Abstract
Despite intensive efforts to eradicate the disease, tuberculosis continues to be a major threat to Indian society, with an estimated prevalence of 3.45 million cases in 2006. Emergence of multidrug-resistant tuberculosis has complicated eradication attempts in recent years. Incomplete and/inadequate treatment are the main causes for development of drug resistance. Directly observed therapy, short-course (DOTS) is the World Health Organization (WHO) strategy for worldwide eradication of tuberculosis, and our country achieved 100% coverage for DOTS through the Revised National Tuberculosis Control Program in 2006. For patients with multidrug-resistant tuberculosis, the WHO recommends a DOTS-Plus treatment strategy. Early detection and prompt treatment of multidrug-resistant tuberculosis is crucial to avoid spread of the disease and also because of the chances of development of potentially incurable extensively drug-resistant tuberculosis in these cases. This review discusses the epidemiologic, diagnostic, and therapeutic aspects of multidrug-resistant tuberculosis, and also outlines the role of primary care doctors in the management of this dangerous disease.
Keywords: multidrug-resistant, extensively drug-resistant, tuberculosis, general practice
Introduction
Tuberculosis is among the top three causes of death from a single infectious agent, along with malaria and human immunodeficiency virus (HIV). One-third of the world’s population is infected with Mycobacterium tuberculosis, the etiologic agent of tuberculosis.1 Of the 9.2 million tuberculosis cases reported worldwide in 2006, 0.7 million were HIV-positive and 1.7 million cases were fatal, of which 0.2 million occurred in HIV-positive people.2 India continues to occupy a unique position with respect to the global tuberculosis burden. In 2006, of the 9.2 million new cases of tuberculosis reported globally, 1.93 million were reported in India, which constitutes approximately one-fifth of the total global burden. This included 0.87 million smear-positive cases of the total estimated prevalence and incidence of tuberculosis of 3.45 million and 1.93 million, respectively, in India for 2006.2
Pulmonary tuberculosis is a highly contagious and life-threatening infection, and the mode of spread is droplet infection. The usual source of infection is a human case whose sputum is positive for tuberculous bacilli and who has received no treatment or has not been treated fully.1 The World Health Organization (WHO) classifies tuberculous infection into four categories for the purpose of treating cases effectively and in a uniform manner worldwide. In recent years, emergence of multidrug-resistant tuberculosis has become a major issue in the successful management and eradication of the disease.
Multidrug-resistant tuberculosis has now reached an approximate prevalence rate of 5% of the total global tuberculosis burden,3 amounting to half a million cases, of which most cases are from Indian, China, and Russia.4,5 Although the measures adopted by various countries have resulted in a 51% case detection rate for tuberculosis and a 70% successful treatment rate worldwide, there is still a long way to go to achieve a 70% case detection rate and 85% successful treatment rate for nonresistant tuberculosis, as suggested by the WHO.6 Also, the WHO has suggested 70% case detection and treatment rates for multidrug-resistant tuberculosis to curb the development of extensively drug-resistant tuberculosis.4 If an adequate drug regimen is followed, tuberculosis is considered to be 100% curable, as proven to some extent by reduction in the case fatality rate from 29% to 4%, under the Revised National Tuberculosis Control Program (RNTCP) in India which adopted directly observed therapy, short-course (DOTS) in 1993,7 but tuberculosis still has a high mortality rate, as evidenced by nearly two million deaths every year worldwide.8 To reduce this burden further, India is planning to implement DOTS-Plus by 2010.7 The incidence of extensively drug-resistant tuberculosis is about 6%–7.3% in patients treated for multidrug-resistant tuberculosis worldwide.9,10 Table 1 shows the WHO categories of tuberculosis treatment.
Table 1.
