Abstract
Background
Ovules as developmental precursors of seeds are organs of central importance in angiosperm flowers and can be traced back in evolution to the earliest seed plants. Angiosperm ovules are diverse in their position in the ovary, nucellus thickness, number and thickness of integuments, degree and direction of curvature, and histological differentiations. There is a large body of literature on this diversity, and various views on its evolution have been proposed over the course of time. Most recently evo–devo studies have been concentrated on molecular developmental genetics in ovules of model plants.
Scope
The present review provides a synthetic treatment of several aspects of the sporophytic part of ovule diversity, development and evolution, based on extensive research on the vast original literature and on experience from my own comparative studies in a broad range of angiosperm clades.
Conclusions
In angiosperms the presence of an outer integument appears to be instrumental for ovule curvature, as indicated from studies on ovule diversity through the major clades of angiosperms, molecular developmental genetics in model species, abnormal ovules in a broad range of angiosperms, and comparison with gymnosperms with curved ovules. Lobation of integuments is not an atavism indicating evolution from telomes, but simply a morphogenetic constraint from the necessity of closure of the micropyle. Ovule shape is partly dependent on locule architecture, which is especially indicated by the occurrence of orthotropous ovules. Some ovule features are even more conservative than earlier assumed and thus of special interest in angiosperm macrosystematics.
Keywords: Angiosperms, development, diversity, evo–devo, evolution, integuments, macrosystematics, micropyle, nucellus, ovules, seed plants
INTRODUCTION
Ovules, the developmental precursors of seeds, are the organs in angiosperm flowers that can be traced back farthest in time, back to early seed plants almost 400 million years ago. In spite of their relatively stable basic structure, ovules have attained a broad diversity of forms. The early evolution of ovules in angiosperms has been much under discussion in comparative structural studies and embryology on extant and fossil plants, and recently ovules became prominent in molecular developmental genetic studies.
Thus, information on ovules relies on sources from different fields, and a synthetic review needs to draw from all of them. There is a plethora of descriptive studies on embryology of single angiosperm species, also including the sporophytic part of the ovules, especially from Indian botanists in the time between 1930 and 1980. Each by itself may not be of special interest, but taken together they are a treasure trove of information on ovule diversity, the value of which continuously increases with each new study. Another field encompasses studies on the development of ovules in model species, especially Arabidopsis, from the past 20 years. There are a number of comparative studies and reviews in which the sporophytic part of ovules and its diversity was considered (Brongniart, 1827; Mirbel, 1829; Agardh, 1858; Warming, 1878, 1913; van Tieghem, 1901; Schnarf, 1929, 1931, 1933; Mauritzon, 1939b; Maheshwari, 1950, 1963; Johri, 1963, 1967, 1984; Kapil and Vasil, 1963; Puri, 1970; Bouman, 1974, 1984a, b; Philipson, 1974, 1977; Hamann, 1977; Yakovlev and Batygina, 1981–1990; Tobe, 1989; Dahlgren, 1991; Kapil and Bhatnagar, 1991; Johri et al., 1992; Rudall, 1997; Shamrov, 1998, 2002b, 2003, 2006; Rangan and Rangaswamy, 1999; Batygina, 2002). Currently we are able to discuss the diversity and evolution of ovules based on molecular phylogenetic results (APG, 2009). In addition, molecular developmental studies on ovules brought to light new evolutionary facets over the past 20 years. This review focuses mainly on (a) evolution of ovules within angiosperms as seen in the current phylogenetic framework; (b) understanding of certain specific features of angiosperm ovules from patterns and trends in a broad range of angiosperm ovules; (c) evolution of angiosperm ovules from gymnosperm ovules; and (d) the role of ovules in angiosperm macrosystematics.
In the studies from my laboratory, carpel and ovule diversity was compared through all families of extant basal angiosperms, including the ANITA grade, magnoliids and the basal grades of monocots and of eudicots (Endress, 1986; Endress and Igersheim 1997, 1999, 2000a, b; Endress et al., 2000; Igersheim and Endress, 1997, 1998; Igersheim et al., 2001), as well as several orders of rosids (Matthews and Endress, 2002, 2004, 2005a, b, 2008, 2011; Endress and Matthews, 2006; Bachelier and Endress, 2007, 2008, 2009). In addition, data on floral structure, including ovules, were compiled from >3300 original publications (see Endress, 2011). Although ovules have their own developmental dynamics, some structural properties of ovules, such as curvature and symmetry, are dependent on their position in the ovary. Thus, ovule structure cannot be fully understood if the architecture of their surroundings is not considered in the discussion.
Most of the figures are original. Collections used for figures are listed in the Appendix.
BASIC STRUCTURE AND DEVELOPMENT OF ANGIOSPERM OVULES
Angiosperm ovules basically consist of a nucellus and two integuments and may be sessile on the placenta or attached to it by a stalk, the funiculus (survey by Bouman, 1984a). Most commonly a vascular bundle extends from the placenta through the funiculus to the chalaza, i.e. the area right below the base of the nucellus where the integuments depart. The funiculus and the chalaza are intercalary structures and thus less well demarcated than the nucellus and integuments. The nucellus represents the megasporangium, in which a meiocyte undergoes meiosis forming four megaspores, typically only one of which develops into an embryo sac representing the megagametophyte. The embryo sac contains basically four or eight nuclei, organized into four or seven cells, depending on whether there are two or three rounds of mitotic divisions in the developing embryo sac (Maheshwari, 1950; Friedman and Williams, 2003; Friedman, 2006). These cells are the egg cell, associated with two synergids, all three forming the egg apparatus, a large central cell with one or two nuclei, and, if seven cells are present, three antipodals opposite the egg apparatus. The inner or both integuments form the micropyle, a narrow canal through which a pollen tube reaches the nucellus, grows into the nucellus and the embryo sac, and there into one of the synergids. One of the two sperm cells conveyed with the pollen tube fertilizes the egg cell resulting in the zygote, and the other fuses with the nucleus of the central cell (double fertilization), which then gives rise to the endosperm. In typical embryo sacs with seven cells, the central cell contains two nuclei, which fuse into a diploid nucleus and the endosperm becomes triploid; this is the most common type of embryo sac in angiosperms (Polygonum type). In embryo sacs with four cells, the central cell has only one nucleus and the endosperm is diploid.
Ovules begin development from the inner morphological surface of the carpels (Endress, 2006). They first appear as a mound, similar to other floral organs. The mound elongates and, close to the apex, the two integument primordia appear almost simultaneously, but often the inner slightly earlier than the outer (Figs 1A and 2B). The site where the integuments are initiated is the prospective chalaza. Most angiosperm ovules are curved so that the micropyle is directed toward the placenta, the direction from which pollen tubes arrive. In young ovules that later become curved (anatropous or campylotropous; see ‘Basic diversity of ovules in extant angiosperms’), the integument primordia are more conspicuous on the convex side; the outer may even be lacking on the concave side (Figs 2E' and 3B). The part of the ovule above the inner integument primordium will develop into the nucellus. Thus the nucellus is only delimited with the initiation of the integuments. If there are no integuments there is consequently no nucellus, and the ovule is morphologically undifferentiated. The inner integument always forms a tubular sheath around the nucellus. The outer integument is more variable. In orthotropous ovules it also forms a tubular sheath. In anatropous or campylotropous ovules it either also forms a tubular sheath (annular outer integument) or it is incomplete on the concave side (semi-annular, hood-shaped outer integument). Whether it becomes annular or semi-annular depends on the speed of ovule curvature or the speed of progression of the outer integument primordium from the convex towards the concave side. The greater the speed of ovule curvature and the lower the speed of progression of the outer integument, the more the development is towards semi-annular. Basic developmental processes in ovules of the model species Arabidopsis thaliana were described by Bowman (1993) and Schneitz et al. (1995). Curved ovules have a raphe, a sometimes conspicuous area through which the vascular bundle runs from the funiculus to the chalaza. It is not useful to describe the raphe as a product of ‘congenital fusion of the outer integument with the funiculus’ (Tilton and Lersten, 1981a, p. 452). The raphe develops by the extension of the ovule on one side beyond the funiculus and below the outer integument and is merely a developmental by-product of ovule curvature (Fig. 2E).
Fig. 1.
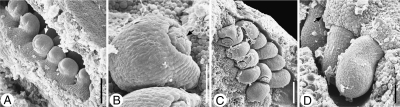
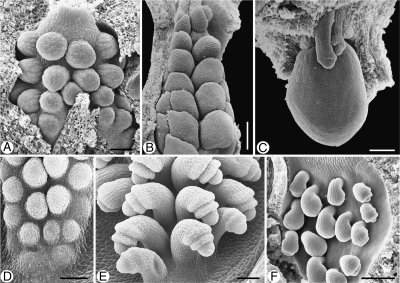
Anatropous ovules in Tasmannia piperita (Winteraceae). SEM micrographs. (A) Young ovules in one series on a placenta, showing the nucellar apex and initiation of the inner integument (arrow). (B) Ovule before anthesis, antiraphal side, micropyle curved toward the pollen tube transmitting tissue on the placenta, inner integument lobed (arrow). (C) Ovules at anthesis, in two series, curved away from each other, micropylar area partly covered with secretion from micropyle. (D) Three ovules at anthesis, seen from raphal side, the funiculus surrounded by papillate pollen tube transmitting tissue (arrow). Scale bars: (A, B, C) = 0·1 mm; (D) = 0·3 mm.
Fig. 2.
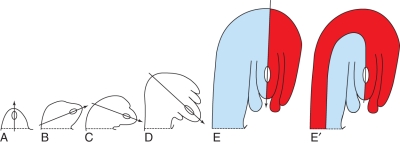
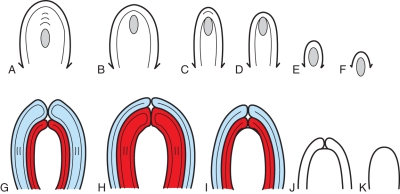
Development of an anatropous bitegmic angiosperm ovule. Median longitudinal sections (schematized and augmented from Bouman, 1984a). (A–E) Straight thin line drawn through the middle of the nucellus. Arrowhead indicating successive ovule curvature from 0 to 180 °. (E, E′) Two possible ways of designation of sides of a mature ovule are shown with colours. In (E′) the thin line is not straight but follows the curvature of the ovule. (A) Ovule before integument initiation. (B) Ovule at initiation of inner integument. (C) Slightly older ovule. (D) Ovule with both integuments formed. (E) Mature ovule. Raphal side blue, antiraphal side red. (E′) Mature ovule. Concave side blue, convex side red.
Fig. 3.
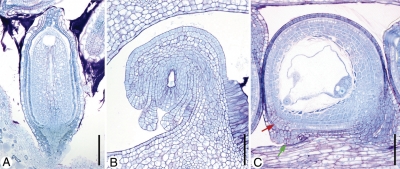
Diversity in ovule curvature. Median longitudinal microtome sections. (A) Orthotropous ovule (Barclaya rotundifolia). (B) Anatropous ovule (Asimina triloba). (C) Campylotropous ovule (Hypecoum pendulum). In the zig-zag micropyle (C) the part formed by the outer integument is marked with a green arrow, the part formed by the inner integument with a red arrow. Scale bars: (A) = 0·2 mm; (B, C) = 0·1 mm.
In terms of developmental genetics and the ‘ABC of floral development’, an additional class D MADS-box gene was assumed to determine ovule identity (Angenent et al., 1995; Colombo et al., 1995; Dreni et al., 2007). From subsequent studies, ovule identity appears to be promoted by the shared activity of C and D class genes (Favaro et al., 2003; Pinyopich et al., 2003). The D gene lineage originated from duplication of the C gene lineage; the C lineage may originally have operated in female organ identity (including ovules) and, following duplication, underwent sub-functionalization by which the D lineage specialized in ovule morphology (Kramer et al., 2004). A crucial event in ovule morphogenesis is integument initiation. As mentioned above, with integument initiation, the nucellus, chalaza and funiculus also become defined (Schneitz et al., 1995; Schneitz, 1999), and the genetics of this differentiation, in which NOZZLE plays an important role, was first studied in Arabidopsis (Schneitz et al., 1997, 1998a; Balasubramaniam and Schneitz, 2000, 2002). So far, numerous genes have been recovered that are involved in ovule development (Gasser et al., 1998; Schneitz et al., 1998a; Kelley and Gasser, 2009; Skinner and Gasser, 2009). This genetic diversity may reflect part of the morphological diversity of angiosperm ovules.
The putative evolutionary sequence of parts in ovules corresponds to the developmental sequence (nucellus – inner integument – outer integument – funiculus) and is reflected by molecular genetics of development in Arabidopsis, which shows that it is easier to affect the outer integument and funiculus than the nucellus and inner integument (Schneitz et al., 1998b). As an analogy of this developmental sequence, in stamen development the central part, the anther, also develops before the filament. Both ovules and stamens have in common that the part where meiosis takes place differentiates first. It may be assumed that this is functionally important because differentiation of meiocytes involves a highly specialized surrounding tissue which, in turn, requires a relatively long time for development. In contrast, the other (outer, basal) parts have a simpler structure and differentiate more rapidly.
BASIC DIVERSITY OF OVULES IN EXTANT ANGIOSPERMS
Ovule diversity is expressed in several respects. The main aspects of diversity are ovule size, degree of ovule curvature, nucellus thickness, integument number and thickness, formation of the micropyle, funiculus length, degree of vascularization of the ovule and diverse histological differentiation (e.g. hypostase, postament and endothecium).
Ovules are around 0·5 mm long in many angiosperm clades at the time of fertilization. In small-ovuled clades they are approx. 0·15 mm long. Large ovules may reach >2 mm. Diversity of ovule size may be extensive even at the level of orders.
Ovules can be straight or curved in various ways. Straight, uncurved ovules (orthotropous, atropous; Fig. 3A) are radially symmetric (or disymmetric). In curved ovules the nucellus is either straight (anatropous ovule, Fig. 3B) or it is also involved in the curvature (campylotropous ovule, Fig. 3C). Ovules that are only slightly curved are hemitropous (hemianatropous). The three terms ‘orthotrope’, ‘anatrope’ and ‘campulitrope’ (in French) for the different types of expression of curvature were used by Mirbel (1829) (see also Wagenitz, 2003). Curved ovules are either monosymmetric or, when they twist, in addition may be asymmetric. The latter is the case in pendant ovules on a lateral placenta. In extant angiosperms, anatropous ovules are most probably ancestral (Doyle, 2008; Endress and Doyle, 2009). Some other types, in addition to the three most commonly distinguished types (orthotropous, anatropous and campylotropous), have been described (e.g. Bocquet, 1959; Bouman and Boesewinkel, 1991; Taylor, 1991; Batygina 2002), but will not be treated here, as their systematic significance is unexplored.
The nucellus is diverse in thickness and length. van Tieghem (1898) coined the terms ‘plantes crassinucellées’ (plants with crassinucellar ovules) (Fig. 10A, B) and ‘plantes tenuinucellées’ (plants with tenuinucellar ovules) (Fig. 10C–F) to distinguish between thick and thin nucelli. This distinction between crassinucellar and tenuinucellar has long been used in the embryological and morphological literature. In a review by Warming (1913) ‘ovules eusporangiates’ and ‘ovules leptosporangiates’ (in French) were distinguished, corresponding to crassinucellar and tenuinucellar. A more detailed classification was attempted by Shamrov (1997, 1998, 2000, 2002b, 2006), containing developmental aspects but without a phylogenetic framework. A phylogenetic framework for a progressively more refined classification was used by Endress (2003, 2005, 2010, 2011) and Endress and Matthews (2006) (see ‘Nucellus structure in angiosperm ovules’).
Integuments are diverse in number and of differential thickness (Fig. 10G–K). The number can be reduced from the basic two to one or (exceptionally) none. van Tieghem (1898) considered integument number in his ovule classification as ‘plantes bitegminées’ (plants with bitegmic ovules) and ‘plantes unitegminées’ (plants with unitegmic ovules), and also used this distinction in his angiosperm classification (van Tieghem, 1901). Shamrov (2000, 2003) dealt with integument diversity from a developmental point of view. Endress and Matthews (2006) and Endress (2010, 2011) found new correlations in integument thickness with macrosystematics from a phylogenetic point of view. Further, integuments can be lobed or unlobed, and the outer integument can be annular or semi-annular (review of basal angiosperms in Endress and Igersheim, 2000a).
The micropyle may be formed by one or both integuments. In some cases there is no micropyle at the time of ovule maturity, and adjacent parts (the funiculus or obturator) may be in contact with the rim of the integuments or directly with the nucellus.
Ovules are borne on the placentae of the carpels. They may have stalks (funiculi) of different length or may be sessile. When they are sessile they may have a narrow or an extensive attachment area.
Most ovules have a vascular bundle extending from the placenta through the funiculus and raphe to the chalaza. In a number of clades, vascular bundles also reach into one of the integuments. This is mostly in combination with large seeds. At the other extreme, there are ovules with only an undifferentiated procambial strand in the funiculus and raphe or even without any procambial strand at all. Such ovules are small and also otherwise reduced.
EVOLUTIONARY ORIGIN OF OVULES IN SEED PLANTS
Discussion on evolution of ovules needs to incorporate aspects of function, development, differentiation at the key functional stages, extant diversity and fossil record (Haig and Westoby, 1989). The main functions of ovules as developmental precursors of seeds are: (1) production via meiosis of the female gametophyte with the egg cell; (2) collection of pollen (microspores) (in gymnosperms) or attraction of pollen tubes (male gametophytes) (in angiosperms) at the micropyle; (3) canalization of male gametes toward the egg cell via the nucellus and female gametophyte; (4) protection of the nucellus containing the female gametophyte with the egg cell and the developing embryo and endosperm; (5) closure of the pollen chamber (in gymnosperms); and (6) formation of specializations for seed dispersal, such as wings or a sarcotesta (combined with a sclerotesta) in endozoochory (in gymnosperms) and also other devices (in angiosperms). Thus an evolutionary reconstruction needs to take into consideration all these six functions. Function (1) is always provided by a nucellus. Thus it can be expected that nucelli in gymnosperms and angiosperms are all homologous. In contrast, functions (2–4) may not be furnished by the same organs in all seed plants and thus there may be transference of functions.
The egg cell is produced within the nucellus by a multicellular gametophyte in gymnosperms (e.g. Singh, 1978; Friedman and Carmichael, 1998) or by a few-celled gametophyte (the embryo sac) in angiosperms (e.g. Maheshwari, 1950; Friedman, 2006).
In extant gymnosperms, in which the ovules are exposed, attraction of microspore-transporting pollinators (in insect-pollinated clades) is olfactory and/or optical by odour and colour of the integument or organs surrounding the ovules, and the pollination drop presented at the micropyle. There is little known about the mechanism of attraction of the pollen tubes or gametes (spermatozoids) toward the egg cells (Singh, 1978). In contrast, in angiosperms, in which ovules are enclosed in a carpel or a multicarpellate gynoecium, attraction of the pollen through pollinators is by the carpels or other floral organs and attraction of the pollen tubes is chemical within the carpel or gynoecium by compounds secreted from upper parts of the carpels (Kim et al., 2003) and from the synergids of the embryo sac, or also secreted by the nucellus apex or the micropyle (Tilton and Lersten, 1981a, b, c; Franssen-Verheijen et al., 1993; Hülskamp et al., 1995; Smyth, 1997; Shimizu and Okada, 2000; McCormick and Yang, 2005; Dresselhaus and Márton, 2009). Spermatozoids are present in extant gymnosperms only in cycads and Ginkgo but were more common in the early evolutionary history of spermatophytes (e.g. Poort et al., 1996; Nishida et al., 2003; Doyle, 2006). The pollination drop, occurring in most extant gymnosperms, which is presented at the micropyle (see next paragraph) and in which pollen grains are caught, is formed by the integument and from decaying tissue (holocrine secretion) at the nucellus apex (Ziegler, 1959; Singh, 1978; Tomlinson, 1991; Tomlinson et al., 1991; Chesnoy, 1993; Takaso and Owens, 1996; Takaso et al., 1996; Stützel and Röwekamp, 1997; Gelbart and von Aderkas, 2002; Wagner et al., 2007; Nepi et al., 2009). In extant gnetophytes, most of which are insect-pollinated, not only do the fertile ovules present pollination drops but, in addition, the male units are associated with pollen drop-producing sterile ovules (e.g. Endress, 1996). There is little known on attraction by colour or scent in early seed plants, if there were animal pollinators at all. In fossils, such as Elkinsia or Lagenostoma (Lyginopteris) (Rothwell et al., 1989; Rothwell and Serbet, 1992; Taylor and Taylor, 1993), the organs surrounding ovules and forming a cupule are spreading, and in Lagenostoma (Lyginopteris) from the Carboniferous they appear to have had glands, which could have been protective or attractive. For paleozoic pteridosperms pollination drops were inferred by Rothwell (1977).
