Abstract
Connexins form hemichannels at undocked plasma membranes and gap-junction channels (GJCs) at intercellular contacting zones. Under physiological conditions, hemichannels have low open probabilities, but their activation under pathological conditions, such as ischemia, induces and/or accelerates cell death. Connexin 46 (Cx46) is a major connexin of the lens, and mutations of this connexin induce cataracts. Here, we report the effects of linoleic acid (LA) on the electrical properties of Cx46 GJCs and hemichannels expressed in Xenopus laevis oocytes. LA has a biphasic effect, increasing hemichannel current at 0.1 μM and decreasing it at concentrations of 100 μM or higher. The effects of extracellular and microinjected LA conjugated to coenzyme A (LA-CoA) suggest that the current activation site is accessible from the intracellular but not extracellular compartment, whereas the current inhibitory site is either located in a region of the hemichannel pore inaccessible to intracellular LA-CoA, or requires crossing of LA through an organelle membrane. Experiments with other fatty acids demonstrated that the block of hemichannels depends on the presence of a hydrogenated double bond at position 9 and is directly proportional to the number of double bonds. Experiments in paired oocytes expressing Cx46 showed that LA does not affect GJCs. The block by unsaturated fatty acids reported here opens the possibility that increases in the concentration of these lipids in the lens induce cataract formation by blocking Cx46 hemichannels.
Keywords: Gap junction, Cataract, Frog oocyte, Lens fiber, Cx46, Ion channel
Introduction
A gap-junction channel (GJC) is formed by head-to-head docking of two hemichannels, one from each adjacent cell. These hemichannels, also named connexons, are hexamers of proteins called connexins. The presence of undocked hemichannels at the plasma membrane has been demonstrated in several cell types, including lens fibers [16, 50]. However, their role under physiological or pathological conditions is not well defined, although several reports support the idea that controlled hemichannel opening allows autocrine/paracrine cell communication. For example, it has been demonstrated that hemichannels are involved in the release of signaling molecules such as ATP [54], glutamate [58], NAD+ [10], and prostaglandin E2 [12], as well as in the uptake of glucose [46]. In pathological conditions such as inhibition of metabolism, hemichannels contribute to cell damage because their opening induces or accelerates cell death due to loss of metabolites and ion gradients, and Ca2+ entry [5, 50, 51, 55].
Lacking a vascular system, lens fibers use GJC as a nutritional and excretory pathway, where the flows of ions, water, and metabolites between the cells are determined by their chemical or electrochemical gradients [23, 38]. At least three connexin isoforms are expressed in the lens: Cx43 [6], Cx46 [45], and Cx50 [32], and it has been recently proposed that Cx46 and Cx50 hemichannels play a role in Na+ and Ca2+ influx in lens fibers [16]. The importance of connexins in cataract formation is well documented, with Cx46- and Cx50-knockout mice developing cataracts at early ages, suggesting that connexin function is essential to maintain lens transparency [22, 43]. Increased levels of unsaturated fatty acids in the lens have been shown to induce and/or accelerate the development of cataracts [27, 33], but the mechanism of the damage of lens fibers is unknown. Although generation of radicals and lipid peroxidation products likely plays a role, they are not sufficient to explain the cytotoxicity [see 27]. Linoleic acid (LA), an unsaturated omega-6 fatty acid essential for biosynthesis of arachidonic acid [53], induces cataract formation in human [37] and bovine lens [21, 40]. Although there is no information on lens connexin isoforms, some isoforms are known to be sensitive to fatty acids. For example, oleamide-derived molecules inhibit Cx26 GJC [41].
Since Cx46 is essential for normal lens function, fatty acids are known to modulate connexins, and LA induces cataract formation, we decided to test whether LA alters the function of Cx46, one of the major connexins in the lens. In two-electrode voltage-clamp studies, we found a biphasic effect of LA on Cx46 hemichannels: low concentrations increased Cx46 hemichannel currents, whereas higher concentrations decreased them. We also observed a direct correlation between the number of fatty acid double bonds and the magnitude of the hemichannel current block, and found that a double bond at C9 is essential for hemichannel current inhibition. Unexpectedly, LA at low or high concentration did not affect Cx46 GJC.
Methods
Chemicals
Linoleic acid, palmitic acid, linoleoyl-CoA, and calphostin C were purchased from Sigma-Aldrich (Schnelldorf, Germany). Oleic acid, arachidonic acid, 9-thiastearic acid, and 9-nitrooleate were purchased from Cayman (Ann Arbor, Michigan, USA), and BAPTA-AM from Calbiochem (San Diego, California, USA).
Plasmid engineering, cRNA preparation, and injection into Xenopus laevis oocytes
The plasmid pSP64T-Cx46 containing rat Cx46 DNA was obtained from Dr. Lisa Ebihara (Rosalind Franklin University) [45]. Oocytes were injected with 12.5 ng of antisense Cx38 oligonucleotide alone or in combination with 25 ng of cRNA coding for Cx46. After cRNA injection, oocytes were maintained in Barth’s solution (88 mM NaCl, 1 mM KCl, 5 mM CaCl2, 0.8 mM MgCl2, and 10 mM HEPES/NaOH; pH 7.4, supplemented with 0.1 mg/ml gentamycin and 20 U/ml penicillin–streptomycin each) for 24–48 h, to allow for a good expression level. Additional details have been published [2].