WHO treatment categories for tuberculosis1
| Category of treatment | Patient |
|---|---|
| Category I | New sputum smear-positive |
| Seriously ill, sputum smear-negative | |
| Seriously ill, extrapulmonary | |
| Category II | Sputum smear-positive, relapse |
| Sputum smear-positive, failure | |
| Sputum smear-positive treatment after default | |
| Category III | Sputum smear-negative, not seriously ill |
| Extrapulmonary, not seriously ill | |
| Category IV | Multidrug-resistant tuberculosis |
Definitions and epidemiology
Multidrug resistance is defined as resistance of M. tuberculosis to two or more first-line antituberculous drugs that include isoniazid and rifampicin.11 Extensively drug-resistant tuberculosis refers to cases of multidrug-resistant tuberculosis which show resistance to second-line antituberculous drugs including any one of the fluoroquinolones and one of the three injectable drugs (amikacin, kanamycin, or capreomycin).12 The most recent estimates suggest that globally there were about 489,000 cases of multidrug-resistant tuberculosis in 2006, of which 110,132 cases were in India.2 This is about 4.9% of the total number of tuberculosis cases in the country. About 0.9%–1.5% of multidrug-resistant cases in India may fall into the category of extensively drug-resistant tuberculosis.12
Multidrug resistance can be primary or acquired. Primary resistance is defined as resistance in patients without a history or other evidence of previous antituberculous drug treatment. Acquired drug resistance occurs in those who have previously received antituberculous treatment for at least one month and in those with treatment failures and relapses. The prevalence of primary multidrug resistance is 3% and that of acquired multidrug resistance is 12% in India. Although drug resistance started appearing in patients with M. tuberculosis infection soon after the introduction of effective antituberculous drugs, multidrug-resistant tuberculosis was not a major public health problem until the early 1990s13 when HIV infection became a global epidemic.
Causes of multidrug-resistant tuberculosis
A history of tuberculosis and previous antituberculous treatment are the most important factors identified in the causation of multidrug-resistant tuberculosis.14 Factors which predispose a patient to development of multidrug-resistant tuberculosis include:
Incomplete treatment
Inadequate treatment
Errors in tuberculosis management, such as use of a single antituberculous drug
Addition of a single drug to a failing regimen
Failure to identify pre-existing resistance
Initiation of an inadequate primary regimen
Failure to identify and address nonadherence to treatment
Noncompliance
Inappropriate isoniazid preventive therapy
Variations in bioavailability of antituberculous drugs.15
There are various other factors favoring the spread of tuberculosis, which include increasing high-risk groups (eg, the elderly, prisoners, immigrants, drug addicts6), recurrent defaulters, multidrug-resistant and extensively drug-resistant treatment failures,3 increasing age of the general population, increasing number of patients with immunosuppression due to bone marrow and solid organ transplantation,16 and lack of optimal recommended duration of treatment for multidrug-resistant and extensively drug-resistant tuberculosis in controlled clinical trials.4 In addition, certain host genetic factors and coexistent HIV infection may also predispose to the development of multidrug-resistant tuberculosis.11,17
Diagnosis of drug resistance
Drug-resistant tuberculosis needs to be suspected based on history of prior treatment (smear-positive cases after repeated treatment courses and category II treatment failures), and such cases should undergo drug susceptibility testing. Lowenstein-Jensen culture is the method traditionally used for drug sensitivity testing in tuberculosis.17 A duration of 6–8 weeks is required before sensitivity results are known with this method. Several new methods are now available for testing drug sensitivity patterns, but they are costlier and are not widely available. The methods for diagnosis of multidrug-resistant tuberculosis include:
Conventional (Lowenstein-Jensen) culture, ie, proportional, resistance ratio, absolute concentration
BACTEC-460
Mycobacterial growth indicator tube
E test
Mycobacteriophage method
Molecular methods, ie, polymerase chain reaction, ligase chain reaction, restriction fragment length polymorphism.11
Identification and proper management
Drug marketing surveys done in India for 2006 have shown that 75% of the total cost for antituberculous drugs was spent outside of the RNTCP, mainly in the private sector.12 The vast majority of multidrug-resistant tuberculosis cases might have been managed in the private sector in recent years. Improper supervision of treatment in the private sector might lead to treatment defaulting and hence incomplete management of cases. This may result in development of extensively drug-resistant tuberculosis, which is a major public health challenge.12
Multidrug-resistant tuberculosis cases are difficult to manage in view of the need for prolonged treatment (up to 24 months), high rates of treatment failures, higher costs and potential toxicities of medications, need for surgery in locally confined untreatable cases, and risk of transmission to unexposed individuals, including health care workers. Cases of extensively drug-resistant tuberculosis may be incurable, making the situation worse. A comparison of multidrug-resistant and extensively drug-resistant tuberculosis is made in Table 2.
