Abstract
Systemic lupus erythematosus (SLE) is a classic antibody-mediated systemic autoimmune disease characterised by the development of autoantibodies to ubiquitous self-antigens (such as antinuclear antibodies and antidouble-stranded DNA antibodies) and widespread deposition of immune complexes in affected tissues. Deposition of immune complexes in the kidney results in glomerular damage and occurs in all forms of lupus nephritis. The development of nephritis carries a poor prognosis and high risk of developing end-stage renal failure despite recent therapeutic advances. Here we review the role of DNA-anti-DNA immune complexes in the pathogenesis of lupus nephritis and possible new treatment strategies aimed at their control.
Keywords: immune complex, systemic lupus erythematosus, nephritis, therapy
Introduction
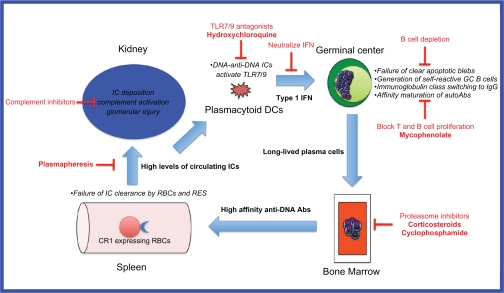
Systemic lupus erythematosus (SLE) is a complex, heterogeneous disease of multi-factorial etiology where multiple genetic, environmental and sex hormonal influences converge to break down B cell tolerance to self-antigens normally sequestered inside the cell nucleus.1 Recent insights obtained from genetic mouse models and genome-wide association scans in large patient cohorts have enabled the identification of several key players in the multistep pathogenesis of lupus (Figure 1). These studies reveal a positive feedback loop whereby inefficient clearance of apoptotic blebs by macrophages results in positive selection of germinal center B cells, which have self-reactivity against nuclear antigens exposed on these blebs. These self-reactive B cells undergo T cell-dependent affinity maturation and isotype switching,2 and differentiate into long-lived plasma cells which reside in the bone marrow. The high affinity IgG anti-DNA antibodies secreted by these cells bind to the DNA to form immune complexes which activate plasmacytoid dendritic cells (pDCs) via toll-like receptor- (TLR-) 9 to produce inflammatory cytokines such as interferon-alpha. These cytokines augment the humoral immune response and lead to further autoantibody production. The high levels of circulating DNA-anti-DNA immune complexes overwhelm the capacity of the reticuloendothelial system (RES) to clear them, and they are deposited in various tissues including glomeruli where local complement activation results in glomerular injury.3
Figure 1.
Model of DNA-anti-DNA immune complex generation and glomerular damage in lupus nephritis and potential therapeutic targets.
Abbreviations: Abs, antibodies; DCs, dendritic cells; GC, germinal center; ICs, immune complexes; RBCs, red blood cells; RES, reticuloendothelial system.
Nephritis is a common complication of SLE, occurring in 14% to 55% of patients, with higher rates seen in Asian, African, and Hispanic populations.4 Histological patterns of lupus nephritis have been classified by the World Health Organization and, more recently, by the International Society of Nephrology/Renal Pathology Society (ISN/RPS) (Table 1).5 These histologic patterns are predictive of prognosis6 and provide a basis for treatment guidelines to prevent end-organ damage and improve mortality and morbidity. Despite improvements in the long-term survival of patients with SLE,7 patients who develop nephritis still have a worse prognosis with a 10-year survival of only 88% compared with 94% for patients without nephritis.8
Table 1.
Classification of lupus nephritis
| Class | WHO (1982) | ISN/RPSb (2003) |
|---|---|---|
| I | Normal glomeruli
|
Minimal mesangial
|
| II | Pure mesangial
|
Mesangial proliferative |
| III | Focal segmentala
|
Focal (<50% glomeruli)c A. Active lesions A/C. Active and chronic lesions C. Chronic inactive lesions with scars |
| IV | Diffusea
|
Diffuse segmental (IV-S) or global (IV-G)d (≥50% glomeruli segmental or global lesions). A, A/C, C as above |
| V | Diffuse membranous
|
Membranous May occur in combination with class III or IV, in which case both will be diagnosed |
| VI | Advanced sclerosinga | Advanced sclerosing (≥90% of glomeruli globally sclerosed without residual activity) |
Notes:
Percentage was not stipulated in the 1982 modification of the WHO criteria;
Indicate and grade (mild, moderate, severe) tubular atrophy, interstitial inflammation and fibrosis, severity of arteriosclerosis or other vascular lesions;
Indicate the proportion of glomeruli with active and with sclerotic lesions;
Indicate the proportion of glomeruli with fibrinoid necrosis and cellular crescents.
Abbreviations: EM, electron microscopy; IF, immunofluorescence; ISN/RPS, International Society of Nephrology/Renal Pathology Society; LM, light microscopy; WHO, World Health Organization.
The mainstay of treatment for lupus nephritis has been corticosteroids, azathioprine, cyclophosphamide and, more recently, mycophenolate. These drugs are toxic with significant side effects and, despite their use, up to 20% of patients with nephritis will still progress to end-stage renal failure and require renal replacement therapy. It is timely therefore to re-examine the role of immune complexes in the pathogenesis of lupus nephritis and update the current status of new therapeutic strategies that target immune complexes.
DNA-anti-DNA immune complexes in the pathogenesis of lupus nephritis
Raised serum levels of circulating immune complexes have long been described in lupus, and correlate with disease activity.9 The role of anti-DNA antibodies in lupus nephritis is also well documented, and the evidence for the involvement of complexes containing these autoantibodies is summarized in Table 2. Despite the evidence linking DNA-anti-DNA immune complexes to lupus nephritis, the precise mechanism of renal damage is still unknown. In the prevailing hypothesis, nucleosomes released from apoptotic cells bind to autoanti-bodies and deposit in glomeruli, resulting in complement activation and thus tissue injury. An alternative hypothesis is that anti-DNA antibodies cross-react with non-DNA components in glomeruli, but this is thought to be less likely.10
Table 2.
