Abstract
Memory Island and the Novel-Image Novel-Location are recently developed measures of spatial learning and recognition-memory modeled after the Morris water maze and the novel object-recognition tests. The goal of this study was to characterize how sex, age, and handedness contribute to Memory Island and Novel-Image Novel-Location performance. Volunteers (N=287, ages 6 to 67) from a local science museum completed four Memory Island trials containing a visible target and four trials containing a hidden target. A pronounced sex difference favoring males was noted in all measures of hidden trial performance. The total latency during the hidden trials among older-adults was longer than younger-adults or adolescents. Faster and more efficient performance by males was also identified during the visible trials, particularly among children. Adolescents and younger-adults outperformed children and older ages. Sinistrals had a lower cumulative distance to the target. Novel-Image Novel-Location behavior was examined in a separate sample (N=128, ages 6 to 86). Females had higher Novel-Image and Novel-Location scores than males. Novel-Image performance was independent of age while sinistrals had elevated Novel-Image scores relative to dextrals. Together, these findings identify how sex, age, and handedness uniquely contribute to performance on these tasks.
Keywords: aging, female, learning, left-handedness, memory, sinistrality
1. Introduction
Animal models have been integral in determining the anatomy, biochemistry, and molecular substrates of learning and memory [1,2]. The degree of experimental control over the animal’s environment and experiences throughout their lifespan cannot even begin to be approximated in human investigations. However, one fundamental challenge is the development of human neurocognitive tasks that are firmly connected to the substantial preclinical knowledge base which has been established by behavioral neuroscientists conducting preclinical investigations with rats and mice. This report examines performance across the lifespan on two human neurobehavioral measures developed in the laboratory [3–6], Memory Island (Experiment I) and the Novel-Image, Novel-Location test (Experiment II).
1.1. Memory Island (MI)
Several research groups have recently developed and validated virtual water mazes [3,4,7–10]. These computerized assessments are based on the Morris water maze to varying degrees. A male advantage in spatial-learning has been identified as well as reduced performance during senescence in rodents [2]. The Morris water maze has substantially advanced our understanding of the neural substrates of spatial learning and memory in rodents [1,11] and, although the many discoveries with this test are crucial, their elaboration to humans was hampered by the absence of a paradigm that extends upon the foundation these rodent studies have provided.
Computerized water mazes are practical to conduct and may provide an ecologically valid index of spatial learning and memory, which complements other more traditional neurobehavioral measures (e.g. Spatial Span). Faster and more efficient performance by college-aged males on virtual water mazes is a robust phenomenon that has been identified by different research teams [3,7,10,12–14]. Some evidence of a male advantage has also been noted in pre-pubescent children [6,15]. However, sex differences in the elderly have been relatively modest [5]. In terms of age differences, Driscoll and colleagues found a decrease in the elderly (age 60+) relative to younger adults (20–39) in both spatial learning and memory [16]. As the virtual environments used across laboratories are quite different in terms of their dependent measures, number and sequence of trials, and how they account for group differences in sensory and motor capacities, the primary objective of the first experiment was to determine when sex differences emerge using a moderately large sample completing a single instrument. As ten-year olds had superior spatial memory on MI compared to younger ages [4], a secondary objective was to use a wider range of ages (6 to 67) and to determine performance on this task across the lifespan. Finally, this community based sample allowed to ascertain whether right and left-handers differ in their virtual maze performance. Although no prior research has addressed this issue, learning disordered populations have a disproportionate number of left-handers (sinistrals) [17]. Therefore we anticipated a right-handed advantage in MI performance.
1.2. Novel-Image Novel-Location (NINL)
Habituation to familiar stimuli and a heightened response to unfamiliar ones is a fundamental phenomenon across species. The one trial object-recognition test [18] utilizes habituation to further understand the neurobiology of learning and memory [2]. Rodents will show preferential exploration of an unfamiliar object relative to a previously encountered one. Similarly, human infants will readily spend more time looking at an unfamiliar picture relative to a previously observed one [19]. This measure has also been expanded to include a location component in which a familiar object is moved to a novel place. The novel location element is much more challenging than the novel object one and has different neuroanatomical substrates [2]. NINL has broad similarities to the novel-object novel-location measure of exploratory behavior in rodents [2,18], the delayed non-matching to sample test used with non-human primates [20], and the Fagan Test used with human infants [19]. In the original non-computerized version of the NINL test, participants viewed twelve panels that each contained three pictures arranged in one of four locations on a page. After being instructed to learn the identity and placement of these thirty-six pictures, participants viewed another twelve panels in which one of the three images was either moved to a novel location (the Novel-Location or NL condition) or one image was replaced with an unfamiliar one (the Novel-Image or NI condition). The NINL total score showed a moderate correlation (r = 0.52) among adults (ages 20–44) with a standardized facial recognition test [3].
The objective of Experiment II was to examine NINL behavior across the lifespan and to further characterize the potential contributions of age, sex, and handedness to performance on this test. In addition, NINL test performance was compared with behavior on the Spatial Span Backward (SSB), a classic measure of visuospatial working memory [21,22]. The SSB was selected based on the known age profile [23]. It was also determined whether the internal consistency [24] and split-half reliability of the NINL instrument was acceptable.
2. Experiment I: Material and Methods
2.1. Subjects
Subjects were recruited at Oregon Museum of Science Industry (OMSI) (N=287, ages 6 to 67). Participants were first asked their age and hand preference to write/use the joystick and then the task was explained. Exclusion criteria included those younger than 6 years of age, children without proper parental approval, with conditions effecting eye sight and those without sufficient time to complete the task. All methods and materials were approved by Oregon Health and Science University Institutional Review Board.
2.2. Memory Island (MI)
The virtual testing environment of MI simulates an island, 347 × 287 m, comprised of four quadrants, each containing a different target object (sculpture, seagull, seal, or fountain). Participants were instructed to navigate using a joystick to a target location visibly marked with a flag adjacent to the target (visible trials 1–4). A Microsoft Sidewinder joystick was used which is equally comfortable for right and left-handed participants. After completing the visible target training with a flag adjacent to each target (Fig. 1 shows a panoramic view of the island; for screen shots of the targets see [3,4,6]), the subject navigated to a hidden target (i.e., no flag located beside the target item, but otherwise indistinguishable from the visible target trials) on trials 5–8. The target items (one/trial) were located in the same locations for all participants. Their instructions were as follows:
Fig. 1.
Memory Island panoramic view from the starting location. Note that all the flags are depicted here for comparative purposes, but in the actual test only one flag/target is present in a visible trial.
You will cruise on a virtual island. In each trial, you will start in the same position, but you may be looking in a different direction. Your mission is to find a mysterious object hidden somewhere on the island. To do that you need to look closely at what’s on the island. Try to make a map in your head of the island and where the mysterious objects are located on the island. If you cannot find the mysterious object within two minutes, an arrow will help guide you to it. Once you have found it, you must stand next to it and wait for the game to end.