Electrophysiological recordings and calculations
Whole-cell hemichannel currents were measured as described [47]. Briefly, oocytes were placed in a 1-ml recording chamber and superfused with ND96 solution (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, and 5 mM HEPES/NaOH; pH 7.4) at room temperature. Glass microelectrodes were filled with 3 M KCl and had tip resistances of 0.5–1.5 MΩ when immersed in ND96. For data acquisition and analysis, we used a voltage-clamp amplifier (OC-725C, Warner Instruments, Hamden, CT) with pCLAMP 10 and a Digidata 1440A A/D Board (Molecular Devices, Foster City, CA). Currents were measured following 15-s rectangular voltage pulses, ranging from −50 to +60 mV, in 10-mV steps, with a holding potential of −60 mV and 10-s intervals between pulses. Current–voltage (I–V) relationships were calculated from the peak current values. Currents through GJCs were measured in paired oocytes. Briefly, both cells of the pair were clamped at −40 mV, and junctional currents were measured after changing the cell-membrane voltage of one cell to values between −140 and +60 mV (20-mV steps, 15-s intervals between pulses), while holding constant the voltage of the other cell (used as reference). The current supplied to the cell clamped at −40 mV is equal in amplitude, but opposite in sign, to the transjunctional current. To evaluate the effects of fatty acids, 500 μl of lipids dissolved in ND96 was carefully added to the 1-ml recording chamber, to final concentrations ranging from 0.1 to 1,000 μM. Unless otherwise indicated, the recordings were performed after 5 min of incubation with the fatty acids
Statistical analysis
Results are expressed as means ± SEM, and n refers to the number of independent experiments. For statistical analysis, each treatment was compared to its respective control, and significance was determined using a one-way ANOVA or paired Student’s t test, as appropriate. Differences were considered significant at P<0.05.
Results
Linoleic acid affects Cx46 hemichannel currents
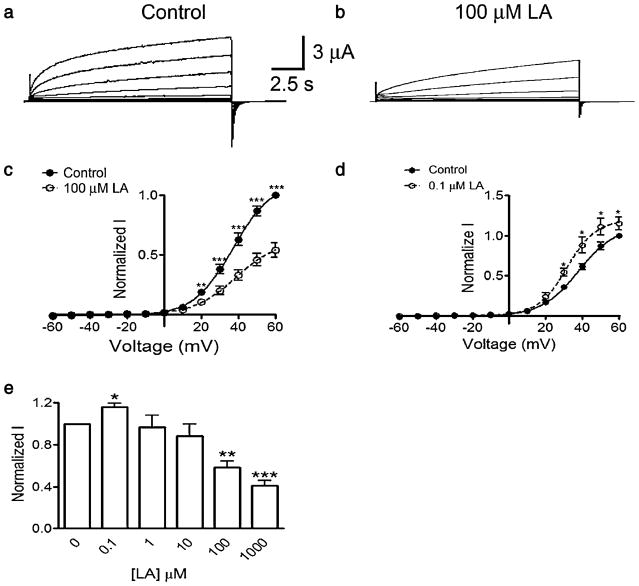
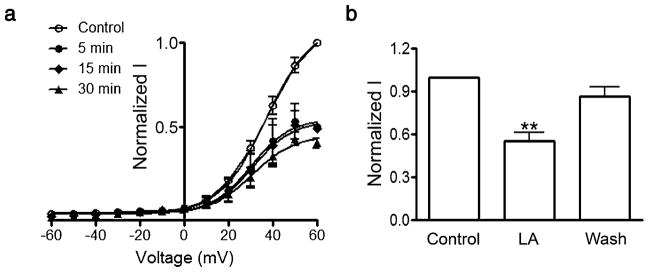
As previously described [47, 48], membrane depolarization of oocytes expressing rat Cx46 hemichannels induces slow outward currents that do not show inactivation (Fig. 1a). The maximum current at +60 mV was 5.5±0.3 μA (P<0.001, n=10). Upon returning to the holding potential of −60 mV, small tail currents were observed (Fig. 1a). Addition of LA to the bath solution produced a biphasic response. At 0.1 μM LA, the currents increased ~15% (Fig. 1d), whereas at concentrations equal to or greater than 100 μM LA, both outward and tail currents were significantly reduced (Fig. 1b). With 100 μM LA, the current was reduced by ~40% (Fig. 1e), with >90% of the inhibition within 2 min of exposure (not shown). The inhibitory effect of LA was sustained for at least 30 min (Fig. 2a). To study the reversibility of the effect, we exposed oocytes for 5 min to 100 μM LA and then washed them for 5 min with ND96 without LA. The current, reduced 45% by LA, recovered to ~90% of the control value after washing (Fig. 2b). In four of the 12 experiments, the hemichannel current after wash actually increased to values similar to those elicited by 0.1 μM LA (not shown). The simplest explanation for this phenomenon and the dose–response effect in Fig. 1e is that LA has at least two mechanisms of action, one with higher affinity (that increases hemichannel currents) and other with lower affinity (that decreases hemichannel currents). Fits to the Boltzmann equation of the current data before and after 0.1 or 100 μM LA did not show changes in the voltage dependency of Cx46 hemichannels, suggesting that LA modifies the open probability and/or the hemichannel conductance. In summary, LA has a biphasic effect, with a current increase at lower concentration and a decrease at higher concentrations. The inhibitory effect is complete in approximately 2 min and is reversible.
Fig. 1.
Cx46 hemichannels are inhibited by linoleic acid. a Representative whole-cell current records from Xenopus oocytes injected with Cx46 cRNA under control conditions. Oocytes were depolarized from −60 to +60 mV in steps of 10 mV for 15 s. At the end of each depolarizing step, the membrane was clamped to −60 mV for additional 10 s. b Same as a but after a 5-min exposure to 100 μM linoleic acid (LA). c Average effect of 100 μM LA (n=10). d Average effect of 0.1 μM LA (n=10). Currents were normalized to that under control conditions, at +60 mV, in the absence of LA. The symbols ** and *** correspond to P<0.01 and P<0.001 compared to the corresponding control values. e LA concentration dependence of Cx46 hemichannel currents. Results from ten oocytes were obtained at +60 mV and were normalized as described for c. The symbols *, **, and *** correspond to P<0.05, P<0.01, and P<0.001, respectively, compared to control condition, in the absence of LA. Average data in c–e are means ± SEM
Fig. 2.
