Abstract
Nuclear lamins A and C are encoded by LMNA and are present in terminally differentiated cells. Lamins participate in DNA replication, chromatin organization, arrangement of nuclear pores, nuclear growth, and anchorage of nuclear membranes. In several Canadian probands with partial lipodystrophy, since found to have a common ancestor, we identified a rare novel LMNA mutation, R482Q, that completely cosegregated with the partial lipodystrophy phenotype. We evaluated the relationship between quantitative metabolic phenotypes in both diabetic and nondiabetic carriers of LMNA R482Q and family controls, who were LMNA R482/R482 homozygotes. We found that when compared with LMNA R482/R482 homozygotes: (1) diabetic LMNA Q482/R482 heterozygotes had significantly higher glucose, glycosylated hemoglobin, triglycerides, insulin and C-peptide, and significantly lower HDL cholesterol; and (2) nondiabetic LMNA Q482/R482 heterozygotes had significantly higher triglycerides, insulin and C-peptide, and significantly lower HDL cholesterol. We also found that diabetic LMNA Q482/R482 heterozygotes were older and more likely to take antihypertensive medications. Thus, LMNA R482Q was associated with lipodystrophy, hyperinsulinemia, dyslipidemia, diabetes, and hypertension. The results indicate that perturbations in plasma lipids precede the plasma glucose abnormalities in LMNA Q482-associated hyperinsulinemia. Thus, rare mutations in a nuclear structural protein can be associated with markedly abnormal qualitative and quantitative metabolic phenotypes
Nuclear lamins are members of the intermediate filament multigene family, and participate in DNA replication, chromatin organization, spatial arrangement of nuclear pores, nuclear growth, and anchorage of nuclear membranes (Stuurman et al. 1998). Lamins A and C arise from differential splicing of the LMNA gene message (Stuurman et al. 1998). Both lamins A and C are absent from early embryos and undifferentiated cells, but are present in most terminally differentiated cells (Stuurman et al. 1998). Lamins A and C polymerize to form part of the nuclear lamina, a structural meshwork of 10 nm filaments on the nucleoplasmic side of the inner nuclear membrane (Stuurman et al. 1998). Recently, mutations in LMNA were found in families with autosomal-dominant Emery-Dreifuss muscular dystrophy (EDMD-AD), which is characterized by regional and progressive degeneration of skeletal and cardiac myocytes (Bonne et al. 1999) and also in families with an autosomal-dominant form of dilated cardiomyopathy (DCM-AD) (Fatkin et al. 1999). In addition, we have recently identified a rare, novel mutation in LMNA in individuals with Dunnigan-type familial partial lipodystrophy (FPLD; OMIM 151660) (Cao and Hegele 2000). Our rationale to focus on LMNA was based upon deductive reasoning: FPLD has been mapped to chromosome 1q21-q22 (Peters et al. 1998) and there was analogy between the highly specific anatomical site involvement in these diseases (Cao and Hegele 2000; Hegele 2000).
FPLD is a rare autosomal-dominant disease, which is a member of a heterogeneous family of disorders characterized by complete or partial absence of adipose tissue (Burn and Baraitser 1986; Kobberling and Dunnigan 1986). Patients with FPLD are born with normal fat distribution, but begin to lose subcutaneous fat from their extremities, trunk, and gluteal region after the onset of puberty (Burn and Baraitser 1986; Kobberling and Dunnigan 1986; Garg et al. 1999). Also, excess fat may become deposited within the face, neck, back, and labia majora (Burn and Baraitser 1986; Kobberling and Dunnigan 1986; Garg et al. 1999). Furthermore, patients with FPLD have normal stores of intermuscular, intra-abdominal, intrathoracic, and bone marrow fat (Burn and Baraitser 1986; Kobberling and Dunnigan 1986; Garg et al. 1999). Affected subjects often appear extremely muscular and the phenotype is somewhat easier to detect in women than in men. Additional phenotypic findings variably include acanthosis nigricans, hirsutism, menstrual abnormalities and polycystic ovarian disease (Burn and Baraitser 1986; Kobberling and Dunnigan 1986). A biochemical hallmark of affected subjects is severe insulin resistance with hyperinsulinemia. FPLD subjects often develop diabetes later in life, which can require extremely large doses of insulin in order to maintain glycemic control. FPLD subjects can also present with dyslipidemia and early coronary heart disease (Kobberling and Dunnigan 1986; Burn and Baraitser 1986).
