Abstract
Background and Aims
Homeotic transitions are usually dismissed by population geneticists as credible modes of evolution due to their assumed negative impact on fitness. However, several lines of evidence suggest that such changes in organ identity have played an important role during the origin and subsequent evolution of the angiosperm flower. Better understanding of the performance of wild populations of floral homeotic varieties should help to clarify the evolutionary potential of homeotic mutants. Wild populations of plants with changes in floral symmetry, or with reproductive organs replacing perianth organs or sepals replacing petals have already been documented. However, although double-flowered varieties are quite popular as ornamental and garden plants, they are rarely found in the wild and, if they are, usually occur only as rare mutant individuals, probably because of their low fitness relative to the wild-type. We therefore investigated a double-flowered variety of lesser periwinkle, Vinca minor flore pleno (fl. pl.), that is reported to have existed in the wild for at least 160 years. To assess the merits of this plant as a new model system for investigations on the evolutionary potential of double-flowered varieties we explored the morphological details and distribution of the mutant phenotype.
Methods
The floral morphology of the double-flowered variety and of a nearby population of wild-type plants was investigated by means of visual inspection and light microscopy of flowers, the latter involving dissected or sectioned floral organs.
Key Results
The double-flowered variety was found in several patches covering dozens of square metres in a forest within the city limits of Jena (Germany). It appears to produce fewer flowers than the wild-type, and its flowers are purple rather than blue. Most sepals in the first floral whorl resemble those in the wild-type, although occasionally one sepal is broadened and twisted. The structure of second-whorl petals is very similar to that of the wild-type, but their number per flower is more variable. The double-flowered character is due to partial or complete transformation of stamens in the third whorl into petaloid organs. Occasionally, ‘flowers within flowers’ also develop on elongated pedicels in the double-flowered variety.
Conclusions
The flowers of V. minor fl. pl. show meristic as well as homeotic changes, and occasionally other developmental abnormalities such as mis-shaped sepals or loss of floral determinacy. V. minor fl. pl. thus adds to a growing list of natural floral homeotic varieties that have established persistent populations in the wild. Our case study documents that even mutant varieties that have reproductive organs partially transformed into perianth organs can persist in the wild for centuries. This finding makes it at least conceivable that even double-flowered varieties have the potential to establish new evolutionary lineages, and hence may contribute to macroevolutionary transitions and cladogenesis.
Keywords: Double-flowered variety, homeosis, lesser periwinkle, macroevolution, Vinca minor fl. pl
INTRODUCTION
Compared with the wild-type, homeotic mutants have organs replaced by those of a contrasting identity, thus displaying dramatic yet coordinated changes in their body plans (Lewis, 1994). Homeotic mutants are well known from the fruit fly Drosophila melanogaster and other model animals, but they are also frequent in plants (e.g. Meyerowitz et al., 1989), where naturally occurring homeotic mutants have been proposed to play important roles during evolution (e.g. Sattler, 1988; Ronse De Craene, 2003; Rudall and Bateman, 2003). Especially prominent are floral homeotic mutants. In the model plant thale-cress (Arabidopsis thaliana) many of such mutants fall into one of three classes: A, B or C. Ideal class A mutants have carpels replacing sepals in the first whorl of the flower, and produce stamens rather than petals in the second whorl. Class B mutants have sepals instead of petals in the second whorl and carpels rather than stamens in the third whorl. Class C mutants have flowers in which stamens and carpels (i.e. the reproductive organs) are replaced by petals and sepals, respectively (perianth organs); moreover, the determinacy of floral growth is lost, so that the mutant flowers produce a larger number of floral organs than wild-type flowers (Meyerowitz et al., 1989; Theißen, 2001).
The three classes of floral homeotic mutants have been explained by simple genetic models, the most widely known of which is the ABC model (Coen and Meyerowitz, 1991). Inspired by the three classes of homeotic mutants, it proposes three different floral homeotic functions, termed A, B and C, to explain how the different floral organs develop their unique identities. Specifically, the model maintains that class A genes specify sepals in the first floral whorl, the combined activity of class A and class B genes specifies petals in the second whorl, class B together with class C genes specify stamens in the third whorl, and a class C gene specifies carpels in the fourth whorl. Cloning of the floral homeotic genes in several model plants such as Arabidopsis thaliana, Antirrhinum majus (snapdragon) and Petunia × hybrida (petunia) revealed that they all encode transcription factors, i.e. proteins that recognize specific DNA motifs of other genes and influence their transcription. In contrast with the homeotic genes of animals, however, they do not encode homeodomain proteins but rather (with few exceptions) produce MIKC-type MADS-domain proteins (reviewed by Becker and Theißen, 2003; Krizek and Fletcher, 2005). The combinatorial interaction of floral homeotic genes is probably based on the formation of multimeric transcription factor complexes that also include class E (or SEPALLATA-like) proteins, as outlined by the ‘floral quartet model’ (Theißen, 2001).
There is no doubt that floral homeotic mutants have been invaluable as an entry point into studies of the molecular basis of flower development. However, whether floral homeotic mutants also play a meaningful role in evolution is a highly controversial topic. Some floral homeotic mutants have phenotypes resembling differences in character states between major flowering plant lineages, such as petaloid rather than sepaloid first-whorl floral organs, or actinomorphic rather than zygomorphic flowers. One can therefore assume that the loci underlying such ‘phylo-mimicking mutants’ indicate genes that play essential roles during the evolution of major morphological characters, an observation that is compatible with the view that floral homeotic transitions occurred during evolution (Haag and True, 2001). Along these lines, phylogenetic and morphological analyses provide support for the claim of homeotic transitions in several groups of angiosperms. For example, there is evidence that in the flowers of several Zingiberales, some stamens have been replaced by a lip, others by petaloid staminodes (Kirchoff, 1991), and that the petals of Rosaceae (including roses) originated from stamens (Ronse De Craene, 2003).