The linoleic acid effect is sustained and reversible. a Rapid and stable current block by 100 μM LA. Oocytes were exposed to 100 μM LA for 5, 15, or 30 min, and hemichannel currents were measured. Data were normalized and expressed as described in the legend to Fig. 1c. The solid lines are the fits of the Boltzmann equation to the data (n=3 each). b Reversibility of the inhibition by LA. Oocytes expressing Cx46 hemichannels (n=12) were exposed to LA for 5 min (100 μM LA), and hemichannel currents were recorded. Currents were measured again after washing without LA for 5 min (Wash). Currents were normalized to control values. A P<0.001 compared to control is denoted by **
The inhibitory LA effect does not depend on an increase in intracellular free-[Ca2+] or PKC-mediated phosphorylation
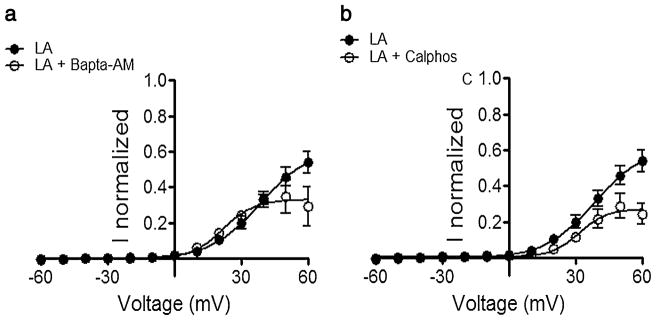
Since LA can increase intracellular [Ca2+] [17] and also activate protein kinase C [31], we tested the effects of the Ca2+ chelator BAPTA-AM (100 μM) and the PKC inhibitor calphostin C (1 μM) on the LA effect. Oocytes were pre-incubated for 1 h with one of these agents, briefly washed, exposed to 100 μM LA for 5 min, and then Cx46 hemichannel currents were recorded. Neither BAPTA-AM nor calphostin C by themselves affected Cx46 hemichannel currents (data not shown, n=6), nor reduced the inhibitory effect of 100 μM LA (Fig. 3). If anything, the LA effect was increased at the more positive clamping voltages. We did not examine further the increase in the response to LA, but one possibility is that BAPTA and calphostin C block the stimulatory effect elicited by lower LA concentrations (see Fig. 1, panels d and e). Nevertheless, the data strongly suggest that intracellular [Ca2+] or PKC-mediated phosphorylation is not necessary for the LA inhibitory effect.
Fig. 3.
The effect of linoleic acid is not due to PKC- or Ca2+-activated pathways. a Response to LA in oocytes incubated with 100 μM BAPTA-AM for 1 h. Immediately after removing the Ca2+ chelator, the response to a 5-min exposure to 100 μM LA was recorded and compared to control data from oocytes not treated with BAPTA-AM. b Response to 100 μM LA in oocytes incubated with 1 μM calphostin C (Calphos) for 1 h. Immediately after removing the PKC blocker, the response to a 5-min exposure to 100 μM LA was recorded and compared to control data from oocytes not treated with calphostin C. See legend to Fig. 1 for additional details
Unsaturated fatty acids other than linoleic acid also inhibit Cx46 hemichannels
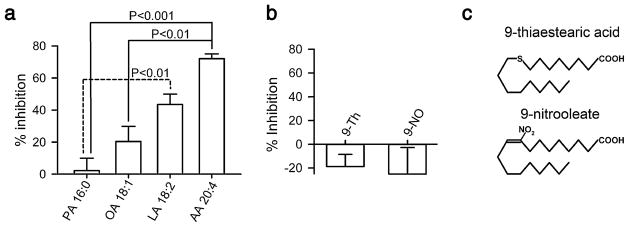
Oleamide and certain derivatives need specific chemical features, i.e., number and positions of double bonds, to affect GJC expressed in rat astrocytes [7]. Therefore, we tested the effects of fatty acids with different number of double bonds on Cx46 hemichannel currents. We used 100 μM of each fatty acid and compared their effects to that of LA (18 carbons, two double bonds) at the same concentration. The choice of 100 μM is based on concentrations of free C18:2 fatty acids of 50–100 μM reported in human plasma [20, 28, 30]. The best Cx46 hemichannel inhibitor was arachidonic acid (AA) which is a polyunsaturated fatty acid with four double bonds and 20 carbons. The current reduction with AA was >70% (Fig. 4a). Oleic acid (OA), with one double bond and 18 carbons, reduced the Cx46 current by 20% (Fig. 4a). Palmitic acid (PA), with 16 carbons and no double bonds, had no effect on the current (Fig. 4a). These results suggest that the inhibition by fatty acids depends on the presence of double bonds and seems to be proportional to the degree of unsaturation.
Fig. 4.
