Abstract
Micafungin is an echinocandin antifungal agent available for clinical use in Japan, Europe, and the United States. Through inhibition of β-1,3-glucan production, an essential component of the fungal cell wall, micafungin exhibits potent antifungal activity against key pathogenic fungi, including Candida and Aspergillus species, while contributing minimal toxicity to mammalian cells. This activity is maintained against polyene and azole-resistant isolates. Pharmacokinetic and pharmacodynamic studies have demonstrated linear kinetics both in adults and children with concentration-dependent activity observed both in vitro and in vivo. Dosage escalation studies have also demonstrated that doses much higher than those currently recommended may be administered without serious adverse effects. Clinically, micafungin has been shown to be efficacious for the treatment of invasive candidiasis and invasive aspergillosis. Furthermore, the clinical effectiveness of micafungin against these infections occurs without the drug interactions that occur with the azoles and the nephrotoxicity observed with amphotericin B formulations. This review will focus on the pharmacology, clinical microbiology, mechanisms of resistance, safety, and clinical efficacy of micafungin in the treatment of invasive candidiasis and invasive aspergillosis.
Keywords: micafungin, echinocandin, Candida, Aspergillus, invasive candidiasis, invasive aspergillosis
Introduction
During the past 15 years, the number of clinically available antifungal agents has increased substantially. Many of these newer agents have a broader spectrum of activity than fluconazole and reduced toxicities compared to amphotericin B deoxycholate. One reason for the increased number of available antifungal agents is the recognition that invasive fungal infections remain a significant clinical problem. In the United States, Candida species are the fourth most common cause of nosocomial bloodstream infections (Wisplinghoff et al 2004), and are associated with significant morbidity and mortality, with studies reporting crude mortality rates of approximately 40% (Wisplinghoff et al 2004; Pfaller and Diekema 2007). Reports have also demonstrated increased frequency of non-Candida albicans species, which may have clinical consequences as some species such as C. krusei and C. glabrata have reduced susceptibility to fluconazole and other azoles (Bodey et al 2002; Malani et al 2005; Pfaller and Diekema 2007). In addition to invasive candidiasis, the incidence of infections caused by Aspergillus species has increased over the last decade at major cancer centers and it is a major cause of infectious disease-related morbidity and mortality in immunocompromised patients, including those with hematologic malignancies, solid organ transplant recipients, and those undergoing hematopoietic stem cell transplantation (Baddley et al 2001; Lin et al 2001; Marr et al 2002; Garcia-Vidal et al 2008; Marr 2008).
The development and introduction of new azoles and lipid formulations of amphotericin B have not fully overcome the toxicity and drug interaction limitations associated with these antifungal classes (Wiederhold and Lewis 2003). Recently, the echinocandins, including micafungin, caspofungin, and anidulafungin, have become available for clinical use. Echinocandins inhibit (1→3)-β-D-glucan synthase, limiting the production of (1→3)-β-D-glucan, an essential component of the fungal cell wall (Douglas et al 1997). This is an attractive target for antifungal activity as human cells lack a homologous enzyme, thereby avoiding significant collateral toxicities and drug interactions associated with the azoles and amphotericin B. In the United States, micafungin is currently approved for the treatment of esophageal candidiasis, candidemia, acute disseminated candidiasis, Candida peritonitis and abscesses, and as prophylaxis against Candida infections in patients undergoing hematopoietic stell cell transplantation (Mycamine 2008). The purpose of this review is to discuss the pharmacology, clinical microbiology, mechanisms of resistance, safety, and clinical efficacy of micafungin in the treatment of invasive candidiasis and invasive aspergillosis.
Pharmacology
Mechanism of action
(1→3)-β-D-glucans are polysaccharides that are synthesized by the glucan synthase complex bound to the fungal cell membrane. These polysaccharides are a major component of the fungal cell wall contributing to its shape and integrity. Through non-competitive inhibition of the glucan synthase enzyme complex, the echinocandins lead to depletion of (1→3)-β-D-glucan within the cell wall, resulting in osmostic instability and cell wall lysis (Kurtz et al 1994; Douglas et al 1997; Bowman et al 2002). Against Candida species, exposure to micafungin may result in fungicidal activity (Ernst et al 2002). In this setting the minimum inhibitory concentration (MIC), which measures reductions in visible growth, may be used to assess in vitro drug activity. The echinocandins display unique patterns of growth inhibition against filamentous fungi, including Aspergillus and Scedosporium species, due to the glucan synthase complex being located at the growing apical tips (Beauvais et al 2001). In these organisms, exposure to an echinocandin results in aberrant hyphal growth with an abundance of short, stubby branches (Watabe et al 2003; Bowman et al 2002). Thus, the minimum effective concentration (MEC) has been used as an alternative to the MIC for measuring in vitro echinocandin activity against filamentous fungi. In vitro studies have demonstrated that the MEC, defined as the lowest concentration of an echinocandin resulting in abnormally branched stubby hyphae, is a consistent measure of in vitro echinocandin activity (Arikan et al 2001; Arikan et al 2003).
Clinical microbiology
Micafungin is a relatively broad-spectrum antifungal agent with in vitro activity against Candida and Aspergillus species, as well as the mycelial forms of dimorphic fungi. The most potent activity of micafungin is against Candida, including non-Candida albicans species (Table 1), and is conserved against clinically invasive isolates resistant to fluconazole (Espinel-Ingroff 2003; Ostrosky-Zeichner et al 2005; Messer et al 2006; Pfaller et al 2006; Pfaller and Diekema 2007). Micafungin and the other available echinocandins lack in vitro activity against Cryptococcus neoformans, Fusarium species, Trichosporon species, and the Zygomycetes (Goodman et al 2002; Espinel-Ingroff 2003; Heyn et al 2005; Matsue et al 2006). Against most Candida species, the MIC90 ranges from 0.015 to 0.5 μg/mL (Table 1). However, similar to anidulafungin and caspofungin, the potency of micafungin is reduced against C. parapsilosis, C. orthopsilosis, C. metapsilosis, C. guilliermondii, and C. lusitaniae, with MIC90 values ranging from 0.25 to 8 μg/mL (Tawara et al 2000; Ostrosky-Zeichner et al 2005; Pfaller et al 2006; Garcia-Effron et al 2008a). Although some patients with infections caused by these species have been reported to respond to echinocandin therapy, the clinical significance of this reduced potency is unclear as clinical trials have not been powered to assess efficacy against these species (Mora-Duarte et al 2002; Kuse et al 2007; Pappas et al 2007; Reboli et al 2007).
Table 1.
Microdilution minimum inhibitory concentration and minimum effective concentration (μg/mL) for the clinically available echinocandins against Candida and Aspergillus species (Tawara et al 2000; Arikan et al 2003; Espinel-Ingroff 2003; Heyn et al 2005; Ostrosky-Zeichner et al 2005; Pfaller et al 2006; Messer et al 2006; Pfaller et al 2006; Pfaller and Diekema 2007; Garcia-Effron et al 2008a)
| Species | Micafungin | Anidulafungin | Caspofungin | |||
|---|---|---|---|---|---|---|
| MIC range | MIC90range | MIC range | MIC90 range | MIC range | MIC90 range | |
| Candida species | ||||||
| C. albicans | 0.01 to ≥8 | 0.01–0.5 | 0.01 to ≥8 | 0.01–0.5 | 0.01 to ≥8 | 0.12–1 |
| C. glabrata | 0.01 to ≥8 | 0.01–0.5 | 0.01–8 | 0.12–0.5 | 0.01 to ≥8 | 0.06–1 |
| C. parapsilosis | 0.03 to ≥8 | 1 to >8 | 0.01 to ≥8 | 2 to >8 | 0.03 to ≥8 | 1–4 |
| C. tropicalis | 0.01 to ≥8 | 0.03–2 | 0.03 to ≥8 | 0.06–2 | 0.03 to ≥8 | 0.06–1 |
| C. krusei | 0.06–4 | 0.12–0.25 | 0.01–8 | 0.03–1 | 0.12 to ≥4 | 0.25– 2 |
| Species | MEC range | MEC90 range | MEC range | MEC90range | MEC range | MEC90 range |
| Aspergillus species | ||||||
| A. fumigatus | 0.007–0.125 | 0.015–0.125 | 0.06 | 0.06 | 0.06–0.125 | 0.125 |
| A. terreus | 0.004–0.008 | 0.004–0.008 | 0.03 | 0.03 | 0.125–2 | 1 |
| A. flavus | 0.003–0.125 | 0.015–0.125 | 0.03–0.125 | 0.03–0.125 | 0.03–0.125 | 0.125 |
| A. niger | 0.007–0.0125 | 0.008–.125 | 0.03–0.125 | 0.06–0.125 | 0.125–2 | 0.125–1 |
Abbreviations: MEC, minimum effective concentration; MIC, minimum inhibitory concentration.
