Abstract
There is a growing understanding of the pathophysiology of secondary hyperparathyroidism (SHPT) and a recent emergence of new agents for SHPT treatment in patients with advanced kidney disease. At the same time, appreciation that mineral metabolic derangements promote vascular calcification and contribute to excess mortality, along with recognition of potentially important “non-classical” actions of vitamin D, have prompted the nephrology community to reexamine the use of various SHPT treatments, such as activated vitamin D sterols, phosphate binders, and calcimimetics. In this review, the evidence for treatment of SHPT with calcimimetics and vitamin D analogs is evaluated, with particular consideration given to recent clinical trials that have reported encouraging findings with cinacalcet use. Additionally, several controversies in the pathogenesis and treatment of SHPT are explored. The proposition that calcitriol deficiency is a true pathological state is challenged, the relative importance of the vitamin D receptor and the calcium sensing receptor in parathyroid gland function is summarized, and the potential relevance of non-classical actions of vitamin D for patients with advanced renal disease is examined. Taken collectively, the balance of evidence now supports a treatment paradigm in which calcimimetics are the most appropriate primary treatment for SHPT in the majority of end stage renal disease patients, but which nevertheless acknowledges an important role for modest doses of activated vitamin D sterols.
Keywords: secondary hyperparathyroidism, vitamin D, vitamin D receptor, calcium sensing receptor, calcimimetics, kidney disease
Introduction
Several cardinal findings over the past decade have thrust derangements of mineral metabolism to the forefront of nephrology. Studies demonstrating the association of phosphorous with mortality in end stage renal disease (ESRD) patients,1,2 the prevalence of vascular calcification even in young ESRD patients,3 and the association of calcium-based binders with increased vascular calcification relative to non-calcium based binders4–7 have prompted a re-examination of traditional therapies for secondary hyperparathyroidism (SHPT) of advanced renal disease.
Accompanying increased awareness of the importance of mineral metabolic dysregulation have been numerous clinical and scientific advancements. The development and introduction of calcimimetics for the treatment of SHPT stands as a major clinical milestone. Calcimimetics, which are allosteric modulators of the calcium sensing receptor (CASR), have rapidly achieved widespread use in ESRD patients with SHPT. In parallel, there have also been major advancements in understanding the pathogenesis of SHPT, with a greater appreciation of the biological functions of the CASR, the vitamin D receptor (VDR), the regulation of 1,25(OH)2 D production and metabolism by the 1α-hydroxylase/24-hydroxylase (Cyp17b1/Cyp24) enzyme system, and the role of fibroblast growth factor-23 (FGF23), the principle phosphaturic factor. Additionally, there is growing interest in potential “non-classical” actions of vitamin D, ie, those actions beyond its traditional role in the maintenance of mineral homeostasis. Unfortunately, the clinician’s success in reaching targets for control of calcium (Ca), phosphate (P), calcium × phosphorous product (Ca × P), and parathyroid hormone (PTH) remains suboptimal.
In this review, we explore in detail the body of the evidence for treatment of SHPT with both calcimimetics and vitamin D analogs, also known as activated vitamin D compounds, vitamin D sterols, or calcitriol analogs, and discuss the clinical study evidence as well as the experimental basis for these treatments. We also consider several emerging controversies with direct relevance to SHPT treatment, specifically (1) whether reduced 1,25(OH)2 D in chronic kidney disease (CKD) is pathological condition requiring treatment or an adaptive response to decreased renal function, (2) whether the CASR or the VDR is relatively more important in parathyroid gland (PTG) function, and (3) whether “non-classical” actions of vitamin D have relevance for patients with advanced kidney disease. We conclude with a conceptual framework for the treatment of SHPT in end stage renal disease (ESRD).
Review criteria
A PubMed search of English-language articles from 1988 to the present was undertaken. The search strategy utilizing a first term of “calcimimetics”, “cinacalcet”, “calcium sensing receptor”, “vitamin D”, “vitamin D receptor”, “FGF23”, or “fibroblast growth factor”, coupled by the operator AND with a second term of “secondary hyperparathyroidism”, “end stage renal disease”, “chronic kidney disease”, “chronic kidney failure”, or “chronic renal failure.” Original reports as well as reviews were examined. For all reviews, references to original reports were sought. Bibliographies of each report were examined for additional relevant articles not captured by the initial search strategy. This search strategy was augmented by hand-searches for publications of interest in the authors’ personal libraries.
Discussion
What evidence exists that cinacalcet improves mineral metabolic control in SHPT?
A relatively large number of clinical studies have been undertaken to investigate the effects of cinacalcet on the control of SHPT in ESRD patients. There have been eight published randomized controlled trials (RCTs) plus the ACHIEVE trial, which is currently in submission. Initial trials were designed to test the effects of cinacalcet when added to standard-of-care therapy (consisting of either no treatment other than oral phosphorous binders or, much more commonly, vitamin D analogs), while later trials utilized designs in which cinacalcet was tested in combination with low-dose vitamin D analogs against higher-dose vitamin D “monotherapy.” More recently, five “single-treatment-arm” studies, in which cohorts of subjects were compared to themselves pre- and post-treatment with cinacalcet, have been published. While not true RCTs, these latter studies provide invaluable real-world clinical insights.
Randomized controlled trials of cinacalcet
In 2002, the first evidence that cinacalcet lowered PTH levels emerged.8 When cinacalcet, then known as AMG 073, or placebo was given to 30 hemodialysis (HD) patients for 8 days, treated subjects demonstrated a statistically significant improvement in PTH levels, measured as percent change from baseline. Calcium, P and Ca × P also fell, although p-values were not reported. Soon thereafter, Quarles et al9 and Lindberg et al10 reported on true placebo-controlled RCTs of cinacalcet. Both studies consisted of 12-week titration phases and 6-week assessment phases. Quarles et al studied 71 HD patients, approximately two-thirds of whom were taking vitamin D analogs, and found that cinacalcet treatment resulted in robustly significant decreases in PTH, Ca, and Ca × P, when measured as percent change from baseline. Phosphorus did not change. Vitamin D analog use did not affect the results, because when subjects were divided into those who had an increase, decrease, or no change in vitamin D analog dose over the course of the study, reductions in percent change in PTH remained significant (p = 0.008). The study by Lindberg et al involving 78 HD patients found that cinacalcet-treated subjects achieved statistically-significant improvements in percent change of PTH, Ca, P, and Ca × P (p < 0.001 for all). Again, about two-thirds of subjects were using vitamin D analogs at the start of the study; these investigators used logistic regression to determine that its use was not associated with reductions in PTH.
Larger studies, with increased power to examine multiple biochemical outcomes, were subsequently conducted. Lindberg et al studied 395 HD and peritoneal dialysis (PD) patients, and found that cinacalcet was associated with statistically-significant decreases in both mean and percent change of PTH, Ca, and Ca × P, as well as the percent of subjects achieving PTH ≤ 300 pg/mL and Ca × P < 55 mg2/dL2.11 The largest study of cinacalcet versus placebo was that of Block et al,12 which consisted of two identical parallel trials (in North America and Europe) enrolling a total of 741 HD patients. The percent of subjects reaching PTH ≤ 250 pg/mL (the primary endpoint), the percent change in PTH from baseline, and the difference in final mean PTH value were all statistically significant, as were decreases in Ca and P. In stratified analyses of sex, age, race (African versus European descent), dialysis duration, strata of initial PTH (using cutoffs of 300, 500, and 800 pg/mL), and use of vitamin D analogs, cinacalet was superior in the attainment of the primary endpoint.
All of the above studies were designed as “real world” studies in which providers had the liberty to adjust vitamin D analog doses as they saw fit, albeit according to a study-specific standardized protocols. This was done to allow the subjects to continue to receive standard-of-care therapy, which at that time included vitamin D analogs for SHPT. In each of the studies, vitamin D analog doses remained relatively constant within each arm as well as comparable across arms at the baseline and final timepoints. While this type of design allows for the isolation of the effects of cinacalcet, it does not permit a determination of whether one agent is more effective than another for control of SHPT, or yield insights as to whether one therapy (cinacalet) could replace another (vitamin D analogs) as primary therapy.
