil with epoprostenol significantly reduced MPAP by 2.8 mmHg and increased cardiac output by 0.6 L/min over baseline (P < 0.05). In the placebo group, cardiac output and pulmonary artery pressures were stable over the study duration.
The PHIRST (Pulmonary Arterial Hypertension and Response to Tadalafil) study was the largest randomized trial of tadalafil for the treatment of PAH to date.35 Although patients treated with epoprostenol, iloprost, or treprostinil were excluded, patients were allowed to continue concomitant bosentan therapy. Placebo-corrected change in 6MWD from baseline to 16 weeks was the primary efficacy outcome. Over 400 subjects were randomized to receive one of five treatments, ie, tadalafil 2.5 mg, 10 mg, 20 mg, 40 mg, or placebo once daily. The 10, 20, and 40 mg groups significantly increased the 6MWD compared with placebo in a dose-dependent manner by 20 m (P = 0.047), 27 m (P = 0.028), and 33 m (P < 0.001), respectively. The 40 mg dose was the only regimen achieving any significant impact. Multiple predefined subgroups were also analyzed to compare 6MWD distance from baseline to week 16. Significant improvements in 6MWD were observed in treatment-naive patients (P < 0.01), WHO Class I/II (P = 0.04), and WHO Class III/IV patients (P = 0.02). The onset and incidence of clinical worsening improved only in the 40 mg group compared with placebo (P = 0.041 and P = 0.038, respectively). No significant changes were observed between any treatment group and placebo in Borg dyspnea score or improvements in WHO classification. Quality of life was assessed using two survey instruments. A significant improvement was shown in the 40 mg group from baseline to week 16 in all sections of the EuroQoL-5D questionnaire (P < 0.02) and six of eight domains of the Medical Outcomes Study 36-item short form version 2 questionnaire (P < 0.01). Pulmonary hemodynamics were measured in 93 patients and showed improvement with tadalafil. In the 20 mg and 40 mg groups compared with baseline, MPAP was reduced (−8.5 mmHg, 95% CI −13 to −4, P < 0.001 and −4.3 mmHg, 95% CI −8 to −1; respectively, P = 0.01). Pulmonary vascular resistance also decreased in the 20 and 40 mg groups compared with baseline (−254 dyne/s/cm−5, 95% CI −388 to −120, P = 0.001, and −209 dyne/s/cm−5, 95% CI −406 to −13, P = 0.039, respectively). A significant increase in cardiac index by 0.6 L/min/m2 was observed in the 40 mg group compared with baseline (P = 0.028). An extension of the study evaluated the 20 mg and 40 mg doses beyond the initial 16-week study period. The long-term follow-up showed tadalafil had sustained favorable effects on exercise capacity for at least 10 months.
Small randomized clinical studies
Sildenafil
One randomized study evaluated sildenafil’s effects on exercise capacity, functional class, and resting pulmonary artery pressures.29 Although this small trial included New York Heart Association (NYHA) Class II–IV subjects, only one subject was classified as Class IV at baseline. Furthermore, one subject had pulmonary hypertension secondary to thromboembolism. All subjects underwent physical, laboratory, and electrocardiogram evaluations at baseline. Pulmonary pressures were estimated using Doppler studies. All vasodilator medications were discontinued one week prior to study entry. Subjects were then randomized to either sildenafil or placebo groups for two weeks prior. Following a two-week washout period, subjects were crossed over to the other study group to complete an additional two-week evaluation. A significant improvement in the 6MWD from baseline (163.89 ± 110.73 m) was found with sildenafil compared with placebo (266.67 ± 131.45 versus 170.0 ± 105.0 m, respectively, P < 0.005). Also, sildenafil was associated with a significant reduction in the MPAP from baseline (80.78 ± 21.30 mmHg) after two weeks of treatment over placebo (55.33 ± 16.52 versus 75.33 ± 19.75 mmHg, respectively, P < 0.005). Although no significant improvement was observed with sildenafil on functional class, the lack of benefit may be explained by the low number of study subjects.