We first identified the LMNA R482Q mutation in several Canadian FPLD index cases (Cao and Hegele 2000). The genetic arguments in favor of a causal relationship between LMNA R482Q and FPLD include its complete cosegregation with the FPLD phenotype, its total absence from 2000 alleles from normal subjects, and the conservation of the R482 residue throughout evolution (Cao and Hegele 2000). We now report that thorough evaluation of genealogical records has revealed that these probands are members of the same large, multigenerational extended family, and all share a common ancestral pair. We can now use the LMNA R482Q genotype to stratify affected family members using molecular rather than clinical criteria to better evaluate and understand the impact of LMNA R482Q on quantitative and qualitative metabolic traits.
RESULTS
Composition of the Study Sample
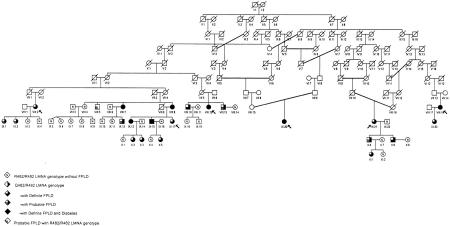
There were six probands in this study; each had been independently referred for clinical assessment on different occasions. Their relationship to one another was reconstructed using archival genealogical records (Fig. 1). The 47 adult subjects (aged 18 and above) from the FPLD kindred who had sufficient clinical data and adequate plasma and cellular material available for all analyses could be placed into six nuclear families (Fig. 1). Each subject studied was fewer than 10 generations removed from a common ancestral husband-wife pair (Fig. 1). Twenty-two subjects carried the mutant allele and 25 subjects, almost all of whom were within a second-degree relationship of a carrier, did not carry the mutant allele. Thus, there was an adequate number of genotypically normal adult subjects within the family to serve as controls for LMNA R482Q heterozygotes in pairwise nonparametric comparisons.
Figure 1.
Pedigree structure of FPLD kindred. Arrows indicate probands. The right half of a symbol in solid indicates heterozygosity for LMNA Q482. The lower left quarter of a symbol in solid indicates a definite diagnosis of FPLD. The lower left quarter of a symbol shaded indicates probable FPLD. The upper left quarter of a symbol in solid indicates a confirmed diagnosis of diabetes. N within a symbol indicates a subject who was definitely unaffected and was also homozygous for LMNA R482/R482.
Association of Genotype with Variation in Biochemical Variables
Table 1 shows the means (±S.D.) of the clinical and biochemical traits of seven adult family members with diabetes who were LMNA Q482/R482 heterozygotes, 15 adult family members who were LMNA Q482/R482 heterozygotes, and 25 family-based control subjects who were LMNA R482/R482 homozygotes. Table 2 summarizes the P-values from the nonparametric pairwise comparisons between groups in Table 1. There were numerous significant differences between the three categories of subjects. First, there was an extremely strong association of the LMNA R482Q variant with a definite diagnosis of FPLD in both diabetic and nondiabetic LMNA Q482/R482 heterozygotes. There were fewer nondiabetic heterozygotes with a definite diagnosis of FPLD, but the remaining five heterozygotes were all classified as possibly having FPLD. No heterozygote was classified as being definitely normal.
Table 1.
