Abstract
Background and Aims
The family of MADS box genes is involved in a number of processes besides controlling floral development. In addition to supplying homeotic functions defined by the ABC model, they influence flowering time and transformation of vegetative meristem into inflorescence meristem, and have functions in roots and leaves. Three Gerbera hybrida At-SOC1-like genes (Gh-SOC1–Gh-SOC3) were identified among gerbera expressed sequence tags.
Methods
Evolutionary relationships between SOC1-like genes from gerbera and other plants were studied by phylogenetic analysis. The function of the gerbera gene Gh-SOC1 in gerbera floral development was studied using expression analysis, protein–protein interaction assays and reverse genetics. Transgenic gerbera lines over-expressing or downregulated for Gh-SOC1 were obtained using Agrobacterium transformation and investigated for their floral phenotype.
Key Results
Phylogenetic analysis revealed that the closest paralogues of At-SOC1 are Gh-SOC2 and Gh-SOC3. Gh-SOC1 is a more distantly related paralogue, grouping together with a number of other At-SOC1 paralogues from arabidopsis and other plant species. Gh-SOC1 is inflorescence abundant and no expression was seen in vegetative parts of the plant. Ectopic expression of Gh-SOC1 did not promote flowering, but disturbed the development of floral organs. The epidermal cells of ray flower petals appeared shorter and their shape was altered. The colour of ray flower petals differed from that of the wild-type petals by being darker red on the adaxial side and greenish on the abaxial surface. Several protein–protein interactions with other gerbera MADS domain proteins were identified.
Conclusions
The At-SOC1 paralogue in gerbera shows a floral abundant expression pattern. A late petal expression might indicate a role in the final stages of flower development. Over-expression of Gh-SOC1 led to partial loss of floral identity, but did not affect flowering time. Lines where Gh-SOC1 was downregulated did not show a phenotype. Several gerbera MADS domain proteins interacted with Gh-SOC1.
Keywords: Gerbera hybrida, MADS box gene, Gh-SOC1, flower development, floral organ identity, gene transfer
INTRODUCTION
MADS box genes form a gene superfamily coding for transcription factors and are best known for their involvement in determining floral organ identity in flowering plants (Becker and Theissen, 2003). In addition to their function as floral homeotic genes, MADS box genes are also involved in other flowering-related processes, such as floral induction (Mandel and Yanofsky, 1995; Immink et al., 1999; Borner et al., 2000; Ferrario et al., 2004; Ruokolainen et al., 2010a). Furthermore, aside from participation in reproductive developmental processes, these genes have functions in roots and leaves, and are involved in vernalization and senescence processes (Zhang and Forde, 1998; Samach et al., 2000; Ratcliffe et al., 2001, 2003; Fang and Fernandez, 2002; Vrebalov et al., 2002; Trevaskis et al., 2003).
SOC1-like genes, MADS box genes similar to the arabidopsis (Arabidopsis thaliana) gene SUPPRESSOR OF OVEREXPRESSION OF CO1 (At-SOC1), were first described in tomato (Sl-TM3; Pnueli et al., 1991). They have been isolated in many plant species, but apart for At-SOC1 (see below), the group has remained largely unknown in terms of function (Becker and Theissen, 2003). Some authors have suggested that this class of MADS box genes should be called F class genes, as a further extension to the ABC and ABCDE models of flower development (Coen and Meyerowitz, 1991; Theissen and Saedler, 2001; Nam et al., 2003, 2004). They are preferentially expressed in vegetative parts of both angiosperms and gymnosperms (Tandre et al., 1995; Walden et al., 1998; Winter et al., 1999; Watson and Brill, 2004), but examples of SOC1-like genes expressed in reproductive organs have also been reported (Heuer et al., 2001; Münster et al., 2002). Some subfamily members have been associated with vascular development (Decroocq et al., 1999; Alvarez-Buylla et al., 2000) or involvement in vascular cambium or expanding xylem at wood formation (Cseke et al., 2003). Over-expression of At-SOC1-like genes causes early flowering in several plant species (Tadege et al., 2003; Ferrario et al., 2004; Smykal et al., 2007).
The best characterized member of this subfamily is the arabidopsis flowering time integrator gene At-SOC1. At-SOC1 plays a central role in arabidopsis reproduction since it integrates the photoperiodic, the autonomous, the vernalization, the energy and the gibberellin pathways to flowering (Borner et al., 2000; Samach et al., 2000; Moon et al., 2003, 2005). At-SOC1 is expressed in arabidopsis roots, but the strongest expression is seen in leaves and flowers (Borner et al., 2000). Arabidopsis contains a number of At-SOC1-like genes, but few of them have been studied in detail (Becker and Theissen, 2003). Some of them, such as At-AGL19 and At-AGL14, are expressed solely in roots (Rounsley et al., 1995; Alvarez-Buylla et al., 2000). At-AGL42 is also mainly expressed in the arabidopsis root, where its expression can be used as a marker for the quiescent centre (Nawy et al., 2005). At-AGL71 is expressed in germinating arabidopsis seedlings (Ma et al., 2005). Both At-AGL71 and At-AGL72 were tested in the comprehensive arabidopsis MADS domain protein–protein interaction mapping assay (de Folter et al., 2005), but At-AGL72 has not been investigated further.
Another important model species for flowering studies, petunia (Petunia hybrida), also has several genes which belong to the Sl-TM3 subfamily of MADS box genes. The best characterized is UNSHAVEN (Ph-UNS, formerly known as Ph-FBP20) (Ferrario et al., 2004). Other related genes are Ph-FBP21, Ph-FBP22 and Ph-FBP28 (Immink et al., 2003). All the At-SOC1-like petunia genes are expressed mainly in green tissues (including sepals), but Ph-UNS and Ph-FBP21 are also expressed in roots, Ph-FBP28 in stamens, and Ph-FBP21 and Ph-FBP22 at a low level in petals (Immink et al., 2003). Mutations in Ph-UNS or Ph-FBP28 did not lead to phenotypes, but ectopic expression of Ph-UNS in petunia caused early flowering (Vandenbussche et al., 2003a; Ferrario et al., 2004).