Inhibition of Cx46 hemichannel currents by other unsaturated fatty acids. a Dependence of the inhibitory effect on the number of unsaturated bonds. The effects of exposure to palmitic acid (PA, n=5), oleic acid (OA, n=5), linoleic acid (LA, n=6), or arachidonic acid (AA, n=6) for 5 min at 100 μM were determined. Protocol as described for Fig. 1d. Data are presented as means ± SEM. The labeling of the bars shows, for each fatty acid, the number of carbons followed by the number of double bonds; e.g., AA 20:4 for arachidonic acid, with 20 carbons and four double bonds. b Importance of the double bond at position 9. The experimental protocol was identical to that in a, but the fatty acids used were 9-thiastearic acid (9-Th) and 9-nitrooleate (9-NO). No statistically significant effects on the Cx46 hemichannel current were detected (n=7 for each compound). c Chemical structures of 9-thiastearic acid and 9-nitrooleate
A common characteristic between OA, LA, and AA is that all of them have a double bond at position 9, which seems to be important in the GJC inhibition by oleamide [7]. To test the importance of the double bond at position 9, we determined the effects of 100 μM of 9-thiastearic acid (9-Th, C17H34O2S) and 9-nitrooleate (9-NO, C18H33NO4). The former has a sulfur atom at position 9, and the latter has a NO2 at position 9, with the double bond not fully hydrogenated. The Cx46 hemichannel current was not affected by 5-min incubation with either of these fatty acids (Fig. 4b). These results suggest that an unblocked double bond at carbon 9 is essential for the effect of fatty acids on Cx46 hemichannels.
Impermeable LA has no effects on hemichannel currents
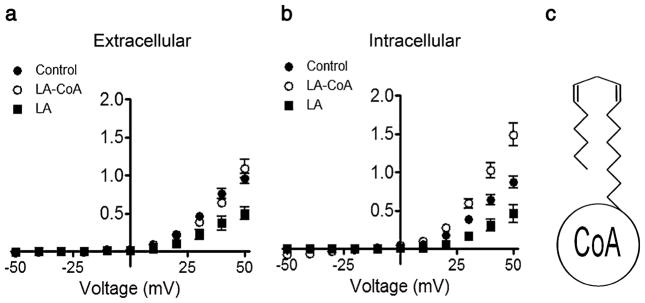
Fatty acids are present in the inner and outer leaflets of the membrane bilayer because of fast flip-flop of the protonated species [29]. To test whether LA incorporation into the membrane inner leaflet is necessary for the fatty acid inhibitory effect, we studied the hemichannel response to LA attached to coenzyme A (LA-CoA, MW 1,029). LA-CoA is inserted in the outer leaflet of the plasma membrane, but as other CoA-linked fatty acids, it is not expected to flip to the internal monolayer [9]. The addition of 100 μM LA-CoA to the bath solution for 5 min had no effect on Cx46 hemichannel currents (Fig. 5a). However, when LA-CoA was microinjected into the oocytes to a final estimated concentration of ca. 250 μM, it did not decrease, but increased Cx46 hemichannel currents (Fig. 5b). In contrast, a decrease was observed when LA was micro-injected to reach ca. 250 μM (Fig. 5b).
Fig. 5.
Microinjection of linoleic acid-CoA increases Cx46 hemichannel currents. a Current–voltage relationship of Cx46 hemichannel currents under control conditions and after a 5-min exposure to 100 μM linoleic acid attached to coenzyme A (LA-CoA) or LA alone added to the extracellular solution (n=8 for each group). b Current–voltage relationship of hemichannel currents under control conditions and 5 min after microinjection of LA-CoA (n=15) or LA (n=14) to reach final concentrations of ca. 250 μM. Intracellular concentrations were estimated assuming a 1-μl oocyte volume. Data are presented as means ± SEM of currents normalized to the control value at +60 mV
LA does not affect Cx46 GJC currents
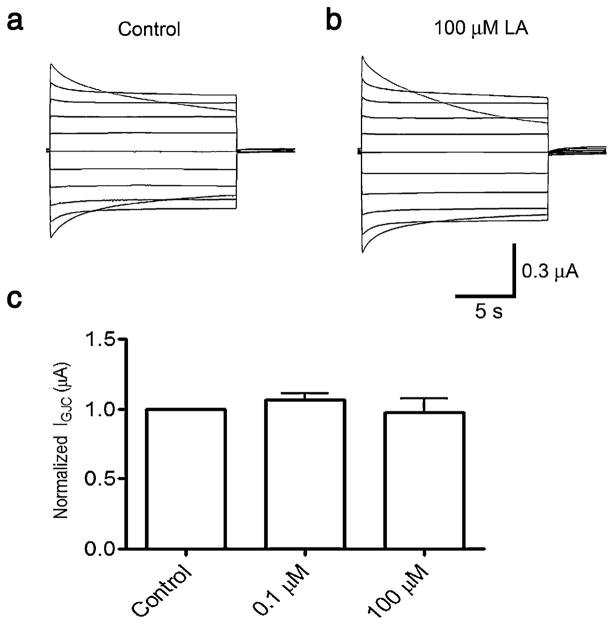
The data presented earlier showed that LA at 0.1 μM activates and at 100 μM inhibits Cx46 hemichannel currents. Here, we tested the effects of both concentrations on GJC currents in paired oocytes expressing Cx46. Under control conditions, Cx46 GJC exhibit currents of about 300 nA (283±63 nA at Vj −100 mV, and 361±36 nA at Vj ±100 mV), with a clear relaxation at Vj over 80 mV (Fig. 6a). A 5-min exposure to 0.1 or 100 μM LA did not affect the GJC currents (data at +60 mV are shown in Fig. 6b). These results indicate that LA, at the same concentration and exposure time that affects hemichannel currents, does not alter the electrical properties of GJC.
Fig. 6.