A recent metaanalysis by Strippoli et al formally examined the above studies.13 These investigators concluded that, for absolute levels of PTH, Ca, P, and Ca × P, cinacalcet resulted in significantly different outcomes relative to placebo. However, several other RCTs were either not included in the above analysis, or were published subsequent to it. Martin et al randomized 410 HD patients to cinacalcet or placebo and found that cinacalcet-treated subjects had statistically-significant decreases in several biochemical endpoints, including percent change in PTH, mean concentrations of PTH, Ca, P, and Ca × P, and the proportion of subjects reaching PTH < 250 pg/mL.14 The percentage of subjects receiving vitamin D analogs was no different between groups at baseline and at study completion. Fukugawa et al used lower doses of cinacalcet (100 mg/day being the highest allowable dose, versus 180 mg for most other trials) to randomize Japanese patients to cinacalet versus placebo and found significant decreases in Ca, P and Ca × P, as well as a significantly greater number of subjects reaching 30% reductions in PTH and PTH ≤ 250 pg/mL.15 Vitamin D analog use at baseline was reported to have no effect on attainment of goals, but a full description of activated vitamin D usage in the two groups was not reported.
Only two studies, to our knowledge, have attempted to minimize vitamin D analog dose in an attempt to determine whether cinacalcet might be used in lieu of, rather than in addition to, vitamin D analogs. Messa et al recently published the results of the OPTIMA study.16 These investigators deliberately attempted to titrate down vitamin D analog dose, while preserving the provider’s ability to dose as they saw fit. A total of 552 subjects were enrolled in an open-label study. These authors found cinacalcet to be superior in achieving goals for PTH (≤300 pg/mL), Ca (<9.5 mg/dL), P (<5.5 pg/mL), Ca × P (≤55 mg2/dL2), and Ca × P plus PTH endpoints. While there was only a modest relative dose change in vitamin D between the groups (a 6% decrease in the cinacalcet group versus a 14% increase in the vitamin D-alone group), in the subgroup of subjects receiving vitamin D analogs at baseline (68% of the total study sample), there was a 22% decrease in relative vitamin D analog dose in the cinacalcet arm as opposed to a 3% increase in the control arm; whether this was statistically significant was not reported. Finally, the ACHIEVE study, currently under peer review, randomized approximately 178 HD patients to cinacacet and low-dose vitamin D analogs or vitamin D alone. This study showed that cinacalcet-treated subjects resulted in significantly higher numbers of subjects reaching targets for PTH, P, and Ca × P KDOQI targets, although there was more hypocalcemia in the cinacalcet arm.
The majority of evidence now demonstrates that cinacalcet is an extremely effective agent for the suppression of PTH, and that it has a clear role in therapy for SHPT. Such conclusions emerge readily upon collectively reading individual reports, while confirmation of this is provided in the findings of the recent metaanalysis. Even more significantly, the OPTIMA and ACHIEVE studies provide convincing evidence that cinacalcet can have a vitamin D analog-minimizing effect while achieving important clinical targets, suggesting that cinacalcet is a viable candidate for primary treatment for SHPT.
Other clinical studies of cinacalcet
As cinacalcet is increasingly used in clinical practice, other investigators are reporting on their long-term experiences with this agent, particularly in situations where cinacalcet-based regimens have supplanted vitamin D analog-based ones in clinical practices. Moe et al reported on 59 subjects who completed 100 weeks of an open-label trial of cinacalcet.17 They demonstrated long-term control of PTH, in which the level of PTH at 52 weeks was little changed at 100 weeks, with PTH at both timepoints being significantly better than at baseline. Sterrett et al reported on 1 year of cinacalcet use in 210 HD patients, demonstrating a significantly greater decrease in percent change of PTH, percent change of Ca × P, attainment of PTH ≤ 250 pg/mL, and ≥30% reduction in PTH.18
Two recent studies involved paradigms in which deliberate attempts were made to reduce vitamin D analog dose. Chertow et al examined 72 subjects with elevated Ca × P, all of whom were taking vitamin D analogs, in an open-label prospective study.19 Subjects had their vitamin D analog dose reduced to “physiologic” levels of calcitriol (eg, paricalcitol 2 μg thrice weekly) while cinacalcet was initiated and subsequently titrated upwards as needed. Percent change in PTH, Ca, P, and Ca × P, and percent of subjects reaching Ca × P goal were all significantly greater in the cinacalcet arm (p < 0.0001 for all), while mean vitamin D dose was halved (and discontinued outright in 21% of subjects), demonstrating the ability of cinacalcet to “spare” vitamin D analogs. An open-label study by Block et al involved 375 subjects in which doses were reduced to low levels at study initiation.20 Mean vitamin D dose was also halved in this study, and percent change in PTH, Ca, P, and Ca × P were significantly different between arms (p < 0.0001), again demonstrating that cinacalcet can be effective therapy for SHPT even in the face of lowering vitamin D dose.
Cinacalcet has also been tested in patients with extremely high levels of PTH. Arenas et al treated 28 HD patients with refractory SHPT (mean PTH 829 pg/mL), in whom treatment with vitamin D analogs was compromised by hypercalcemia or hyperphosphatemia, with cinacalcet.21 They observed robust decreases in PTH, Ca, and Ca × P product, while P did not change. No decrement in vitamin D dose was necessarily sought, since the patients were by definition difficult to control with vitamin D analogs alone. After 9 months, all subjects achieved PTH ≤ 500 pg/mL, demonstrating cinacalcet’s usefulness is refractory SHPT.
Finally, in analyses of small single-center cohorts, other investigators have studied the effects of changes in treatment protocols. Lazar et al22 and Speigel et al23 initiated new cincacalcet-based paradigms, and compared attainment of KDOQI Clinical Practice Guideline targets before and after conversion of their treatment paradigms. While the studies were modest in size (35 and 61 subjects, respectively), and statistical power therefore limited, the former study demonstrated a significant increase in subjects attaining at least three mineral metabolic targets, and the latter a significant improvement in subjects attaining PTH goals. In the former study, the investigators reported that there was a 33% increase in subjects administered paricalcitol, presumably due to either hypocalcemia or because lowered cinacalcet-induced Ca × P permitted reintroduction of vitamin D analogs. In the latter study, vitamin D doses did not change in the cinacalcet-treated subjects.
Other evidence for benefits of cinacalcet
As reviewed definitively by Brown and MacLeod,24 the CASR is found in a variety of tissues. Many of these are unlikely to play a major role in mineral metabolism, such as the brain, pancreas, bone marrow, pituitary, skin (keratinocytes) and breast (ductal cells). However, the CASR is found in other locations which may have direct impact on mineral metabolism, and, as such, morbidity in dialysis patients. Three such tissues are the parathyroid gland (PTG), the bone, and the vasculature.
In the PTG, the CASR regulates PTH secretion acutely and PTH gene transcription chronically.24,25 It also regulates PTG cell growth.26 In both humans with neonatal severe hyperparathyroidism27 and in the analogous homozygous casr animal knockouts,28 there are increases in PTH, serum Ca, and hyperplasia of the PTG, while in uremia-induced SHPT, the CASR is downregulated in humans and animals.29–32 As reviewed by Drueke at al,33 the effects of calcimimetcs have been extensively studied in rodents, where cinacalcet has consistently been shown to prevent to development of, or mitigate the effects of, PTG hyperplasia.34,35 Cinacalcet has even been shown to reverse hyperplasia.35 Calcimimetics have also been shown to upregulate the CASR in rodent models of renal failure.36
The effects of cinacalcet on calcium-mediated PTH release have been examined by de Francisco et al, who studied 10 subjects with extreme SHPT (mean PTH 1116 pg/mL).37 Subjects were placed on alternating low-Ca and high-Ca dialysate baths to maximally stimulate and suppress the PTG, exposed to a mean of 13 weeks of cinacalcet, then tested again in the same manner to determine if there was any effect of cinacalcet on the PTH release setpoint (the Ca level associated with 50% maximal PTH stimulation). Cinacalcet reduced the setpoint and the maximal PTH release, an important finding because this setpoint may be a marker for the severity of SHPT and of PTG mass. More direct, but still rather preliminary, evidence of a potential association between cinacalcet and PTG histology comes from Lomonte et al.38 These investigators examined PTG glands and found that nodular hyperplasia was more common in vitamin D analog-treated and in cinacalcet plus vitamin D-treated individuals than in individuals treated with binders alone. Because no subjects were exposed to cincacalcet monotherapy, they relied on linear regression to attempt to isolate the independent effects of cinacalcet, and found that the drug was associated with a significant increase in oxyphil to chief cell ratio. Oxyphil cells have been reported to proliferate at a lower rate than chief cells,39 perhaps indicating that cinacalcet could slow the histologic progression of SPTH. However, the study was small, and the results should be considered hypothesis-generating.