The complex inflorescence structure of gerbera (Gerbera hybrida, Asteraceae) consists of three morphologically distinct types of flowers. The marginal ray flowers develop showy petals and are female, while the central disc flowers are inconspicuous and hermaphrodite. The third flower type between these two, the trans flowers, is female like the ray flowers, but their corolla size varies and the flowers in many varieties are inconspicuous like the disc flowers. The presence of different flower morphs with different roles raises many intriguing questions of how such a structure has evolved and how it is controlled at the molecular level. Gerbera has several features in inflorescence and floral development which are consistent with other model species. The B and C function of floral development can be applied to gerbera (Yu et al., 1999; Broholm et al., 2010). True A function genes in the strict sense of the ABC model are apparently not present in gerbera, but several genes related to arabidopsis At-AP1 and At-FUL have been described (Ruokolainen et al., 2010a). In contrast to the arabidopsis At-SEP genes (Ma et al., 1991; Mandel and Yanofsky, 1998; Pelaz et al., 2000; Ditta et al., 2004), which are redundant to a great degree, the E function of gerbera is provided both by specialized and general, apparently partially redundant, genes (Gh-GRCD1, Gh-GRCD2, Gh-GRCD4 and Gh-GRCD5) (Kotilainen et al., 2000; Uimari et al., 2004; Ruokolainen et al., 2010b).
Here we report on the functional characterization of the Gerbera hybrida At-SOC1-like1 MADS box gene Gh-SOC1. Ectopic expression of Gh-SOC1 in gerbera led to a partial loss of floral organ identity. Phenotypic changes, some of them affecting petal epidermal cell size and surface structure, were observed in all floral whorls. In gerbera, over-expression of Gh-SOC1 did not, however, accelerate flowering as At-SOC1 and Ph-UNS do in arabidopsis and petunia, respectively (Borner et al., 2000; Ferrario et al., 2004).
MATERIALS AND METHODS
Isolation, sequence and phylogenetic analysis of Gh-SOC1–Gh-SOC3
Gh-SOC1 sequences were identified from the gerbera expressed sequence tag (EST) collection (Laitinen et al., 2005) and Gh-SOC2 and Gh-SOC3 sequences by 454 pyrosequencing of gerbera cDNA molecules (P. Elomaa and T. H. Teeri, unpubl. res.). Searches were done using the tblastn algorithm (Altschul et al., 1997) locally on our gerbera sequence collections using At-SOC1 as the initial query, followed by incremental cross-searching with Gh-SOC1–Gh-SOC3. A search with chrysanthemum (Dendranthema grandiflorum) and sunflower (Helianthus annuus) sequences Cm-SOC1 and Ha-SOC1 did not yield further SOC1-like sequences. Gh-SOC1 was retrieved as a full-length cDNA clone of 867 bp from the gerbera EST clone collection (Laitinen et al., 2005). The Gh-SOC2 cluster codes for a nearly full-length polypeptide with few amino acids missing from the C-terminus. The Gh-SOC3 cluster codes for a polypeptide lacking the MADS domain, half of the K domain and the C-terminal end including most of the conserved domain found in At-SOC1-like proteins. Amino acid sequence alignment with similar deduced polypeptides from arabidopsis, petunia, chrysanthemum and sunflower was performed using the ClustalW multiple sequence alignment program (Thompson et al., 1994). The alignments spanning the conserved MADS and K domains were subsequently converted, codon by codon, to a corresponding nucleotide alignment. These sequence alignments were used to create the phylogenetic tree using parsimony analysis (Goloboff et al., 2003). A total of 1000 bootstrap replicates were performed with ten random addition sequences for each replicate, saving one tree per replicate. There was only a single most-parsimonious tree.
Expression analysis of Gh-SOC1
RNA was isolated from different plant organs and from different stages of petal development (stages 1–11, see Helariutta et al., 1993) using Trizol reagent (Invitrogen). The amount of RNA was measured by spectrophotometry and rRNA bands were stained with ethidium bromide to verify equal amounts of RNA per lane (10 µg). The RNA gels were blotted on Hybond-N membrane (Amersham Biosciences) and hybridized in the UltraHyb hybridization buffer (Ambion) with a gene-specific probe [275 bp, encoding the last 30 amino acids, and including the 3′ untranslated region (UTR) and the poly(A) sequence from the 3′ end of Gh-SOC1]. The probe was labelled with [32P]dCTP and hybridized at +42 °C for 16 h. The membranes were washed with 1× SSC, 0·1 % SDS at +42 °C for 20 min. Subsequent washes were performed at +65 °C for 15 min, 1–2 times. Films were exposed at –80 °C.
In situ hybridization analysis was performed as described in Ruokolainen et al. (2010a, b). Gh-SOC1 gene-specific sense and antisense probes (275 bp from the 3′ end) were prepared and quantitated using a DIG RNA labelling kit (Boehringer Mannheim) according to the manufacturer's instructions. Sections (10 µm thick) were mounted in 50 % glycerol after hybridization.
Scanning electron microscopy
Scanning electron microscopy was performed as previously described in Kotilainen et al. (2000).
Protein–protein interaction studies
Yeast two- and three-hybrid assays were performed as described in Ruokolainen et al. (2010b). In brief, full-length gerbera MADS-box genes were introduced into the Gateway system as cDNAs, recombined with the activation and binding domain-containing plasmids pDEST22 and pDEST32 (Invitrogen) and transformed into yeast PJ69-4A and PJ69-4α (James et al., 1996). Autoactivation was tested and, when observed (for Gh-RCD4 and Gh-RCD5, see Ruokolainen et al., 2010b), C-terminal deletions were introduced into the constructs. For yeast three-hybrid assays, the plasmid pARC351 was used to express the third protein in yeast cells. Three-hybrid assays were designed so that the proteins fused to the activation and binding domains did not interact (Ruokolainen et al., 2010b). Protein–protein interaction was scored as growth on minimal medium lacking appropriate amino acids for plasmid selection, and adenine to score for strong interactions, or histidine [with 1–25 mM 3-aminotriazole (3-AT)] to score for weak interactions. Plates were incubated at +22 °C for 7 d. For yeast two-hybrid assays, all reciprocal tests were performed and showed the same interactions. For yeast three-hybrid assays, Gh-SOC1 was in each case introduced as the unfused partner expressed from pARC351.
Plant material and transformation
Gerbera hybrida ‘Terra Regina’ was obtained from the commercial producer Terra Nigra, The Netherlands. Plants were grown under standard growth chamber conditions as described in Ruokolainen et al. (2010a, b). The full-length cDNA for Gh-SOC1 was inserted into the T-DNA vector pHTT602 (Elomaa and Teeri, 2001) under the transcriptional control of the cauliflower mosaic virus (CaMV) 35S promoter in both sense and antisense orientation. Vector sequences add about 200 nucleotides to the transcript, making it possible to distinguish the natural and the transgene product on an RNA gel blot. Gerbera transformation was performed using the Agrobacterium-mediated gene transfer method as previously described (Elomaa et al., 1993; Elomaa and Teeri, 2001).