Linoleic acid does not affect GJC currents. a Representative transjunctional current records from paired oocytes under control conditions. Both cells of the pair were clamped at −40 mV, and junctional currents were measured after changing the cell-membrane voltage of one cell to values between −140 and +60 mV, in 20-mV steps. b Lack of effect of LA on transjunctional conductance. GJC currents measured at +60 mV in the presence of 0.1 or 100 μM LA were normalized to the current at +60 mV under control conditions. No differences were observed when 0.1 μM or 100 μM LA were used. Data are means ± SEM (n=6 for each concentration used)
Discussion
Mice expressing certain Cx46 mutants [44] and a Cx46 knockout [22] develop cataracts, clearly pointing to an essential role of Cx46 in maintaining lens transparency. Cataracts are frequent in diabetes [8, 56], and their incidence increases with age [34]; however, few studies have correlated cataract formation with potential alterations in Cx46 function induced by aging or diabetes. The studies available focused mostly on Cx46 modifications due to increases in free radicals [4, 47] and changes in phosphorylation state [19], but there are no studies on the effects of fatty acids on lens connexins, even though high concentrations of unsaturated fatty acids are toxic to human lens epithelial cells [27, 37]. In the present studies, we show the effect of physiological levels of unsaturated fatty acids on Cx46 hemichannels and GJCs. We focused our work on LA because it is present at the plasma membrane of lens epithelial cells and elevations of its concentration in the lens produce or accelerate cataract formation [37]. LA had a biphasic effect, increasing Cx46-hemichannel currents at low concentrations and decreasing the currents at higher concentrations. This is consistent with a high-affinity stimulatory effect and a low-affinity inhibitory effect. Dual effects of arachidonic acid on plasma-membrane Ca2+-ATPase [42] and of oleic acid on gap-junction coupling in A7r5 cells [25] have also been reported.
In order to investigate the mechanism of action of LA, we evaluated the roles of increases in intracellular free-[Ca2 +] and stimulation of PKC, two known responses to LA in other systems [17, 31]. We found no involvement of either signaling pathway in the hemichannel inhibitory response to LA. Modulation of BKCa2+ channels [13], voltage-gated K+ channels [18], TRP channels [39], and voltage-gated Ca2+ channels [49] by fatty acids has been reported, and in most cases, it has been suggested that there is a direct interaction between lipid and ion channel [14, 15, 49]. For example, arachidonic acid appears to bind to Thr250 and Val275, in the pore of Ca2+-activated K+ channels [24].
Polyunsaturated fatty acids increase BK channel activity by shifting the voltage dependence of the open probability [15]. In this study, the magnitudes of the effects of OA, LA, and AA were in the same order as our inhibitory results on Cx46 hemichannels. Cis-unsaturated fatty acids disorder the bilayer interior and order the head-group region [31], and therefore, the effects on membrane proteins could be mediated by changes in the biophysical properties of the phospholipid membrane. Several facts argue against this interpretation of our results. In a study using 100 μM concentrations, the oil/water partition coefficients were PA > OA > LA > AA [1], i.e., the opposite sequence to their inhibition of hemichannel conductance. In addition, in the BK channel study quoted above [15], there was no correlation between membrane fluidity and BK channel activation in response to the fatty acids. In sum, our results do not support an effect of LA and other unsaturated fatty acids mediated by changes in the properties of the plasma membrane. Nevertheless, additional experiments will be needed to determine whether the effects of fatty acids on Cx46 are due to direct binding to the hemichannels or are mediated by more complex signaling mechanisms, as suggested by the LA-CoA results discussed below. In addition, since Cx46 and Cx50 are the main lens connexin [35] and they form heteromeric hemichannels [26], further studies will be needed to determine whether hemichannels containing Cx50 are also affected by unsaturated fatty acids.
The absence of response to extracellular LA-CoA, not expected to flip across to the internal monolayer and therefore not to cross lipid bilayers [9], suggests that fatty acids need to access the inner leaflet of the plasma membrane and/or enter the intracellular compartment to affect hemichannels. Since the intracellular injection of sufficient LA-CoA to reach inhibitory concentrations produces an increase in Cx46 hemichannel currents, it is obvious that the mechanisms of stimulation and inhibition by LA are distinct. The current-activation site is accessible from the intracellular compartment to both LA and LA-CoA, whereas the inhibitory site is only accessible to LA, suggesting that it is located in a region of the hemichannel pore inaccessible to LA-CoA or that the signaling mechanism involved requires crossing of LA through an organelle membrane. Understanding of the complex mechanisms of the fatty acid effects on hemichannels will require additional studies.
Hemichannels are affected by changes in membrane phospholipid composition [36]. GJCs formed by Cx26 or Cx32 associate preferentially with certain phospholipids; phosphatidylcholine does not associate with Cx26 hemi-channels, whereas phosphatidylserine does not associate with Cx32 hemichannels [11, 36]. In contrast, phosphatidylcholine and phosphatidyl serine are associated with both isoforms in junctional plaques [36]. The concept of specific lipid–connexin interaction is also supported by Boger and co-workers [7], who found that molecules derived from the fatty acid primary amide oleamide affect GJCs when they contain 16–24 carbon atoms, a hydrophobic methyl terminus, a polarized carboxy-terminal and a cis double bond at position 9. In agreement with these observations, our results with fatty acids containing varied number of double bonds, with and without an unsaturated cis double bond at position 9, support the idea that the inhibitory effect of the fatty acids requires the unsaturated cis double bond at position 9 and is increased by the degree of unsaturation. As mentioned above, some phospholipids interact differentially with hemichannel or GJCs formed by Cx26 and Cx32 [36]. Our results showing that LA affects hemichannels, but not the GJCs formed by Cx46, extend previous studies to a lens connexin and show differences in functional responses between GJCs and hemichannels. We have not determined whether the selective effect on hemichannels vs. GJCs is due to differences in affinity for LA of hemichannels and GJCs. However, since LA is present on both leaflets of the plasma membrane, the absence of effect on GJCs may be due to structural differences between hemichannels and GJCs.
