Abstract
Background and Aims
The expression of floral symmetry genes is examined in the CYCLOIDEA lineage following duplication, and these are linked to changes in flower morphology. The study focuses on Dipsacales, comparing DipsCYC2 gene expression in Viburnum (radially symmetrical Adoxaceae) to members of early-diverging lineages of the bilaterally symmetrical Caprifoliaceae (Diervilla and Lonicera).
Methods
Floral tissue from six species, which included dorsal, lateral and ventral regions of the corolla, was dissected. RNA was extracted from these tissues and each copy of DipsCYC2 was amplified with reverse transcriptase PCR.
Key Results
Members of DipsCYC2 were expressed across the corolla in the radially symmetrical Viburnum plicatum. A shift to bilaterally symmetrical flowers at the base of the Caprifoliaceae was accompanied by a duplication of the DipsCYC2 gene, resulting in DipsCYC2A and DipsCYC2B, and by loss of expression of both of these copies in the ventral petal. In Lonicera (Caprifolieae), there is a shift from flowers with two dorsally and three ventrally oriented corolla lobes to a clear differentiation of dorsal, lateral and ventral lobes. This shift entailed a decoupling of expression of DipsCYC2A and DipsCYC2B; DipsCYC2B continues to be expressed in the dorsal and lateral lobes, while DipsCYC2A expression is restricted to just the two dorsal lobes. A reversion to more radially symmetrical flowers within Lonicera was accompanied by a re-expansion of expression of both DipsCYC2A and DipsCYC2B.
Conclusions
The transition to bilateral symmetry in Caprifoliaceae involved: (a) duplication of an ancestral DipsCYC2 gene; (b) the loss of expression of both of these copies in the ventral petal; and (c) changes in the zone of expression, with one copy continuing to be expressed across the dorsal and lateral petals, and the other copy becoming restricted in expression to the dorsal corolla lobes.
Keywords: Flower symmetry, CYCLOIDEA, Dipsacales, Caprifoliaceae, Lonicera, RNA expression
INTRODUCTION
Shifts between radially symmetrical (polysymmetrical, actinomorphic) and bilaterally symmetrical (monosymmetrical, zygomorphic) flowers have been common within angiosperms (Weberling, 1989; Endress, 1996, 1999). Such morphological shifts are of special interest in relation to shifts in pollination (e.g. see Neal, 1998) and perhaps to shifts in rates of diversification (Sargent, 2004). A shift from radial to bilateral symmetry entails a transition of a single petal or corolla lobe form to the individualization, ultimately, of three types: a medially positioned (abaxial or ventral) petal, two lateral petals and two adaxial or dorsal petals (Donoghue et al., 1998). To tease apart the mechanisms underpinning the evolutionary transition from radial to bilateral symmetry it is helpful to compare representatives of early-diverging bilaterally symmetrical lineages within a clade to their closest relatives with radial symmetry, with the aim of identifying any changes in the number of genes and/or shifts in the expression of genes that may underlie the morphological differences.
Most studies of floral symmetry genes have focused on species with highly derived morphologies (e.g. Luo et al., 1996; Citerne et al., 2006; Feng et al., 2006; Busch and Zachgo, 2007; Broholm et al., 2008; Chapman et al., 2008; Kim et al., 2008; Wang et al., 2008). A few studies have correlated gene duplications in CYCLOIDEA (CYC) with morphological shifts in floral symmetry (e.g. Howarth and Donoghue, 2005; Chapman et al., 2008; Kim et al., 2008; Zhang et al., 2010). Here, gene expression patterns following duplication are examined, and shifts in expression linked to changes in flower morphology. Additionally, different forms of symmetry are examined, including radially symmetrical flowers in Viburnum, and several forms of bilateral symmetry in Diervilla and Lonicera. It is shown how changes in the zones of expression of duplicated copies of CYC within the corolla appear to be related to morphological changes and how a series of changes in the deployment of duplicated genes serves to developmentally individuate different regions of the corolla along the dorsoventral axis.
Background on CYCLOIDEA and related genes
Understanding the evolution of floral symmetry has been greatly advanced by the study of three transcription factors from two gene families: the TCP family, including CYCLOIDEA (Luo et al., 1996) and the MYB family, including DIVARICATA (Almeida et al., 1997; Galego and Almeida, 2002) and RADIALIS (Corley et al., 2005; Costa et al., 2005). CYCLOIDEA (CYC) is the most thoroughly studied to date and has been shown to be involved in specifying dorsal flower identity (Luo et al., 1996, 1999; Almeida et al., 1997). A duplication in Antirrhineae (which includes the snapdragon, Antirrhinum majus; Hileman and Baum, 2003), resulted in two closely related copies, CYC and DICH (DICHOTOMA), which have overlapping expression patterns in floral meristems (Luo et al., 1996, 1999; Hileman et al., 2003). In Antirrhinum, a fully radial and ventralized flower (a peloric form) is produced only in CYC/DICH double mutants (Luo et al., 1996; Almeida et al., 1997). Additionally, CYC and DICH both inhibit stamen growth in A. majus, with expression in stamen primordia resulting in abortion (Luo et al., 1996, 1999).
Similar CYC function has been corroborated in the closely related Linaria (Cubas et al., 1999), as well as in two legumes, Lotus japonica and Pisum sativum (Feng et al., 2006; Wang et al., 2008). Additionally, CYC orthologues in Asteraceae play a role in specifying disk versus ray florets (Broholm et al., 2008; Kim et al., 2008). CYC has also been shown to be dorsally expressed in an array of other core eudicots: Arabidopsis, Bournea, Iberis, Lonicera, Lupinus and Malpighiaceae (Cubas et al., 2001; Citerne et al., 2006; Howarth and Donoghue, 2006; Busch and Zachgo, 2007; Zhou et al., 2008; Zhang et al., 2010). An exception is the radially symmetrical Cadia, where one CYC-like copy (LegCYC1A) has an apparently derived, expanded expression across the corolla (Citerne et al., 2006). These data suggest that CYC expression and function may be similar across rosid and asterid angiosperms.
Previously it has been shown that CYC-like genes duplicated twice around the divergence of the core eudicots, resulting in three paralogues (Howarth and Donoghue, 2006). These three paralogues fall into a clade of TCP genes that contain an ECE motif (Howarth and Donoghue, 2006). All of the studies of the role of CYC-like genes in floral symmetry have focused on a single core eudicot copy, members of the CYC2 clade (Howarth and Donoghue, 2006). This gene clade also contains the greatest number of secondary gene duplications, with a pattern emerging of extra independent duplications in bilaterally symmetrical groups. Duplications in CYC-like genes have been common in the Asteridae (e.g. Reeves and Olmstead, 2003). In Antirrhinum and its relatives there is one clear duplication within the CYC2 clade at the level of the Antirrhineae (resulting in both CYC and DICH (Gübitz et al., 2003; Hileman and Baum, 2003). Digitalis (also in Plantaginaceae) lacks this duplication, but has three additional copies (Hileman and Baum, 2003). In Gesneriaceae, three broad duplications have been hypothesized across Cyrtandroideae (Citerne et al., 2000; Gao et al., 2008; Song et al., 2009; Pang et al., 2010), and one more within Gesnerioideae (Smith et al., 2004). Chapman et al. (2008) documented ten separate copies in Helianthus (Asteraceae), with one copy exclusive to bilaterally symmetrical ray florets. Previously extra duplications have also been noted in Dipsacaceae, Morinaceae and Valerianceae (Dipsacales), as well as in Scaevola (Goodeniaceae) (Howarth and Donoghue, 2005, 2006; S. Carlson and M. J. Donoghue, University of Neuchâtel, Switzerland, unpubl. res.). Outside the Asteridae, the same pattern has also been documented in legumes, with three or four copies present in many of the groups that have been examined (Citerne et al., 2003; Fukuda et al., 2003; Ree et al., 2004; Feng et al., 2006; Wang et al., 2008). Additionally, in Malpighiaceae, a duplication appears to have occurred near the origin of bilaterally symmetrical flowers (Zhang et al., 2010).