Table 2.
Comparison of multidrug-resistant and extensively drug-resistant tuberculosis
| Multidrug-resistant tuberculosis | Extensively drug-resistant tuberculosis | |
|---|---|---|
| Clinical suspicion | First time treated, culture-positive at three months, or microscopy-positive at five months. | Treated multidrug-resistant patients, with no improvement |
| Category II failure cases. | ||
| Default and relapse cases | ||
| Resistance pattern | Isoniazid, rifampicin ± other first-line drugs | Multidrug-resistant tuberculosis + any fluoroquinolone + at least one of the three injectable drugs (amikacin, kanamycin, capreomycin) |
Treatment
Tuberculosis is a disease with high cure rates if managed properly, and an effective national control program can reduce the risk of transmission to the unexposed public. The Indian tuberculosis control program is now one of the largest public health programs in the world.18 The RNTCP was established under WHO guidance in 1993. The strategy of the RNTCP for control of tuberculosis is DOTS, which is familiar to most clinicians now. With extensive efforts on the part of government and health departments, India achieved a DOTS coverage of 100% in 2006.2 The reported cure rate with DOTS treatment was 86% in 2005.2
Management of multidrug-resistant tuberculosis is a challenge for clinicians. Adherence to appropriate and prolonged drug therapy is the cornerstone of treatment success in multidrug-resistant tuberculosis. Hence, the standard DOTS regimen may not be helpful for treating these cases.
In low- and middle-income countries that have adopted the DOTS strategy, the WHO and its international partners have emphasized DOTS-Plus for multidrug resistant tuberculosis programs since 1998, in order to promote effective treatment of multidrug-resistant tuberculosis.19,20
A comparison between DOTS and DOTS-Plus is shown in Table 3. The major differences between DOTS and DOTS-Plus are that DOTS employs sputum microscopy for diagnosis of tuberculosis cases and multidrug-resistant tuberculosis cases, and uses first-line antituberculosis drugs over a short period of treatment, while DOTS-Plus uses sputum culture and drug sensitivity testing for diagnosis of multidrug-resistant tuberculosis cases and uses both first- and second-line antituberculous drugs over a longer period of time, ie, up to 24 months.11
Table 3.
Comparison of DOTS and DOTS-Plus11
| DOTS | DOTS-Plus | |
|---|---|---|
| Political and administrative commitment | Yes | Yes |
| Diagnosis by | Sputum microscopy | Sputum microscopy |
| Sputum culture and drug susceptibility testing | ||
| Drugs used | First-line antituberculous | First-line and second-line antituberculous |
| Duration of therapy | Short course | 18–24 months |
| Duration of intensive phase of treatment | 2–3 months | Six months |
| Individualized treatment | No | Yes |
| Directly observed | Yes | Yes |
| Purpose of therapy | Cure tuberculosis and prevent multidrug resistance | Treat multidrug resistance |
| Monitoring of treatment | Monitoring and evaluation of treatment outcome | Monitoring, reporting, and evaluation of treatment outcome |
Only experienced clinicians at centers equipped with reliable laboratory services for mycobacterial culture and in vitro sensitivity testing should treat multidrug-resistant tuberculosis cases.17,19 Sputum samples from all suspected cases of multidrug-resistant tuberculosis, should be subjected to culture sensitivity and antituberculous drug sensitivity testing.11,15,17 Pending results, these patients may then be started on WHO category II treatment (under program conditions) or the regimens employing various drugs (DOTS-Plus).11 Further therapy is guided by the culture and sensitivity report.11 A single drug should never be added to a failing regimen and, when initiating treatment, at least three previously unused drugs with in vitro susceptibility must be used.15,21
We need novel drugs and vaccines to be able to face the challenges in the treatment of multidrug-resistant and extensively drug-resistant tuberculosis. Some of the new drugs and vaccines having the potential to cure multidrug-resistant and extensively drug-resistant tuberculosis are linezolid,16,22,23 thioridazine (a neuroleptic phenothiazine),24–26 myrtle leaf extracts (used as an antiseptic in Sardinian traditional medicine),27 medicinal plants having antituberculous activity (Acalypha indica, Adhatoda vasica, Allium cepa, Allium sativum, Aloe vera28), and HSP65+IL-12/HVJ vaccine.29 HSP65+IL-12/HVJ is a combination of DNA vaccines expressing mycobacterial heat-shock protein 65 and interleukin-12, delivered by the hemagglutinating