Evidence for role of DNA-containing immune complexes in the pathogenesis of lupus nephritis
| Murine models |
|
| Human studies |
|
Doubts about the importance of DNA-anti-DNA immune complexes arise because not all patients with anti-DNA antibodies develop lupus nephritis. Furthermore, glomerular immune complex deposition may be seen without clinically overt renal disease,11 suggesting that additional factors are necessary for the development of renal pathology. Particular characteristics of anti-DNA antibodies may make some more nephritogenic than others. For example, it has been postulated that the isotype and subclass of the antibody is important. In particular, the IgG isotype12 and specifically the IgG313,14 or IgG214 subclasses present a higher risk of clinical nephritis. Although there is some evidence that avidity of anti-dsDNA antibodies may also play a role in vitro,15,16 their role in vivo has been questioned.10,17
The specificity of anti-DNA antibodies is another important factor in pathogenicity. A specificity for nucleosomes rather than DNA,10 the presence of cationic moieties that bind to negatively charged glycosaminoglycans such as heparan sulfate,18 and cross-reactivity of antibodies with alpha-actinin19 are linked to an increased likelihood of renal pathology. Consistent with the idea of immune complex-mediated damage being central to the pathogenesis of lupus nephritis, the availability of extra-cellular chromatin17 has been identified as another factor linked to the development of nephritis. Abnormalities in DNA fragmentation as a result of reduced levels of the endonuclease DNase1 have been identified in mouse models of lupus nephritis, perhaps predisposing to the deposition of chromatin in glomeruli.20
Once DNA-anti-dsDNA immune complexes have been formed, they are normally cleared by the RES but defects of some of the clearance mechanisms have been described in SLE, including aberrant interactions with Fcγ receptors (FcγRs), complement and complement receptors, and anti-C1q antibodies. With respect to the first of these interactions, a particular polymorphism in FcγRIIB is associated with SLE in Asian populations.21 FcγRIIB has a cytoplasmic tail which mediates inhibitory functions. Therefore, FcγRIIB signaling is important in controlling the immune response, and deficiency may predispose to autoimmunity.21 The activating FcγRs are also involved in the pathogenesis of lupus nephritis. Immune complex binding to FcγRI and FcγRIII trigger monocytes and macrophages to release proinflammatory mediators and chemokines which recruit immune effector cells that contribute to renal damage.22–24 Increased expression of FcγRI on monocytes has been found to correlate with the presence of active lupus nephritis.23 Secondly, genetic variations in C4 can affect the handling of immune complexes. Deficiency in C4A relative to C4B is common in SLE, and has also been associated with the development of lupus nephritis. C4A prevents immune complex precipitation, and therefore deficiency could result in increased deposition. A number of other variants of C4 may promote or protect against immune complex damage.25 Thirdly, immune complexes that bind and activate complement can also be cleared by high affinity complement binding receptor type 1 (CR1, CD35). SLE patients have reduced expression of CR1 on their erythrocytes, perhaps contributing to defective clearance of immune complexes.26 Fourthly, the presence of anti-C1q antibodies can influence the handling of immune complexes. These autoantibodies have been associated with the presence and activity of lupus nephritis.27,28 Infusion of anti-C1q antibodies results in deposition in glomeruli in mice, which are not pathogenic unless C1q-fixing antiglomerular basement membrane antibodies (at subnephritogenic doses) are also present.29 It is thought that anti-C1q antibodies amplify the complement cascade by themselves fixing and activating complement, recruiting further anti-C1q antibodies, and thus increasing the risk of renal damage.
Immune complexes containing DNA signal via TLR-9 and can activate plasmacytoid dendritic cells,30 which then process and present chromatin-derived peptides to costimulate T helper cells. Chromatin-specific T cells interact with dsDNA-specific B cells, facilitating the secretion of anti-DNA antibodies. Renal damage results from a combination of complement activation and cellular inflammation. A Th1 response is associated with diffuse proliferative lupus nephritis, while a Th2 response is associated with the membranous form.31 Thrombotic microangiopathy caused by antiphospholipid antibodies may also contribute to the final pathology.
Therapies of lupus nephritis targeting immune complex formation
The mainstays of treatment of lupus nephritis are corticosteroid therapy combined with cyclophosphamide or mycophenolate for induction therapy, and corticosteroids combined with azathioprine or mycophenolate for maintenance therapy. The evidence for the use of these agents is summarized in Tables 3 and 4. These agents are not specifically targeted at the reduction of DNA-anti-DNA immune complexes per se. However, a reduction in autoantibody formation and hence immune complex generation occurs after the broad immunosuppression caused by these agents, and a decrease in serum anti-dsDNA antibody levels accompanied clinical improvement in most studies listed.
Table 3.
Trials of induction therapies for lupus nephritis
| Class of nephritis5 | Study design | Intervention 1 | Intervention 2 | Number of LN patients (intervention 1 vs 2) | Duration of follow-up | Outcome | Reference |
|---|---|---|---|---|---|---|---|
| Trials of various cyclophosphamide regimens | |||||||
| IV | RCT | Prednisone (av 40 mg/d). Maintenance prednisone |
Prednisone (av 29 mg/day) plus PO CYC (average 107 mg/day) for 6 months. Maintenance prednisone | 26 vs 24 | 4 years | Lower relapse rate in PO CYC group (48% vs 14%) with steroid sparing effect of PO CYC | Donadio et al77 |
| WHO III, IV, Vc, Vd | RCT | IV CYC 0.5 g/m2 monthly (increased acc to nadir WBC to max 1.5 g) for 6 months followed by 2 quarterly pulses. AZA 2 weeks after last CYC | Low dose IV CYC: 500 mg fortnightly × 6 doses. AZA 2 weeks after last CYC | 46 vs 44 | 10 years | Similar outcomes for renal remissions, renal flares, death, doubling of creatinine (12%), ESRD (7%) | Houssiau et al (Euro-Lupus Nephritis Trial)78,79 |
| Not classified | RCT | Monthly IV CYC 750 mg/m2 for 6 months followed by quarterly IV CYC for 2 years | High dose (50 mg/kg) IV CYC for 4 days | 26 vs 21 | 30 months | 64% vs 20% complete renal response (P = 0.08) | Petri et al80 |
| Proliferative | RCT | IV CYC 10 mg/kg every 3 weeks for 4 doses. Then PO CYC 5 mg/kg for 2 days every 4 weeks for 9 months; then every 6 weeks for 12 months | PO CYC 2 mg/kg/day for 3 months then AZA 1.5 mg/kg/day | 16 vs 16 | 3.3 years | No difference in efficacy | Yee et al81 |
| Proliferative | Phase I/II pilot study | PO CYC 0.5 g/m2 BSA monthly with SC fludarabine 30 mg/m2 on days 1–3 for 3–6 cycles | – | 13 | 2.6 to 6.7 years | Severe myelosuppression – study terminated | Illei et al82 |