Another small study investigated exercise capacity, cardiac function, pulmonary pressures, and quality of life scores associated with sildenafil over a six-week period.30 Although this study included NYHA Class II and III subjects, over 80% were Class II at baseline. Also, the median duration of PAH symptoms was about 30 months prior to study enrollment. After baseline assessments, subjects were randomized to either sildenafil or placebo. The sildenafil regimens were weight-based, ie, 25 mg orally three times daily (≤25 kg), 50 mg orally three times daily (26–50 kg), and 100 mg orally three times daily (≥51 kg). Patient assessments were performed every two weeks for six weeks in both groups. Subjects were crossed over to the other treatment group and again followed up for another six weeks. Although digoxin, diuretics, and oral anticoagulants were allowed, all other PAH-specific therapies were prohibited during the study period. Exercise capacity was measured as time on the treadmill rather than the 6MWD. Exercise time significantly improved with sildenafil compared with placebo (686.82 ± 224.02 versus 475.05 ± 168.02 seconds, respectively, P < 0.0001) at the end of six weeks over baseline (440.09 ± 172.17 seconds). Cardiac index also significantly improved from baseline (2.83 ± 1.06 L/m2) with sildenafil compared with placebo (3.45 ± 1.16 versus 2.80 ± 0.90 L/m2, respectively, P < 0.0001). However, systolic pulmonary artery pressures did not significantly improve from baseline with sildenafil (107.36 ± 24.98 versus 98.50 ± 24.38 mmHg, respectively, P = 0.09). A significant improvement in quality of life scores were also observed with sildenafil over placebo. One major limitation to this study was the lack of a washout period due to the crossover design. Despite the effects of sildenafil possibly being carried into the placebo group after subjects finished the active treatment, it is doubtful those effects would have been present at the end of the six-week study period.
The SERAPH (Sildenafil versus Endothelin Receptor Antagonist for Pulmonary Hypertension) study was a randomized, double-blind trial directly comparing the effects of sildenafil and bosentan.31 Patients were included if the MPAP was >25 mmHg at rest, 6MWD was 150–450 m, and if they were symptomatic despite the use of diuretics, digoxin, and anticoagulants. The primary outcome was change in right ventricular mass from baseline, with secondary outcomes of exercise capacity, cardiac index, and quality of life. Subjects were randomized to receive active treatment for a total of 16 weeks. Both sildenafil 50 mg and bosentan 62.5 mg were administered orally twice daily for four weeks. Sildenafil subjects were titrated to 50 mg three times daily and bosentan subjects were titrated up to 125 mg twice daily. All subjects entered the open-label bosentan phase of the study during the fifth and final month. Right ventricular mass at baseline for the sildenafil and bosentan groups was 159.8 ± 17.2 and 134 ± 13.1 g, respectively. No significant difference was found between sildenafil and bosentan in regard to right ventricular mass reduction from baseline on an intention-to-treat analysis. However, per protocol analysis found that sildenafil was superior to bosentan in reducing right ventricular mass 8.1 g reduction with sildenafil (P = 0.016) compared with no change with bosentan (P = 0.172). No significant differences were observed between treatment groups for exercise capacity and cardiac function. However, both treatment groups had significantly improved 6MWD and quality of life scores over baseline. Although pulmonary pressures were not directly measured in this study, right ventricular mass reduction may indicate a physiologic response from lowered pulmonary vascular resistance. The clinical significance of the primary endpoint remains unknown, since right ventricular mass is not a commonly measured endpoint in PAH studies. Nonetheless, both interventions resulted in similar improvement in exercise capacity.