Using a well-resolved species tree for Dipsacales (see Donoghue et al., 2003), and a thorough sample of CYC-like genes in this group (Howarth and Donoghue, 2005), it was possible to map clearly CYC-like duplications within this group. Previously it has been shown that the radially symmetrical clade of Dipsacales, the Adoxaceae, have only a single base copy of DipsCYC2, with a duplication within the Viburnum lineage (Howarth and Donoghue, 2005), and that a duplication occurred along the branch leading to the bilaterally symmetrical Caprifoliaceae. This duplication in Caprifoliaceae is concurrent with the shift to bilateral flower symmetry.
Background on Dipsacales, Caprifoliaceae and Lonicera
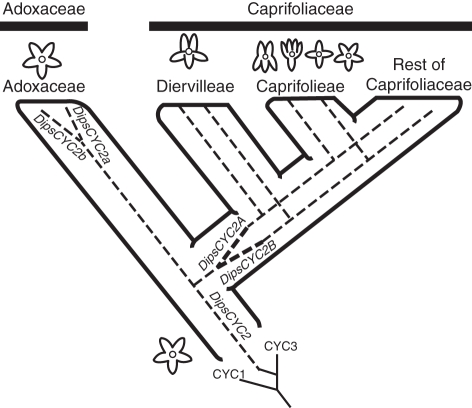
The Dipsacales phylogeny is now quite well resolved, based on a series of molecular and morphological phylogenetic analyses (Judd et al., 1994; Backlund and Donoghue, 1996; Bell et al., 2001; Donoghue et al., 2001b, 2003; Pyck, 2001; Zhang et al., 2003; Smith and Donoghue, 2008). This understanding provides a solid basis for inferring the location of evolutionary changes in flower characters (Donoghue et al., 2003), as well as a secure framework within which to infer the evolution of CYC and related genes. A variety of floral forms are found within Dipsacales, including polysymmetric, monosymmetric and asymmetric flowers (Donoghue et al., 2003). Recent phylogenetic analyses of campanulid angiosperms support the radially symmetrical Paracryphiaceae as sister to Dipsacales (Winkworth et al., 2008b; Tank and Donoghue, 2010), which supports an ancestral condition of radial symmetry in the entire clade. The primary split within Dipsacales separates the Adoxaceae from the Caprifoliaceae (Fig. 1). Adoxaceae (including Viburnum, Sambucus and Adoxa and its relatives) have radially symmetrical flowers, with small calyx lobes, and rotate corollas, whereas Caprifoliaceae (including Diervilleae, Caprifolieae, Linnaeeae, Morinaceae, Dipsacaceae and Valerianaceae) typically have bilaterally symmetrical flowers, larger calyx lobes, and tubular corollas (Fukuoka, 1972; Donoghue et al., 2003). Caprifoliaceae appear to represent an independent derivation of bilaterally symmetrical flowers within the Asteridae (Fig. 1).
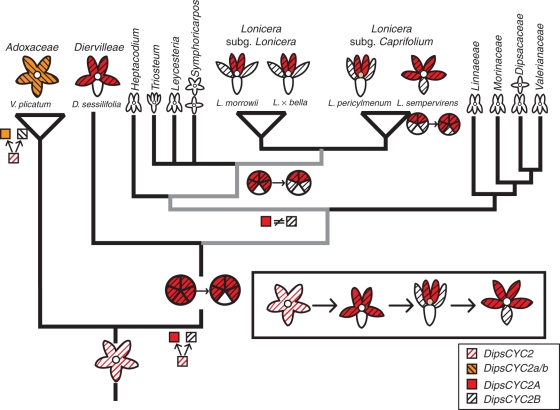
Fig. 1.
Floral symmetry shifts and DipsCYC2 duplication events. The ancestor of Dipsacales is hypothesized to have been radially symmetrical. This radial symmetry is maintained in Adoxaceae. Within Viburnum (Adoxaceae), a duplication event resulted in two copies, DipsCYC2a and DipsCYC2b. The shift to bilateral symmetry in the ancestor of Caprifoliaceae is correlated with a duplication of DipsCYC2 into DipsCYC2A and DipsCYC2B.
There are several forms of bilateral symmetry present within the Caprifoliaceae. Two-lipped flowers, in which the two dorsal petals are differentiated from the two lateral petals and the medial ventral petal (the 2:3 form), are widespread within the clade and appear to be ancestral. Members of the sister group of the rest of the Caprifoliaceae, the Diervilleae, are only weakly bilaterally symmetrical with the 2:3 form. In Diervilla, for example, the corolla lobes are more or less indistinguishable except for a modified ventral petal. Another early-diverging lineage, the Caprifolieae, includes species with two-lipped flowers in which the two dorsal and the two lateral petals are differentiated from the medial ventral petal (the 4:1 form); such flowers are found in Lonicera and Triosteum, and radially symmetrical flowers appear to have re-evolved in Symphoricarpos. There have also been several shifts in the number of corolla lobes, most notably the reduction from five to four lobes in Symphoricarpos and in Dipsacaceae. Stamen number became reduced from five to four in the remainder of the Caprifoliaceae (excluding Diervilleae and Caprifolieae) through loss of the medial dorsal stamen. Further reductions in stamen number within this group occurred within Morinaceae, and multiple times within the Valerianaceae (Donoghue et al., 2003).
This study is focused on two early-diverging lineages within the Caprifoliaceae, the Diervilleae and the Caprifolieae. Diervilleae is made up of two sublineages, Diervilla (three species) and Weigela (ten species). Caprifolieae is a much larger group, containing Leycesteria (six species, 2:3 floral form), Lonicera (approx. 180 species, 4:1 floral form), Symphoricarpos (approx. 15 species, radial flower) and Triosteum (six species, 4:1 floral form). Caprifolieae is well supported as a clade and Heptacodium (one species, 2:3 floral from) is typically reconstructed as sister to these four genera (Bell et al., 2001; Donoghue et al., 2001a, b; Zhang et al., 2003; Theis et al., 2008; Winkworth et al., 2008a). However, relationships among these four genera have remained problematic, possibly owing to rapid radiation (Bell and Donoghue, 2005). The best-supported results to date suggest that Triosteum is sister to the other three genera, and that Leycesteria and Symphoricarpos form a clade that is sister to Lonicera (Theis et al., 2008; Smith, 2009). This has implications for the evolution of bilateral symmetry; specifically, the 4:1 floral form may have arisen separately in Lonicera and in Triosteum within the Caprifolieae.
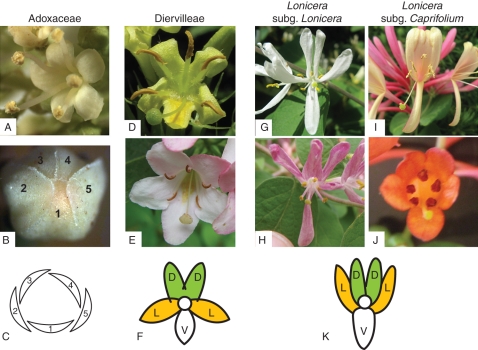
Lonicera is divided into two clades, traditionally recognized as the subgenera Lonicera and Caprifolium (Rehder, 1903; Theis et al., 2008; Smith, 2009). The two groups are morphologically distinctive; members of subgenus Lonicera have two-flowered cymes and free leaves, while species of Caprifolium have three-flowered cymes in whorls and perfoliate leaves subtending the inflorescences. Although Lonicera species all have the 4:1 flower form, they vary from being weakly bilaterally symmetrical (see L. sempervirens; Fig. 2J) to very strongly bilatiate, with the two dorsal and two lateral petals nearly entirely fused and pointed upward (see L. periclymenum; Fig. 2I). In contrast, in L. morrowii and L. × bella (studied here) the two dorsal lobes are distinct in orientation from the two lateral lobes, though all are similar in size and shape (Fig. 2G, H).
Fig. 2.
Images of Dipsacales examined in this study: V. plicatum (A–C), V. plicatum aestivation in bud (B) and floral diagram (C), D. sessilifolia (D), W. florida (E), petal arrangement of Diervilleae (F), L. morrowii (G), L. × bella (H), L. periclymenum (I), L. sempervirens (J), and general petal arrangement in Lonicera (K). In (B) and (C), numbers indicate the order of dissected petals. In (F) and (K), D = dorsal, L = lateral, V = ventral. Photos taken by D. G. Howarth (A, B, G, H, J), M. J. Donoghue (E, I) and Alan Cressler (D).