Only one report, to our knowledge, has investigated the potential effects of calcimimetics in bone histology of humans.40 Cinacalet use was associated with improved bone histomorphometry. While vitamin D analog use was not controlled for, the investigators attempted to minimize the dose. Cinacalcet-treated subjects had reduced markers of bone turnover and bone turnover rates by histology. However, they also experienced improvement in PTH, so improvements in bone histology may have been the result of better mineral metabolic control, rather than of any specific benefit attributable to cinacalcet per se.
Of great importance in ESRD patients are the potential effects of calcimimetics on the cardiovascular system. In vitro models demonstrate that functional CASR is present in rat cardiac myocytes.41,42 When such cells were exposed to calcium, intracellular inosotol phosphate concentrations increased, while exposure to cinacalcet shifted the curve to the left, implicating the CASR in this pathway.42 Cinacalcet also reduced DNA synthesis in proliferating neonatal cardiac myocytes, indicating that the CASR may play a role in cardiac hypertrophy.42 The CASR also appears to be present in blood vessels. While not all studies have demonstrated this,43 several investigators have reported the CASR in adventitia44 and endothelium.45,46 In human aorta, the CASR agonist spermine increased intracellular Ca and NO production.47 Taken together with data showing that dietary Ca causes vascular relaxation, it is plausible to suggest that the CASR plays a role in vascular relaxation.48 In vivo models are also promising in this regard. In the uremic rodent model, calcimimetics were associated with decreased cardiac remodeling,49 raising the possibility that cinacalcet could provide protection against cardiac remodeling and vascular calcification in humans.
Whether calcimimetics might demonstrate survival beneficial effects in ESRD patients though these actions on the PTG, bone, or the vasculature, requires much future investigation. However, some tantalizing evidence for this possibility has recently emerged. In study of 4 similarly-designed trials comprising 1184 subjects, Cunningham et al found significant associations between use of cinacalcet and decreased risk of parathyroidectomy and cardiovascular hospitalization, although not with mortality risk.50 While the study was a retrospective and relied on pooled data, it provides intriguing preliminary evidence that cinacalcet might be a useful in reducing the need parathyroidectomy, and, more profoundly, might also have cardioprotective effects.
What evidence exists that vitamin D analogs improve mineral metabolic control in SHPT?
The clinical trial evidence for the ability of vitamin D analogs to improve mineral metabolic parameters is somewhat less than that for cinacalcet, which is probably a reflection that the former’s demonstrable ability to lower PTH allowed it to become standard-of-care without evidence from multiple RCTs. There have been a few RCTs which have tested the efficacy of vitamin D analogs versus placebo for treatment of SHPT. For example, Martin et al51 and Delmez et al52 tested paricalcitol and calcitriol, respectively, against placebo, and found significant improvements in PTH in response to exposure to the activated vitamin D compound. Several trials have been designed to test one (ie, a non-selective) agent against another (ie, a selective agent) over timeframes ranging from a few days53,54 to 32 weeks,55 although longer-term studies of cohorts exposed to single agents in non-placebo-controlled studies have been conducted over a year or more.56–58 Control of mineral metabolic parameters, and not mortality, was their principle endpoint.
However, there are been several large observational studies that have shown a consistent positive association between treatment of ESRD patients with vitamin D analogs and survival. Using the Fresenius Medical Care database, Teng et al examined over 67,000 HD patients to compare the effects of paricalcitol, a selective vitamin D analog, with calcitriol, a first-generation non-selective agent.59 They observed an adjusted hazard ratio (HR) of 0.84 (95% confidence intervals [CI] 0.79–0.90) for paricalcitol relative to calcitriol. In a subsequent study, these investigators investigated whether use of any vitamin D analog provided survival benefits relative to no use. Over 50,000 Fresenius Medical Care dialysis patients were examined for 2-year survival.60 They observed a 20% survival improvement for vitamin D analogs (HR 0.80, 95% CI 0.76–0.83). The investigators used sophisticated statistical techniques, including treatment of the exposure as time-dependent (to appropriately credit exposure risks and benefits to actual exposure), use of Cox regression models augmented by stratum-specific hazard ratios, sensitivity analyses to test for consistency of results under various assumptions, and, in a secondary analysis, the relatively novel technique of inverse probability of treatment weighting (IPTW), in which subjects are assigned a probability of receiving an exposure (vitamin D analogs) in a time-dependent fashion. Through these numerous analyses, the survival benefit attributable to vitamin D analogs was generally in the 20% to 27% range; that multiple statistical approachs yielded a similar magnitude of benefit strengthens their conclusions.
Kalentar-Zadeh et al examined over 58,000 incident and prevalent HD patients, and also found a significant survival advantage associated with paricalcitol use.61 These investigators also used time-dependent Cox models, and used models adjusted for both case mix (a surrogate for baseline comorbidites, to which the authors did not have access) and a malnutrition-inflammation index created by the authors. This study, however, identified dose-dependent effects of vitamin D analogs, such that individuals receiving high (≥15 μg of paricalcitol per week) had worse survival, consistent with possible toxic effects of high dose vitamin D analogs. An alternative possibility, which cannot be discounted, is that patients with refractory SHPT, in whom large doses of vitamin D analogs were prescribed as a result, had a much higher mortality than those with less florid SHPT. The other large study examining this issue was that of Tentori et al who studied over 7700 prevalent HD patients from the Dialysis Clinics Incorporated database.62 While median followup was relatively short (37 weeks), these investigators also found a 20% improvement in mortality associated with use of any vitamin D analog.
Despite this evidence, caution should be used in extrapolating these results too broadly. No technique of statistical adjustment is likely to remove all residual confounding, and the results could also be explained by confounding by intent (non-random treatment allocation bias). In many cases, decisions to treat or not to treat with vitamin D analogs in ESRD involves a physician’s bedside anecdotal knowledge of the patient’s medical history and comorbidities, and is unlikely to be fully captured by knowledge of either comorbidites (as recorded in databases) or through case-mix adjustment. Given the disappointing history of the discordance between observational studies and RCTs in the dialysis population, a RCT is needed to demonstrate the survival advantage of vitamin D analogs.
Controversies in therapy for SHPT: Is there a biologically plausible rationale to select one class of agents over another?
In the absence of prospective trials of dialysis patients demonstrating whether either calcimimetics or vitamin D analogs improve mortality, a variety of other considerations should inform prescribing practices. Here we examine several controversies in SHPT treatment, including whether reduced 1,25(OH)2 D in advanced kidney disease is pathological or an adaptive condition requiring treatment, whether either agent could be expected to have direct effects on PTG function through their respective receptors, and whether “non-classical” actions of vitamin D have relevance for patients with advanced kidney disease.
Is calcitriol deficiency in kidney disease a pathological state or an adaptive response?
The focus of the traditional understanding of SHPT has centered on diminished renal synthesis of 1,25(OH)2 D. According to this view, chronic kidney disease (CKD) represents a functional vitamin D-deficient state,63,64 resulting from the inability of the decreased renal mass to convert 25(OH) D (calcidiol) to 1,25(OH)2 D (calcitriol). This traditional framework has been shaped by an understanding of “true” vitamin D-deficient states, and holds that impaired gastrointestinal Ca absorption results from a reduction in 1,25(OH)2 D levels, and also results in stimulated PTH secretion. PTH has several targets: (1) bone, to increase Ca efflux; and (2) kidney, to stimulate 1,25(OH)2 D production, increase tubular Ca absorption, and inhibit P reabsorption. Elevated PTH levels maintain circulating Ca and P levels until compensatory mechanisms fail, during which time PTG hypertrophy and hyperplasia ensue. The role of diminished calcidiol availability is controversial: it may or may not be due to nutritional factors,65 dysregulated liver metabolism,66 or decreased substrate delivery to the kidney resulting from low glomerular filtration rate.63 Diminished calcidiol likely contributes to decreased 1,25(OH)2 D levels in CKD. This view, which centers around the cardinal importance of vitamin D, would suggest that primary treatment of SHPT should consist of active vitamin D analogs to suppress PTH, possibly in conjunction with supplementation by nutritional vitamin D calcidiol.
The traditional view that CKD is a state of true vitamin D deficiency may be challenged by several observations. First, phosphate retention in CKD appears to be the initial abnormality inciting the development of SHPT. This is evidenced by the decreased capacity of the kidney to excrete phosphate, as well as the observation that reduction in dietary phosphate alone, in proportion to declining GFR, can prevent the development of SHPT in models of CKD.67 Second, nutritional vitamin D deficiency is more commonly associated with hypophosphatemia,68 rather than hyperphosphatemia resulting from impaired renal phosphate clearance. Third, the principal function of the vitamin D/PTH axis is not to prevent hyperphosphatemia, but rather to protect the organism from hypocalcemia by stimulating bone Ca efflux, 1,25(OH)2 D production, and renal Ca reabsorption. The role of PTH on P excretion is a secondary, mainly designed to excrete the P accompanying gastrointestinal Ca absorption and Ca efflux from the bone. Together, these strongly suggest that elevated PTH secretion in both vitamin D deficiency and advanced kidney disease have more relevance to the maintenance of circulating Ca levels than with P homeostasis.