virus of Japan envelope and liposome).29 The HSP65+IL-12/HVJ vaccine has been shown to have protective and therapeutic efficacy in murine models and in Cynomolgus monkeys (which are considered to be the best animal model of human tuberculosis). It has also been observed that this vaccine exerted therapeutic efficacy (100% survival in Cynomolgus monkeys infected with tuberculosis) and showed a synergistic effect after an initial priming Bacillus Calmette-Guérin dose. The implications of this study are that this vaccine might be useful against M. tuberculosis, including multidrug-resistant and extensively drug-resistant tuberculosis in humans participating in therapeutic clinical trials.29 Other agents shown to be effective in animal models include a dry powder PA-824 cyclodextrin-lecithin aerosol suspension.30
Two newer classes of drugs targeting mycobacterial cell wall synthesis have been discovered recently, ie, benzothiazinones and dinitrobenzamide, which are highly active against M. tuberculosis, including extensively drug-resistant tuberculosis strains.31
Future inventions include drugs with activity on different bacterial cell wall components, virulence genes, small guanosine triphosphatases etc,32 and inhibitors of the aspartate kinases involved in bacterial cell wall synthesis.33 Mutations at hotspots are responsible for the development of extensively drug-resistant tuberculosis strains, as observed in a study involving 194 M. tuberculosis strains isolated from patients.34 Early detection of these extensively drug-resistant cases could be done using a rapid test called MTBDRsI, which detects resistance to ethambutol, fluoroquinolones, second-line aminoglycosides (amikacin and kanamycin), and cyclic peptide (capreomycin), and this could be confirmed by drug susceptibility testing.35
The principles of treatment (and poor prognostic indicators) for multidrug-resistant tuberculosis include:
Use of as many first-line agents to which the organism is sensitive
At least one parenteral drug should be part of the regimen
At least three agents to which the isolate is sensitive should be used
Administration of oral agents should be directly observed
Bactericidal agents should be used whenever possible
Single drugs should never be added to a failing regimen.36
Poor prognostic indicators are:
Category II failures given intermittent therapy
Cavity at presentation
HIV positivity
Other factors that predict progression to extensively drug-resistant tuberculosis in multidrug-resistant patients are bilateral cavitating lesions, prior exposure to a second-line injectable antibiotic, and each additional month in which a patient has failed to take at least 80% of their prescribed drugs.9 In a recent study, the success rate for treatment was 87% for multidrug-resistant but not extensively drug-resistant tuberculosis, and 41% for extensively drug-resistant cases. In this study, resistance to ciprofloxacin, ofloxacin, and amikacin is associated with an unsuccessful outcome.37 However, DOTS-Plus, which has been found to be an effective strategy for treatment of multidrug-resistant tuberculosis showed the best cure rates at 70%.2 Monitoring with serial cultures should be done at months 4, 6, 12, 18, and 24 of treatment.12 Treatment for 18–24 months is mandatory in multidrug-resistant tuberculosis, and the drugs used are costly and have higher toxicity rates. Important drugs used for treatment of multidrug-resistant tuberculosis and their main adverse effects are shown in Table 4.
Table 4.
Drugs used in the treatment of multidrug-resistant tuberculosis11
| Group | Drugs | Important adverse effects |
|---|---|---|
| Aminoglycosides | Streptomycin | Nephrotoxicity |
| Kanamycin | Ototoxicity | |
| Amikacin | Capreomycin is hepatotoxic too | |
| Capreomycin | ||
| Thioamides | Ethionamide | Psychosis |
| Prothionamide | Hepatotoxicity | |
| Fluoroquinolones | Ofloxacin | Gastrointestinal disturbances |
| Levofloxacin | Central nervous system symptoms | |
| Moxafloxacin | ||
| Others | Pyrazinamide | Hepatotoxicity, hyperuricemia |
| Cycloserine | Convulsions, suicidal tendency | |
| Para-amino salicylic acid | Hepatotoxicity, hypersensitivity | |
| Ethambutol | Optic neuritis, peripheral neuritis |
Prevention strategies
It has been noted worldwide that only 20% of patients with tuberculosis receive treatment according to current WHO recommendations. The WHO emphasizes and warns that if immediate action is not taken to persuade more public health authorities and doctors to use the recommended treatment, the window of opportunity to prevent the spread of drug-resistant strains will be missed.