Micafungin also exhibits significant in vitro activity against Aspergillus species, with MEC values reported to be ≤0.125 μg/mL (Table 1) (Arikan et al 2003). In contrast, higher values have been reported against Aspergillus species when the MIC endpoint has been used (Arikan et al 2003; Heyn et al 2005). This may reflect the limitation of using the MIC endpoint as a measure of echinocandin in vitro activity against filamentous fungi. Similarly, MEC values of the echinocandins against Scedosporium are lower than the corresponding MIC values (Arikan et al 2001; Arikan et al 2003). The activity of this class against Scedosporium species is markedly reduced compared to that reported against Aspergillus. In addition to Candida and Aspergillus species, micafungin also has potent in vitro activity against the mycelial forms of dimorphic fungi, such as Histoplasma capsulatum and Blastomyces dermatitidis, but limited activity against the yeast morphology of these pathogens encountered clinically (Nakai et al 2003). This difference in potency has been attributed to the limited amount of (1→3)-β-D-glucan within the cell walls of the yeast forms (Domer 1971; Kanetsuna and Carbonell 1971; Kanetsuna et al 1972; Nakai et al 2003). This activity against the mycelial forms of dimorphic fungi has limited clinical relevance, as it is the yeast morphology that is associated with clinical disease. Indeed, data from animal models have demonstrated poor activity of the echinocandins against infections caused by dimorphic fungi (Graybill et al 1998; Kohler et al 2000).
Recently, the Clinical and Laboratory Standards Institute (CLSI) Antifungal Subcommittee has developed recommendations for echinocandin susceptibility testing, including breakpoints for classifying Candida isolates as susceptible to members of this antifungal class (Pfaller et al 2008a). Briefly, the CLSI-endorsed broth microdilution method for echinocandins is similar to that for azoles. One difference is that for the echinocandins the visual endpoint is read after 24 hours of incubation as opposed to 48 hours for the azoles. Good correlation between clinical outcomes and the echinocandin breakpoint for susceptible isolates (MIC of ≤2 μg/mL) has been observed using these methods (Pfaller et al 2008b). Isolates with an echinocandin MIC of >2 μg/mL are classified as non-susceptible.
A previous multi-center study has demonstrated that this methodology has high interlaboratory reproducibility (Odds et al 2004). Interestingly, some in vitro studies have reported enhanced potency of anidulafungin and micafungin against Candida species compared to caspofungin (Ostrosky-Zeichner et al 2003; Cota et al 2006), while others have not noted differences in potency among the echinocandins (Pfaller et al 2008a). The differences in in vitro potency observed by some investigators have not translated into improved efficacy in vivo. Two studies have reported similar efficacy among members of this class in murine models of invasive fungal infections, including candidiasis and aspergillosis, despite the greater in vitro potency of anidulafungin and micafungin (Paderu et al 2007; Wiederhold et al 2007). In these studies, the in vivo activity correlated better with the in vitro potency of these agents when tested in the presence of human serum. The effect of serum on the activity of the echinocandins is not fully understood. One potential explanation is that the observed reduction in potency is due to the significant protein binding associated with each of these agents (Andes et al 2008b). Although this reduction in echinocandin potency may be secondary to the high degree of protein binding, a previous study with micafungin reported that the activity of this echinocandin remained greater than 50-fold higher than the free drug concentrations predicted by the protein binding ratio (Mochizuki et al 2006). Thus, although micafungin may demonstrate enhanced in vitro potency compared to caspofungin against clinical isolates, this may not translate into enhanced clinical efficacy. Indeed, a clinical trial directly comparing micafungin to caspofungin for the treatment of candidemia and invasive candidiasis demonstrated similar clinical outcomes among the various treatment groups (Pappas et al 2007).
Mechanisms of resistance
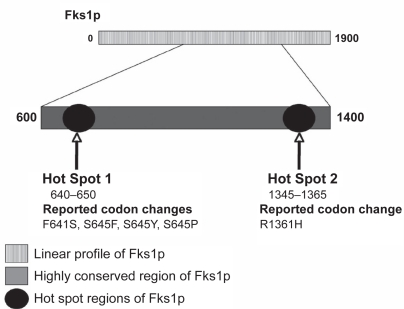
As the clinical use of the echinocandins continues to grow, reports of echinocandin failure associated with elevated MIC values continue to appear in the literature. Currently, the most established mechanism for reduced echinocandin susceptibility is an alteration in the glucan synthase enzyme complex. Genetic studies in Saccharomyces cerevisiae and C. albicans first suggested that alterations within this essential component of glucan synthase cause a reduced affinity of the echinocandins to this enzyme and could account for elevated MICs to this class of agents (Douglas et al 1994; Douglas et al 1997). These studies identified several single nucleotide polymorphisms (SNPs) that may occur within FKS1, the gene encoding for Fks1p, a subunit of the glucan synthase enzyme complex that is inhibited by the echinocandins (Balashov et al 2006). These SNPs occur within two regions of FKS1, and result in single amino acid substitutions within highly conserved regions of Fks1p. The first region associated with decreased echinocandin activity spans approximately 10 amino acids while the second region comprises 20 amino acids (Figure 1). Substitutions within these regions have been associated with increased echinocandin MICs in spontaneous laboratory C. albicans mutants and clinical isolates (Park et al 2005). These amino acid changes also resulted in a significant increase in the echinocandin 50% inhibitory concentration (IC50) against glucan synthase activity (Douglas et al 1997). In C. albicans, mutations within the highly conserved hot spot regions of FKS1 have been reported to lead to codon changes F641S, S645F, S645Y, S645P, and R1361H (Park et al 2005; Balashov et al 2006). Similar changes have been reported in a number of clinical non-C. albicans isolates with reduced echinocandin susceptibility (Table 2) collected from patients who experienced clinical failure while receiving an echinocandin (Kahn et al 2007; Cleary et al 2008; Garcia-Effron et al 2008b). In C. glabrata, SNPs in FKS1 and FKS2 result in amino acid changes that confer reduced susceptibility and have been documented in patients failing echinocandin therapy (Cleary et al 2008; Thompson et al 2008). Interestingly, a recent study has demonstrated that the reduced potency of echinocandins against C. parapsilosis and the closely related species C. orthopsilosis and C. metopsilosisis due to a naturally occurring proline-to-alanine substitution at amino acid 660 (P660A) at the end of one of the highly conserved hot spot regions within Fks1p (Garcia-Effron et al 2008a). Other mechanisms that may result in reduced echinocandin susceptibility include overexpression of the Golgi protein Sbe2p, which is involved in cell wall component transport (Osherov et al 2002), up-regulation of the cell wall integrity pathway (Wiederhold et al 2005), and increases in cell wall chitin content (Pfaller et al 1989; Stevens et al 2006; Cota et al 2008). The clinical significance of these other mechanisms is unknown.
Figure 1.
Linear profile of the Fks1p subunit in Candida albicans and loci containing amino acid substitutions associated with reduced echinocandin susceptibility. Adapted with permission from Park S, Kelly R, Kahn JN, et al. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob Agents Chemother, 49:3264–73, and from Balashov SV, Park S, Perlin DS. 2006. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1. Antimicrob Agents Chemother, 50:2058–63. Copyright © 2005 and 2006 American Society for Microbiology.
Table 2.
Amino acid sequences and nucleotide changes in susceptible and resistant isolates from various Candida species reported in the literature
| Isolates | Protein sequence | Nucleotide change | Caspofungin MIC (μg/mL) |
|---|---|---|---|
| Candida albicans (Wiederhold et al 2008) | |||
| SC5314 | FLTLSLRDP | – | 0.125 |
| 2762 | SLTLSLRDP | T1922C | 4 |
| 53264 | FLTLPLRDP | T1933C | 4 |
| Candida glabrata (Thompson et al 2008; Cleary et al 2008) | |||
| 7754 | FFLILSLRDP | – | 0.25 |
| 7755 | FVLILSLRDP (Hot Spot 1) | T1975G | 2 |
| 06-3169 | FLILSLREP (Hot Spot 2) | T1896G | >2 |
| Candida krusei (Kahn et al 2007) | |||
| Ck-98 | FLILSIRDP | – | 0.25 |
| Ck-100 | CLILSIRDP | T2080K | 8 |
| Candida tropicalis (Garcia-Effron et al 2008b) | |||
| ATCC 750 | FLTSLRDP | – | 0.25 |
| T3 | FLTLS/PLRDP | T1935C | 4 |
| T26 | LLTLSLRDP | T1923C | 1 |
| Candida parapsilosis (Codon Change) (Garcia-Effron et al 2008a) | |||
| ATCC 22019 | FLTLSLRDA | P660A | 1.4 |
| H4 | FLTSLRDA | P660A | 2.24 |
Note: Bolded and italicized letters refer to amino acid changes within protein sequences as a result of a nucleic acid point mutation.
Abbreviation: MIC, minimum inhibitory concentration.
In many of the case reports detailing clinical failure associated with reductions in echinocandin susceptibility, reduced potency occurred over a prolonged period of echinocandin therapy or in patients with an untreated focus of infection (ie, central venous catheter, prosthesis) (Pelletier et al 2005; Hakki et al 2006; Cleary et al 2008). In one case report of a patient with esophageal candidiasis who failed micafungin, the MIC values rose significantly over 6-week period of therapy from a baseline value of 0.06 μg/mL with similar decreases in susceptibility also observed for anidulafungin and caspofungin (Laverdiere et al 2006). In some reports, the MIC values for anidulafungin and micafungin remained below the recently established CLSI breakpoint for echinocandin susceptibility, despite a caspofungin MIC of >2 μg/mL, but normalized when tested in the presence of serum (Wiederhold et al 2007; Thompson et al 2008). Similar results have been demonstrated in vivo and correlated to in vitro susceptibility testing done in the presence of serum (Wiederhold et al 2007; Maki et al 2008), suggesting that switching to a different echinocandin when resistance is noted for another member of this class may not be appropriate (Pfaller et al 2008a). Despite the publication of numerous case reports of echinocandin clinical failure associated with reduced in vitro susceptibility, clinical failure due to echinocandin resistance appears to be of limited clinical scope as surveillance studies have not revealed significant changes in the activity of these agents (Pfaller et al 2006; Pfaller et al 2008a).