For canalization of male gametes a narrow tube is needed, the micropyle, which is formed by the single integument in extant gymnosperms and, within the carpel in angiosperms, by one or two integuments. The evolution of the integument in gymnosperms is unclear. An integument may have evolved several times (Li et al., 1997). Evolution from a group of branches of dichotomous branching systems (telomes in the terminology of Zimmermann, 1952) that became associated with megasporangia has most often been suggested as a first step (e.g. Andrews, 1963; Smith, 1964; Long, 1967; Gillespie et al., 1981; Retallack and Dilcher, 1988; Rothwell and Scheckler, 1988; Galtier and Rowe, 1989; Rothwell et al., 1989; Stewart and Rothwell, 1993; Hilton and Edwards, 1999; Kelley and Gasser, 2009), and such ovule precursors without a micropyle are called ‘pre-ovules’ (e.g. Stewart and Rothwell, 1993; Hilton and Edwards, 1996). Kenrick and Crane (1997) suggested a derivation of this megasporangium envelope from a group of sterile megasporangia. In some cases, the integument may be derived from two units, depending on symmetry and the number of vascular bundles in fossils, such as in the early Carboniferous Mitrospermum of cordaitean affinity (Long, 1977), the Late Carboniferous Stephanospermum (Drinnan et al., 1990) and Callospermarion of potential medullosan affinity (Eggert and Delevoryas, 1960), or the Permian Choanostoma of unknown affinity (Klavins et al., 2001). Such an envelope of several branches may function in catching microspores from the air by producing specific local airflows, if they were wind-pollinated, but not in exact canalization of microspores to the nucellus apex (Niklas, 1981a, b). Alternatively, it may be that the integument developed from the outer wall of a differentiation of the ovule apex, the pollen chamber (including the lagenostome and salpinx) (Meeuse and Bouman, 1974), as in extinct early seed plants, which probably functioned in canalization (called a ‘pollen-receiving mechanism’ in Taylor et al., 2009). However, from descriptions it is often not clear whether the pollen chamber and its wall in extinct seed plants is a structure at the morphological level, i.e. by direct origin from the ovule apex, or merely at the histological level, i.e. by differential decay of tissue (e.g. Long, 1960; Hilton and Bateman, 2006), similar to the pollen chamber of some living gymnosperms (e.g. Friedman, 1987; Douglas et al., 2007, for Ginkgo). Such a hypothesis, derivation of the integument from the pollen chamber wall, would only make sense if the pollen chamber was a structure at the morphological level, a problem not considered by Meeuse and Bouman (1974).
Protection of the nucellus in gymnosperms is by the integument. In early seed plants sterile branches could have functioned for protection (see preceding paragraph). In more advanced gymnosperms protection is more complex. In Bennettitales the so-called interseminal scales could have played this role (e.g. Crane, 1985; Stockey and Rothwell, 2003; Crane and Herendeen, 2009; Rothwell et al., 2009). In Ephedra (Gnetales), it is a pair or a whorl of 3–4 fused bracts (‘seed envelope’), which may be free in the upper part (Rydin et al., 2010), perhaps also in the fossil gnetophyte Siphonospermum (Rydin and Friis, 2010). Perhaps the triangular seeds of Doylea (Stockey and Rothwell, 2009) and Rugonella (Friis et al., 2009) and the quadrangular seeds of Ephedrispermum, Buarcospermum, Lignierispermum and Lobospermum (Friis et al., 2009) also have a similar structure with an outer envelope of three or four units. Among gymnospermous ovules/seeds, such an outer envelope, commonly with layers of sclerified tissue, is known from Gnetales, Erdtmanithecales and Bennettitales (Friis et al., 2007, 2009). Whether it is homologous in all three orders has not been resolved. In angiosperms it is not only the integuments that protect the nucellus but also the carpel or syncarpous gynoecium in which the ovules are enclosed. In addition, angiosperms ancestrally probably have two integuments (e.g. Doyle and Endress, 2000; Endress and Doyle, 2009).
Protection of the young sporophyte in gymnosperms is provided by closure (sealing) of the pollen chamber and/or integument (Takaso and Bouman, 1986; Serbet and Rothwell, 1995). Some earlier information is summarized in Singh (1978).
The presence of wings and potential anemochory (in gymnosperms) was described in seeds of the Late Devonian (Rowe, 1992, 1997) and Permian (Dilcher et al., 1997), and they occur in some extant conifers and in some gnetophytes (Welwitschia, some Ephedra species). A sarcotesta was described for some Carboniferous (Taylor, 1965) and Permian seeds (Klavins et al., 2001; Hilton et al., 2003), and is present, at least in part, in all major extant gymnosperm clades. In gymnosperm seeds, commonly also a sclerified (‘mechanical’) layer is developed. In angiosperms, the position of this layer is diverse but macrosystematically significant (inner or outer surface or central area of the integument or of both integuments; Corner, 1976). A detailed historical survey on the use of terms for different layers was given by Schmid (1986), and surveys on the diversity of seed coats were given by Corner (1976) and Bouman (1984b).
SYMMETRY OF OVULES IN GYMNOSPERMS AND ANGIOSPERMS
Curvature directly affects the symmetry of ovules. Orthotropous ovules are radially symmetric or disymmetric, whereas curved ovules are monosymmetric or asymmetric. However, curvature is not the only factor influencing ovule symmetry.
In early spermatophytes the distinction between radiospermic (radially symmetric) and platyspermic (disymmetric) ovules/seeds (Rothwell, 1986; Stewart and Rothwell, 1993) appears to be phylogenetically important at the macro-level (Rothwell and Serbet, 1994; Doyle, 1996, 2006; Hilton and Bateman, 2006). Both radiospermic and platyspermic seeds appear in the Devonian (Chaloner et al., 1977; Gillespie et al., 1981). In contrast, in angiosperms, radial and flattened ovules may occur in closely related groups, and flattened ovules may simply be understood by space constraints in the ovary locule.
Angiosperm ovules are probably derived from radiospermic seeds among gymnosperms (Meyen, 1982). However, this does not preclude that ancestral angiosperm ovules were anatropous and, thus, monosymmetric. Changes in the symmetry of ovules occurred multiple times in angiosperms and at different systematic levels. Araceae are an example in which there are multiple changes even within a single family (French, 1986; Mayo et al., 1997). Thus radial symmetry, disymmetry and monosymmetry in gymnosperms and angiosperms are not directly related; their significance is not at the same levels.
EVOLUTION OF BITEGMY FROM UNITEGMY ON THE WAY TO ANGIOSPERM EVOLUTION
The inner integument of angiosperms is probably homologous to the single integument in their gymnospermous ancestors (Reinheimer and Kellogg, 2009), and the outer integument may have been derived from the wall of a cupule and reduction of ovule number to one per cupule in angiosperm ancestors [e.g. Glossopteridales or Caytoniales (e.g. Gaussen, 1946; Stebbins, 1974, 1976; Doyle 1978, 1994, 2008)]. In Caytoniales the cupules are curved and could have given rise in this way directly to an anatropous bitegmic ovule (Gaussen, 1946; Doyle, 1978, 2008). Curved cupules are also known from some other fossil gymnosperms [Ktalenia, Umkomasia, Corystospermales; Petriellaea, Petriellaeales (e.g. Taylor and Archangelsky, 1985; Taylor et al., 1994, 2006, 2009; Klavins et al., 2002; Frohlich, 2003; Taylor and Taylor, 2009)]. Curvature in these various gymnosperms and the crown-group angiosperms could also have independently arisen several times.
There is little information on ovule evolution from the perspective of molecular developmental genetics. In a study on the interaction of NOZZLE and INNER NO OUTER, and that of PHABULOSA and WUSCHEL, Sieber et al. (2004, p. 333) are ‘tempted to speculate that bitegmic ovules of extant angiosperms might have been derived through the ‘splitting’ of an integument in a unitegmic precursor'. They hesitate to acknowledge an interpretation of other authors, of the outer integument being derived from a leaf simply because the YABBY gene INO is expressed on its outer side as other YABBY genes are on the outer (abaxial) side of leaves. It should be taken into consideration that the activity of these genes may not be organ specific but pattern or symmetry specific, i.e. promoting dorsiventrality (Eshed et al., 2001). However, this hypothesis of origin of the second integument is of interest as it is at variance with the common paleobotanical hypothesis that the second (the outer integument) in angiosperms was co-opted from an already existing organ, such as the cupule (see ‘Evolutionary origin of ovules in seed plants’ point 4).
DEVELOPMENT OF CURVATURE IN OVULES
Ovule curvature is a predominant feature in angiosperms, in contrast to the commonly uncurved ovules in gymnosperms. Curvature ensures a position of the micropyle close to the attachment site of the ovule and thus close to the placenta for an easy uptake of pollen tubes (Figs 1A–D, 2A–E, E′). It was first discussed by Agardh (1858) that ovule curvature is functionally important to take up pollen tubes by the micropyle. That the micropyle (called ‘mamelon d'imprégnation’) plays a role for the development of the seed was already described by Brongniart (1827) and Mirbel (1829), who observed the pollen tube (called ‘tube conducteur’ and ‘filet’) from the style to the micropyle in some Cucurbitaceae, but the exact function of pollen tubes was not recognized until the ground-breaking work of Hofmeister (1849).
I contend that curvature and the advent of bitegmy are intimately functionally connected and that the development of the outer integument is responsible for curvature. There is evidence from several sources: (1) differential thickness of the outer integument in curved and uncurved angiosperm ovules; (2) structure of the outer integument in abnormally uncurved ovules in angiosperms; (3) behaviour of ovule mutants in model organisms without normal curvature; and (4) development of ovules in the rare gymnosperms that have curved ovules.
The outer integument is often thinner in orthotropous ovules than in anatropous ovules, or is even lacking. For instance, it is only two cell layers thick in the orthotropous Barclaya of Nymphaeaceae, in contrast to more layers in the other, anatropous Nymphaeaceae; only two in the orthotropous family Piperaceae and Hydnoraceae, in contrast to more in most other magnoliids; and also only two(to three) in the orthotropous Proteaceae and Platanaceae, in contrast to the anatropous Nelumbonaceae among Proteales; and even unitegmy in the orthotropous Sabiaceae (Igersheim and Endress, 1998; Endress and Igersheim, 1999). In ovules that have an early forming aril, full ovule curvature seems to be slightly hindered. Often such ovules are not fully anatropous but more or less hemianatropous. Examples are Myristicaceae (Endress, 1973; Igersheim and Endress, 1997), Xanthorrhoeaceae (Steyn and Smith, 1998), and in Sapindaceae ovules are also sometimes hemianatropous but later they become campylotropous without going through an anatropous stage. In Mauloutchia (Myristicaceae), in which a well-developed aril is lacking, the ovule appears to be more anatropous, or at least the outer integument appears to be semi-annular (Sauquet et al., 2003).
Abnormal orthotropous (or hemianatropous) ovules often occur in plants that normally have anatropous ovules, especially in ovaries with numerous ovules. These are found in various major clades of angiosperms. Interestingly, in these abnormal ovules that failed to develop a normal curvature, the outer integument is often reduced; it is shorter or completely lacking. Another concomitant trait is that the funiculus is often longer than in the normal anatropous ovules. Such ovules were described and drawn in a number of publications for single species. Both features together (reduced outer integument and long funiculus) were documented for Takhtajania (Winteraceae, Fig. 4A; Endress et al., 2000), Butomus (Butomaceae, Fig. 4B; pers. obs.; Fernando and Cass, 1996), Burmannia (Burmanniaceae, one integument completely lacking; Rübsamen, 1986), Berberis (Berberidaceae; Schleiden, 1839; Mauritzon, 1938), Caltha (Ranunculaceae, outer integument growing backward; Kapil and Jalan, 1962), Holoptelea (Ulmaceae; Capoor, 1937), Parnassia (Parnassiaceae, one integument completely lacking; Saxena, 1964b), Dalechampia (Euphorbiaceae, outer integument growing backward in one case; Singh and Pal, 1968), Podostemon (Podostemaceae, funiculus length not indicated; Hammond, 1937), Jussieua (Onagraceae; Khan, 1942) and Foeniculum (Apiaceae, integument growing backward; Gupta, 1964). In the following taxa, the outer integument was not reduced, but the funiculus was longer: Hitchenia (Zingiberaceae; Panchaksharappa, 1962), Platystemon (Papaveraceae; Bocquet and Bersier, 1960), Bergenia (Saxifragaceae; Saxena, 1969), Heuchera (Saxifragaceae; Mauritzon, 1933), Saxifraga (Saxifragaceae; Saxena, 1964a), Neptunia (Fabaceae; Singh and Shivapuri, 1935) and Rhodamnia (Myrtaceae; Mauritzon, 1939a). In the following taxa the outer integument was misshapen, but the funiculus was not longer: Podophyllum emodi (Berberidaceae, Fig. 4C; pers. obs.) and Pterospermum (Malvaceae; Venkata Rao, 1954). In some Myrtaceae the pluriovulate placenta regularly contains some reduced ovules (‘ovulodes’) (see also ‘Direction of the ovule initiation squence in placentae with numerous ovules’). In Angophora, ovulodes have only one integument (Prakash, 1969).
The behaviour of mutants in the model species A. thaliana strongly supports the role of the outer integument in ovule curvature. In ant (aintegumenta) (Elliott et al., 1996; Schneitz et al., 1997, 1998b; Skinner and Gasser, 2009) and hll (huellenlos) (Schneitz et al., 1997, 1998b) both integuments are lacking and the ovule is not curved, and the same occurs in double, triple and quadruple mutants involving ant, stk (seedstick), shatterproof1 (shp1) and shp2 (shatterproof2) (Losa et al., 2010) and in triple mutants with cna (corona), phb (phabulosa) and phv (phavoluta) (Kelley et al., 2009). In wus (wuschel) ovules both integuments are lacking and there is no normal curvature (Gross-Hardt et al., 2002). In ino (inner no outer), the outer integument is almost lacking (initiated but not further developed) but the inner is well developed, and there is no curvature (Baker et al., 1997; Schneitz et al., 1997; Villanueva et al., 1999; Gallagher and Gasser, 2008; Skinner and Gasser, 2009); the same was found in an ino mutant of the basal angiosperm Annona squamosa (Lora et al., 2011). In pfs2 (pretty few seeds2) mutants with the PFS2 transgene some ovules are normal; however, in some ovules the outer integument is reduced (shorter than the inner) and the ovule is only halfway curved (Park et al., 2004, 2005). In kan1 (kanadi1) and kan2 (kanadi2) double mutants the outer integument remains short and the ovules are not curved (Eshed et al., 2001); the same is the case in kan1, kan2, kan3 triple mutants (McAbee et al., 2006) and in seu, cyp85A2-1 double mutants (Nole-Wilson et al., 2010). In sin1 (short integuments 1) (Robinson-Beers et al., 1992; Baker et al., 1997) and sin2 mutants (Broadhvest et al., 2000) both integuments remain short and the ovules are only weakly curved, and similarly in ag/AG stk shp1 shp2 mutants (Brambilla et al., 2007). In bel1 (bell) the two integuments are not distinct, forming 2–4 irregular mounds, and there is no regular curvature (Robinson-Beers et al., 1992, Schneitz et al., 1997). Taking these results from several mutants together, there is a distinct pattern: the shorter the outer integument, the less the ovule is curved. If the outer integument is not formed at all, there is no curvature. For the formation of curvature, apparently an outer integument needs to be present and it must have a slant in order to develop asymmetrically from early on. A seemingly contradictory case was described and interpreted for the ovules of rice, in which curvature is said to be ‘associated closely with the extent of inner integument growth’ (Yamaki et al., 2005, p. 408). However, these ovules are almost uncurved and not easily compared with anatropous or campylotropous ovules.
Among extant gymnosperms, Podocarpaceae are the only group that shows prominent ovule curvature during development. Although Podocarpaceae do not have an outer integument, they have a special structure, the ‘epimatium’. The epimatium looks like the hood-shaped outer integument of an anatropous ovule in angiosperms. The epimatium is involved with curvature (‘anatropy’) of the ovule (Doyle, 1945; Tomlinson et al., 1991; Tomlinson, 1992; Del Fueyo, 1999; Mill et al., 2001; Tomlinson and Takaso, 2002). Thus it is in some way functionally analogous to an outer integument. However, morphologically it corresponds to the ovuliferous scale in other conifers (Tomlinson, 1992). This difference in homology is also reflected in the precocious developmental appearance of the epimatium compared with the ovule (Tomlinson, 1992). The function of this ‘anatropy’ in Podocarpaceae is ‘pollen scavenging’: the pollination drop spreads in the area around the micropyle, and pollen grains trapped in the pollination drop will then float into the micropylar cavity (Tomlinson, 1991; Tomlinson et al., 1991). The epimatium is also involved in seed dispersal as it becomes fleshy and brightly coloured.
Fig. 4.
Abnormal orthotropous ovules on a multiovulate placenta (asterisks). (A) Takhtajania perrieri. (B) Butomus umbellatus. (C) Podophyllum emodi. Abnormal hemitropous ovule with short outer integument marked with an arrow. Scale bars: (A) = 0·15 mm; (B) = 0·1 mm; (C) = 0·5 mm.
DIVERSITY OF OVULE POSITION IN THE GYNOECIUM AND REPERCUSSIONS OF OVARY ARCHITECTURE ON OVULE SHAPE
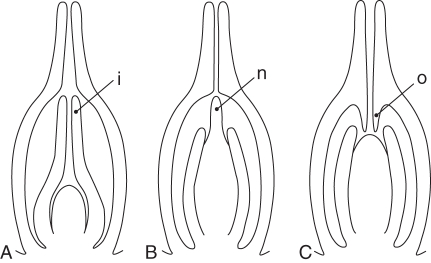
The position of the ovules in the gynoecium and ovary locule architecture have repercussions on details of ovule structure, especially ovule curvature and symmetry. Therefore, this aspect is important to understand details of ovule shape (Endress, 1994a, 2008). In most locule architectures, anatropous or campylotropous ovules direct their micropyle close to the placenta by their curvature, which facilitates pollen tube growth from the carpels directly into the micropyles (Fig. 1). For this reason, curved ovules are so common in angiosperms and appear to be the basal state for extant angiosperms (see ‘Basic diversity of ovules in extant angiosperms’; Doyle, 2008; Endress and Doyle, 2009). Completely orthotropous ovules occur in four different placenta positions or locule architectures. (1) A single ovule on a basal placenta in a narrow locule (single ascidiate carpel or syncarpous gynoecium) (Figs 6A, 7). The micropyle is directed towards the stylar canal and is connected with it either through secretion or by contiguity. This is a relatively widespread situation in a number of unrelated angiosperm groups [e.g. Piperales of magnoliids, some Araceae of basal monocots, Juglandaceae, Myricaceae, and Urticaceae of rosids, Polygonaceae of the asterid alliance; Endress, 2008]. (2) Numerous ovules on laminar-diffuse or protruding-diffuse placentae in a spacious locule (locule much larger than the ovules), filled with secretion [Barclaya of the ANITA grade, Fig. 3A; Hydnora of magnoliids; Acorus (Rudall and Furness, 1997; Buzgo and Endress, 2000); Pistia, Fig. 6D (Buzgo, 1994); and Hydrocharis of basal monocots (Igersheim et al., 2001); Xiphidium coeruleum of core monocots (slightly curved), Figs 5B, 6E; Akebia of basal eudicots (slightly curved), Figs 5C, 6F (Endress and Igersheim, 1999); and Cytinus of core eudicots (Igersheim and Endress, 1998)]. (3) Several ovules on parietal placentae, the micropyles being contiguous with an adjacent placenta, Fig. 6B [e.g. Houttuynia cordata of Piperales, Fig. 5A (Endress, 1994b); Mayacaceae of monocots, Casearia of rosids (Endress, 2008); Scaphocalyx of rosids (van Heel, 1973)]. (4) Ovules with a long, curved funiculus, which directs the micropyle to their own placenta [e.g. Helianthemum of core eudicots, Fig. 6C (Nandi, 1998)].
Fig. 5.
Orthotropous ovules and ovary locule architecture (arrows point to micropyles). (A) Houttuynia cordata. (B) Xiphidium coeruleum. (C) Akebia quinata (with secretory hairs and secretions between the ovules). Scale bars: (A) = 0·2 mm; (B) = 0·3 mm; (C) = 0·1 mm.
Fig. 6.
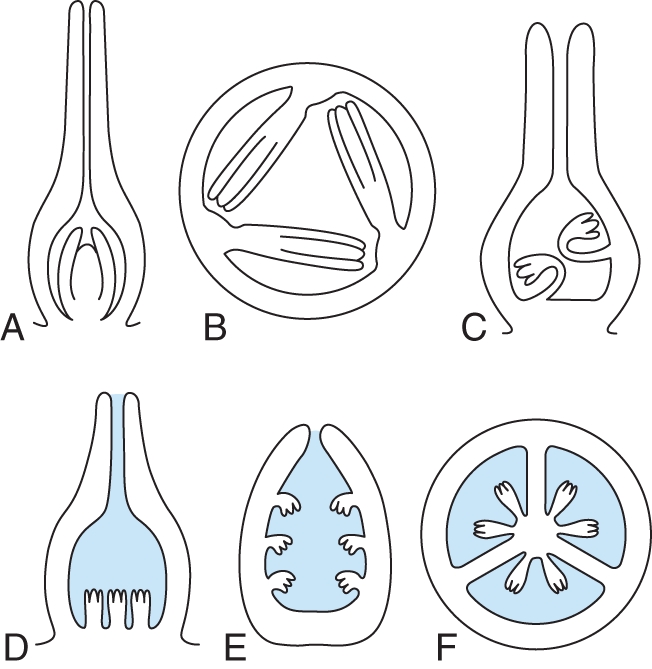
Orthotropous ovules and conditions of ovary locule architectures under which they occur (schematic, only one integument is drawn in each ovule for simplicity; augmented and modified from Endress, 1994a). (A–C) Ovary or locules not filled with secretion. (A) Single ovule with basal placenta (LS gynoecium) (e.g. Piperaceae, Juglandaceae, Urticaceae). (B) Ovules on parietal placentae with the micropyle directed toward another placenta (TS ovary) (e.g. Casearia, Salicaceae; Mayaca, Mayacaceae). (C) Ovules with long funiculi curved to their own placenta (LS gynoecium) (e.g. Helianthemum, Cistaceae). (D–F) Ovary or locules filled with secretion (secretion shaded blue). (D) Basal diffuse placenta (LS gynoecium) (e.g. Pistia, Araceae). (E) Laminar-diffuse placenta (TS carpel/ovary) (e.g. Barclaya, Nymphaeaceae; Hydrocharis, Hydrocharitaceae; Akebia, Lardizabalaceae, shown, in the latter ovules slightly curved at anthesis). (F) Axile placenta (TS ovary) (e.g. Acorus, Acoraceae; Xiphidium, Haemodoraceae).