Plasma Lipoproteins and Related Biochemical Variables in Nondiabetic FPLD Family Members According to LMNA R482Q Genotype
| Q482/R482 | R482/R482 | ||
|---|---|---|---|
| with diabetes | without diabetes | ||
| Number/females | 7/6 | 15/10 | 25/15 |
| Definite FPLD (%) | 100 | 66.7 | 0.0 |
| Age (years) | 49.1 ± 10.0 | 33.8 ± 15.4 | 39.3 ± 15.4 |
| BMI (kg/m2) | 25.2 ± 3.5 | 26.4 ± 4.6 | 26.7 ± 5.4 |
| Cholesterol (mmoles/liter) | |||
| total | 5.90 ± 1.67 | 5.34 ± 0.75 | 5.24 ± 1.17 |
| LDL | 2.92 ± 0.31 | 3.54 ± 0.68 | 3.55 ± 1.13 |
| HDL | 0.75 ± 0.20 | 1.07 ± 0.20 | 1.32 ± 0.32 |
| Triglycerides (mmoles/liter) | 6.65 ± 5.55 | 2.19 ± 1.44 | 1.20 ± 0.60 |
| Insulin (U/liter) | 20.8 ± 9.3 | 19.2 ± 13.1 | 10.0 ± 13.1 |
| C-peptide (pmoles/liter) | 1219 ± 262 | 977 ± 439 | 610 ± 414 |
| Glucose (mmoles/liter) | 8.73 ± 3.00 | 4.94 ± 0.78 | 4.72 ± 0.47 |
| Hemoglobin A1C (%) | 7.74 ± 1.25 | 5.68 ± 0.35 | 5.70 ± 0.44 |
| Medication use | |||
| diabetes (%) | 57.1 | 0.0 | 0.0 |
| hypertension (%) | 42.9 | 6.7 | 8.0 |
| dyslipidemia (%) | 14.3 | 6.7 | 4.0 |
Table 2.
Summary of P-Values of Pairwise Comparisons
| Q482/R482a | Q482/R482 with diabetes vs.Q482/R482 without diabetesa | ||
|---|---|---|---|
| with diabetes vs. R482/R482 | without diabetes vs. R482/R482 | ||
| Definite FPLD (%) | 0.0000004 | 0.000000008 | N.S. |
| Age (years) | 0.047 | N.S. | 0.026 |
| BMI (kg/m2) | N.S. | N.S. | N.S. |
| Cholesterol (mmol/liter) | |||
| total | N.S. | N.S. | N.S. |
| LDL | N.S. | N.S. | N.S. |
| HDL | 0.0003 | 0.0038 | 0.0081 |
| Triglycerides (mmoles/liter) | 0.0007 | 0.011 | 0.022 |
| Insulin (U/liter) | 0.0056 | 0.0027 | N.S. |
| C-peptide (pmoles/liter) | 0.0014 | 0.0017 | N.S. |
| Glucose (mmoles/liter) | 0.003 | N.S. | 0.01 |
| Hemoglobin A1C (%) | 0.0003 | N.S. | 0.0017 |
| Medication use | |||
| hypertension (%) | 0.025 | N.S. | 0.04 |
| dyslipidemia (%) | N.S. | N.S. | N.S. |
Comparisons are from Table 1.
(N.S.) Not significant.
The only significant difference among the genotypic classes in baseline clinical attributes was a higher mean age in LMNA Q482/R482 heterozygotes with diabetes. Notably, there was no difference between the groups with respect to body mass index.
Compared with control LMNA R482/R482 homozygotes, LMNA Q482/R482 heterozygotes with diabetes had significantly higher mean concentrations of triglycerides, insulin, C-peptide, glucose, and hemoglobin A1C and had lower mean concentrations of high density lipoprotein (HDL) cholesterol (Tables 1 and 2). A significantly greater proportion of LMNA Q482/R482 heterozygotes with diabetes received treatment for hypertension. However, there was no difference in treatment for hypertension between control LMNA R482/R482 homozygotes and LMNA Q482/R482 heterozygotes with diabetes, suggesting that hypertension was related to aging and/or the presence of diabetes in LMNA Q482/R482 heterozygotes with diabetes. There was no difference in the mean concentration of total and LDL cholesterol in LMNA Q482/R482 heterozygotes with diabetes compared with control LMNA R482/R482 homozygotes.
Compared with control LMNA R482/R482 homozygotes, LMNA Q482/R482 heterozygotes without diabetes had significantly higher mean concentrations of triglycerides, insulin, and C-peptide and had lower mean concentrations of HDL cholesterol (Tables 1 and 2). There was no difference in the mean concentration of glucose and hemoglobin A1C, total, and LDL cholesterol in LMNA Q482/R482 heterozygotes without diabetes compared with control LMNA R482/R482 homozygotes.