Fourth, there is some evidence of refractoriness to treatment of calcidiol deficiency in CKD. While this issue is an active area of investigation in which more study evidence is likely to come to light, provision of nutritional vitamin D supplementation in the form of ergocalciferol to many CKD individuals with calcidiol deficiency leads to disappointing results. Some CKD patients appear refractory to nutritional vitamin D supplementation, as evidenced by the difficulty achieving “normal” levels of calcidiol using ergocalciferol in these patients. For example, Zisman et al administered ergocalciferol to deficient stage 3 and 4 CKD subjects according to the KDOQI suggested protocol.69 After a long mean treatment period of over 7 months, subjects increased their calcidiol levels only to the 30 to 35 ng/mL range, with about one-third of subjects failing to reach levels >30 ng/mL. In another study, insufficient and deficient stage 3 and 4 CKD subjects were treated with an intense ergocalciferol regimen (50,000 IU weekly for 12 weeks, then 50,000 IU monthly for 3 months).70 Calcidiol levels increased from an average of 16.6 ng/mL to only 27.2 ng/mL, and 45% demonstrated trivial increases of calcidiol (<5 ng/mL). It is certainly possible that ergocalciferol is not the optimal form of nutritional vitamin D supplementation. However, while one study demonstrated greater success by using cholecalciferol, resulting in a greater improvement in calcidiol levels, this was not accompanied by a statistically-significant suppression of PTH.71
The discovery of FGF23, and its function as a 1,25(OH)2 D counter-regulatory hormone,72,73 provides a new conceptual framework for understanding the pathogenesis of SHPT. Made in bone, FGF23 is involved in adaptive responses which have evolved to protect the organism from hyperphosphatemia and vitamin D intoxication. Both PTH and FGF23 have phosphaturic actions, but they have opposite effects on 1α-hydroxylase/24-hydroxylase enzyme systems: PTH stimulates 1,25(OH)2 D production and inhibits its degradation, whereas FGF23 inhibits production74–76 and increases degradation. FGF23 acts as a “counter-regulatory” hormone, to decrease 1α-hydroxylase and increase 24-hydroxylase (diminishing calcitriol levels). In this way, the FGF23-bone-kidney axis may be an effector of a phosphate “trade-off” that compensates for limited renal P excretion. Reduced renal P excretion may be the initial stimulus for a cascade of events, in which FGF23-dependent suppression of renal 1,25(OH)2 D production is an adaptive response, limiting gastrointestinal P absorption, rather than a functionally-deficient state requiring treatment. Thus, small increases in FGF23 are a very early event in CKD to maintain neutral P balance.77–79 FGF23 also has direct effects on the PTG to suppress PTH secretion,74 which leads to the removal of PTH-mediated stimulatory effects on 1α-hydroxylase, and to further endogenous suppression of 1,25(OH)2 D production. As such, PTH elevation is almost certainly a later event following FGF23-mediated reductions in 1,25(OH)2 D.
Therapeutic approaches to SHPT would therefore differ depending on which conceptualization of SHPT pathogenesis is correct. If kidney disease represents functional calcitriol deficiency, then administration of vitamin D analogs to suppress PTH is rational. However, if P retention leads to increases in FGF23, suppression of 1α-hydroxylase, and stimulation of 24-hydroxylase leading to a fall of calcitrol levels, then the primary treatment of SHPT would be P restriction rather than vitamin D analog administration, since the latter would serve to increase Ca and P absorption, hyperphosphatemia, and further stimulation of FGF23. If this is true, it is fair to ask why treatment with vitamin D analogs in stage 4 and 5 CKD suppresses PTH without raising serum P.80 The answer may be that vitamin D itself stimulates production of FGF23 levels, in turn increasing phosphaturia in the setting of decreasing renal function. In ESRD patients, in contrast, treatment with active vitamin D analogs worsens hyperphosphatemia, probably reflecting the unopposed effect of increase gastrointestinal phosphate absorption,62 while cinacalcet results in a slight decrease in serum phosphate levels.12,16 The mechanism of this latter effect is uncertain, but may be due to a decrement in PTH-mediated P efflux from bone.
What is the relative importance of CASR and VDR in regulating PTG function?
The CASR regulates PTH gene transcription and secretion24,25 and cell proliferation of the PTG.26 In humans with neonatal severe hyperparathyroidism27 and, analogously, in homozygous casr knockout mice,28 there are increases in PTH and serum Ca, as well as hyperplasia of the PTG, in spite elevations in circulating 1,25(OH)2 D.33,81–83 In contrast, while ablation of the VDR also results in severe hyperparathyroidism, normalization of serum Ca (leading to activation of the CASR) is sufficient to normalize PTH secretion and suppress PTG hyperplasia.84 That calcitriol is ineffective in suppressing PTH in the absence of the CASR, while Ca is sufficient to normalize PTG function in the absence of VDR, is evidence that the CASR is the dominant regulator of PTG function. The principal direct function of the VDR in the PTG, then, is to suppress PTH gene transcription; the VDR has an indirect function on the PTG via its actions in the gastrointestinal tract to increase Ca absorption and elevate serum Ca levels, which serves to affect PTG function via the CASR.
That the CASR appears to have a more dominant role over VDR in PTG hyperplasia does not trivialize the role of the VDR is unimportant. Complex interrelationships between the CASR and VDR exist. Both the CASR29–32 and the VDR85–89 can be downregulated in humans and animals with severe SHPT, making the gland resistant to treatment with vitamin D analogs. In rodent models of renal failure, calcimimetics upregulate the CASR.36 There is also data that the VDR upregulates the CASR.90–92 While in the setting of adequate Ca the VDR is not necessary for the short-term survival of the organism, in the setting of more complex environments and over the long-term, a physiologically replete vitamin D state may be important.
Putative actions of vitamin D beyond mineral metabolism: relevance to patients with kidney disease
There is a growing body of literature on potential non-classical actions of vitamin D, ie, actions beyond those directly involved in mineral metabolism. Much of this data are derived from observational studies of individuals with normal renal function who are nutritionally vitamin D-deficiency (and who therefore have presumed impairments of VDR-dependent signaling), and from studies that investigate the extra-renal production of 1,25(OH)2 D and its role in innate immunity. Of particular relevance to ESRD patients are the putative effects of extrarenal production of 1,25(OH)2 D on innate immunity.93 As reviewed by Peterlik et al,94 activated vitamin D (calcidiol) is involved in many immune functions, such as induction of monocyte chemotaxis,95 macrophage differentiation,96,97 upregulation of Fc receptors,98 and production of the respiratory burst99 and of nitric oxide.100 Perhaps the most compelling evidence for the role of calcidiol emerges from studies of tuberculosis, where both in vitro and in vivo studies, as well as in epidemiologic investigations, calcidiol status is important in defense against this organism.101 While tuberculosis is uncommon in ESRD patients in developed countries, other types of infections are extremely common; whether a state of physiologic 1,25(OH)2 D repletion would be a defense against infection is uncertain.
However, in addition to substrate, extrarenal production of 1,25(OH)2 D requires activation of Toll-like receptors necessary for upregulation of 1α-hydroxylase and downregulation of 24-hydroxylase.93 Given the low levels of peripheral conversion of 25(OH) D to 1,25(OH)2 D,102 it is not clear if the extrarenal production of 1,25(OH)2 D has the same significance in the setting of renal failure, where the vitamin D axis is suppressed by FGF23, as it does in nutritionally vitamin D-insufficient patients with normal kidney function.
Also of great potential importance is the possible role of vitamin D and its analogs in cardioprotection. Levels of calcidiol have been shown to be correlated with insulin sensitivity in epidemiologic studies,103–105 and may be associated with the metabolic syndrome. Additionally, there may be direct cardioprotective effects of calcitriol on the cardiovascular system, with evidence that calcitriol antagonizes cardiac myocyte hypertrophy in rats106 and down-regulates the renin-angiotensin system.107 Again, whether these findings have clinical relevance in humans requires further study.
Given the collective body of evidence, what therapeutic approaches might be most suitable for the treatment of SHPT?