If orthotropous ovules are present in narrow locules and not in a central basal position, they cannot be completely radially symmetrical on architectural grounds, but are somewhat curved at their base. This needs to be emphasized because this is the case in some of the basal angiosperms, such as Amborella (Endress, 1986, 1994b), Chloranthaceae (Endress, 1987, 1994b) and Ceratophyllum (Igersheim and Endress, 1998). There has been debate in the literature as to whether these ovules are orthotropous or anatropous, without fully realizing the problem of architectural constraint. The question is still open in most cases of whether they are basically anatropous but could not complete the curvature because of limited space in the locule or, vice versa, whether they are basically orthotropous but were forced to make a slight curve at the base because of spatial constraint. Thus the curvature may be considered to be a superimposed restriction. This question is especially interesting in Amborella, the sister of all other extant angiosperms, which has been described both as anatropous and as orthotropous (see, for example, Bailey and Swamy, 1948; Endress, 1986; Endress and Igersheim, 1997, 2000b; Doyle and Endress, 2000; Tobe et al., 2000; Yamada et al., 2001b; Endress and Doyle, 2009). Similar cases also occur in basal monocots (Potamogeton, Igersheim et al., 2001; Shamrov, 2006) and basal eudicots (Platanus, Endress and Igersheim, 1999). An example of nearly orthotropous ovules due to developmental constraint on original anatropy occurs in Avicennia (Acanthaceae; Borg and Schönenberger, 2011).
An especially obvious case of dependence of ovule shape on locule architecture are ascending orthotropous ovules with the micropyle contiguous with the transition area of the stylar canal into the locule. In some groups with this architecture one or both integuments elongate to keep pace with the elongation of the locule (Fig. 7A) [Elatostema and Myriocarpa of Urticaceae, Rosales (Fagerlind, 1944); Didymeles of Buxales (ovules hemitropous) (von Balthazar et al., 2003)]. In these Urticaceae the reverse situation also occurs: instead of an elongation of the integuments, hairs from the pollen tube transmitting tissue grow into the micropyle or onto the nucellus (Fig. 7C) (Fagerlind, 1944). Thus either the integument(s) or the pollen tube transmitting tract grows actively toward its functional counterpart. In some Polygonaceae (Caryophyllales) with the same gynoecium architecture, a third possibility occurs: growth of a nucellar beak into the stylar canal (Fig. 7B) (Edman, 1929).
Fig. 7.
Different patterns of contact between the ovule and pollen tube transmitting tissue of the stylar canal in gynoecia with a single orthotropous ovule on a basal placenta (schematic, only one integument drawn in each ovule for simplicity). (A) Integument(s) protruding into the stylar canal (i, e.g. Didymeles, Didymelaceae, schematized after von Balthazar et al., 2003; Elatostema, Urticaceae, schematized after Fagerlind, 1944). (B) Nucellus (nucellar beak, n) protruding into the stylar canal (e.g. Polygonum, Polygonaceae, schematized after Edman, 1929). (C) Carpel forming an obturator (o, e.g. Elatostema, Urticaceae, schematized after Fagerlind, 1944).
DIRECTION OF OVULE CURVATURE AND CARPEL CURVATURE
Ovules are commonly formed at or close to the margin of carpels (in a marginal or sub-marginal position). To reach angiospermy, carpel margins curve inward so that the ventral carpel surface and the ovules become enclosed. The direction of carpel curvature is always the same in angiosperms. However, this is not the case for the direction of ovule curvature. Although in most cases the direction of curvature in anatropous or campylotropous ovules is the same as that of carpel curvature, there are other cases in which ovule curvature is in the opposite direction. These opposite patterns were earlier distinguished as apotropous and epitropous (introduced in the Latin form by Agardh, 1858; used, for example, by Engler, 1931). They were defined in a complicated and impractical way, as the relationship with carpel curvature was not considered. To make this connection and thus have a simpler definition, the terms ‘syntropous’ (Fig. 8A) and ‘antitropous’ (Fig. 8B) were introduced (Endress, 1994a). Syntropous ovules are common, e.g. in basal angiosperms (Endress and Igersheim, 2000a), whereas antitropous ovules are well known, e.g. from many Malpighiales (Sutter and Endress, 1995; Merino Sutter et al., 2006; Matthews and Endress, 2008, 2011), from Anacardiaceae (Bachelier and Endress, 2007, 2009) and some Rosaceae (Juel, 1918).
Fig. 8.
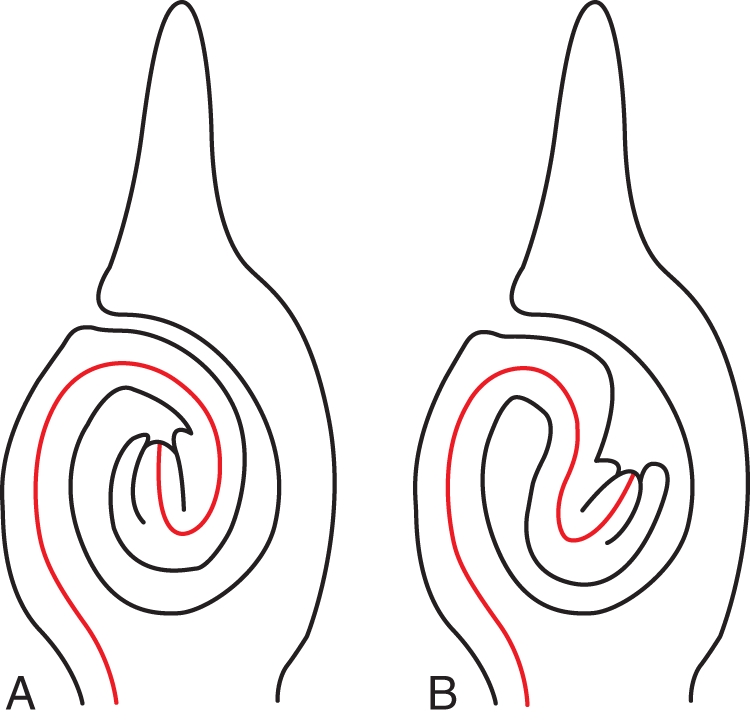
Different orientations of curved ovules with respect to carpel curvature (denoted by a red line). (A) Syntropous. Curvature of the ovule in the same direction as the curvature of the carpel. (B) Antitropous. Curvature of the ovule in the opposite direction to that of the carpel.
The predominant syntropous direction is optimal for ovules arranged in two parallel series (the most common pattern in angiosperms) because it leaves enough space between the opposite ovules for curvature to bring the micropyle close to the placenta and thus to form a direct passage for pollen tubes. Pollen tubes grow from the placenta around the funiculus to the other side of the ovule where the micropyle is located (Fig. 1C, D). With the antitropous pattern this would not be the case; the antitropous pattern commonly occurs in carpels with only one or two ovules. Associated with antitropous ovules is often a special auxiliary structure, an obturator, which is necessary for pollen tubes to bridge the gap between the pollen tube transmitting tract and the micropyle. For instance, in Malpighiales, many clades have antitropous ovules with obturators, and in clades with syntropous ovules within the order obturators are lacking. As seen throughout the angiosperms, obturators may have different developmental origins. Often they are formed from the carpel flanks above the placenta.
In multiovulate carpels or gynoecia, the curvature is syntropous. In linear (axile or parietal) placentae the longitudinal series of ovules are curved away from each other (Fig. 1C). In median placentae the ovules are curved downwards (and also outwards, if there are many). In laminar-diffuse placentae they are curved downwards if in the ascidiate zone (Fig. 6F) and sideways if in the plicate zone. In free central placentae (which represent part of the ascidiate zone), the ovules are also curved downwards.
DIRECTION OF THE OVULE INITIATION SEQUENCE IN PLACENTAE WITH NUMEROUS OVULES
In long, linear placentae or in diffuse placentae (with a number of ovules side by side), there is often a gradient in ovule development. Payer (1857), based on >30 examples, reported three patterns of ovule initiation: (1) basipetal in axile placentae (Fig. 9A); (2) acropetal in parietal placentae; and (3) bidirectional in placentae that are axile at the base and parietal on top. A fourth type, intercalation of new ovules between older ones, was described later (Kaplan, 1968). Okamoto (1984) hypothesized that initiation begins closest to the site of the former floral apex which, to some extent, conforms with Payer's axile and parietal placentation initiation types. However, Okamoto (1984) considered only nine genera. Unfortunately, he did not take into account that axile placentae can be present in both the symplicate and synascidiate zone of the ovary and that only in the latter would the site of the former floral apex be on top of the placenta. Whereas this correlation between placenta form and direction of ovule development appears to be a trend in the material studied by Payer (1857), there are also cases in which parietal placentae show basipetal ovule initiation (such as Dicentra and Mentzelia, Payer, 1857; and Berberidopsis, Ronse De Craene, 2004). As expected, the direction of initiation is also commonly basipetal in flowers with a free central placenta (e.g. Sundberg, 1982; Caris et al., 2000; Caris and Smets, 2004). Payer's (1857) study is as yet the largest comparative study. A limited number of species was described by Sattler (1973), and mostly only single species by other authors. Thus the problem of the direction of ovule initiation needs more critical study.
Fig. 9.
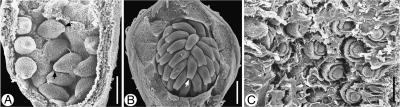
Gradients in ovule development on multiovulate placentae. (A–C) Acropetal and basipetal development of ovules on the placenta (and reduction of last formed ovules in some cases). (A) Basipetal. Solanum sisymbrifolium. (B) Acropetal, upper ovules reduced. Liquidambar orientalis. (C) Acropetal, upper ovules greatly reduced. Corylopsis willmottiae. (D–F) Peripheral delay in development. (D) Passiflora holosericea. After ovule initiation. (E) Passiflora holosericea. After integument initiation. (F) Nymphaea tetragona. Scale bars: (A, D, E) = 0·05 mm; (B, F) = 0·2 mm; (C) = 0·1 mm.
The pattern of ovule initiation in multiovulate placentae apparently depends on the direction of elongation/maturation of the ovary locules. In ovaries with basipetal ovule initiation there is intercalary locule elongation mainly at the base. The pattern of elongation and expansion is especially complex in Orchidaceae. Although ovule development was studied in a number of species, generally the authors focused on single ovules and did not study the development of the placenta and the sequence of initiation of numerous ovules. The parietal placenta may be multiply branched or crested, and ovule initiation begins in the centre of each branch or crest (Abe, 1972; Yeung and Law, 1997; Tsai et al., 2008); the crests may be convoluted (Zhang and O'Neill, 1993). From my experience, there is often a centrifugal direction of ovule appearance on a placenta. The ovules in the centre of an extended placenta appear the most developed, whereas the ovules at the periphery are less developed (Fig. 9D–F). However, whether this reflects the initiation sequence or a post-initiation delay of the peripheral ovules remains an open question. In some groups with linear placentae and acropetal succession of ovule development, the later formed ovules may remain small and be sterile (e.g. Altingiaceae, Fig. 9B; Hamamelidaceae, Fig. 9C; Anemoneae, Ren et al., 2010). In groups with diffuse placentae, the peripheral ovules may be sterile, such as in some Myrtaceae (e.g. Eucalyptus, Davis, 1968).
OVULE BASE AND VASCULARIZATION
Commonly, ovules have a short funiculus at the base, marking the transition from the placenta. Long funiculi, as an extreme, are uncommon and scattered in angiosperms. They show some concentration in Caryophyllales (notably in both the core clade, e.g. Cactaceae, Leins and Schwitalla, 1985; and the extended clade, e.g. Plumbaginaceae, De Laet et al., 1995) and Brassicales (Brassicaceae, Shamrov, 2002a; and Caricaceae, Ronse Decraene and Smets, 1999). In basal angiosperms long funiculi are present, e.g. in Monodora (Annonaceae) (Igersheim and Endress, 1997), and in basal monocots, e.g. in Hydrocleys (Limnocharitaceae) (Igersheim et al., 2001). As another extreme, sessile ovules with an extensive attachment area occur, e.g. among monocots in palms (Robertson, 1976) and among core eudicots in some Malpighiales (Irvingiaceae and Caryocaraceae, Matthews and Endress, 2011; and Achariaceae, van Heel, 1973).
The chalaza also belongs to the ovule base, as it is located below the nucellus and the integuments. In pachychalazal ovules the chalaza is relatively long compared with the nucellus and integuments, and the embryo sac becomes partly ‘inferior’ (e.g. Lauraceae; Endress, 1972). In perichalazal ovules the chalaza is long only in the median symmetry plane of the ovule but shorter in the transverse areas (e.g. Austrobaileyaceae; Endress, 1980). Both pachychalazal and perichalazal ovules occur scattered in several angiosperm groups.
Anatomically, ovules are connected with the placenta by a vascular bundle, which commonly extends through the funiculus and raphe and ends in the chalaza. However, in many cases the vascular bundle branches in the chalaza and branches extend into the outer, inner or both integuments. In some cases, branching begins in the raphe, and such vascular branches reach the outer integument by by-passing the chalaza. This is especially the case in ovules with an extensive attachment area (Matthews and Endress, 2011), but not only there (Tokuoka and Tobe, 2003). Vascularized integuments occur especially in massive ovules and ovules that develop into large seeds (Kühn, 1928; Corner, 1976; Bouman, 1984a). This may be seen in Araceae with vascularized and non-vascularized taxa (French, 1986) or Rhizophoraceae, where the large-seeded mangrove genera are more vascularized than non-mangrove genera (Matthews and Endress, 2011). Other clades with extensive vascularization in the integument(s) are Fagales and Euphorbiaceae (e.g. Tokuoka and Tobe, 2003). Among extant basal angiosperms (ANITA grade) ovules with a vascular bundle in the (outer) integument occur in Trimeniaceae (Endress and Sampson, 1983). As another extreme, there are ovules without a vascular bundle or with only an undifferentiated procambial strand, e.g. in Orchidaceae (Asparagales, monocots; Shamrov, 2006), the parasitic Cytinaceae (Malvales, rosids; Teryokhin, 1981; Shamrov, 2003, 2006) and some asterids, such as in Lamiales and Gentianaceae (Gentianales; Shamrov, 1990, 2003). Such ovules without differentiated vascular bundles are generally restricted to groups with numerous small seeds (Endress, 2010, 2011).
NUCELLUS STRUCTURE IN ANGIOSPERM OVULES
Nucellus thickness (measured in the number of cell layers above and around the meiocyte) greatly varies in angiosperms, but is relatively constant in larger clades. Nucellus structure is best compared at the time of prophase of meiosis. At this stage, the tissues of the nucellus are still intact. Later, during meiosis and embryo sac formation, tissue adjacent to the gametophytic parts is generally crushed and it becomes difficult to determine the number of cell layers around the embryo sac. The classical distinction between crassinucellar (with one or more hypodermal cell layers between the meiocyte and nucellus apex) and tenuinucellar (with no hypodermal cell layer between the meiocyte and nucellus apex) has been modified into a finer grid of six types based on the recognition that they characterize larger clades.
In surveys on floral diversity at the levels of eudicots (Endress, 2010) and angiosperms (Endress, 2011), the following ovule classification according to nucellus thickness was tentatively used: (a) crassinucellar (with more than one hypodermal cell layer between meiocyte and nucellus apex; Fig. 10A) (e.g. Cinnamomum, Lauraceae; Endress, 1972); (b) weakly crassinucellar (with just one hypodermal cell layer between the meiocyte and nucellus apex; Fig. 10B) (e.g. Dichelostemma, Asparagaceae, Berg, 1996); (c) pseudocrassinucellar (without a hypodermal cell layer between the meiocyte and nucellus apex, but with periclinal cell divisions in the epidermis of the nucellus apex; Fig. 10C) (e.g. Sagittaria, Alismataceae, Johri, 1935); (d) incompletely tenuinucellar (without a hypodermal cell layer between the meiocyte and nucellus apex, but with hypodermal tissue at the nucellus flanks and/or below the meiocyte) (e.g. Nemophila, Boraginaceae; Berg, 2009; Fig. 10D); (e) tenuinucellar (without any hypodermal tissue in the nucellus; Fig. 10E) (e.g. Orphium, Gentianaceae; Hakki, 1997); and (f) reduced tenuinucellar (as in tenuinucellar but meiocyte partly extending below the nucellus, thus with a partly ‘inferior’ position; Fig. 10F) (e.g. Phyllis, Rubiaceae; Fagerlind, 1936).
Fig. 10.
Diversity of nucellus thickness and integument number and thickness (thick lines, morphological surfaces; thin lines, boundaries between cell layers; epidermal layer drawn in full, other layers only partially drawn; modified from Endress, 2011). (A–F) Different nucellus shapes. Meiocytes are shaded grey. (A) Crassinucellar. (B) Weakly crassinucellar. (C) Pseudocrassinucellar. (D) Incompletely tenuinucellar. (E) Tenuinucellar. (F) Reduced tenuinucellar. (G–K) Different integument differentiation. In bitegmic ovules, the inner integument is shaded red, the outer blue. (G) The outer integument is thicker than the inner. (H) The inner integument is thicker than the outer. (I) Both integuments are equally thick. (J) Unitegmic. (K) Ategmic.
The terms in Endress (2010, 2011) had been used in part earlier by other authors, such as ‘pseudocrassinucellar’ (Davis, 1966) and ‘reduced tenuinucellar’ (as ‘reduced variation of tenuinucellate’) (Shamrov, 1998). Shamrov (1998) divised an elaborate classification with types and sub-types, primarily based on histogenesis, which is sensible. However, a drawback is that a type may change during development into another: the ovules of Butomus are at first crassinucellate and then become medionucellate (Shamrov, 1998, p. 403). A practicable typology should be based on a fixed developmental stage. Also, it would be premature to make too elaborate a typology before its systematic relevance has been tested.
Other nucellus differentiations of systematic interest are a nucellus cap and a nucellus beak. They are sometimes confused in the literature. A cap refers to the anatomical/histological structural level and a beak to the morphological level. A cap is formed by multiple periclinal divisions in the epidermis of the nucellus apex, sometimes, in addition, in the originally hypodermal tissue, whereas a beak is an acuminate protrusion of the nucellar apex, which can grow partly or entirely through the micropyle (Merino Sutter et al., 2006). A beak is usually associated with a cap, but not vice versa.
In thin ovules (tenuinucellar, incompletely tenuinucellar and weakly crassinucellar), often an endothelium is formed on the inside of the inner integument (Kapil and Tiwari, 1978; Endress, 2010, 2011). In such ovules, during embryo sac formation the nucellus dissolves around the embryo sac and the embryo sac becomes adjacent to and contiguous with the inside of the inner integument. The endothelium appears to supply the embryo sac with certain substances. An endothelium is especially present in certain rosids and in asterids (see ‘Features of ovules and macrosystematics of angiosperms’). It is noteworthy that an endothelium is also present in Lactoris, a magnoliid with exceptionally thin (incompletely tenuinucellar) ovules (Tobe et al., 1993) and in Canrightia, a Lower Cretaceous magnoliid fossil (Friis and Pedersen, 2011). In both these magnoliids the endothelium appears to be persistent during seed development, in contrast to those core eudicots in which it occurs.
INTEGUMENT THICKNESS
Integuments are two or more cell layers thick. Two-cell-layered integuments are developmentally derived from the dermatogen (‘dermal integuments’, Bouman, 1984a). Integuments of more than two cell layers are either derived from the dermatogen and become thicker later in development by periclinal cell divisions in the epidermis or they are derived from both dermatogen and sub-dermatogen (‘subdermal integuments’, Bouman, 1984a). Whether integuments are dermal or sub-dermal is correlated with their thickness at anthesis and later. It cannot be used for any deduction of homology (in contrast to Tilton and Lersten, 1981a).
Integument thickness is a relatively stable character, and therefore of interest at the macrosystematic level. This is especially so for the relative thickness of outer and inner integument (Fig. 10G–I; Endress and Matthews, 2006; Endress, 2010, 2011), which are constrained by the subsequent differentiation of the seed coat (for systematic significance, see ‘Features of ovules and macrosystematics of angiosperms’). In wild-type Arabidopsis the outer integument is regularly two cell layers thick and the inner three. However, in the mutant ats (aberrant testa shape), which has an abnormal seed coat, the entire cover is only three cell layers thick (Léon-Kloosterziel et al., 1994).
The inner integument appears to be constrained in thickness by the outer integument. This can be deduced from two observations. (1) If the inner integument is longer than the outer, it is considerably thicker in the micropyle where it is not surrounded by the outer. (2) In abnormal ovules with an exceedingly long inner integument forming the micropyle (in species in which the micropyle is normally formed by both integuments), the exposed rim of the inner integument becomes much thicker than in normal ovules (Eschscholzia; Sachar and Mohan Ram, 1958).
HOODED, SEMI-ANNULAR VS. CUP-SHAPED, ANNULAR OUTER INTEGUMENT
In curved (anatropous) ovules the outer integument is either hooded or cup-shaped, developmentally derived from a semi-annular or annular early stage, respectively (Yamada et al., 2001a). Hooded vs. cup-shaped outer integuments have been believed to represent two fundamentally different organizations by some authors (Kato and Imaichi, 1993). However, as it looks now, this difference is rather a consequence of minor differences in the speed of developmental curvature of the ovule, and not a fundamental difference.