Compared with nondiabetic LMNA Q482/R482 heterozygotes, diabetic LMNA Q482/R482 heterozygotes had significantly higher glucose and hemoglobin A1C (by definition), higher mean triglycerides, and lower mean HDL cholesterol (Tables 1 and 2). Subject VIII-5 had been diagnosed as possibly affected based on increased plasma insulin and C-peptide. He had none of the typical morphological features of FPLD. Thus, the high plasma insulin in subject VIII-5 was very likely to simply have been the common form related to abdominal obesity (Lamarche 1998). There was no effect on any analysis when this individual was excluded.
DISCUSSION
The availability of a genetic marker for FPLD has allowed us to stratify family members according to LMNA genotype. This has eliminated the need to deduce carrier status from clinical criteria, which can be a problem for a trait that is variably penetrant, such as FPLD, especially at younger ages. This improved diagnostic discrimination has aided in the evaluation of the association of the LMNA genotype with quantitative and qualitative metabolic traits in the extended Canadian FPLD family. For example, we have found that, when compared with LMNA R482/R482 homozygotes, diabetic LMNA Q482/R482 heterozygotes had significantly higher concentrations of glucose, hemoglobin A1C, triglycerides, insulin, and C-peptide and significantly lower concentrations of HDL cholesterol. We also found that, when compared with LMNA R482/R482 homozygotes, nondiabetic LMNA Q482/R482 heterozygotes had significantly higher concentrations of triglycerides, insulin, and C-peptide and significantly lower concentrations of HDL cholesterol. We also found that diabetic LMNA Q482/R482 heterozygotes were older, had more severe dyslipidemia, and were more likely to receive antihypertensive medication. Thus, LMNA R482Q was strongly associated with lipodystrophy, hyperinsulinemia, dyslipidemia, hypertension, and diabetes.
Because the onset of the FPLD phenotype occurs during puberty, it may be possible to use the LMNA genotype to diagnose at-risk individuals prepubertally. There is presently no information regarding the potential efficacy of any intervention to delay or avert the development of the metabolic phenotype and clinical complications in FPLD. However, there are some newer therapeutic modalities, such as the thiazoladinedione family of drugs, which have a theoretical benefit for the treatment of insulin-resistance syndromes. Continuous low doses of leptin have been shown to reverse insulin resistance in mice with congenital lipodystrophy (Shimomura et al. 1999). Being able to identify carriers presymptomatically using LMNA genotype at least raises the possibility of pharmacogenetic stratification of subjects for therapeutic intervention protocols.
The proatherogenic lipoprotein abnormalities associated with LMNA R482Q included elevated plasma triglycerides and reduced plasma HDL cholesterol, with no difference in plasma total or LDL cholesterol. These are the typical lipoprotein abnormalities seen in the insulin-resistance syndrome related to obesity in the general population (Lamarche 1998). It is now generally accepted that the visceral fat is the best correlate of most of the highly atherogenic metabolic complications seen in individuals with abdominal obesity in the general population (Lamarche 1998). These include the features seen in LMNA R482Q carriers, such as insulin resistance and hyperinsulinemia, hypertriglyceridaemia, and reduced plasma HDL cholesterol. Abdominal obesity may be the most prevalent common denominator of highly atherogenic dyslipidemia and hyperinsulinemia/insulin-resistant states in affluent, sedentary societies (Lamarche 1998). The abdominal obesity in LMNA R482Q carriers with FPLD is a genetic and clinically extreme form, with all adipocytes located centrally owing to complete absence of subcutaneous peripheral fat. The lipoprotein abnormalities in the FPLD kindred appeared to be specifically related to differences in body fat distribution, and not to differences in age or body mass index. The results suggest that hyperinsulinemia and insulin resistance underlie a proatherogenic lipoprotein profile, regardless of whether they are associated with abdominal obesity in the general population or with LMNA R482Q-associated peripheral adipose wasting in FPLD.