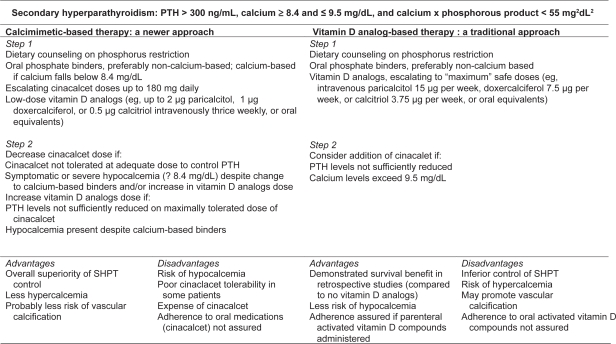
In Figure 1, we illustrate two potential therapeutic approaches to SHPT (one calcimimetic-based, the other vitamin D analog-based), and list relevant advantages and disadvantages of each. The Figure represents less a specific protocol than an overall conceptualization of possible treatment strategies. Patients with low calcium levels (ie, <8.4 mg/dL) might best avoid the potential for cinacalcet-induced hypocalcemia, at least until low doses of activated vitamin D compounds increase serum calcium to the normal range, while those with high serum calcium ought to avoid large doses of activated vitamin D compounds. For patients whose calcium levels fall within the “acceptable” NKF K/DOQI range, namely 8.4 to 9.5 mg/dL, the approaches have many similarities. In both, dietary phosphorous restriction is important, and oral phosphate binders critical. Non-calcium-based binders are generally preferred in either approach, given the mounting evidence that non-calcium based binders are associated with less vascular calcification4–7,108 and perhaps improved survival,109 although the latter finding requires replication and extension in true blinded RCTs.
Figure 1.
Competing therapeutic strategies for treatment of secondary hyperparathyroidism, with advantages and disadvantages.
Abbreviations: PTH, parathyroid hormone; SHPT, secondary hyperparathyroidism.
However, the cinacalcet-based approach relies on calcimimetics as the principal agent for PTH reduction, while still maintaining a role for low doses of vitamin D analogs to defend against hypocalcemia while contributing potential non-mineral-metabolism-related survival benefits that may be responsible for the findings of other studies.60,61 In a cinacalcet-based approach, doses of activated vitamin D analogs can be increased if cinacalcet is ineffective or not tolerated at adequate doses. Because of poor tolerability in some patients as well as the expense for individuals without drug insurance coverage, cinacalcet treatment poses adherence challenges. Nevertheless, the potential benefits of a cinacalcet-based strategy, specifically better overall control of SHPT, as demonstrated in multiple RCTs, and the likelihood of less hypercalcemia, make this the preferred therapeutic approach at the current time. A vitamin D analog-based strategy, in which cinacalet is added only if large doses of activated vitamin D is ineffective at controlling PTH or if hypercalcemia results, is less likely to lead to optimal SHPT control and may lead to a higher risk of vascular calcification and, ultimately, mortality. As such, the latter is a less attractive therapeutic approach for most patients.
Conclusions: time for a new treatment paradigm of SHPT in ESRD?
Treatment paradigms for SHPT must consider the pathogenesis of the disease as well as the results of clinical studies. In general, we believe that a cinacalcet-based approach is superior for most patients with SHPT because (1) clinical trials have demonstrated the superior suppression of PTH and control of Ca × P product in ESRD patients with calcimimetics, relative to vitamin D analogs, both when used as adjunctive therapy superimposed on vitamin D analogs and also as “primary” therapy with low doses of vitamin D analogs; (2) decreased levels of calcitriol appear to be an adaptive response in advanced renal disease to limit the toxic effects of hyperphosphatemia; (3) molecular human and mouse genetic studies demonstrate the greater importance of the CASR, relative to the VDR, in the regulation of PTG function; and (4) the use of cinacalcet may result in less calcium loading than therapy with vitamin D analogs. Calcium loading with calcium-based binders, in the setting of concurrent activated vitamin D compound therapy, has been associated with increased vascular calcifications.4
Although survival benefits of vitamin D analogs in ESRD have not been demonstrated in an RCT, such benefits are biologically plausible, and physiological “replacement” doses of vitamin D analogs (eg, paricalcitol 2 μg or doxercalciferol 1 μg thrice weekly) appear warranted in the majority of patients without obvious contraindications. In contrast, long-term use of high-dose vitamin D analogs probably results in a more positive Ca balance in ESRD than similar treatment with cinacalcet, although studies that assess the risk reduction of cinacalcet currently exist solely in animal models.110 Low doses of vitamin D analogs, as adjunctive therapy to cinacalet, are likely to emerge as the standard of care. These tonic doses of activated vitamin D therapy could be increased when (1) calcimimetics have been tried at maximal doses and failed to control SHPT, (2) calcimimetics have intolerable side effects at effective doses, or (3) calcimimetic use results in hypocalcemia, an effect which can frequently be remedied by vitamin D analogs. Whether nutritional vitamin D supplementation, in addition to treatment with activated vitamin D compounds, is of benefit is uncertain at the current time.
The nephrology community has dire need for data from well-designed RCTs to determine whether calcimimetics provide mortality benefits relative to vitamin D analogs. While a study in which cinacalcet is tested directly against a vitamin D analog would provides for a more rigorous experimental design, widespread use of vitamin D analogs, as well as their benefit in protection against cinacalcet-induced hypocalcemia, probably makes employment of such a design impractical. However, a trial in which low-dose vitamin D analogs are administered in the cinacalet arm would reflect clinical practice realities and increase the likelihood that subjects randomized to cinacalcet would be consistently exposed to the drug over a lengthy treatment period, a likely requirement if the trial is to show unambiguous differences in cumulative event rates. In the absence of clinical trial data, calcimimetics, along with dietary phosphate control, should be considered primary therapy in most ESRD patients with SHPT.
Footnotes
Disclosures
JBW receives honoraria and research funding from Amgen, Inc. LDQ receives honoraria from, and serves on the advisory board of, Amgen, Inc.
References
- 1.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 2.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca × PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12(10):2131–2138. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 3.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342(20):1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62(1):245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 5.Chertow GM, Raggi P, McCarthy JT, Schulman G, Silberzweig J, Kuhlik A, et al. The effects of sevelamer and calcium acetate on proxies of atherosclerotic and arteriosclerotic vascular disease in hemodialysis patients. Am J Nephrol. 2003;23(5):307–314. doi: 10.1159/000072822. [DOI] [PubMed] [Google Scholar]
- 6.Ferramosca E, Burke S, Chasan-Taber S, Ratti C, Chertow GM, Raggi P. Potential antiatherogenic and anti-inflammatory properties of sevelamer in maintenance hemodialysis patients. Am Heart J. 2005;149(5):820–825. doi: 10.1016/j.ahj.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68(4):1815–1824. doi: 10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 8.Goodman WG, Hladik GA, Turner SA, Blaisdell PW, Goodkin DA, Liu W, et al. The Calcimimetic agent AMG 073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism. J Am Soc Nephrol. 2002;13(4):1017–1024. doi: 10.1681/ASN.V1341017. [DOI] [PubMed] [Google Scholar]
- 9.Quarles LD, Sherrard DJ, Adler S, Rosansky SJ, McCary LC, Liu W, et al. The calcimimetic AMG 073 as a potential treatment for secondary hyperparathyroidism of end-stage renal disease. J Am Soc Nephrol. 2003;14(3):575–583. doi: 10.1097/01.asn.0000050224.03126.ad. [DOI] [PubMed] [Google Scholar]