The starting location was always the center of the island (X, Y coordinate 0, 0, also see Fig. 1). The target locations were as follows: sculpture −276.1, −295.7; seal +285, −328; seagull −224.0, +303.0, and fountain +327.0, +229.1. Visual distractions were limited by having participants complete testing in a private indoor tent. Participants also wore headphones which played the audio associated with MI (nature sounds) which further limited any distractions. Notably, preliminary testing identified a ceiling effect when a probe trial was completed immediately after the last hidden trial. As even a short retention (e.g. 15 to 30 minute) interval was not feasible due to time constraints with this community sample of volunteers, this trial was not completed. Additional details regarding this program are available elsewhere [3,4,6].
2.3. Statistical Analysis
All analyses were conducted using SPSS, version 16.0 (SPSS Inc., Chicago, IL). In each trial, movement was recorded in time-stamped coordinate files, which were used to calculate latency until the first movement (sec), latency to reach the target (sec), distance traveled (virtual units), and velocity (virtual units/second). Cumulative distance to the target, a running total of distance to the target obtained twice per second, was also recorded. If Mauchly’s sphericity test was significant on repeated measures ANOVAs, then results of the Greenhouse-Geisser were reported with the corresponding reduction in the degrees of freedom. Mixed (Trial x Sex) ANOVAs were conducted separately for the visible and hidden trials separately. Analysis of covariance was completed to determine if group differences on latency and cumulative distance to the target during the hidden trials were retained when accounting for the variance attributable to speed. Given that the group sizes, particularly for comparisons of right versus left-handers, were unequal, Levene’s test assessed whether the homogeneity of variance assumption was violated. The sample was subdivided into four age groups (children, 6–11; adolescents, 12–17; younger-adults, 18–39; and older-adults, 40–67) to further explore sex differences. Pearson correlations were completed among MI measures. For all figures, data is presented as mean ±SEM.
3. Results
3.1. Sex Differences in Memory Island Performance
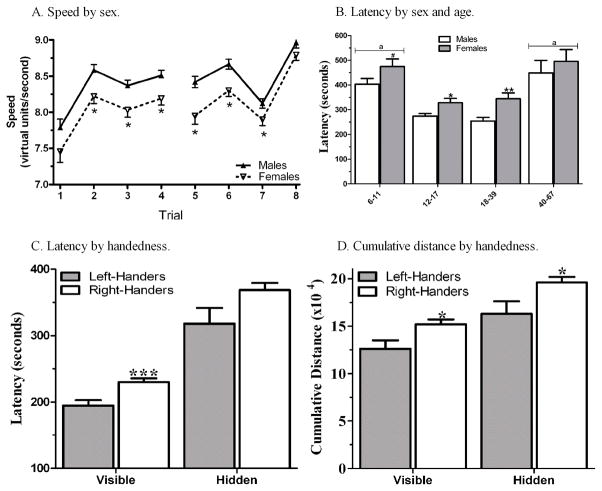
The sample (N=287) consisted of a similar number of males (N=151, 52.6%) and females (N=136, 47.4%). During the visible trials (participants find four different targets in four trials), males traveled significantly faster on second, third, and fourth trials (Fig. 2A). The cumulative distance to the target was 16.5% longer for females (Total for Males = 13.8 (±0.6) × 104 virtual units, Females= 16.1 (± 0.7), t(285) = 2.55, P < 0.025; Cohen’s d = 0.30). The same general pattern was also identified in the hidden trials with females traveling slower on the first, second, and third trials (Fig. 2A) and having an 18.7% longer cumulative distance to the target (Males=17.5(±0.7), Females=21.1 (±0.8), t(285) = 3.26, P ≤ 0.001; d = 0.39). Notably, the sex difference in cumulative distance to the target during the hidden trials was also retained with the average visible speed included as a covariate (F(1,284) = 8.26, P < 0.005).
Fig. 2.
Performance on Memory Island varies by sex, age, and handedness. A) Speed (virtual units/sec) on the visible (1–4) and hidden (5–8) trials, *p < .05 versus males. B) Sex and age differences in total latency to complete the hidden trials, #p = .07, *p < .05 or **p < .01 versus males; ap ≤ .0005 versus adolescents (age 12–17) or younger-adults (age 18–39). C) Latency by handedness, ***p ≤ .0005 versus right-handers. D) Cumulative distance to the target by handedness, *p < .05 versus right-handers.
Table 1 shows the results of the Trial x Sex ANOVA for the entire sample. Importantly, as a sex difference in age was observed (Males = 17.9 ±1.0, Females = 21.7±1.3, t(264.2) = 2.34, P < .025), further analyses were conducted with the sample stratified into four groups, children (ages 6–11, N = 85, 57.6% male), adolescents (ages 12–17, N=107, 59.8% male), younger-adults (age 18–39, N = 59, 39.0% male), and older-adults (age 40–67, N = 36, 41.7% male). Note that this division in age groups is admittedly arbitrary and other categories could be made, but for this data-set, these demarcations offer the advantage of an equivalent sex ratio within groupings for the minors (<18) as well as adults (≥18). On the visible trials, no sex differences were present in younger-adults on MI performance. In contrast, sex differences were noted in the latency to reach the targets and speed among children. On the hidden trials, a very different pattern was evident with younger-adults showing clear evidence for sex differences on the primary measures of interest, cumulative distance to the target and latency (Fig. 2B), as well as speed. Sex differences were also present among adolescents, but were less pronounced for children. However, the performance of male and female older-adults was indistinguishable.
Table 1.
Mixed (Trial x Sex) ANOVA main effect p values during the visible and hidden trials of Memory Island for the total sample (N = 287).
| Performance Measures | All | Children | Adolescents | Younger-Adults | Older-Adults | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (6–11 yrs) | (12–17 yrs) | (18–39 yrs) | (40–67 yrs) | |||||||
| Sex | Trial | Sex | Trial | Sex | Trial | Sex | Trial | Sex | Trial | |
| Visible | ||||||||||
| Start Latency (sec) | Ns | .0005 | .059 | .004 | ns | .0005 | ns | .088 | ns | .003 |
| Speed (virtual units/sec) | .014 | .0005 | .041 | .0005 | .062 | .0005 | ns | .0005 | ns | .0005 |
| Total Trial Latency (sec) | .011 | .0005 | ns | .0005 | .081 | .0005 | ns | .005 | ns | .013 |
| Cumulative Distance to the Target (virtual units) | .003 | .0005 | .002 | .0005 | ns | .0005 | ns | ns | ns | ns |
| Hidden | ||||||||||
| Start Latency (sec) | .004 | ns | nsi | ns | .062 | .028 | ns | ns | ns | ns |
| Speed (virtual units/sec) | .001s | .0005 | .065 | .0005 | .006i,s | .0005 | .007s | .0005 | ns | .001 |
| Total Trial Latency (sec) | .001s | .0005 | ns | .0005 | .010s | .0005 | .004s | .0005 | ns | .0005 |
| Cumulative Distance to the Target (virtual units) | .001 | .0005 | .049i | .0005 | .010 | .0005 | .004 | .0005 | ns | .003 |
ns = not significant,
p<.05 for the Sex x Trial interaction,
main effect retained with visible speed as a covariate.