Pharmacodynamics
Micafungin demonstrates concentration-dependent activity against Candida and Aspergillus species in vitro and in preclinical animal models. Against C. albicans, C. glabrata, and C. krusei, micafungin demonstrated fungicidal activity in time-kill studies, defined as a ≥3 log10 reduction in colony-forming units per milliliter from the starting inoculum, with improved activity at higher concentrations (Ernst et al 2002). However, against C. tropicalis, fungistatic activity was observed. In animal studies of invasive candidiasis, increases in survival and reductions in tissue fungal burden have been described at doses of ≥0.125 mg/kg (Ikeda et al 2000; Maesaki et al 2000; Petraitis et al 2002). Free drug area under the concentration curve to MIC ratios (AUC/MIC) of 10 and 20 have been associated with stasis and a 1 log10 reduction in tissue fungal burden (CFU per gram of kidney tissue), respectively, against susceptible C. albicans and C. glabrata isolates in a murine model of invasive candidiasis (Andes et al 2008b). These values for stasis and a 1-log reduction in fungal burden were similar to the AUC/MIC ratios for anidulafungin reported by the same investigators (Andes et al 2008a). Because of the long half-lives achieved clinically and the tolerability observed in dose escalation trials (Cancidas 2005; Hiemenz et al 2005; Sirohi et al 2006), extended-interval dosing of the echinocandins has been suggested as a means of overcoming the need for daily intravenous therapy. This strategy has been demonstrated to be effective in a murine model of disseminated candidiasis in which a single large dose of micafungin (100 mg/kg) effectively reduced tissue fungal burden with no evidence of regrowth after 7 days in mice inoculated with C. glabrata (Gumbo et al 2007).
In animal models of invasive aspergillosis, increasing doses of micafungin have been shown to improve survival (Ikeda et al 2000; Petraitis et al 2002; Ichiyasu et al 2006). However, reductions in tissue fungal burden have not been consistently reported with increasing doses. In fact, a paradoxical attenuation of of echinocandin activity at higher concentrations despite an inhibitory effect at lower drug levels has been reported both in vitro and in vivo. In Candida species, the attenuated activity occurs within a range of 4 to 32 μg/mL (Ramage et al 2002; Stevens et al 2004; Wiederhold et al 2005), and is both echinocandin and Candida species specific. In a study of 60 Candida bloodstream isolates, the highest frequency of this phenomenon was observed with caspofungin, occurring in 90% of C. parapsilosis, 60% of C. albicans, 40% of C. tropicalis, and in 1 C. krusei isolate (Chamilos et al 2007). For anidulafungin, this effect was reported in 40% of C. albicans and 20% of the C. tropicalis isolates tested. In contrast, a paradoxical effect was not observed for micafungin against any C. albicans or C. parapsilosis isolates, but did occur in C. tropicalis (70%) and C. krusei (60%) isolates. Paradoxical increases in markers of disease burden have been reported with higher doses of the ecinocandins in animal models of invasive aspergillosis. For micafungin, an increase in serum galactomannan antigenemia was reported at a dose of 2 mg/kg/day compared to 1 mg/kg/day reported in a neutropenic rabbit model of invasive pulmonary aspergillosis (Petraitis et al 2002). In addition, the mean total lung weight of animals that received dosages of 2 mg/kg/day of micafungin did not differ from that of infected controls even though a reduction was reported with a lower dosage. However, higher doses of micafungin did result in further damage to the hyphae and this was associated with significant reductions in pulmonary infarct scores. Similarly, in a murine model of central nervous system aspergillosis, micafungin dosages of 10 mg/kg/day did not result in significant clearance of residual fungal burden as measured by colony-forming units from the secondary site of infection, the kidneys, despite a significant reduction in animals treated with 5 mg/kg/day (Clemons et al 2005). In contrast, a recent study comparing micafungin to caspofungin in a murine model of invasive pulmonary aspergillosis reported no increases in pulmonary fungal burden as measured by quantitative real-time PCR with higher doses of micafungin compared to a modest paradoxical effect observed with caspofungin (Lewis et al 2008a). Although the paradoxical effect has been inconsistently reported in animal studies, it is important to remember that this effect appears to be echinocandin-dependent for the specific species in question for both Candida and Aspergillus species (Chamilos et al 2007; Antachopoulos et al 2008). Where this phenomenon may impact therapy is with extended interval dosing of the echinocandins as therapy or prophylaxis against invasive aspergillosis. Indeed, one study reported worse survival, increased pulmonary fungal burden, and persistent hyphal clusters within the lungs as measured by histopathology in mice that received a single dose of micafungin 20 mg/kg as prophylaxis compared to a lower dose of 10 mg/kg (Lewis et al 2008b).
Recently, attention has focused on the immunomodulatory effects of the echinocandins against fungi secondary to the effects of these agents on (1→3)-β-D-glucan. Besides having an important role on fungal cell wall structure, (1→3)-β-D-glucan exhibits immunostimulatory properties secondary to recognition by the innate immune receptor Dectin-1 on alveolar macrophages, neutrophils, and dendritic cells (Brown 2006; Taylor et al 2007). This binding of (1→3)-β-D-glucan by Dectin-1 triggers phagocytosis, the release of pro-inflammatory cytokines, and the generation of reactive oxygen intermediates (Brown 2006). Recent studies have demonstrated that echinocandin-induced morphological changes in hyphae of filamentous fungi result in increased cell wall exposure of (1→3)-β-D-glucan and Dectin-1 mediated pro-inflammatory responses by macrophages and neutrophils (Hohl et al 2008; Lamaris et al 2008). It is noteworthy that this pro-inflammatory response has been primarily studied upon exposure of hyphae to caspofungin. However, enhanced polymorphonuclear neutrophil-mediated damage has also been observed when A. fumigatus hyphae are preincubated with anidulafungin and micafungin (Lamaris et al 2008). Previous studies have demonstrated A. fumigatus hyphae that have not been exposed to an echinocandin illicit a Th2 mediated inflammatory response in dendritic cells resulting in poor outcomes in an animal model of invasive aspergillosis (Bozza et al 2002; Bozza et al 2003). Further work in experimental models of invasive fungal infections using a wide range of echinocandin doses are needed to fully determine the clinical relevance of the immunomodulatory effects of these agents.
Pharmacokinetics
Micafungin has linear pharmacokinetics over a wide range of doses (Hebert et al 2005b; Hiemenz et al 2005). In healthy adult volunteers the mean peak plasma concentration (Cmax) and area under the concentration curve (AUC) were 8.8 μg/mL and 125.9 μg × h/mL, respectively, following a single 100 mg dose (Hebert et al 2005b). In a randomized, double-blind dose escalation study similar pharmacokinetic values were observed for micafungin doses ranging from 12.5 to 200 mg/day in adult patients undergoing bone marrow or peripheral stem cell transplantation (Hiemenz et al 2005). In this study, micafungin exposure was proportional to the dose administered with accumulation of the drug from days 1 to 7 following linear pharmacokinetics. In adults, the volume of distribution of micafungin approximates that of the extracellular fluid (0.26 to 0.39 L/kg) (Hebert et al 2005b; Heresi et al 2006). Similar to anidulafungin and caspofungin, micafungin is extensively protein-bound in the plasma (>99.5%) (Mycamine 2008). The effects of protein binding on the activity of echinocandins are not fully understood as studies on the effects of protein binding on echinocandin activity have reported conflicting results. While one early study demonstrated enhanced activity of caspofungin against A. fumigatus in the presence of sera (Chiller et al 2000), other studies have reported reduced potency of members of this class in the presence of human sera. Mochizuki et al reported that the MIC of micafungin against C. albicans increased 8-fold in the presence of inactivated sera (Mochizuki et al 2006). However, the activity of micafungin was 50-fold higher than that predicted by the free drug concentration profile. Another recent study comparing each of the three available echinocandins reported a reduction in the activity of each agent with the addition of 50% human sera, resulting in the neutralization of the enhanced potency of micafungin relative to caspofungin both in vitro and in vivo (Paderu et al 2007).
The pharmacokinetics of micafungin have also been evaluated in pediatric patients. Micafungin was administered at doses ranging from 0.5 to 4 mg/kg/day in an open-label phase I study of patients with febrile neutropenia ranging from age 2 to 17 years (Seibel et al 2005). Similar to adults, Cmax and AUC values increased in a linear fashion. However, consistent with previous studies of antifungal agents that have demonstrated greater clearance and reduced exposure in pediatric patients compared to adolescents and adults, a higher clearance and volume of distribution and shorter half-life were observed in patients age 2 to 8 years compared to those 9 to 17 years old (Table 3) (Lee et al 1992; Walsh et al 2004; Walsh et al 2005). In neonates less than or equal to 40 weeks gestational age and weighing at least 1000 g, linear increases in Cmax and AUC values were also observed following single doses of 0.75, 1.5, and 3 mg/kg (Heresi et al 2006). Micafungin clearance was also increased in this group (1.7-fold higher than that of children age 2–8 years, and 2.6-fold higher than adolescents age 9–17 years).
Table 3.