It has been suggested that a hood-shaped (semi-annular) outer integument is primitive in angiosperms (Kato and Imaichi, 1993; Matsui et al., 1993; Umeda et al., 1994; Imaichi et al., 1995; Yamada et al., 2003a, b). In our comparative study on carpels and ovules through all families of basal angiosperms (as mentioned in the Introduction), we found a diversity of anatropous ovules with semi-annular (hood-shaped) and annular (cup-shaped) outer integument. Often both co-occur at low systematic levels. This indicates that there is no fundamental difference between the two forms. If anatropous ovules are primitive at the level of crown-group angiosperms, which is likely (discussion in Endress and Doyle, 2009), this does not automatically mean that the hood shape is also primitive. The hood shape is probably only a consequence of the early developmental curvature. Thus it is only the propensity to form hood-shaped outer integuments that is primitive. For instance, the outer integument is not semi-annular but annular in Illiciaceae, Canellaceae, Myristicaceae, Degeneriaceae and Himantandraceae (Igersheim and Endress, 1997). It has also been repeatedly found that ovules with both a semi-annular and an annular outer integument occur in the same family or even the same genus or species (e.g. Calycanthus, Peumus, Siparuna; Endress and Igersheim, 1997; Nuphar, Nymphaea, Aristolochia, Thottea; Igersheim and Endress, 1998), indicating that the two features are not of fundamental evolutionary difference but may merely depend on subtle developmental differences. The earlier the curvature begins, the more pronounced will the semi-annular form become. It may be assumed that if a certain threshold of retardation on one side is surpassed, instead of a complete ring, a partial ring and a compensatory additional lobe are formed. Thus the additional lobe is probably not a fundamentally different part as assumed by Matsui et al. (1993) or Umeda et al. (1994). This interpretation is also supported by those species in which abnormal orthotropous ovules were found, which always had a cup-shaped (annular) outer integument, as opposed to the normal anatropous ovules (see ‘Development of curvature in ovules’).
MULTIPLE EVOLUTION OF UNITEGMY FROM BITEGMY WITHIN ANGIOSPERMS
If bitegmy was so important for curvature in angiosperms, why was it possible that unitegmy (Fig. 10J) evolved secondarily within angiosperms several times, and yet in many cases the ovules did not give up their curvature? In anatropous unitegmic ovules (as found in most asterids and some other eudicots), the single integument probably does not correspond to an outer or an inner integument but is an evolutionarily complex structure in which both participate, although they can no longer be distinguished morphologically (as discussed by Bouman and Calis, 1977). This process of amalgamation of the two integuments is shown by those rare genera in which both bitegmic and unitegmic conditions are present [e.g. Impatiens (McAbee et al., 2005; Colombo et al., 2008, Kelley and Gasser, 2009) and Coriaria (Matthews and Endress, 2004)]. In contrast, there is some evidence that unitegmy in basal angiosperms evolved by reduction and loss of the outer integument (Igersheim and Endress, 1998).
Whether in orthotropous unitegmic ovules of core eudicots the only integument corresponds to the inner integument is unknown, but would be interesting to study (e.g. in Fagales and Rosales). That in several cases unitegmy goes together with orthotropy is plausible if the outer integument, which is responsible for curvature, is reduced. This is likely to be the case in (unitegmic) Peperomia, as in other (bitegmic) Piperaceae the outer integument is already shortened. In Rafflesiaceae and Cytinaceae the outer integument is still present but highly reduced, and Ceratophyllum has only one integument (Igersheim and Endress, 1998). Also in the (orthotropous) Urticaceae the outer integument is shortened (Fagerlind, 1944).
FURTHER REDUCTION OF INTEGUMENTS AND ENTIRE OVULES
Ovules also became reduced in other respects in some angiosperm clades. Integuments and then entire ovules were successively reduced in the parasitic order Santalales (Fagerlind, 1947, 1948; Shamrov et al., 2001, Brown et al., 2010; Endress, 2010, 2011). Brown et al. (2010) found in Santalales that lack differentiation into nucellus and integuments that integument-associated genes were expressed in the periphery of the ovule. It is not necessary to assume congenital fusion between the nucellus and integument(s). Under the assumption of non-differentiation (i.e. lack of nucellus and integuments) this evolutionary process can be seen as transference of function, in which the peripheral zone of the ovule that is normally formed by the (outer) integument is now formed by the periphery of the undifferentiated ovule. Some mycotrophic Gentianaceae also evolved highly reduced ovules without differentiation into nucellus and integument (ategmic) (Fig. 10K; Goebel, 1933; Bouman et al., 2002).
LOBATION OF INTEGUMENTS IN ANGIOSPERM OVULES
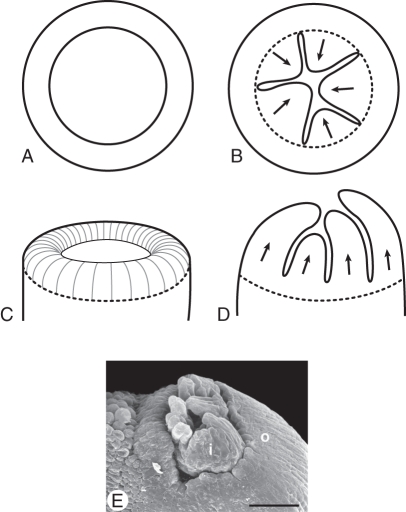
It has been argued that the lobes on the rim of the inner integument in some basal angiosperms (Magnolia) (Figs 1B, 11E; Umeda et al., 1994; Herr, 1995), in some other angiosperms (van Heel, 1970, 1976) or in some mutants of Arabidopsis (Park et al., 2004) may represent remnants or atavisms of ancient telomic structures. In earlier publications we expressed doubts concerning this interpretation (Endress and Igersheim, 1997; Igersheim and Endress, 1997). We argued that if an annular young plant part that spans an opening of a certain diameter in early development needs to close in later development, i.e. to form a closed pore, it can do this only by lobation (in the longitudinal direction) (Fig. 11C, D) or by irregular thickening, which also leads to a sort of lobation (in the transverse direction) (Fig. 13 in Igersheim and Endress, 1997), or by both processes in combination (Fig. 11A, B).
Fig. 11.
Two possibilities for morphological closure of a tubular structure through differential directional growth: irregular thickening or lobation. (A, B) End of a tube seen from above. (A) Tube open. (B) Tube partially closed by irregular thickening (arrows). (C, D) End of the tube seen from the side. (C) Tube open. (D) Tube partially closed by lobe formation (arrows). (E) Micropyle with lobed inner (i) and outer (o) integuments. Illicium floridanum (from Igersheim and Endress, 1997, Fig. 75). Scale bar: (E) = 0·1 mm.
From these principles of plant growth and development, several hypotheses can be derived. These hypotheses can be tested with the wide array of species studied in our laboratory, covering all families of basal angiosperms, including the ANITA grade, magnoliids and the basal grades of monocots and eudicots (Igersheim and Endress, 1997, 1998; Endress and Igersheim, 1998, 1999, 2000a; Igersheim et al., 2001).
According to our studies, lobed integuments are widespread in basal angiosperms, not only in inner, but also in outer integuments. Of 131 taxa in which the lobation of the integuments was studied [from all families of the ANITA grade (except Hydatellaceae), magnoliids, basal eudicots and basal monocots] 124 have two integuments. Of these 124 taxa, in 57 the inner integument is lobed and in 40 the outer integument is lobed. We tested the following three hypotheses with our material.
The outer integument is relatively more often lobed than the inner if both integuments form the micropyle, because the circumference of the outer integument is larger than that of the inner. Result: of the 124 taxa with two integuments studied, in 34 taxa both integuments form the micropyle. Of those, in 14 the inner integument is lobed, in 13 the outer integument is lobed (38 % of all). However, in the 85 taxa in which the micropyle is formed by the inner integument alone, the inner integument is lobed in 40 taxa, but the outer only in 21 taxa (only 25 % of all). Thus hypothesis 1 receives some support.
Ovules in which the integuments do not form a micropyle more often have unlobed integuments (inner integuments) than those that have a micropyle, because they do not have to compensate for their initially wide circumference. Result: of the seven taxa studied without a micropyle (five bitegmic, two unitegmic), both integuments or the only integument are lobed in only two (one bitegmic, one unitegmic). The number of cases is too small but does not speak against the hypothesis.
Integuments are more often lobed in ovules with thick nucelli than in ovules with thin nucelli, because they need to make a closure with an initially wider circumference. Result: two measurements were taken, always from anthetic flowers: (a) nucelli were measured at the broadest diameter at anthesis (ANITA grade, magnoliids, basal monocots and basal eudicots); (b) nucelli were measured at their base (only ANITA grade and magnoliids). In both cases the result was not clear. Ovules with broad nucelli did not show integument lobation more often than ovules with narrow nucelli. Of course, to use the size of mature nucelli is a very crude measure for a first approximation. For more useful results it would be important to measure the size of nucelli at a stage shortly after integument initiation. The size difference of nucelli between these two stages is considerable, and thus mature nucelli may not be suitable for meaningful deductions in this matter.
However, these preliminary results on basal angiosperms lend some support to the view that lobes on the rim of integuments in angiosperms are not remnants of ancient organs but merely the result of a developmental necessity for the closure of the micropyle, as discussed above. The peculiar deep lobation of the single integument in Juglandaceae that puzzled van Heel and Bouman (1972) may have a different cause. The ovule has an unusual position in the centre of the unilocular ovary formed by two carpels. The two integument lobes develop in the median symmetry plane of the carpels. There may be two explanations that have not been discussed by von Heel and Bouman (1972): (1) the lobation originates by space constraint in the slit-shaped two-carpellate locule; or (2) ovule growth is controlled by the two carpels, which is reflected in the two-parted integument.
EVOLUTION OF ADDITIONAL ‘INTEGUMENTS’ IN ANGIOSPERMS
Here and there in angiosperms there are ovules with a third envelope, which is called a ‘third integument’ if similar to the two normal integuments, or an ‘aril’, if it is more different and especially if delayed in development and functional as an attractive organ in fruit. Such extra envelopes can be present between the normal integuments, such as the third integument in some Annonaceae (Christmann, 1989), but commonly they appear on the outside of the two normal integuments, such as the arils in Myristicaceae (Endress, 1973) and many other groups. As they appear later than the normal integuments, their position is commonly distanced from the integuments and closer to the funiculus. More often than previously assumed, there are ‘reduced arils’, the role of which is unknown in most cases. They appear as small mounds at anthesis and do not develop further [e.g. among basal angiosperms, in Nymphaeaceae (Igersheim and Endress, 1998); in Sarcandra of Chloranthaceae (Endress and Igersheim, 1997) and Canellaceae (Igersheim and Endress, 1997); in Ranunculales, in Nandina of Berberidaceae (Endress and Igersheim, 1997); and in Buxales, in Buxus and Notobuxus of Buxaceae (von Balthazar and Endress, 2002) and Didymelaceae (von Balthazar et al., 2003); in the latter two families, this inconspicuous feature supports their relationship]. In Arabidopsis a small extra mound is formed in ovules of ucn (unicorn) mutants, which could represent a partial supernumerary integument (Schneitz et al., 1997; Schneitz, 1998b). To what extent third integuments and arils in angiosperms are homologous is uncertain.
MICROPYLE
The preponderance of narrow micropyles at the time around fertilization indicates that it is important for a successful functioning. In a few taxa a micropyle is not formed so that the nucellus apex is exposed. It is expected that in these taxa the architecture of the ovary is such that the exposed nucellus touches the funiculus, the placenta or the inner ovary wall. In this way a narrow gate above the nucellus apex may also be provided, e.g. in Cassytha (Lauraceae; Endress and Igersheim, 1997), Hernandia (Hernandiaceae; Heo and Tobe, 1995), Quisqualis (Combretaceae; Fagerlind, 1941), various Euphorbiaceae s.l. (Sutter and Endress, 1995; Merino Sutter et al., 2006), and Hiptage and Stigmatophyllon (Malpighiaceae; Rao, 1940). In the mentioned Hernandia, Euphorbiaceae s.l. and Malpighiaceae a nucellar beak is contiguous with the ovary roof.
In bitegmic ovules micropyles are commonly formed by both integuments (amphistomic) (Fig. 3C) or the inner alone (endostomic) (Fig. 3A, B), and only rarely by the outer alone (exostomic). If both integuments participate in micropyle formation, they may form a straight or undulating canal or, if the integuments are not aligned straight, a zig-zag-shaped canal (‘zig-zag micropyle’, Fig. 3C).
Micropyles may be open, forming an open canal (Fig. 3B), or closed (Fig. 3A, C). In the former the micropyle may be sealed by a secretion (e.g. Annona, Igersheim and Endress, 1997; Ornithogalum, Tilton and Lersten, 1981a, b; Beta, Olesen and Bruun, 1990). In the latter a closed pollen tube transmitting tract is formed by post-genital fusion (Helianthus, Yan et al., 1991). Thus this diversity is analogous to that of the carpels sealed by secretion or by post-genital fusion (Endress and Igersheim, 2000a). However, details of the histology of mature micropyles have only rarely been studied.
For some time, it had been suggested that a zig-zag micropyle is a basal feature of angiosperm ovules. This would fit the hypothesis by Gaussen (1946) (see ‘Evolution of bitegmy from unitegmy on the way to angiosperm evolution’) of a derivation of the angiosperm ovule from a cupule. In addition, a zig-zag micropyle is pronouncedly differentiated in Dilleniaceae, a family earlier thought to be basal in angiosperms (Stebbins, 1974). However, since in the meantime the phylogenetic position of Dilleniaceae has been found not to be basal, the zig-zag micropyle can be considered to be merely a consequence of excessive elongation of the outer integument concomitant with excessive curvature of the ovule. (This view does not contradict the hypothesis by Gaussen, 1946.) The hypothesis that a zig-zag micropyle is not basal is corroborated from two different sides, the systematic distribution and the strong association with camplyotropous ovules. (1) Zig-zag micropyle and campylotropous ovules are mainly present in more derived groups; this combination is lacking in the ANITA grade, and in magnoliids it is restricted to Canellaceae (Igersheim and Endress, 1997); in Magnoliaceae zig-zag micropyles have been reported in anatropous ovules. (2) In campylotropus ovules the antiraphal peripheral side of the ovule develops comparatively strongly; an expression of this strong development is also that the outer integument becomes exceedingly long and may far overtop the inner one; thus a zig-zag micropyle is formed. The combination of a zig-zag micropyle and campylotropy tends to be present in some Ranunculales (Papaveraceae, Berberidaceae, weakly in Menispermaceae; Endress and Igersheim, 1999) and in a number of major sub-clades of core eudicots, such as various Dilleniaceae (Svedelius 1911; Swamy and Periasamy, 1955; Rao, 1957; Sastri, 1958; Stebbins, 1974; Imaichi and Kato, 1996); Fabales (various Fabaceae, Rau, 1951; Prakash and Chan, 1976; Lakshmi et al., 1987; Ashrafunnisa and Pullaiah, 1999); Oxalidales (Elaeocarpaceae: Aristotelia, Mauritzon, 1934); Myrtales (Lythraceae: Sonneratia, Venkateswarlu, 1937; Kamelina, 1985; Melastomataceae: Rhexia, Etheridge and Herr, 1968; Monochaetum, Ziegler, 1925; Myrtaceae: Acca, Pescador et al., 2009; Baeckea, Mauritzon, 1939a; Psidium, Narayanaswami and Roy, 1960); Crossosomatales (Crossosomataceae: Crossosoma, Kapil and Vani, 1963); Brassicales (various Brassicaceae; Hakki, 1974; Prasad, 1974; Bouman, 1975; Febulans and Pullaiah, 1990; Beeckman et al., 2000; Capparaceae: Cadaba, Narayana, 1965; Niehburia, Arunalakshmi, 1985; Resedaceae: Reseda, Chaban and Yakovlev, 1974); and Malvales (various Malvaceae; Venkata Rao, 1954; Singh, 1967). However, in Caryophyllales, although the ovules are prominently campylotropous, there is no zig-zag micropyle; there the prominent inner integument forms the micropyle. There are also cases of anatropous ovules with zig-zag micropyle, but they are more rare. Examples are in Oxalidales (Elaeocarpaceae: Elaeocarpus, Venkata Rao, 1953); Geraniales (Geraniaceae: Geranium, Boesewinkel and Been, 1979); and Sapindales (Rutaceae: Aegle, Johri and Ahuja, 1957; Poincirus, Boesewinkel, 1978).
PATHWAY OF POLLEN TUBES FROM CARPELS TO OVULES
It was mentioned above that pollen tubes are attracted to the micropyle by secretions of the micropyle, nucellus and embryo sac (Hülskamp et al., 1995). That the embryo sac plays an important role in this attraction has long been known (e.g. Jensen, 1974). In ovules with degenerated embryo sacs the pollen tubes do not grow into the micropyle but down to the chalaza (Elodea; Ernst-Schwarzenbach, 1945). On the other hand, ovules with more than one embryo sac attract more pollen tubes (Persea; Sedgley, 1976). In Arabidopsis mutants with delayed embryo sac maturation, pollen tubes lose their way on the funiculus just before entering the micropyle (Shimizu and Okada, 2000). However, in normal Arabidopsis ovules there is a mechanism to prevent reception of more than one pollen tube (Shimizu and Okada, 2000; Shimizu, 2002; Palanivelu et al., 2003).
In plant groups with delayed ovule development, in which the ovules are far from fully developed at the time of pollination, pollen tubes do not enter the ovules via the micropyle. This was first described as chalazogamy in Casuarina (Treub, 1891), and later found in many other Fagales (Sogo et al., 2004; Sogo and Tobe, 2006a, c, 2008) and in Garryales (Eucommia, Sogo and Tobe, 2006b). A more neutral term, non-porogamy, considers that the entrance of the pollen tube to the ovules is not always via the chalaza in such cases. Luza and Polito (1991) showed that in Juglans, a genus with delayed ovule maturation and non-porogamy under natural conditions, the pollen tubes grew through the micropyle in post-anthetic flowers experimentally pollinated at the time of embryo sac maturity.
Immaturity of ovules at the time of pollination has also been found in other groups, but in such cases either non-porogamy does not occur or the pathway of the pollen tubes has not been studied (survey in Sogo and Tobe, 2006a). Examples are in magnoliids (Magnoliaceae; Igersheim and Endress, 1997), monocots (Orchidaceae; Yeung and Law, 1997), basal eudicots [Eupteleaceae (Endress, 1969), Circaeasteraceae, some Ranunculaceae and Lardizabalaceae, (Endress and Igersheim, 1999). Platanaceae, Myrothamnaceae (Endress and Igersheim, 1999) Buxaceae: Sarcococca, Pachysandra (von Balthazar and Endress, 2002)], Saxifragales [Altingiaceae, Cercidiphyllaceae, Daphniphyllaceae (Endress and Igersheim, 1999), some Hamamelidaceae (Endress, 1967, 1977)] and Sapindales [some Anacardiaceae (Bachelier and Endress, 2007)]. Mutants with precocious stigma development, such as ettin in Arabidopsis (Sessions, 1997), may give a clue to the easy and multiple evolution of this displacement of stigma and ovule maturity.
FEATURES OF OVULES AND MACROSYSTEMATICS OF ANGIOSPERMS
As angiosperm phylogeny becomes increasingly resolved, the evolution of ovules can be traced in ever more detail. Large clades can be better characterized by their ovule features and we learn more about evolutionary idiosyncrasies of ovules. This was still not possible or not attempted when the last round of big reviews on embryological features was published (Johri et al., 1992; Batygina et al., 2002). First attempts in the new era were made by Endress (2003, 2005, 2010, 2011) and Endress and Matthews (2006), and are briefly summarized here. The names used here for the major clades of angiosperms are those in APG (2009).
ANITA grade
Ovules are crassinucellar. Only in Hydatellaceae, highly specialized, miniaturized wetland plants, are they reduced to pseudocrassinucellar or incompletely tenuinucellar (Hamann, 1975; Rudall et al., 2008), and in the water plant family Cabombaceae they are weakly crassinucellar (Ramji and Padmanabhan, 1965; Igersheim and Endress, 1998). The ovules are predominantly anatropous, but they are almost orthotropous in Amborellaceae, Chloranthaceae and Ceratophyllaceae (see above) (position of Chloranthaceae and Ceratophyllaceae in the ANITA grade uncertain, see, for example, Endress and Doyle, 2009) and completely orthotropous in Barclaya of Nymphaeaceae (Schneider, 1978; Igersheim and Endress, 1998). They are bitegmic, except for the unitegmic Ceratophyllaceae, another reduced water plant family. The outer integument is predominantly more than two cell layers thick [only 2–3 in Cabombaceae (Endress and Igersheim, 1997, 2000a; Igersheim and Endress, 1997, 1998) and two in Hydatellaceae (Rudall et al., 2007) Barclaya of Nymphaeaceae (Igersheim and Endress, 1998) and Ascarina of Chloranthaceae (Endress and Igersheim, 1997)]. The inner integument is predominantly 2–3 cell layers thick. The micropyle is commonly formed by the inner integument. However, in Hydatellaceae, some derived Nymphaeaceae, in Trimeniaceae and in some Chloranthaceae both integuments participate in the formation of the micropyle (Endress and Igersheim, 1997; Igersheim and Endress, 1997, 1998; Rudall et al., 2007).