Despite considerable circumstantial evidence that homeotic changes played an important role during the evolution of the flower, it is still widely believed by most evolutionary biologists and population geneticists that homeotic transitions, due to their assumed negative impact on fitness in the wild, generate nothing but ‘hopeless monsters’ and thus are not reasonable modes of evolution. However, overly simplistic theoretical arguments have often been used that may not reflect the situation in natural habitats (reviewed by Theißen, 2006, 2009). Unfortunately, we know very little about the performance of homeotic mutants in natural populations, which should be changed to better understand their evolutionary potential (Bateman and DiMichele, 2002; Rudall and Bateman, 2003, 2004; Vergara-Silva, 2003; Theißen, 2006, 2009).
Like all other mutants, homeotic mutants first appear as rare individuals in populations of wild-type organisms. To establish new evolutionary lineages, such mutants must therefore survive many years under conditions of natural selection until the mutant homeotic allele obtains fixation, presumably accompanied by modifying mutations that fine-tune the new ‘body plan’. There is little doubt that most floral homeotic mutants have sufficiently reduced fitness so that their long-term survival in nature is seriously hampered (Nutt et al., 2006). Only a small fraction of homeotic mutants is likely to have sufficient fitness under natural growth conditions to establish new evolutionary lineages. A plausible approach to identify candidates for such mutants is thus to seek populations of floral homeotic varieties in the wild that have existed for considerable time and thus have already documented viable levels of fitness. However, few of such populations have been described in the literature. One case is bicalyx, a variant of Clarkia concinna (Onagraceae) in which the petals are transformed into sepaloid organs (Ford and Gottlieb, 1992). Another example is a peloric version of common toadflax (Linaria vulgaris) with actinomorphic rather than zygomorphic flowers (Cubas et al., 1999). Both these examples are mutated at a single, recessive locus. It became evident that the toadflax variant is affected in a CYCLOIDEA-like gene by methylation of DNA (epimutation, Cubas et al., 1999), but for the bicalyx variant no molecular data have been reported so far. A third case that is being intensively studied is Stamenoid petals (Spe), a shepherd's purse (Capsella bursa-pastoris) variety that has petals completely replaced by stamens, whereas all other organs are unchanged (Hintz et al., 2006; Nutt et al., 2006; Ziermann et al., 2009). The mutant allele of the Spe locus proved to be semi-dominant rather than recessive, suggesting a gain-of-function mutation.
In none of these cases are reproductive organs (stamens or carpels) transformed into non-reproductive organs, which may at least partly explain their reasonable performance in the wild. This is often different in the case of ‘double-flowered’ or ‘full-flowered’ flore pleno varieties, loosely defined as varieties that have flowers with extra petals, often displaying ‘flower within flower’ characteristics. Class C floral homeotic mutants such as the null mutant of the AGAMOUS gene of Arabidopsis thaliana are thus an extreme case of ‘double-flowered’ mutants.
Double-flowered varieties have been known for a long time. For example, double-flowered roses were mentioned by Theophrastus in his Enquiry into Plants, written before 286 BC; also, double-flowered roses and peonies were cultivated in ancient China (Meyerowitz et al., 1989). Double-flowered varieties of many species became well known among herbalists of the Renaissance. Today double-flowered varieties of diverse ornamental plants, including carnations, camellias, peonies, lilacs, tulips and marsh marigolds, to mention but a few, remain fashionable. In the case of roses, the vast majority of cultivated varieties exhibit a double-flowered trait.
However, whereas double-flowered varieties have been popular as ornamental and garden plants, they are rarely found in the wild and, if they are, usually only as few or even single individuals (mutants), presumably because of their low fitness. Therefore, we became intrigued by a double-flowered variety of lesser periwinkle described as Vinca minor fl.Plena (henceforth, we use the more common term V. minor flore pleno) that was reported to exist in the wild for at least 160 years within the city limits of Jena (Bogenhard, 1850; Dietrich and Heinrich, 2008). To explore the perspectives of the plant as a new model system for investigating the evolutionary potential of double-flowered varieties, we estimated the distribution and assessed details of the mutant phenotype of this plant.
MATERIALS AND METHODS
Sampling of plant material
Specimens of Vinca minor flore pleno were sampled in a shady forest within the city limits of Jena (Germany) at a locality whose approximate position is described by Dietrich and Heinrich (2008). Two plants were transplanted to a sunny place in a garden in 2009 and photographed in spring 2010. Material of wild-type plants for comparison was collected from a reference population located in the same forest, about 500 m from the double-flowered population.
Morphological analyses
Dissected floral buds and flowers were observed under a binocular microscope (Leica MZ FLIII) with a CCD digital camera (JVC KY F70B). Fixation, dehydration, embedding and sectioning of plant material were performed using standard procedures (www.its.caltech.edu/~plantlab/protocols/insitu.pdf), except that FAA (3·7 % formaldehyde, 5 % glacial acetic acid, 50 % ethanol) was used as fixative. After deparaffinization and rehydration, sections were stained with 0·1 % Toluidine blue (pH 2·0–2·5). Dried sections were mounted with Canada balsam and examined under a microscope (Leica CTR5500).