Anthocyanin determination
Intact ray flowers (stage 8) of over-expression lines (TR1, TR6 and TR9) were collected and ground in liquid nitrogen. The wild-type cultivar ‘Terra Regina’ was used as control. The powder was freeze-dried for 48 h. Samples of 10 mg were weighed, extraction buffer (70 % methanol, 20 % MQ water, 10 % 1 m HCl) was added and then they were incubated overnight at +4 °C. After 20 h of extraction, samples were centrifuged for 20 min at 10 000 rpm at +4 °C and absorbance was measured at 530 nm. Chlorophyll content (measured at 657 nm) was found to be absent in the extracts.
RESULTS
Sequence and phylogenetic analysis of gerbera Gh-SOC1–Gh-SOC3
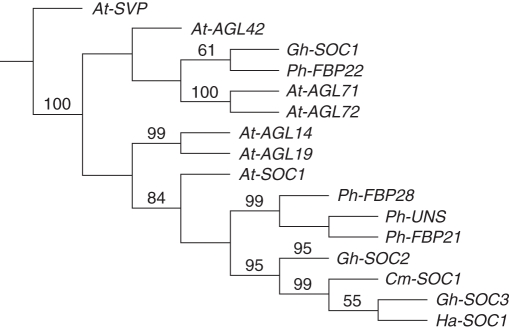
The search for At-SOC1-like sequences from our gerbera EST collections (Laitinen et al., 2005, and unpubl. res.) identified three different transcripts. Gh-SOC1 is represented by three Sanger reads originating from a cDNA library made from gerbera petals collected from fully opened flowers. Gh-SOC2 and Gh-SOC3 are both represented by 454 reads where mRNA from small (1–3 mm wide) capitula or inflorescence stem (scape) was used as a template. The Gh-SOC1 cDNA encodes an apparently full-length putative polypeptide of 211 amino acids, Gh-SOC2 a polypeptide of 209 amino acids, possibly not full length at the C-terminus, and Gh-SOC3 a fragment of 80 amino acids including half of the conserved K domain and part of the C-terminus. All deduced amino acid sequences have high sequence similarity to the arabidopsis At-SOC1 and SOC1-like proteins from other species (see Supplementary Data Fig. S1, available online). The deduced Gh-SOC1 and Gh-SOC2 polypeptides contained the SOC1 protein motif at their C-terminal end (Vandenbussche et al., 2003b; Nakamura et al., 2005), highlighted in red in Supplementary Fig. S1. Phylogenetic analysis places Gh-SOC2 and Gh-SOC3 within the clade that holds At-SOC1, Ph-UNS, Cm-SOC1 and Ha-SOC1. Gh-SOC1, analysed in more detail in this study, groups in phylogeny with Ph-FBP22 (Fig. 1).
Fig. 1.
Phylogenetic relationships of SOC1-like genes from Gerbera hybrida (Gh-SOC1, FR716023; Gh-SOC2, FR846140; Gh-SOC3, FR846141), Arabidopsis thaliana (At-SVP, At2g22540; At-SOC1, At2g45660; At-AGL71, At5g51870; At-AGL72, At5g51860; At-AGL42, At5g62165; At-AGL14, At4g11880; At-AGL19, At4g22950), Petunia hybrida (Ph-UNS, AF335238; Ph-FBP21, AF335239; Ph-FBP22, AF335240; Ph-FBP28, AF335244), Dendranthema grandiflorum (Cm-SOC1, AB481225) and Helianthus annuus (Ha-SOC1, GU985593). Gh-SOC2 and Gh-SOC3 are the putative orthologues of At-SOC1. Bootstrap support is shown when it exceeds 50 %.
Expression pattern of gerbera Gh-SOC1
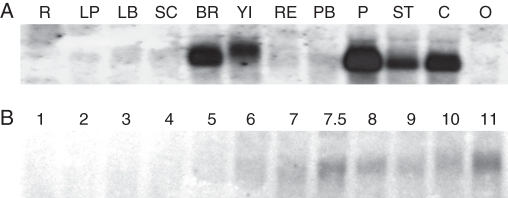
An RNA gel blot hybridized with a short gene-specific probe showed Gh-SOC1 expression in gerbera floral organs. Strong expression was seen in involucral bracts, young inflorescence, petals, stamen and carpel, but no expression was detected in vegetative parts of gerbera, which included roots, leaf blade and leaf petiole. Also the receptacle, scape, pappus bristles and ovary showed no expression of Gh-SOC1 (Fig. 2A). An RNA gel blot consisting of samples from different petal developmental stages (for stages, see Helariutta et al., 1993) was probed to see whether Gh-SOC1 was differentially expressed during gerbera inflorescence development. Gh-SOC1 expression was found to be stronger at stages 6–11, relatively late in ray flower petal development (Fig. 2B).
Fig. 2.
Expression pattern of Gh-SOC1 in different gerbera organs (A) and at different petal developmental stages (B) (for stages, see Helariutta et al., 1993). R, roots; LP, leaf petiole; LB, leaf blade; SC, scape; BR, involucral bracts; YI, young inflorescence (6–16 mm in diameter); RE, receptacle; PB, pappus bristles; P, petals; ST, stamens; C, carpel; O, ovary; RF, ray flower; DF, disc flower.
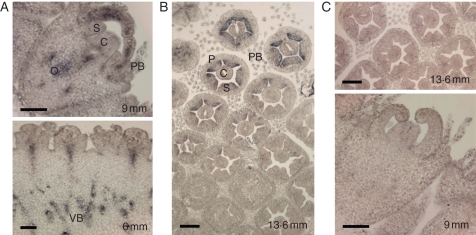
In situ hybridization of young developing gerbera inflorescences (inflorescence diameter 9–14 mm) showed Gh-SOC1 expression in ray flower petals, reproductive organs (ovary area, ovule, carpel and stamen) and pappus bristles (Fig. 3A). Also the vascular bundles of developing inflorescence expressed Gh-SOC1 relatively strongly (Fig. 3A), as did developing gerbera disc flowers (Fig. 3B).
Fig. 3.
RNA in situ expression analysis of Gh-SOC1 in developing gerbera inflorescences (inflorescence diameter 6–13·6 mm, antisense probe) showing ray flowers (A) and disc flowers (B). Abbreviations: P, petal; S, stamen; C, carpel; O, ovary; VB, vascular bundles; PB, pappus bristles. Expression is seen in petals, ovary, carpel, stamen and pappus bristles. Controls were hybridized with a Gh-SOC1 sense probe (C). Scale bars = 100 µm.