Another study was conducted to investigate sildenafil therapy in Eisenmenger’s syndrome and idiopathic PAH patients.32 This study included 20 patients, half with idiopathic PAH and half with Eisenmenger’s syndrome. Some of the patients were children, although the breakdown by age was not provided. This crossover design study included a six-week, randomized, placebo-controlled portion followed by a two-week washout and then a crossover for another six weeks. Adults randomized to sildenafil were administered 25 mg with another 25 mg given six hours later. Sildenafil was continued at 100 mg orally three times daily for six weeks in those subjects not experiencing hypotension following the two initial doses. Children were given sildenafil starting at 3.125 mg and titrated to a final dose of 25 mg three times daily or 6.25 mg titrated to a final dose of 50 mg three times daily for those weighing <30 kg and >30 kg, respectively. The 6MWD at baseline was 262 ± 99 m and increased significantly both with placebo (293.4 ± 89.4 m, P < 0.009) and sildenafil (358.0 ± 96.5 m, P < 0.0001). Pulmonary artery systolic pressure was estimated by echocardiography, and fell significantly after six weeks of active treatment (98.9 ± 20.5 mmHg versus 78.3 ± 15.3 mmHg, respectively, P < 0.0001). In addition, sildenafil significantly improved the 6MWD, functional class, and exercise duration compared with placebo in both the idiopathic PAH and Eisenmenger groups.
Adjunctive sildenafil therapy
The acute effects of sildenafil combined with inhaled iloprost have been studied in severe pulmonary hypertension patients.33 Although the functional class of these subjects was not specified as an inclusion criteria nor was it disclosed as part of baseline characteristics, the investigators defined “severe” pulmonary hypertension as MPAP > 40 mmHg. All subjects received short-term vasodilator testing, which required inhaled NO (iNO) 20–40 ppm to produce a maximum response. Following the return of hemodynamic variables to baseline, all subjects received nebulized 2.8 μg of iloprost over four minutes, with hemodynamics measured at five intervals up to 90 minutes after administration. All subjects were then randomized to the following treatment groups after hemodynamic variables returned to baseline, ie, oral sildenafil 12.5 mg alone, inhaled iloprost 2.8 μg an hour after oral sildenafil 12.5 mg, oral sildenafil 50 mg alone, or inhaled iloprost 2.8 μg an hour after oral sildenafil 50 mg. Hemodynamics as well as laboratory sampling was measured at multiple intervals up to 180 minutes following administration. The greatest improvement in cardiac index and pulmonary pressures was found with the combination of sildenafil 50 mg and iloprost. There appeared to be a dose-response relationship with sildenafil at higher doses, resulting in greater improvement. iNO without the addition of other agents had the smallest improvement.
Tadalafil
A small randomized, double-blind, crossover study evaluated tadalafil in PAH patients with congenital left to right shunts.36 Eleven patients received tadalafil 20 mg once daily or placebo for four weeks, followed by a minimum two-week washout period, and concluded with four weeks of placebo. The primary outcome measure was change in 6MWD from baseline to four weeks after tadalafil and after placebo. Patients on tadalafil showed a significant increase in 6MWD, walking 136.38 m versus 46.5 m on placebo (P = 0.001). Borg dypsnea score decreased significantly in the tadalafil group compared with placebo (P = 0.021). No significant changes in WHO class were observed, but six of eight patients who completed the study improved by at least one WHO class on tadalafil. The sole hemodynamic measure evaluated was pulmonary artery systolic pressure estimated by echocardiography. Pulmonary artery systolic pressure decreased 25.38 mmHg in the tadalafil group compared with 4.63 mmHg in the placebo group (P = 0.003).
Direct comparison among sildenafil, tadalafil, and vardenafil
One study directly compared the short-term effects on cardiopulmonary hemodynamics and gas exchange of the three currently available PDE-5 inhibitors in PAH.34 All subjects were administered short-term iNO 20–40 ppm to produce maximum pulmonary vasodilator responses. Once hemodynamic measurements returned to baseline, subjects were randomized into one of six treatment groups, ie, sildenafil 50 mg, tadalafil 20 mg, tadalafil 40 mg, tadalafil 60 mg, vardenafil 10 mg, and vardenafil 20 mg. Only one dose of the study medication was administered in each treatment group, with observation up to 120 minutes. All study groups as well as iNO significantly decreased MPAP from baseline except in the tadalafil 60 mg group. iNO and sildenafil reduced MPAP by 9.8 (95% CI 7.2–13.3) and 16.2 mmHg (95% CI 11.6–21.4), respectively. Both the vardenafil 10 mg and 20 mg doses significantly decreased MPAP from baseline by 14.3 (95% CI 5.6–23.1) and 12.1 mmHg (95% CI 7.3–15.8), respectively. Tadalafil was found to decrease MPAP significantly in the 20 mg (−12.6 mmHg, 95% CI −2.8 to −24.4, P < 0.01) and 40 mg groups (−18.3 mmHg, 95% CI −13.3 to −21.8, P < 0.001), whereas the 60 mg dose did not show benefit (−10.0 mmHg, 95% CI −22.2–2.0, P = NS). Furthermore, sildenafil and iNO significantly improved arterial oxygenation as well as mixed venous oxygen saturation, while vardenafil and tadalafil did not.