RESULTS
Location and distribution of double-flowered lesser periwinkle
Based on the approximate location described by Dietrich and Heinrich (2008), the double-flowered variant of Vinca minor, V. minor fl. pl., was found in the upper part of a small valley named ‘Rautal’ near Closewitz village, within the city limits of Jena. The habitat is a forest composed of large trees of Tilia platyphyllos, Fagus sylvatica, Carpinus betulus, Fraxinus excelsior and diverse Acer species (e.g. A. pseudoplatanus, A. platanoides), with a rich herbaceous layer covering the ground, including rare orchid species such as lady's slipper Cypripedium calceolus. The site is of local fame due to a large wild population of winter aconite (Eranthis hyemalis) comprising roughly about 2 million plants together covering about 5000 m2 (Dietrich and Heinrich, 2008). Several patches of the double-flowered variant V. minor fl. pl. ranging from a few to several dozens of square metres, and each covering the ground almost completely, were identified in May 2009 and May 2010. No other site where this variety occurs has thus far been recognized in the whole Jena area or elsewhere during several years of observations by us, whereas the wild-type of V. minor is frequent in the forests around Jena, including the environs of the double-flowered variety (Dietrich and Heinrich, 2008, and our own observations).
Phenotype of double-flowered lesser periwinkle
Vinca minor is a plant native to central and southern Europe that develops solitary flowers in the leaf axils, mainly from early spring to the middle of summer, although flowering can extend late into autumn. Phylogenetically, V. minor is an asterid belonging to the Apocynaceae family (Endress and Bruyns, 2000; Simoes et al., 2007). Although this family possesses species with diverse flower morphologies, species from the subfamily Rauvolfioideae, to which V. minor belongs, have flowers relatively similar in morphology (Endress and Bruyns, 2000; Simoes et al., 2007). In wild-type V. minor flowers, the first floral whorl consists of a calyx with five erect sepals (Fig. 1A). In the second whorl there is a blue salverform corolla consisting of a cylindrical tube subtending five lobes that spread horizontally, together representing five basally fused petals (Figs 1A, B and 2A). Five stamens are attached halfway up the corolla tube, consisting of short filaments that bend abruptly at the base and anthers that are crowned with a hairy flap-like appendage (Fig. 2A, B). The fourth floral whorl consists of two adpressed carpels (Fig. 2C), each developing several ovules. The style of Vinca is similar to that of other Apocynaceae in that it possesses a collar with which the stigmatic tissue is associated (Fig. 2B) (Darwin, 1861; Simoes et al., 2007).
Fig. 1.
Flowers of Vinca minor. (A, B) Wild-type. (C–F) Spectrum of floral phenotypes of the flore pleno variety (C, E and F photographed after transplantation into a garden) with an extra (inner) whorl of petals visible in (D) and (E). In (F), a stalked second flower arises from within the flower. Abbreviations: s, sepal; p, petal; ip, extra (inner) petals; if, stalked flower within flower.
Fig. 2.
Anatomy of V. minor wild-type (A–C) and flore pleno (D–K) flowers. (A) Petals and stamens; (B) growth positions of stamens and the collar-bearing style in situ; (C) gynoecium; (D) mis-shaped broadend sepal; (E–G) different organs from a single flower with intermediate mutant phenotype; in (G), stamens with purple (petaloid) areas are shown; (H–K) organs from flower with severe mutant phenotype; (I) upper part: petals of the second whorl; lower part: petals from the third whorl; right bottom: organs from inner whorls; (J) organs from inner whorls as shown at right bottom of (I); (K) elongated ovary of a mutant flower. Scale bars = 1 mm. Abbreviations: p, petal; st, stamen; c, collar of the style; ca, carpel; bs, broadened sepal; pa, purple (petal-like) areas on stamens of mutant plants; op, second whorl (outer) petals; ip, extra (inner) petals; il, leaf-like organ inside the third whorl; eo, elongated ovary.
Although the floral phenotype of the V. minor fl. pl. variety is somewhat variable, petals are always reddish purple (Figs 1C–F and 2D–F, H, I) in contrast to the blue petals of wild-type plants. We observed only five petal lobes in all wild-type flowers (Figs 1A, B and 2A), whereas the number of petal lobes in the second whorl appeared more variable in the flore pleno variety, ranging from five to eight, with six in the majority of cases (Figs 1C–F and 2E). Concerning organ structure and identity, five sepals were generally found in the first whorl that usually appeared normal, but in some flowers one sepal was found to be broadened and twisted (Fig. 2D).
The double-flowered characteristics of V. minor fl. pl. were expressed to different degrees among flowers. Whereas some proved to be very similar to wild-type flowers, except for a tiny petal-like structure growing out of the corolla tube (Figs 1C and 2E), others had developed obvious ‘second flowers’ within the outer corolla tube (Fig. 1E). Many other flowers showed mutant phenotypes that lie between these extremes (e.g. Fig. 1D). A special case is the development of a subsiding flower on an elongated pedicel within the original flower (Fig. 1F), indicating at least partial indeterminacy of floral development. This trait has so far been observed only in plants transplanted to a sunny environment.