Magnoliids
Crassinucellar ovules are by far predominant. They are incompletely tenuinucellar in a few Piperales, such as Lactoridaceae, Hydnoraceae, and Houttuynia of Saururaceae (Tobe et al., 1993; Igersheim and Endress, 1998). The ovules are predominantly anatropous, but orthotropous in Saururaceae, Piperaceae and Hydnoraceae (Piperales) (Igersheim and Endress, 1998), and almost orthotropous in Gomortegaceae (Endress and Igersheim, 1997). They are bitegmic, except for Siparunaceae (Laurales) (Endress, 1972; Renner et al., 1997), and Peperomia and Hydnoraceae (Piperales) (Igersheim and Endress, 1998). The outer integument is more than two cell layers thick (Endress and Igersheim, 1997, 2000a; Igersheim and Endress, 1997; Heo et al., 1998), except for Piperales (Igersheim and Endress, 1998). The inner integument is mostly 2–3 cell layers thick. The micropyle is predominantly formed by the inner integument, but by both integuments in Magnoliaceae, Canellaceae, Gomortegaceae, some Calycanthaceae, a few Monimiaceae and several Piperales (Endress and Igersheim, 1997; Igersheim and Endress, 1997, 1998).
Monocots
Crassinucellar and weakly crassinucellar ovules are common, the latter here and there in most orders and prominent in Commelinales and Zingiberales of commelinids. Pseudocrassinucellar ovules appear to be more common in basal groups (Acorales, some Alismatales) than in more derived groups (a few Asparagales and Poales) (Rudall, 1997; Igersheim et al., 2001; Endress, 2011). Incompletely tenuinucellar ovules are widespread, with increased frequency in Dioscoreales, Pandanales and Poales (see Endress, 2011). Tenuinucellar ovules are only known from Orchidaceae (Asparagales) and Triuridaceae (Pandanales): both families mycotrophic (see Endress, 2011). Reduced tenuinucellar ovules appear to be absent. Ovules are predominantly anatropous, and only rarely campylotropous. However, they are orthotropous in Acorales, several Alismatales, some Asparagales, Commelinales and Poales (see Endress, 2011). Ovules are bitegmic (Bouman, 1984a), with a few exceptions of unitegmy in Alismatales (Igersheim et al., 2001). The inner integument is almost always only two cell layers thick (see Endress, 2011). The same is also very often the case in the outer integument, but in Zingiberales and mostly in Asparagales and Liliales it is thicker (see Endress, 2011). Thus thin integuments are a conspicuous general trend in monocots, as compared with the other angiosperm groups. The micropyle is mostly formed by the inner integument (only in Poales more often by both integuments) (see Endress, 2011).
Basal grade of eudicots
Ovules are basically crassinucellar, except for some reduced Ranunculales (Circaeasteraceae, Anemone and Clematis in Ranunculaceae; reviewed in Endress and Igersheim, 1999). They are almost always curved, mostly anatropous (in some Ranunculales campylotropous), but orthotropous in Sabiaceae, Platanaceae and many Proteaceae (Endress and Igersheim, 1999). They are almost always bitegmic, but with a conspicuous trend to unitegmy in core Ranunculales [Circaeasteraceae, some Menispermaceae and some Ranunculaceae (Wang and Ren, 2008); derived from ‘integumentary shifting’ at least in Menispermaceae and Ranunculaceae, as shown by intermediate forms (Bouman and Calis, 1977)]; Sabiaceae are also unitegmic (Raju, 1952; Endress and Igersheim, 1999). The inner integument is mostly around 2–3 cell layers thick, the outer between two and up to 13 (Endress and Igersheim, 1999). The outer integument is notably thinner than the inner in Papaveraceae, Platanaceae, Proteaceae and Trochodendraceae (Endress and Igersheim, 1999). The micropyle is formed by the inner integument in some Ranunculales, most Proteales and in Gunneraceae, otherwise by both integuments (Endress and Igersheim, 1999). In several basal eudicots ovule development is delayed at anthesis (see ‘Pathway of pollen tubes from carpels to ovules’).
Core eudicots: rosid alliance
Crassinucellar ovules are common in Saxifragales, Vitales, the nitrogen-fixing clade, Geraniales, Myrtales, Crossosomatales, Sapindales, Malvales and many Brassicales. However, the ovules are weakly crassinucellar in Zygophyllales, and tend to be incompletely tenuinucellar or weakly crassinucellar in the COM clade (Celastrales, Oxalidales, Malpighiales) and some Brassicales (Endress, 2010). There is a strong tendency to form an endothelium in incompletely tenuinucellar and weakly crassinucellar (and even some crassinucellar) ovules of the COM clade and malvids. Tenuinucellar ovules (in the new, restricted sense) are lacking, as seen from my literature search. Ovules are predominantly curved, with a tendency to campylotropy and zig-zag micropyle in Fabales (many Fabaceae, Surianaceae) and malvids (some Geraniales, Myrtales, Crossosomatales, core Brassicales, a few Malvales) (Endress, 2010). A trend towards orthotropy is present in the nitrogen-fixing clade, especially in some Fagales and Rosales (former members of Urticales), and some families of Malvales (Nandi, 1998; Endress, 2010). Ovules are almost always bitegmic, but there is a trend to unitegmy in some families of the Cucurbitales–Fagales clade (Endress, 2010). The outer integument is commonly thicker than the inner or equally thick. However, there is a strong trend to differentiate an inner integument that is thicker than the outer in the COM clade and malvids (Endress and Matthews, 2006). The micropyle is mostly formed by both integuments or by the inner integument alone, but there are no conspicuous trends.
Core eudicots: asterid alliance
Asterids are well known to be characterized by tenuinucellar ovules (tenuinucellar in the traditional sense), associated with an endothelium. A recent review showed that also in the Caryophyllales and Santalales, newly acquired orders of the asterid alliance, the nucelli are relatively thin, even if they are crassinucellar, such as in Caryophyllales (Endress, 2010, 2011). In Santalales there is stepwise reduction of ovules to forms without integuments; there are also carpels without differentiated ovules or without an ovary locule and then with embryo sac formation in the compact gynoecium (Dahlgren, 1927; Fagerlind, 1948; Shamrov et al., 2001; Brown et al., 2010). Among the classical sub-clades of asterids, incompletely tenuinucellar, tenuinucellar and reduced tenuinucellar ovules abound in euasterids, whereas in the basal orders of asterids, crassinucellar, weakly crassinucellar and incompletely tenuinucellar ovules are predominant (Endress, 2010, 2011). In euasterids, tenuinucellar ovules (in the new, restricted sense) characterize some species-rich clades, such as Asteraceae, Gentianales and Lamiales, whereas reduced tenuinucellar ovules are largely restricted to asclepiads (Apocynaceae), some Gentianaceae and Rubiaceae, a few families of Lamiales, and Convolvulaceae of Solanales (Endress, 2011). Notably, in Gentianales, an endothelium is absent (Endress, 2010). In some mycotrophic Gentianaceae even integumentless ovules occur (Goebel, 1933). Ovules are always anatropous (or campylotropous), never orthotropous (except for reduced members of Santalales, see above). Unitegmic ovules dominate, and are almost exclusive in euasterids. However, ovules are bitegmic in Berberidopsidales, Caryophyllales and in the only weakly reduced, basal sub-clades of Santalales and in some Ericales. Among bitegmic Ericales the micropyle is more often formed by the inner integument alone than by both integuments, and the inner integument is thicker than the outer in the clade of the former Primulales, but the other way around in the other bitegmic families (Endress, 2010).
CONCLUSIONS
A synthetic look at ovule structure from several points of view (structure, development, diversity, fossils, evo–devo and systematic distribution) allowed the elucidation of some trends in angiosperm ovules: (1) the co-occurrence of bitegmic and anatropous ovules; (2) lobed integuments; (3) hood-shaped vs. cup-shaped outer integuments; (4) zig-zag micropyles; (5) the evolution of unitegmy within angiosperms; (6) the partial dependence of ovule structure on locular architecture; and (7) relatively stable features of ovules.
The two most salient differences between ovules of angiosperms and other seed plants are that they are basically and predominantly anatropous and bitegmic (vs. orthotropous and unitegmic). The combination of these two features is not a coincidence. Evidence from four independent sources indicates that curvature is functionally dependent on bitegmy: (a) in orthotropous ovules the outer integument is often thin (only two cell layers thick), short or even absent; (b) in normally bitegmic, anatropous clades, abnormal orthotropous ovules mostly have a reduced outer integument; (c) in mutants of Arabidopsis without ovule curvature, the outer integument is mostly reduced, with the same pattern arising with different genes and gene combinations; (d) in Podocarpaceae, the only extant gymnosperm clade with a conspicuous developmental ovule curvature, an outer envelope (but not homologous with the outer integument in angiosperms) also appears instrumental for ovule curvature.
Lobation of inner (and outer) integuments in angiosperms does not represent remnants of telomes as in fossil gymnosperms but is simply a morphogenetic necessity in forming a narrow micropyle.
Hood-shaped and cup-shaped outer integuments are not fundamentally different in angiosperms. They co-occur within closely related clades. It is likely that the hood shape is just an extreme form resulting from rapid early curvature.
Zig-zag micropyles in angiosperms are not basal, but rather the result of developmental overgrowth in ovules with excessive development of the antiraphal side. (a) Zig-zag micropyles are rare in basal angiosperms but more common in more derived groups; and (b) zig-zag micropyles and campylotropous ovules show a high correlation of occurrence. Campylotropy results from especially strong growth of the antiraphal side.
The evolutionary pathway to unitegmy from bitegmy in angiosperms is not uniform: in unitegmic basal angiosperms and some derived groups it probably evolved mainly by loss of the outer integument, often associated with orthotropous ovules, but in derived eudicots (asterids) it is mainly by incorporation of the outer into the inner integument, associated with anatropous ovules.
Ovule structure is partly shaped by locule architecture and also associated with the presence or absence of massive locular secretions. This is especially pronounced in the distribution of orthotropous ovules through the angiosperms.
A closer look at nucellus and integument thickness shows a clear association of some newly recognized types with larger clades in angiosperms. It indicates that certain aspects of ovule diversity are relatively stable in evolution. However, stability has changed over time, e.g. ovule symmetry has become less stable from gymnosperms to angiosperms, whereas ovule size has become more stable from basal to more advanced angiosperms.
OUTLOOK
Some new questions derived from the ‘Conclusions’ and unanswered old questions are as follows. (a) How does the interdependence of ovule shape and locule architecture work developmentally in detail? (b) It has been shown that the thickness of the nucelli and the relative thickness of the two integuments are relatively stable at the macrosystematic level. How is this stable thickness developmentally regulated? (c) The diversity of the micropyle differentiation (fusion or non-fusion) across angiosperms is little known. Is there a systematic pattern? (d) There is no comparative study on the development of different ovule curvature patterns. How exactly do these different patterns come about in development? (e) The evolution of the outer integument in angiosperms is still uncertain. Does it correspond to a cupular wall or does it have a different origin? (f) How was the evolution of ancestral anatropy in angiosperms co-ordinated with the evolutionary advent of the closed carpel?
ACKNOWLEDGEMENTS
I thank George Schatz, Missouri Botanical Garden, and Edward Schneider, Botanical Garden, University of California, Santa Barbara, for valuable plant material. Anton Igersheim and Rosemarie Siegrist are acknowledged for microtome sections, Urs Jauch for support with the SEM, and Alex Bernhard for graphic work. Mary Endress is thanked for commenting on the manuscript. Two anonymous reviewers are acknowledged for their valuable suggestions.
APPENDIX
List of collections used for figures
| Species | Collection |
|---|---|
| Akebia quinata Decne. (Lardizabalaceae) | P. K. Endress 4625, Botanic Garden, University of Zurich. |
| Barclaya rotundifolia M. Hotta (Nymphaeaceae) | E. L. Schneider, s.n., 1992, Malaysia. |
| Asimina triloba (L.) Dunal (Annonaceae) | P. K. Endress 5160, Botanic Garden, University of Zurich. |
| Butomus umbellatus L. (Butomaceae) | P. K. Endress, s.n., s.d., Botanic Garden, University of Zurich. |
| Corylopsis willmottiae Rehder & E. H. Wilson (Hamamelidaceae) | P. K. Endress 3577, Botanic Garden, University of Zurich. |
| Hypecoum pendulum L. (Papaveraceae) | P. K. Endress 4488, Botanic Garden, University of Zurich. |
| Houttuynia cordata Thunb. (Saururaceae) | P. K. Endress 7259, Botanic Garden, University of Zurich. |
| Liquidambar orientalis Mill. (Altingiaceae) | P. K. Endress 2221, Rhodos, Greece. |
| Nymphaea tetragona Georgi (Nymphaeaceae) | P. K. Endress 4901, Botanic Garden, University of Zurich. |
| Passiflora holosericea Ruiz & Pav. ex Mast. (Passifloraceae) | P. K. Endress, s.n., 27 IX 1990, Botanic Garden, University of Zurich. |
| Podophyllum emodi Wall. ex Hook. f. & Thomson (Berberidaceae) | P. K. Endress 4606, Botanic Garden, University of Zurich. |
| Solanum sisymbrifolium Lam. (Solanaceae) | P. K. Endress 7281, Botanic Garden, University of Zurich. |
| Takhtajania perrieri (Capuron) M. Baranova & J.-F. Leroy (Winteraceae) | P. J. Rakotomalaza et al. 1342, Madagascar. |
| Tasmannia piperita Miers (Winteraceae) | P. K. Endress 4137, Papua New Guinea. |
| Xiphidium coeruleum Aubl. (Haemodoraceae) | P. K. Endress 3764, Botanic Garden, University of Zurich. |
LITERATURE CITED
- Abe K. Contributions to the embryology of the family Orchidaceae. VII. A comparative study of the orchid embryo sac. Science Reports of the Tôhoku University, Series IV. 1972;36:179–201. [Google Scholar]
- Agardh JG. Theoria systematis plantarum. Lund: Gleerup; 1858. [Google Scholar]
- Andrews HN. Early seed plants: recent fossil discoveries shed light on the seed and on seed plant progenitors. Science. 1963;142:925–931. doi: 10.1126/science.142.3594.925. [DOI] [PubMed] [Google Scholar]
- Angenent GC, Franken J, Busscher M, et al. A novel class of MADS box genes is involved in ovule development in Petunia. Plant Cell. 1995;7:1569–1582. doi: 10.1105/tpc.7.10.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APG (The Angiosperm Phylogeny Group) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society. 2009;161:105–121. [Google Scholar]
- Arunalakshmi V. Embryological studies in Capparidaceae – life history of Niebuhria apetala DC. Journal of the Indian Botanical Society. 1985;64:17–24. [Google Scholar]
- Pullaiah T Ashrafunnisa [without first name] Embryology of Teramnus labialis (Fabaceae) Phytomorphology. 1999;49:197–202. [Google Scholar]
- Bachelier JB, Endress PK. Development of inflorescences, cupules, and flowers in Amphipterygium, and comparison with Pistacia (Anacardiaceae) International Journal of Plant Sciences. 2007;168:1237–1253. [Google Scholar]
- Bachelier JB, Endress PK. Floral structure of Kirkia (Kirkiaceae) and its position in Sapindales. Annals of Botany. 2008;102:539–550. doi: 10.1093/aob/mcn139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelier JB, Endress PK. Comparative floral morphology and anatomy in Anacardiaceae and Burseraceae (Sapindales), with a special focus on gynoecium structure and evolution. Botanical Journal of the Linnean Society. 2009;159:499–571. [Google Scholar]
- Bailey IW, Swamy BGL. Amborella trichopoda Baill., a new morphological type of vesselless dicotyledon. Journal of the Arnold Arboretum. 1948;29:245–254. [Google Scholar]
- Baker SC, Robinson-Beers K, Villanueva JM, Gaiser JC, Gasser CS. Interactions among genes regulating ovule development in Arabidopsis thaliana. Genetics. 1997;145:1109–1124. doi: 10.1093/genetics/145.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S, Schneitz K. NOZZLE regulates proximal–distal pattern formation, cell proliferation and early sporogenesis during ovule development in Arabidopsis thaliana. Development. 2000;127:4227–4238. doi: 10.1242/dev.127.19.4227. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Schneitz K. NOZZLE links proximal–distal and adaxial–abaxial pattern formation during ovule development in Arabidopsis thaliana. Development. 2002;129:4291–4300. doi: 10.1242/dev.129.18.4291. [DOI] [PubMed] [Google Scholar]
- von Balthazar M, Endress PK. Reproductive structures and systematics of Buxaceae. Botanical Journal of the Linnean Society. 2002;140:193–228. [Google Scholar]
- von Balthazar M, Schatz GE, Endress PK. Female flowers and inflorescences of Didymelaceae. Plant Systematics and Evolution. 2003;237:199–208. [Google Scholar]
- Batygina TB, editor. Embryology of flowering plants. Terminology and concepts, Vol. 1. Generative organs of flower. Enfield, NH: Science Publishers; 2002. [Google Scholar]
- Beekman T, De Rycke R, Viane R, Inzé D. Histological study of seed coat development in Arabidopsis thaliana. Journal of Plant Research. 2000;113:139–148. [Google Scholar]
- Berg RY. Development of ovule, embryo sac, and endosperm in Dipterostemon and Dichelostemma (Alliaceae) relative to taxonomy. American Journal of Botany. 1996;83:790–801. doi: 10.3732/ajb.90.6.937. [DOI] [PubMed] [Google Scholar]
- Berg RY. Embryo sac, endosperm, and seed of Nemophila (Boraginaceae) relative to taxonomy, with a remark on embryogeny in Pholistoma. American Journal of Botany. 2009;96:565–579. doi: 10.3732/ajb.0800208. [DOI] [PubMed] [Google Scholar]
- Bocquet G. The campylotropous ovule. Phytomorphology. 1959;9:222–227. [Google Scholar]
- Bocquet G, Bersier JD. La valeur systématique de l'ovule: développements tératologiques. Archives des Sciences. 1960;13:475–496. [Google Scholar]
- Boesewinkel FD. Development of ovule and testa in Rutaceae III. Some representatives of the Aurantioideae. Acta Botanica Neerlandica. 1978;27:341–354. [Google Scholar]
- Boesewinkel FD, Been W. Development of ovule and testa of Geranium pratense L. and some other representatives of the Geraniaceae. Acta Botanica Neerlandica. 1979;28:335–348. [Google Scholar]
- Borg AJ, Schönenberger J. Comparative floral development and structure of the black mangrove genus Avicennia L. and related taxa in the Acanthaceae. International Journal of Plant Sciences. 2011;172:330–344. [Google Scholar]
- Bouman F. Developmental studies of the ovule, integuments and seed in some angiosperms. Naarden: Los; 1974. PhD Thesis, University of Amsterdam. [Google Scholar]
- Bouman F. Integument initiation and testa development in some Cruciferae. Botanical Journal of the Linnean Society. 1975;70:213–229. [Google Scholar]
- Bouman F. The ovule. In: Johri BM, editor. Embryology of angiosperms. Berlin: Springer; 1984a. pp. 123–157. [Google Scholar]
- Bouman F. The seed: structure. In: Johri BM, editor. Embryology of angiosperms. Berlin: Springer; 1984b. pp. 567–610. [Google Scholar]
- Bouman F, Boesewinkel FD. The campylotropous ovules and seeds, their structure and functions. Botanische Jahrbücher für Systematik. 1991;113:255–270. [Google Scholar]
- Bouman F, Calis JIM. Integumentary shifting – a third way to unitegmy. Berichte der Deutschen Botanischen Gesellschaft. 1977;90:15–28. [Google Scholar]
- Bouman F, Cobb L, Devente N, Goethals V, Maas PJM, Smets E. The seeds of Gentianaceae. In: Struwe L, Albert VA, editors. Gentianaceae: systematics and natural history. Cambridge: Cambridge University Press; 2002. pp. 498–572. [Google Scholar]