3.2. Handedness Differences in Memory Island Performance
Left-handedness (sinistrality) was relatively common in this sample (37/287 or 12.9%). The percentage of sinistrals who were also female (45.9%) did not differ from right-handers (dextrals) (47.6%). The average age of sinistrals (20.4±2.6) was also equivalent to that of dextrals (19.6±0.8). The total latency to reach the visible targets was over a half-minute sooner for sinistrals than for dextrals (d = 0.51; Fig. 2C). Similarly, the total cumulative distance to the target was significantly shorter for sinistrals (d = 0.40). In the hidden trials, a non-significant left-handed advantage was noted for latency (P = 0.08). The cumulative distance to the target was also smaller for sinistrals (d = 0.38; Fig. 2D). However, handedness had no effect on the speed during the visible or hidden trials.
3.3. Age Differences in Memory Island Performance
A general pattern of extreme (young or old) age groups performing less well than those in the middle was noted. Older-adults and children showed less efficient performance (slower speeds, longer latencies, higher cumulative distances to the target) relative to adolescents and young-adults on the visible trials (Table 2).
Table 2.
Age differences in Memory Island performance (N = 287).
| Performance Measures | Children | Adolescents | Younger-Adults | Older-Adults |
|---|---|---|---|---|
| (6–11 yrs) | (12–17 yrs) | (18–39 yrs) | (40–67 yrs) | |
| Visible | ||||
| Start Latency (sec) | 11.1 (±1.1) | 11.1 (±0.9) | 15.2 (±2.0) | 16.0 (±2.2)c,a |
| Speed (virtual units/sec) | 8.1 (±0.1)aa | 8.4 (±0.9) | 8.2 (±0.1) | 7.6 (±0.2)aa,y |
| Total Trial Latency (sec) | 236.1 (±9.5)a | 206.8 (±7.1) | 217.1 (±9.8) | 267.7 (±20.8)aa,y |
| Cumulative Distance to the Target (virtual units) | 15.5 (±0.8) | 13.8 (±0.7) | 14.0 (±0.9) | 18.1 (±1.7)a,y |
| Hidden | ||||
| Start Latency (sec) | 10.5 (±1.2) | 8.3 (±0.7) | 13.2 (±1.6)aa | 11.5 (±2.0) |
| Speed (virtual units/sec) | 8.1 (±0.1)aaa,yy | 8.6 (±0.1) | 8.5 (±0.1) | 8.1 (±0.2)aa,y |
| Total Trial Latency (sec) | 433.4 (±19.1)aaa,yyy | 296.2 (±9.8)s | 309.3 (±16.4)s | 476.1 (±34.7)aaa,y |
| Cumulative Distance to the Target (virtual units) | 23.0 (±1.0)aaa,yyy | 15.6 (±0.7)s | 16.4 (±1.1)s | 25.5 (±1.9)aaa,y |
p < .05 versus children;
p < .05,
p < .01, or
p ≤ .0005 versus adolescents;
p < .05,
p < .01, or
p ≤ .0005 versus younger-adults;
p ≤ .0005 versus children or older-adults with mean speed during the visible trials included as a covariate.
The total latency during the hidden trials did not differ between children and older adults. In contrast, children took significantly longer than either adolescents or younger-adults. Older adults also had a longer latency relative to either adolescents or younger-adults (Fig. 2B). These age differences in latency during the hidden trials were retained with average visible speed included as a covariate. An identical pattern of age differences was also identified for cumulative distance to the target.
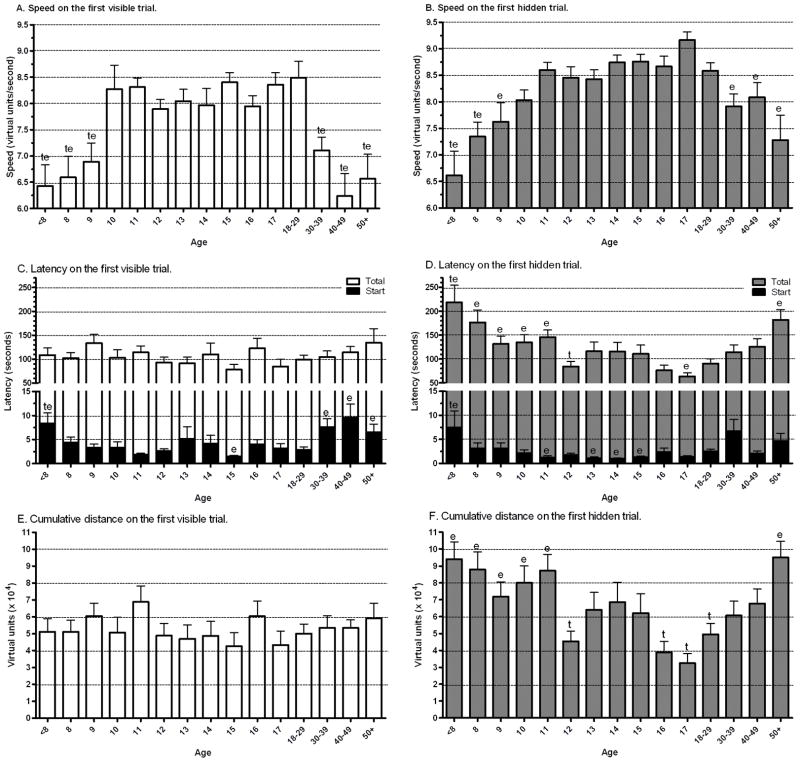
As there are substantial changes in performance across the eight MI trials, also indicated by the significant trial (i.e. learning) effects in Table 1, further examination for age differences was completed using the first visible and first hidden trials. A step-like increase in speed during childhood was identified with 10 year-olds traveling faster than all younger ages. Early-adults (ages 18–29) also traveled quicker than people in the fifties and sixties, forties, or thirties (P < .005, Fig. 3A). On the hidden trial, early-adults also traveled faster than the very young or very old, but the age-related progression and regression was much more gradual (Fig. 3B). No age differences were noted in latency to reach the target (Fig. 3C). However, start latency did show several age differences with early-adults having a shorter latency than very young children (age 6–7) or people in their thirties, forties, or fifties. The start latency of fifteen-year olds (1.5±0.2 sec) was approximately half of early-adults (2.9±0.6). The latency to reach the first hidden target was quicker among early-adults than children (≤11) or older-adults (50+). Start latency was shorter in early-adults compared to very young children, but longer relative to eleven, thirteen, fourteen, or fifteen year-olds (Fig. 3D). The cumulative distance to the target during the visible trial showed no age differences (Fig. 3E). In contrast, early-adults had a lower cumulative distance to the target relative to children or older-adults (Fig. 3F).