Mean micafungin pharmacokinetic parameters (±SD) by different age groups and different doses in pediatric and adult patients (Hebert et al 2005b; Hiemenz et al 2005; Seibel et al 2005; Heresi et al 2006)
| Age group | Clearance (mL/h/kg) | Half-life (h) | Volume distribution (L/kg) |
|---|---|---|---|
| Neonates >1000 g | 38.9 (12.1) | 8.3 (1.8) | 0.435 (0.11) |
| Children 2–8 yrs | 22.5 (8.6) | 11.5 (2.9) | 0.335 (0.16) |
| Adolescents 9–17 yrs | 15.1 (6.3) | 13.4 (3.8) | 0.243 (0.07) |
| Adults | 14.6 (3.4) | 13.1 (3.0) | 0.256 (0.01) |
| Adult PK parameters by dose | Cmax (μg/mL) | AUC (μg × h/mL) | |
| 50 mg | 5.1 (1.0) | 54 (13) | |
| 100 mg | 10.1 (2.6) | 115 (25) | |
| 150 mg | 16.4 (16.4) | 167 (167) |
Abbreviations: PK, pharmacokinetic.
Micafungin undergoes limited phase I metabolism to three metabolites (M1, M2, and M5). The M1 metabolite is formed by metabolism of the parent drug by arylsulfatase, and this metabolite is further degraded by cathechol-O-methyltransferase to M2. The third metabolite, M5, is formed by hydroxylation of the side chain by CYP450 isoenzymes. However, the cumulative AUC and Cmax of these three metabolites have been reported to be approximately 8% and 4%, respectively, of the parent compound in healthy volunteers at steady state (Keirns et al 2007). The primary route of micafungin elimination is through fecal excretion (Mycamine 2008). In a health volunteer study 14C-radiolabeled micafungin was administered as a 25 mg dose to healthy volunteers. In this study, 71% of micafungin clearance occurred via biliary elimination via the fecal route as either unchanged parent drug or metabolite.
Because micafungin is not cleared renally and undergoes limited hepatic metabolism, its pharmacokinetic parameters appear to be unchanged in patients with hepatic or renal dysfunction. In a phase I, open-label, 100 mg single-dose pharmacokinetic study no differences in peak plasma concentration, clearance, volume of distribution, or half-life were observed among patients with: 1) moderate hepatic impairment (Child-Pugh score 7–9), 2) creatinine clearance <30 mL/min, and 3) age and gender matched healthy controls (Hebert et al 2005b). No differences in AUC were noted between patients with renal dysfunction and healthy controls. Patients with moderate hepatic impairment did have a lower micafungin AUC (97.5 μg × h/mL) compared to healthy controls (125.9 μg × h/mL), although this may have been due to a significantly higher mean body weight in this group compared to healthy controls. Hypoalbuminemia resulting in alterations in protein binding may also be a potential explanation for the reduced micafungin AUC in patients with hepatic impairment due to the high protein binding of this drug. Reductions in albumin may increase the volume of distribution as more drug is able to distribute out of the bloodstream, which has been shown to occur for micafungin in animals with acute hepatic failure (Konishi et al 2005). However, no changes in the weight-adjusted volume of distribution were observed between healthy subjects and those with moderate hepatic impairment in the study by Hebert et al (2005b). A subsequent study also reported no influence of hypoalbuminemia on micafungin plasma concentrations (Nakagawa et al 2007). Currently, no dosage adjustments are recommended for patients with mild to moderate hepatic or mild, moderate, or severe renal impairment (Mycamine 2008).
Interestingly, a recent study has demonstrated that patient weight influences the clearance of micafungin. Using a population pharmacokinetic analysis with serum micafungin concentration data from a previous study of bone marrow transplant recipients, Gumbo et al identified patient weight to be a significant covariate on the serum clearance of this echinocandin (Gumbo et al 2008). The clearance of micafungin in patients weighing 66.3 kg or greater was increased by 50% compared to those weighing less than this amount. Because increased clearance impacts the overall drug exposure, as measured by the AUC, and the pharmacokinetic/pharmacodynamic parameter associated with the in vivo activity of micafungin is the AUC/MIC ratio (Gumbo et al 2007; Andes et al 2008b), the authors speculated that this may negatively affect heavier patients infected with Candida isolates with higher MIC values if the dose is not increased. However, it is unknown if this finding indeed has clinical implications.
Adverse effects
Micafungin is well tolerated with few drug-related adverse effects reported in clinical trials. No differences in safety profiles were observed between micafungin and fluconazole, a relatively well-tolerated antifungal, in head-to-head trials of these two agents (van Burik et al 2004; de Wet et al 2004; de Wet et al 2005). Rash, chills, nausea, vomiting, diarrhea, headache, and injection site reactions have been the most commonly reported adverse events (de Wet et al 2004; de Wet et al 2005; Ostrosky-Zeichner et al 2005; Denning et al 2006). Pruritus, facial swelling, and vasodilation have also been reported and may be due to histamine release that occurs with the intravenous administration (Mycamine 2008). Adverse laboratory effects associated with micafungin are generally mild and include elevated transaminases, alkaline phosphatase, and serum bilirubin (van Burik et al 2004; de Wet et al 2005; Mycamine 2008). Overall, few patients enrolled in clinical trials have discontinued micafungin due to adverse effects related to this drug.
The excellent safety profile, favorable pharmacokinetics, and concentration-dependent activity of micafungin have led several investigators to evaluate the safety of dosage escalation with this agent. In adult bone-marrow or peripheral stem cell transplant patients randomized to either micafungin or placebo for up to 4 weeks, grade 3 or higher toxicities thought to be related to this echinocandin were observed in 4 patients at dosages of 150 or 200 mg/day (Hiemenz et al 2005). These included atrial fibrillation, hypokalemia, pancreatitis, and maculopapuler rash. However, since the same grade 3 or higher toxicity was not observed in at least 3 patients in the same dosage group, the criterion for the maximum-tolerated dose was not met. A maximum-tolerated dose of micafungin was also not observed in an open-label study of hematopoietic stem cell transplant recipients who received dosages of 3, 4, 6, or 8 mg/kg/day for a period of 8 days to 4 weeks (Sirohi et al 2006). No patients developed grade 3 or 4 toxicities related to micafungin, and one patient was able to tolerate a dose of 900 mg/day. In addition, no significant changes in transaminase levels were reported, and the adverse effects observed were mild to moderate in severity. Similarly, a maximum tolerated dose was not observed in neutropenic patients age 2 to 17 years, including autologous and allogeneic hematopoietic stem cell transplant recipients, who received micafungin at 0.5 to 4 mg/kg/day up to a maximum of 200 mg/day (Seibel et al 2005).
Drug interactions
One of the advantages of micafungin over the azole antifungals is that few clinically significant drug interactions are associated with this echinocandin. Although micafungin appears to be a mild inhibitor of cytochrome P450 3A4 in vitro, this appears to be of minimal clinical significance as no interactions have been found with the known 3A4 inhibitors fluconazole, voriconazole, and ritonavir or with the potent inducer rifampin (Hiemenz et al 2005; Keirns et al 2007; Mycamine 2008). Healthy volunteer studies have demonstrated increases in nifedipine AUC (18%) and Cmax (42%) values in the presence of steady-state micafungin concentrations (Sakaeda et al 2005; Mycamine 2008). Similarly, micafungin has been shown to increase the AUC of sirolimus by 21%. In addition, the AUC and Cmax of itraconazole may be increased with co-administration of micafungin (Mycamine 2008). Thus, it is recommended that patients receiving sirolimus, nifedipine, or itraconazole in combination with micafungin be monitored for signs and symptoms of toxicity, and that dosage reductions of these medications may be necessary (Mycamine 2008).
In healthy volunteers, micafungin AUC values have been reported to increase between 10% and 14% with concurrent administration of a single dose of cyclosporine (Hebert et al 2005c). Conversely, micafungin appears to be a mild inhibitor of cyclosporine metabolism, resulting in a decrease in the oral clearance and an increase in the half-life (Hebert et al 2005c). Although the clinical relevance of this interaction is unknown, careful monitoring of cyclosporine concentrations may be warranted as one-fifth of the volunteers in this study had potentially clinically significant increases in cyclosporine concentrations when coadministered with micafungin. Interestingly, no changes in tacrolimus or micafungin pharmacokinetic parameters were observed in a similar drug interaction study (Hebert et al 2005a).
Clinical efficacy
Esophageal candidiasis
The echinocandins have a useful role in the treatment of esophageal candidiasis, including azole-refractory infections, secondary to their excellent safety profile and limited number of clinically significant drug interactions. Micafungin has been shown in clinical studies to be as effective as fluconazole for the treatment of esophageal candidiasis. In a double-blind dose-ranging study of HIV-positive patients comparing different doses of micafungin to fluconazole (200 mg/day), a clear dose-response was noted in patients randomized to micafungin 50 mg (69%), 100 mg (77%), or 150 mg (90%) as measured by endoscopy (de Wet et al 2004). Interestingly, despite similar endoscopic cure rates and clinical responses at the end of therapy, mycological eradication occurred in a higher percentage of patients who received micafungin 100 mg compared to those randomized to 150 mg (78% vs 57%, respectively; p = 0.031). However, successful treatment, defined as no evidence of esophageal candidiasis associated plaques at the end of therapy, did not differ significantly among patients randomized to receive micafungin 100 mg or 150 mg compared to fluconazole (87%). Similar outcomes were reported in a second trial comparing micafungin (150 mg/day) to fluconazole (200 mg/day) for the treatment of endoscopy confirmed esophageal candidiasis (de Wet et al 2005). In this study, endoscopic cure at the end of therapy was similar between patients randomized to micafungin and fluconazole (88% in both treatment groups). In each study, micafungin was reported to be well tolerated with rash, fever, and phlebitis the most commonly observed adverse effects.