- Bowman JL, editor. Arabidopsis: an atlas of morphology and development. New York: Springer; 1993. [Google Scholar]
- Brambilla V, Battaglia R, Colombo M, et al. Genetic and molecular interactions between BELL1 and MADS box factors support ovule development in Arabidopsis. The Plant Cell. 2007;19:2544–2556. doi: 10.1105/tpc.107.051797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadhvest J, Baker SC, Gasser CS. SHORT INTEGUMENTS 2 promotes growth during Arabidopsis reproductive development. Genetics. 2000;155:899–907. doi: 10.1093/genetics/155.2.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brongniart AT. Mémoire sur la génération et le développement de l'embryon dans les végétaux phanérogames. Annales des Sciences Naturelles. 1827;12:14–53. 145–172, 225–298. [Google Scholar]
- Brown RH, Nickrent DL, Gasser CS. Expression of ovule and integument-associated genes in reduced ovules of Santalales. Evolution and Development. 2010;12:231–240. doi: 10.1111/j.1525-142X.2010.00407.x. [DOI] [PubMed] [Google Scholar]
- Buzgo M. Inflorescence development of Pistia stratiotes (Araceae) Botanische Jahrbücher für Systematik. 1994;115:557–570. [Google Scholar]
- Buzgo M, Endress PK. Floral structure and development of Acoraceae and its systematic relationships with basal angiosperms. International Journal of Plant Sciences. 2000;161:23–41. doi: 10.1086/314241. [DOI] [PubMed] [Google Scholar]
- Capoor SP. The life history of Holoptelea integrifolia Planch. (Ulmaceae) Beihefte zum Botanischen Centralblatt A. 1937;57:233–249. [Google Scholar]
- Caris PL, Smets EF. A floral ontogenetic study on the sister group relationship between the genus Samolus (Primulaceae) and the Theophrastaceae. American Journal of Botany. 2004;91:627–643. doi: 10.3732/ajb.91.5.627. [DOI] [PubMed] [Google Scholar]
- Caris P, Ronse Decraene LP, Smets E, Clinckemaillie D. Floral development of three Maesa species, with special emphasis on the position of the genus within Primulales. Annals of Botany. 2000;86:87–97. [Google Scholar]
- Chaban IA, Yakovlev MS. The embryology of Reseda lutea L. I. Megasporogenesis and development of embryo sac. Botaniceskij Zhurnal (Moscow & Leningrad) 1974;59:24–37. [Google Scholar]
- Chaloner WG, Hill AJ, Lacy WS. First Devonian platyspermic seed and its implications in gymnosperm evolution. Nature. 1977;293:462–464. [Google Scholar]
- Chesnoy L. Les sécrétions dans la pollinisation des gymnospermes. Acta Botanica Gallica. 1993;140:145–156. [Google Scholar]
- Christmann M. Die tritegmischen Annonaceen-Samen. Botanische Jahrbücher für Systematik. 1989;110:433–439. [Google Scholar]
- Colombo L, Franken J, Koetje E, et al. The Petunia MADS box gene FBP11 determines ovule identity. The Plant Cell. 1995;7:1859–1868. doi: 10.1105/tpc.7.11.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo L, Battaglia R, Kater MM. Arabidopsis ovule development and its evolutionary conservation. Trends in Plant Science. 2008;13:444–450. doi: 10.1016/j.tplants.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Corner EJH. The seeds of dicotyledons. Cambridge: Cambridge University Press; 1976. 1, 2. [Google Scholar]
- Crane PR. Phylogenetic analysis of seed plants and the origin of angiosperms. Annals of the Missouri Botanical Garden. 1985;72:716–793. [Google Scholar]
- Crane PR, Herendeen PS. Bennettitales from the Grisethorpe Bed (Middle Jurassic) at Cayton Bay, Yorkshire, UK. American Journal of Botany. 2009;96:284–295. doi: 10.3732/ajb.0800193. [DOI] [PubMed] [Google Scholar]
- Dahlgren G. Steps toward a natural system of the dicotyledons: embryological characters. Aliso. 1991;13:107–165. [Google Scholar]
- Dahlgren KVO. Die Morphologie des Nuzellus mit besonderer Berücksichtigung der deckzellosen Typen. Jahrbücher für Wissenschaftliche Botanik. 1927;67:347–426. [Google Scholar]
- Davis GL. Systematic embryology of the angiosperms. New York: Wiley; 1966. [Google Scholar]
- Davis GL. Floral morphology and the development of gametophytes in Eucalyptus melliodora A. Cunn. Australian Journal of Botany. 1968;16:19–35. [Google Scholar]
- De Laet J, Clinckemaillie D, Jansen S, Smets E. Floral ontogeny in the Plumbaginaceae. Journal of Plant Research. 1995;108:289–304. [Google Scholar]
- Del Fueyo GM. Cone and ovule development in the Podocarpus species from Argentina. Phytomorphology. 1999;49:49–60. [Google Scholar]
- Dilcher D, Mei M, Du M. A new winged seed from the Permian of China. Review of Palaeobotany and Palynology. 1997;98:247–256. [Google Scholar]
- Douglas AW, Stevenson DW, Little DP. Ovule development in Ginkgo biloba L., with emphasis on the collar and nucellus. International Journal of Plant Sciences. 2007;168:1207–1236. [Google Scholar]
- Doyle J. Developmental lines in pollination mechanisms in the Coniferales. Scientific Proceedings of the Royal Dublin Society. 1945;23:43–62. [Google Scholar]
- Doyle JA. Origin of angiosperms. Annual Reviews in Ecology and Systematics. 1978;9:365–392. [Google Scholar]
- Doyle JA. Origin of the angiosperm flower: a phylogenetic perspective. Plant Systematics and Evolution Supplement. 1994;8:7–29. [Google Scholar]
- Doyle JA. Seed plant phylogeny and the relationships of Gnetales. International Journal of Plant Sciences. 1996;157:S3–S39. [Google Scholar]
- Doyle JA. Seed ferns and the origin of angiosperms. Journal of the Torrey Botanical Society. 2006;133:169–209. [Google Scholar]
- Doyle JA. Integrating molecular phylogenetic and paleobotanical evidence on origin of the flower. International Journal of Plant Sciences. 2008;169:816–843. [Google Scholar]
- Doyle JA, Endress PK. Morphological phylogenetic analysis of basal angiosperms: comparison and combination with molecular data. International Journal of Plant Sciences. 2000;161:S121–S153. doi: 10.1086/314241. [DOI] [PubMed] [Google Scholar]
- Dreni L, Jacchia S, Fornara F, et al. The D-lineage MADS-box gene OsMADS13 controls ovule identity in rice. The Plant Journal. 2007;52:690–699. doi: 10.1111/j.1365-313X.2007.03272.x. [DOI] [PubMed] [Google Scholar]
- Dresselhaus T, Márton ML. Micropylar pollen tube guidance and burst: adapted from defense mechanisms? Current Opinion in Plant Biology. 2009;12:773–780. doi: 10.1016/j.pbi.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Drinnan AN, Schramke JM, Crane PR. Stephanospermum konopeonus (Langford) comb. nov.: a medullosan ovule from the Middle Pennsylvanian Mazon Creek flora of northeastern Illinois, U.S.A. Botanical Gazette. 1990;151:385–401. [Google Scholar]
- Edman G. Zur Entwicklungsgeschichte der Gattung Oxyria Hill, nebst zytologischen, embryologischen und systematischen Bemerkungen über einige andere Polygonaceen. Acta Horti Bergiani. 1929;9:165–291. [Google Scholar]
- Eggert DA, Delevoryas T. Callospermarion – a new seed genus from the upper Pennsylvanian of Illinois. Phytomorphology. 1960;10:131–138. [Google Scholar]
- Elliott RC, Betzner AS, Huttner E, et al. AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. The Plant Cell. 1996;8:155–168. doi: 10.1105/tpc.8.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endress PK. Systematische Studie über die verwandtschaftlichen Beziehungen zwischen den Hamamelidaceen und Betulaceen. Botanische Jahrbücher für Systematik. 1967;87:431–525. [Google Scholar]
- Endress PK. Gesichtspunkte zur systematischen Stellung der Eupteleaceen (Magnoliales). Untersuchungen über Bau und Entwicklung der generativen Region bei Euptelea polyandra Sieb. et Zucc. Berichte der Schweizerischen Botanischen Gesellschaft. 1969;79:229–278. [Google Scholar]
- Endress PK. Zur vergleichenden Entwicklungsmorphologie, Embryologie und Systematik bei Laurales. Botanische Jahrbücher für Systematik. 1972;92:331–428. [Google Scholar]
- Endress PK. Arils and aril-like structures in woody Ranales. New Phytologist. 1973;72:1159–1171. [Google Scholar]
- Endress PK. Evolutionary trends in the Hamamelidales–Fagales group. Plant Systematics and Evolution Supplement. 1977;1:321–347. [Google Scholar]
- Endress PK. The reproductive structures and systematic position of the Austrobaileyaceae. Botanische Jahrbücher für Systematik. 1980;101:393–433. [Google Scholar]
- Endress PK. Reproductive structures and phylogenetic significance of extant primitive angiosperms. Plant Systematics and Evolution. 1986;152:1–28. [Google Scholar]
- Endress PK. The Chloranthaceae: reproductive structures and phylogenetic position. Botanische Jahrbücher für Systematik. 1987;109:153–226. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994a. [Google Scholar]
- Endress PK. Floral structure and evolution of primitive angiosperms. Plant Systematics and Evolution. 1994b;192:79–97. [Google Scholar]
- Endress PK. Structure and function of female and bisexual organ complexes in Gnetales. International Journal of Plant Sciences. 1996;157:S113–S125. [Google Scholar]
- Endress PK. What should a ‘complete’ morphological phylogenetic analysis entail? In. In: Stuessy TF, Hörandl E, Mayer V, editors. Deep morphology: towards a renaissance of morphology in plant systematics. Regnum Vegetabile. Vol. 141. Ruggell: Gantner; 2003. pp. 131–164. [Google Scholar]
- Endress PK. Links between embryology and evolutionary floral morphology. Current Science. 2005;89:749–754. [Google Scholar]
- Endress PK. Angiosperm floral evolution: morphological developmental framework. Advances in Botanical Research. 2006;44:1–61. [Google Scholar]
- Endress PK. The whole and the parts: relationships between floral architecture and floral organ shape, and their repercussions on the interpretation of fragmentary floral fossils. Annals of the Missouri Botanical Garden. 2008;95:101–120. [Google Scholar]
- Endress PK. Flower structure and trends of evolution in eudicots and their major subclades. Annals of the Missouri Botanical Garden. 2010;97:541–583. [Google Scholar]
- Endress PK. Evolutionary diversification of the flowers in angiosperms. American Journal of Botany. 2011;98:370–396. doi: 10.3732/ajb.1000299. [DOI] [PubMed] [Google Scholar]
- Endress PK, Doyle JA. Reconstructing the ancestral flower and its initial specializations. American Journal of Botany. 2009;96:22–66. doi: 10.3732/ajb.0800047. [DOI] [PubMed] [Google Scholar]
- Endress PK, Igersheim A. Gynoecium diversity and systematics of the Laurales. Botanical Journal of the Linnean Society. 1997;125:93–168. [Google Scholar]
- Endress PK, Igersheim A. Gynoecium diversity and systematics of the basal eudicots. Botanical Journal of the Linnean Society. 1999;130:305–393. [Google Scholar]
- Endress PK, Igersheim A. Gynoecium structure and evolution in basal angiosperms. International Journal of Plant Sciences. 2000a;161:S211–S223. doi: 10.1086/314241. [DOI] [PubMed] [Google Scholar]
- Endress PK, Igersheim A. The reproductive structures of the basal angiosperm Amborella trichopoda (Amborellaceae) International Journal of Plant Sciences. 2000b;161:S237–S248. [Google Scholar]
- Endress PK, Matthews ML. First steps towards a floral structural characterization of the major rosid subclades. Plant Systematics and Evolution. 2006;260:223–251. [Google Scholar]
- Endress PK, Sampson FB. Floral structure and relationships of the Trimeniaceae (Laurales) Journal of the Arnold Arboretum. 1983;64:447–473. [Google Scholar]
- Endress PK, Igersheim A, Sampson FB, Schatz GE. Floral structure of Takhtajania and its systematic position in Winteraceae. Annals of the Missouri Botanical Garden. 2000;87:347–365. [Google Scholar]
- Engler A. Übersicht der in den Bänden 19a, 19b und 19c bearbeiteten Familien. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 2nd edn. Leipzig: Engelmann; 1931. pp. 7–10. 19a. [Google Scholar]
- Ernst-Schwarzenbach M. Kreuzungsversuche an Hydrocharitaceen. Archiv der Julius Klaus-Stiftung. 1945;20:22–41. [Google Scholar]
- Eshed Y, Baum SF, Perea JV, Bowman JL. Establishment of polarity in lateral organs of plants. Current Biology. 2001;11:1251–1260. doi: 10.1016/s0960-9822(01)00392-x. [DOI] [PubMed] [Google Scholar]
- Etheridge AL, Herr JM., Jr. The development of the ovule and megagametophyte in Rhexia virginica (Melastomataceae) Rhodora. 1968;69:163–178. [Google Scholar]
- Fagerlind F. Embryologische Beobachtungen über die Gattung Phyllis. Botaniska Notiser. 1936;1936:577–584. [Google Scholar]
- Fagerlind F. Der Bau der Samenanlage und des Makrogametophyten bei Quisqualis indica. Botaniska Notiser. 1941;1941:216–222. [Google Scholar]
- Fagerlind F. Die Samenbildung und die Zytologie bei agamospermischen und sexuellen Arten von Elatostema und einigen nahestehenden Gattungen nebst Beleuchtung einiger damit zusammenhängender Probleme. Kungliga Svenska Vetenskapsakademiens Handlingar, Ser. 321 (4) 1944:1–130. [Google Scholar]
- Fagerlind F. Gynöceummorphologische und embryologische Studien in der Familie Olacaceae. Botaniska Notiser. 1947;1947:207–230. [Google Scholar]
- Fagerlind F. Beiträge zur Kenntnis der Gynöceummorphologie und Phylogenie der Santalales-Familien. Svensk Botanisk Tidskrift. 1948;42:195–229. [Google Scholar]
- Favaro R, Pinyopich A, Battaglia R, et al. MADS-box protein complexes control carpel and ovule development in Arabidopsis. The Plant Cell. 2003;15:2603–2611. doi: 10.1105/tpc.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febulans GNV, Pullaiah T. Embryology of Schouwia (Brassicaceae) Phytomorphology. 1990;40:377–382. [Google Scholar]
- Fernando DD, Cass DD. Development and structure of ovule, embryo sac, embryo, and endosperm in Butomus umbellatus (Butomaceae) International Journal of Plant Sciences. 1996;157:269–279. [Google Scholar]
- Franssen-Verheijen MAW, Willemse MTM. Micropylar exudate in Gasteria (Aloaceae) and its possible function in pollen tube growth. American Journal of Botany. 1993;80:253–262. [Google Scholar]
- French JC. Ovule vasculature in Araceae. Botanical Gazette. 1986;147:478–495. [Google Scholar]
- Friedman WE. Growth and development of the male gametophyte of Ginkgo biloba within the ovule (in vivo) American Journal of Botany. 1987;74:1797–1815. [Google Scholar]
- Friedman WE. Embryological evidence for developmental lability during early angiosperm evolution. Nature. 2006;441:337–340. doi: 10.1038/nature04690. [DOI] [PubMed] [Google Scholar]
- Friedman WE, Carmichael JS. Heterochrony and developmental innovation: evolution of female gametophyte ontogeny in Gnetum, a highly apomorphic seed plant. Evolution. 1998;52:1016–1030. doi: 10.1111/j.1558-5646.1998.tb01830.x. [DOI] [PubMed] [Google Scholar]
- Friedman WE, Williams JH. Modularity in the angiosperm female gametophyte and its bearing on the early evolution of flowering plants. Evolution. 2003;57:216–230. doi: 10.1111/j.0014-3820.2003.tb00257.x. [DOI] [PubMed] [Google Scholar]
- Friis EM, Pedersen KR. Canrightia resinifera gen. et sp. nov., a new extinct angiosperm with Retimonocolpites-type pollen from the Early Cretaceous of Portugal: missing link in the eumagnoliid tree? Grana. 2011;50:3–29. [Google Scholar]
- Friis EM, Crane PR, Pedersen KR, et al. Phase-contrast X-ray microtomography links Cretaceous seeds with Gnetales and Bennettitales. Nature. 2007;450:549–552. doi: 10.1038/nature06278. [DOI] [PubMed] [Google Scholar]
- Friis EM, Pedersen KR, Crane PR. Early Cretaceous microfossils from Portugal and Eastern North America related to the Bennettitales–Erdtmanithecales–Gnetales group. American Journal of Botany. 2009;96:252–283. doi: 10.3732/ajb.0800113. [DOI] [PubMed] [Google Scholar]
- Frohlich MW. An evolutionary scenario for the origin of flowers. Nature Reviews Genetics. 2003;4:559–566. doi: 10.1038/nrg1114. [DOI] [PubMed] [Google Scholar]
- Galtier J, Rowe NP. A primitive seed-like structure and its implications for early gymnosperm evolution. Nature. 1989;340:225–227. [Google Scholar]
- Gallagher TL, Gasser CS. Independence and interaction of regions of the INNER NO OUTER protein in growth control during ovule development. Plant Physiology. 2008;147:306–315. doi: 10.1104/pp.107.114603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser CS, Broadhvest J, Hauser BA. Genetic analysis of ovule development. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:1–24. doi: 10.1146/annurev.arplant.49.1.1. [DOI] [PubMed] [Google Scholar]
- Gaussen H. Les Gymnospermes, actuelles et fossils. Travaux du Laboratoire Forestier Toulouse, Tome II, Études Dendrologiques. 1946:1–26. Sect. 1, Vol. 1. Fasc. 3, Chap. 5. [Google Scholar]
- Gelbart G, von Aderkas P. Ovular secretions as part of pollination mechanisms in conifers. Annals of Forest Science. 2002;59:345–357. [Google Scholar]
- Gillespie WH, Rothwell GW, Scheckler SE. The earliest seeds. Nature. 1981;293:462–464. [Google Scholar]
- Goebel K. Organographie der Pflanzen, insbesondere der Archegoniaten und Samenpflanzen. 3rd edn. Jena: Fischer; 1933. 3. [Google Scholar]
- Gross-Hardt R, Lenhard M, Laux T. WUSCHEL signalling functions in interregional communication during Arabidopsis ovule development. Genes and Development. 2002;16:1129–1138. doi: 10.1101/gad.225202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC. The embryology of Coriandrum sativum L. and Foeniculum vulgare Mill. Phytomorphology. 1964;14:530–547. [Google Scholar]
- Haig D, Westoby M. Selective forces in the emergence of the seed habit. Biological Journal of the Linnean Society. 1989;38:215–238. [Google Scholar]
- Hakki MI. Embryologische und morphologische Beobachtungen an Succowia balearica (L.) Medik. (Brassicaceae) Botanische Jahrbücher für Systematik. 1974;94:360–382. [Google Scholar]
- Hakki MI. On floral morphology and embryology of Orphium frutescens (L.) E. Meyer (Gentianaceae) Botanische Jahrbücher für Systematik. 1997;119:337–383. [Google Scholar]
- Hamann U. Neue Untersuchungen zur Embryologie und Systematik der Centrolepidaceae. Botanische Jahrbücher für Systematik. 1975;96:154–191. [Google Scholar]
- Hamann U. Über Konvergenzen bei embryologischen Merkmalen der Angiospermen. Berichte der Deutschen Botanischen Gesellschaft. 1977;90:369–384. [Google Scholar]
- Hammond BL. Development of Podostemon ceratophyllum. Bulletin of the Torrey Botanical Club. 1937;64:17–36. [Google Scholar]
- van Heel WA. Distally lobed integuments in some angiosperm ovules. Blumea. 1970;18:67–70. [Google Scholar]
- van Heel WA. Flowers and fruits in Flacourtiaceae I. Scaphocalyx spathacea Ridl. Blumea. 1973;21:259–279. [Google Scholar]
- van Heel WA. Distally-lobed integuments in Exochorda, Juglans, Leontice and Bongardia. Phytomorphology. 1976;26:1–4. [Google Scholar]
- van Heel WA, Bouman F. Note on the early development of the integument in some Juglandaceae, together with some general questions on the structure of angiosperm ovules. Blumea. 1972;20:157–159. [Google Scholar]
- Heo K, Tobe H. Embryology and relationships of Gyrocarpus and Hernandia (Hernandiaceae) Journal of Plant Research. 1995;108:327–341. [Google Scholar]
- Heo K, Tobe H, van der Werff H. Embryology and relationships of Lauraceae (Laurales) Botanical Journal of the Linnean Society. 1998;126:295–322. [Google Scholar]
- Herr JM., Jr The origin of the ovule. American Journal of Botany. 1995;82:547–564. [Google Scholar]
- Hilton J, Bateman RM. Pteridosperms are the backbone of seed-plant phylogeny. Journal of the Torrey Botanical Society. 2006;133:119–168. [Google Scholar]
- Hilton J, Edwards D. A new Late Devonian acupulate preovule from Taffs Well, South Wales. Review of Palaeobotany and Palynology. 1996;93:235–252. [Google Scholar]
- Hilton J, Edwards D. New data on Xenotheca devonica (Arber and Goode), an enigmatic early seed plant cupule bearing preovules. In: Kurmann MH, Hemsley AR, editors. The evolution of plant architecture. Kew: Royal Botanical Gardens; 1999. pp. 75–90. [Google Scholar]
- Hilton J, Wang S-J, Tian B. Reinvestigation of Cardiocarpus minor (Wang) Li nomen nudum from the Lower Permian of China and its implications for seed plant taxonomy, systematics and phylogeny. Botanical Journal of the Linnean Society. 2003;141:151–175. [Google Scholar]
- Hofmeister W. Die Entstehung des Embryos der Phanerogamen. Leipzig: Hofmeister; 1849. [Google Scholar]