Fig. 3.
Developmental differences in Memory Island performance on the first visible and hidden trials. The N/age=10–37, ep < .05 versus 18–29 year olds, tp < .05 versus 10 year olds.
3.4. Correlations among Memory Island Measures
There was a strong positive correlation between total latency and cumulative distance to the target in the visible (r(285) = +0.79, P ≤ .0005) and hidden (r(285) = +0.95, P ≤ 0.0005) trials. Faster speeds were associated with lower start latencies on the visible (r(285)= −0.59, P ≤ 0.0005) and hidden (r(285)=−0.50, P ≤ 0.0005) trials. Similarly, individuals that traveled faster on the visible trials performed likewise on the hidden trials (r(285) = +0.61, P ≤ 0.0005). Start latencies on the visible trials were moderately associated with start latencies on the hidden trials (r(285) = 0.42, P ≤ 0.0005). However, the latency to reach the visible targets was only weakly correlated with the latencies to find the hidden targets (r(285)= +0.22, P ≤ 0.0005) and cumulative distance to the target during the visible trials was only modestly associated with that measure on the hidden trials (r(285) = +0.25, P ≤ 0.0005).
4. Discussion
There are three key findings of this study with MI. First, there were pronounced sex differences across MI measures. A male advantage for speed during the target visible trials has been documented previously among the elderly, as well as in adults and children [3,5,6]. In addition to verifying these velocity differences, this investigation also determined that males had shorter latencies and cumulative distances to the target. These sex differences were retained even when accounting for speed differences in the visible trials. Interestingly, if sex hormones were the single factor mediating sexually dimorphic functional differences, one might anticipate the most pronounced differences would be found in younger-adults with less clear differences in pre-pubescent or older-adult groups. This general pattern was identified in the hidden trials with significant differences in latency as well as cumulative distance to the target in younger-adults and adolescents and no significant differences on these measures in children or older-adults. However, in the visible trials, male children had shorter latencies than female children while no significant sex difference was obtained among any of the older ages. This data might indicate that although the changes in androgens and estrogens during puberty could contribute to the magnitude of sex differences, other factors including genetics, the in utero organizational effects of sex hormones, or task difficulty, could also influence whether sex differences are observed. Notably, inferior performance by female rodents has also generally been documented in spatial learning with the Morris Water Maze [2].
Second, contrary to our expectations, left-handers displayed better MI performance. Sinistrals had a shorter visible latency and lower cumulative distances to the target during the visible and hidden trials, relative to right-handers. A contribution of handedness to virtual water maze performance is, to our knowledge, a novel outcome. However, it should be emphasized that prior research has not been sufficiently powered to detect such a difference based on handedness. Mental rotation, a task that is moderately correlated with the ability to find a hidden water maze target [25] does not appreciably differ based on handedness [26]. A neurobiological origin for the present findings is not immediately apparent as even the well known handedness difference in language production is actually rather modest with the vast majority of both right and left-handers having language lateralized to their left-hemispheres [27,28]. A disproportionate number (43.9%) of children that met the criteria for Developmental Coordination Disorder were either left-handed or ambidextrous [29] which extends upon an earlier report that learning disorders were over nine-fold more common in left-handers [17] although see [30]. Regardless of whether the left-handers in this community based sample had other subtle neuropsychological conditions, based on the biased proportioning of neuropsychological conditions within the left-handed population, these would be expected to lower, not enhance, their spatial function. Notably, a genetic basis of handedness has long-been suspected based on twin and pedigree studies [31]. Although specific genes mediating sinistrality have yet to be conclusively identified, some incremental progress has been made [32,33]. Additional behavioral genetic research is currently ongoing to extend upon this intriguing outcome.
Thirdly, age, independent of sex, influenced overall MI performance across both the visible and hidden trials. A pronounced impairment was identified among older-adults relative to either younger-adults or adolescents. This was identified for trial latency and cumulative distance to the target, the primary measures of MI performance, as well as start latency. Start latency is the duration between when the trial begins and the subject first moves from the center of the virtual world. Although some brief (500–1,000 ms) hesitancy at trial onset might reflect a combination of simple reaction time and orientating, particularly during the latter trials, longer start latencies (>2 sec/trial) may be indicative of other processes like attention. Children also showed slower speeds and longer cumulative distances and latencies on the hidden trials relative to adolescents or younger-adults. Importantly, there was an absence of age differences between relatively young children and older-adults. Across the ages studied, there was a clear “U shaped” (curvilinear), or an inverted U depending on the measure, relationship between age and MI performance. The finding of a decline in spatial function during late-adulthood is concordant with rodent investigations [2].
A more detailed evaluation of age differences was made by examining MI behavior on the first visible and first hidden trials. These trials both involve searching for the same target item, but differ based on the presence or absence of a large flag to facilitate navigation. As noted earlier, ten-year olds had better memory on the probe trial relative to ages seven or eight [4]. This report identified a stage-like progression in visible speed with ten-year olds moving faster than eight or even nine year-olds. In contrast, the profile of age related progression was more gradual during the hidden trial. In addition, a comparison with early adults (ages 18–29), revealed reductions in speed on the visible and hidden trials for individuals beginning in their thirties. Early-adults reached the target during the hidden trials quicker and took a more direct route than individuals in their fifties. There were no appreciable age differences on either the cumulative distance to the target or latency during the visible trials, which suggests that the visible trials are sufficiently challenging so that this instrument can be employed across a wide range of developmental periods. This pattern of MI results is generally congruent with, and also extends upon, prior investigations of how age contributes to a progression in spatial learning and memory performance in childhood and a decline that begins early in adulthood [16,34,35].
Structural and functional neuroimaging studies have begun to provide a framework for understanding individual differences in spatial navigation. Macguire and colleagues discovered that taxi-cab drivers had increased gray matter volumes of their posterior hippocampus [36]. A subsequent report determined that the ability to successfully navigate through a virtual town was uncorrelated with hippocampal volumes [37]. However, strong correlations were observed between hippocampal fractional anisotropy, an index of microstructural integrity, and the ability to find targets in a virtual city among adults [38]. The hemodynamic activity in younger-adults was greater in the posterior hippocampus and parahippocampal gyrus than in older-adults during spatial navigation. The reverse pattern was also identified with older-adults having greater activity in the anterior cingulate [39]. The neural networks mediating navigation are quite similar in the sexes [40], but men have been shown to engage the hippocampus more than women while women rely more on frontal and parietal regions [41]. In contrast, the neural underpinnings of handedness differences in spatial navigation abilities must await further study using a more detailed index of hand preferences than was employed in the present endeavor.