| Trials of mycophenolate vs IV cyclophosphamide | |||||||
| V | Pooled analysis of pure class V nephritis from two studies83,84 | MMF 2.5–3.0 g/day | IV CYC as per NIH protocol | 42 vs 42 | 6 months | Similar outcomes for urine protein, change in urine protein, complete and partial remission rates | Radhakrishnan et al85 |
| III, IV or V | RCT | MMF target dose 3 g/d | IV CYC NIH protocol; median dose received 0.75 g/m2 | 185 vs 185 | 24 weeks, maintenance phase reported below | Similar response rate (56% vs 53%) | Appel et al (ALMS group)84 |
| III, IV or V | Meta-analysis of Ginzler 200583 and Ong 200586 | MMF 1 g bid for 6 months86. MMF pushed up to 3 g daily if tolerated83 | IV CYC 0.75–1.0 g/m2 monthly for 6 months.86 NIH IV CYC83 | 90 vs 94 | 6 months86 | Complete remission rate after induction therapy higher in MMF group | Zhu et al87 |
| Miscellaneous trials of conventional immunosuppressant agents | |||||||
| Various | Retrospective review of Hopkins Lupus Cohort | Addition of tacrolimus to MMF in those failing MMF | – | 7 | 2–54 months | Frequent toxicity, infrequent success (1 patient achieved complete renal remission) | Lanata et al88 |
| WHO III, IV, Vc, Vd | RCT | AZA 2 mg/kg/day and pulse MP (3 × 3 pulses of 1 g over 2 years) | IV CYC 750 mg/m2 (13 doses over 2 years) | 37 vs 50 | 5.7 years | Relapses more frequent in AZA group (RR8.8). Higher chronicity and activity indices on repeat biopsy in AZA group | Grootscholten (Dutch Working Party on SLE)89,90; Chan91 |
| III or IV | RCT | CSA 4–5 mg/kg/d for 9 months, gradually decreasing (3.75–1.25 mg/kg/d) over next 9 months | IV CYC 8 doses of 10 mg/kg IV over 9 months, then 4–5 × PO at same dose ever 6–8 weeks | 19 vs 21 | 18 months | CSA as effective as CYC | Zavada et al (Cyclofa-Lune study)92 |
| Trials of rituximab | |||||||
| III, IV, V | Systematic review including 9 uncontrolled studies and 26 case reports (not including other papers listed in this table) | Various regimens of RTX. 52% had concomitant IV CYC | – | 103 with lupus nephritis (188 SLE in total) | 17 months | Renal response 91%. CRR 67%, PRR 33%. Higher response rate in those having concomitant CYC than those who did not. Lymphoma regimen (375 mg/m2 × 4 doses) appeared more effective | Ramos-Casals et al93 |
| III or IV | RCT | RTX monotherapy. 1000 mg IV 2 doses 2 weeks apart | RTX + IV CYC. As for group1 but with IV CYC 750 mg following the first dose of RTX | 9 vs 10 | 48 weeks | No difference in CRR (21%) or PRR (58%). Rituximab effective as induction therapy |
Li et al94 |
| WHO IV or V | Retrospective study of refractory LN | RTX 375 mg/m2 2 doses 2 weeks apart accompanied by IV CYC 500 mg each time | – | 7 with refractory LN | 18 months | 3/7 had CRR, 4/7 had PRR. Most had disease flares 6–12 months after B cell repopulation | Lateef et al95 |
| WHO III or IV (not all biopsied) | Observational | RTX 1000 mg days 1 and 15. Added to current immunosuppressive treatment | – | 13 Hispanic with active lupus nephritis | 6 months | 38% CRR, 38% PRR | Garcia-Carrasco et al96 |
| WHO III–V | Retrospective | RTX 275 mg/m2 weekly for 4 doses; IV CYC 500–100 mg 3 weeks apart for 2 doses | – | 28 (WHO III and IV) and 15 (WHO V) | 12 months | Membranous and proliferative LN respond similarly to rituximab | Jonsdottir et al97 |
| ISN III or IV | RDBPCT | RTX 1000 mg on days 1 and 15; repeated at 6 months. Background MMF target dose 3 g/day | Placebo + MMF target dose 3 g/day | 72 vs 72 | – | No difference in renal response despite better serological response in rituximab group | Furie et al (LUNAR)33; Looney34 |
| ISN III-V | Prospective observational registry | RTX, various protocols | – | 42 | >3 months | CRR in 45%, PRR in 29% (total renal response rate 74%) | Terrier et al (French AutoImmunity and Rituximab Registry)36 |
Note: All studies are with corticosteroids in both arms, unless specified.
Abbreviations: AZA, azathioprine; bid, twice daily; CRF, chronic renal failure; CRR, complete renal response; CSA, cyclosporine A; CYC, cyclophosphamide; ESRD, end stage renal disease; IV, intravenous; LN, lupus nephritis; MMF, mycophenolate mofetil; PO, per oral; PRR, partial renal response; RCT, randomized controlled trial; RDBPCT, randomized double-blinded placebo-controlled trial; RTX, rituximab.
Table 4.
Trials of maintenance therapies in lupus nephritis. All received glucocorticoids unless otherwise specified
| Class of nephritis5 | Study design | Induction | Maintenance strategy 1 | Maintenance strategy 2 | Number of LN patients (strategy 1 vs 2 vs 3) | Duration of follow-up | Outcome | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| WHO III, IV, Vb | RCT | IV CYC 0.5–1.0 g/m2 monthly for 7 doses | AZA 1–3 mg/kg day | MMF 1.5 g/day for 12 months then weaned | Maintenance strategy 3: IV CYC 0.5–1.0 g/m2 every 3 months | 19 vs 20 vs 20 | 72 months | Relapse free survival highest in MMF group (77%) vs AZA (57%) and IV CYC (43%). MMF and AZA better for composite endpoint of death or CRF | Contreras et al98,99 |
| III–V | Retrospective study | IV CYC | AZA 2 mg/kg/day | MMF 1.5–2.0 g/day | 15 vs 17 | 41 months | CRR similar (60% vs 58%) | Sahin et al100 | |
| Proliferative | RCT | Eurolupus IV CYC (500 mg × 6 fortnightly doses). Maintenance Rx started at week 12. Renal response not required prior to commencing maintenance | AZA 2 mg/kg/day target dose | MMF 2 g/day target dose | 52 vs 53 | 14 months | Renal relapse rate similar (25% vs 19%) | Houssiau et al (MAINTAIN trial)101 | |
| III–V | RDBPCT | MMF vs IV CYC. Patients who achieved partial or complete response re-randomized at week 24 | AZA 2 mg/kg/day | MMF 2 g/day | 227 | Not yet published in full | MMF superior to AZA in delaying time to treatment failure (composite of death, serious renal damage, renal relapse) | Wofsy et al (ALMS group)102 | |
| WHO IV,Vc,Vd | RCT | PO CYC 1–2 mg/kg/day for 3 months | AZA 2 mg/kg/day for 1 month then optional reduction to 1.5 mg/kg/day if well controlled | CSA 4 mg/kg/day for 1 month then weaned to 2.5–3.0 mg/kg/day keeping trough level of 75–200 ng/mL | 33 vs 36 | 4 years | Similar rates of SLE flare (13.4 vs 10.6 flares per 100 patient years), proteinuria and creatinine clearance | Moroni et al103 | |
Abbreviations: see Table 3.
Specific strategies for targeting immune complex formation include: reducing autoantibody production (targeting B cells), reducing the binding of autoantibodies, reducing the availability of nucleosomal material, increasing the clearance of immune complexes, and interfering in the feedback loop (Figure 1). This is a theoretical framework, and while the mechanisms of action of some of the currently used treatments for lupus nephritis may fall into these categories, further research is necessary in each of these areas to understand their mechanisms and potential clinical efficacy.
Reducing autoantibody production (targeting B cells and plasma cells)
Theoretically, autoantibody production may be reduced by depletion of B cells (either by targeting B cell surface molecules or by removing factors required for B cell survival); interfering with the development or function of plasma cells; or by inducing B-cell tolerance.