In nondiabetic carriers of LMNA R482Q, the lipoprotein abnormalities were more apparent than the abnormalities in glucose and hemoglobin A1C. This suggests that the lipoprotein perturbations occur earlier in the natural history of LMNA R482Q-associated insulin resistance. Conversely, it would appear that diabetes and hypertension, with markedly worse dyslipidemia occur later in the course of LMNA R482Q-associated insulin resistance. This suggests that there might be a greater compensatory capacity within the pathways that maintain glucose homeostasis. The results indicate that the lipoprotein abnormalities antedate the development of diabetes but then become worse after the decompensation of glucose homeostasis and development of diabetes.
The findings confirm our previous conclusion that rare mutations in a nuclear structural protein can be associated with markedly abnormal qualitative and quantitative metabolic phenotypes (Cao and Hegele 2000). Different mutations in LMNA can underlie the disparate clinical entities of EDMD-AD and FPLD, analogous to the relationship between different mutations in the RET proto-oncogene and the disparate clinical entities of multiple endocrine neoplasia type 2, related sporadic tumors, and Hirschsprung disease (Eng and Mulligan 1997). The position of the mutant residue within LMNA appears to be a crucial determinant of both the affected cell type and the anatomical distribution of the affected cells. This suggests a high degree of functional specificity for particular lamin A/C residues and raises the possibility that LMNA mutations could underlie other diseases characterized by degeneration of specific cell types in particular anatomical distributions.
Lamins A and C are members of the intermediate filament multigene family, which form part of the nuclear lamina, a structural meshwork of 10-nm filaments on the nucleoplasmic side of the inner nuclear membrane (Stuurman et al. 1998). The nonconservative Arg→Gln change at LMNA codon 482 occurs within the carboxy-terminal tail sequence that is common to both lamins A and C and is conserved in lamin A/C across species (Stuurman et al. 1998). However, it is not conserved among other members of the lamin multigene family. The importance of residue 482 in LMNA as being specific for FPLD was supported by the subsequent identification of two more mutations affecting this residue in European FPLD kindreds (Shackleton et al. 2000). In contrast, the mutated residues in EDMD-AD are each conserved not just in lamin A/C across species but also among other members of the lamin multigene family (Bonne et al. 1999). This might explain why LMNA R482 underlies a different phenotype than the EDMD-AD mutations.
The LMNA disease mutations in FPLD, EDMD-AD, and DCM-AD, by affecting charge or hydrophobicity, could simply destabilize lamin dimers and multimers, thereby disrupting the integrity of the nuclear lamina (Hegele 2000). The specificity of affected tissues might simply reflect the domain altered by the mutation: LMNA mutations in DCM-AD tend to be within the α-helical rod domain, which might affect polymerization, whereas the LMNA mutations in EDMD-AD and FPLD tend to be within the carboxy-terminal domain, which might affect dimer stability or interfere with assembly of filaments in a head-to-tail orientation. Conversely, any mechanism that so fundamentally undermines the nuclear envelope might be expected to have widespread consequences, which would be difficult to reconcile with the specific, progressive tissue involvement in the diseases due to mutant LMNA.
There are tissue-specific differences in the distribution of the other nuclear laminar proteins, such as lamin B1, which has been suggested to be another determinant of the specificity of the impact of particular LMNA mutations (Morris and Manilal 1999). It is possible that a function lost due to a LMNA mutation could be rescued as a result of the redundant interactions involving another lamin and lamin-associated proteins (Hegele 2000). A complicating attribute in FPLD is that puberty is clearly related to the onset of adipocyte degeneration. This suggests that changes in the hormonal or metabolic milieu trigger the expression of the specific histological and anatomical changes in carriers of the LMNA R482Q mutation. Such complexity might overwhelm the capacity of current in vitro functional assays of lamin interactions with other laminar proteins to fully explain the phenotypic changes observed in FPLD; other in vitro models of lamin A/C function might be required.
The availability of a genetic marker for FPLD will be helpful to evaluate such attributes as epitasis or gene-environment interactions that could affect penetrance of the FPLD phenotype in LMNA Q482 carriers. So far, only probands heterozygous for LMNA Q482/R482 have been ascertained because of the striking FPLD phenotype. By systematic extension of the family, it might be possible to identify LMNA Q482 carriers who are not obviously clinically affected and would have been missed through ascertainment based upon clinical features. The putative existence of carriers with a less severe clinical phenotype should create an opportunity to evaluate factors that can affect penetrance of quantitative and qualitative phenotypes, because carriers can now be unequivocally identified using LMNA genotyping.