Although MI was designed as a human model of the rodent Morris water maze in terms of the underlying logic of the trial structure and dependent measures, we recognize that this objective, but relatively brief, test may provide a useful measure for other purposes (e.g. further exploring structures important for topographical learning like the hippocampus, parahippocampal gyrus, parietal cortex, and cerebellum in lesion studies). The use of different strategies (e.g. head toward a prominent local landmark and then turn left or the formation of a spatial map using distal landmarks) may result in a combination of both egocentric and allocentric navigation. The availability of more than one technique to efficiently solve MI may emulate “real-world” navigation more closely than that offered by other virtual mazes that encourage only an allocentric approach. However, it should also be emphasized that MI as well as other virtual mazes, for example, the radial arm maze used by Astur and colleagues [25], are unlike more naturalistic navigation in several non-trivial ways. The exclusive reliance on a visual modality offers clear conceptual benefits, but the absence of vestibular [43] and proprioceptive feedback is unlike real-world way-finding. Further study is needed to determine whether head direction and place cells [42] respond similarly in virtual and non-virtual environments.
5. Experiment II: Material and Methods
5.1. Subjects
Participants consisted of patrons of the Oregon Museum of Science Industry. The findings of Experiment I prompted inclusion of a wider age-range (6–86 year-old) and more detailed information regarding handedness (described below). The sample (N=128, 52.4% male, 12.5% Sinistrals) included Children (6–11, N=40), Adolescents (12–19, N=20), Early-Adults (20–49, N=39) and Late-Adults (50–86, N=29). Study participants received verbal and visual instructions prior to each test. Handedness was determined using an interview modeled after the content of the Edinburgh Handedness Inventory [44]. Participants were asked which hand they used to complete several activities (e.g. writing, drawing, throwing, using spoon). Many younger children did not have experience with “strike a match” and this item was omitted for younger children. A Laterality Index was calculated by adding up the number of checks with each hand and using the equation [(R−L)/(R+L)]*100.
5.2. Novel-Image Novel-Location (NINL)
The NINL test, version 0.2, was a modification and extension of the original measure described earlier [3,5]. The differences relative to the original version were: 1) more detailed instructions and a practice trial so that the test could be more readily completed by children; 2) use of images imported into a Microsoft Power Point slide show to precisely regulate the display time; 3) an increase in the number of images from forty to eighty; 4) use of high resolution pictures from the International Affective Picture System [45]; and 5) only scoring the NI and NL items but not the eight unchanged items. In keeping with how the rodent object recognition test is typically completed [2,18,46], only neutral images (e.g. household objects, clothes, plants) were selected (see Table 3 for further details). These images can be obtained for research purposes by contacting [45]. Each of the 48 slides (24 Learn and 24 Test) consisted of 4 quadrants containing 3 images and one empty space. Each slide was shown for eight-seconds during the learn phase. After viewing the learn slides, testing from Set A commenced (twelve slides: four NI, four NL, and four unchanged slides). A computerized version of the SSB (described below) was then conducted [22] and subsequently the remainder of the NINL test was completed (Set B: also four NI, four NL, and four unchanged slides). The quadrant of the changed images and locations were randomized across the four areas. NINL scoring consisted of 0–3 points earned per item. No points were awarded if the participant was unable to identify if a change had occurred, one point was awarded for correctly identifying that a change had taken place, two points were awarded for also identifying the correct ‘type’ of change (NI or NL), and three points were awarded for correctly identifying the ‘type’ of change and the quadrant with the change (maximum = 24 points for NI or NL). As a secondary analysis, the percentage of the 16 items with a one-point score was determined.
Table 3.
Visual stimuli for the Novel-Image, Novel-Location test, Learn (L) and Test (Set A & B). Image numbers are neutral pictures from the International Affective Picture System (2007). Image numbers are shown in parentheses or are empty (-). Quadrants are upper-left (UL), upper-right (UR), lower-right (LR) and lower-left (LL). Type is Novel Image (NI), Novel Location (NL) or No Change (NC).
| Slide | Quadrant | Slide | ||||||
|---|---|---|---|---|---|---|---|---|
| # | UL | UR | LL | LR | # | Basis | Type | Description |
| L01 | - | outlet (6150) | mushroom (5531) | train (7039) | A01 | L07 | NL | car (8531) to UR |
| L02 | dust pan (7040) | pocket watch (7190) | - | towel (7002) | A02 | L16 | NC | |
| L03 | coffee cup (7057) | dark clouds (5594) | cube (7185) | - | A03 | L04 | NI | clock (7211) at LR |
| L04 | hammer (7034) | book (7090) | - | abstract painting (7830) | A04 | L11 | NI | crimps (7056) at UL |
| L05 | yard (5130) | - | shoes (7031) | woven basket (7010) | A05 | L10 | NC | |
| L06 | set table (5849) | canyon (5661) | - | row boats (5390) | A06 | L24 | NL | pins (7052) to UL |
| L07 | hair dryer (7050) | - | convertible car (8531) | fan (7020) | ||||
| L08 | - | leaves (5750) | fork (7080) | parking lot (7595) | A08 | L17 | NI | stool (7025) at LL |
| L09 | fireworks (5480) | - | unlit light bulb (7055) | waste can (7060) | A09 | L18 | NC | |
| L10 | - | satellites (5471) | blue cup (7009) | headlight (7095) | A10 | L02 | NL | pocket (7190) to LL |
| L11 | rolling pin (7000) | bus (7140) | tiger lilly (5030) | - | A11 | L08 | NI | clothes (7242) at LL |
| L12 | drill (7043) | latch (7059) | - | Native Amer pattern (7179) | A12 | L03 | NL | cube (7185) to LR |
| L13 | blue door (5731) | bridge (7547) | pink flower(1604) | - | B13 | L06 | NC | |
| L14 | - | power lines (9080) | spoon (7004) | red-yellow abstract (7161) | B14 | L15 | NL | bulb (7170) to LR |
| L15 | lit bulb (7170) | ship (5395) | large baskets (7041) | - | B15 | L19 | NC | |
| L16 | orchid (5010) | - | leaves (5740) | empty pool (9360) | B16 | L09 | NI | rack (7217) at UL |
| L17 | earth (5890) | mountain top (5660) | air plane (7620) | - | B17 | L23 | NI | field (5250) at UL |
| L18 | - | white bowl (7006) | tissue (7950) | sports car (8510) | B18 | L22 | NC | |
| L19 | clear glass (7035) | hydrant (7100) | gold bars (8500) | - | B19 | L13 | NC | |
| L20 | - | ferris wheel (7508) | orange flower (5020) | file cabinet (7705) | B20 | L20 | NL | wheel (7508) to UL |
| L21 | dumbells (7042) | - | lamp (7175) | yellow sail boat (8210) | B21 | L21 | NI | universe (5300) at LR |
| L22 | shoes (7038) | scarf (7205) | - | building (7491) | B22 | L12 | NL | Native (7179) to LL |
| L23 | flowers (5000) | semi truck (7130) | - | freeway (7560) | B23 | L14 | NI | plate (7233) at UR |
| L24 | - | clothes pins (7052) | umbrella (7150) | snow day (5635) | B24 | L01 | NL | train (7039) to UR |
5.3. Spatial Span Backward (SSB)
Visual-spatial working memory was assessed using a computerized version of the Corsi’s block tapping task [21,22], programmed in E-Prime 1.1 (Psychology Software Tools, Pittsburgh, PA). Briefly, an array of ten grey squares on a black background was presented on the computer screen (Supplementary Figure 1). Each trial consisted of a smiley face appearing in one of the squares at a rate of one square per second. Participants used a mouse to click on the squares in the opposite order in which the smiley face appeared, with two trials at each level of difficulty (ascending from two to eight location sequences). The software required that participants successfully completed a practice item prior to beginning the test. The SSB test terminated when both trials within a difficulty level were incorrect. The dependent measure was the number of trials completed correctly.