Pathophysiological significance
Unsaturated fatty acids modulate a wide range of cellular processes [52], and in the lens they induce or accelerate cataract formation by unknown mechanisms [21, 27, 33, 37, 40]. The cytotoxic effects of fatty acids on lens fibers depend on unsaturated fatty acid uptake by the cells and increase with the fatty acid-to-albumin ratio [27, 57]. In aqueous humor, the concentration of albumin is very low compared to that in plasma [27], and increases in the fatty acid-to-albumin ratio occur in a number of conditions associated with cataracts, including diabetes, lipid disorders, nephritic syndrome, and liver diseases [see 27]. Under these conditions, unsaturated fatty acid damage of the lens fibers can occur, particularly in the elderly, where there is a significant loss of the blood-aqueous humor barrier [see 27].
The block of Cx46 hemichannel currents by LA and other unsaturated fatty acids at concentrations found in the lens, without affecting Cx46 GJCs, may contribute to the induction of cataracts by: (a) reducing the uptake of physiological molecules from the extracellular space, as has been proposed for astrocytes [46], (b) reducing the release of signaling molecules, as it has been shown in many cell types [50], and/or (c) diminishing cell-to-cell communication. It has been proposed that the activity of Cx46 hemichannels mediates GJC formation [3], and therefore, reduced hemichannel activity may decrease the number of GJCs.
Acknowledgments
This work was supported by the National Institutes of Health grants GM068586, GM79629, and R01GM079629-03S1; Texas Advanced Research Program grant 010674-0046-2007; Fondecyt de Iniciación 11080061; and Proyecto Interno Universidad del Desarrollo 23.400.012.
Contributor Information
Mauricio A. Retamal, Email: mretamal@udd.cl, Laboratorio de Fisiología, Facultad de Medicina, Clínica, Alemana-Universidad del Desarrollo, Avenida Las Condes 12438, Santiago, Chile. Department of Cell Physiology and Molecular Biophysics and Center for Membrane Protein Research, Texas Tech University Health Sciences Center, Lubbock, TX 79430-6551, USA
Flavio Evangelista-Martínez, Laboratorio de Fisiología, Facultad de Medicina, Clínica, Alemana-Universidad del Desarrollo, Avenida Las Condes 12438, Santiago, Chile.
Carmen G. León-Paravic, Laboratorio de Fisiología, Facultad de Medicina, Clínica, Alemana-Universidad del Desarrollo, Avenida Las Condes 12438, Santiago, Chile
Guillermo A. Altenberg, Department of Cell Physiology and Molecular Biophysics and Center for Membrane Protein Research, Texas Tech University Health Sciences Center, Lubbock, TX 79430-6551, USA
Luis Reuss, Department of Cell Physiology and Molecular Biophysics and Center for Membrane Protein Research, Texas Tech University Health Sciences Center, Lubbock, TX 79430-6551, USA.
References
- 1.Anel A, Richieri GV, Kleinfeld AM. Membrane partition of fatty acids and inhibition of T cell function. Biochemistry. 1993;32:530–536. doi: 10.1021/bi00053a018. [DOI] [PubMed] [Google Scholar]
- 2.Bao X, Altenberg GA, Reuss L. Mechanism of regulation of the gap junction protein connexin 43 by protein kinase C-mediated phosphorylation. Am J Physiol Cell Physiol. 2004;286:C647–C654. doi: 10.1152/ajpcell.00295.2003. [DOI] [PubMed] [Google Scholar]
- 3.Beahm DL, Hall JE. Opening hemichannels in nonjunctional membrane stimulates gap junction formation. Biophys J. 2004;86:781–796. doi: 10.1016/S0006-3495(04)74154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthoud VM, Beyer EC. Oxidative stress, lens gap junctions, and cataracts. Antioxid Redox Signal. 2009;11:339–353. doi: 10.1089/ars.2008.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besancon E, Guo S, Lok J, Tymianski M, Lo EH. Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci. 2008;29:268–275. doi: 10.1016/j.tips.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Beyer EC, Kistler J, Paul DL, Goodenough DA. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989;108:595–605. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boger DL, Patterson JE, Guan X, Cravatt BF, Lerner RA, Gilula NB. Chemical requirements for inhibition of gap junction communication by the biologically active lipid oleamide. Proc Natl Acad Sci USA. 1998;95:4810–4815. doi: 10.1073/pnas.95.9.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borenshtein D, Ofri R, Werman M, Stark A, Tritschler HJ, Moeller W, Madar Z. Cataract development in diabetic sand rats treated with alpha-lipoic acid and its gamma-linolenic acid conjugate. Diabetes/Metab Res Rev. 2001;17:44–50. doi: 10.1002/1520-7560(0000)9999:9999<::aid-dmrr153>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 9.Boylan JG, Hamilton JA. Interactions of acyl-coenzyme A with phosphatidylcholine bilayers and serum albumin. Biochemistry. 1992;31:557–567. doi: 10.1021/bi00117a037. [DOI] [PubMed] [Google Scholar]