B cell depletion
Anti-nucleosome and anti-dsDNA antibodies are modestly reduced by anti-CD20 mAbs such as rituximab, which effect B cell depletion. The reduction in these titers suggests that these autoantibodies are produced by a B cell population with more rapid turnover than cells that produce anti-ENA, anti-tetanus or antipneumococcal antibodies, which persist. However, their incomplete reduction may reflect the presence of longer-lived plasma cells which do not express CD20.32 Trials of rituximab, however, have yielded conflicting results on clinical endpoints (see Table 3). Uncontrolled, observational, and retrospective studies seemed to demonstrate benefit in lupus nephritis, but two major randomized trials failed to find benefit. The LUpus Nephritis Assessment with Rituximab (LUNAR) trial, which specifically included patients with proliferative lupus nephritis, did not demonstrate any difference in the proportion of patients obtaining a renal response to rituximab compared with placebo.33 However, the use of mycophenolate rather than cyclophosphamide as background therapy in this trial has been criticized, as it is thought that the effects of rituximab may be enhanced, or synergistic, with cyclophosphamide.34 Further, given that all participants were treated with mycophenolate, any effect of rituximab may have been masked. The other major trial, Rituximab in patients with Severe Systemic Lupus Erythematosus (EXPLORER),35 excluded major organ threatening disease and thus <2% of the patients had renal involvement. This trial found no difference in the rituximab compared with placebo-treated groups, but given its patient characteristics, this finding cannot be applied to patients with lupus nephritis. Contrary to these findings, prospective follow-up of 31 patients with lupus nephritis from a cohort of 136 patients entered in the French Autoimmunity and Rituximab registry,36 demonstrated renal response in 74% of patients, with complete response in 45%. Unfortunately, trials of another anti-CD20 agent, ocrelizumab (BELONG), for lupus nephritis have been halted due to concerns over serious and opportunistic infections.37
A plethora of other anti-B cell therapies is on the horizon, targeting all aspects important for B cell existence and function, such as survival factors, differentiation factors, co-stimulatory factors, cell-signaling pathways, and homing factors.38 Most of these studies are in preliminary phases, or have not been evaluated in human lupus nephritis. Belimumab, a fully human recombinant monoclonal antibody that binds to and inhibits B lymphocyte stimulator (BLyS, also known as B cell activating factor or BAFF) has been shown to reduce anti-dsDNA titers by 29%, but patients with lupus nephritis were excluded from early trials.39,40 More recently, the large phase III studies, BLISS-52 and BLISS-76, have shown promise with improvements in the SLE responder index, though more information on lupus nephritis and belimumab is awaited. The Food and Drug Administration has granted this drug a priority review designation as a potential treatment for SLE (GSK press release August 19, 2010). Epratuzumab, targets CD22, a surface molecule involved in regulating B cell receptor signaling, and modifies B cell function. A phase II study has had promising results, with improvements in BILAG scores despite lack of reduction in anti-dsDNA levels, although there were too few patients with lupus nephritis to draw any conclusions about efficacy in this domain.41
Targeting plasma cells
If B cell depletion does indeed reduce immune complexes it may do so indirectly by killing the precursor germinal center B cells that give rise to antibody-secreting plasma cells. To reduce autoantibody production more effectively, agents targeting plasma cells specifically may be more useful. Indeed, corticosteroids may well exert their beneficial effect by this mechanism, among others. Proteasome inhibitors have been introduced into the therapeutic armamentarium for multiple myeloma due to their ability to cause apoptosis of plasma cells.42 The use of proteasome inhibitors in SLE has been promising in mouse models, eliminating autoreactive plasma cells, reducing anti-dsDNA antibody levels, and preventing nephritis;43 human trials are underway.
Induction of B cell tolerance
Induction of tolerance would be the ultimate way to reduce anti-dsDNA antibody concentrations. Although murine models have provided hope, human trials have again been unimpressive. Regular injections of nucleosomal peptide autoepitopes in lupus-prone mice reduced autoantibody levels and delayed the onset of nephritis by the induction of TGF-producing regulatory T cells.44 However, abetimus, a conjugate composed of 4 identical strands of dsDNA, did not show any benefit in reducing renal flares in human SLE. Interestingly, abetimus did reduce the level of anti-dsDNA antibodies, possibly due to the formation of soluble complexes that were rapidly eliminated and, possibly, by tolerizing B cells and reducing autoantibody production.45
Reducing the binding of autoantibodies
The mechanism of action of the antimalarials chloroquine and hydroxychloroquine in SLE has recently been revisited, because of the recognition of their inhibition of TLR-9 binding to DNA, by preventing acidification of the lysosome. However, hydroxychloroquine, as one of its many mechanisms of action, also affects the affinity of binding of antibodies to their targets. Hydroxychloroquine interferes with the binding of antiphospholipid antibodies in vitro, and causes a reduction in the levels of these autoantibodies as measured by commercially available ELISAs.46 We recently demonstrated that the binding of anti-dsDNA antibodies as measured by the modified Farr assay is reduced by the addition of hydroxychloroquine in vitro.47 This effect is likely to be due to the high protein-binding capacity of hydroxychloroquine,48 and intercalation of DNA (if sharing this property with chloroquine),49,50 potentially modifying critical autoepitopes. Whether this affects the pathogenesis of human lupus nephritis is unknown.
Reducing the availability of DNA and nucleosomal material
Material for anti-dsDNA and antinucleosome antibodies to bind may originate from tissue damage in the kidneys, resulting in situ formation of complexes or, alternatively, from damage remotely, resulting in the formation of circulating immune complexes, which then deposit in glomeruli (reviewed by Fismen et al51). A phase Ib trial of recombinant human DNase I (rhDNase) to hydrolyze extracellular DNA in patients with lupus did not reduce anti-dsDNA levels, the concentrations of circulating immune complexes, nor change other serological markers.52 No further studies of rhDNase have been published.
Increasing the clearance of immune complexes
Plasmapheresis is able to lower the titer of anti-dsDNA antibodies but does not necessarily result in sustained clinical remission once withdrawn, possibly due to compensatory increased production by pathogenic B cell clones (rebound effect).53 Removal of pathogenic anti-dsDNA antibodies physically by plasmapheresis may improve outcomes for those receiving intravenous (IV) cyclophosphamide. A combination of plasmapheresis and IV cyclophosphamide results in higher rates of complete renal remission than IV cyclophosphamide alone.54,55 However, not all trials have found benefit, and larger randomized trials are required to confirm these findings. Other small studies and case reports have also demonstrated benefit with immunoadsorption plasmapheresis,56,57 but further investigation is required to clarify the role of this treatment.