MATERIALS AND METHODS
Plant collections
Dissections were performed on the following six taxa: Viburnum plicatum, Diervilla sessilifolia, Lonicera sempervirens, Lonicera periclymenum, Lonicera morrowii and Lonicera × bella. Viburnum plicatum was collected on Raff Ave. in Floral Park, NY. Diervilla sessilifolia and L. periclymenum were collected from propagated plants in the greenhouses of the Marsh Botanical Gardens, Yale University, New Haven, CT. Lonicera sempervirens was collected near the Stewart Manor train station in Garden City, NY. Lonicera morrowii was collected in two locations. One was in Guilford, CT and the other was on The Grand Central Pkwy access road, where it crosses with the Midland Pkwy, in Jamaica, NY. Lonicera × bella is a horticultural hybrid between Lonicera tatarica and L. morrowii. It was also collected in Jamaica, NY in the same location as L. morrowii. Vouchers for these species have been deposited at Yale University Herbarium, New Haven, CT.
Plants were collected by cutting off large branches that contained flower buds at multiple stages. The branches were taken to the laboratory and immediately dissected. The dissected material was housed in a freezer at –80 °C until RNA extraction could be performed.
Dissections
Whenever possible, dissections were performed on multiple accessions of each species. Every accession contained three separate corolla lobe dissections (Fig. 2F, K) consisting of dorsal (two dorsal petals), lateral (two lateral petals) and ventral (single ventral petal). Corolla lobes were separated from the corolla tubes of multiple flower buds from early stage (first stage possible to dissect) to nearly open flower buds. The floral parts were already well developed and only elongating through all of these dissectible bud stages. The bud stages were mixed because the comparisons were across multiple taxa that do not have comparable stages, CYC2 clade members are expressed throughout corolla development, and, in several species, there was a limited supply of buds from plants that flower for only a short window of time. Late-stage buds alone (from buds about to open) were also used in accessions of three of the taxa. Dissections targeted only the lobes, without any of the fused corolla tube tissue. At least one dissection from each species also included whole bud, tube only (no petal lobes) and leaf for comparison.
In Adoxaceae, the petals of V. plicatum were dissected individually based on aestivation. Petal 1 was determined in each flower as the lone innermost petal of the bud (see Fig. 2B, C). Petals 2–5 were then dissected in a clockwise pattern. We hypothesize that petal 1 is the ventral petal; however, this is extremely difficult to determine in these small radially symmetrical flowers. Whole bud dissections from central reproductive flowers and sterile marginal flowers were also included, as well as leaf tissue. The extractions required significant tissue, requiring petal lobes from at least 40 buds.
Reverse transcriptase PCR (rtPCR)
Frozen tissues were pulverized with the Bio101 FastPrep system and were subsequently extracted using an RNeasy kit with the optional DNase step (Qiagen). When necessary, the samples were subsequently treated with DNase I (NEB), to eliminate all DNA contamination. For most samples, the cDNA was generated with gene-specific primers using the one-step RT-PCR kit (Invitrogen). The rtPCR used the standard protocol suggested with the kit, with a 55 °C annealing temperature. Reactions amplifying DipsCYC2A and DipsCYC2B were done in 25-μL reactions (half reactions), while GAPDH controls were done in 12·5-μL reactions (quarter reactions). The exception was L. morrowii from Madison, CT, in which cDNA was generated from RNA using Superscript III (Invitrogen). The genes were subsequently amplified from total cDNA utilizing the following cycling programme: 95 °C for 45 s, 50–56 °C for 1 min and 72 °C for 1 min 30 s, repeated for 39 cycles. Reactions were performed using Taq DNA polymerase (Qiagen, Valencia, CA or Promega) in 25 µL, with final concentrations of 2·5 mm MgCl2, 0·5 µm of each primer, 0·8 mm dNTPs and 0·5 × Q Solution (Qiagen). The above PCR parameters were also used to test the total RNA for DNA contamination (data not shown).
To amplify DipsCYC2A the following gene-specific primers were used: DierCYC2AF30 (5′-CCAAGGACCCCGAGACCGGAGGGTAAGATTGTCCATTGAAATC) and DierCYC2AR19 (5'-CCCTCGACTCTCTAGCAACAAGATCAATGGCAGATTTATCTGTCTG) for Diervilla, and CaprCYC2AF (5'-GRACWCTTGATTGGCTACTCAAC) and LoniCYC2AR (5'-GATTTATSTGTWGGCTTTCTCAAC) for all four Lonicera species. To amplify DipsCYC2B the following gene-specific primers were used: DierCYC2BF27 (5'-GGCACACGGTCCTAGGGATCGGAGAGTAAGGTTATCAATGGAG) and DierCYC2BR22 (5' CGATTCTCTCGAACTACTATCAAATGCATCCCTGATCTTCTCG) for Diervilla and LoniCYC2BF (5'-GATGAAAATCAACTGCACTACTGG) and LoniCYC2BR (5'-AGCATCCCTCTTCTCGTTCCCAAC) for all four Lonicera species. A GAPDH control was also included, using primers from Strand et al. (1997). These results were run on a 1 % agarose gel in sodium borate buffer and copies were verified by sequencing. New sequences were submitted to Genbank (JF431004-13).
A modification of the above RNA-isolation protocol was used in V. plicatum, which was recalcitrant to extraction due to secondary compounds. Standard methods, such as trizol, urea and SDS (Gough, 1988), or with Qiagen RNeasy kits with either RLT or RLC buffers, were not successful. Modifications such as adding 1–2 % polyvinylpyrrolidone 360000, 2 % beta mercaptoethanol (β-ME), 10 mg mL−1 proteinase K and 0·5 % DMSO yielded no RNA. Ultimately, a standard Qiagen RNeasy extraction kit with RLC buffer (including β-ME) and 1 % high molecular weight polyethylene glycol (PEG 20000) was utilized as outlined by Gehrig et al. (2000). The use of PEG 20000 provided limited but observable amounts of RNA. Using DNA extracted from DNeasy Qiagen Kit, all members of the CYC2 clade from V. plicatum were amplified. Two copies (Genbank numbers: JF431004-5) were uncovered, which were similar in sequence to each of the two previous copies published from Viburnum rhytidophyllum (Howarth and Donoghue, 2005). These two copies, DipsCYC2a and DipsCYC2b, are supported as sister to each other by 0·95 Bayesian posterior probabilities and 81 % parsimony bootstrap, and therefore represent an independent duplication from the two Caprifoliaceae copies, DipsCYC2A and DipsCYC2B (Howarth and Donoghue, 2005). Primers were designed to specifically amplify each V. plicatum copy, DipsCYC2a (F: 5'-CAAGGCAAGTAAAACCCTAGAATGGTTG and R: 5'-GCTCATCTTTTTCTCTTTGTTAACGC) and DipsCYC2b (F: 5'-CAAACCTAGCAAAACGCTAGATTGGCTGC and R: 5'-GCTTTACGTTGTCGCCTCAACAACCCG). rtPCR reaction protocols were as above.
RESULTS
Within the CYC2 clade of ECE-TCP genes, the Adoxaceae ancestrally contains only a single copy. In the Caprifoliaceae, on the other hand, CYC2 was duplicated co-incident with the divergence of the group and a shift to bilateral symmetry. One of the first diverging lineages, the Diervilleae, are weakly bilaterally symmetrical with a 2:3 form. Lonicera all have a 4:1 floral form, however, there is variability in how strongly bilaterally symmetrical they are. It was observed that all Lonicera species had three different slit sizes. The slit between the ventral and lateral petals is always the deepest, the slit between the lateral and dorsal petals is the next deepest and the single slit between the two dorsal petals is always the most shallow (see Fig. 2I).