Safety and tolerability
Overall, the PDE-5 inhibitors have been shown to be well tolerated with minimal adverse events. The tadalafil discontinuation rate was about 16% across all study groups in the PHIRST trial.35 Three deaths occurred during the study period, one in the placebo group due to worsening PAH, one in the 10 mg (sudden death), and one in the 20 mg (hematophagic histiocytosis syndrome) group. The most frequently reported adverse events were headache, myalgia, and flushing. More frequent adverse events occurred in the higher-dose groups, with headache being more prominent.35 There were no deaths and no significant adverse events related to the study drug in patients with PAH associated with congenital heart disease.36
The majority of the smaller sildenafil studies reported no major adverse events and none of the subjects discontinued the study drug due to side effects.29–34 However, in the two largest clinical trials with sildenafil, 1.4% and 5% of subjects withdrew from treatment with sildenafil due to adverse events.27,28 In the latter study, the withdrawal rate in the sildenafil group was not statistically different than the placebo group.28 Only three subjects experienced serious adverse events related to sildenafil, which included hypoxia (n = 1) and hypotension (n = 2). The serious adverse event rate was similar to the placebo group (n = 2). Most of the side effects with sildenafil treatment were considered mild to moderate in nature, consisting of headache, nausea, extremity pain and numbness, insomnia, and back pain.28,30 The only study evaluating vardenafil showed the 20 mg dose was associated with flushing and light headache in three of the nine patients, whereas none were reported with the 10 mg regimen.34 Although the PDE-5 inhibitors studies for PAH did not report any adverse events with vision or hearing, several reports of serious side effects with these medications have been observed in the erectile dysfunction trials. Specifically, anterior nonischemic optic neuropathy and decreased hearing have been reported.13
Discussion
In summary, there are many smaller studies and two well-designed larger studies supporting the safety and efficacy of sildenafil in PAH. A single, large, well-designed study and a small crossover study showed that tadalafil is also safe and effective. All studies investigating sildenafil’s effect on exercise capacity consistently demonstrated significant improvements in walk distance and time.27–32 MPAP reduction was shown in most studies.27–29,32–34 In contrast with the vast majority of studies reported, only a single poorly designed study of sildenafil in a mixed adult and pediatric population did not show improvements in hemodynamics.30 Among studies reporting invasively measured hemodynamics, beneficial effects were consistently seen. The absolute increase in cardiac index was about 0.3 L/min for most of these studies with the greatest increase reported in one study of about 0.6 L/min.27,30,33 However, comparisons among trials are particularly difficult because the sample size and study design were very different in the various studies described.
A key clinical issue that remains unanswered is the optimal dose of sildenafil. Several studies used higher sildenafil doses than those recommended by the FDA (ie, 20 mg three times daily). Subjects were titrated to sildenafil 80 mg orally three times daily in two studies and 100 mg orally three times daily in two more trials.27,28,30,32 The other studies either evaluated the effects of only one dose administration or used lower dosing regimens (25 mg twice daily or 50 mg three times daily).29,31,33,34 Some evidence suggests that the 80 mg three times daily regimen may offer added benefit over lower doses.27 Thus, the optimal dosing regimen for sildenafil remains controversial. Although there are inadequate data to draw firm conclusions, it is now common practice among many experts to increase the sildenafil dose over time in PAH patients.