Relapses following discontinuation of therapy have been reported in clinical trails for each member of the echinocandin class, raising concerns about the use of these agents for the treatment of esophageal candidiasis. In the first study discussed above, relapses were reported in 9 patients randomized to micafungin and in none who received fluconazole (de Wet et al 2004). Similarly, in the second study approximately 15% of patients who received micafungin relapsed following discontinuation of therapy (de Wet et al 2005). However, 11% of patients randomized to fluconazole also relapsed after the end of treatment. Similar relapse rates have also been reported for caspofungin and fluconazole during the post-treatment period (11% and 8%, respectively) (Villanueva et al 2002) as well as for anidulafungin (35%) (Krause et al 2004). Thus, it is unknown if the relapses following discontinuation of treatment for esophageal candidiasis is a property specific for the echinocandins.
Candidemia/invasive candidiasis
A primary use of the echinocandins is for the treatment of invasive candidiasis. Micafungin has been evaluated for the treatment of patients with invasive candidiasis/candidemia in three clinical trials. In an open-label, noncomparative study, micafungin, either alone or in combination with another antifungal agent, was shown to be effective in patients with newly diagnosed or refractory candidemia (Ostrosky-Zeichner et al 2005). The initial dose of micafungin was 50 mg/day (1 mg/kg for patients less than 40 kg) for the treatment of C. albicans infections, and 100 mg per day for infections caused by other Candida species. Overall treatment success, defined as a complete or partial response based on the global assessment of clinical and mycological response at the end of therapy, was 76% in patients who received at least one dose of micafungin, and increased to 83% in patients who received at least five doses. Response rates were similar among patients who received micafungin in combination for refractory disease (79%), alone for newly diagnosed candidemia (87%), or alone for refractory candidemia (76%). As this trial was a preliminary study to obtain data on the efficacy of micafungin for the treatment of candidemia, the dose used for the treatment of infections caused by C. albicans (50 mg/day) was lower than in subsequent studies, where the minimum dose was 100 mg/day regardless of the infection.
The efficacy of micafungin for invasive candidiasis/candidemia has also been compared to that of liposomal amphotericin B. In a double-blind, non-inferiority study patients were randomized to receive either micafungin 100 mg/day or liposomal amphotericin B administered as 3 mg/kg/day (Kuse et al 2007). The majority of patients enrolled in this study had candidemia, and the causative agent of infection was frequently a non-albicans species: 62% in micafungin patients and 59% in those randomized to liposomal amphotericin B. In the intent-to-treat analysis overall treatment success, defined as clinical and mycological response at the end of therapy, was observed in 72% of patients in the micafungin group and 68% of those who received liposomal amphotericin B. No differences were observed among patients infected with different Candida species. Similar to a previous study that compared caspofungin to amphotericin B deoxycholate (Mora-Duarte et al 2002), micafungin was better tolerated with a lower incidence of nephrotoxicity and infusion-related reactions than those observed with liposomal amphotericin B.
Results from a pediatric sub-study as part of the micafungin versus liposomal amphotericin B study have recently been published (Queiroz-Telles et al 2008). In this study, micafungin was administered at a dose of 2 mg/kg/day and liposomal amphotericin B at 3 mg/kg/day. This dose of micafungin was used secondary to the shorter half-life and faster clearance observed in premature infants and young children (Seibel et al 2005; Heresi et al 2006). Overall treatment success was similar between patients who received micafungin (73%) and those that received liposomal amphotericin B (76%). The treatment success rates were consistent across age groups and in patients who were born prematurely, and mycologic persistence at the end of therapy was observed in 16% of patients in both treatment groups. Similar to the adult study, discontinuation of therapy due to adverse effects was lower in the micafungin group compared to liposomal amphotericin B (3.8% vs 17%, respectively).
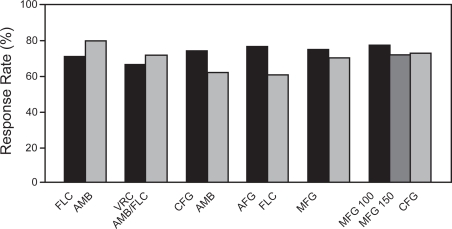
In the two previously described studies, as well as other studies that have evaluated caspofungin and anidulafungin for the treatment of invasive fungal infections, a member of another antifungal class has been used as the active comparator. To date, only one clinical trial has directly compared two echinocandins for the treatment of invasive fungal infections. In this double-blind, non-inferiority study, patients were randomized to receive either micafungin 100 mg/day or 150 mg/day, or caspofungin, administered at 70 mg on the first day and 50 mg/day thereafter, for the treatment of invasive candidiasis/candidemia (Pappas et al 2007). Overall success, again defined as a favorable clinical and mycological response at the end of therapy, was similar among the three groups with 74% of patients randomized to micafungin 100 mg, 70% to micafungin 150 mg, and 71% in those who received caspofungin having a favorable outcome. Interestingly, a higher response rate was reported for the higher dose of micafungin in patients with infections caused by C. glabrata. However, this study was not sufficiently powered to assess differences among Candida species. These results demonstrate that micafungin is as effective as caspofungin for the treatment of invasive candidiasis/candidemia. Similar safety profiles among the treatment groups were also reported. Although a clear dose-response was observed in an early study that compared micafungin to fluconazole for the treatment of esophageal candidiasis, such an effect was not observed in this study as micafungin 100 mg/day was as effective as the higher dose of 150 mg/day. Overall, the response rates reported in the studies that have evaluated micafungin for invasive candidiasis/candidemia are similar to those observed for caspofungin and anidulafungin as well as other antifungal agents in clinical studies (Figure 2) (Rex et al 1994; Mora-Duarte et al 2002; Kullberg et al 2005; Reboli et al 2007).
Figure 2.
Response rates of antifungal agents in clinical trials of invasive candidiasis. FLC: fluconazole, AMB: amphotercin B deoxycholate, CFG: caspofungin, AFG: anidulafungin, MFG: micafungin, LAMB: liposomal amphotericin B, MFG 100: micafungin 100 mg, MFG 150: micafungin 150 mg (Rex et al 1994; Mora-Duarte et al 2002; Kullberg et al 2005; Kuse et al 2007; Pappas et al 2007; Reboli et al 2007).
Antifungal prophylaxis
Antifungal prophylaxis has been shown to reduce the incidence of invasive fungal infections and in some studies to improve survival (Goodman et al 2002; Winston et al 2003; Marr et al 2004; Cornely et al 2007; Ullmann et al 2007). However, this strategy is often limited by the drug interactions and adverse effects associated with the azoles and amphotericin B formulations. The echinocandins offer an alternative strategy as antifungal prophylaxis secondary to their favorable adverse effect profile and lack of clinically relevant drug interactions. In a prospective, double-blind, multicenter study that included autologous and allogeneic stem cell transplant recipients, patients were randomized to receive either micafungin 50 mg/day (1 mg/kg for those weighing less than 50 kg) or fluconazole 400 mg/day (8 mg/kg in those weighing less than 50 kg) (van Burik et al 2004). Prophylaxis was initiated in the pre-engraftment period and continued until approximately 5 days after engraftment or up to 42 days post-transplant. Treatment success was defined as the absence of proven, probable, or suspected systemic fungal infections at the end of prophylaxis and the absence of proven or probable systemic fungal infections at the end of the 4-week follow-up period. Patients randomized to micafungin experienced higher treatment success compared to those who received fluconazole (80% vs 74%; p = 0.03). No difference in breakthrough candidiasis was observed between the two groups. Instead, this difference was primarily due to fewer cases of breakthrough invasive aspergillosis in the micafungin group (1 patient) compared to fluconazole (7 patients). Less empiric antifungal therapy was also required in the micafungin group compared to fluconazole (15% vs 21%, respectively; p = 0.024). One of the limitations of this study is that the median duration of prophylaxis was only 18 days. Thus, it is unclear how effective micafungin prophylaxis would be in the setting of graft-versus host disease and corticosteroid therapy in allogeneic stem cell transplant recipients, as observational studies have demonstrated an increased risk of invasive aspergillosis during the post-engraftment period (Wald et al 1997).
Invasive aspergillosis
Micafungin does not currently have an indication for the treatment of invasive aspergillosis and data on its use against this opportunistic infection are limited. In one open-label, non-comparative study, micafungin was evaluated as primary therapy or as salvage therapy in patients who had failed previous therapy or were intolerant of other antifungal agents (Denning et al 2006). Patients were initially dosed at 75 mg/day with dosage escalation permitted if disease progression occurred or if cultures remained positive. A favorable response, defined as either a complete or partial response at the end of therapy based on radiological, mycological, and clinical data, was observed in 36% of patients who received at least one dose of micafungin. However, disease progression was observed in 53% of patients during the course of treatment. Patients in whom neutrophil recovery occurred had a favorable response rate of 50% compared to only 17% in those who remained neutropenic. During the 6-week follow-up period, overall mortality was 56%, of which 59% was considered to be attributable to invasive aspergillosis. Overall, the results of micafungin monotherapy in this study (41% favorable response rate) are similar to those reported for caspofungin monotherapy in patients receiving this echinocandin as salvage therapy for invasive aspergillosis (45%) (Maertens et al 2004). In patients with refractory disease who received micafungin in combination with other antifungal therapy, response rates were less favorable (35% with a favorable response; 60 of 174). Responses were especially poor in allogeneic stem cell transplant recipients who were receiving salvage antifungal combination therapy (25% with a favorable response; 21 of 83) (Kontoyiannis et al 2008). Similarly low response rates have been reported for caspofungin in combination with other antifungal therapy for the salvage treatment of invasive aspergillosis (Kontoyiannis et al 2003), highlighting the difficulty in treating patients with refractory disease.