Breaking the feedback (amplification) loop
Signaling of immune complexes containing RNA and DNA via TLRs 7 and 9, respectively, activates plasmacytoid dendritic cells to produce large amounts of type I interferon. Type I interferons activate B cells and enhance antibody responses to soluble proteins, thereby completing a feedback loop resulting in the increased production of immune complexes.58 One of the mechanisms of action for antimalarial drugs such as hydroxychloroquine in lupus is thought to be the inhibition of nucleic acid interaction with intracellular TLRs 7 and 9, possibly as a result of an increase in pH in microsomal compartments.59 Novel treatments aimed at blocking TLR7 and TLR9 are being developed.60 A phase I study of an anti-interferon-α monoclonal antibody has been completed,61 and a phase II study is underway.
Conclusion
Immune complexes containing IgG anti-dsDNA antibodies and DNA play a significant role in the complex pathogenesis of lupus nephritis. Although strategies specifically aimed at reducing immune complexes in SLE are mostly novel, they provide a fertile area for further research. Disappointments with early trials of new therapeutics strengthen the argument that a combination of strategies aimed at different pathogenic mechanisms is likely to be necessary to improve the prognosis of this disease.
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- 1.Rahman A, Isenberg DA. Mechanisms of disease: systemic lupus erythematosus. N Engl J Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 2.Radic M, Marion T, Monestier M. Nucleosomes are exposed at the cell surface in apoptosis. J Immunol. 2004;172:6692–6700. doi: 10.4049/jimmunol.172.11.6692. [DOI] [PubMed] [Google Scholar]
- 3.Biesecker G, Katz S, Koffler D. Renal localization of the membrane attack complex in systemic lupus erythematosus nephritis. J Exp Med. 1981;154:1779–1794. doi: 10.1084/jem.154.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ortega LM, Schultz DR, Lenz O, et al. Lupus nephritis: pathologic features, epidemiology, and a guide to therapeutic decisions. Lupus. 2010;19:557–574. doi: 10.1177/0961203309358187. [DOI] [PubMed] [Google Scholar]
- 5.Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–530. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 6.Najafi CC, Korbet SM, Lewis EJ, et al. Significance of histologic patterns of glomerular injury upon long-term prognosis in severe lupus glomerulonephritis. Kidney Int. 2001;59:2156–2163. doi: 10.1046/j.1523-1755.2001.00730.x. [DOI] [PubMed] [Google Scholar]
- 7.Borchers AT, Keen CL, Shoenfeld Y, et al. Surviving the butterfly and the wolf: mortality trends in systemic lupus erythematosus. Autoimmun Rev. 2004;3:423–453. doi: 10.1016/j.autrev.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Cervera R, Khamashta MA, Font J, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003;82:299–308. doi: 10.1097/01.md.0000091181.93122.55. [DOI] [PubMed] [Google Scholar]
- 9.Levinsky RJ, Cameron JS, Soothill JF. Serum immune complexes and disease activity in lupus nephritis. Lancet. 1977;1:564–567. doi: 10.1016/s0140-6736(77)91998-5. [DOI] [PubMed] [Google Scholar]
- 10.Mortensen ES, Rekvig OP. Nephritogenic potential of anti-DNA antibodies against necrotic nucleosomes. J Am Soc Nephrol. 2009;20:696–704. doi: 10.1681/ASN.2008010112. [DOI] [PubMed] [Google Scholar]
- 11.Cruchaud A, Chenais F, Fournie GJ, et al. Immune complex deposits in systemic lupus erythematosus kidney without histological or functional alterations. Eur J Clin Invest. 1975;1975:297–309. doi: 10.1111/j.1365-2362.1975.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 12.Forger F, Matthias T, Oppermann M, et al. Clinical significance of anti-dsDNA antibody isotypes: IgG/IgM ratio of anti-dsDNA antibodies as a prognostic marker for lupus nephritis. Lupus. 2004;13:36–44. doi: 10.1191/0961203304lu485oa. [DOI] [PubMed] [Google Scholar]
- 13.Amoura Z, Koutouzov S, Chabre H, et al. Antinucleosome antibodies of the IgG3 subclass are markers of renal pathogenicity in systemic lupus erythematosus. Arthritis Rheum. 2000;43:76–84. doi: 10.1002/1529-0131(200001)43:1<76::AID-ANR10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 14.Bijl M, Dijstelbloem HM, Oost WW, et al. IgG subclass distribution of autoantibodies differs between renal and extra-renal relapses in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2002;41:62–67. doi: 10.1093/rheumatology/41.1.62. [DOI] [PubMed] [Google Scholar]
- 15.Villalta D, Romelli PB, Savina C, et al. Anti-dsDNA antibody avidity determination by a simple reliable ELISA method for SLE diagnosis and monitoring. Lupus. 2003;12:31–36. doi: 10.1191/0961203303lu277oa. [DOI] [PubMed] [Google Scholar]
- 16.Jaekel H-P, Trabandt A, Grobe N, et al. Anti-dsDNA antibody subtypes and anti-C1q antibodies: toward a more reliable diagnosis and monitoring of systemic lupus erythematosus and lupus nephritis. Lupus. 2006;15:335–345. doi: 10.1191/0961203306lu2308oa. [DOI] [PubMed] [Google Scholar]
- 17.Fenton KA, Tommeras B, Marion TN, et al. Pure anti-dsDNA mAbs need chromatin structures to promote glomerular mesangial deposits in BALB/c mice. Autoimmunity. 2010;43:179–188. doi: 10.3109/08916930903305633. [DOI] [PubMed] [Google Scholar]
- 18.Kohro-Kawata J, Wang P, Kawata Y, et al. Highly cationic anti-DNA antibodies in patients with lupus nephritis analyzed by two-dimensional electrophoresis and immunoblotting. Electrophoresis. 1998;19:1511–1515. doi: 10.1002/elps.1150190849. [DOI] [PubMed] [Google Scholar]
- 19.Mostoslavsky G, Fischel R, Yachimovich N, et al. Lupus anti-DNA autoantibodies cross-react with a glomerular structural protein: a case for tissue injury by molecular mimicry. Eur J Immunol. 2001;31:1221–1227. doi: 10.1002/1521-4141(200104)31:4<1221::aid-immu1221>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 20.Zykova SN, Seredkina N, Benjaminsen J, et al. Reduced fragmentation of apoptotic chromatin is associated with nephritis in lupus-prone (NZB x NZW)F(1) mice. Arthritis Rheum. 2008;58:813–825. doi: 10.1002/art.23276. [DOI] [PubMed] [Google Scholar]
- 21.Lee YH, Ji JD, Song GG. Fcgamma receptor IIB and IIIB polymorphisms and susceptibility to systemic lupus erythematosus and lupus nephritis: a meta-analysis. Lupus. 2009;18(8):727–734. doi: 10.1177/0961203309104020. [DOI] [PubMed] [Google Scholar]