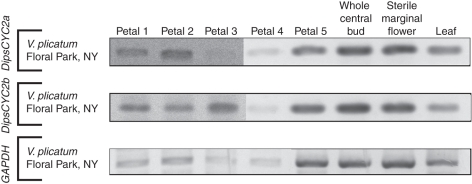
In the Adoxaceae, two copies of CYC2 in V. plicatum, DipsCYC2a and DipsCYC2b, which resulted from a duplication independent from those of Caprifoliaceae, were uncovered. The expression of both of these copies was found in whole buds of the central (fertile) flowers, in sterile marginal flowers (opened but still growing), and in leaves (Fig. 3). Expression was also seen in all five petals. Although expression in a few of these dissections was weak, this can be attributed to minimal quantities of RNA, especially given that expression of GAPDH was similarly weak (Fig. 3).
Fig. 3.
rtPCR of V. plicatum tissues, including each of the five separate petals, whole buds of the central fertile flowers, sterile marginal flowers and leaves. Petals 1–5 are described in Fig. 2. Both DipsCYC2 copies were present in all tissues, including across the entire corolla.
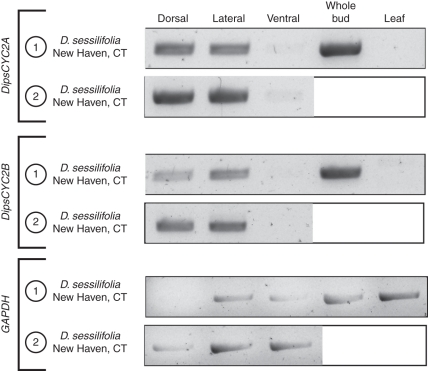
In D. sessilifolia of the Caprifoliaceae both DipsCYC2A and DipsCYC2B are similarly expressed (Fig. 4). Two accessions of D. sessilifolia were used, which show the same pattern. Both accessions had mixed ages of flower buds (from the youngest that were dissectible to nearly mature). DipsCYC2A and DipsCYC2B are both strongly expressed in both dorsal and lateral petal lobes. There is extremely weak, or no, expression in the ventral petals of either gene copy. The mixed age whole buds also exhibited strong expression and there is no expression in leaf tissue.
Fig. 4.
rtPCR of Diervilla sessilifolia tissues from two separate dissections, including dorsal, lateral and ventral corolla lobes. One dissection also included whole bud and leaf tissue. Both DipsCYC2A and DipsCYC2B are expressed in dorsal and lateral corolla lobes and are absent from ventral lobes. There was also strong expression of both copies in whole buds and absence from leaves. Boxed white areas were not sampled in those dissections.
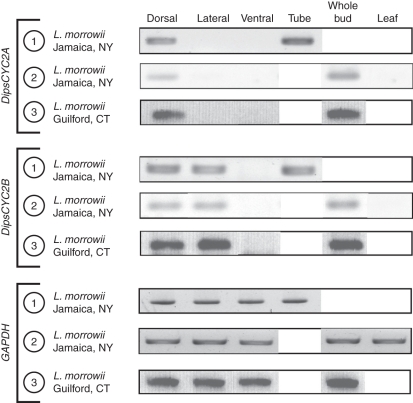
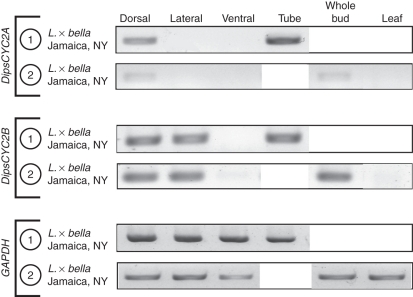
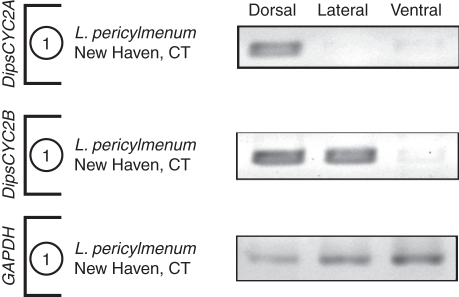
In all of the Lonicera species, by contrast, DipsCYC2A and DipsCYC2B diverge in expression. Lonicera morrowii, L. × bella and L. periclymenum all had a similar pattern of expression across all samples. DipsCYC2A was expressed in the dorsal petals, but not in the lateral or ventral petals (Figs 5–7). DipsCYC2B was expressed in dorsal and lateral petals but not in the ventral petal. This pattern holds in the mixed ages and the nearly mature bud dissections of L. morrowii (Fig. 5) and L. × bella (Fig. 6), and the mixed ages of L. pericylmenum (Fig. 7). Lonicera pericylmenum is strongly bilaterally symmetrical with deep slits between the ventral petal and the upper four petals. There is also strong expression of both DipsCYC2A and DipsCYC2B in all three taxa in whole bud extractions and in the floral tube. No expression of either gene was detected in leaves in any of the taxa.
Fig. 5.
rtPCR of Lonicera morrowii tissues from three separate dissections from two separate collection sites. All collections included dorsal, lateral and ventral dissections of corolla lobes. Dissection 1 also contained corolla tube tissue. Dissection 2 contained whole bud and leaf tissue. Dissection 3 contained whole bud tissue. DipsCYC2A was expressed in dorsal lobes, but not in lateral or ventral lobes. It was also strongly expressed in corolla tubes and whole buds. DipsCYC2B was expressed in dorsal and lateral corolla lobes but was absent from ventral lobes. DipsCYC2B was also found in corolla tube tissue and whole buds. Both DipsCYC2A and DipsCYC2B were absent in leaves. Boxed white areas were not sampled in those dissections.
Fig. 6.
rtPCR of Lonicera × bella tissues from two separate dissections. All collections included dorsal, lateral and ventral dissections of corolla lobes. Dissection 1 included corolla tube tissue. Dissection 2 included whole bud and leaf tissue. DipsCYC2A was expressed in dorsal lobes, but not in lateral or ventral lobes. It was also expressed in corolla tubes and whole buds. DipsCYC2B was expressed in dorsal and lateral corolla lobes but was absent from ventral lobes. DipsCYC2B was also found in corolla tube tissue and whole buds. Both DipsCYC2A and DipsCYC2B were absent in leaves. Boxed white areas were not sampled in those dissections.
Fig. 7.
rtPCR of a single dissection of Lonicera periclymenum. A single dissection from L. periclymenum included dorsal, lateral and ventral corolla lobes. DipsCYC2A was expressed in dorsal lobes, but not in lateral or ventral lobes. DipsCYC2B was expressed in dorsal and lateral corolla lobes but was absent from ventral lobes.
Lonicera sempervirens flowers have three different slit depths, like the other species, but differences are minor and the flowers are nearly radial in appearance. In L. sempervirens, all three samples showed strong expression in dorsal and lateral petals for DipsCYC2A, although all three were weaker in the lateral petals than in the dorsal petals (Fig. 8). There was also strong expression in whole buds at both younger and older stages and in the corolla tube. The same pattern is seen in the mixed bud stages as well as the nearly mature buds (Fig. 8, sample 3). DipsCYC2B, by contrast, is strongly expressed throughout the flower, although arguably weaker in ventral petals (Fig. 8). DipsCYC2B is also strongly expressed in whole-bud dissections and in the corolla tube. Unlike any of the other sampled Caprifoliaceae, L. sempervirens showed expression of both DipsCYC2A and DipsCYC2B in leaf tissue.
Fig. 8.
rtPCR of Lonicera sempervirens tissues from three separate dissections. All collections included dorsal, lateral, and ventral dissections of corolla lobes. Dissection 1 also included three differently aged whole buds (10-, 25- and 40-mm-long buds), an early inflorescence, and leaf tissue. Dissection 2 included corolla tube tissue. Dissection 3 included a whole young bud (10 mm). DipsCYC2A was expressed in dorsal and lateral lobes, and was absent from ventral lobes. By contrast, DipsCYC2B was expressed across all corolla lobes. Both copies were also found in all stages of flower buds, in corolla tube tissue, and weakly in leaves. Boxed white areas were not sampled in those dissections.