On the basis of small-scale studies that have used the DOTS strategy to prevent the emergence of resistance in tuberculosis patients, the WHO has been emphasizing and arguing for DOTS for years.38 Measures to prevent the spread of multidrug-resistant tuberculosis include:
Cough hygiene
Well-ventilated wards
Segregation of suspected cases
Negative pressure exhausts
Air exchangers and high efficiency particulate arresting filters
Personal protection with N95 masks
Apart from these, other measures important in the control of the spread of multidrug-resistant and extensively drug-resistant tuberculosis include major improvements in laboratory facilities and infection control, improving the performance of tuberculosis control programs, better treatment for both drug-susceptible and drug-resistant disease, massive scale-up in diagnosis and treatment of all types of tuberculosis, newer diagnostic tests, newer vaccines, early pulmonary resection combined with chemotherapy in multidrug-resistant and extensively drug-resistant tuberculosis cases, and commitment on a national scale by countries with the highest tuberculosis burden (ie, India, China, and Russia) by improving national policies and health systems that remove financial barriers to treatment, encourage rational drug usage, and create the relevant infrastructure.39
Multidrug-resistant tuberculosis in general practice
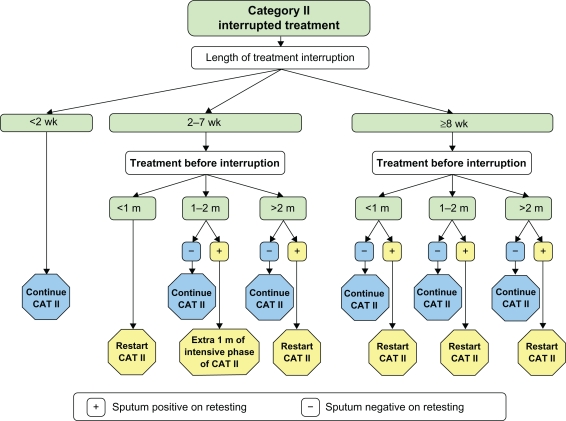
General practitioners and primary care physicians have key roles in the successful management of tuberculosis and thus preventing emergence of multidrug resistance in patients. Implementation of DOTS in India should be mainly done in the primary care setting because most of the population reside in rural areas. Timely identification and prompt referral of suspected cases to specialty care facilities for DOTS-Plus are the main roles of primary care doctors in the management of multidrug-resistant tuberculosis. Default from drug treatment is one of the major causes of multidrug resistance, and hence vigilance should be exercised in handling such cases. Figure 1 shows the approach to cases with interrupted category II treatment to prevent emergence of multidrug-resistant tuberculosis.
Figure 1.
Approach to cases with interrupted category II treatment to avoid multidrug-resistant tuberculosis.1
Factors that are important in making treatment decisions for defaulters include duration of treatment interruption (less than two weeks, 2–7 weeks, or more than eight weeks), duration of treatment before interruption (less than one month, 1–2 months, or more than two months), and the sputum report (positive or negative). In all sputum-negative cases, irrespective of the length of interruption and duration of treatment before interruption, it is advisable to continue the remaining months of WHO category II treatment. Patients who are sputum-positive during treatment of 1–2 months and who had 2–7 weeks of interrupted treatment, should take an extra month of intensive WHO category II treatment. All remaining sputum-positive cases, while on treatment, irrespective of length of interruption and/or duration of treatment before interruption, should receive a fresh course of WHO category II treatment.1
Conclusion
Emergence of multidrug-resistant and extensively drug-resistant tuberculosis is a major threat to successful control of tuberculosis worldwide. Prompt implementation of DOTS reduces the chances for development of multidrug-resistant tuberculosis. Early identification and timely referral of suspected multidrug-resistant tuberculosis cases by primary care doctors for DOTS-Plus treatment will help in the appropriate management of multidrug-resistant tuberculosis and thereby prevent development of life-threatening extensively drug-resistant disease.