- Hülskamp M, Schneitz K, Pruitt RE. Genetic evidence for a long-range activity that directs pollen tube guidance in Arabidopsis. The Plant Cell. 1995;7:57–64. doi: 10.1105/tpc.7.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igersheim A, Endress PK. Gynoecium diversity and systematics of the Magnoliales and winteroids. Botanical Journal of the Linnean Society. 1997;124:213–271. [Google Scholar]
- Igersheim A, Endress PK. Gynoecium diversity and systematics of the paleoherbs. Botanical Journal of the Linnean Society. 1998;127:289–370. [Google Scholar]
- Igersheim A, Buzgo M, Endress PK. Gynoecium diversity and systematics of the basal monocots. Botanical Journal of the Linnean Society. 2001;136:1–65. [Google Scholar]
- Imaichi R, Kato M. A scanning electron microscopic study of ovule development in Dillenia suffruticosa (Dilleniaceae) Phytomorphology. 1996;46:45–51. [Google Scholar]
- Imaichi R, Kato M, Okada H. Morphology of the outer integument in three primitive angiosperm families. Canadian Journal of Botany. 1995;73:1242–1249. [Google Scholar]
- Jensen WA. Reproduction in flowering plants. In: Robards AW, editor. Dynamic aspects of plant ultrastructure. London: McGraw-Hill; 1974. pp. 481–503. [Google Scholar]
- Johri BM. Studies in the family Alismaceae. III. Sagittaria guyanensis H.B.K. and S. latifolia Willd. Proceedings of the Indian Academy of Sciences. 1935;2:33–48. [Google Scholar]
- Johri BM. Embryology and taxonomy. In: Maheshwari P, editor. Recent advances in the embryology of angiosperms. Delhi: International Society of Plant Morphologists, Department of Botany, University of Delhi; 1963. pp. 395–444. [Google Scholar]
- Johri BM, editor. Symposium on comparative embryology of angiosperms. New Delhi: Indian National Science Academy; 1967. [Google Scholar]
- Johri BM, editor. Embryology of angiosperms. Berlin: Springer; 1984. [Google Scholar]
- Johri BM, Ahuja MR. A contribution to the floral morphology and embryology of Aegle marmelos Correa. Phytomorphology. 1957;7:10–24. [Google Scholar]
- Johri BM, Ambegoakar KB, Srivastava PS. Comparative embryology of angiosperms 1,2. Berlin: Springer; 1992. [Google Scholar]
- Juel HO. Beiträge zur Blütenanatomie und zur Systematik der Rosaceen. Kungliga Svenska Vetenskapsakademiens Handlinger. 1918;58(5):1–81. [Google Scholar]
- Kamelina OP. Sonneratiaceae. In: Batygina TB, Yakovlev MS, editors. Comparative embryology of flowering plants – Brunelliaceae–Tremandraceae. Leningrad: Nauka; 1985. pp. 88–93. [Google Scholar]
- Kapil RN, Bhatnagar AK. Embryological evidence in angiosperm classification and phylogeny. Botanische Jahrbücher für Systematik. 1991;113:309–338. [Google Scholar]
- Kapil RN, Jalan S. Studies in the family Ranunculaceae: I. The embryology of Caltha palustris L. In: Maheshwari P, editor. Plant embryology. A symposium. New Delhi: CSIR; 1962. pp. 205–214. [Google Scholar]
- Kapil RN, Tiwari SC. The integumentary tapetum. Botanical Review. 1978;44:457–490. [Google Scholar]
- Kapil RN, Vani RS. Embryology and systematic position of Crossosoma californicum Nutt. Current Science. 1963;32:493–495. [Google Scholar]
- Kapil RN, Vasil IK. Ovule. In: Maheshwari P, editor. Recent advances in the embryology of angiosperms. Delhi: International Society of Plant Morphologists; 1963. pp. 41–67. [Google Scholar]
- Kaplan DR. Histogenesis of the androecium and gynoecium in Downingia bacigalupii. American Journal of Botany. 1968;55:933–950. [Google Scholar]
- Kato M, Imaichi R. Morphology and origin of the outer integument. Proceedings of the Japanese Society of Plant Taxonomists. 1993;9:67–72. [Google Scholar]
- Kelley DR, Gasser CS. Ovule development: genetic trends and evolutionary considerations. Sexual Plant Reproduction. 2009;22:229–234. doi: 10.1007/s00497-009-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DR, Skinner DJ, Gasser CS. Roles of polarity determinants in ovule development. Plant Journal 57. 2009:1054–1064. doi: 10.1111/j.1365-313X.2008.03752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenrick P, Crane PR. The origin and early diversification of land plants. A cladistic study. Washington, DC: Smithsonian Institution Press; 1997. [Google Scholar]
- Khan R. A contribution to the embryology of Jussieua repens Linn. Journal of the Indian Botanical Society. 1942;21:267–282. [Google Scholar]
- Kim S, Mollet J-C, Dong J, Zhang K, Park S-Y, Lord EM. Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proceedings of the National Academy of Sciences, USA. 2003;100:16125–16130. doi: 10.1073/pnas.2533800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavins SD, Taylor EL, Krings M, Taylor TN. An unusual, structurally preserved ovule from the Permian of Antarctica. Review of Palaeobotany and Palynology. 2001;115:107–117. doi: 10.1016/s0034-6667(01)00052-5. [DOI] [PubMed] [Google Scholar]
- Klavins SD, Taylor TN, Taylor EL. Anatomy of Umkomasia (Corystospermales) from the Triassic of Antarctica. American Journal of Botany. 2002;89:664–676. doi: 10.3732/ajb.89.4.664. [DOI] [PubMed] [Google Scholar]
- Kramer EM, Jaramillo MA, Di Stilio VS. Patterns of gene duplication and functional evolution during the diversification of the AGAMOUS subfamily of MADS box genes in angiosperms. Genetics. 2004;166:1011–1023. doi: 10.1534/genetics.166.2.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn G. Beiträge zur Kenntnis der intraseminalen Leitbündel bei den Angiospermen. Botanische Jahrbücher für Systematik. 1928;61:325–379. [Google Scholar]
- Lakshmi PS, Kumari KN, Pullaiah T. Embryology of Macroptilium (Fabaceae) Phytomorphology. 1987;37:201–207. [Google Scholar]
- Leins P, Schwitalla S. Studien an Cactaceen-Blüten I. Einige Bemerkungen zur Blütenentwicklung von Pereskia. Beiträge zur Biologie der Pflanzen. 1985;60:313–323. [Google Scholar]
- Léon-Kloosterziel KM, Keijzer CJ, Koorneef M. A seed shape mutant of Arabidopsis that is affected in integument development. The Plant Cell. 1994;6:385–392. doi: 10.1105/tpc.6.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-S, Hilton J, Hemsley AR. Frasnian (Upper Devonian) evidence for multiple origins of seed-like structures. Botanical Journal of the Linnean Society. 1997;123:133–146. [Google Scholar]
- Long AG. On the structure of Calymmatotheca kidstoni (emended) and Genomosperma latens gen. et sp. nov. from the Calciferous Sandstone Series of Berwickshire. Transactions of the Royal Society of Edinburgh. 1960;64:29–48. [Google Scholar]
- Long AG. Some Lower Carboniferous fructifications from Berwickshire, together with a theoretical account of the evolution of ovules, cupules, and carpels. Transactions of the Royal Society of Edinburgh. 1967;66:345–375. [Google Scholar]
- Long AG. Observations on Carboniferous seeds of Mitrospermum, Conostoma and Lagenostoma. Transactions of the Royal Society of Edinburgh. 1977;70:37–61. [Google Scholar]
- Lora J, Hormaza JI, Herrero M, Gasser CS. Seedless fruits and the disruption of a conserved genetic pathway in angiosperm ovule development. Proceedings of the National Academy of Sciences, USA. 2011;108:5461–5465. doi: 10.1073/pnas.1014514108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losa A, Colombo M, Brambilla V, Colombo L. Genetic interaction between AINTEGUMENTA (ANT) and the ovule identity genes SEEDSTICK (STK), SHATTERPROOF1 (SHP1) and SHATTERPROOF2 (SHP2) Sexual Plant Reproduction. 2010;23:115–121. doi: 10.1007/s00497-009-0130-3. [DOI] [PubMed] [Google Scholar]
- Luza JG, Polito VS. Porogamy and chalazogamy in walnut (Juglans regia L.) Botanical Gazette. 1991;152:100–106. [Google Scholar]
- Maheshwari P. An introduction to the embryology of angiosperms. New York: McGraw-Hill; 1950. [Google Scholar]
- Maheshwari P. Embryology in relation to taxonomy. In: Turrill WB, editor. Vistas in botany IV. Oxford: Pergamon Press; 1963. pp. 55–97. [Google Scholar]
- Matsui M, Imaichi R, Kato M. Ovular development and morphology in some Magnoliaceae species. Journal of Plant Research. 1993;106:297–304. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure in Oxalidales (Oxalidaceae, Connaraceae, Cephalotaceae, Brunelliaceae, Cunoniaceae, Elaeocarpaceae, Tremandraceae) Botanical Journal of the Linnean Society. 2002;140:321–381. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Cucurbitales (Corynocarpaceae, Coriariaceae, Datiscaceae, Tetramelaceae, Begoniaceae, Cucurbitaceae, Anisophylleaceae) Botanical Journal of the Linnean Society. 2004;145:129–185. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Celastrales (Celastraceae, Parnassiaceae, Lepidobotryaceae) Botanical Journal of the Linnean Society. 2005a;149:129–194. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Crossosomatales (Crossosomataceae, Stachyuraceae, Staphyleaceae, Aphloiaceae, Geissolomataceae, Ixerbaceae, Strasburgeriaceae) Botanical Journal of the Linnean Society. 2005b;147:1–46. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Chrysobalanaceae s.l. (Chrysobalanaceae, Dichapetalaceae, Euphroniaceae, Trigoniaceae; Malpighiales) Botanical Journal of the Linnean Society. 2008;157:249–309. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Rhizophoraceae, Erythroxylaceae, and the potentially related Ctenolophonaceae, Linaceae, Irvingiaceae, and Caryocaraceae (Malpighiales) Botanical Journal of the Linnean Society. 2011 (in press) [Google Scholar]
- Mauritzon J. Studien über die Embryologie der Familien Crassulaceae und Saxifragaceae. Lund: Ohlsson; 1933. Doctoral dissertation, University of Lund. [Google Scholar]
- Mauritzon J. Zur Embryologie der Elaeocarpaceae. Arkiv för Botanik. 1934;26A(10):1–8. [Google Scholar]
- Mauritzon J. Zur Embryologie der Berberidaceen. Acta Horti Gothoburgensis. 1938;11:1–18. [Google Scholar]
- Mauritzon J. Contributions to the embryology of the orders Rosales and Myrtales. Lunds Universitets Arsskrift, n.F., Avd. 2. 1939a;35(2):1–121. [Google Scholar]
- Mauritzon J. Die Bedeutung der embryologischen Forschung für das natürliche System der Pflanzen. Lunds Universitets Arsskrift, n.F., Avd. 2. 1939b;35(15):1–70. [Google Scholar]
- Mayo SJ, Bogner J, Boyce PC. The genera of Araceae. Kew: Royal Botanic Gardens; 1997. [Google Scholar]
- McAbee JM, Kuzoff RK, Gasser CS. Mechanisms of derived unitegmy among Impatiens species. The Plant Cell. 2005;17:1674–1684. doi: 10.1105/tpc.104.029207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAbee JM, Hill TA, Skinner DJ, et al. ABERRANT TESTA SHAPE encodes a KANADI family member, linking polarity determination to separation and growth of Arabidopsis ovule integuments. The Plant Journal. 2006;46:522–532. doi: 10.1111/j.1365-313X.2006.02717.x. [DOI] [PubMed] [Google Scholar]
- McCormick S, Yang H. Is there more than one way to attract a pollen tube? Trends in Plant Science. 2005;10:260–263. doi: 10.1016/j.tplants.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Meeuse ADJ, Bouman F. The inner integument – its probable origin and homology. Acta Botanica Neerlandica. 1974;23:237–249. [Google Scholar]
- Merino Sutter D, Forster PI, Endress PK. Female flowers and systematic position of Picrodendraceae (Euphorbiaceae s.l., Malpighiales) Plant Systematics and Evolution. 2006;261:187–215. [Google Scholar]
- Meyen SV. Gymnosperm fructifications and their evolution as evidenced by palaeobotany. Zhurnal Obshchei Biologii. 1982;43:303–323. [Google Scholar]
- Mill RR, Möller M, Christie F, Glidewell SM, Masson D, Williamson B. Morphology, anatomy and ontogeny of female cones in Acmopyle pancheri (Brongn. & Gris) Pilg. (Podocarpaceae) Annals of Botany. 2001;88:55–67. [Google Scholar]
- Mirbel CFB. Nouvelles recherches sur la structure et les développemens de l'ovule végétale. Annales des Sciences Naturelles. 1829;17:302–318. [Google Scholar]
- Nandi OI. Ovule and seed anatomy of Cistaceae and related Malvanae. Plant Systematics and Evolution. 1998;209:239–264. [Google Scholar]
- Narayana HS. Studies in Capparidaceae. II. Floral morphology and embryology of Cadaba indica Lamk. and Crataeva nurvala Buch.-Ham. Phytomorphology. 1965;15:158–175. [Google Scholar]
- Narayanaswami S, Roy SK. Embryology of the genus Psidium. Journal of the Indian Botanical Society. 1960;39:35–45. [Google Scholar]
- Nepi M, von Aderkas P, Wagner R, Mugnaini S, Coulter A, Pacini E. Nectar and pollination drops: how different are they? Annals of Botany. 2009;104:205–219. doi: 10.1093/aob/mcp124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas KJ. Airflow patterns around some early seed plant ovules and cupules: implications concerning efficiency in wind pollination. American Journal of Botany. 1981a;68:635–650. [Google Scholar]
- Niklas KJ. Simulated wind pollination and airflow around ovules of some early seed plants. Science. 1981b;211:275–277. doi: 10.1126/science.211.4479.275. [DOI] [PubMed] [Google Scholar]
- Nishida H, Pigg KB, Rigby JF. Swimming sperm in an extinct Gondwanan plant. Nature. 2003;422:396–397. doi: 10.1038/422396a. [DOI] [PubMed] [Google Scholar]
- Nole-Wilson S, Rueschhoff EE, Bhatti H, Franks RG. Synergistic disruptions in seuss cyp85A2 double mutants reveal a role for brassinolide synthesis during gynoecium and ovule development. BMC Plant Biology. 2010;10:198. doi: 10.1186/1471-2229-10-198. doi:10.1186/1471-2229-10-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M. Centrifugal ovule inception I. Sequence of ovule inception in Silene cucubalus. Botanical Magazine Tokyo. 1984;97:345–353. [Google Scholar]
- Olesen P, Bruun L. A structural investigation of the ovule in sugar beet, Beta vulgaris: integuments and micropyle. Nordic Journal of Botany. 1990;9:499–506. [Google Scholar]
- Palanivelu R, Brass L, Edlund AF, Preuss D. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell. 2003;114:47–59. doi: 10.1016/s0092-8674(03)00479-3. [DOI] [PubMed] [Google Scholar]
- Panchaksharappa MG. Embryological studies in some members of the Zingiberaceae. In: Maheshwari P, editor. Plant embryology, a symposium. New Delhi: Council of Scientific and Industrial Research; 1962. pp. 224–238. [Google Scholar]
- Park SO, Hwang S, Hauser BA. The phenotype of Arabidopsis ovule mutants mimics the morphology of primitive seed plants. Proceedings of the Royal Society B: Biological Sciences. 2004;271:311–316. doi: 10.1098/rspb.2003.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SO, Zeng Z, Oppenheimer DG, Hauser BA. The PRETTY FEW SEEDS2 gene encodes an Arabidopsis homeodomain protein that regulates ovule development. Development. 2005;132:841–849. doi: 10.1242/dev.01654. [DOI] [PubMed] [Google Scholar]
- Payer J-B. Traité d'organogénie comparée de la fleur. Paris: Masson; 1857. [Google Scholar]
- Pescador R, Barbante-Kerbauy G, Constantino-Strassburg R, Kraus JE. Structural aspects of the zygotic embryogenesis of Acca sellowiana (O. Berg) Burret (Myrtaceae) Acta Botanica Brasilica. 2009;23:136–144. [Google Scholar]
- Philipson WR. Ovular morphology and the major classification of the dicotyledons. Botanical Journal of the Linnean Society. 1974;68:89–108. [Google Scholar]
- Philipson WR. Ovular morphology and the classification of dicotyledons. Plant Systematics and Evolution Supplement. 1977;1:123–140. [Google Scholar]
- Pinyopich A, Ditta GS, Savidge B, et al. Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature. 2003;424:85–88. doi: 10.1038/nature01741. [DOI] [PubMed] [Google Scholar]
- Poort RJ, Visscher H, Dilcher DL. Zoidogamy [sic!] in fossil gymnosperms: the centenary of a concept, with special reference to prepollen of late Paleozoic conifers. Proceedings of the National Academy of Sciences, USA. 1996;93:11713–11717. doi: 10.1073/pnas.93.21.11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash N. A contribution to the life history of Angophora floribunda (Sm.) Sweet (Myrtaceae) Australian Journal of Botany. 1969;17:457–469. [Google Scholar]
- Prakash N, Chan YY. Embryology of Glycine max. Phytomorphology. 1976;26:302–309. [Google Scholar]
- Prasad K. Studies in the Cruciferae. Gametophytes, structure and development of seed in Eruca sativa Mill. Journal of the Indian Botanical Society. 1974;53:24–33. [Google Scholar]
- Puri V. The angiosperm ovule. Proceedings of the Indian Science Congress, Section of Botany. 1970;57:1–36. [Google Scholar]
- Raju MVS. Embryology of Sabiaceae. Current Science. 1952;21:107–108. [Google Scholar]
- Ramji MV, Padmanabhan D. Developmental studies on Cabomba caroliniana Gray. I. Ovule and carpel. Proceedings of the Indian Academy of Sciences B. 1965;62:215–223. [Google Scholar]
- Rangan TS, Rangaswamy NS. Nucellus – a unique embryologic system. Phytomorphology. 1999;49:337–376. [Google Scholar]
- Rao AMS. Studies in the Malpighiaceae I. Embryo-sac development and embryogeny in the genera Hiptage, Banisteria and Stigmatophyllum. Journal of the Indian Botanical Society. 1940;18:145–156. [Google Scholar]
- Rao N. A contribution to the embryology of Dilleniaceae. Proceedings of the Iowa Academy of Science. 1957;64:172–176. [Google Scholar]
- Rau MA. The endosperm in some of the Papilionaceae. Phytomorphology. 1951;1:153–158. [Google Scholar]
- Reinheimer R, Kellogg EA. Evolution of AGL6-like MADS box genes in grasses (Poaceae): ovule expression is ancient and palea expression is new. The Plant Cell. 2009;21:2591–2605. doi: 10.1105/tpc.109.068239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Chang H-L, Endress PK. Floral development in Anemoneae (Ranunculaceae) Botanical Journal of the Linnean Society. 2010;162:77–100. [Google Scholar]
- Renner SS, Schwarzbach AE, Lohmann L. Phylogenetic position and floral function of Siparuna (Siparunaceae: Laurales) International Journal of Plant Sciences. 1997;158:S89–S98. [Google Scholar]
- Retallack GJ, Dilcher DL. Reconstructions of selected seed ferns. Annals of the Missouri Botanical Garden. 1988;75:1010–1057. [Google Scholar]
- Robertson BL. Embryology of Jubaeopsis caffra Becc.: 2. Megasporangium, megasporogenesis and megagametogenesis. Journal of South African Botany. 1976;42:173–184. [Google Scholar]
- Robinson-Beers K, Pruitt RE, Gasser CS. Ovule development in wild-type Arabidopsis and two female-sterile mutants. The Plant Cell. 1992;4:1237–1249. doi: 10.1105/tpc.4.10.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronse De Craene LP. Floral development of Berberidopsis corallina: a crucial link in the evolution of flowers in the core eudicots. Annals of Botany. 2004;94:741–751. doi: 10.1093/aob/mch199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronse Decraene LP, Smets EF. The floral development and anatomy of Carica papaya (Caricaceae) Canadian Journal of Botany. 1999;77:582–598. [Google Scholar]
- Rothwell GW. Evidence for a pollination drop mechanism in Paleozoic pteridosperms. Science. 1977;198:1251–1252. doi: 10.1126/science.198.4323.1251. [DOI] [PubMed] [Google Scholar]
- Rothwell GW. Classifying the earliest gymnosperms. In: Spicer RA, Thomas BA, editors. Systematic and taxonomic approaches in palaeobotany. Oxford: Clarendon Press; 1986. pp. 137–162. [Google Scholar]
- Rothwell GW, Scheckler SE. Biology of ancestral gymnosperms. In: Beck CB, editor. Origin and evolution of gymnosperms. New York: Columbia University Press; 1988. pp. 85–134. [Google Scholar]
- Rothwell GW, Serbet R. Pollination biology of Elkinsia polymorpha, implications for the origin of the gymnosperms. Courier Forschungs-Institut Senckenberg. 1992;147:225–231. [Google Scholar]
- Rothwell GW, Serbet R. Lignophyte phylogeny and the evolution of spermatophytes: a numerical cladistic analysis. Systematic Botany. 1994;19:443–482. [Google Scholar]
- Rothwell GW, Scheckler SE, Gillespie WH. Elkinsia gen. nov., a Late Devonian gymnosperm with cupulate ovules. Botanical Gazette. 1989;150:170–189. [Google Scholar]
- Rothwell GW, Crepet WL, Stockey RA. Is the anthophyte hypothesis alive and well? New evidence from the reproductive structures of Bennettitales. American Journal of Botany. 2009;96:296–322. doi: 10.3732/ajb.0800209. [DOI] [PubMed] [Google Scholar]
- Rowe NP. Winged Late Devonian seeds. Nature. 1992;359(682) [Google Scholar]
- Rowe NP. Late Devonian winged preovules and their implications for the adaptive radiation of early seed plants. Palaeontology. 1997;40:575–595. [Google Scholar]