Phenotypic changes in over-expression lines of Gh-SOC1
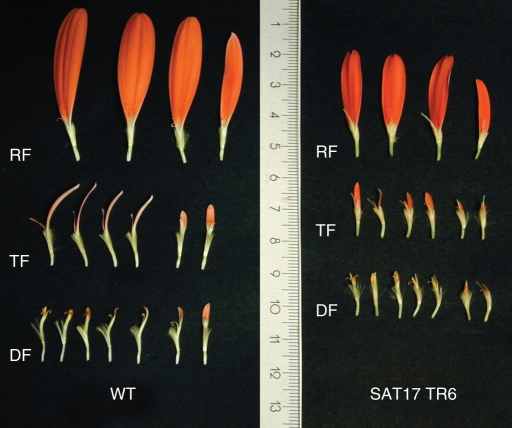
The over-expression plants for Gh-SOC1 (Supplementary Data Fig. S2A) showed consistent phenotypic changes in three individual lines (TR1, TR6 and TR9). In contrast, lines where Gh-SOC1 expression was downregulated (Supplementary Data Fig. S2B) showed no phenotype. The petal colour of Gh-SOC1 over-expression lines differed from that of the wild-type cultivar ‘Terra Regina’ in all flower types, and the petals were shorter. The abaxial side of petals in ray and trans flowers had a greenish tint and the adaxial side a deeper shade of coral than the control inflorescences. The disc flowers were lighter in colour than the trans and ray flowers (Fig. 4). The greatest reduction in length was seen in ray flower petals, which were visibly shorter in Gh-SOC1 over-expression lines (Fig. 5).
Fig. 4.
Gerbera hybrida wild-type ‘Terra Regina’ disc flower vs. Gh-SOC1 over-expression line SAT17 TR6 disc flower. The petals of the over-expression line lack anthocyanin and the stamen structure is abnormal.
Fig. 5.
Gerbera hybrida wild-type ‘Terra Regina’ flower types vs. Gh-SOC1 over-expression line SAT17 TR6 flower types. All the flower types of the over-expression line were shorter than the respective flower types in the wild type. Abbreviations: RF, ray flower; TR, trans flower; DF, disc flower. The scale is in cm.
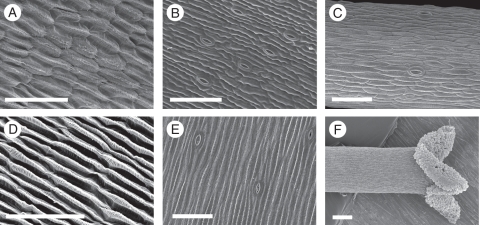
Scanning electron micrographs showed a different cell shape in ray flower petal epidermal cells in over-expression lines for Gh-SOC1 (Fig. 6). The adaxial side of these petals had epidermal cells, which appeared flatter and shorter than those of the wild-type controls. The ray flower petal adaxial epidermal cells of transformant lines were not as strongly ridged as in normal gerbera ray flower petal epidermal cells (Fig. 6A, D). Also the ray flower petal epidermal abaxial cells were shorter and irregularly shaped, compared with those of the wild-type petal (Fig. 6B, E). The carpel epidermis of Gh-SOC1 over-expression lines showed cell structures which resembled guard cells of stomata, normally located on the abaxial surface of leaves and petals in gerbera (Fig. 6C). This cell type is not normally found in gerbera wild-type carpels (Fig. 6F) in any of the flower types.
Fig. 6.
Scanning electron microscopy analysis of the Gh-SOC1 over-expression line SAT17 TR6. Petal adaxial epidermal cells (A, D), petal abaxial epidermal cells (B, E) and carpel epidermal cells (C, F) of disc flowers from theGh-SOC1 over-expression line (A–C) and non-transgenic control plants (D–F). The epidermal cell structure of both the adaxial and abaxial petal surfaces of the Gh-SOC1 over-expression line differs from the wild-type petal epidermal cell structure. The carpel of the Gh-SOC1 over-expression line displays guard cell-like structures not normally found in wild-type carpels. Scale bars = 100 µm.
Petal epidermal cells appear shorter and are rich in anthocyanin in Gh-SOC1 over-expression plants
About 40 epidermal cells in ray flower petals of the over-expression line SAT17 TR6 were measured and found to be 8 % shorter compared with the same cell type in the wild-type controls (81 ± 3 vs. 88 ± 3 µm). However, since this difference is smaller than the difference in petal length and not statistically significant, we cannot conclude that the shorter ray flower petals of Gh-SOC1 over-expression lines are due to shorter petal epidermal cells.
The inflorescences of Gh-SOC1 over-expression lines had a deeper hue of orange than the control inflorescences. To test whether the difference perceived in colour was due to elevated anthocyanin content, the absorbance of ray flower extracts was measured at 530 nm. Gh-SOC1 over-expression lines contained nearly twice as much anthocyanin as the control inflorescences (Supplementary Data Table S1, available online).
Gh-SOC1 interactions with other MADS domain proteins
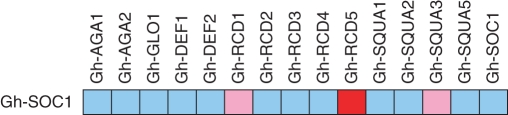
In a pairwise yeast assay with 15 gerbera MADS domain proteins, Gh-SOC1 interacted with Gh-SQUA1, Gh-SQUA3 and Gh-RCD5 (Fig. 7). Both Gh-SQUA1 and Gh-SQUA3 belong to the euAP1-like protein group (Ruokolainen et al., 2010a). Gh-RCD5 in gerbera, together with Gh-RCD4, provides the general E function necessary for the formation of all floral organs (Ruokolainen et al., 2010b).
Fig. 7.
Yeast two-hybrid analysis of protein–protein interactions between Gh-SOC1 and other gerbera MADS domain proteins. Red, strong interaction; pink, weak interaction; blue, no interaction detected.