DISCUSSION
CYCLOIDEA duplications
The DipsCYC2 genes of Dipsacales all fall into the CYC2 clade of ECE-containing TCP genes (Howarth and Donoghue, 2006). These ECE-TCP genes duplicated twice around the divergence of the Pentapetalae (core eudicots excluding Gunnerales; Cantino et al., 2007), into the paralogous CYC1, CYC2 and CYC3 clades (Howarth and Donoghue, 2006). The predominately studied copies are orthologues that all fall into the CYC2 clade (including CYC and DICH paralogues from Antirrhinum and TCP1 from arabidopsis) (Howarth and Donoghue, 2006). There is growing evidence that CYC-like genes function similarly across rosids and asterids, with CYC2 members playing a role in dorsal identity of the flower. Although their expression is not examined here, there is evidence that members of the CYC3 clade may also play a role in floral symmetry, especially given that genes in the CYC2 and the CYC3 clades are tandemly duplicated in many lineages, including Caprifoliaceae (Howarth and Donoghue, 2005, 2006). Possible dorsoventral asymmetry to their expression has been shown previously (Howarth and Donoghue, 2006); however, CYC3 members have not yet been explored in any group. CYC1 members have not been implicated in floral symmetry, although they have been shown to play a role in plant branching in arabidopsis (Finlayson, 2007). Outside Pentapetalae, the ECE-containing TCP genes (which are orthologous to the clade containing CYC1, CYC2 and CYC3) appear to have been co-opted in the floral symmetry pathway independently multiple times. Specifically, independent duplications in Papaveraceae have correlative expression with shifts to monosymmetry (Damerval et al., 2007). A paralogous TCP gene has been implicated in patterning the monosymmetry of the Oryza palea (Yuan et al., 2009). TB1, the orthologue to all three copies in Zea, plays a role in both inflorescence branching and stamen abortion (Doebley et al., 1995), the latter also being a recognized function of CYC2 members (Luo et al., 1996).
Duplications in the CYC2 gene clade have been found in nearly every group examined, spanning core eudicots (Citerne et al., 2000, 2003; Fukuda et al., 2003; Gübitz et al., 2003; Hileman and Baum, 2003; Zhang et al., 2003; Ree et al., 2004; Smith et al., 2004; Feng et al., 2006; Chapman et al., 2008; Wang et al., 2008). In Dipsacales, the bilaterally symmetrical Caprifoliaceae have been explicitly compared with the radially symmetrical Adoxaceae and evidence of a duplication near the divergence of the Caprifoliaceae has been shown (Howarth and Donoghue, 2005). Other duplications within the Caprifoliaceae resulted in two copies of DipCYC2B in Morinaceae (which may be concurrent with a shift to calyx monosymmetry) and multiple copies of DipCYC2B in various Valerianaceae (Howarth and Donoghue, 2005; G. Boyden and D. G. Howarth, unpubl. res.) and Dipsacaceae (S. Carlson and M. J. Donoghue, unpubl. res.). Previously these genes have been cloned from Diervilla and Weigela (Diervilleae) and from Leycesteria, Triosteum, Symphoricarpos and multiple species of Lonicera but no evidence for additional duplications of DipsCYC2A or DipsCYC2B has been found within these groups (Howarth and Donoghue, 2005). Therefore, in this study, it was possible to examine shifts in gene expression following duplication, focusing directly on the location in the phylogeny where gene duplication and divergence occurred and where there were relevant shifts in flower morphology.
Flower symmetry evolution
Studies of floral symmetry have generally focused on species with derived morphologies, nested well within bilaterally symmetrical groups. Antirrhinum, Mohavea and Linaria are all nested within the bilaterally symmetrical Antirrhineae (Luo et al., 1996; Cubas et al., 1999; Hileman et al., 2003; Oyama and Baum, 2004). Lupinus, Lotus and Pisum are typical papilionoid legumes, and are nested well within this major clade (Citerne et al., 2006; Feng et al., 2006; Wang et al., 2008). Helianthus, Gerbera and Senecio are highly derived Asteraceae containing both radial disk and bilateral ray flowers (Broholm et al., 2008; Chapman et al., 2008; Kim et al., 2008). Iberis is nested within a small bilaterally symmetrical clade of Brassicaceae (Busch and Zachgo, 2007). Zhang et al. (2010) compared the expression of CYC2-like genes of outgroups to morphological shifts in old-world and new-world Malpighiaceae. A few studies have examined radially symmetrical species that are nested within bilaterally symmetrical clades, such as in Bournea, Cadia, Plantago and Tengia (Citerne et al., 2006; Zhou et al., 2008; Reardon et al., 2009; Pang et al., 2010). The present study attempts to understand the independent evolution of bilaterally symmetrical flowers within the Dipsacales, focusing specifically on the primary gene duplication event subtending the Caprifoliaceae, and subsequent evolutionary shifts in gene expression associated with flower form in its early-diverging lineages.
Character state reconstructions using likely outgroups (Paracryphiaceae) support the view that the first Dipsacales had radially symmetrical flowers (Donoghue et al., 2003; Winkworth et al., 2008b; Tank and Donoghue, 2010). The transition to bilateral symmetry occurred along the line leading to Caprifoliaceae, after divergence from its radially symmetrical sister group, Adoxaceae. Concurrent with that morphological transition there was a duplication in the CYC2-like gene lineage, yielding DipsCYC2A and DipsCYC2B (Fig. 1). Most Adoxaceae have only a single form of a CYC2-like gene, DipsCYC2 (Howarth and Donoghue, 2005). However, the present analyses confirm that a separate duplication occurred within Viburnum, resulting in two CYC2 copies, DipsCYC2a and DipsCYC2b, both of which are expressed throughout the corolla (Fig. 3). In other radially symmetrical flowers, CYC2 genes are either expressed across the entire corolla (as in Viburnum) or are entirely absent. Elatinaceae, Cadia and Tengia show CYC expression across the entire corolla (Citerne et al., 2006; Pang et al., 2010; Zhang et al., 2010), whereas in Antirrhinum peloric mutants, Bournea (late expression), Centroplacaceae and Plantago, floral CYC expression is lacking (Luo et al., 1996; Zhou et al., 2008; Reardon et al., 2009; Zhang et al., 2010). Based on the discovery that CYC2 genes are expressed across the entire corolla in Viburnum, and that they are widely expressed in Diervilla, we hypothesize that the first Dipsacales expressed DipsCYC2 throughout the corolla.
Within Caprifoliaceae, the first split separated Diervilleae from all of the rest (see Donoghue et al., 2003). Diervilleae contains two genera, Diervilla and Weigela, which are both weakly bilaterally symmetrical with the 2:3 floral form, and marked by a morphologically distinctive ventral corolla lobe (Fig. 2). The present expression data for Diervilla indicate that DipsCYC2A and DipsCYC2B are expressed in a similar pattern to each other; both are strongly expressed in the dorsal and lateral petals, and are nearly completely absent from the ventral petal (Fig. 4). Based on this observation we hypothesize that the expression of Caprifoliaceae DipsCYC2 orthologues shifted out of the ventral petal. This shift could have occurred before or after the duplication (Fig. 9).
Fig. 9.
A model for transitions in gene expression in Diervilleae and Lonicera placed on a phylogeny and in the order that we hypothesize that they occurred (boxed). Striped orange lines indicate expression of both DipsCYC2a and DipsCYC2b in V. plicatum. Red shading shows expression of DipsCYC2A and black striped lines show the expression of DipsCYC2B in Caprifoliaceae. The first step occurred around the divergence of Caprifoliaceae and the duplication event leading to two copies. This transition is correlated with the loss of expression in the ventral corolla lobe (Diervilleae). The second transition involved the decoupling of DipsCYC2A and DipsCYC2B expression, with DipsCYC2A only in dorsal corolla lobes, and DipsCYC2B in dorsal and lateral lobes (Lonicera). This transition could have happened anywhere along the grayed nodes. A third shift occurred in the nearly radial L. sempervirens, in which both copies shifted ventrally.
Within the large clade including the remainder of the Caprifoliaceae, the basal split separated the Caprifolieae lineage from all the rest (Figs 1 and 9). While the relationships are still uncertain, Heptacodium (with a 2:3 floral form) appears as sister to the Caprifolieae in most analyses (reviewed in Winkworth et al., 2008a), and the best-supported result to date shows Triosteum as sister to the other three genera, with Leycesteria and Symphoricarpos forming a clade that is sister to Lonicera (Theis et al., 2008; Smith, 2009). These data suggest that the 4:1 form may have evolved independently in Lonicera and Triosteum, implying that shifts in the type of bilateral symmetry are labile in Caprifolieae. Even within Lonicera, the degree of separation between the ventral petal and the four upper petals varies greatly.