METHODS
FPLD Kindred
The kindred is designated FPLD, and was ascertained through six separate probands whose relationship to each other was reconstructed after complete pedigree data were obtained. After informed consent was obtained, we performed clinical evaluations and drew blood samples from six probands and members of their families, shown in Figure 1. A total of 55 subjects over age 18 were studied: Medical histories and clinical examinations were performed in the field. Each family member was assessed for characteristic physical attributes of FPLD and provided a fasting serum sample for biochemical determinations, including serum insulin and C-peptide. The phenotype was classified as definitely affected, probably affected or definitely unaffected based upon clinical and biochemical criteria. The absence of subcutaneous fat tissue from upper and lower extremities and an extremely muscular appearance commencing in adolescence was the essential criterion for a definitive diagnosis of FPLD. Other important phenotypic criteria for a definite diagnosis included the presence of grossly excessive adipose tissue in the face and neck, giving a pseudo-Cushingoid appearance and/or acanthosis nigricans. Subjects in whom anthropometric features were equivocal, but who had at least two additional supportive criteria, including the presence of hirsutism, menstrual abnormalities, and/or laboratory data confirming the presence of diabetes-elevated insulin and/or elevated C-peptide were called probably affected.
A priori exclusion criteria included an inadequate blood sample available for all determinations and incomplete phenotypic information. After these exclusions, 47 subjects remained; 22 were carriers of the mutant LMNA Q482 allele, of whom seven had diabetes; and 25 were homozygous for the wild-type LMNA R482 allele. Of the 22 carriers, 17 had a definite diagnosis of FPLD, whereas none of the LMNA R482/R482 homozygotes was definitely affected (P<10−9).
Biochemical and Genetic Determinations
Plasma lipoproteins and serum glucose, hemoglobin A1C, insulin, and C-peptide were measured using established procedures (Hegele et al. 1998a,b). Amplification using the oligonucleotide primers 5′-GCAAGATACACCCAAGAGCC-3′ and 5′-ACACCTGGGTTCCCTGTTC-3′ produced a 1069-bp product. This amplification product was digested with MspI, and the digestion products were electrophoresed in 2% agarose gels. Digestion of the amplification product from the wild-type LMNA allele R482 produced two variant fragments with size 480 and 69bp, in addition to invariant fragments with sizes 381, 81 and 59bp. Digestion of the product from the mutant LMNA allele Q482 produced a single fragment with size 549bp, in addition to the invariant fragments.
Statistical Analyses
All statistical analyses were carried out with the Statistical Analysis Software (SAS) version 6.12 (SAS Institute, Cary, NC). We wished to test the hypothesis that within the extended kindred, the heterozygotes for LMNA Q482, both with and without diabetes, had a different biochemical phenotype than subjects who did not carry the mutation. Thus, biochemical traits for LMNA Q482/R482 heterozygotes were compared with control LMNA R482/R482 homozygote subjects from the pedigree. Because of the small numbers of subjects and the non-normal distribution of the biochemical variables, nonparametric analysis was carried out using the Kruskal-Wallis χ2 approximation test of significance of the Wilcoxon rank sums, as was reported previously (Hegele et al. 1998b). By convention, a P<0.05 was taken as the nominal level of significance for a difference in the pairwise comparisons.
Acknowledgments
We thank Drs. N. Forbath, T.J. McDonald, W. Dykeman, M.A. Carlos, and M.C. McSween for referring their patients. Pearl Campbell provided excellent technical assistance. Novel concepts and materials derived from this work have been embodied in U.S. patent application no. 60/154825 (September 20, 1999). This work was supported by grants from MRC Canada (MT13430) and the Canadian Genetic Diseases Network. R.A.H. is a Career Investigator of the Heart and Stroke Foundation of Ontario.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL robert.hegele@rri.on.ca; FAX (519) 663-3789.