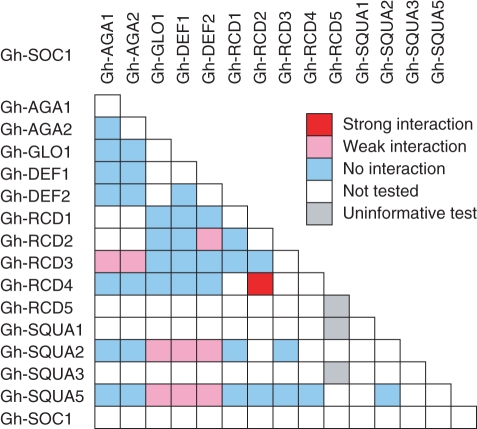
In the yeast three-hybrid analysis, Gh-SOC1 showed bridging capacity with several other MADS domain proteins that did not interact in a pairwise fashion (Fig. 8). Gh-SOC1 was capable of mediating interaction between the At-SEP-like gerbera proteins Gh-RCD2 (Uimari et al., 2004) or Gh-RCD4 (Laitinen et al., 2005, Ruokolainen et al., 2010b), and the B function protein Gh-DEF2 (Yu et al., 1999). Another gerbera At-SEP-like protein, Gh-RCD3 (Kotilainen et al., 2000; Laitinen et al., 2005) could be bridged by Gh-SOC1 to both Gh-AGA1 and Gh-AGA2, the two gerbera C function proteins (Yu et al., 1999). Additionally, gerbera Gh-SQUA2 (Ruokolainen et al., 2010a), which belongs to paleoAP1/FUL-like proteins, and strongly promotes flowering in gerbera, reacted with Gh-SOC1 in combination with gerbera B class proteins Gh-DEF2 or Gh-GLO1 (Yu et al., 1999). Interaction was also seen in combination with Gh-DEF1 which, despite its close sequence similarity to Gh-DEF2, is not a B function protein (Broholm et al., 2010).
Fig. 8.
Yeast three-hybrid analysis of ternary protein complex formation among Gh-SOC1 and other gerbera MADS domain proteins. The strength of interactions is indicated.
DISCUSSION
Gerbera Gh-SOC1 is a paralogue of At-SOC1
The gerbera genome harbours at least three expressed SOC1-like genes. Gh-SOC2 and Gh-SOC3 were identified by RNA sequencing in very young capitula and the inflorescence stem, and group phylogenetically with At-SOC1. Gh-SOC1, obtained from a late petal cDNA library, groups outside of At-SOC1, together with the petunia gene Ph-FBP22. Arabidopsis has six SOC1-like genes altogether. Although Gh-SOC1 is clearly paralogous to At-SOC1, the phylogenetic analysis does not resolve if it is orthologous to At-SOC1, At-AGL71/At-AGL72 or At-AGL14/At-AGL19 (see Fig. 1).
The amino acid sequences of all three gerbera SOC1-like proteins are highly similar to those of other SOC1-like proteins. The consensus sequence of the SOC1 protein motif (Vandenbussche et al., 2003b; Nakamura et al., 2005) is present at the C-terminal end of gerbera Gh-SOC1 and Gh-SOC2; Gh-SOC3 is not full length at the 3′ end (Supplementary Data Fig. S1). All homologous sequences, both in gymnosperms and in angiosperms, contain the SOC1 motif. Nakamura et al. (2005) speculated that the conservation of the motif across long phylogenetic distances might be linked to partner specificity determination in higher order complex formation. They also suggested the sequence to be subject to post-translational modification, influencing DNA binding specificity, subcellular localization or the ability to attract binding protein partners. Specific C-terminal sequences have been shown to influence higher order complex formation with MADS domain proteins (Egea-Cortines et al., 1999; Lamb and Irish, 2003). However, a more recent study shows that at least for arabidopsis B class genes, the C-terminal motifs are dispensable for floral organ identity function (Piwarzyk et al., 2007). Obviously, the conservation of the SOC1 motif must reflect its importance for the function of this group of proteins.
Gh-SOC1 is expressed in gerbera floral organs
The expression pattern of Gh-SOC1 differs from the expression patterns of other SOC1-like genes, including arabidopsis At-SOC1 and petunia Ph-UNS (Borner et al., 2000; Ferrario et al., 2004). Gerbera Gh-SOC1 is expressed only in the inflorescence and in organs derived from the inflorescence (involucre). Typically, SOC1-like genes are expressed in vegetative organs such as leaves and roots, and so far none of the genes in this subfamily has been described as inflorescence abundant. Petunia Ph-UNS (Ferrario et al., 2004) is expressed in vegetative parts of the plant and only at a very low level in inflorescence meristems. No expression was detected in floral meristems and emerging floral organ primordia. Arabidopsis At-SOC1 transcript was detected in vegetative as well as floral parts of the plant. Strong expression was seen in leaves and bolting inflorescences and slightly weaker expression in roots and flowers (Borner et al., 2000). In contrast to At-SOC1, Gh-SOC1 is expressed late in the corollas, which may indicate a role in later developmental stages of gerbera petals. Expressed Gh-SOC1 sequences were obtained from late stages of petals, and also microarray experiments revealed Gh-SOC1 expression in late petal developmental stages (Laitinen et al., 2005). Interestingly, in situ hybridization analysis shows clear expression at earlier stages of floral development as well. However, RNA gel blot and in situ hybridization are difficult to compare in quantitative terms.
Ectopic expression of Gh-SOC1 leads to partial loss of floral organ identity but does not affect flowering time
Transgenic gerbera lines over-expressing Gh-SOC1 show several changes at the inflorescence level. The colour of the inflorescence was altered, and the adaxial side of the ray flower petals was a darker shade than the wild-type petals due to increased anthocyanin content of the cells. The abaxial surface of the ray flower petals had a greenish tint. The ray flower petals were shorter than the wild-type petals with slightly, but not significantly, shorter epidermal cells. Also, the surface structure of the cells was altered.
Greenish sepaloid petals have been reported for other over-expression phenotypes of genes sharing close sequence similarity with Gh-SOC1. Both arabidopsis At-SOC1 and petunia Ph-UNS display similar characteristics when over-expressed (Borner et al., 2000; Ferrario et al., 2004). Especially under short day conditions, the arabidopsis petals were small and sepaloid, with a light green colour. Elongation of pistils in over-expression lines was also observed (Borner et al., 2000). Petunia 35S::Ph-UNS flowers were reported to have a greenish hue, which was maintained until full maturity of the flower (Ferrario et al., 2004). The flattened epidermal cells of petals were considered to be a feature characteristic to leaves and bracts. Ph-UNS over-expression lines featured trichomes on both the adaxial and abaxial side of petals, in the ovaries and in the carpel. While trichomes are most commonly found in leaves and stems, the growth of trichomes on organs where they are not normally found in petunia suggests leaf-like characteristics extending to floral organs by the function of Ph-UNS over-expression. Based on the altered morphology of petunia organs in Ph-UNS over-expression lines and differential expression of other MADS box genes, the authors conclude that, taken together, these observations suggest petals and carpels lose their floral identity (Ferrario et al., 2004).
The effects of ectopic Ph-UNS expression on floral organ identity in petunia may be due to a dominant-negative interference with other factors necessary for proper floral development. A deletion version of Ph-UNS lacking the MADS domain retained both protein–protein interaction and the dominant-negative capacity, but lost its ability to promote flowering (Ferrario et al., 2004). Interestingly, Gh-SOC1 that is expressed in normally developing flowers, unlike Ph-UNS, has a similar negative effect on floral identity when expressed under the 35S promoter. Apparently the role of Gh-SOC1 in normal development of floral organs requires very precisely controlled expression. Its natural expression domain is late in organ development, and ectopic (heterochronic) expression during early stages seems to have an interfering effect in normal development.