5.4. Statistical Analysis
In order to determine if the developmental profile (progression during childhood and regression during senescence) was equivalent on each test, two complementary analyses were completed. First, the standardized (Z) scores for each test were compared across four age groups (Children, 6 to 11; Adolescents, 12–19; Early-adults, 20–49; and Late-adults, 50–86). Second, a nonlinear quadratic function using Prism version 4.0 (Graphpad Inc., La Jolla, CA) was used for the regression analysis between age and test performance. Pearson correlations were performed to assess reliability in NI and NL performance in sets A and B (Table 3). Cronbach’s alpha was calculated as an index of the internal consistency [24] of the NI and NL test items. Finally, participants were divided into two handedness groups to assess the potential effect of handedness on NINL test performance (Dextrals: Laterality Index > 0 and Sinistrals: Laterality Index ≤ 0).
6. Results
There were no age differences based on sex (Males = 28.6 ± 2.6, Females = 31.5 ±2.9) or handedness (Dextrals = 30.4 ± 2.1, Sinistrals = 28.7 ± 5.1). The number of males and females did not differ based on handedness (ratio of Males:Females for Dextrals 55:55; Sinistrals 11:5; χ2(1) = 1.97, P = 0.16).
Overall performance on the SSB test was 7.3 ± 0.3 (Minimum = 0, Maximum = 13, Median = 8) and on the NINL test was 31.0 ± 0.9 (Minimum = 2, Maximum = 47, Median = 32). The internal consistency of the complete sixteen-item NINL test was 0.811. Similarly, NI (Cronbach’s alpha = 0.773) and NL (alpha = 0.716) scale values were reasonable. There was a moderate correlation between items in Sets A and B (r(126) = 0.72, P < 0.005). Performance scores of Set B (14.9 ± 0.5), which was completed after Set A and the SSB test, were slightly, but significantly, lower than those of Set A (16.2 ± 0.5, t(127) = 3.62, P < 0.0005, d = 0.32). The scores were higher on NI (17.6±0.5) than NL (13.5 ±0.5, t(127) = 7.62, P < 0.0005, d = 0.67).
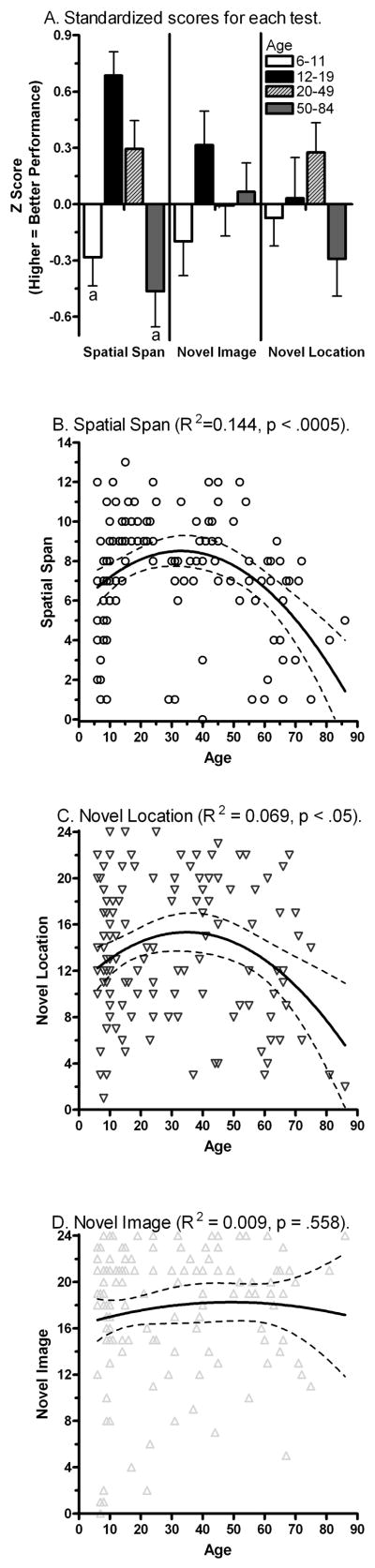
An ANOVA revealed an effect of Age on the SSB test (F(3,122) = 8.54, P < 0.0005). Adolescents and Early-Adults had significantly higher scores than either children or late-adults. However, the effect of age was not significant for NI (F(3,124) = 1.22, P = 0.30) or NL (F(3,124) = 1.94, P = 0.13, Figure 4A) performance. Similarly, non-linear regression was applied to determine the relationship between age and test performance. A polynomial or inverted U function accounted for a significant proportion of the variance for SSB test performance (F(2,123) = 10.36, P < 0.0005, R2 = 0.144, SSB = −0.511+ 0.56*Age − 0.0008487*Age2, Figure 4B). A polynomial function was statistically significant for the NL test (F(2,125) = 4.64, P < 0.05, R2 = 0.069, NL = −0.446 + 0.4372* −0.0006273*Age2, Figure 4C), but not the NI test (F(2,125) = 0.59, P = 0.56, Figure 4D).
Figure 4.
Standardized scores on the Spatial Span Backward and the Novel-Image Novel-Location tests (A). Scatterplots of performance by age in the Spatial Span (B), Novel-Location (C), and Novel-Image (D) tests. ap < .05 versus Ages 12–19 or 20–49.
There were sex and handedness group differences in performance on the NINL but not SSB test. Females had higher scores than males on the NI test (d = 0.68) and NL (d = 0.41). Sinistrals had higher NI scores than Dextrals (Table 4, d = 0.47). The percentage of the sixteen NINL items with a score of one point, i.e. correct identification of a change, but incorrect recognition of the type of change that occurred, was 13.8 ± 0.9% for all respondents. This response type showed indications of a sex difference (Males = 15.2 ± 1.3%, Females = 11.9 ± 1.1%, t(122.7) = 1.94, P = 0.054), but this measure did not differ appreciably based on age or handedness. The correlation between performance on the NI and NL tests was moderate (r(126) = 0.46, P < 0.0005). Performance on the SSB test also showed a modest, but significant, association with performance on the NL test (r(124) = 0.26, P < 0.005) and NI (r(124) = 0.20, P < 0.05).