- 22.Bergtold A, Gavhane A, D’Agati V, et al. FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis. J Immunol. 2006;177:7287–7295. doi: 10.4049/jimmunol.177.10.7287. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Lee PY, Sobel ES, et al. Increased expression of FcγRI/CD64 on circulating monocytes parallels ongoing inflammation and nephritis in lupus. Arthritis Res Ther. 2009;11:R6. doi: 10.1186/ar2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clynes R, Calvani N, Croker BP, et al. Modulation of immune response in pristane-induced lupus by expression of activation and inhibitory Fc receptors. Clin Exp Immunol. 2005;141:230–237. doi: 10.1111/j.1365-2249.2005.02847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karp DR. Complement and systemic lupus erythematosus. Curr Opin Rheumatol. 2005;17:538–542. doi: 10.1097/01.bor.0000172799.03379.86. [DOI] [PubMed] [Google Scholar]
- 26.Kavai M. Immune complex clearance by complement receptor type 1 in SLE. Autoimmun Rev. 2008;8:160–164. doi: 10.1016/j.autrev.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Sinico RA, Ikehata M, Giammarresi G, et al. Anti-C1q autoantibodies in lupus nephritis: prevalence and clinical significance. Ann N Y Acad Sci. 2005;1050:193–200. doi: 10.1196/annals.1313.020. [DOI] [PubMed] [Google Scholar]
- 28.Trendelenburg M, Lopez-Trascasa M, Potlukova E, et al. High prevalence of anti-C1q antibodies in biopsy-proven active lupus nephritis. Nephrol Dial Transplant. 2006;21:3115–3121. doi: 10.1093/ndt/gfl436. [DOI] [PubMed] [Google Scholar]
- 29.Trouw LA, Groeneveld TWL, Seelen MA, et al. Anti-C1q autoantibodies desposit in glomeruli but are only pathogenic in combination with glomerular C1q-containing immune complexes. J Clin Invest. 2004;114:679–688. doi: 10.1172/JCI21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boule MW, Broughton C, Mackay F, et al. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaveri SV, Mouthon L, Bayry J. Basophils and nephritis in lupus. N Engl J Med. 2010;363:1080–1082. doi: 10.1056/NEJMcibr1006936. [DOI] [PubMed] [Google Scholar]
- 32.Cambridge G, Leandro MJ, Teodorescu M, et al. B cell depletion therapy in systemic lupus erythematosus: effect on autoantibody and antimicrobial antibody profiles. Arthritis Rheum. 2006;54:3612–3622. doi: 10.1002/art.22211. [DOI] [PubMed] [Google Scholar]
- 33.Furie R, Looney R, Rovin B, et al., editors. Efficacy and safety of ritux-imab in subjects with active proliferative lupus nephritis (LN): results from the randomized, double-blind phase III LUNAR study. American College of Rheumatology National Meeting 2009; Philadelphia (PA). 2009 Oct 17–21; Abstract 1149. [Google Scholar]
- 34.Looney RJ. B cell-targeted therapies for systemic lupus erythematosus: an update on clinical trial data. Drugs. 2010;70:529–540. doi: 10.2165/11535420-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 62:222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terrier B, Amoura Z, Ravaud P, et al. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum. 62:2458–2466. doi: 10.1002/art.27541. [DOI] [PubMed] [Google Scholar]
- 37.Biogen Idec; 2010. Roche and Biogen Idec decide to suspend ocrelizumab treatment – rheumatoid arthritis development programme on hold. http://www.biogenidec.com/PRESS_RELEASE_DETAILS.aspx?ID=5981&ReqId=1400446. Accessed 2010 Oct 12. [Google Scholar]
- 38.Sanz I, Lee FE. B cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6:326–337. doi: 10.1038/nrrheum.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallace DJ, Stohl W, Furie RA, et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Care Res. 2009;61:1168–1178. doi: 10.1002/art.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobi AM, Huang W, Wang T, et al. Effect of long-term belimumab treatment on B cells in systemic lupus erythematosus: extension of a phase II, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2010;62:201–210. doi: 10.1002/art.27189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorner T, Kaufmann J, Wegener WA, et al. Initial clinical trial of epratuzumab (humanized anti-CD22 antibody) for immunotherapy of systemic lupus erythematosus. Arthritis Res Ther. 2006;8:R74. doi: 10.1186/ar1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dingli D, Rajkumar SV. How best to use new therapies in multiple myeloma. Blood Rev. 2010;24:91–100. doi: 10.1016/j.blre.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neubert K, Meister S, Moser K, et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14:748–755. doi: 10.1038/nm1763. [DOI] [PubMed] [Google Scholar]
- 44.Kang H-K, Michaels MA, Berner BR, et al. Very low-dose tolerance with nucleosomal peptides controls lupus and induces regulatory T cell subsets. J Immunol. 2005;174:3247–3255. doi: 10.4049/jimmunol.174.6.3247. [DOI] [PubMed] [Google Scholar]
- 45.Cardiel MH, Tumlin JA, Furie RA, et al. Abetimus sodium for renal flare in systemic lupus erythematosus: results of a randomized, controlled phase III trial. Arthritis Rheum. 2008;58:2470–2480. doi: 10.1002/art.23673. [DOI] [PubMed] [Google Scholar]
- 46.Rand JH, Wu X-X, Quinn AS, et al. Hydroxychloroquine directly reduces the binding of antiphospholipid antibody-β2-glycoprotein I complexes to phospholipid bilayers. Blood. 2008;112:1687–1695. doi: 10.1182/blood-2008-03-144204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toong C, Wienholt L, Adelstein S. Hydroxychloroquine reduces binding of anti-double stranded DNA antibodies in vitro. Intern Med J. 2010;40(Suppl 4):17. [Google Scholar]
- 48.McLachlan AJ, Cutler DJ, Tett SE. Plasma protein binding of the enantiomers of hydroxychloroquine and metabolites. Eur J Clin Pharmacol. 1993;44:481–484. doi: 10.1007/BF00315548. [DOI] [PubMed] [Google Scholar]
- 49.Scaria PV, Craig JC, Shafer RH. Differential binding of the enantiomers of chloroquine and quinacrine to polynucleotides: implications for stereoselective metabolism. Biopolymers. 1993;33:887–895. doi: 10.1002/bip.360330604. [DOI] [PubMed] [Google Scholar]
- 50.Kwakye-Berko F, Meshnick S. Sequence preference of chloroquine binding to DNA and prevention of Z-DNA formation. Mol Biochem Paristol. 1990;39:275–278. doi: 10.1016/0166-6851(90)90066-u. [DOI] [PubMed] [Google Scholar]