At-SOC1 strongly affects flowering time of arabidopsis (Borner et al., 2000). Likewise, petunia lines expressing the full-length Ph-UNS flower early (Ferrario et al., 2004). In gerbera, over-expression of Gh-SOC1 did not suggest any changes in flowering time compared with the wild-type plants. The single case where we have observed accelerated flowering in gerbera thus far has been in lines over-expressing the gerbera MADS box gene Gh-SQUA2 (Ruokolainen et al., 2010a), but GhSOC2 and GhSOC3 are obviously further candidates for obtaining this phenotype when over-expressed.
Despite successful downregulation of Gh-SOC1 expression in gerbera (Supplementary Data Fig. S2), no phenotypic changes were observed.
Higher order interactions with other MADS domain proteins
In pairwise assays, Gh-SOC1 interacted with a few other gerbera MADS domain proteins. However, in higher order assays, Gh-SOC1 showed multiple interactions with several other gerbera MADS domain proteins.
Arabidopsis At-SOC1 is an active player in floral development, converging several developmental pathways. Such a gene is likely to interact with many other proteins, as was indeed shown by de Folter et al. (2005) in their exhaustive yeast two-hybrid screen of arabidopsis MADS domain proteins. The interaction of At-SOC1 with At-CAULIFLOWER (At-CAL) and At-APETALA1 (At-AP1) was earlier reported by Pelaz et al. (2001). In the pairwise assay of all arabidopsis MADS domain proteins, At-SOC1 had >20 interacting protein partners among MADS domain proteins (de Folter et al., 2005).
Gerbera Gh-SOC1 has few interaction partners in yeast two-hybrid assays. The protein shows a pairwise interaction with Gh-SQUA1 and Gh-SQUA3, both of which are euAP1-like proteins like arabidopsis At-AP1 (Ruokolainen et al., 2010a), but no interaction was observed with paleoAP1/FUL-like proteins Gh-SQUA2 and Gh-SQUA5, which are homologous to arabidopsis At-FRUITFULL (At-FUL). Gerbera Gh-RCD5, but not the other gerbera At-SEP-like proteins (Gh-RCD1–Gh-RCD4), interacted with Gh-SOC1. These interactions have counterparts in interactions of the other arabidopsis SOC1-like proteins. At-AGL42 interacts with At-SEP1 (as well as with At-AGL6 and At-SOC1) and At-AGL71 with At-SEP1 (and At-AGL6). At-AGL14, on the other hand, interacts with At-FUL (de Folter et al., 2005).
Petunia Ph-UNS has also been tested for protein–protein interactions (Ferrario et al., 2004). Dimerization partners include Ph-FBP2 and Ph-FBP5, the At-SEP-like proteins of petunia.
In their large-scale study of arabidopsis MADS domain protein ternary complexes, Immink et al. (2009) identified several higher order complexes containing At-SOC1. In the yeast three-hybrid assay Gh-SOC1 protein exhibited rather weak interactions with other gerbera MADS domain proteins compared with other higher order protein–protein interactions seen with different sets of gerbera MADS proteins (Ruokolainen et al., 2010b). These interactions may still be of importance. Our functional analysis (downregulation) could not show where Gh-SOC1 is needed in flower development, but transgenic lines showed that ectopically expressed Gh-SOC1 disturbs floral development. As shown for Ph-UNS (Ferrario et al., 2004), this feature may be a passive dominant-negative effect, reflected by the proteins' capacity to participate in a network of floral regulators.
Conclusions
Gh-SOC1 shares several features with At-SOC1 and Ph-UNS, but its floral abundant expression pattern separates it from these homologous sequences. Analysis of a larger set of expressed gerbera sequences uncovered two additional SOC1-like genes in gerbera, grouping phylogenetically much closer to At-SOC1 and Ph-UNS. Gh-SOC1 appears to be paralogous to At-SOC1, and more closely related to the less studied arabidopsis SOC1-like genes At-AGL14, At-AGL19, At-AGL42, At-AGL71 and At-AGL72.
Gh-SOC1 is expressed most strongly during late petal development, indicating a possible role in late stages of flower organ development. Over-expression of Gh-SOC1 leads to partial loss of floral identity, but does not affect flowering time. Several gerbera MADS domain proteins were identified in yeast two- and three-hybrid analyses for interaction, but Gh-SOC1 did not participate in higher order protein complexes of dimers very actively.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
Eija Takala is thanked for excellent technical assistance, and Sanna Peltola for taking care of transgenic gerbera lines in the greenhouse. We are grateful for Dr Richard Immink of Plant Research International, Wageningen,The Netherlands, for sharing material and information concerning the yeast assays. Professor Pieter Ouwerkeerk of University of Leiden, The Netherlands granted permission to use the pRED-NLSa plasmid derivative pARC351. This work was supported by the Academy of Finland (grant no. 207410).