Dissection of flowers and closer inspection of floral organs revealed that the double-flowered appearance is brought about by a partial or complete transformation of third-whorl organs (stamens) into petals. Stamens of wild-type flowers are hairy, greenish and grow in the middle of the corolla tube (Fig. 2A, B). Some flowers of the flore pleno variety still develop stamens that are morphologically normal except that the side facing the petals is purple in colour (stamen on the left side of Fig. 2G). In more severely affected flowers, stamens are petaloid; whereas the lower part still resembles a stamen except being purple, the upper part is flattened and of petal-like appearance (stamen on the right side of Fig. 2G). In the most severely affected flowers, third-whorl organs are completely transformed into petals (Fig. 2H, I). Inside the third whorl there can develop leaf-like structures, coloured either uniform green or green suffused with purple (Fig. 2I, J). In the centre of the flower, the carpels occasionally grew with a normal stigma but only one elongated ovary (Fig. 2K).
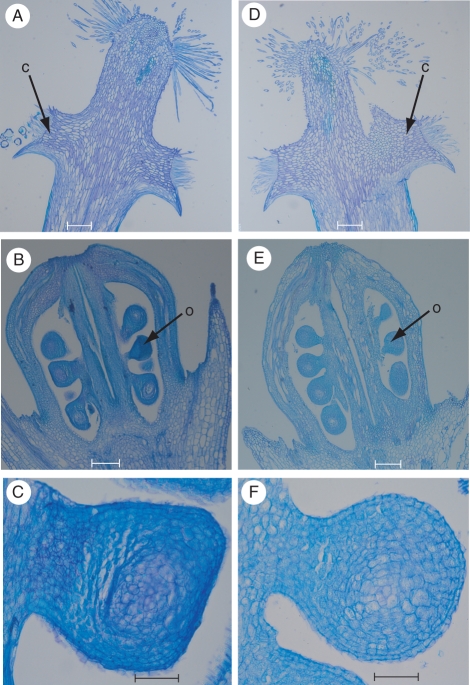
Sectioning of wild-type (Fig. 3A–C) and mutant (Fig. 3D–F) gynoecia revealed that almost all flowers of the flore pleno variety had no obvious aberrations in tissue structure in the pistils (compare Fig. 3A and Fig. 3D), ovaries (compare Fig. 3B and Fig. 3E) and ovules/seeds (compare Fig. 3C and Fig. 3F).
Fig. 3.
Sections of V. minor flowers. (A–C) wild-type and (D–F) flore pleno variety: (A, D) style head with collar, scale bars = 200 µm; (B, E) ovary with ovules, scale bars = 200 µm; (C, F) ovule, scale bars = 50 µm. Abbreviations: c, collar of the style; o, ovule.
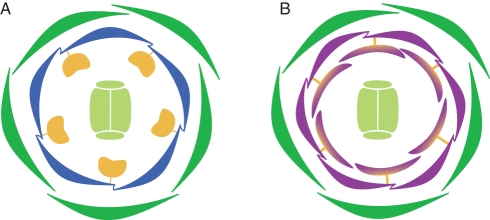
Taken together, the most obvious and consistent phenotypic aberrations of the flore pleno variety compared with V. minor wild-type flowers are third whorl organs of the flore pleno variety that possess a phenotypic range from being almost indistinguishable from wild-type stamens to complete transformation into petal-like organs. Furthermore, flore pleno plants develop purple instead of blue petals. To aid comparisons the typical flower structures of a V. minor wild-type plant and a flore pleno variety are depicted schematically in Fig. 4.
Fig. 4.
Floral diagram of V. minor wild-type (A) and flore pleno (B) flowers. Note that the diagram of the mutant flower is just one example among the range of phenotypes detected. Sepals are shown in dark green; petals in blue and purple, respectively; stamens in yellow and the gynoecium in pale green. Stamens that are partly transformed into petals are shown in purple that runs into yellow.
We also observed that the V. minor fl. pl. variety produced far fewer flowers than wild-type plants, but we did not quantify this effect and did not determine to what extent this divergence depends on the season or other growth conditions.
DISCUSSION
On the origin and persistence of double-flowered V. minor in the wild
There are reports of a double-flowered variety of V. minor in the Rautal valley from the year 1850 (Bogenhard, 1850), the year 1908 (published by Hergt, 1909) and the 21st century (Dietrich and Heinrich, 2008; present study), whereas no other populations of these mutant varieties are known, even though wild-type V. minor is frequent in the forests around Jena. Therefore, we consider it far more likely that the population of V. minor fl. pl. has existed for at least 160 years in the Rautal valley, rather originating or migrating there several times independently. How could a variety with such dramatic changes in its floral architecture have survived in the wild for so long? We assume that the general growth habit of V. minor contributes significantly to its survival in the wild. Both V. minor wild-type and the double-flowered variety are trailing, viny sub-shrubs that have an efficient method of vegetative propagation by spreading along the ground and rooting along the stems to form clonal colonies of considerable size. Sexual reproduction may thus play only a minor role in the persistence of a local population at short and intermediate timescales. Indeed, wild-type plants of V. minor are reported to set few to no seeds under natural growth conditions in Germany (Jäger and Werner, 2002). Therefore, seed-set and other classical fitness proxies may not be appropriate parameters to infer the degree of persistence in the wild.