- 10.Lindberg JS, Moe SM, Goodman WG, Coburn JW, Sprague SM, Liu W, et al. The calcimimetic AMG 073 reduces parathyroid hormone and calcium × phosphorus in secondary hyperparathyroidism. Kidney Int. 2003;63(1):248–254. doi: 10.1046/j.1523-1755.2003.00720.x. [DOI] [PubMed] [Google Scholar]
- 11.Lindberg JS, Culleton B, Wong G, Borah MF, Clark RV, Shapiro WB, et al. Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter study. J Am Soc Nephrol. 2005;16(3):800–807. doi: 10.1681/ASN.2004060512. [DOI] [PubMed] [Google Scholar]
- 12.Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350(15):1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 13.Strippoli GF, Palmer S, Tong A, Elder G, Messa P, Craig JC. Meta-analysis of biochemical and patient-level effects of calcimimetic therapy. Am J Kidney Dis. 2006;47(5):715–726. doi: 10.1053/j.ajkd.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Martin KJ, Juppner H, Sherrard DJ, Goodman WG, Kaplan MR, Nassar G, et al. First- and second-generation immunometric PTH assays during treatment of hyperparathyroidism with cinacalcet HCl. Kidney Int. 2005;68(3):1236–1243. doi: 10.1111/j.1523-1755.2005.00517.x. [DOI] [PubMed] [Google Scholar]
- 15.Fukagawa M, Yumita S, Akizawa T, Uchida E, Tsukamoto Y, Iwasaki M, et al. Cinacalcet (KRN1493) effectively decreases the serum intact PTH level with favorable control of the serum phosphorus and calcium levels in Japanese dialysis patients. Nephrol Dial Transplant. 2008;23(1):328–335. doi: 10.1093/ndt/gfm534. [DOI] [PubMed] [Google Scholar]
- 16.Messa P, Macario F, Yaqoob M, Bouman K, Braun J, von Albertini B, et al. The OPTIMA study: assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2008;3(1):36–45. doi: 10.2215/CJN.03591006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moe SM, Cunningham J, Bommer J, Adler S, Rosansky SJ, Urena-Torres P, et al. Long-term treatment of secondary hyperparathyroidism with the calcimimetic cinacalcet HCl. Nephrol Dial Transplant. 2005;20(10):2186–2193. doi: 10.1093/ndt/gfh966. [DOI] [PubMed] [Google Scholar]
- 18.Sterrett JR, Strom J, Stummvoll HK, Bahner U, Disney A, Soroka SD, et al. Cinacalcet HCI (Sensipar/Mimpara) is an effective chronic therapy for hemodialysis patients with secondary hyperparathyroidism. Clin Nephrol. 2007;68(1):10–17. doi: 10.5414/cnp68010. [DOI] [PubMed] [Google Scholar]
- 19.Chertow GM, Blumenthal S, Turner S, Roppolo M, Stern L, Chi EM, et al. Cinacalcet hydrochloride (Sensipar) in hemodialysis patients on active vitamin D derivatives with controlled PTH and elevated calcium × phosphate. Clin J Am Soc Nephrol. 2006;1(2):305–312. doi: 10.2215/CJN.00870805. [DOI] [PubMed] [Google Scholar]
- 20.Block GA, Zeig S, Sugihara J, Chertow GM, Chi EM, Turner SA, et al. Combined therapy with cinacalcet and low doses of vitamin D sterols in patients with moderate to severe secondary hyperparathyroidism. Nephrol Dial Transplant. 2008 doi: 10.1093/ndt/gfn026. In press. [DOI] [PubMed] [Google Scholar]
- 21.Arenas MD, Alvarez-Ude F, Gil MT, Moledous A, Malek T, Nunez C, et al. Implementation of ‘K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease’ after the introduction of cinacalcet in a population of patients on chronic haemodialysis. Nephrol Dial Transplant. 2007;22(6):1639–1644. doi: 10.1093/ndt/gfl840. [DOI] [PubMed] [Google Scholar]
- 22.Lazar E, Hebert K, Poma T, Stankus N. Long-term outcomes of cinacalcet and paricalcitol titration protocol for treatment of secondary hyperparathyroidism. Am J Nephrol. 2007;27(3):274–278. doi: 10.1159/000101727. [DOI] [PubMed] [Google Scholar]
- 23.Spiegel DM, Casey L, Bell S, Parker M, Chonchol M. Achieving targets for bone and mineral metabolism: the impact of cinacalcet HCl in clinical practice. Hemodial Int. 2006;10(Suppl 2):S24–27. doi: 10.1111/j.1542-4758.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- 24.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81(1):2392–97. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 25.Brown EM. Clinical lessons from the calcium-sensing receptor. Nat Clin Pract Endocrinol Metab. 2007;3(2):122–133. doi: 10.1038/ncpendmet0388. [DOI] [PubMed] [Google Scholar]
- 26.Silver J, Levi R. Regulation of PTH synthesis and secretion relevant to the management of secondary hyperparathyroidism in chronic kidney disease. Kidney Int Suppl. 2005;(95):S8–12. doi: 10.1111/j.1523-1755.2005.09501.x. [DOI] [PubMed] [Google Scholar]
- 27.Pollak MR, Brown EM, Chou YH, Hebert SC, Marx SJ, Steinmann B, et al. Mutations in the human Ca(2+)−sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell. 1993;75(7):1297–1303. doi: 10.1016/0092-8674(93)90617-y. [DOI] [PubMed] [Google Scholar]
- 28.Ho C, Conner DA, Pollak MR, Ladd DJ, Kifor O, Warren HB, et al. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet. 1995;11(4):389–394. doi: 10.1038/ng1295-389. [DOI] [PubMed] [Google Scholar]
- 29.Gogusev J, Duchambon P, Hory B, Giovannini M, Goureau Y, Sarfati E, et al. Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int. 1997;51(1):328–336. doi: 10.1038/ki.1997.41. [DOI] [PubMed] [Google Scholar]
- 30.Kifor O, Moore FD, Jr, Wang P, Goldstein M, Vassilev P, Kifor I, et al. Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J Clin Endocrinol Metab. 1996;81(4):1598–1606. doi: 10.1210/jcem.81.4.8636374. [DOI] [PubMed] [Google Scholar]
- 31.Brown AJ, Ritter CS, Finch JL, Slatopolsky EA. Decreased calcium-sensing receptor expression in hyperplastic parathyroid glands of uremic rats: role of dietary phosphate. Kidney Int. 1999;55(4):1284–1292. doi: 10.1046/j.1523-1755.1999.00386.x. [DOI] [PubMed] [Google Scholar]
- 32.Ritter CS, Finch JL, Slatopolsky EA, Brown AJ. Parathyroid hyperplasia in uremic rats precedes down-regulation of the calcium receptor. Kidney Int. 2001;60(5):1737–1744. doi: 10.1046/j.1523-1755.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 33.Drueke T, Martin D, Rodriguez M. Can calcimimetics inhibit parathyroid hyperplasia? Evidence from preclinical studies. Nephrol Dial Transplant. 2007;22(7):1828–1839. doi: 10.1093/ndt/gfm177. [DOI] [PubMed] [Google Scholar]
- 34.Colloton M, Shatzen E, Miller G, Stehman-Breen C, Wada M, Lacey D, et al. Cinacalcet HCl attenuates parathyroid hyperplasia in a rat model of secondary hyperparathyroidism. Kidney Int. 2005;67(2):467–476. doi: 10.1111/j.1523-1755.2005.67103.x. [DOI] [PubMed] [Google Scholar]
- 35.Chin J, Miller SC, Wada M, Nagano N, Nemeth EF, Fox J. Activation of the calcium receptor by a calcimimetic compound halts the progression of secondary hyperparathyroidism in uremic rats. J Am Soc Nephrol. 2000;11(5):903–911. doi: 10.1681/ASN.V115903. [DOI] [PubMed] [Google Scholar]
- 36.Mizobuchi M, Hatamura I, Ogata H, Saji F, Uda S, Shiizaki K, et al. Calcimimetic compound upregulates decreased calcium-sensing receptor expression level in parathyroid glands of rats with chronic renal insufficiency. J Am Soc Nephrol. 2004;15(10):2579–2587. doi: 10.1097/01.ASN.0000141016.20133.33. [DOI] [PubMed] [Google Scholar]
- 37.de Francisco AL, Izquierdo M, Cunningham J, Pinera C, Palomar R, Fresnedo GF, et al. Calcium-mediated parathyroid hormone release changes in patients treated with the calcimimetic agent cinacalcet. Nephrol Dial Transplant. 2008 Apr 23; doi: 10.1093/ndt/gfn191. [DOI] [PubMed] [Google Scholar]
- 38.Lomonte C, Vernaglione L, Chimienti D, Bruno A, Cocola S, Teutonico A, et al. Does vitamin D receptor and calcium receptor activation therapy play a role in the histopathologic alterations of parathyroid glands in refractory uremic hyperparathyroidism? Clin J Am Soc Nephrol. 2008;3(3):794–799. doi: 10.2215/CJN.04150907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamaguchi S, Yachiku S, Morikawa M. Analysis of proliferative activity of the parathyroid glands using proliferating cell nuclear antigen in patients with hyperparathyroidism. J Clin Endocrinol Metab. 1997;82(8):2681–268. doi: 10.1210/jcem.82.8.4117. [DOI] [PubMed] [Google Scholar]