- 10.Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 2001;15:10–12. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- 11.Cascio M. Connexins and their environment: effects of lipids composition on ion channels. Biochim Biophys Acta. 2005;1711:142–153. doi: 10.1016/j.bbamem.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, Jiang JX. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke AL, Petrou S, Walsh JV, Jr, Singer JJ. Modulation of BK(Ca) channel activity by fatty acids: structural requirements and mechanism of action. Am J Physiol Cell Physiol. 2002;283:C1441–C1453. doi: 10.1152/ajpcell.00035.2002. [DOI] [PubMed] [Google Scholar]
- 14.Clarke AL, Petrou S, Walsh JV, Jr, Singer JJ. Site of action of fatty acids and other charged lipids on BKCa channels from arterial smooth muscle cells. Am J Physiol Cell Physiol. 2003;284:C607–C619. doi: 10.1152/ajpcell.00364.2002. [DOI] [PubMed] [Google Scholar]
- 15.Denson DD, Wang X, Worrell RT, Eaton DC. Effects of fatty acids on BK channels in GH(3) cells. Am J Physiol Cell Physiol. 2000;279:C1211–C1219. doi: 10.1152/ajpcell.2000.279.4.C1211. [DOI] [PubMed] [Google Scholar]
- 16.Ebihara L, Tong JJ, Vertel B, White TW, Chen TL. Properties of connexin 46 hemichannels in dissociated lens fiber cells. Invest Ophthalmol Vis Sci. 2011;52:882–889. doi: 10.1167/iovs.10-6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Yassimi A, Hichami A, Besnard P, Khan NA. Linoleic acid induces calcium signaling, Src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J Biol Chem. 2008;283:12949–12959. doi: 10.1074/jbc.M707478200. [DOI] [PubMed] [Google Scholar]
- 18.Feng DD, Luo Z, Roh SG, Hernandez M, Tawadros N, Keating DJ, Chen C. Reduction in voltage-gated K+ currents in primary cultured rat pancreatic beta-cells by linoleic acids. Endocrinology. 2006;147:674–682. doi: 10.1210/en.2005-0225. [DOI] [PubMed] [Google Scholar]
- 19.Fleschner CR. Connexin 46 and connexin 50 in selenite cataract. Ophthalmic Res. 2006;38:24–28. doi: 10.1159/000088527. [DOI] [PubMed] [Google Scholar]
- 20.Fraser DA, Thoen J, Rustan AC, Førre O, Kjeldsen-Kragh J. Changes in plasma free fatty acid concentrations in rheumatoid arthritis patients during fasting and their effects upon T-lymphocyte proliferation. Rheumatol Oxf. 1999;38:948–952. doi: 10.1093/rheumatology/38.10.948. [DOI] [PubMed] [Google Scholar]
- 21.Glanz D, Lennarz B, Glaesser D. Fatty acid cytotoxicity to organ-cultured bovine lenses. Ophthalmic Res. 2006;38:62–65. doi: 10.1159/000091389. [DOI] [PubMed] [Google Scholar]
- 22.Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar NM, Horwitz J, Gilula NB. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–843. doi: 10.1016/s0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- 23.Goodenough DA. Lens gap junctions: a structural hypothesis for nonregulated low-resistance intercellular pathways. Invest Ophthalmol Vis Sci. 1979;18:1104–1122. [PubMed] [Google Scholar]
- 24.Hamilton KL, Syme CA, Devor DC. Molecular localization of the inhibitory arachidonic acid binding site to the pore of hIK1. J Biol Chem. 2003;278:16690–16697. doi: 10.1074/jbc.M212959200. [DOI] [PubMed] [Google Scholar]
- 25.Hirschi KK, Minnich BN, Moore LK, Burt JM. Oleic acid differentially affects gap junctionmediated communication in heart and vascular smooth muscle cells. Am J Physiol Cell Physiol. 1993;265:C1517–C1526. doi: 10.1152/ajpcell.1993.265.6.C1517. [DOI] [PubMed] [Google Scholar]
- 26.Hopperstad MG, Srinivas M, Spray DC. Properties of gap junction channels formed by Cx46 alone and in combination with Cx50. Biophys J. 2000;79:1954–1966. doi: 10.1016/S0006-3495(00)76444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwig M, Glaesser D, Fass U, Struck HG. Fatty acid cytotoxicity to human lens epithelial cells. Exp Eye Res. 2004;79:689–704. doi: 10.1016/j.exer.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Jahn CE, Leiss O, von Bergmann K. Lipid composition of human aqueous humor. Ophthalmic Res. 1983;15:220–224. doi: 10.1159/000265263. [DOI] [PubMed] [Google Scholar]
- 29.Kamp F, Westerhoff HV, Hamilton JA. pH gradients across phospholipid membranes caused by fast flip-flop of un-ionized fatty acids. Proc Natl Acad Sci USA. 1992;89:11367–11370. doi: 10.1073/pnas.89.23.11367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kangani CO, Kelley DE, DeLany JP. New method for GC/FID and GC-C-IRMS analysis of plasma free fatty acid concentration and isotopic enrichment. J Chromatogr B. 2008;873:95–101. doi: 10.1016/j.jchromb.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim MH, Kim MO, Kim YH, Kim JS, Han HJ. Linoleic acid induces mouse embryonic stem cell proliferation via Ca2+/PKC, PI3K/Akt, and MAPKs. Cell Physiol Biochem. 2009;23:53–64. doi: 10.1159/000204090. [DOI] [PubMed] [Google Scholar]
- 32.Kistler J, Kirkland B, Bullivant S. Identification of a 70, 000-D protein in lens membrane junctional domains. J Cell Biol. 1985;101:28–35. doi: 10.1083/jcb.101.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kojima K, Okochi Y, Yagi K. The opacity of the rat lens caused by fatty acid peroxide. Nippon Ganka Gakkai Zasshi. 1968;72:1733–1739. [PubMed] [Google Scholar]
- 34.Kronfeld PC. Eye changes due to advanced age. Ill Med J. 1953;103:104–107. [PubMed] [Google Scholar]