More reliable and detailed answers to our question will require comparative studies of the reproductive biology and fitness of V. minor wild-type and the flore pleno variety under natural growth conditions in the Rautal, including determination of the mode of pollination and the relative importance of vegetative versus sexual reproduction. We hypothesize that the different patches of the double-flowered variety represent just one clone of vegetatively propagated plants, an assumption that would readily be tested by DNA fingerprinting techniques such as amplified fragment-length polymorphism. This approach could also be used to investigate whether the double-flowered variety originated from the populations of wild-type plants in close vicinity or migrated there from a more remote place. As some cultivars of V. minor, such as ‘Rubra Plena’ and ‘Multiplex’, resemble the double-flowered variety from the Rautal in floral architecture and colour, it is also conceivable that it was deliberately brought into the Jena area as an ornamental plant before the mid-19th century and subsequently ‘escaped’ from garden culture. This scenario is especially intriguing as a description of V. minor varieties with ‘filled’ flowers cultivated in gardens in Jena appeared as early as 1824 (Graumüller, 1824).
On flower colour
Many flower colours are due to anthocyanin pigments, which are products of the highly conserved anthocyanin biosynthetic pathway. Anthocyanin pigments are derived from six common types of anthocyanidin by the addition of sugar and/or methyl groups (Holton and Cornish, 1995; Hopkins and Rausher, 2011). Generally, less hydroxylated anthocyanidins give rise to redder pigments, while more hydroxylated anthocyanidins give rise to bluer pigments (Holton and Cornish, 1995; Hopkins and Rausher, 2011). Flavonoid 3′,5′-hydroxylase (F3′5′H), a member of the cytochrome P450 family, is generally a key enzyme for the synthesis of blue flower-specific 3′,5′-hydroxylated anthocyanidins such as delphinidin (Holton and Cornish, 1995). Experimental evidence suggests that this also applies to the blue petals of wild-type Vinca major, where delphinidin is the only anthocyanidin that accumulates in petals (Mori et al., 2004). As V. major is closely related to V. minor (Endress et al., 2007), we assume that delphinidin is also the predominant cause of blue colour in V. minor. Besides changes in the flavonoid biosynthetic pathway, aspects of the cellular milieu such as pH and the presence of metallic cations such as iron or aluminium can substantially influence flower colour. Clearly, clarifying the cause of the different colours of wild-type and the double-flowered variety will require further investigations, such as determination of anthocyanidins by high-performance liquid chromatogrpahy, and sequence, expression and functional analyses of the conserved genes and gene products known to act in the anthocyanin biosynthetic pathway (Mori et al., 2004). Besides the gene coding for F3′5′H, mutations in some other genes such as those coding for dihydroflavonol 4-reductase or anthocyanidin synthase can cause the transition from blue to red flowers (Hopkins and Rausher, 2011). It will be interesting to see whether any of these loci is affected in V. minor fl. pl., and whether a cis-regulatory region or the coding region of the mutated gene is affected. As there are cultivars of V. minor such as ‘Atropurpurea’ that show a reddish purple flower colour without double-flowered characteristics (http://pss.uvm.edu/pss123/pervinca.html), we hypothesize that the two traits have different genetic bases and originated independently in the lineage that led to the Rautal variety.
On the molecular genetic basis of the change in floral structure
With its specific combination of homeotic, meristic and other developmental abnormalities, the double-flowered variety of V. minor does not perfectly match any of the well-known mutants of model plants. Nevertheless, the Rautal variety resembles phenotypes produced by some alleles of class C floral homeotic genes such as PLENA from Antirrhinum majus (Stubbe, 1966), now known to be a close relative of the AGAMOUS (AG) gene of Arabidopsis thaliana (Carpenter and Coen, 1990; Yanofsky et al., 1990; Bradley et al., 1993). A. majus is an especially instructive example because in this plant sub-functionalization of AG-like genes occurred in a way that PLENA (PLE) confers carpel and stamen identity while its paralogue FARINELLI (FAR) has a more minor function that is mainly restricted to stamen development (Davies et al., 1999). The duplication giving rise to PLE and FAR is relatively ancient and occurred before asterids and rosids diverged (Kramer et al., 2004; Zahn et al., 2006). Thus, two functionally diverged AG-like genes are probably also present in V. minor, and mutation in only one of these may account for the incomplete class C loss-of-function phenotype observed in V. minor fl. pl.
However, phenotypes similar to that observed in the flore pleno variety can also result from mutations in class E floral homeotic genes, which, like class C genes, also encode MADS-domain transcription factors. For example, down-regulation of GRCD1 in Gerbera hybrida caused homeotic changes only in one whorl: sterile staminodes, which normally develop in whorl 3 of female florets marginal to the capitulum, were transformed into petals (Kotilainen et al., 2000). Likewise, down-regulation of FBP2 in petunia caused transformation of stamens into petaloid structures (Angenent et al., 1994) similar to those observed in the double-flowered V. minor variety in which stamens became petaloid in the upper part (Fig. 2G). Similarly, down-regulation of TM5 caused changes in organ number and incomplete homeotic transformations in tomato (Pnueli et al., 1994). We made analogous observations on the double-flowered V. minor, where some stamens remain more or less normal (Fig. 2F, G), but petal number is often increased (compare Fig. 1A, B with Fig. 1C–F). Thus, a loss-of-function mutation in a class C or class E floral homeotic MADS-box gene may underlie the double-flowered phenotype of the Rautal variety. Also, loss of floral determinacy and development of a stalked flower or inflorescence from within the fourth whorl (‘flower in flower’) (Fig. 1F) is a well-known characteristic of class E gene loss-of-function (see, for example, Angenent et al., 1994; Pelaz et al., 2000).