In the species examined that have a clearly recognizable 4:1 form (L. periclymenum, L. morrowii and L. × bella), DipsCYC2A is expressed in only the dorsal petals, whereas DipsCYC2B is expressed in both dorsal and the lateral petals (Figs 5–7). In view of the observation that these genes are expressed in the dorsal and the lateral petals in Diervilla, we hypothesize that DipsCYC2A expression became further restricted in Lonicera to the dorsal petals (Fig. 9). This is a more parsimonious explanation than the expansion of DipsCYC2B expression. As the present sample includes representatives of the two major lineages within Lonicera (Theis et al., 2008; Smith, 2009), it is inferred that the restriction of DipsCYC2A occurred at some point prior to the origin of crown group Lonicera. It is likely, therefore, that the expression pattern of the nearly radial L. sempervirens is derived, with DipsCYC2A in dorsal and lateral petals and DipsCYC2B in all petals (Fig. 8). In this case, it would appear that both DipsCYC2A and DipsCYC2B shifted their expression ventrally.
Hypothesized shifts
It has been argued that the diversification of plants relates to gene duplication and the subsequent adaptation of the duplicate genes (Flagel and Wendel, 2009; Van de Peer et al., 2009). When a gene duplicates, its primary fate is to return to one copy (Lynch and Conery, 2000). One way that both gene copies can be maintained, however, is by undergoing subfunctionalization, in which the two duplicates take on different, complementary, roles (Lynch and Force, 2000). An alternative is when one paralogous copy undergoes neofunctionalization, taking on a brand new role relative to the original gene (Ohno, 1970). Accordingly, duplication can promote the rapid modification of expression patterns (Adams et al., 2003). Here, it has been shown that DipsCYC2a and DipsCYC2b are expressed throughout the corolla in the radially symmetrical flowers of Viburnum (Adoxaceae) and that a first step toward bilateral symmetry entailed a shift in both duplicates out of the ventral region of the corolla. Subsequently, during the divergence of the Caprifolieae, we hypothesize that one of the duplicates, DipsCYC2A, became further restricted in its expression to the two dorsal corolla lobes. At this point, DipsCYC2A and DipsCYC2B diverged in their zones of expression. We view this as a form of subfunctionalization, in which one copy retains the ancestral expression pattern, and one becomes restricted in its expression to just a portion of the ancestral zone. However, judging by their coupled expansion in expression in L. sempervirens, the expressions of the two gene copies appear not to be completely decoupled from one another (Fig. 9).
The major transitions in the evolution of bilateral symmetry involve the individualization of distinct zones of the corolla along the dorsoventral axis. Within Caprifoliaceae, the first step entailed the individuation of the ventral corolla lobe from the other four corolla lobes. This is seen at the morphological level (differentiation of the ventral corolla lobe in Diervilleae) and genetically (the loss of expression of both DipCYC2 copies in the ventral lobe). The second step resulted in the individuation of three separate corolla zones: ventral, lateral and dorsal. We hypothesize that this was underlain by the decoupling of the two DipsCYC2 copies, such that one became expressed only in dorsal tissue while the other maintained expression in the lateral and dorsal tissues. This partial subfunctionalization may have set the stage for the two dorsal petals to operate independently of the two lateral petals.
Nearly all bilaterally symmetrical groups examined have multiple copies of CYC2-like genes [one clear exception is Iberis (Busch and Zachgo, 2007), which falls into a small bilaterally symmetrical group of the radially symmetrical Brassicaceae]. Caprifoliaceae, Malpighiaceae and Asteraceae all have duplications in CYC2-like genes that correlate with shifts in floral symmetry (Howarth and Donoghue, 2006; Chapman et al., 2008; Zhang et al., 2010). In bilaterally symmetrical flowers in which the expression of multiple copies has been documented, one copy is nearly always dorsally restricted with respect to the other copy. It is perhaps not surprising that duplicate gene copies are showing evidence of partial subfunctionalization, with one copy expanding or restricting expression with respect to the other. What is striking, however, is that these shifts in expression are happening again and again in the same way in widely separated plant groups. In Lonicera, it is found that the expression of DipsCYC2A is restricted to just the dorsal petals, while that of DipsCYC2B remains in dorsal and lateral petals. A similar pattern has been found in Malpighiaceae, with at least one gene duplicate expressed in just the dorsal petal, and at least one gene duplicate expressed in both the dorsal and lateral petals (Zhang et al., 2010). In Papilionoid legumes, the same pattern exists in Pisum and Lotus (Feng et al., 2006; Wang et al., 2008). In Pisum, PsCYC2 (LST1) is expressed only in the dorsal petal and mutations of this gene result in dorsal petals that are lateralized. PsCYC3 (K) is expressed in both dorsal and lateral petals and mutations result in lateral petals that are ventralized. Mutants at both loci result in fully ventralized flowers. Differential expression in petals has also been documented in Opithandra (Gesneriaceae) in which one gene copy is restricted to just the dorsal petals, while a separate duplicate is expressed throughout the corolla (Song et al., 2009). Even in Antirrhinum, in which CYC and DICH are both restricted to the dorsal petals, DICH is restricted to the dorsal portion of the dorsal lobes, while CYC is expanded over most of the dorsal portion of the corolla (Luo et al., 1996). These data, taken together, imply that differential expression of duplicated CYC2 genes may play a role in setting up petal identity with respect to the axis; i.e. whether petals will have dorsal, lateral or ventral identity.
Alternatively, the decoupling of the expression of CYC2 duplicates may play a role in the orientation of the lateral petals. Three plants groups have been shown to have one CYC2 duplicate expressed in dorsal petals and a second CYC2 duplicate expressed in dorsal and lateral petals: Lonicera, Pisum (Wang et al., 2008) and Malpighiaceae (Zhang et al., 2010). All three of these groups also share an uncommon morphology in which the lateral petals are oriented more dorsally than they would be in a radially symmetric flower. Most bilaterally symmetrical groups exhibit the 2:3 form, with the two lateral petals oriented ventrally. It is possible, therefore, that CYC2 members play a role in the orientation of the lateral petals, possibly through the expression of fewer copies in the lateral petals than in the dorsal petals. This could be controlled through either differential growth, changes in slit depth that can determine petal orientation, or a gradient of expression.
In summary, we hypothesize that the first Dipsacales had radially symmetrical flowers with ubiquitous corolla expression of CYC2. Following the split between the Adoxaceae and the Caprifoliaceae, CYC2 was duplicated along the Caprifoliaceae branch and both copies lost expression in the ventral corolla lobe (Fig. 9). This shift in expression was accompanied by a shift in the morphology of the ventral corolla lobe relative to the dorsal and lateral lobes. Subsequently, a shift in DipsCYC2A expression along the line leading to Lonicera (partial subfunctionalization) resulted in a distinction between dorsal and lateral petal identities, and may have affected corolla lobe orientation, yielding the 4:1 morphology. Coupled ventral expansion of both DipsCYC2 paralogues in L. sempervirens was associated with reversion to a more radially symmetrical flower. We hypothesize that the more dorsally restricted the expression of CYC2 paralogues in the corolla, the more extreme the resulting bilateral symmetry.
ACKNOWLEDGEMENTS
We thank members of the laboratories of D.G.H. and M.J.D., and M. P. Dunn for helpful discussions during this study. We also thank Alan Cressler for the use of his photo. D.G.H. is grateful for the Forest B. H. and Elizabeth D. W. Brown Postdoctoral Fellowships from Yale University. The work of M.J.D. on angiosperm phylogeny was supported by an Assembling the Tree of Life (ATOL) grant from the National Science Foundation.