- 51.Fismen S, Mortensen ES, Rekvig OP. Nuclease deficiencies promote end-stage lupus nephritis but not nephritogenic autoimmunity in (NZBxNZW) F1 mice. Immunol Cell Biol. 2010 Jun 15; doi: 10.1038/icb.2010.75. [DOI] [PubMed] [Google Scholar]
- 52.Davis JC, Jr, Manzi S, Yarboro C, et al. Recombinant human Dnase I (rhDNase) in patients with lupus nephritis. Lupus. 1999;8:68–76. doi: 10.1191/096120399678847380. [DOI] [PubMed] [Google Scholar]
- 53.Mistry-Burchardi N, Schonermark U, Samtleben W. Apheresis in lupus nephritis. Ther Apher. 2001;5:161–170. [PubMed] [Google Scholar]
- 54.Yamaji K, Kim YJ, Tsuda H, et al. Long-term clinical outcomes of synchronized therapy with plasmapheresis and intravenous cyclophosphamide pulse therapy in the treatment of steroid-resistant lupus nephritis. Ther Apher Dial. 2008;12(4):298–305. doi: 10.1111/j.1744-9987.2008.00591.x. [DOI] [PubMed] [Google Scholar]
- 55.Danieli MG, Palmieri C, Salvi A, et al. Synchronised therapy and high-dose cyclophosphamide in proliferative lupus nephritis. J Clin Apher. 2002;17:72–77. doi: 10.1002/jca.10020. [DOI] [PubMed] [Google Scholar]
- 56.Sugimoto K, Yamaji K, Yang KS, et al. Immunoadsorption plasmapheresis using a phenylalanine column as an effective treatment for lupus nephritis. Ther Apher Dial. 2006;10:187–192. doi: 10.1111/j.1744-9987.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi S, Wada N, Harada K. Immunoadsorbent apheresis eliminates pathogenic IgG in childhood lupus nephritis. Pediatr Int. 2007;49:817–821. doi: 10.1111/j.1442-200X.2007.02452.x. [DOI] [PubMed] [Google Scholar]
- 58.Pascual V, Farkas L, Banchereau J. Systemic lupus erythematosus: all roads lead to type I interferons. Curr Opin Immunol. 2006;18:676–682. doi: 10.1016/j.coi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 59.Lafyatis R, York M, Marshak-Rothstein A. Antimalarial agents: closing the gate on Toll-like receptors? Arthritis Rheum. 2006;54:3068–3070. doi: 10.1002/art.22157. [DOI] [PubMed] [Google Scholar]
- 60.Fulmer T. Sparing steroids in lupus. SciBX. 2010;3:1–2. [Google Scholar]
- 61.Yao Y, Richman L, Higgs BW, et al. Neutralizaiton of interferon-α/β-inducible genes and downstream effect in a phase I trial of an anti-inteferon-α monoclonal antibody in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1785–1796. doi: 10.1002/art.24557. [DOI] [PubMed] [Google Scholar]
- 62.Madaio MP, Carlson J, Cataldo J, et al. Murine monoclonal anti-DNA antibodies bind directly to glomerular antigens and form immune deposits. J Immunol. 1987;138:2883–2889. [PubMed] [Google Scholar]
- 63.Chan TM, Leung JK, Ho SK, et al. Mesangial cell-binding anti-DNA antibodies in patients with systemic lupus erythematosus. J Am Soc Nephrol. 2002;13:1219–1229. doi: 10.1097/01.asn.0000014223.71109.13. [DOI] [PubMed] [Google Scholar]
- 64.Du H, Chen M, Zhang Y, et al. Cross-reaction of anti-DNA autoantibodies with membrane proteins of human glomerular mesangial cells in sera from patients with lupus nephritis. Clin Exp Immunol. 2006;145:21–27. doi: 10.1111/j.1365-2249.2006.03102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kramers C, Hylkema MN, van Bruggen MCJ, et al. Anti-nucleosome antibodies complexed to nucleosomal antigens show anti-DNA reactivity and bind to rat glomerular basement membrane in vivo. J Clin Invest. 1994;94:568–577. doi: 10.1172/JCI117371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morioka T, Fujigaki Y, Batsford SR, et al. Anti-DNA antibody derived from a systemic lupus erythematosus (SLE) patient forms histone-DNA-anti-DNA complexes that bind to rat glomeruli in vivo. Clin Exp Immunol. 1996;104:92–96. doi: 10.1046/j.1365-2249.1996.d01-658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsao BP, Ohnishi K, Cheroutre H, et al. Failed self-tolerance and autoimmunity in IgG anti-DNA transgenic mice. J Immunol. 1992;149:350–358. [PubMed] [Google Scholar]
- 68.Raz E, Brezis M, Rosenmann E, et al. Anti-DNA antibodies bind directly to renal antigens and induce kidney dysfunction in the isolated perfused rat kidney. J Immunol. 1989;142:3076–3082. [PubMed] [Google Scholar]
- 69.Ehrenstein MR, Katz DR, Griffiths MH, et al. Human IgG anti-DNA antibodies deposit in kidneys and induce proteinuria in SCID mice. Kidney Int. 1995;48:705–711. doi: 10.1038/ki.1995.341. [DOI] [PubMed] [Google Scholar]
- 70.Ravirajan CT, Rahman MA, Papadaki L, et al. Genetic, structural and functional properties of an IgG DNA-binding monoclonal antibody from a lupus patient with nephritis. Eur J Immunol. 1998;28:339–350. doi: 10.1002/(SICI)1521-4141(199801)28:01<339::AID-IMMU339>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 71.Kalaaji M, Fenton KA, Mortensen ES, et al. Glomerular apoptotic nucleosomes are central target structures for nephritogenic antibodies in human SLE nephritis. Kidney Int. 2007;71:664–672. doi: 10.1038/sj.ki.5002133. [DOI] [PubMed] [Google Scholar]
- 72.Casals SP, Friou GJ, Myers LL. Significance of antibody to DNA in systemic lupus erythematosus. Arthritis Rheum. 1964;7:379–390. doi: 10.1002/art.1780070404. [DOI] [PubMed] [Google Scholar]
- 73.Cervera R, Vinas O, Ramos-Casals M, et al. Anti-chromatin antibodies in systemic lupus erythematosus: a useful marker for lupus nephropathy. Ann Rheum Dis. 2003;62:431–434. doi: 10.1136/ard.62.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.ter Borg EJ, Horst G, Hummel EJ, et al. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus: A long-term, prospective study. Arthritis Rheum. 1990;33(5):634–643. doi: 10.1002/art.1780330505. [DOI] [PubMed] [Google Scholar]
- 75.Adler MK, Baumgarten A, Hecht B. Prognostic significance of DNA-binding capacity patterns in patients with lupus nephritis. Ann Rheum Dis. 1975;34:444–450. doi: 10.1136/ard.34.5.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Horowitz DM, Furie RA. Abetimus sodium: a medication for the prevention of lupus nephritis flares. Expert Opin Pharmacother. 2009;10:1501–1507. doi: 10.1517/14656560902946419. [DOI] [PubMed] [Google Scholar]
- 77.Donadio JVJ, Holley KE, Ferguson RH, et al. Treatment of diffuse proliferative lupus nephritis with prednisone and combined prednisone and cyclophosphamide. N Engl J Med. 1978;299:1151–1155. doi: 10.1056/NEJM197811232992102. [DOI] [PubMed] [Google Scholar]