Role in therapy
Selection of the specific therapies in PAH has become more complex as more medications are now available. Clinicians attempt to balance efficacy, safety, toxicity, and ease of administration in crafting regimens. Recently published guidelines offer evidence-based suggestions based on functional class.48 However, data comparing various active regimens are lacking.4 The PDE-5 inhibitors remain an attractive option for initial therapy in Class II and III patients. Furthermore, the lower cost and lack of required monitoring makes them particularly attractive. In patients with difficult phlebotomy, such as pediatric and chronically ill patients, the lack of laboratory monitoring is an important advantage. In more advanced disease, most experts still rely on continuously infused prostacyclins as the preferred first-line agents.
An ample body of evidence exists to support the use of both sildenafil and tadalafil in PAH. However, there are inadequate data to recommend vardenafil. Sildenafil and tadalafil are recommended as first-line therapy (Grade A and B, respectively) for Class II PAH patients over the prostacyclins.48 Sildenafil and tadalafil are also options as first-line therapy (Grade A and B, respectively) in Class III patients.48 Sildenafil’s role as monotherapy is less certain in Class IV patients.48 Based on the PACES study, the addition of sildenafil to epoprostenol in severe PAH may be recommended.28
Future studies
Compared with where we were 20 years ago, the field of PAH has made tremendous progress. However, there remains no cure and most patients will die from their disease. As we look ahead, new studies will hopefully introduce new medications active at different targets. Furthermore, the optimal combination of the different agents remains unclear. Currently enrolling and planned studies will hopefully shed further light on the optimal management of PAH.
Conclusion
Among the therapies currently available for the treatment of PAH, the PDE-5 inhibitors are a safe, effective, and well-tolerated therapeutic option. Sildenafil and tadalafil are FDA-approved for the treatment of PAH without respect to functional class. However, there is a paucity of data regarding vardenafil in PAH, and its use cannot be currently recommended. Whether as upfront therapy or add-on therapy, a solid body of research now supports sildenafil and tadalafil as key treatments in PAH.
Footnotes
Disclosure
Dr Feldman serves as a consultant to United Therapeutics and Gilead and has participated in advisory boards for United Therapeutics and Gilead.
References
- 1.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 2.Chin KM, Rubin LJ. Pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51:527–538. doi: 10.1016/j.jacc.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Benedict N, Seybert A, Mathier MA. Evidence-based pharmacologic management of pulmonary arterial hypertension. Clin Ther. 2007;29:2134–2153. doi: 10.1016/j.clinthera.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Badesch DB, Abman SH, Simonnequ G, Rubin LJ, McLaughlin VV. Medical therapy of pulmonary arterial hypertension. Chest. 2007;131:1917–1928. doi: 10.1378/chest.06-2674. [DOI] [PubMed] [Google Scholar]
- 5.Simonneau G, Galie N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 6.Simonneau G, Robbins IM, Geghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Humbert M, Morrell NW, Archer SL, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12 Suppl S):13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 8.Traiger GL. Pulmonary arterial hypertension. Crit Care Nurs Q. 2007;30:20–43. doi: 10.1097/00002727-200701000-00004. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- 10.Hackman AM, Lackner TE. Pharmacotherapy for idiopathic pulmonary arterial hypertension during the past 25 years. Pharmacotherapy. 2006;26:68–94. doi: 10.1592/phco.2006.26.1.68. [DOI] [PubMed] [Google Scholar]