However, MADS-box genes may not be the only credible candidates. Conceptually, genes repressing class C gene expression in the first and second whorl of the flower may have extended to the third whorl, thus leading to the development of petaloid organs in the place of stamens. In A. thaliana, regulation of class C gene expression is complex and involves transcription factors such as AP2, but also proteins involved in mRNA processing such as HUA1 and HEN4 (Liu and Mara, 2010). However, it is unclear to what extent these regulatory circuits are conserved in other eudicots. For example, the repressive function of AP2-like genes on class C genes may be restricted to A. thaliana and close relatives (Keck et al., 2003; Litt, 2007). However, in petunia as well as in A. majus, the microRNAs miRBL and miRFIS, respectively, have been shown to be involved in the regulation of class C gene expression domains (Cartolano et al., 2007). Loss-of-function mutations in these microRNAs led to the development of stamenoid organs in the second whorl (McSteen et al., 1998; Cartolano et al., 2007). If a similar regulatory mechanism also exists in V. minor, enhanced expression of an orthologue of miRBL may lead to repression of class C gene function in the third whorl and thus to a flore pleno phenotype.
Another allusion on potential candidate genes causing the flore pleno phenotype is provided by the heterologous and ectopic expression of the homeobox genes ZmHox1a and ZmHox1b from maize (Zea mays ssp. mays) in tobacco (Nicotiana tabacum). This also caused the development of petaloid stamens (Überlacker et al., 1996) very similar to those of the V. minor variety (Fig. 2G–I). Thus, the ectopic expression of some homeobox genes could be responsible for the emergence of the double-flowered trait in V. minor. However, because in the transgenic plants generated by Überlacker et al. (1996) vegetative development was also severely affected, we consider this scenario less likely than the mutation of canonical floral homeotic genes.
Clearly, the molecular genetic basis of the double-flowered phenotype remains to be clarified. Orthologues of AGAMOUS (class C), SEPALLATA (class E), ZmHox1 and miRBL genes constitute plausible candidate genes. Investigating the structure and expression of these genes in wild-type and double-flowered V. minor should provide clues to the mutational basis of the phenotype of interest. Furthermore, crossing experiments may indicate whether the ‘flore pleno’ allele is dominant or recessive, yielding further clues to whether a loss- or gain-of-function mutation is responsible for the double-flowered phenotype and thus also confining the pool of candidate genes. Also, it should be considered that the molecular basis of the mutant phenotype traces back to epigenetic events such as variations in DNA methylation. This scenario could also explain the morphological variance in organ structure observed in the flore pleno flowers.
Future perspectives
One of the most important steps towards a better understanding of V. minor fl. pl. is identification of the locus responsible for the flore pleno phenotype. Several candidates have been suggested above, of which we consider either a class C gene or, perhaps more probably, a repressor of a class C gene (such as a miRBL-related miRNA) to be the most likely.
Detailed studies on plants such as the double-flowered V. minor may help to understand better the modes and the proximate and ultimate causes of homeotic transitions during evolution. As arguably even more drastic changes in plant body plans than homeotic transitions occurred during evolution, for instance such that require fuzzy rather than clear-cut concepts of organ identity for description (Rutishauser and Isler, 2001), even such seemingly ambitious endeavours may only represent a small step towards a comprehensive understanding of macroevolution.
ACKNOWLEDGEMENTS
G.T. thanks Annette Becker (University of Bremen) for her invitation to contribute to this Special Issue of the Annals of Botany, and for her patience. We thank Susanne Schilling and Heidi Kreßler (our own lab) for help with histological analyses, Laetitia Bradaczek (Jena) for help with some figures, and Helga Dietrich (FSU Jena) and Wolfgang Heinrich (Jena) for valuable information about the Vinca minor fl. pl. population in Jena. We are indebted to Richard Bateman, Paula Rudall (both Royal Botanic Gardens Kew, UK), Helga Dietrich and an anonymous reviewer for helpful and encouraging comments on the manuscript. Y.-Q.W. was supported by the International Leibniz Research School for Microbial and Biomolecular Interactions (ILRS) as part of the Jena School of Microbial Communication (JSMC). R.M. received a postdoctoral fellowship from the Carl-Zeiss-Stiftung. We thank the Friedrich Schiller University Jena for general support.