LITERATURE CITED
- Adams K, Cronn R, Percifield R, Wendel J. Genes duplicated by polyploidy show unequal contributions to the transcriptome and organ-specific reciprocal silencing. Proceedings of the National Academy of Sciences of the USA. 2003;100:4649–4654. doi: 10.1073/pnas.0630618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida J, Rocheta M, Galego L. Genetic control of flower shape in Antirrhinum majus. Development. 1997;124:1387–1392. doi: 10.1242/dev.124.7.1387. [DOI] [PubMed] [Google Scholar]
- Backlund A, Donoghue MJ. Morphology and phylogeny of the order Dipsacales. 1996 PhD Dissertation, Uppsala University, Sweden. [Google Scholar]
- Bell CD, Donoghue MJ. Dating the Dipsacales: comparing models, genes, and evolutionary implications. American Journal of Botany. 2005;92:284–296. doi: 10.3732/ajb.92.2.284. [DOI] [PubMed] [Google Scholar]
- Bell CD, Edwards EJ, Kim ST, Donoghue MJ. Dipsacales phylogeny based on chloroplast DNA sequences. Harvard Papers in Botany. 2001;6:481–499. [Google Scholar]
- Broholm SK, Tähtiharju S, Laitinen RAE, Albert VA, Teeri TH, Elmaa P. A TCP domain transcription factor controls flower type specification along the radial axis of the Gerbera (Asteraceae) inflorescence. Proceedings of the National Academy of Arts and Sciences. 2008;105:9117–9122. doi: 10.1073/pnas.0801359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch A, Zachgo S. Control of corolla monosymmetry in the Brassicaceae Iberis amara. Proceedings of the National Academy of Arts and Sciences. 2007;104:16714–16719. doi: 10.1073/pnas.0705338104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantino PD, Doyle JA, Graham SW, et al. Towards a phylogenetic nomenclature of Tracheophyta. Taxon. 2007;56:822–846. [Google Scholar]
- Chapman MA, Leebens-Mack JH, Burke JM. Positive selection and expression divergence following gene duplication in the sunflower CYCLOIDEA gene family. Molecular Biology and Evolution. 2008;25:1260–1273. doi: 10.1093/molbev/msn001. [DOI] [PubMed] [Google Scholar]
- Citerne HL, Möller M, Cronk QCB. Diversity of cycloidea-like genes in Gesneriaceae in relation to floral symmetry. Annals of Botany. 2000;86:167–176. [Google Scholar]
- Citerne HL, Luo D, Pennington RT, Coen E, Cronk QCB. A phylgenomic investigation of CYCLOIDEA-like TCP genes in the Leguminosae. Plant Physiology. 2003;131:1042–1053. doi: 10.1104/pp.102.016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citerne HL, Pennington RT, Cronk QCB. An apparent reversal in floral symmetry in the legume Cadia is a homeotic transformation. Proceedings of the National Academy of Arts and Sciences. 2006;103:12017–12020. doi: 10.1073/pnas.0600986103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corley SB, Carpenter R, Copsey L, Coen E. Floral asymmetry involves an interplay between TCP and MYB transcription factors in Antirrhinum. Proceedings of the National Academy of Sciences of the USA. 2005;102:5068–5073. doi: 10.1073/pnas.0501340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MMR, Fox S, Hanna Ai, Baxter C, Coen E. Evolution of regulatory interactions controlling floral asymmetry. Development. 2005;132:5093–5101. doi: 10.1242/dev.02085. [DOI] [PubMed] [Google Scholar]
- Cubas P, Vincent C, Coen E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature. 1999;401:157–161. doi: 10.1038/43657. [DOI] [PubMed] [Google Scholar]
- Cubas P, Coen E, Zapater JMM. Ancient asymmetries in the evolution of flowers. Current Biology. 2001;11:1050–1052. doi: 10.1016/s0960-9822(01)00295-0. [DOI] [PubMed] [Google Scholar]
- Damerval C, Le Guilloux M, Jager M, Charon C. Diversity and evolution of CYCLOIDEA-like TCP genes in relation to flower development in Papaveraceae. Plant Physiology. 2007;143:759–772. doi: 10.1104/pp.106.090324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebley J, Stec A, Gustus C. Teosinte branched1 and the origin of maize: evidence for epistasis and the evolution of dominance. Genetics. 1995;141:333–346. doi: 10.1093/genetics/141.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue MJ, Ree RH, Baum DA. Phylogeny and the evolution of flower symmetry in the Asteridae. Trends in Plant Science. 1998;3:311–317. [Google Scholar]
- Donoghue MJ, Bell CD, Li JH. Phylogenetic patterns in Northern Hemisphere plant geography. International Journal of Plant Sciences. 2001a;162:S41–S52. [Google Scholar]
- Donoghue MJ, Eriksson T, Reeves PA, Olmstead RG. Phylogeny and phylogenetic taxonomy of Dipsacales, with special reference to Sinadoxa and Tetradoxa (Adoxaceae) Harvard Papers in Botany. 2001b;6:459–479. [Google Scholar]
- Donoghue MJ, Bell CD, Winkworth RC. The evolution of reproductive characters in Dipsacales. International Journal of Plant Sciences. 2003;164(5 Suppl.):S453–S464. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- Endress PK. Symmetry in flowers: diversity and evolution. International Journal of Plant Sciences. 1999;160:S3–S23. doi: 10.1086/314211. [DOI] [PubMed] [Google Scholar]
- Feng X, Zhao Z, Tian Z, et al. Control of petal shape and floral zygomorphy in Lotus japonicus. Proceedings of the National Academy of Arts and Sciences. 2006;103:4970–4975. doi: 10.1073/pnas.0600681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson SA. Arabidopsis TEOSINTE BRANCHED1-LIKE 1 regulates axillary bud outgrowth and is homologous to monocot TEOSINTE BRANCHED1. Plant Cell Physiology. 2007;48:667–677. doi: 10.1093/pcp/pcm044. [DOI] [PubMed] [Google Scholar]
- Flagel LE, Wendel JF. Genome duplication and evolutionary novelty in plants. New Phytologist. 2009;183:557–564. doi: 10.1111/j.1469-8137.2009.02923.x. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Yokoyama J, Maki M. Molecular evolution of cycloidea-like genes in Fabaceae. Journal of Molecular Evolution. 2003;57:588–597. doi: 10.1007/s00239-003-2498-2. [DOI] [PubMed] [Google Scholar]
- Fukuoka N. Taxonomic study of Caprifoliaceae. Memoirs of the Faculty of Science Kyoto University Series of Biology. 1972;6:15–58. [Google Scholar]
- Galego L, Almeida J. Role of DIVARICATA in the control of dorsoventral asymmetry in Antirrhinum flowers. Genes and Development. 2002;16:880–891. doi: 10.1101/gad.221002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Tao J-H, Yan D, Wang Y-Z, Li Z-Y. Expression differentiation of CYC-like floral symmetry genes correlated with their protein sequence divergence in Chirita heterotricha (Gesneriaceae) Development, Genes & Evolution. 2008;218:341–351. doi: 10.1007/s00427-008-0227-y. [DOI] [PubMed] [Google Scholar]
- Gehrig HH, Winter K, Cushman J, Borland A, Taybi T. An improved RNA isolation method for succulent plant species rich in polyphenols and polysaccharides. Plant Molecular Biology Reporter. 2000;18:369–376. [Google Scholar]
- Gough NM. Rapid and quantitative preparation of cytoplasmic RNA from small numbers of cells. Analytical Biochemistry. 1988;173:93–95. doi: 10.1016/0003-2697(88)90164-9. [DOI] [PubMed] [Google Scholar]
- Gübitz T, Caldwell A, Hudson A. Rapid molecular evolution of CYCLOIDEA-like genes in Antirrhinum and its relatives. Molecular Biology and Evolution. 2003;20:1537–1544. doi: 10.1093/molbev/msg166. [DOI] [PubMed] [Google Scholar]
- Hileman LC, Baum DA. Why do paralogs persist? Molecular evolution of CYCLOIDEA and related floral symmetry genes in Antirrhineae (Veroniacaceae) Molecular Biology and Evolution. 2003;20:591–600. doi: 10.1093/molbev/msg063. [DOI] [PubMed] [Google Scholar]