Conclusion
The echinocandins have been a welcome addition to the antifungal armamentarium. Major advantages of this class of agents include the potent activity against Candida and Aspergillus species, as well as the excellent safety compared to amphotericn B formulations and fewer drug interactions than the azoles. Clinical studies have demonstrated micafungin to be as safe and as effective as fluconazole for the treatment of esophageal candidiasis. In addition, micafungin is as effective as liposomal amphotericin B and caspofungin for the treatment of invasive candidiasis/candidemia. Notably, fewer adverse effects were observed for micafungin compared to liposomal amphotericin B in both adults and pediatric patients. The utility of micafungin against invasive aspergillosis is less clear. Although potent in vitro activity is observed against Aspergillus species, and these results are supported by preclinical in vivo studies, clinical data supporting the use of micafungin against invasive aspergillosis are scarce. Thus, while micafungin appears to be a suitable option for the treatment of invasive candidiasis/candidemia, further studies are needed to accurately judge its role in the treatment of invasive aspergillosis.
Footnotes
Disclosures
NPW has received research support from Pfizer, Schering-Plough, and Cydex Pharmaceuticals.
References
- Andes D, Diekema DJ, Pfaller MA, et al. In vivo pharmacodynamic characterization of anidulafungin in a neutropenic murine candidiasis model. Antimicrob Agents Chemother. 2008a;52:539–50. doi: 10.1128/AAC.01061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andes DR, Diekema DJ, Pfaller MA, et al. In vivo pharmacodynamic target investigation for micafungin against Candida albicans and C. g1abrata in a neutropenic murine candidiasis model. Antimicrob Agents Chemother. 2008b;52:3497–503. doi: 10.1128/AAC.00478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antachopoulos C, Meletiadis J, Sein T, et al. Comparative In Vitro Pharmacodynamics of Caspofungin, Micafungin, and Anidulafungin against Germinated and Nongerminated Aspergillus Conidia. Antimicrob Agents Chemother. 2008;52:321–8. doi: 10.1128/AAC.00699-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikan S, Lozano-Chiu M, Paetznick V, et al. In vitro susceptibility testing methods for caspofungin against Aspergillus and Fusarium isolates. Antimicrob Agents Chemother. 2001;45:327–30. doi: 10.1128/AAC.45.1.327-330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikan S, Yurdakul P, Hascelik G. Comparison of two methods and three end points in determination of in vitro activity of micafungin against Aspergillus spp. Antimicrob Agents Chemother. 2003;47:2640–3. doi: 10.1128/AAC.47.8.2640-2643.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddley JW, Stroud TP, Salzman D, et al. Invasive mold infections in allogeneic bone marrow transplant recipients. Clin Infect Dis. 2001;32:1319–24. doi: 10.1086/319985. [DOI] [PubMed] [Google Scholar]
- Balashov SV, Park S, Perlin DS. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1. Antimicrob Agents Chemother. 2006;50:2058–63. doi: 10.1128/AAC.01653-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais A, Bruneau JM, Mol PC, et al. Glucan synthase complex of Aspergillus fumigatus. J Bacteriol. 2001;183:2273–9. doi: 10.1128/JB.183.7.2273-2279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey GP, Mardani M, Hanna HA, et al. The epidemiology of Candida glabrata and Candida albicans fungemia in immunocompromised patients with cancer. Am J Med. 2002;112:380–5. doi: 10.1016/s0002-9343(01)01130-5. [DOI] [PubMed] [Google Scholar]
- Bowman JC, Hicks PS, Kurtz MB, et al. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob Agents Chemother. 2002;46:3001–12. doi: 10.1128/AAC.46.9.3001-3012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza S, Gaziano R, Spreca A, et al. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J Immunol. 2002;168:1362–71. doi: 10.4049/jimmunol.168.3.1362. [DOI] [PubMed] [Google Scholar]
- Bozza S, Perruccio K, Montagnoli C, et al. A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood. 2003;102:3807–14. doi: 10.1182/blood-2003-03-0748. [DOI] [PubMed] [Google Scholar]
- Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- Cancidas . Cancidas Package Insert. Merck and Co., Inc.; Whitehouse Station, NJ, USA: 2005. [Google Scholar]
- Chamilos G, Lewis RE, Albert N, et al. Paradoxical effect of Echinocandins across Candida species in vitro: evidence for echinocandin-specific and candida species-related differences. Antimicrob Agents Chemother. 2007;51:2257–9. doi: 10.1128/AAC.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiller T, Farrokhshad K, Brummer E, et al. Influence of human sera on the in vitro activity of the echinocandin caspofungin (MK-0991) against Aspergillus fumigatus. Antimicrob Agents Chemother. 2000;44:3302–5. doi: 10.1128/aac.44.12.3302-3305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JD, Garcia-Effron G, Chapman SW, et al. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob Agents Chemother. 2008;52:2263–5. doi: 10.1128/AAC.01568-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons KV, Espiritu M, Parmar R, et al. Comparative efficacies of conventional amphotericin b, liposomal amphotericin B (AmBisome), caspofungin, micafungin, and voriconazole alone and in combination against experimental murine central nervous system aspergillosis. Antimicrob Agents Chemother. 2005;49:4867–75. doi: 10.1128/AAC.49.12.4867-4875.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348–59. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- Cota J, Carden M, Graybill JR, et al. In vitro pharmacodynamics of anidulafungin and caspofungin against Candida glabrata isolates, including strains with decreased caspofungin susceptibility. Antimicrob Agents Chemother. 2006;50:3926–8. doi: 10.1128/AAC.00538-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota JM, Grabinski JL, Talbert RL, et al. Increases in SLT2 expression and chitin content are associated with incomplete killing of Candida glabrata by caspofungin. Antimicrob Agents Chemother. 2008;52:1144–6. doi: 10.1128/AAC.01542-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wet N, Llanos-Cuentas A, Suleiman J, et al. A randomized, double-blind, parallel-group, dose-response study of micafungin compared with fluconazole for the treatment of esophageal candidiasis in HIV-positive patients. Clin Infect Dis. 2004;39:842–9. doi: 10.1086/423377. [DOI] [PubMed] [Google Scholar]
- De Wet NT, Bester AJ, Viljoen JJ, et al. A randomized, double blind, comparative trial of micafungin (FK463) vs fluconazole for the treatment of oesophageal candidiasis. Aliment Pharmacol Ther. 2005;21:899–907. doi: 10.1111/j.1365-2036.2005.02427.x. [DOI] [PubMed] [Google Scholar]
- Denning DW, Marr KA, Lau WM, et al. Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis. J Infect. 2006;53:337–49. doi: 10.1016/j.jinf.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domer JE. Monosaccharide and chitin content of cell walls of Histoplasma capsulatum and Blastomyces dermatitidis. J Bacteriol. 1971;107:870–7. doi: 10.1128/jb.107.3.870-877.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas CM, D’ippolito JA, Shei GJ, et al. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-beta-D-glucan synthase inhibitors. Antimicrob Agents Chemother. 1997;41:2471–9. doi: 10.1128/aac.41.11.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas CM, Marrinan JA, Li W, et al. A Saccharomyces cerevisiae mutant with echinocandin-resistant 1,3-beta-D-glucan synthase. J Bacteriol. 1994;176:5686–96. doi: 10.1128/jb.176.18.5686-5696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst EJ, Roling EE, Petzold CR, et al. In vitro activity of micafungin (FK-463) against Candida spp.: microdilution, time-kill, and postantifungal-effect studies. Antimicrob Agents Chemother. 2002;46:3846–53. doi: 10.1128/AAC.46.12.3846-3853.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinel-Ingroff A. In vitro antifungal activities of anidulafungin and micafungin, licensed agents and the investigational triazole posaconazole as determined by NCCLS methods for 12,052 fungal isolates: review of the literature. Rev Iberoam Micol. 2003;20:121–36. [PubMed] [Google Scholar]
- Garcia-Effron G, Katiyar SK, Park S, et al. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob Agents Chemother. 2008a;52:2305–12. doi: 10.1128/AAC.00262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Effron G, Kontoyiannis DP, Lewis RE, et al. Caspofungin-resistant Candida tropicalis strains causing breakthrough fungemia in patients at high risk for hematologic malignancies. Antimicrob Agents Chemother. 2008b;52:4181–3. doi: 10.1128/AAC.00802-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Vidal C, Upton A, Kirby KA, et al. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47:1041–50. doi: 10.1086/591969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D, Pamer E, Jakubowski A, et al. Breakthrough trichosporonosis in a bone marrow transplant recipient receiving caspofungin acetate. Clin Infect Dis. 2002;35:E35–6. doi: 10.1086/341305. [DOI] [PubMed] [Google Scholar]