- 35.Lo WK, Shaw AP, Takemoto LJ, Grossniklaus HE, Tigges M. Gap junction structures and distribution patterns of immunoreactive connexins 46 and 50 in lens regrowths of Rhesus monkeys. Exp Eye Res. 1996;62:171–180. doi: 10.1006/exer.1996.0021. [DOI] [PubMed] [Google Scholar]
- 36.Locke D, Harris AL. Connexin channels and phospholipids: association and modulation. BMC Biol. 2009;17:7–52. doi: 10.1186/1741-7007-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu M, Taylor A, Chylack LT, Jr, Rogers G, Hankinson SE, Willett WC, Jacques PF. Dietary fat intake and early age-related lens opacities. Am J Clin Nutr. 2005;81:773–779. doi: 10.1093/ajcn/81.4.773. [DOI] [PubMed] [Google Scholar]
- 38.Mathias RT, Rae JL, Baldo GJ. Physiological properties of the normal lens. Physiol Rev. 1997;77:21–50. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- 39.Matta JA, Miyaresm RL, Ahern GP. TRPV1 is a novel target for omega-3 polyunsaturated fatty acids. J Physiol. 2007;578:397–411. doi: 10.1113/jphysiol.2006.121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen N, Glanz D, Glaesser D. Fatty acid cytotoxicity to bovine lens epithelial cells: investigations on cell viability, ecto-ATPase, Na(+), K(+)-ATPase and intracellular sodium concentrations. Exp Eye Res. 2000;71:405–413. doi: 10.1006/exer.2000.0896. [DOI] [PubMed] [Google Scholar]
- 41.Nojima H, Ohba Y, Kita Y. Oleamide derivatives are prototypical anti-metastasis drugs that act by inhibiting Connexin 26. Curr Drug Saf. 2007;2:204–211. doi: 10.2174/157488607781668837. [DOI] [PubMed] [Google Scholar]
- 42.Oliveira VH, Nascimento KS, Freire MM, Moreira OC, Scofano HM, Barrabin H, Mignaco JA. Mechanism of modulation of the plasma membrane Ca(2+)-ATPase by arachidonic acid. Prostaglandins Other Lipid Mediat. 2008;87:47–53. doi: 10.1016/j.prostaglandins.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Pal JD, Berthoud VM, Beyer EC, Mackay D, Shiels A, Ebihara L. Molecular mechanism underlying a Cx50-linked congenital cataract. Am J Physiol Cell Physiol. 1999;276:C1443–C1446. doi: 10.1152/ajpcell.1999.276.6.C1443. [DOI] [PubMed] [Google Scholar]
- 44.Pal JD, Liu X, Mackay D, Shiels A, Berthoud VM, Beyer EC, Ebihara L. Connexin46 mutations linked to congenital cataract show loss of gap junction channel function. Am J Physiol Cell Physiol. 2000;279:C596–C602. doi: 10.1152/ajpcell.2000.279.3.C596. [DOI] [PubMed] [Google Scholar]
- 45.Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Retamal MA, Froger N, Palacios-Prado N, Ezan P, Sáez PJ, Sáez JC, Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci. 2007;27:13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Retamal MA, Yin S, Altenberg GA, Reuss L. Modulation of Cx46 hemichannels by nitric oxide. Am J Physiol Cell Physiol. 2009;296:C1356–C1363. doi: 10.1152/ajpcell.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Retamal MA, Yin S, Altenberg GA, Reuss L. Voltage-dependent facilitation of Cx46 hemichannels. Am J Physiol Cell Physiol. 2010;298:C132–C139. doi: 10.1152/ajpcell.00258.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts-Crowley ML, Rittenhouse AR. Arachidonic acid inhibition of L-type calcium (CaV1.3b) channels varies with accessory CaVbeta subunits. J Gen Physiol. 2009;133:387–403. doi: 10.1085/jgp.200810047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sáez JC, Schalper KA, Retamal MA, Orellana JA, Shoji KF, Bennett MV. Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp Cell Res. 2010;316:2377–2389. doi: 10.1016/j.yexcr.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 51.Schalper KA, Palacios-Prado N, Retamal MA, Shoji KF, Martínez AD, Sáez JC. Connexin hemichannel composition determines the FGF-1-induced membrane permeability and free [Ca2+]i responses. Mol Biol Cell. 2008;19:3501–3513. doi: 10.1091/mbc.E07-12-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Steinberg G, Slaton WH, Jr, Howton DR, Mead JF. Metabolism of essential fatty acids. IV. Incorporation of linoleate into arachidonic acid. J Biol Chem. 1956;220:257–264. [PubMed] [Google Scholar]
- 54.Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 55.Stridh MH, Tranberg M, Weber SG, Blomstrand F, Sandberg M. Stimulated efflux of amino acids and glutathione from cultured hippocampal slices by omission of extracellular calcium: likely involvement of connexin hemichannels. J Biol Chem. 2008;283:10347–10356. doi: 10.1074/jbc.M704153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan JS, Wang JJ, Mitchell P. Influence of diabetes and cardiovascular disease on the long-term incidence of cataract: the Blue Mountains eye study. Ophthalmic Epidemiol. 2008;15:317–327. doi: 10.1080/09286580802105806. [DOI] [PubMed] [Google Scholar]
- 57.Trimborn M, Iwig M, Glanz D, Gruner M, Glaesser D. Linoleic acid cytotoxicity to bovine lens epithelial cells: influence of albumin on linoleic acid uptake and cytotoxicity. Ophtalmic Res. 2000;32:87–93. doi: 10.1159/000055595. [DOI] [PubMed] [Google Scholar]
- 58.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]