- Hileman LC, Kramer EM, Baum DA. Differential regulation of symmetry genes and the evolution of floral morphologies. Proceedings of the National Academy of Sciences of the USA. 2003;100:12814–12819. doi: 10.1073/pnas.1835725100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth DG, Donoghue MJ. Duplications in CYC-like genes from Dipsacales correlate with floral form. International Journal of Plant Sciences. 2005;166:357–370. [Google Scholar]
- Howarth DG, Donoghue MJ. Phylogenetic analysis of the “ECE” (CYC TB1) clade reveals duplications predating the core eudicots. Proceedings of the National Academy of Arts and Sciences. 2006;103:9101–9106. doi: 10.1073/pnas.0602827103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd WS, Sanders RW, Donoghue MJ. Angiosperm family pairs – preliminary phylogenetic analyses. Harvard Papers in Botany. 1994;5:1–51. [Google Scholar]
- Kim M, Cui M-L, Cubas P, et al. Regulatory genes control a key morphological and ecological trait transferred between species. Science. 2008;322:1116–1119. doi: 10.1126/science.1164371. [DOI] [PubMed] [Google Scholar]
- Luo D, Carpenter R, Vincent C, Copsey L, Coen E. Origin of floral asymmetry in Antirrhinum. Nature. 1996;383:794–799. doi: 10.1038/383794a0. [DOI] [PubMed] [Google Scholar]
- Luo D, Carpenter R, Copsey L, Vincent C, Clark J, Coen E. Control of organ asymmetry in flowers of Antirrhinum. Cell. 1999;99:367–376. doi: 10.1016/s0092-8674(00)81523-8. [DOI] [PubMed] [Google Scholar]
- Lynch M, Conery J. The evolutionary fate and consequences of duplicate genes. Science. 2000;290:1151–1155. doi: 10.1126/science.290.5494.1151. [DOI] [PubMed] [Google Scholar]
- Lynch M, Force A. The probability of duplicate gene preservation by subfunctionalization. Genetics. 2000;154:459–473. doi: 10.1093/genetics/154.1.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal PR. Floral symmetry and its role in plant–pollinator systems: terminology, distribution, and hypotheses. Annual Review of Ecology and Systematics. 1998;29:345–373. [Google Scholar]
- Ohno S. Evolution by gene duplication. New York, NY: Springer-Verlag; 1970. [Google Scholar]
- Oyama RK, Baum DA. Phylogenetic relationships of North American Antirrhinum (Veronicaceae) American Journal of Botany. 2004;91:918–925. doi: 10.3732/ajb.91.6.918. [DOI] [PubMed] [Google Scholar]
- Pang H-B, Sun Q-W, He S-Z, Wang Y-Z. Expression pattern of CYC-like genes relating to a dorsalized actinomorphic flower in Tengia (Gesneriaceae) Journal of Systematics and Evolution. 2010;48:309–317. [Google Scholar]
- Pyck N. Phylogenetic relationships within Dipsacales: a combined molecular and morphological approach. 2001 PhD Dissertation, Katholieke University, Leuven, Belgium. [Google Scholar]
- Reardon W, Fitzpatrick DA, Fares MA, Nugent JM. Evolution of flower shape in Plantago lanceolata. Plant Molecular Biology. 2009;71:241–250. doi: 10.1007/s11103-009-9520-z. [DOI] [PubMed] [Google Scholar]
- Ree RH, Citerne HL, Lavin M, Cronk QCB. Heterogeneous selection on LEGCYC paralogs in relation to flower morphology and the phylogeny of Lupinus (Leguminosae) Molecular Biology and Evolution. 2004;21:321–331. doi: 10.1093/molbev/msh022. [DOI] [PubMed] [Google Scholar]
- Reeves PA, Olmstead RG. Evolution of the TCP gene family in Asteridae: cladistic and network approaches to understanding regulatory gene family diversification and its impact on morphological evolution. Molecular Biology and Evolution. 2003;20:1997–2009. doi: 10.1093/molbev/msg211. [DOI] [PubMed] [Google Scholar]
- Rehder A. Synopsis of the genus Lonicera. Missouri Botanical Garden Annual Report. 1903;14:27–232. [Google Scholar]
- Sargent RD. Floral symmetry affects speciation rates in angiosperms. Proceedings of the Royal Society of London, Series B. 2004;271:603–608. doi: 10.1098/rspb.2003.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JF, Hileman LC, Powell MP, Baum DA. Evolution of GCYC, a Gesneriaceae homolog of CYCLOIDEA, within Gesnerioideae (Gesneriaceae) Molecular Phylogenetics and Evolution. 2004;31:765–779. doi: 10.1016/j.ympev.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Smith SA. Taking into account phylogenetic and divergence-time uncertainty in a parametric biogeographical analysis of the Northern Hemisphere plant clade Caprifolieae. Journal of Biogeography. 2009;36:2324–2337. [Google Scholar]
- Smith SA, Donoghue MJ. Rates of molecular evolution are linked to life history in flowering plants. Science. 2008;322:86–89. doi: 10.1126/science.1163197. [DOI] [PubMed] [Google Scholar]
- Song C-F, Lin Q-B, Liang R-H, Wang Y-Z. Expressions of ECE-CYC2 clade genes relating to abortion of both dorsal and ventral stamens in Opithandra (Gesneriaceae) BMC Evolutionary Biology. 2009;9:244. doi: 10.1186/1471-2148-9-244. doi:10.1186/1471-2148-9-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand AE, Leebens-Mack J, Milligan BG. Nuclear DNA-based markers for plant evolutionary biology. Molecular Ecology. 1997;6:113–118. doi: 10.1046/j.1365-294x.1997.00153.x. [DOI] [PubMed] [Google Scholar]
- Tank DC, Donoghue MJ. Phylogeny and phylogenetic nomenclature of the Campanulidae based on an expanded sample of genes and taxa. Systematic Botany. 2010;35:425–441. [Google Scholar]
- Theis N, Donoghue MJ, Li J. Phylogenetics of the Caprifolieae and Lonicera (Dipsacales) based on nuclear and chloroplast DNA sequences. Systematic Botany. 2008;33:776–783. [Google Scholar]
- Van De Peer Y, Maere S, Meyer A. The evolutionary significance of ancient genome duplications. Nature Reviews Genetics. 2009;10:724–732. doi: 10.1038/nrg2600. [DOI] [PubMed] [Google Scholar]
- Wang Z, Luo Y, Li X, et al. Genetic control of floral zygomorphy in pea (Pisum sativum L.) Proceedings of the National Academy of Sciences of the USA. 2008;105:10414–10419. doi: 10.1073/pnas.0803291105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weberling F. Morphology of flowers and inflorescences. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- Winkworth RC, Bell CD, Donoghue MJ. Mitochondrial sequence data and Dipsacales phylogeny: mixed models, partitioned Bayesian analyses, and model selection. Molecular Phylogenetics and Evolution. 2008a;46:830–843. doi: 10.1016/j.ympev.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Winkworth RC, Lundberg J, Donoghue MJ. Toward a resolution of Campanulid phylogeny, with special reference to the placement of Dipsacales. Taxon. 2008b;57:53–65. [Google Scholar]
- Yuan Z, Gao S, Xue D-W, et al. RETARDED PALEA1 controls palea development and floral zygomorphy in rice. Plant Physiology. 2009;149:235–244. doi: 10.1104/pp.108.128231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Kramer EM, Davis CC. Floral symmetry genes and the origin and maintenance of zygomorphy in a plant pollinator mutualism. Proceedings of the National Academy of Sciences of the USA. 2010;107:6388–6393. doi: 10.1073/pnas.0910155107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WH, Chen JD, Li J, Chen HB, Tang YC. Phylogeny of the dipsacales s.l. based on chloroplast trnL-F and ndhF sequences. Molecular Phylogenetics and Evolution. 2003;26:176–189. doi: 10.1016/s1055-7903(02)00303-2. [DOI] [PubMed] [Google Scholar]
- Zhou X-R, Wang Y-Z, Smith JF, Chen R. Altered expression patterns of TCP and MYB genes relating to the floral developmental transition from initial zygomorphy to actinomorphy in Bournea (Gesneriaceae) New Phytologist. 2008;178:532–543. doi: 10.1111/j.1469-8137.2008.02384.x. [DOI] [PubMed] [Google Scholar]