LITERATURE CITED
- Angenent GC, Franken J, Busscher M, Weiss D, Vantunen AJ. Co-suppression of the petunia homeotic gene FBP2 affects the identity of the generative meristem. Plant Journal. 1994;5:33–44. doi: 10.1046/j.1365-313x.1994.5010033.x. [DOI] [PubMed] [Google Scholar]
- Bateman RM, DiMichele WA. Generating and filtering major phenotypic novelties: neoGoldschmidtian saltation revisited. In: Cronk QC, Bateman RM, Hawkins JA, editors. Developmental genetics and plant evolution. London: Taylor & Francis; 2002. pp. 109–159. [Google Scholar]
- Becker A, Theißen G. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Molecular Phylogenetics and Evolution. 2003;29:464–489. doi: 10.1016/s1055-7903(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Bogenhard C. Taschenbuch der Flora von Jena. Leipzig: Wilhelm Engelmann; 1850. [Google Scholar]
- Bradley D, Carpenter R, Sommer H, Hartley N, Coen E. Complementary floral homeotic phenotypes result from opposite orientations of a transposon at the PLENA Locus of Antirrhinum. Cell. 1993;72:85–95. doi: 10.1016/0092-8674(93)90052-r. [DOI] [PubMed] [Google Scholar]
- Carpenter R, Coen ES. Floral homeotic mutations produced by transposon mutagenesis in Antirrhinum majus. Genes & Development. 1990;4:1483–1493. doi: 10.1101/gad.4.9.1483. [DOI] [PubMed] [Google Scholar]
- Cartolano M, Castillo R, Efremova N, et al. A conserved microRNA module exerts homeotic control over Petunia hybrida and Antirrhinum majus floral organ identity. Nature Genetics. 2007;39:901–905. doi: 10.1038/ng2056. [DOI] [PubMed] [Google Scholar]
- Coen ES, Meyerowitz EM. The war of the whorls – genetic interactions controlling flower development. Nature. 1991;353:31–37. doi: 10.1038/353031a0. [DOI] [PubMed] [Google Scholar]
- Cubas P, Vincent C, Coen E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature. 1999;401:157–161. doi: 10.1038/43657. [DOI] [PubMed] [Google Scholar]
- Darwin C. Fertilisation of Vincas. Gardeners' Chronicle and Agricultural Gazette. 1861;24:552. [Google Scholar]
- Davies B, Motte P, Keck E, Saedler H, Sommer H, Schwarz-Sommer Z. PLENA and FARINELLI: redundancy and regulatory interactions between two Antirrhinum MADS-box factors controlling flower development. EMBO Journal. 1999;18:4023–4034. doi: 10.1093/emboj/18.14.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich H, Heinrich W. Frühblüher um Jena: Aus der Flora Thüringens. Bürgel: EchinoMedia Verlag; 2008. [Google Scholar]
- Endress ME, Bruyns PV. A revised classification of the Apocynaceae s.l. Botanical Review. 2000;66:1–56. [Google Scholar]
- Endress ME, van der Ham RWJM, Nilsson S, et al. A phylogenetic analysis of Alyxieae (Apocynaceae) based on rbcL, matK, trnL intron, trnL-F spacer sequences, and morphological characters. Annals of the Missouri Botanical Garden. 2007;94:1–35. [Google Scholar]
- Ford VS, Gottlieb LD. Bicalyx is a natural homeotic floral variant. Nature. 1992;358:671–673. [Google Scholar]
- Graumüller JCF. Flora Jenensis. Eisenberg: Verlag der Schöneschen Buchhandlung; 1824. [Google Scholar]
- Haag ES, True JR. Perspective: From mutants to mechanisms? Assessing the candidate gene paradigm in evolutionary biology. Evolution. 2001;55:1077–1084. doi: 10.1111/j.0014-3820.2001.tb00627.x. [DOI] [PubMed] [Google Scholar]
- Hergt B. Exkursion im Rautal. 1909;25:62. Bericht über die Frühjahrshauptversammlung in Jena am 14. und 15. Juni 1908. Mitteilungen des Thüringischen Botanischen Vereins N.F. [Google Scholar]
- Hintz M, Bartholmes C, Nutt P, Ziermann J, Hameister S, Neuffer B, Theissen G. Catching a ‘hopeful monster’: shepherd's purse (Capsella bursa-pastoris) as a model system to study the evolution of flower development. Journal of Experimental Botany. 2006;57:3531–3542. doi: 10.1093/jxb/erl158. [DOI] [PubMed] [Google Scholar]
- Holton TA, Cornish EC. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell. 1995;7:1071–1083. doi: 10.1105/tpc.7.7.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins R, Rausher MD. Identification of two genes causing reinforcement in the Texas wildflower Phlox drummondii. Nature. 2011;469:411–414. doi: 10.1038/nature09641. [DOI] [PubMed] [Google Scholar]
- Jäger E, Werner K. Rothmaler, Exkursionsflora von Deutschland, Band 4, Gefäßpflanzen: Kritischer Band. Heidelberg: Spektrum Akademischer Verlag; 2002. [Google Scholar]
- Keck E, McSteen P, Carpenter R, Coen E. Separation of genetic functions controlling organ identity in flowers. EMBO Journal. 2003;22:1058–1066. doi: 10.1093/emboj/cdg097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchoff BK. Homeosis in the flowers of the Zingiberales. American Journal of Botany. 1991;78:833–837. [Google Scholar]
- Kotilainen M, Elomaa P, Uimari A, Albert VA, Yu DY, Teeri TH. GRCD1, an AGL2-like MADS box gene, participates in the C function during stamen development in Gerbera hybrida. Plant Cell. 2000;12:1893–1902. doi: 10.1105/tpc.12.10.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer EM, Jaramillo MA, Di Stilio VS. Patterns of gene duplication and functional evolution during the diversification of the AGAMOUS subfamily of MADS box genes in angiosperms. Genetics. 2004;166:1011–1023. doi: 10.1093/genetics/166.2.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek BA, Fletcher JC. Molecular mechanisms of flower development: an armchair guide. Nature Reviews Genetics. 2005;6:688–698. doi: 10.1038/nrg1675. [DOI] [PubMed] [Google Scholar]