- 40.Malluche HH, Monier-Faugere MC, Wang G, Fraza OJ, Charytan C, Coburn JW, et al. An assessment of cinacalcet HCl effects on bone histology in dialysis patients with secondary hyperparathyroidism. Clin Nephrol. 2008;69(4):269–278. doi: 10.5414/cnp69269. [DOI] [PubMed] [Google Scholar]
- 41.Wang R, Xu C, Zhao W, Zhang J, Cao K, Yang B, et al. Calcium and polyamine regulated calcium-sensing receptors in cardiac tissues. Eur J Biochem. 2003;270(12):2680–268. doi: 10.1046/j.1432-1033.2003.03645.x. [DOI] [PubMed] [Google Scholar]
- 42.Tfelt-Hansen J, Hansen JL, Smajilovic S, Terwilliger EF, Haunso S, Sheikh SP. Calcium receptor is functionally expressed in rat neonatal ventricular cardiomyocytes. Am J Physiol Heart Circ Physiol. 2006;290(3):H1165–1171. doi: 10.1152/ajpheart.00821.2005. [DOI] [PubMed] [Google Scholar]
- 43.Farzaneh-Far A, Proudfoot D, Weissberg PL, Shanahan CM. Matrix gla protein is regulated by a mechanism functionally related to the calcium-sensing receptor. Biochem Biophys Res Commun. 2000;277(3):736–740. doi: 10.1006/bbrc.2000.3747. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Bukoski RD. Distribution of the perivascular nerve Ca2+ receptor in rat arteries. Br J Pharmacol. 1998;125(7):1397–1404. doi: 10.1038/sj.bjp.0702195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wonneberger K, Scofield MA, Wangemann P. Evidence for a calcium-sensing receptor in the vascular smooth muscle cells of the spiral modiolar artery. J Membr Biol. 2000;175(3):203–212. doi: 10.1007/s00232001068. [DOI] [PubMed] [Google Scholar]
- 46.Smajilovic S, Hansen JL, Christoffersen TE, Lewin E, Sheikh SP, Terwilliger EF, et al. Extracellular calcium sensing in rat aortic vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;348(4):1215–1223. doi: 10.1016/j.bbrc.2006.07.192. [DOI] [PubMed] [Google Scholar]
- 47.Ziegelstein RC, Xiong Y, He C, Hu Q. Expression of a functional extracellular calcium-sensing receptor in human aortic endothelial cells. Biochem Biophys Res Commun. 2006;342(1):153–163. doi: 10.1016/j.bbrc.2006.01.135. [DOI] [PubMed] [Google Scholar]
- 48.Smajilovic S, Tfelt-Hansen J. Calcium acts as a first messenger through the calcium-sensing receptor in the cardiovascular system. Cardiovasc Res. 2007;75(3):457–467. doi: 10.1016/j.cardiores.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 49.Ogata H, Ritz E, Odoni G, Amann K, Orth SR. Beneficial effects of calcimimetics on progression of renal failure and cardiovascular risk factors. J Am Soc Nephrol. 2003;14(4):959–967. doi: 10.1097/01.asn.0000056188.23717.e5. [DOI] [PubMed] [Google Scholar]
- 50.Cunningham J, Danese M, Olson K, Klassen P, Chertow GM. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int. 2005;68(4):1793–1800. doi: 10.1111/j.1523-1755.2005.00596.x. [DOI] [PubMed] [Google Scholar]
- 51.Martin KJ, Gonzalez EA, Gellens M, Hamm LL, Abboud H, Lindberg J. 19-Nor-1-alpha-25-dihydroxyvitamin D2 (Paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol. 1998;9(8):1427–1432. doi: 10.1681/ASN.V981427. [DOI] [PubMed] [Google Scholar]
- 52.Delmez JA, Kelber J, Norwood KY, Giles KS, Slatopolsky E. A controlled trial of the early treatment of secondary hyperparathyroidism with calcitriol in hemodialysis patients. Clin Nephrol. 2000;54(4):301–308. [PubMed] [Google Scholar]
- 53.Coyne DW, Grieff M, Ahya SN, Giles K, Norwood K, Slatopolsky E. Differential effects of acute administration of 19-Nor-1,25-dihydroxy-vitamin D2 and 1,25-dihydroxy-vitamin D3 on serum calcium and phosphorus in hemodialysis patients. Am J Kidney Dis. 2002;40(6):1283–1288. doi: 10.1053/ajkd.2002.36899. [DOI] [PubMed] [Google Scholar]
- 54.Joist HE, Ahya SN, Giles K, Norwood K, Slatopolsky E, Coyne DW. Differential effects of very high doses of doxercalciferol and paricalcitol on serum phosphorus in hemodialysis patients. Clin Nephrol. 2006;65(5):335–341. doi: 10.5414/cnp65335. [DOI] [PubMed] [Google Scholar]
- 55.Sprague SM, Llach F, Amdahl M, Taccetta C, Batlle D. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int. 2003;63(4):1483–1490. doi: 10.1046/j.1523-1755.2003.00878.x. [DOI] [PubMed] [Google Scholar]
- 56.Brandi L, Daugaard H, Tvedegaard E, Nielsen PK, Egsmose C, Storm T, et al. Long-term suppression of secondary hyperparathyroidism by intravenous 1 alpha-hydroxyvitamin D3 in patients on chronic hemodialysis. Am J Nephrol. 1992;12(5):311–318. doi: 10.1159/000168465. [DOI] [PubMed] [Google Scholar]
- 57.Lindberg J, Martin KJ, Gonzalez EA, Acchiardo SR, Valdin JR, Soltanek C. A long-term, multicenter study of the efficacy and safety of paricalcitol in end-stage renal disease. Clin Nephrol. 2001;56(4):315–323. [PubMed] [Google Scholar]
- 58.Akizawa T, Suzuki M, Akiba T, Nishizawa Y, Ohashi Y, Ogata E, et al. Long-term effect of 1,25-dihydroxy-22-oxavitamin D(3) on secondary hyperparathyroidism in haemodialysis patients. One-year administration study. Nephrol Dial Transplant. 2002;(17 Suppl 10):28–36. doi: 10.1093/ndt/17.suppl_10.28. [DOI] [PubMed] [Google Scholar]
- 59.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349(5):446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 60.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA, Jr, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16(4):1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 61.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70(4):771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 62.Tentori F, Hunt WC, Stidley CA, Rohrscheib MR, Bedrick EJ, Meyer KB, et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70(10):1858–1865. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- 63.Al-Badr W, Martin KJ. Vitamin D and Kidney Disease. Clin J Am Soc Nephrol. 2008 May 1; doi: 10.2215/CJN.01150308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown AJ, Slatopolsky E. Drug insight: vitamin D analogs in the treatment of secondary hyperparathyroidism in patients with chronic kidney disease. Nat Clin Pract Endocrinol Metab. 2007 Feb;3(2):134–144. doi: 10.1038/ncpendmet0394. [DOI] [PubMed] [Google Scholar]
- 65.Mehrotra R, Kermah D, Budoff M, Salusky IB, Mao SS, Gao YL, et al. Hypovitaminosis D in Chronic Kidney Disease. Clin J Am Soc Nephrol. 2008 Apr 16; doi: 10.2215/CJN.05781207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zehnder D, Landray MJ, Wheeler DC, Fraser W, Blackwell L, Nuttall S, et al. Cross-sectional analysis of abnormalities of mineral homeostasis, vitamin D and parathyroid hormone in a cohort of pre-dialysis patients. The chronic renal impairment in Birmingham (CRIB) study. Nephron Clin Pract. 2007;107(3):c109–16. doi: 10.1159/000108652. [DOI] [PubMed] [Google Scholar]
- 67.Slatopolsky E, Brown A, Dusso A. Role of phosphorus in the pathogenesis of secondary hyperparathyroidism. Am J Kidney Dis. 2001 Jan;37(1 Suppl 2):S54–7. doi: 10.1053/ajkd.2001.20740. [DOI] [PubMed] [Google Scholar]
- 68.Rowe PS. The wrickkened pathways of FGF23, MEPE and PHEX. Crit Rev Oral Biol Med. 2004;15(5):264–281. doi: 10.1177/154411130401500503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zisman AL, Hristova M, Ho LT, Sprague SM. Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol. 2007;27(1):36–43. doi: 10.1159/000098561. [DOI] [PubMed] [Google Scholar]
- 70.Al-Aly Z, Qazi RA, Gonzalez EA, Zeringue A, Martin KJ. Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis. 2007;50(1):59–68. doi: 10.1053/j.ajkd.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 71.Chandra P, Binongo JN, Ziegler TR, Schlanger LE, Wang W, Someren JT, et al. Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: a randomized controlled pilot study. Endocr Pract. 2008;14(1):10–17. doi: 10.4158/EP.14.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saito H, Kusano K, Kinosaki M, Ito H, Hirata M, Segawa H, et al. Human fibroblast growth factor-23 mutants suppress Na+−dependent phosphate co-transport activity and 1alpha, 25-dihydroxyvitamin D3 production. J Biol Chem. 2003;278(4):2206–2211. doi: 10.1074/jbc.M207872200. [DOI] [PubMed] [Google Scholar]