REFERENCES
- Bonne G, DiBarletta MR, Varnous S, Becane HM, Hammouda EH, Merlini L, Muntoni F, Greenberg CR, Gary F, Urtizberea JA, et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet. 1999;21:285–288. doi: 10.1038/6799. [DOI] [PubMed] [Google Scholar]
- Burn J, Baraitser M. Partial lipoatrophy with insulin resistant diabetes and hyperlipidemia (Dunnigan syndrome) J Med Genet. 1986;23:128–130. doi: 10.1136/jmg.23.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Hegele RA. Nuclear Lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet. 2000;9:109–112. doi: 10.1093/hmg/9.1.109. [DOI] [PubMed] [Google Scholar]
- Eng C, Mulligan LM. Mutations of the RET proto-oncogene in the multiple endocrine neoplasia type 2 syndromes, related sporadic tumours, and Hirschsprung disease. Hum Mutat. 1997;9:97–109. doi: 10.1002/(SICI)1098-1004(1997)9:2<97::AID-HUMU1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M, Atherton J, Vidaillet HJ, Jr, Spudich S, De Girolami U, et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med. 1999;341:1715–1724. doi: 10.1056/NEJM199912023412302. [DOI] [PubMed] [Google Scholar]
- Garg A, Peshock RM, Fleckenstein JL. Adipose tissue distribution pattern in patients with familial partial lipodystrophy (Dunnigan variety) J Clin Endocrinol Metab. 1999;84:170–174. doi: 10.1210/jcem.84.1.5383. [DOI] [PubMed] [Google Scholar]
- Hegele RA. The envelope, please: Nuclear lamins and disease. Nat Med. 2000;6:136–137. doi: 10.1038/72221. [DOI] [PubMed] [Google Scholar]
- Hegele RA, Harris SB, Zinman B, Wang J, Cao H, Hanley AJG, Tsui L-C, Scherer SW. Variation in the AU(AT)-rich element within the 3′-untranslated region of PPP1R3 is associated with variation in plasma glucose in aboriginal Canadians. J Clin Endocrinol Metab. 1998a;83:3980–3983. doi: 10.1210/jcem.83.11.5219. [DOI] [PubMed] [Google Scholar]
- Hegele RA, Breckenridge WC, Cox DW, Maguire GF, Little JA, Connelly PW. Elevated low-density lipoprotein triglyceride concentrations in subjects heterozygous for hepatic lipase S267F variant. Arterioscler Thromb Vasc Biol. 1998b;18:1212–1216. doi: 10.1161/01.atv.18.8.1212. [DOI] [PubMed] [Google Scholar]
- Kobberling J, Dunnigan MF. Familial partial lipodystrophy. J Med Genet. 1986;23:120–127. doi: 10.1136/jmg.23.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche B. Abdominal obesity and its metabolic complications: Implicationsfor the risk of ischaemic heart disease. Coron Artery Dis. 1998;9:473–481. doi: 10.1097/00019501-199809080-00002. [DOI] [PubMed] [Google Scholar]
- Morris GE, Manilal S. Heart to heart: From nuclear proteins to Emery-Dreifuss muscular dystrophy. Hum Mol Genet. 1999;8:1847–1851. doi: 10.1093/hmg/8.10.1847. [DOI] [PubMed] [Google Scholar]
- Peters JM, Barnes R, Bennett L, Gitomer WM, Bowcock AM, Garg A. Localization of the gene for familial partial lipodystrophy (Dunnigan variety) to chromosome 1q21–22. Nat Genet. 1998;18:292–295. doi: 10.1038/ng0398-292. [DOI] [PubMed] [Google Scholar]
- Shackleton S, Lloyd DJ, Jackson SN, Evans R, Niermeijer MF, Singh BM, Schmidt H, Brabant G, Kumar S, Durrington PN, et al. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet. 2000;24:153–156. doi: 10.1038/72807. [DOI] [PubMed] [Google Scholar]
- Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- Stuurman N, Heins S, Aebi U. Nuclear lamins: Their structure, assembly and interactions. J Struct Biol. 1998;122:42–66. doi: 10.1006/jsbi.1998.3987. [DOI] [PubMed] [Google Scholar]