- Rübsamen T. Morphologische, embryologische und systematische Untersuchungen an Burmanniaceae und Corsiaceae (Mit Ausblick auf die Orchidaceae-Apostasioideae) Dissertationes Botanicae. 1986;92:1–310. [Google Scholar]
- Rudall J. The nucellus and chalaza in monocotyledons: structure and systematics. Botanical Review. 1997;63:140–181. [Google Scholar]
- Rudall PJ, Furness CA. Systematics of Acorus: ovule and anther. International Journal of Plant Sciences. 1997;158:640–651. [Google Scholar]
- Rudall PJ, Remizowa MV, Beer AS, et al. Comparative ovule and megagametophyte development in Hydatellaceae and water lilies reveal a mosaic of features among the earliest angiosperms. Annals of Botany. 2008;101:941–956. doi: 10.1093/aob/mcn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydin C, Friis EM. A new Early Cretaceous relative of Gnetales: Siphonospermum simplex gen. et sp. nov. from the Yixian Formation of Northeast China. BMC Evolutionary Biology. 2010;10:183. doi: 10.1186/1471-2148-10-183. doi:10.1186/1471-2148-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydin C, Khodabandeh A, Endress PK. The female reproductive unit of Ephedra (Gnetales): comparative morphology and evolutionary perspectives. Botanical Journal of the Linnean Society. 2010;163:387–430. doi: 10.1111/j.1095-8339.2010.01066.x. [DOI] [PubMed] [Google Scholar]
- Sachar RC, Mohan Ram HY. The embryology of Eschscholzia californica Cham. Phytomorphology. 1958;8:114–124. [Google Scholar]
- Sastri RLN. Floral morphology and embryology of some Dilleniaceae. Botaniska Notiser. 1958;111:495–511. [Google Scholar]
- Sattler R. Organogenesis of flowers. A photographic text-atlas. Toronto: University of Toronto Press; 1973. [Google Scholar]
- Sauquet H, Doyle JA, Scharaschkin T, et al. Phylogenetic analysis of Magnoliales and Myristicaceae based on multiple data sets: implications for character evolution. Botanical Journal of the Linnean Society. 2003;142:125–186. [Google Scholar]
- Saxena NP. Studies in the family Saxifragaceae. I. A contribution to the morphology and embryology of Saxifraga diversifolia Wall. Proceedings of the Indian Academy of Sciences B. 1964a;60:38–51. [Google Scholar]
- Saxena NP. Studies in the family Saxifragaceae II. Development of ovule and megagametophyte in Parnassia nubicola Wall. Proceedings of the Indian Academy of Sciences B. 1964b;60:196–202. [Google Scholar]
- Saxena NP. Studies in the family Saxifragaceae IV. A contribution to the embryology of Bergenia ciliata (Royle) Raizada. Proceedings of the Indian Academy of Sciences B. 1969;70:104–110. [Google Scholar]
- Schleiden MJ. Über Bildung des Eichens und Entstehung des Embryo's bei den Phanerogamen. Nova Acta Leopoldina. 1839;19(1):27–58. [Google Scholar]
- Schmid R. On Cornerian and other terminology of angiospermous and gymnospermous seed coats: historical perspective and terminological recommendations. Taxon. 1986;35:476–491. [Google Scholar]
- Schnarf K. Embryologie der Angiospermen. Berlin: Borntraeger; 1929. [Google Scholar]
- Schnarf K. Vergleichende Embryologie der Angiospermen. Berlin: Borntraeger; 1931. [Google Scholar]
- Schnarf K. Die Bedeutung der embryologischen Forschung für das natürliche System der Pflanzen. Biologia Generalis. 1933;9:271–288. [Google Scholar]
- Schneider EL. Morphological studies on the Nymphaeaceae. IX. The seed of Barclaya longifolia Wall. Botanical Gazette. 1978;139:223–230. [Google Scholar]
- Schneitz K. The molecular and genetic control of ovule development. Current Opinion in Plant Biology. 1999;2:13–17. doi: 10.1016/s1369-5266(99)80003-x. [DOI] [PubMed] [Google Scholar]
- Schneitz K, Hülskamp M, Pruitt RE. Wild-type ovule development in Arabidopsis thaliana: a light microscope study of cleared whole-mount tissue. The Plant Journal. 1995;7:731–749. [Google Scholar]
- Schneitz K, Hülskamp M, Kopczak SD, Pruitt RE. Dissection of sexual organ ontogenesis: a genetic analysis of ovule development in Arabidopsis thaliana. Development. 1997;124:1367–1376. doi: 10.1242/dev.124.7.1367. [DOI] [PubMed] [Google Scholar]
- Schneitz K, Balasubramanian S, Schiefthaler U. Organogenesis in plants: the molecular and genetic control of ovule development. Trends in Plant Science. 1998a;3:468–472. [Google Scholar]
- Schneitz K, Baker SC, Gasser CS, Redweijk A. Pattern formation and growth during floral organogenesis: HUELLENLOS and AINTEGUMENTA are required for the formation of the proximal region of the ovule primordium in Arabidopsis thaliana. Development. 1998b;125:2555–2563. doi: 10.1242/dev.125.14.2555. [DOI] [PubMed] [Google Scholar]
- Sedgley M. Control by the embryo sac over pollen tube growth in the style of the avocado (Persea americana Mill.) New Phytologist. 1976;77:149–152. [Google Scholar]
- Serbet R, Rothwell GW. Functional morphology and homologies of gymnospermous ovules: evidence from a new species of Stephanospermum (Medullosales) Canadian Journal of Botany. 1995;73:650–661. [Google Scholar]
- Sessions RA. Arabidopsis (Brassicaceae) flower development and gynoecium patterning in wild type and ettin mutants. American Journal of Botany. 1997;84:1179–1191. [PubMed] [Google Scholar]
- Shamrov II. The ovule of Gentiana cruciata (Gentianaceae): structural–functional aspects of development. Botanicheskij Zhurnal (Moscow & Leningrad) 1990;75:1363–1379. [Google Scholar]
- Shamrov II. Nucellus typification and ovule classification. Bulletin of the Polish Academy of Sciences, Biological Sciences. 1997;45:65–74. [Google Scholar]
- Shamrov II. Ovule classification in flowering plants – new approaches and concepts. Botanische Jahrbücher für Systematik. 1998;120:377–407. [Google Scholar]
- Shamrov II. The integument of flowering plants: developmental patterns and evolutionary trends. Acta Biologica Cracoviensia, Series Botanica. 2000;42/2:9–20. [Google Scholar]
- Shamrov II. Ovule and seed morphogenesis in Capsella bursa-pastoris (Brassicaceae) in connection with peculiar mode of endothelium formation. Botanicheskij Zhurnal (St. Petersburg) 87. 2002a;(2):1–18. [Google Scholar]
- Shamrov II. Ovule nucellus: its origin, differentiation, structure and functions. Botaniceskji Zhurnal (St. Petersburg) 2002b;87(10):1–30. [Google Scholar]
- Shamrov II. The integument of flowering plants: origin, differentiation, structure and functions. Botanicheskij Zhurnal (St. Petersburg) 2003;88(6):1–30. [Google Scholar]
- Shamrov II. Morphological nature of ovule and its evolutionary lineages in flowering plants. Botanicheskij Zhurnal (St. Petersburg) 2006;91:1601–1635. [Google Scholar]
- Shamrov II, Anisimova GM, Batygina TB, Sita GL. The types and morphological evolution of the ovule in the order Santalales. Botanicheskij Zhurnal (St. Petersburg) 2001;86 (7):1–14. [Google Scholar]
- Shimizu KK. Ecology meets molecular genetics in Arabidopsis. Population Ecology. 2002;44:221–233. [Google Scholar]
- Shimizu KK, Okada K. Attractive and repulsive interactions between female and male gametophytes in Arabidopsis pollen tube guidance. Development. 2000;127:4511–4518. doi: 10.1242/dev.127.20.4511. [DOI] [PubMed] [Google Scholar]
- Sieber P, Gheyselinck J, Gross-Hardt R, Laux T, Grossniklaus U, Schneitz K. Pattern formation during early ovule development in Arabidopsis thaliana. Developmental Biology. 2004;273:321–334. doi: 10.1016/j.ydbio.2004.05.037. [DOI] [PubMed] [Google Scholar]
- Singh B. The structure and development of Abelmoschus moschatus Medic. seed. Phytomorphology. 1967;17:282–299. [Google Scholar]
- Singh B, Shivapuri TN. The gametophytes of Neptunia oleracea Lour. Proceedings of the Indian Academy of Sciences B. 1935;1:423–434. [Google Scholar]
- Singh H. Embryology of gymnosperms. Berlin: Borntraeger; 1978. [Google Scholar]
- Singh RP, Pal A. Structure and development of seeds in Euphorbiaceae: Dalechampia roezliana Muell.-Arg. National Botanic Gardens Lucknow, Technical Communication. 1968;1968:65–74. [Google Scholar]
- Skinner DJ, Gasser CS. Expression-based discovery of candidate ovule development regulators through transcriptional profiling of ovule mutants. BMC Plant Biology. 2009;9(29) doi: 10.1186/1471-2229-9-29. doi:10.1186/1471-2229-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL. The evolution of the ovule. Biological Review. 1964;39:137–159. [Google Scholar]
- Smyth DR. Plant development: attractive ovules. Current Biology. 1997;7:R64–R66. doi: 10.1016/s0960-9822(06)00037-6. [DOI] [PubMed] [Google Scholar]
- Sogo A, Tobe H. Delayed fertilization and pollen-tube growth in pistils of Fagus japonica (Fagaceae) American Journal of Botany. 2006a;93:1748–1756. doi: 10.3732/ajb.93.12.1748. [DOI] [PubMed] [Google Scholar]
- Sogo A, Tobe H. Mode of pollen tube growth in pistils of Eucommia ulmoides (Eucommiaceae, Garryales) International Journal of Plant Sciences. 2006b;167:933–941. [Google Scholar]
- Sogo A, Tobe H. The evolution of fertilization modes independent of the micropyle in Fagales and ‘pseudoporogamy’. Plant Systematics and Evolution. 2006c;259:73–80. [Google Scholar]
- Sogo A, Tobe H. Mode of pollen tube growth in pistils of Ticodendron incognitum (Ticodendraceae, Fagales) and the evolution of chalazogamy. Botanical Journal of the Linnean Society. 2008;157:621–631. [Google Scholar]
- Sogo A, Noguchi J, Jaffre T, Tobe H. Pollen-tube growth pattern and chalazogamy in Casuarina equisetifolia (Casuarinaceae) Journal of Plant Research. 2004;117:37–46. doi: 10.1007/s10265-003-0129-z. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. Flowering plants. Evolution above the species level. Cambridge, MA: Belknap Press of Harvard University Press; 1974. [Google Scholar]
- Stebbins GL. Seeds, seedlings and the origin of the angiosperms. In: Beck CB, editor. Origin and early evolution of angiosperms. New York: Columbia University Press; 1976. pp. 300–311. [Google Scholar]
- Stewart WN, Rothwell GW. Paleobotany and the evolution of plants. 2nd edn. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Steyn EAM, Smith GF. Ovule orientation, curvature and internal structure in the Aloaceae. South African Journal of Botany. 1998;64:192–197. [Google Scholar]
- Stockey RA, Rothwell GW. Anatomically preserved Williamsonia (Williamsoniaceae): evidence for bennettitalean reproduction in the Late Cretaceous of Western North America. International Journal of Plant Science. 2003;164:251–262. [Google Scholar]
- Stockey RA, Rothwell GW. Distinguishing angiophytes from the earliest angiosperms: a Lower Cretaceous (Valanginian-Hauterivian) fruit-like reproductive structure. American Journal of Botany. 2009;96:323–335. doi: 10.3732/ajb.0800295. [DOI] [PubMed] [Google Scholar]
- Stützel T, Röwekamp I. Bestäubungsbiologie bei Nacktsamern. Der Palmengarten. 1997;61(2):100–109. [Google Scholar]
- Sundberg MD. Floral ontogeny in Cyclamen persicum ‘F-1 Rosemunde Rose’ (Primulaceae) American Journal of Botany. 1982;69:380–388. [Google Scholar]
- Sutter D, Endress PK. Aspects of gynoecium structure and macrosystematics in Euphorbiaceae. Botanische Jahrbücher für Systematik. 1995;116:517–536. [Google Scholar]
- Svedelius N. Om fröbyggnaden hos släktena Wormia och Dillenia. Ett bidrag till Dilleniacéernas morfologi. Svensk Botanisk Tidskrift. 1911;5:152–173. [Google Scholar]
- Swamy BGL, Periasamy K. Contributions to the embryology of Acrotrema arnottianum. Phytomorphology. 1955;5:301–314. [Google Scholar]
- Takaso T, Bouman F. Ovule and seed ontogeny in Gnetum gnemon L. Botanical Magazine Tokyo. 1986;99:241–266. [Google Scholar]
- Takaso T, Owens JN. Postpollination–prezygotic ovular secretions into the micropylar canal in Pseudotsuga menziesii (Pinaceae) Journal of Plant Research. 1996;109:147–160. [Google Scholar]
- Takaso T, von Aderkas P, Owens JN. Prefertilization events in ovules of Pseudotsuga: ovular secretion and its influence on pollen tubes. Canadian Journal of Botany. 1996;74:1214–1219. [Google Scholar]
- Taylor DW. Angiosperm ovules and carpels: their character and polarities, distribution in basal clades, and structural evolution. Postilla. 1991;208:1–40. [Google Scholar]
- Taylor EL, Taylor TN. Seed ferns from the late Paleozoic and Mesozoic: any angiosperm ancestors lurking there? American Journal of Botany. 2009;96:237–251. doi: 10.3732/ajb.0800202. [DOI] [PubMed] [Google Scholar]
- Taylor EL, Taylor TN, Kerp H, Hermsen EJ. Mesozoic seed ferns: old paradigms, new discoveries. Journal of the Torrey Botanical Society. 2006;133:62–82. [Google Scholar]
- Taylor TN. Palaeozoic seed studies: a monograph of the American species of Pachytesta. Palaeontographica B. 1965;117:1–46. [Google Scholar]
- Taylor TN, Archangelsky S. The Cretaceous pteridosperms Ruflorinia and Ktalenia and implications on cupule and carpel evolution. American Journal of Botany. 1985;72:1842–1853. [Google Scholar]
- Taylor TN, Taylor EL. The biology and evolution of fossil plants. Englewood Cliffs, NJ: Prentice Hall; 1993. [Google Scholar]
- Taylor TN, Del Fueyo GM, Taylor EL. Permineralized seed fern cupules from the Triassic of Antarctica: implications for cupule and carpel evolution. American Journal of Botany. 1994;81:666–677. [Google Scholar]
- Taylor TN, Taylor EL, Krings M. Palaeobotany. The biology and evolution of fossil plants. Amsterdam: Academic Press; 2009. [Google Scholar]
- Teryokhin ES. Rafflesiaceae. In: Yakovlev MS, editor. Comparative embryology of flowering plants: Winteraceae-Junglandaceae. Leningrad: Nauka; 1981. pp. 96–100. [Google Scholar]
- van Tieghem P. Structure de quelques ovules et parti qu'on peut tirer pour améliorer la classification. Journal de Botanique. 1898;12:197–220. [Google Scholar]
- van Tieghem P. L'oeuf des plantes considéré comme base de leur classification. Annales des Sciences Naturelles, Botanique, Sér. 8. 1901;14:213–390. [Google Scholar]
- Tilton VR, Lersten NR. Ovule development in Ornithogalum caudatum (Liliaceae) with a review of selected papers on angiosperm reproduction. I. Integuments, funiculus, and vascular tissue. New Phytologist. 1981a;88:439–457. [Google Scholar]
- Tilton VR, Lersten NR. Ovule development in Ornithogalum caudatum (Liliaceae) with a review of selected papers on angiosperm reproduction. III. Nucellus and megagametophyte. New Phytologist. 1981b;88:477–504. [Google Scholar]
- Tilton VR, Lersten NR. Ovule development in Ornithogalum caudatum (Liliaceae) with a review of selected papers on angiosperm reproduction. IV. Egg apparatus structure and function. New Phytologist. 1981c;88:505–531. [Google Scholar]
- Tobe H. The embryology of angiosperms: its broad application to the systematic and evolutionary study. Botanical Magazine Tokyo. 1989;102:351–367. [Google Scholar]
- Tobe H, Jaffré T, Raven PH. Embryology of Amborella (Amborellaceae): Descriptions and polarity of character states. Journal of Plant Research. 2000;111:271–280. [Google Scholar]
- Tobe H, Stuessy TF, Raven PH, Oginuma K. Embryology and karyomorphology of Lactoridaceae. American Journal of Botany. 1993;80:933–946. [Google Scholar]
- Tokuoka T, Tobe T. Ovules and seeds in Acalyphoideae (Euphorbiaceae): structure and systematic implications. Journal of Plant Research. 2003;116:355–380. doi: 10.1007/s10265-003-0116-4. [DOI] [PubMed] [Google Scholar]
- Tomlinson PB. Pollen scavenging. National Geographic Research and Exploration. 1991;7:188–195. [Google Scholar]
- Tomlinson PB. Aspects of cone morphology and development in Podocarpaceae (Coniferales) International Journal of Plant Sciences. 1992;153:572–588. [Google Scholar]
- Tomlinson PB, Takaso T. Seed cone structure in conifers in relation to development and pollination: a biological approach. Canadian Journal of Botany. 2002;80:1250–1273. [Google Scholar]
- Tomlinson PB, Braggins JE, Rattenbury JA. Pollination drop in relation to cone morphology in Podocarpaceae: a novel reproductive mechanism. American Journal of Botany. 1991;78:1289–1303. [Google Scholar]
- Treub M. Sur les Casuarinées et leur place dans le système naturel. Annales du Jardin Botanique de Buitenzorg. 1891;10:145–231. [Google Scholar]
- Tsai W-C, Hsiao Y-Y, Pan Z-J, Kuoh C-S, Chen W-H, Chen H-H. The role of ethylene in orchid ovule development. Plant Science. 2008;175:98–105. [Google Scholar]
- Umeda A, Imaichi R, Kato M. Ovular development and morphology of the outer integument of Magnolia grandiflora (Magnoliaceae) American Journal of Botany. 1994;81:361–367. [Google Scholar]
- Venkata Rao C. Floral anatomy and embryology of two species of Elaeocarpus. Journal of the Indian Botanical Society. 1953;32:21–33. [Google Scholar]
- Venkata Rao C. Embryological studies in Malvaceae. I. Development of gametophytes. Proceedings of the National Institute of Sciences of India. 1954;20:127–150. [Google Scholar]
- Venkateswarlu J. A contribution to the embryology of Sonneratiaceae. Proceedings of the Indian Academy of Sciences B. 1937;5:206–223. [Google Scholar]
- Villanueva JM, Broadhvest J, Hauser BA, Meister RJ, Schneitz K, Gasser CS. INNER NO OUTER regulates abaxial–adaxial patterning in Arabidopsis ovules. Genes and Development. 1999;13:3160–3169. doi: 10.1101/gad.13.23.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenitz G. Wörterbuch der Botanik. 2nd edn. Heidelberg: Spektrum; 2003. [Google Scholar]
- Wagner RE, Mugnaini S, Sniezko R, et al. Proteomic evaluation of gymnosperm pollination drop proteins indicates highly conserved and complex biological functions. Sexual Plant Reproduction. 2007;20:181–189. [Google Scholar]
- Wang Z-F, Ren Y. Ovule morphogenesis in Ranunculaceae and its systematic significance. Annals of Botany. 2008;101:447–462. doi: 10.1093/aob/mcm298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warming E. De l'ovule. Annales des Sciences Naturelles, Botanique, Sér. 6. 1878;5:177–266. [Google Scholar]
- Warming E. Observations sur la valeur systématique de l'ovule. Mindeskrift Japetus Steenstrup. 1913:1–45. [Google Scholar]
- Yakovlev MS, Batygina TB. Comparative embryology of flowering plants, 1–5. Leningrad: Nauka; 1981–1990. [Google Scholar]
- Yamada T, Imaichi R, Kato M. Developmental morphology of ovules and seeds of Nymphaeales. American Journal of Botany. 2001a;88:963–974. [PubMed] [Google Scholar]
- Yamada T, Tobe H, Imaichi R, Kato M. Developmental morphology of the ovules of Amborella trichopoda (Amborellaceae) and Chloranthus serratus (Chloranthaceae) Botanical Journal of the Linnean Society. 2001b;137:277–290. [Google Scholar]
- Yamada T, Imaichi R, Prakash N, Kato M. Developmental morphology of ovules and seeds of Austrobaileyales. Australian Journal of Botany. 2003a;51:555–564. [Google Scholar]
- Yamada T, Imaichi R, Kato M. The outer integument and funicular outgrowth complex in the ovule of Magnolia grandiflora (Magnoliaceae) Journal of Plant Research. 2003b;116:189–198. doi: 10.1007/s10265-003-0086-6. [DOI] [PubMed] [Google Scholar]
- Yamaki S, Satoh H, Nagato Y. Gypsy embryo specifies ovule curvature by regulating ovule/integument development in rice. Planta. 2005;222:408–417. doi: 10.1007/s00425-005-1547-z. [DOI] [PubMed] [Google Scholar]
- Yan H, Yang H-Y, Jensen WA. Ultrastructure of the micropyle and its relationship to pollen tube growth and synergid degeneration in sunflower. Sexual Plant Reproduction. 1991;4:166–175. [Google Scholar]
- Yeung EC, Law SK. Ovule and megagametophyte development in orchids. In: Arditti J, Pridgeon AM, editors. Orchid biology: reviews and perspectives. Dordrecht: Kluwer; 1997. pp. 31–73. VII. [Google Scholar]
- Zhang XS, O'Neill SD. Ovary and gametophyte development are co-ordinately regulated by auxin and ethylene following pollination. The Plant Cell. 1993;5:403–418. doi: 10.1105/tpc.5.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler A. Beiträge zur Kenntnis des Androeceums und der Samenentwicklung einiger Melastomataceen. Botanisches Archiv. 1925;9:398–467. [Google Scholar]
- Ziegler H. Über die Zusammensetzung des ‘Bestäubungstropfens’ und den Mechanismus seiner Sekretion. Planta. 1959;52:587–599. [Google Scholar]
- Zimmermann W. Main results of the ‘telome theory’. Paleobotanist. 1952;1:456–470. [Google Scholar]