Also, because tuberculosis is compounded by HIV coinfection, which hastens the emergence of multidrug-resistant and extensively drug-resistant tuberculosis, novel antituberculous drugs are urgently required. In this context, the creation of new drugs that target metabolic pathways essential for survival of the bacteria, without damaging the host, will prove to be vital. It has recently been found that the 2C-methyl-d-erythritol 4-phosphate (MEP) pathway of M. tuberculosis is one of several pathways that are essential for viability of the organism, and that the human host lacks this particular enzyme, and hence treatment targeted to this enzyme would be less toxic to humans.40 Thus, the MEP pathway represents a bacterium-specific drug target and, indeed, fosmidomycin is now known to inhibit the second step in the MEP pathway and may lead us to find other novel antituberculous drugs.40
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
Continuing medical education questions
- Which of the following statements is/are true regarding WHO treatment categories for tuberculosis?
- New, sputum smear-positive sample is WHO category I
- Seriously ill, sputum smear-negative is WHO category I
- Seriously ill, extrapulmonary is WHO category I
- Sputum smear-positive relapse is WHO category I.
- Which of the following statements is/are true regarding methods used to diagnose multidrug-resistant tuberculosis?
- Mycobacteriophage method is conventional culture method
- The mycobacterial growth indicator tube is a conventional culture method
- Polymerase chain reaction is a molecular method
- BACTEC-460 is a molecular method.
- Which of the following statements is/are true regarding the definition of multidrug-resistant tuberculosis?
- It is resistant to isoniazid, rifampicin ± other first-line drugs
- It is resistant to isoniazid, rifampicin ± other first line drugs + an injectable form
- It is resistant to isoniazid, rifampicin ± other first-line drugs + a fluoroquinolone
- It is resistant to a fluoroquinolone and rifampicin.
- Which of the following statements best describes the purpose of DOTS-Plus?
- To cure tuberculosis and prevent multidrug resistance
- To monitor, report, and evaluate treatment outcome
- To make the diagnosis by sputum microscopy
- Duration of intensive phase of treatment is 2–3 months.
- Which of these statements predicts poor prognosis in multidrug-resistant tuberculosis WHO category II failures who received intermittent therapy?
- Cavity at presentation
- HIV positivity
- Sputum culture-positive at three months on optimal therapy.
Answers
True: 1.a, 1.b, 1.c, 2.c, 3.a, 4.b, 5.a, 5.b, 5.c.
False: 1.d, 2.a, 2.b, 2.d,
References
- 1.Park K. Park’s Textbook of Preventive and Social Medicine. 17th ed. Jabalpur, India: Banarsidas Bhanot Publishers; 2007. Tuberculosis; pp. 175–178. [Google Scholar]
- 2.World Health Organization Global tuberculosis control 2008 – surveillance, planning, financing. Available from: http://www.who.int/tb/publications/global_report/2008/pdf/fullreport.pdf. Accessed 2008 Jul 24.
- 3.Dheda K, Warren RM, Zumla A, Grobusch MP. Extensively drug-resistant tuberculosis: Epidemiology and management challenges. Infect Dis Clin North Am. 2010;2:705–725. doi: 10.1016/j.idc.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Chiang CY, Centis R, Migliori GB. Drug-resistant tuberculosis: Past, present, future. Respirology. 2010;15:413–432. doi: 10.1111/j.1440-1843.2010.01738.x. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi NR, Nunn P, Dheda K, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: A threat to global control of tuberculosis. Lancet. 2010;375:1830–1843. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho AC, Migliori GB, Cirillo DM. Tuberculosis in Europe: A problem of drug resistance or much more? Expert Rev Respir Med. 2010;4:189–200. doi: 10.1586/ers.10.7. [DOI] [PubMed] [Google Scholar]