LITERATURE CITED
- Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla ER, Liljegren SJ, Pelaz S, et al. MADS-box gene evolution beyond flowers: expression in pollen, endosperm, guard cells, roots and trichomes. The Plant Journal. 2000;24:457–466. doi: 10.1046/j.1365-313x.2000.00891.x. [DOI] [PubMed] [Google Scholar]
- Becker A, Theissen G. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Molecular Phylogenetics and Evolution. 2003;29:464–489. doi: 10.1016/s1055-7903(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Borner R, Kampmann G, Chandler J, et al. A MADS domain gene involved in the transition to flowering in Arabidopsis. The Plant Journal. 2000;24:591–599. doi: 10.1046/j.1365-313x.2000.00906.x. [DOI] [PubMed] [Google Scholar]
- Broholm SK, Pollanen E, Ruokolainen S, et al. Functional characterization of B class MADS-box transcription factors in Gerbera hybrida. Journal of Experimental Botany. 2010;61:75–85. doi: 10.1093/jxb/erp279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen ES, Meyerowitz EM. The war of the whorls: genetic interactions controlling flower development. Nature. 1991;353:31–37. doi: 10.1038/353031a0. [DOI] [PubMed] [Google Scholar]
- Cseke LJ, Zheng J, Podila GK. Characterization of PTM5 in aspen trees: a MADS-box gene expressed during woody vascular development. Gene. 2003;318:55–67. doi: 10.1016/s0378-1119(03)00765-0. [DOI] [PubMed] [Google Scholar]
- Decroocq V, Zhu X, Kauffman M, et al. A TM3-like MADS-box gene from Eucalyptus expressed in both vegetative and reproductive tissues. Gene. 1999;228:155–160. doi: 10.1016/s0378-1119(98)00613-1. [DOI] [PubMed] [Google Scholar]
- Ditta G, Pinyopich A, Robles P, Pelaz S, Yanofsky MF. The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Current Biology. 2004;14:1935–1940. doi: 10.1016/j.cub.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Egea-Cortines M, Saedler H, Sommer H. Ternary complex formation between the MADS-box proteins SQUAMOSA, DEFICIENS and GLOBOSA is involved in the control of floral architecture in Antirrhinum majus. EMBO Journal. 1999;18:5370–5379. doi: 10.1093/emboj/18.19.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elomaa P, Honkanen J, Puska R, et al. Agrobacterium-mediated transfer of antisense chalcone synthase cDNA to Gerbera hybrida inhibits flower pigmentation. Bio/Technology. 1993;11:508–511. [Google Scholar]
- Elomaa P, Teeri TH. Transgenic Gerbera. In: Bajaj YPS, editor. Biotechnology in agriculture and forestry, transgenic crops III. Berlin: Springer; 2001. pp. 139–154. [Google Scholar]
- Fang SC, Fernandez DE. Effect of regulated overexpression of the MADS domain factor AGL15 on flower senescence and fruit maturation. Plant Physiology. 2002;130:78–89. doi: 10.1104/pp.004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario S, Busscher J, Franken J, et al. Ectopic expression of the petunia MADS box gene UNSHAVEN accelerates flowering and confers leaf-like characteristics to floral organs in a dominant-negative manner. The Plant Cell. 2004;16:1490–1505. doi: 10.1105/tpc.019679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Folter S, Immink RGH, Kieffer M, et al. Comprehensive interaction map of the Arabidopsis MADS box transcription factors. The Plant Cell. 2005;17:1424–1433. doi: 10.1105/tpc.105.031831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goloboff P, Farris S, Nixon K. TNT (Tree analysis using New Technology) 2003 Software published by the authors. Tucumán, Argentina. [Google Scholar]
- Helariutta Y, Elomaa P, Kotilainen M, Seppänen P, Teeri TH. Cloning of cDNA coding for dihydroflavonol-4-reductase (DFR) and characterization of dfr expression in the corollas of Gerbera hybrida var. Regina (Compositae) Plant Molecular Biology. 1993;22:183–193. doi: 10.1007/BF00014927. [DOI] [PubMed] [Google Scholar]
- Heuer S, Hansen S, Bantin J, et al. The maize MADS box gene ZmMADS3 affects node number and spikelet development and is co-expressed with ZmMADS1 during flower development, in egg cells, and early embryogenesis. Plant Physiology. 2001;127:33–45. doi: 10.1104/pp.127.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immink RGH, Ferrario S, Busscher-Lange J, Kooiker M, Busscher M, Angenent GC. Analysis of the petunia MADS-box transcription factor family. Molecular Genetics and Genomics. 2003;268:598–606. doi: 10.1007/s00438-002-0781-3. [DOI] [PubMed] [Google Scholar]
- Immink RGH, Hannapel DJ, Ferrario S, et al. A petunia MADS box gene involved in the transition from vegetative to reproductive development. Development. 1999;126:5117–5126. doi: 10.1242/dev.126.22.5117. [DOI] [PubMed] [Google Scholar]
- Immink R, Tonaco I, de Folter S, et al. SEPALLATA3: the ‘glue’ for MADS box transcription factor complex formation. Genome Biology. 2009;10 doi: 10.1186/gb-2009-10-2-r24. doi:10.1186/gb-2009-10-2-r24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Halladay J, Craig EA. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotilainen M, Elomaa P, Uimari A, Albert VA, Yu D, Teeri TH. GRCD1, an AGL2-like MADS box gene, participates in the C function during stamen development in Gerbera hybrida. The Plant Cell. 2000;12:1893–1902. doi: 10.1105/tpc.12.10.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen RA, Immanen J, Auvinen P, et al. Analysis of the floral transcriptome uncovers new regulators of organ determination and gene families related to flower organ differentiation in Gerbera hybrida (Asteraceae) Genome Research. 2005;15:475–486. doi: 10.1101/gr.3043705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RS, Irish VF. Functional divergence within the APETALA3/PISTILLATA floral homeotic gene lineages. Proceedings of the National Academy of Sciences, USA. 2003;100:6558–6563. doi: 10.1073/pnas.0631708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Yanofsky MF, Meyerowitz EM. AGL1–AGL6, an Arabidopsis gene family with similarity to floral homeotic and transcription factor genes. Genes and Development. 1991;5:484–495. doi: 10.1101/gad.5.3.484. [DOI] [PubMed] [Google Scholar]
- Ma L, Sun N, Liu X, Jiao Y, Zhao H, Deng XW. Organ-specific expression of Arabidopsis genome during development. Plant Physiology. 2005;138:80–91. doi: 10.1104/pp.104.054783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel MA, Yanofsky MF. A gene triggering flower formation in Arabidopsis. Nature. 1995;377:522–524. doi: 10.1038/377522a0. [DOI] [PubMed] [Google Scholar]
- Mandel MA, Yanofsky MF. The Arabidopsis AGL9 MADS box gene is expressed in young flower primordia. Sexual Plant Reproduction. 1998;11:22–28. [Google Scholar]
- Moon J, Suh SS, Lee H, et al. The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. The Plant Journal. 2003;35:613–623. doi: 10.1046/j.1365-313x.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- Moon J, Lee H, Kim M, Lee I. Analysis of flowering pathway integrators in Arabidopsis. Plant and Cell Physiology. 2005;46:292–299. doi: 10.1093/pcp/pci024. [DOI] [PubMed] [Google Scholar]