Table 4.
Role of sex and handedness on performance on the Novel-Image, Novel-Location and Spatial Span Backward tests.
| Sex | Handedness | |||
|---|---|---|---|---|
| Male | Female | Dextral | Sinsistral | |
| Novel Image | 15.7 (0.8) | 19.4 (0.6)a | 17.3 (0.6) | 19.6(0.9)b |
| Novel Location | 12.4 (0.7) | 14.8 (0.7)b | 13.5 (0.6) | 13.0 (1.0) |
| Spatial Span Backward | 7.4 (0.2) | 7.2 (0.4) | 7.3 (0.3) | 6.9 (0.7) |
p < .0005 versus males;
p < .05 versus dextrals or males
7. Discussion
The primary goal of Experiment II was to determine the contributions of age, sex, and, based on Experiment I, handedness to performance on the NINL test. The presence of sex differences on both the NI and NL indices is broadly congruent with a prior report showing better performance on the NL measure by elderly females than males [5]. In contrast, sex differences were not detected in an earlier investigation with adult study participants [3]. The different outcomes in the current study with those in the earlier report [3] might be due to differences in the NINL test methodology, such as differences in the number of images and in the sample size. As a female advantage in verbal processing abilities can result in higher performance on memory tests that are not explicitly verbal [47], females could be employing a verbal strategy for both NI and NL items.
Although there was clear evidence for the anticipated age effects [23,48] for SSB, with higher scores in adolescents and early-adults than children and late adults, performance on the NINL test was largely independent of age in this study. The nonlinear regression analysis for the NL measure was statistically significant although the proportion of variance accounted for by age was modest (7%). While many participants anecdotally recounted that the NINL test was quite difficult, examination of the distribution of scores did not indicate either ceiling or floor effects. Importantly, three-month-old infants can exhibit a clear novelty preference for unfamiliar faces on the Fagan Test of Infant Intelligence when they are presented after a very brief retention interval [19]. One-month old rhesus monkeys are also quite capable on the visual paired comparison test [49]. Similarly, even weanling rats are capable of novel-object recognition, but only at relatively brief periods (≤1 hour) between the stimulus and test sessions [50]. Together, these findings indicate that the NINL test, like analogous tests in rodents and non-human primates, can be employed with a wide range of ages and are generally concordant with prior findings [19,49,50]. For practical reasons with this community-based sample, the total test duration was typically less than twenty minutes and long intervals between the Learn and Test sets were not feasible. Future studies with longer NINL retention intervals might reveal larger contributions of age, particularly during the primary school and senescent periods. The interval has also been shown to be a key variable in characterizing sex differences with a female advantage for the novel-object condition, but a male advantage for the novel-location condition in rats at longer (3 hour) intervals [51].
Sinistrals had higher NI scores than dextrals. An advantage for left-handers was also noted earlier in performance on a spatial navigation test (Experiment I). Similarly, left-handed females, but not males, solved the Tower of Hanoi in fewer moves than right-handers [52]. The Tower of Hanoi, like the NINL, has a working memory component. The neurobiological substrates which could mediate performance differences based on handedness, and selectively on NI, but not NL, are not immediately obvious. Examination of the thickness of the corpus collosum or the asymmetry in size of the left versus right planum temporale as a function of the laterality quotients has identified an exceedingly subtle pattern of results [53,54]. As also noted previously, the frequently cited group differences in language laterality by handedness an oversimplification as the majority of both dextrals and sinistrals have their word generation capacity localized to the left-hemisphere [27–28]. Genetic factors might play a role here. For example, individuals with at least one apolipoprotein E (apoE) ε2 allele were approximately fivefold more likely than apoE ε4 carriers to be left-handed [32]. Increased efforts are warranted for further investigation examining both handedness and APOE genotype to better understand causes for the individual differences in NINL performance.
Two other findings are also noteworthy. The correlation between SSB, a classic test of visual spatial working memory, and NINL, was rather modest. This could be because SSB, but not NINL, includes a fine-motor component. Alternatively, NINL may be more dependent on semantic memory than SSB. Second, a generally accepted guideline for internal consistency is that Cronbach’s α should be between 0.7 and 0.9 [24]. This outcome was achieved for the NINL test in its entirety and each scale also performed satisfactorily, while the consistency of other visual recognition tests is considerably lower [19]. Overall, visual-spatial recognition memory was influenced by sex while visual-spatial memory without recognition was age-dependent.
8. Conclusions
The present findings have identified a neurobehavioral profile that illustrates the importance of sex, development, and handedness factors. Although age and sex differences are frequently taken into account by neuropsychological instruments [55], the present findings, if replicated, suggest that handedness may need to be considered when developing standardized scores for MI and NINL or other similar tests. These results also indicate that the MI and NINL tests, as well as other translational paradigms, will continue to aid in bridging the gap between our understanding of changes in brain function and spatial cognition during aging in human and in non-human animals [19,56,57]. Finally, the MI and NINL tests provide new measures which, in conjunction with more traditional neuropsychological tests, will continue to be useful for furthering our understanding of sexually dimorphic differences in neurocognitive performance.
Supplementary Material
Acknowledgments
We would like to thank Andrea Middleton and the staff at the Oregon Museum of Science and Industry as well as William Cameron, Ph.D. and the Methamphetamine Abuse Research Center for their support of this project, Timothy Pfankuch, Laura Villasanova, Jessica Siegel, Sierra Tittle, and Anthony Bader for data collection in Experiment I, Ted Benice, Ph.D., Reid Olsen, Keri Shiels, Ph.D., and Alex Chu for technical assistance on Experiment II, as well as Larry W. Hawk, Jr., Ph.D. and Meredith Lubow, Ph.D. for feedback on an earlier version of this manuscript. This research was supported by Public Health Service Grant (1 UL1 RR024120-01), National Institute of Drug Abuse (T32DA07262, 1P50DA018165, L30 DA027582), National Institute of Environmental Health Sciences (T32ES007060), Clinical Research Enhancement Fund (90120298), and the Ellison Medical Foundation (AG-NS-0201).