- 11.Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 12.Sirbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: Prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–788. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 13.Croom KF, Curran MP. Sildenafil: A review of its use in pulmonary arterial hypertension. Drugs. 2008;68:383–397. doi: 10.2165/00003495-200868030-00009. [DOI] [PubMed] [Google Scholar]
- 14.Price LC, Howard LS. Endothelin receptor antagonists for pulmonary arterial hypertension: Rationale and place in therapy. Am J Cardiovasc Drugs. 2008;8:171–185. doi: 10.2165/00129784-200808030-00004. [DOI] [PubMed] [Google Scholar]
- 15.Preston IR, Klinger JR, Houtches J, Nelson D, Farber HW, Hill NS. Acute and chronic effects of sildenafil in patients with pulmonary arterial hypertension. Respir Med. 2005;99:1501–1510. doi: 10.1016/j.rmed.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Gomberg-Maitland M, McLaughlin V, Gulati M, Rich S. Efficacy and safety of sildenafil added to treprostinil in pulmonary hypertension. Am J Cardiol. 2005;96:1334–1336. doi: 10.1016/j.amjcard.2005.06.083. [DOI] [PubMed] [Google Scholar]
- 17.Kataoka M, Satoh T, Manabe T, et al. Oral sildenafil improves primary pulmonary hypertension refractory to epoprostenol. Circ J. 2005;69:461–465. doi: 10.1253/circj.69.461. [DOI] [PubMed] [Google Scholar]
- 18.Chau EM, Fan KY, Chow WH. Effects of chronic sildenafil in patients with Eisenmenger syndrome versus idiopathic pulmonary arterial hypertension. Int J Cardiol. 2007;120:301–305. doi: 10.1016/j.ijcard.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Oudiz RJ, Roveran G, Hansen JE, Sun XG, Wasserman K. Effect of sildenafil on ventilatory efficiency and exercise tolerance in pulmonary hypertension. Int J Cardiol. 2007;9:917–921. doi: 10.1016/j.ejheart.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voswinckel R, Reichenberger F, Enke B, et al. Acute effects of the combination of sildenafil and inhaled treprostinil on haemodynamics and gas exchange in pulmonary hypertension. Pulm Pharmacol Ther. 2008;21:824–832. doi: 10.1016/j.pupt.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Chapman TH, Wilde M, Madden SB. Sildenafil therapy in secondary pulmonary hypertension: Is there a benefit in prolonged use? Vascul Pharmacol. 2009;51:90–95. doi: 10.1016/j.vph.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Michelakis E, Tymchak W, Lien D, Webster L, Hashimoto K, Archer S. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: Comparison with inhaled nitric oxide. Circulation. 2002;105:2398–2403. doi: 10.1161/01.cir.0000016641.12984.dc. [DOI] [PubMed] [Google Scholar]
- 23.Michelakis ED, Tymchak W, Noga M, et al. Long-term treatment with oral sildenafil is safe and improves functional capacity and hemodynamics in patients with pulmonary arterial hypertension. Circulation. 2003;108:2066–2069. doi: 10.1161/01.CIR.0000099502.17776.C2. [DOI] [PubMed] [Google Scholar]
- 24.Ghofrani HA, Rose F, Schermuly RT, et al. Oral sildenafil as long-term adjunct therapy to inhaled iloprost in severe pulmonary arterial hypertension. J Am Coll Cardiol. 2003;42:158–164. doi: 10.1016/s0735-1097(03)00555-2. [DOI] [PubMed] [Google Scholar]
- 25.Chcokalingam A, Gnanavelu G, Venkatesan S, et al. Efficacy and optimal dose of sildenafil in primary pulmonary hypertension. Int J Cardiol. 2005;99:91–95. doi: 10.1016/j.ijcard.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Wilkins H, Guth A, Konig J, et al. Effect of inhaled iloprost plus oral sildenafil in patients with primary pulmonary hypertension. Circulation. 2001;104:1218–1222. doi: 10.1161/hc3601.096826. [DOI] [PubMed] [Google Scholar]
- 27.Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 28.Simonneau G, Rubin LJ, Galiè N, et al. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: A randomized trial. Ann Intern Med. 2008;149:521–530. doi: 10.7326/0003-4819-149-8-200810210-00004. [DOI] [PubMed] [Google Scholar]