- 73.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006 May;17(5):1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 74.Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, Jonsson KB, et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195(1):125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 75.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 76.Perwad F, Zhang MY, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293(5):F1577–1583. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- 77.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64(6):2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 78.Shigematsu T, Kazama JJ, Yamashita T, Fukumoto S, Hosoya T, Gejyo F, et al. Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis. 2004;44(2):250–256. doi: 10.1053/j.ajkd.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 79.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16(7):2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 80.Coburn JW, Maung HM, Elangovan L, Germain MJ, Lindberg JS, Sprague SM, et al. Doxercalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney disease stages 3 and 4. Am J Kidney Dis. 2004;43(5):877–890. doi: 10.1053/j.ajkd.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 81.Goodman WG. Calcimimetics: a remedy for all problems of excess parathyroid hormone activity in chronic kidney disease? Curr Opin Nephrol Hypertens. 2005;14(4):355–360. doi: 10.1097/01.mnh.0000172722.52499.71. [DOI] [PubMed] [Google Scholar]
- 82.Kos CH, Karaplis AC, Peng JB, Hediger MA, Goltzman D, Mohammad KS, et al. The calcium-sensing receptor is required for normal calcium homeostasis independent of parathyroid hormone. J Clin Invest. 2003;111(7):1021–1028. doi: 10.1172/JCI17416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tu Q, Pi M, Karsenty G, Simpson L, Liu S, Quarles LD. Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null background. J Clin Invest. 2003;111(7):1029–37. doi: 10.1172/JCI17054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, et al. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology. 1998;139(10):4391–4396. doi: 10.1210/endo.139.10.6262. [DOI] [PubMed] [Google Scholar]
- 85.Martin LN, Kayath MJ, Vieira JG, Nose-Alberti V. Parathyroid glands in uraemic patients with refractory hyperparathyroidism: histopathology and p53 protein expression analysis. Histopathology. 1998;33(1):46–51. [PubMed] [Google Scholar]
- 86.Fukuda N, Tanaka H, Tominaga Y, Fukagawa M, Kurokawa K, Seino Y. Decreased 1,25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J Clin Invest. 1993;92(3):1436–1443. doi: 10.1172/JCI116720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Sun B, Zhou F, Hu J, Yu X, Peng T. Vitamin D receptor and PCNA expression in severe parathyroid hyperplasia of uremic patients. Chin Med J (Engl) 2001;114(4):410–414. [PubMed] [Google Scholar]
- 88.Tokumoto M, Tsuruya K, Fukuda K, Kanai H, Kuroki S, Hirakata H. Reduced p21, p27 and vitamin D receptor in the nodular hyperplasia in patients with advanced secondary hyperparathyroidism. Kidney Int. 2002;62(4):1196–1207. doi: 10.1111/j.1523-1755.2002.kid585.x. [DOI] [PubMed] [Google Scholar]
- 89.Yano S, Sugimoto T, Tsukamoto T, Chihara K, Kobayashi A, Kitazawa S, et al. Decrease in vitamin D receptor and calcium-sensing receptor in highly proliferative parathyroid adenomas. Eur J Endocrinol. 2003;148(4):403–411. doi: 10.1530/eje.0.1480403. [DOI] [PubMed] [Google Scholar]
- 90.Brown AJ, Zhong M, Finch J, Ritter C, McCracken R, Morrissey J, et al. Rat calcium-sensing receptor is regulated by vitamin D but not by calcium. Am J Physiol. 1996;270(3 Pt 2):F454–460. doi: 10.1152/ajprenal.1996.270.3.F454. [DOI] [PubMed] [Google Scholar]
- 91.Canaff L, Hendy GN. Human calcium-sensing receptor gene. Vitamin D response elements in promoters P1 and P2 confer transcriptional responsiveness to 1,25-dihydroxyvitamin D. J Biol Chem. 2002;277(33):30337–30350. doi: 10.1074/jbc.M201804200. [DOI] [PubMed] [Google Scholar]
- 92.Tokumoto M, Taniguchi M, Matsuo D, Tsuruya K, Hirakata H, Iida M. Parathyroid cell growth in patients with advanced secondary hyper-parathyroidism: vitamin D receptor, calcium sensing receptor, and cell cycle regulating factors. Ther Apher Dial. 2005;9(Suppl 1):S27–34. doi: 10.1111/j.1744-9987.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 93.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 94.Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest. 2005;35(5):290–304. doi: 10.1111/j.1365-2362.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 95.Girasole G, Wang JM, Pedrazzoni M, Pioli G, Balotta C, Passeri M, et al. Augmentation of monocyte chemotaxis by 1 alpha, 25-dihydroxyvitamin D3. Stimulation of defective migration of AIDS patients. J Immunol. 1990;145(8):2459–2464. [PubMed] [Google Scholar]
- 96.Provvedini DM, Deftos LJ, Manolagas SC. 1,25-Dihydroxyvitamin D3 promotes in vitro morphologic and enzymatic changes in normal human monocytes consistent with their differentiation into macrophages. Bone. 1986;7(1):23–28. doi: 10.1016/8756-3282(86)90148-1. [DOI] [PubMed] [Google Scholar]
- 97.Orikasa M, Kawase T, Suzuki A. Induction of macrophagic and granulocytic differentiation of murine bone marrow progenitor cells by 1,25-dihydroxyvitamin D3. Calcif Tissue Int. 1993;53(3):193–200. doi: 10.1007/BF01321837. [DOI] [PubMed] [Google Scholar]
- 98.Boltz-Nitulescu G, Willheim M, Spittler A, Leutmezer F, Tempfer C, Winkler S. Modulation of IgA, IgE, and IgG Fc receptor expression on human mononuclear phagocytes by 1 alpha,25-dihydroxyvitamin D3 and cytokines. J Leukoc Biol. 1995;58(2):256–262. doi: 10.1002/jlb.58.2.256. [DOI] [PubMed] [Google Scholar]
- 99.Cohen MS, Mesler DE, Snipes RG, Gray TK. 1,25-Dihydroxyvitamin D3 activates secretion of hydrogen peroxide by human monocytes. J Immunol. 1986;136(3):1049–1053. [PubMed] [Google Scholar]
- 100.Sly LM, Lopez M, Nauseef WM, Reiner NE. 1alpha, 25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J Biol Chem. 2001;276(38):35482–35493. doi: 10.1074/jbc.M102876200. [DOI] [PubMed] [Google Scholar]
- 101.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37(1):113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 102.Gallieni M, Kamimura S, Ahmed A, Bravo E, Delmez J, Slatopolsky E, et al. Kinetics of monocyte 1 alpha-hydroxylase in renal failure. Am J Physiol. 1995;268(4 Pt 2):F746–753. doi: 10.1152/ajprenal.1995.268.4.F746. [DOI] [PubMed] [Google Scholar]
- 103.Pereira MA, Jacobs DR, Jr, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: the CARDIA Study. JAMA. 2002;287(16):2081–2089. doi: 10.1001/jama.287.16.2081. [DOI] [PubMed] [Google Scholar]
- 104.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among US adults. Diabetes Care. 2005;28(5):1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 105.Liu S, Song Y, Ford ES, Manson JE, Buring JE, Ridker PM. Dietary calcium, vitamin D, and the prevalence of metabolic syndrome in middle-aged and older US women. Diabetes Care. 2005;28(12):2926–2932. doi: 10.2337/diacare.28.12.2926. [DOI] [PubMed] [Google Scholar]
- 106.Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest. 1996;97(7):1577–1588. doi: 10.1172/JCI118582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvi-tamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spiegel DM, Raggi P, Smits G, Block GA. Factors associated with mortality in patients new to haemodialysis. Nephrol Dial Transplant. 2007;22(12):3568–3572. doi: 10.1093/ndt/gfm424. [DOI] [PubMed] [Google Scholar]
- 109.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71(5):438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 110.Lopez I, Mendoza FJ, Aguilera-Tejero E, Perez J, Guerrero F, Martin D, et al. The effect of calcitriol, paricalcitol, and a calcimimetic on extraosseous calcifications in uremic rats. Kidney Int. 2008;73(3):300–307. doi: 10.1038/sj.ki.5002675. [DOI] [PubMed] [Google Scholar]