- Lewis EB. Homeosis – the first 100 years. Trends in Genetics. 1994;10:341–343. doi: 10.1016/0168-9525(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Litt A. An evaluation of A-function: evidence from the APETALA1 and APETALA2 gene lineages. International Journal of Plant Sciences. 2007;168:73–91. [Google Scholar]
- Liu ZC, Mara C. Regulatory mechanisms for floral homeotic gene expression. Seminars in Cell & Developmental Biology. 2010;21:80–86. doi: 10.1016/j.semcdb.2009.11.012. [DOI] [PubMed] [Google Scholar]
- McSteen PCM, Vincent CA, Doyle S, Carpenter R, Coen ES. Control of floral homeotic gene expression and organ morphogenesis in Antirrhinum. Development. 1998;125:2359–2369. doi: 10.1242/dev.125.13.2359. [DOI] [PubMed] [Google Scholar]
- Meyerowitz EM, Smyth DR, Bowman JL. Abnormal flowers and pattern-formation in floral development. Development. 1989;106:209–217. [Google Scholar]
- Mori S, Kobayashi H, Hoshi Y, Kondo M, Nakano M. Heterologous expression of the flavonoid 3′,5′-hydroxylase gene of Vinca major alters flower color in transgenic Petunia hybrida. Plant Cell Reports. 2004;22:415–421. doi: 10.1007/s00299-003-0709-3. [DOI] [PubMed] [Google Scholar]
- Nutt P, Ziermann J, Hintz M, Neuffer B, Theißen G. Capsella as a model system to study the evolutionary relevance of floral homeotic mutants. Plant Systematics and Evolution. 2006;259:217–235. [Google Scholar]
- Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature. 2000;405:200–203. doi: 10.1038/35012103. [DOI] [PubMed] [Google Scholar]
- Pnueli L, Hareven D, Broday L, Hurwitz C, Lifschitz E. The TM5 MADS box gene mediates organ differentiation in the 3 inner whorls of tomato flowers. Plant Cell. 1994;6:175–186. doi: 10.1105/tpc.6.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronse De Craene LP. The evolutionary significance of homeosis in flowers: a morphological perspective. International Journal of Plant Sciences. 2003;164:S225–S235. [Google Scholar]
- Rudall PJ, Bateman RM. Evolutionary change in flowers and inflorescences: evidence from naturally occurring terata. Trends in Plant Science. 2003;8:76–82. doi: 10.1016/S1360-1385(02)00026-2. [DOI] [PubMed] [Google Scholar]
- Rudall PJ, Bateman RM. Evolution of zygomorphy in monocot flowers: iterative patterns and developmental constraints. New Phytologist. 2004;162:25–44. [Google Scholar]
- Rutishauser R, Isler B. Developmental genetics and morphological evolution of flowering plants, especially bladderworts (Utricularia): fuzzy arberian morphology complements classical morphology. Annals of Botany. 2001;88:1173–1202. [Google Scholar]
- Sattler R. Homeosis in plants. American Journal of Botany. 1988;75:1606–1617. [Google Scholar]
- Simoes AO, Livshultz T, Conti E, Endress ME. Phylogeny and systematics of the Rauvolfioideae (Apocynaceae) based on molecular and morphological evidence. Annals of the Missouri Botanical Garden. 2007;94:268–297. [Google Scholar]
- Stubbe H. Genetik und Zytologie von Antirrhinum L. sect. Antirrhinum. Jena: VEB Gustav Fischer Verlag; 1966. [Google Scholar]
- Theißen G. Development of floral organ identity: stories from the MADS house. Current Opinion in Plant Biology. 2001;4:75–85. doi: 10.1016/s1369-5266(00)00139-4. [DOI] [PubMed] [Google Scholar]
- Theißen G. The proper place of hopeful monsters in evolutionary biology. Theory in Biosciences. 2006;124:349–369. doi: 10.1016/j.thbio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Theißen G. Saltational evolution: hopeful monsters are here to stay. Theory in Biosciences. 2009;128:43–51. doi: 10.1007/s12064-009-0058-z. [DOI] [PubMed] [Google Scholar]
- Überlacker B, Klinge B, Werr W. Ectopic expression of the maize homeobox genes ZmHox1a or ZmHox1b causes pleiotropic alterations in the vegetative and floral development of transgenic tobacco. Plant Cell. 1996;8:349–362. doi: 10.1105/tpc.8.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara-Silva F. Plants and the conceptual articulation of evolutionary developmental biology. Biology & Philosophy. 2003;18:249–284. [Google Scholar]
- Yanofsky MF, Ma H, Bowman JL, Drews GN, Feldmann KA, Meyerowitz EM. The protein encoded by the Arabidopsis homeotic gene AGAMOUS resembles transcription factors. Nature. 1990;346:35–39. doi: 10.1038/346035a0. [DOI] [PubMed] [Google Scholar]
- Zahn LM, Leebens-Mack JH, Arrington JM, et al. Conservation and divergence in the AGAMOUS subfamily of MADS-box genes: evidence of independent sub- and neofunctionalization events. Evolution & Development. 2006;8:30–45. doi: 10.1111/j.1525-142X.2006.05073.x. [DOI] [PubMed] [Google Scholar]
- Ziermann J, Ritz MS, Hameister S, Abel C, Hoffmann MH, Neuffer B, Theissen G. Floral visitation and reproductive traits of Stamenoid petals, a naturally occurring floral homeotic variant of Capsella bursa-pastoris (Brassicaceae) Planta. 2009;230:1239–1249. doi: 10.1007/s00425-009-1018-z. [DOI] [PubMed] [Google Scholar]