- 29.Bharani A, Mathew V, Sahu A, Lunia B. The efficacy and tolerability of sildenafil in patients with moderate-to-severe pulmonary hypertension. Indian Heart J. 2003;55:55–59. [PubMed] [Google Scholar]
- 30.Sastry BK, Narasimhan C, Reddy NK, Raju BS. Clinical efficacy of sildenafil in primary pulmonary hypertension: A randomized, placebo-controlled, double-blind, crossover study. J Am Coll Cardiol. 2004;43:1149–1153. doi: 10.1016/j.jacc.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 31.Wilkins MR, Paul GA, Strange JW, et al. Sildenafil versus endothelin receptor antagonist for pulmonary hypertension (SERAPH) study. Am J Respir Crit Care Med. 2005;171:1292–1297. doi: 10.1164/rccm.200410-1411OC. [DOI] [PubMed] [Google Scholar]
- 32.Singh TP, Rohit M, Grover A, Malhotra S, Vijayvergiya R. A randomized, placebo-controlled, double-blind, crossover study to evaluate the efficacy of oral sildenafil therapy in severe pulmonary artery hypertension. Am Heart J. 2006;151:851.e1–e5. doi: 10.1016/j.ahj.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Ghofrani HA, Wiedemann R, Rose F, et al. Combination therapy with oral sildenafil and inhaled iloprost for severe pulmonary hypertension. Ann Intern Med. 2002;136:515–522. doi: 10.7326/0003-4819-136-7-200204020-00008. [DOI] [PubMed] [Google Scholar]
- 34.Ghofrani HA, Voswinckel R, Reichenberger F, et al. Differences in hemodynamic and oxygenation responses to three different phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension: A randomized prospective study. J Am Coll Cardiol. 2004;44:1488–496. doi: 10.1016/j.jacc.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 35.Galiè N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 36.Bharani A, Patel A, Saraf J, Jain A, Mehrotra S, Lunia B. Efficacy and safety of PDE-5 inhibitor tadalafil in pulmonary arterial hypertension. Indian Heart J. 2007;59:323–328. [PubMed] [Google Scholar]
- 37.Kass DA, Takimoto E, Nagayuma T, Champion HC. Phosphodiesterase regulation of nitric oxide signaling. Cardiovasc Res. 2007;75:303–314. doi: 10.1016/j.cardiores.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 38.Wilkins MR, Wharton J, Grimminger F, Ghofrani H. Phosphodiesterase inhibitors in the treatment of pulmonary hypertension. Eur Respir J. 2008;32:198–209. doi: 10.1183/09031936.00124007. [DOI] [PubMed] [Google Scholar]
- 39.Archer SL, Michelakis ED. Phosphodiesterase type 5 inhibitors for pulmonary arterial hypertension. N Engl J Med. 2009;361:1864–1871. doi: 10.1056/NEJMct0904473. [DOI] [PubMed] [Google Scholar]
- 40.Corbin JD, Francis SH. Pharmacology of phosphodiesterase-5 inhibitors. Int J Clin Pract. 2002;56:453–459. [PubMed] [Google Scholar]
- 41.Gresser U, Gleiter CH. Erectile dysfunction: Comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil, and tadalafil: Review of the literature. Eur J Med Res. 2002;7:435–446. [PubMed] [Google Scholar]
- 42.Katz SD. Tadalafil: The evidence for its clinical potential in the treatment of pulmonary arterial hypertension. Core Evidence. 2008;2:225–231. [PMC free article] [PubMed] [Google Scholar]
- 43.Francis SH, Corbin JD. Molecular mechanisms and pharmacokinetics of phosphodiesterase-5 antagonists. Curr Urol Rep. 2003;4:457–465. doi: 10.1007/s11934-003-0027-x. [DOI] [PubMed] [Google Scholar]
- 44.New York, NY: Pfizer Labs; 2009. Nov, Revatio® (sildenafil) package insert. Accessed Mar 2010. [Google Scholar]
- 45.Indianapolis, IN: Eli Lilly and Company; May, 2009. Cialis® (tadalafil) package insert. Accessed Mar 2010. [Google Scholar]
- 46.Wayne, NJ: Bayer Healthcare Pharmaceuticals; Dec, 2008. Levitra® (vardenafil) package insert. Accessed Mar 2010. [Google Scholar]
- 47.Giuliano F, Varanese L. Tadalafil: A novel treatment for erectile dysfunction. Eur Heart J Suppl. 2002;4:H24–H31. [Google Scholar]
- 48.Barst RJ, Gibbs JS, Ghofrani HA, et al. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:78–84. doi: 10.1016/j.jacc.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]