- 7.Prasad R. Revised National Tuberculosis Control Programme: Current scenario. J Indian Med Assoc. 2009;107:725–727. [PubMed] [Google Scholar]
- 8.Monedero I, Caminero JA. MDR-/XDR-TB management: What it was, current standards and what is ahead. Expert Rev Respir Med. 2009;3:133–145. doi: 10.1586/ers.09.6. [DOI] [PubMed] [Google Scholar]
- 9.Shin SS, Keshavjee S, Gelmanova IY, et al. Development of extensively drug-resistant tuberculosis during multidrug-resistant tuberculosis treatment. Am J Respir Crit Care Med. 2010;182:426–432. doi: 10.1164/rccm.200911-1768OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ködmön C, Hollo V, Huitric E, Amato-Gauci A, Manissero D. Multidrug- and extensively drug-resistant tuberculosis: A persistent problem in the European Union and European economic area. Euro Surveill. 2010;15:19519. [PubMed] [Google Scholar]
- 11.Sharma SK, Mohan A. Multidrug-resistant tuberculosis: A menace that threatens to destabilize tuberculosis control. Chest. 2006;130:261–272. doi: 10.1378/chest.130.1.261. [DOI] [PubMed] [Google Scholar]
- 12.Arora VK, Singla R, Dhingra VK, Wares Prasad R, Selvakumar S. Panel discussion on MDR and XDR TB. Indian J Tuberc. 2008;55:104–109. [PubMed] [Google Scholar]
- 13.O’Brien RJ, Spigelman M. New drugs for tuberculosis: Current status and future prospects. Clin Chest Med. 2005;26:327–340. doi: 10.1016/j.ccm.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Espinal MA, Laserson K, Camacho M, et al. Determinants of drug-resistant tuberculosis: Analysis of 11 countries. Int J Tuberc Lung Dis. 2001;5:887–893. [PubMed] [Google Scholar]
- 15.Sharma SK, Mohan A. Scientific basis of directly observed treatment, short-course (DOTS) J Indian Med Assoc. 2003;101:157–158. [PubMed] [Google Scholar]
- 16.Manfredi R, Nanetti A, Dal Monte P, Calza L. Increasing pathomorphism of pulmonary tuberculosis: An observational study of slow clinical, microbiological and imaging response of lung tuberculosis to specific treatment. Which role for linezolid? Braz J Infect Dis. 2009;13:297–303. doi: 10.1590/s1413-86702009000400012. [DOI] [PubMed] [Google Scholar]
- 17.Sharma SK, Mohan A. Multidrug-resistant tuberculosis. Indian J Med Res. 2004;120:354–376. [PubMed] [Google Scholar]
- 18.Khatri GR, Frieden TR. Controlling tuberculosis in India. N Engl J Med. 2002;347:1420–1425. doi: 10.1056/NEJMsa020098. [DOI] [PubMed] [Google Scholar]
- 19.Sharma SK, Liu JJ. Progress of DOTS in global tuberculosis control. Lancet. 2006;367:951–952. doi: 10.1016/S0140-6736(06)68391-8. [DOI] [PubMed] [Google Scholar]
- 20.Bastian I, Rigouts L, van Deun A, et al. Directly observed treatment, short-course strategy and multidrug-resistant tuberculosis: Are any modifications required? Bull World Health Organ. 2000;78:238–251. [PMC free article] [PubMed] [Google Scholar]
- 21.Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society, Centers for Disease Control and Prevention and the Infectious Diseases Society: American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America; treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 22.Alffenaar JW, van Altena R, Harmelink IM, et al. Comparison of the pharmacokinetics of two dosage regimens of linezolid in multidrug-resistant and extensively drug-resistant tuberculosis patients. Clin Pharmacokinet. 2010;4:559–565. doi: 10.2165/11532080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Anger HA, Dworkin F, Sharma S, Munsiff SS, Nilsen DM, Ahuja SD. Linezolid use for treatment of multidrug-resistant and extensively drug-resistant tuberculosis, New York City, 2000–2006. J Antimicrob Chemother. 2010;65:775–783. doi: 10.1093/jac/dkq017. [DOI] [PubMed] [Google Scholar]
- 24.Amaral L, Martins A, Molnar J, et al. Phenothiazines, bacterial efflux pumps and targeting the macrophage for enhanced killing of intracellular XDRTB. In Vivo. 2010;24:409–424. [PubMed] [Google Scholar]