- Graybill JR, Najvar LK, Montalbo EM, et al. Treatment of histoplasmosis with MK-991 (L-743,872) Antimicrob Agents Chemother. 1998;42:151–3. doi: 10.1128/aac.42.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbo T, Drusano GL, Liu W, et al. Once-weekly micafungin therapy is as effective as daily therapy for disseminated candidiasis in mice with persistent neutropenia. Antimicrob Agents Chemother. 2007;51:968–74. doi: 10.1128/AAC.01337-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbo T, Hiemenz J, Ma L, et al. Population pharmacokinetics of micafungin in adult patients. Diagn Microbiol Infect Dis. 2008;60:329–31. doi: 10.1016/j.diagmicrobio.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Hakki M, Staab JF, Marr KA. Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrob Agents Chemother. 2006;50:2522–4. doi: 10.1128/AAC.00148-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert MF, Blough DK, Townsend RW, et al. Concomitant tacrolimus and micafungin pharmacokinetics in healthy volunteers. J Clin Pharmacol. 2005a;45:1018–24. doi: 10.1177/0091270005279274. [DOI] [PubMed] [Google Scholar]
- Hebert MF, Smith HE, Marbury TC, et al. Pharmacokinetics of micafungin in healthy volunteers, volunteers with moderate liver disease, and volunteers with renal dysfunction. J Clin Pharmacol. 2005b;45:1145–52. doi: 10.1177/0091270005279580. [DOI] [PubMed] [Google Scholar]
- Hebert MF, Townsend RW, Austin S, et al. Concomitant cyclosporine and micafungin pharmacokinetics in healthy volunteers. J Clin Pharmacol. 2005c;45:954–60. doi: 10.1177/0091270005278601. [DOI] [PubMed] [Google Scholar]
- Heresi GP, Gerstmann DR, Reed MD, et al. The pharmacokinetics and safety of micafungin, a novel echinocandin, in premature infants. Pediatr Infect Dis J. 2006;25:1110–5. doi: 10.1097/01.inf.0000245103.07614.e1. [DOI] [PubMed] [Google Scholar]
- Heyn K, Tredup A, Salvenmoser S, et al. Effect of voriconazole combined with micafungin against Candida, Aspergillus, and Scedosporium spp. and Fusarium solani. Antimicrob Agents Chemother. 2005;49:5157–9. doi: 10.1128/AAC.49.12.5157-5159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiemenz J, Cagnoni P, Simpson D, et al. Pharmacokinetic and maximum tolerated dose study of micafungin in combination with fluconazole versus fluconazole alone for prophylaxis of fungal infections in adult patients undergoing a bone marrow or peripheral stem cell transplant. Antimicrob Agents Chemother. 2005;49:1331–6. doi: 10.1128/AAC.49.4.1331-1336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl TM, Feldmesser M, Perlin DS, et al. Caspofungin modulates inflammatory responses to Aspergillus fumigatus through stage-specific effects on fungal beta-glucan exposure. J Infect Dis. 2008;198:176–85. doi: 10.1086/589304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyasu H, Yamamura A, Honda M, et al. [Successful treatment by voliconazole for pulmonary and adductor magnus muscle aspergillosis induced by immunosuppressive therapy for hypersensitivity pneumonia] Nihon Kokyuki Gakkai Zasshi. 2006;44:754–60. [PubMed] [Google Scholar]
- Ikeda F, Wakai Y, Matsumoto S, et al. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis. Antimicrob Agents Chemother. 2000;44:614–8. doi: 10.1128/aac.44.3.614-618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn JN, Garcia-Effron G, Hsu MJ, et al. Acquired echinocandin resistance in a Candida krusei isolate due to modification of glucan synthase. Antimicrob Agents Chemother. 2007;51:1876–8. doi: 10.1128/AAC.00067-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsuna F, Carbonell LM. Cell wall composition of the yeastlike and mycelial forms of Blastomyces dermatitidis. J Bacteriol. 1971;106:946–8. doi: 10.1128/jb.106.3.946-948.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsuna F, Carbonell LM, Azuma I, et al. Biochemical studies on the thermal dimorphism of Paracoccidioides brasiliensis. J Bacteriol. 1972;110:208–18. doi: 10.1128/jb.110.1.208-218.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirns J, Sawamoto T, Holum M, et al. Steady-state pharmacokinetics of micafungin and voriconazole after separate and concomitant dosing in healthy adults. Antimicrob Agents Chemother. 2007;51:787–90. doi: 10.1128/AAC.00673-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S, Wheat LJ, Connolly P, et al. Comparison of the echinocandin caspofungin with amphotericin B for treatment of histoplasmosis following pulmonary challenge in a murine model. Antimicrob Agents Chemother. 2000;44:1850–4. doi: 10.1128/aac.44.7.1850-1854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H, Sudo M, Sumi M, et al. Pharmacokinetic behavior of micafungin in rats with carbon tetrachloride-induced acute hepatic failure. Biol Pharm Bull. 2005;28:556–9. doi: 10.1248/bpb.28.556. [DOI] [PubMed] [Google Scholar]
- Kontoyiannis DP, Hachem R, Lewis RE, et al. Efficacy and toxicity of caspofungin in combination with liposomal amphotericin B as primary or salvage treatment of invasive aspergillosis in patients with hematologic malignancies. Cancer. 2003;98:292–9. doi: 10.1002/cncr.11479. [DOI] [PubMed] [Google Scholar]
- Kontoyiannis DP, Ratanatharathorn V, Young JA, et al. Micafungin alone or in combination with other systemic antifungal therapies in hematopoietic stem cell transplant recipients with invasive aspergillosis. Transpl Infect Dis. 2008 doi: 10.1111/j.1399-3062.2008.00349.x. DOI: 10.1111/j.1399–3062. 2008. 00349.x. [DOI] [PubMed] [Google Scholar]
- Krause DS, Simjee AE, Van Rensburg C, et al. A randomized, double-blind trial of anidulafungin versus fluconazole for the treatment of esophageal candidiasis. Clin Infect Dis. 2004;39:770–5. doi: 10.1086/423378. [DOI] [PubMed] [Google Scholar]
- Kullberg BJ, Sobel JD, Ruhnke M, et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet. 2005;366:1435–42. doi: 10.1016/S0140-6736(05)67490-9. [DOI] [PubMed] [Google Scholar]
- Kurtz MB, Heath IB, Marrinan J, et al. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-beta-D-glucan synthase. Antimicrob Agents Chemother. 1994;38:1480–9. doi: 10.1128/aac.38.7.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuse ER, Chetchotisakd P, Da Cunha CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet. 2007;369:1519–27. doi: 10.1016/S0140-6736(07)60605-9. [DOI] [PubMed] [Google Scholar]
- Lamaris GA, Lewis RE, Chamilos G, et al. Caspofungin-mediated beta-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae. J Infect Dis. 2008;198:186–92. doi: 10.1086/589305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverdiere M, Lalonde RG, Baril JG, et al. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J Antimicrob Chemother. 2006;57:705–8. doi: 10.1093/jac/dkl022. [DOI] [PubMed] [Google Scholar]
- Lee JW, Seibel NL, Amantea M, et al. Safety and pharmacokinetics of fluconazole in children with neoplastic diseases. J Pediatr. 1992;120:987–93. doi: 10.1016/s0022-3476(05)81975-4. [DOI] [PubMed] [Google Scholar]
- Lewis RE, Albert ND, Kontoyiannis DP. Comparison of the dose-dependent activity and paradoxical effect of caspofungin and micafungin in a neutropenic murine model of invasive pulmonary aspergillosis. J Antimicrob Chemother. 2008a;61:1140–4. doi: 10.1093/jac/dkn069. [DOI] [PubMed] [Google Scholar]
- Lewis RE, Albert ND, Kontoyiannis DP. Efficacy of single-dose liposomal amphotericin B or micafungin prophylaxis in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2008b;52:4178–80. doi: 10.1128/AAC.00715-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis. 2001;32:358–66. doi: 10.1086/318483. [DOI] [PubMed] [Google Scholar]
- Maertens J, Raad I, Petrikkos G, et al. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin Infect Dis. 2004;39:1563–71. doi: 10.1086/423381. [DOI] [PubMed] [Google Scholar]
- Maesaki S, Hossain MA, Miyazaki Y, et al. Efficacy of FK463, a (1,3)-beta-D-glucan synthase inhibitor, in disseminated azole-resistant Candida albicans infection in mice. Antimicrob Agents Chemother. 2000;44:1728–30. doi: 10.1128/aac.44.6.1728-1730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki K, Matsumoto S, Watabe E, et al. Use of a serum-based antifungal susceptibility assay to predict the in vivo efficacy of novel echinocandin compounds. Microbiol Immunol. 2008;52:383–91. doi: 10.1111/j.1348-0421.2008.00053.x. [DOI] [PubMed] [Google Scholar]
- Malani A, Hmoud J, Chiu L, et al. Candida glabrata fungemia: experience in a tertiary care center. Clin Infect Dis. 2005;41:975–81. doi: 10.1086/432939. [DOI] [PubMed] [Google Scholar]
- Marr KA. Fungal infections in hematopoietic stem cell transplant recipients. Med Mycol. 2008;46:293–302. doi: 10.1080/13693780701885552. [DOI] [PubMed] [Google Scholar]
- Marr KA, Carter RA, Crippa F, et al. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–17. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- Marr KA, Crippa F, Leisenring W, et al. Itraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplants. Blood. 2004;103:1527–33. doi: 10.1182/blood-2003-08-2644. [DOI] [PubMed] [Google Scholar]
- Matsue K, Uryu H, Koseki M, et al. Breakthrough trichosporonosis in patients with hematologic malignancies receiving micafungin. Clin Infect Dis. 2006;42:753–7. doi: 10.1086/500323. [DOI] [PubMed] [Google Scholar]
- Messer SA, Diekema DJ, Boyken L, et al. Activities of micafungin against 315 invasive clinical isolates of fluconazole-resistant Candida spp. J Clin Microbiol. 2006;44:324–6. doi: 10.1128/JCM.44.2.324-326.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki N, Aibiki M, Matsumoto Y. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy Abstr M-1598. San Francisco, CA: 2006. [Google Scholar]
- Mora-Duarte J, Betts R, Rotstein C, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med. 2002;347:2020–9. doi: 10.1056/NEJMoa021585. [DOI] [PubMed] [Google Scholar]