- Münster T, Faigl W, Saedler H, Theissen G. Evolutionary aspects of MADS-box genes in the eusporangiate fern Ophioglossum. Plant Biology. 2002;4:474–483. [Google Scholar]
- Nakamura T, Song IJ, Fukuda T, et al. Characterization of TrcMADS1 gene of Trillium camtschatcense (Trilliaceae) reveals functional evolution of the SOC1/TM3-like gene family. Journal of Plant Research. 2005;118:229–234. doi: 10.1007/s10265-005-0215-5. [DOI] [PubMed] [Google Scholar]
- Nam J, dePamphilis CW, Ma H, Nei M. Antiquity and evolution of the MADS-box gene family controlling flower development in plants. Molecular Biology and Evolution. 2003;20:1435–1447. doi: 10.1093/molbev/msg152. [DOI] [PubMed] [Google Scholar]
- Nam J, Kim J, Lee S, An G, Ma H, Nei M. Type I MADS-box genes have experienced faster birth-and-death evolution than type II MADS-box genes in angiosperms. Proceedings of the National Academy of Sciences, USA. 2004;101:1910–1915. doi: 10.1073/pnas.0308430100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy T, Lee JY, Colinas J, et al. Transcriptional profile of the Arabidopsis root quiescent center. The Plant Cell. 2005;17:1908–1925. doi: 10.1105/tpc.105.031724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature. 2000;405:200–203. doi: 10.1038/35012103. [DOI] [PubMed] [Google Scholar]
- Pelaz S, Gustafson-Brown C, Kohalmi SE, Crosby WL, Yanofsky MF. APETALA1 and SEPALLATA3 interact to promote flower development. The Plant Journal. 2001;26:385–394. doi: 10.1046/j.1365-313x.2001.2641042.x. [DOI] [PubMed] [Google Scholar]
- Piwarzyk E, Yang Y, Jack T. Conserved C-terminal motifs of the Arabidopsis proteins APETALA3 and PISTILLATA are dispensable for floral organ identity function. Plant Physiology. 2007;145:1495–1505. doi: 10.1104/pp.107.105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pnueli L, Abu-Abeid M, Zamir D, Nacken W, Schwarz-Sommer Z, Lifschitz E. The MADS box gene family in tomato: temporal expression during floral development, conserved secondary structures and homology with homeotic genes from Antirrhinum and Arabidopsis. The Plant Journal. 1991;1:255–266. [PubMed] [Google Scholar]
- Ratcliffe OJ, Nadzan GC, Reuber TL, Riechmann JL. Regulation of flowering in Arabidopsis by an FLC homologue. Plant Physiology. 2001;126:122–132. doi: 10.1104/pp.126.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe OJ, Kumimoto RW, Wong BJ, Riechmann JL. Analysis of the Arabidopsis MADS AFFECTING FLOWERING gene family: MAF2 prevents vernalization by short periods of cold. The Plant Cell. 2003;15:1159–1169. doi: 10.1105/tpc.009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounsley SD, Ditta GS, Yanofsky MF. Diverse roles for MADS box genes in Arabidopsis development. The Plant Cell. 1995;7:1259–1269. doi: 10.1105/tpc.7.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruokolainen S, Ng YP, Broholm SK, Albert VA, Elomaa P, Teeri TH. Characterization of SQUAMOSA-like genes in Gerbera hybrida, including one involved in reproductive transition. BMC Plant Biology. 2010a;10:128. doi: 10.1186/1471-2229-10-128. doi:10.1186/1471-2229-10-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruokolainen S, Ng YP, Albert VA, Elomaa P, Teeri TH. Large scale interaction analysis predicts that the Gerbera hybrida floral E function is provided both by general and specialized proteins. BMC Plant Biology. 2010b;10:129. doi: 10.1186/1471-2229-10-129. doi:10.1186/1471-2229-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samach A, Onouchi H, Gold SE, et al. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science. 2000;288:1613–1616. doi: 10.1126/science.288.5471.1613. [DOI] [PubMed] [Google Scholar]
- Smykal P, Gennen J, De Bodt S, Ranganath V, Melzer S. Flowering of strict photoperiodic Nicotiana varieties in non-inductive conditions by transgenic approaches. Plant Molecular Biology. 2007;65:233–242. doi: 10.1007/s11103-007-9211-6. [DOI] [PubMed] [Google Scholar]
- Tadege M, Sheldon CC, Helliwell CA, Upadhyaya NM, Dennis ES, Peacock WJ. Reciprocal control of flowering time by OsSOC1 in transgenic Arabidopsis and by FLC in transgenic rice. Plant Biotechnology Journal. 2003;1:361–369. doi: 10.1046/j.1467-7652.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- Tandre K, Albert VA, Sundas A, Engstrom P. Conifer homologues to genes that control floral development in angiosperms. Plant Molecular Biology. 1995;27:69–78. doi: 10.1007/BF00019179. [DOI] [PubMed] [Google Scholar]
- Theissen G, Saedler H. Floral quartets. Nature. 2001;409:469–471. doi: 10.1038/35054172. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevaskis B, Bagnall DJ, Ellis MH, Peacock WJ, Dennis ES. MADS box genes control vernalization-induced flowering in cereals. Proceedings of the National Academy of Sciences, USA. 2003;100:13099–13104. doi: 10.1073/pnas.1635053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uimari A, Kotilainen M, Elomaa P, Yu D, Albert VA, Teeri TH. Integration of reproductive meristem fates by a SEPALLATA-like MADS-box gene. Proceedings of the National Academy of Sciences, USA. 2004;101:15817–15822. doi: 10.1073/pnas.0406844101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche M, Zethof J, Souer E, et al. Toward the analysis of the petunia MADS box gene family by reverse and forward transposon insertion mutagenesis approaches: B, C, and D floral organ identity functions require SEPALLATA-like MADS box genes in petunia. The Plant Cell. 2003a;15:2680–2693. doi: 10.1105/tpc.017376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche M, Theissen G, Van de Peer Y, Gerats T. Structural diversification and neo-functionalization during floral MADS-box gene evolution by C-terminal frameshift mutations. Nucleic Acids Research. 2003b;31:4401–4409. doi: 10.1093/nar/gkg642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, et al. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science. 2002;296:343–346. doi: 10.1126/science.1068181. [DOI] [PubMed] [Google Scholar]
- Walden AR, Wang DY, Walter C, Gardner RC. A large family of TM3 MADS-box cDNAs in Pinus radiata includes two members with deletions of the conserved K domain. Plant Science. 1998;138:167–176. [Google Scholar]
- Watson JM, Brill EM. Eucalyptus grandis has at least two functional SOC1-like floral activator genes. Functional Plant Biology. 2004;31:225–234. doi: 10.1071/FP03181. [DOI] [PubMed] [Google Scholar]
- Winter KU, Becker A, Munster T, Kim JT, Saedler H, Theissen G. MADS-box genes reveal that gnetophytes are more closely related to conifers than to flowering plants. Proceedings of the National Academy of Sciences, USA. 1999;96:7342–7347. doi: 10.1073/pnas.96.13.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DY, Kotilainen M, Pöllänen E, et al. Organ identity genes and modified patterns of flower development in Gerbera hybrida (Asteraceae) The Plant Journal. 1999;17:51–62. doi: 10.1046/j.1365-313x.1999.00351.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Forde BG. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science. 1998;279:407–409. doi: 10.1126/science.279.5349.407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.