- 78.Houssiau FA, Vasconcelos C, D’Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46:2121–2131. doi: 10.1002/art.10461. [DOI] [PubMed] [Google Scholar]
- 79.Houssiau FA, Vasconcelos C, D’Cruz D, et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis. 2010;69:61–64. doi: 10.1136/ard.2008.102533. [DOI] [PubMed] [Google Scholar]
- 80.Petri M, Brodsky RA, Jones RJ, et al. High-dose cyclophosphamide versus monthly intravenous cyclophosphamide for systemic lupus erythematosus: a prospective randomized trial. Arthritis Rheum. 2010;62:1487–1493. doi: 10.1002/art.27371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yee CS, Gordon C, Dostal C, et al. EULAR randomised controlled trial of pulse cyclophosphamide and methylprednisolone versus continuous cyclophosphamide and prednisolone followed by azathioprine and prednisolone in lupus nephritis. Ann Rheum Dis. 2003;63:525–529. doi: 10.1136/ard.2002.003574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Illei GG, Yarboro CH, Kuroiwa T, et al. Long-term effects of combination treatment with fludarabine and low-dose pulse cyclophosphamide in patients with lupus nephritis. Rheumatology (Oxford) 2007;46:952–956. doi: 10.1093/rheumatology/kem001. [DOI] [PubMed] [Google Scholar]
- 83.Ginzler EM, Dooley MA, Aranow C, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353:2219–2228. doi: 10.1056/NEJMoa043731. [DOI] [PubMed] [Google Scholar]
- 84.Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20:1103–1112. doi: 10.1681/ASN.2008101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Radhakrishnan J, Moutzouris D-A, Ginzler EM, et al. Mycophenolate mofetil and intravenous cyclophosphamide are similar as induction therapy for class V lupus nephritis. Kidney Int. 2010;77:152–160. doi: 10.1038/ki.2009.412. [DOI] [PubMed] [Google Scholar]
- 86.Ong LM, Hooi LS, Lim TO, et al. Randomized controlled trial of pulse intravenous cyclophosphamide versus mycophenolate mofetil in the induction therapy of proliferative lupus nephritis. Nephrology. 2005;10:504–510. doi: 10.1111/j.1440-1797.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- 87.Zhu B, Chen N, Lin Y, et al. Mycophenolate mofetil in induction and maintenance therapy of severe lupus nephritis: a meta-analysis of randomized controlled trials. Nephrol Dial Transplant. 2007;22:1933–1942. doi: 10.1093/ndt/gfm066. [DOI] [PubMed] [Google Scholar]
- 88.Lanata CM, Mahmood T, Fine DM, et al. Combination therapy of mycophenolate mofetil and tacrolimus in lupus nephritis. Lupus. 2010;19:935–940. doi: 10.1177/0961203310365714. [DOI] [PubMed] [Google Scholar]
- 89.Grootscholten C, Ligtenberg G, Hagen EC, et al. Azathioprine/methylprednisolone versus cyclophosphamide in proliferative lupus nephritis. A randomized controlled trial. Kidney Int. 2006;70:732–742. doi: 10.1038/sj.ki.5001630. [DOI] [PubMed] [Google Scholar]
- 90.Grootscholten C, Bajema IM, Florquin S, et al. Treatment with cyclophosphamide delays the progression of chronic lesions more effectively than does treatment with azathioprine plus methylprednisolone in patients with proliferative lupus nephritis. Arthritis Rheum. 2007;56:924–937. doi: 10.1002/art.22449. [DOI] [PubMed] [Google Scholar]
- 91.Chan TM. Histologic deterioration and more flares: the case against azathioprine plus methylprednisolone in the treatment of proliferative lupus nephritis. Arthritis Rheum. 2007;56:702–704. doi: 10.1002/art.22448. [DOI] [PubMed] [Google Scholar]
- 92.Zavada J, Pesickova S, Rysava R, et al. Cyclosporine A or intravenous cyclophosphamide for lupus nephritis: the Cyclofa-Lune study. Lupus. 2010;19:1281–1289. doi: 10.1177/0961203310371155. [DOI] [PubMed] [Google Scholar]
- 93.Ramos-Casals M, Soto MJ, Cuadrado MJ, et al. Rituximab in systemic lupus erythematosus: A systematic review of off-label use in 188 cases. Lupus. 2009;18:767–776. doi: 10.1177/0961203309106174. [DOI] [PubMed] [Google Scholar]
- 94.Li EK, Tam LS, Zhu TY, et al. Is combination rituximab with cyclophosphamide better than rituximab alone in the treatment of lupus nephritis? Rheumatology (Oxford) 2009;48:892–898. doi: 10.1093/rheumatology/kep124. [DOI] [PubMed] [Google Scholar]
- 95.Lateef A, Lahiri M, Teng GG, et al. Use of rituximab in the treatment of refractory systemic lupus erythematosus: Singapore experience. Lupus. 2010;19:765–770. doi: 10.1177/0961203309358599. [DOI] [PubMed] [Google Scholar]
- 96.Garcia-Carrasco M, Mendoza-Pinto C, Sandoval-Cruz M, et al. Anti-CD20 therapy in patients with refractory systemic lupus erythematosus: a longitudinal analysis of 52 Hispanic patients. Lupus. 2010;19:213–219. doi: 10.1177/0961203309351541. [DOI] [PubMed] [Google Scholar]
- 97.Jonsdottir T, Gunnarsson I, Mourao AF, et al. Clinical improvements in proliferative vs membranous lupus nephritis following B-cell depletion: pooled data from two cohorts. Rheumatology (Oxford) 2010;49:1502–1504. doi: 10.1093/rheumatology/keq055. [DOI] [PubMed] [Google Scholar]
- 98.Contreras G, Pardo V, Leclercq B, et al. Sequential therapies for proliferative lupus nephritis. N Engl J Med. 2004;350:971–980. doi: 10.1056/NEJMoa031855. [DOI] [PubMed] [Google Scholar]
- 99.Contreras G, Tozman E, Nahar N, et al. Maintenance therapies for proliferative lupus nephritis: mycophenolate mofetil, azathioprine and intravenous cyclophosphamide. Lupus. 2005;14:s33–s38. doi: 10.1191/0961203305lu2115oa. [DOI] [PubMed] [Google Scholar]
- 100.Sahin GM, Sahin S, Kiziltas S, et al. Mycophenolate mofetil versus azathioprine in the maintenance therapy of lupus nephritis. Renal Failure. 2008;30:865–869. doi: 10.1080/08860220802353843. [DOI] [PubMed] [Google Scholar]
- 101.Houssiau F, D’Cruz D, Sangle S, et al. the MAINTAIN Nephritis Trial Group Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial. Ann Rheum Dis. 2010;69:2083–2089. doi: 10.1136/ard.2010.131995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wofsy D, Appel GB, Dooley MA, et al. Aspreva Lupus Management Study maintenance results. Lupus. 2010;19(Suppl):27. (CS12.6). [Google Scholar]
- 103.Moroni G, Doria A, Mosca M, et al. A randomized pilot trial comparing cyclosporine and azathioprine for maintenance therapy in diffuse lupus nephritis over four years. Clin J Am Soc Nephrol. 2006;1:925–932. doi: 10.2215/CJN.02271205. [DOI] [PubMed] [Google Scholar]