- 25.Amaral L, Boeree MJ, Gillespie SH, Udwadia ZF, van Soolingen D. Thioridazine cures extensively drug-resistant tuberculosis (XDR-TB) and the need for global trials is now! Int J Antimicrob Agents. 2010;35:524–526. doi: 10.1016/j.ijantimicag.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Amaral L, Molnar J. Therapy of XDR TB with thioridazine a drug beyond patent protection but eligible for patent “as new use”. Recent Pat Antiinfect Drug Discov. 2010;5:109–114. doi: 10.2174/157489110791233540. [DOI] [PubMed] [Google Scholar]
- 27.Zanetti S, Cannas S, Molicotti P, et al. Evaluation of the antimicrobial properties of the essential oil of Lyrtus communis L. against clinical strains of Mycobacterium spp. Interdiscip Perspect Infect Dis. 2010 Jul 29; doi: 10.1155/2010/931530. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta R, Thakur B, Singh P, et al. Anti-tuberculosis activity of selected medicinal plants against multi-drug resistant Mycobacterium tuberculosis isolates. Indian J Med Res. 2010;131:809–813. [PubMed] [Google Scholar]
- 29.Okada M, Kita Y. Anti-tuberculosis immunity by cytotoxic T cells* granulysin and the development of novel vaccines (HSP-65 DNA+IL-12 DNA) Kekkaku. 2010;85:531–538. Japanese. [PubMed] [Google Scholar]
- 30.Garcia-Contreras L, Sung JC, Muttil P, et al. Dry powder PA-824 aerosols for treatment of tuberculosis in guinea pigs. Antimicrob Agents Chemother. 2010;54:1436–1442. doi: 10.1128/AAC.01471-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manina G, Pasca MR, Buroni S, de Rossi E, Riccardi G. Decaprenylph osphoryl-beta-D-ribose 2′-epimerase from Mycobacterium tuberculosis is a magic drug target. Curr Med Chem. 2010 Jul 14; doi: 10.2174/092986710791959693. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Rajni R, Meena LS. Guanosine triphosphatases as novel therapeutic targets in tuberculosis. Int J Infect Dis. 2010;14:e682–e687. doi: 10.1016/j.ijid.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Chaitanya M, Babajan B, Anuradha CM, et al. Exploring the molecular basis for selective binding of Mycobacterium tuberculosis Asp kinase toward its natural substrates and feedback inhibitors: A docking and molecular dynamics study. J Mol Model. 2010;16:1357–1367. doi: 10.1007/s00894-010-0653-4. [DOI] [PubMed] [Google Scholar]
- 34.Khanna A, Raj VS, Tarai B, et al. Emergence of extensive drug resistant (XDR) Mycobacterium tuberculosis and molecular characterization of these clinical isolates from Delhi Region, India. Antimicrob Agents Chemother. 2010 Aug 16; doi: 10.1128/AAC.00661-10. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brossier F, Veziris N, Aubry A, Jarlier V, Sougakoff W. Detection by genotype MTBDRsl test of complex mechanisms of resistance to second-line drugs and ethambutol in multidrug-resistant Mycobacterium tuberculosis complex isolates. J Clin Microbiol. 2010;48:1683–1689. doi: 10.1128/JCM.01947-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eltringham IJ, Drobniewski F. Multiple drug resistant tuberculosis: Aetiology, diagnosis and outcome. Br Med Bull. 1998;54:569–578. doi: 10.1093/oxfordjournals.bmb.a011711. [DOI] [PubMed] [Google Scholar]
- 37.Tabarsi P, Chitsaz E, Baghaei P, et al. Impact of extensively drug-resistant tuberculosis on treatment outcome of multidrug-resistant tuberculosis patients with standardized regimen: Report from Iran. Microb Drug Resist. 2010;16:81–86. doi: 10.1089/mdr.2009.0073. [DOI] [PubMed] [Google Scholar]
- 38.Brown P. Drug resistant tuberculosis can be controlled, says WHO. BMJ. 2000;320:821. [PMC free article] [PubMed] [Google Scholar]
- 39.Kang MW, Kim HK, Choi YS, et al. Surgical treatment for multidrug-resistant and extensive drug-resistant tuberculosis. Ann Thorac Surg. 2010;89:1597–1602. doi: 10.1016/j.athoracsur.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 40.Eoh H, Brennan PJ, Crick DC. The Mycobacterium tuberculosis MEP (2C- methyl-d-erythritol 4-phosphate) pathway as a new drug target. Tuberculosis (Edinb) 2009;89:1–11. doi: 10.1016/j.tube.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]