- Mycamine . Mycamine Package Insert. Astellas Pharma, US, Inc; 2008. [Google Scholar]
- Nakagawa Y, Ichii Y, Saeki Y, et al. Plasma concentration of micafungin in patients with hematologic malignancies. J Infect Chemother. 2007;13:39–45. doi: 10.1007/s10156-006-0496-1. [DOI] [PubMed] [Google Scholar]
- Nakai T, Uno J, Ikeda F, et al. In vitro antifungal activity of Micafungin (FK463) against dimorphic fungi: comparison of yeast-like and mycelial forms. Antimicrob Agents Chemother. 2003;47:1376–81. doi: 10.1128/AAC.47.4.1376-1381.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds FC, Motyl M, Andrade R, et al. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J Clin Microbiol. 2004;42:3475–82. doi: 10.1128/JCM.42.8.3475-3482.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osherov N, May GS, Albert ND, et al. Overexpression of Sbe2p, a Golgi protein, results in resistance to caspofungin in Saccharomyces cerevisiae. Antimicrob Agents Chemother. 2002;46:2462–9. doi: 10.1128/AAC.46.8.2462-2469.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrosky-Zeichner L, Kontoyiannis D, Raffalli J, et al. International, open-label, noncomparative, clinical trial of micafungin alone and in combination for treatment of newly diagnosed and refractory candidemia. Eur J Clin Microbiol Infect Dis. 2005;24:654–61. doi: 10.1007/s10096-005-0024-8. [DOI] [PubMed] [Google Scholar]
- Ostrosky-Zeichner L, Rex JH, Pappas PG, et al. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob Agents Chemother. 2003;47:3149–54. doi: 10.1128/AAC.47.10.3149-3154.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paderu P, Garcia-Effron G, Balashov S, et al. Serum differentially alters the antifungal properties of echinocandin drugs. Antimicrob Agents Chemother. 2007;51:2253–6. doi: 10.1128/AAC.01536-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas PG, Rotstein CM, Betts RF, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis. 2007;45:883–93. doi: 10.1086/520980. [DOI] [PubMed] [Google Scholar]
- Park S, Kelly R, Kahn JN, et al. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob Agents Chemother. 2005;49:3264–73. doi: 10.1128/AAC.49.8.3264-3273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier R, Alarie I, Lagace R, et al. Emergence of disseminated candidiasis caused by Candida krusei during treatment with caspofungin: case report and review of literature. Med Mycol. 2005;43:559–64. doi: 10.1080/13693780500220415. [DOI] [PubMed] [Google Scholar]
- Petraitis V, Petraitiene R, Groll AH, et al. Comparative antifungal activities and plasma pharmacokinetics of micafungin (FK463) against disseminated candidiasis and invasive pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob Agents Chemother. 2002;46:1857–69. doi: 10.1128/AAC.46.6.1857-1869.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M, Riley J, Koerner T. Effects of cilofungin (LY121019) on carbohydrate and sterol composition of Candida albicans. Eur J Clin Microbiol Infect Dis. 1989;8:1067–70. doi: 10.1007/BF01975172. [DOI] [PubMed] [Google Scholar]
- Pfaller MA, Boyken L, Hollis RJ, et al. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J Clin Microbiol. 2008a;46:150–6. doi: 10.1128/JCM.01901-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Boyken L, Hollis RJ, et al. In vitro susceptibilities of Candida spp. to caspofungin: four years of global surveillance. J Clin Microbiol. 2006;44:760–3. doi: 10.1128/JCM.44.3.760-763.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–63. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ, Ostrosky-Zeichner L, et al. Correlation of MIC with outcome for Candida species tested against caspofungin, anidulafungin, and micafungin: analysis and proposal for interpretive MIC breakpoints. J Clin Microbiol. 2008b;46:2620–9. doi: 10.1128/JCM.00566-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz-Telles F, Berezin E, Leverger G, et al. Micafungin versus liposomal amphotericin B for pediatric patients with invasive candidiasis: substudy of a randomized double-blind trial. Pediatr Infect Dis J. 2008;27:820–6. doi: 10.1097/INF.0b013e31817275e6. [DOI] [PubMed] [Google Scholar]
- Ramage G, Vandewalle K, Bachmann SP, et al. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob Agents Chemother. 2002;46:3634–6. doi: 10.1128/AAC.46.11.3634-3636.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboli AC, Rotstein C, Pappas PG, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007;356:2472–82. doi: 10.1056/NEJMoa066906. [DOI] [PubMed] [Google Scholar]
- Rex JH, Bennett JE, Sugar AM, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National Institute. N Engl J Med. 1994;331:1325–30. doi: 10.1056/NEJM199411173312001. [DOI] [PubMed] [Google Scholar]
- Sakaeda T, Iwaki K, Kakumoto M, et al. Effect of micafungin on cytochrome P450 3A4 and multidrug resistance protein 1 activities, and its comparison with azole antifungal drugs. J Pharm Pharmacol. 2005;57:759–64. doi: 10.1211/0022357056118. [DOI] [PubMed] [Google Scholar]
- Seibel NL, Schwartz C, Arrieta A, et al. Safety, tolerability, and pharmacokinetics of Micafungin (FK463) in febrile neutropenic pediatric patients. Antimicrob Agents Chemother. 2005;49:3317–24. doi: 10.1128/AAC.49.8.3317-3324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi B, Powles RL, Chopra R, et al. A study to determine the safety profile and maximum tolerated dose of micafungin (FK463) in patients undergoing haematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;38:47–51. doi: 10.1038/sj.bmt.1705398. [DOI] [PubMed] [Google Scholar]
- Stevens DA, Espiritu M, Parmar R. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob Agents Chemother. 2004;48:3407–11. doi: 10.1128/AAC.48.9.3407-3411.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens DA, Ichinomiya M, Koshi Y, et al. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin:evidence for beta-1,6-glucan synthesis inhibition by caspofungin. Antimicrob Agents Chemother. 2006;50:3160–1. doi: 10.1128/AAC.00563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawara S, Ikeda F, Maki K, et al. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob Agents Chemother. 2000;44:57–62. doi: 10.1128/aac.44.1.57-62.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PR, Tsoni SV, Willment JA, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–8. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GR, 3rd, Wiederhold NP, Vallor AC, et al. Development of caspofungin resistance following prolonged therapy for invasive candidiasis secondary to Candida glabrata infection. Antimicrob Agents Chemother. 2008;52:3783–5. doi: 10.1128/AAC.00473-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–47. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- Van Burik JA, Ratanatharathorn V, Stepan DE, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004;39:1407–16. doi: 10.1086/422312. [DOI] [PubMed] [Google Scholar]
- Villanueva A, Gotuzzo E, Arathoon EG, et al. A randomized double-blind study of caspofungin versus fluconazole for the treatment of esophageal candidiasis. Am J Med. 2002;113:294–9. doi: 10.1016/s0002-9343(02)01191-9. [DOI] [PubMed] [Google Scholar]
- Wald A, Leisenring W, Van Burik JA, et al. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997;175:1459–66. doi: 10.1086/516480. [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Adamson PC, Seibel NL, et al. Pharmacokinetics, safety, and tolerability of caspofungin in children and adolescents. Antimicrob Agents Chemother. 2005;49:4536–45. doi: 10.1128/AAC.49.11.4536-4545.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh TJ, Karlsson MO, Driscoll T, et al. Pharmacokinetics and safety of intravenous voriconazole in children after single-or multiple-dose administration. Antimicrob Agents Chemother. 2004;48:2166–72. doi: 10.1128/AAC.48.6.2166-2172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe E, Nakai T, Matsumoto S, et al. Killing activity of micafungin against Aspergillus fumigatus hyphae assessed by specific fluorescent staining for cell viability. Antimicrob Agents Chemother. 2003;47:1995–8. doi: 10.1128/AAC.47.6.1995-1998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederhold NP, Kontoyiannis DP, Prince RA, et al. Attenuation of the activity of caspofungin at high concentrations against candida albicans: possible role of cell wall integrity and calcineurin pathways. Antimicrob Agents Chemother. 2005;49:5146–8. doi: 10.1128/AAC.49.12.5146-5148.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederhold NP, Lewis RE. The echinocandin antifungals: an overview of the pharmacology, spectrum and clinical efficacy. Expert Opin Investig Drugs. 2003;12:1313–33. doi: 10.1517/13543784.12.8.1313. [DOI] [PubMed] [Google Scholar]
- Wiederhold NP, Najvar LK, Bocanegra R, et al. In vivo efficacy of anidulafungin and caspofungin against Candida glabrata and association with in vitro potency in the presence of sera. Antimicrob Agents Chemother. 2007;51:1616–20. doi: 10.1128/AAC.00105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston DJ, Maziarz RT, Chandrasekar PH, et al. Intravenous and oral itraconazole versus intravenous and oral fluconazole for long-term antifungal prophylaxis in allogeneic hematopoietic stem-cell transplant recipients. A multicenter, randomized trial. Ann Intern Med. 2003;138:705–13. doi: 10.7326/0003-4819-138-9-200305060-00006. [DOI] [PubMed] [Google Scholar]
- Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]