Role of funding source: The sponsors of this research had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Conflict of Interest: OHSU receives a modest, one-time licensing fee from laboratories that use the Memory Island or Novel-Image, Novel-Location tasks.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 2.Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Rizk-Jackson AM, Acevedo SF, Inman D, Howieson D, Benice TS, Raber J. Effects of sex on object recognition and spatial navigation in humans. Behav Brain Res. 2006;173:181–90. doi: 10.1016/j.bbr.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 4.Piper BJ, Acevedo SF, Craytor MJ, Murray PM, Raber J. Spatial function as assessed by Memory Island: Behavioral development and validation in primary school children. Behav Brain Res. 2010;210:257–62. doi: 10.1016/j.bbr.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berteau-Pavy F, Park B, Raber J. Effects of sex and APOE ε4 on object recognition and spatial navigation in the elderly. Neuroscience. 2007;147:6–17. doi: 10.1016/j.neuroscience.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Acevedo SF, Piper BJ, Craytor MJ, Benice TS, Raber J. Apolipoprotein E and sex alter neurobehavioral performance in primary school children. Pediatr Res. 2010;67:293–9. doi: 10.1203/PDR.0b013e3181cb8e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132:77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- 8.Goodrich-Hunsaker NJ, Livingstone SA, Skelton RW, Hopkins RO. Spatial deficits in a virtual water maze in amnesic participants with hippocampal damage. Hippocampus. 2010;20:481–91. doi: 10.1002/hipo.20651. [DOI] [PubMed] [Google Scholar]
- 9.Laczó J, Andel R, Vyhnalek M, Vlcek K, Magerova H, Varjassyova A, et al. Human analogue of the morris water maze for testing subjects at risk of Alzheimer’s disease. Neurodegener Dis. 2010;7:148–52. doi: 10.1159/000289226. [DOI] [PubMed] [Google Scholar]
- 10.Sandstrom NJ, Kaufman J, Huettel SA. Males and females use different distal cues in a virtual environment navigation task. Cogn Brain Res. 1988;6:351–60. doi: 10.1016/s0926-6410(98)00002-0. [DOI] [PubMed] [Google Scholar]
- 11.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 12.Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: A large and reliable sex difference. Behav Brain Res. 1998;93:185–90. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 13.Mueller SC, Jackson CP, Skelton RW. Sex differences in a virtual water maze: An eye tracking and pupillometry study. Behav Brain Res. 2008;193:209–15. doi: 10.1016/j.bbr.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Woolley DG, Vermaercke B, Op de Beeck H, Wagemans J, Gantois I, D’Hooge R, et al. Sex differences in human virtual water maze performance: novel measures reveal the relative contribution of directional responding and spatial knowledge. Behav Brain Res. 2010;208:408–14. doi: 10.1016/j.bbr.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Newhouse P, Newhouse C, Astur RS. Sex differences in visual-spatial learning using a virtual water maze in pre-pubertal children. Behav Brain Res. 2007;183:1–7. doi: 10.1016/j.bbr.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Driscoll I, Hamilton DA, Yeo RA, Brooks WM, Sutherland RJ. Virtual navigation in humans: The impact of age, sex, and hormones on place learning. Horm Behav. 2005;47:326–35. doi: 10.1016/j.yhbeh.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Geschwind N, Behan P. Left-handedness: Association with immune disease, migraine, and developmental learning disorder. Proc Natl Acad Sci USA. 1982;79:5097–100. doi: 10.1073/pnas.79.16.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 19.Fagan JF, Detterman DK. The Fagan Test of Infant Intelligence: A technical summary. J Appl Dev Psych. 1992;13:173–93. [Google Scholar]
- 20.Bachevalier J. Ontogenetic development of habit and memory formation in primates. Ann NY Acad Sci. 1990;608:457–77. doi: 10.1111/j.1749-6632.1990.tb48906.x. [DOI] [PubMed] [Google Scholar]
- 21.Milner B. Interhemispheric differences in the localization of psychological processes in man. Br Med Bull. 1971;27:272–7. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- 22.Shiels K, Hawk LW, Lysczek CL, Tannock R, Pelham WE, Jr, Spencer SV, et al. The effects of incentives on visual-spatial working memory in children with attention-deficit/hyperactivity disorder. J Abnorm Child Psychol. 2008;36:903–13. doi: 10.1007/s10802-008-9221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hester RL, Kinsella GJ, Ong B. Effect of age on forward and backward span tasks. J Int Neuropsychol Soc. 2004;10:475–81. doi: 10.1017/S1355617704104037. [DOI] [PubMed] [Google Scholar]
- 24.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrica. 1951;16:297–334. [Google Scholar]
- 25.Astur RS, Tropp J, Sava S, Constable RT, Markus EJ. Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behav Brain Res. 2004;151:103–15. doi: 10.1016/j.bbr.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Peters M, Reimers S, Manning JT. Hand preference for writing and associations with selected demographic and behavioral variables in 255,100 subjects: The BBC internet study. Brain Cogn. 2006;62:177–89. doi: 10.1016/j.bandc.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–18. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- 28.Króliczak G, Piper BJ, Frey SH. Atypical lateralization of language predicts cerebral asymmetries in parietal gesture representations. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2011.02.044. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goez H, Zelnik N. Handedness in patients with developmental coordination disorder. J Child Neurol. 2008;23:151–4. doi: 10.1177/0883073807307978. [DOI] [PubMed] [Google Scholar]
- 30.Gilger JW, Pennington BF, Green P, Smith SM, Smith SD. Reading disability, immune disorders, and non-right-handedness: Twin and family studies of their relations. Neuropsychologia. 1992;30:209–27. doi: 10.1016/0028-3932(92)90001-3. [DOI] [PubMed] [Google Scholar]
- 31.Llaurens V, Raymond M, Faurie C. Why are some people left-handed? An evolutionary perspective. Philos Trans R Soc B: Biol Sci. 2009;364:881–94. doi: 10.1098/rstb.2008.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bloss CS, Delis DC, Salmon DP, Bondi MW. APOE genotype is associated with left- handedness and visuospatial skills in children. Neurobiol Aging. 2008;31:787–95. doi: 10.1016/j.neurobiolaging.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francks C, DeLisi LE, Shaw SH, Fisher SE, Richardson AJ, Stein JF, Monaco AP. Parent-of-origin effects on handedness and schizophrenia susceptibility on chromosome 2p12-q11. Hum Mol Genet. 2003;12:3225–30. doi: 10.1093/hmg/ddg362. [DOI] [PubMed] [Google Scholar]
- 34.Moffat SD, Resnick SM. Effects of age on virtual environment place navigation and allocentric cognitive mapping. Behav Neuro. 2002;116:851–859. doi: 10.1037//0735-7044.116.5.851. [DOI] [PubMed] [Google Scholar]
- 35.Overman WH, Pate BJ, Moore K, Peuster A. Ontogeny of place learning in children as measured in the radial arm maze, Morris search task, and open field task. Behav Neurosci. 1996;110:1205–28. doi: 10.1037//0735-7044.110.6.1205. [DOI] [PubMed] [Google Scholar]
- 36.Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak SJ, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA. 2000;97:4398–403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macguire EA, Spiers HJ, Good CD, Hartley T, Frackowiak RS, Burgess N. Navigation expertise and the human hippocampus: A structural brain imaging analysis. Hippocampus. 2003;13:208–17. doi: 10.1002/hipo.10087. [DOI] [PubMed] [Google Scholar]
- 38.Iaria G, Lanyon LJ, Fox CJ, Giaschi D, Barton JJ. Navigational skills correlate with hippocampal fractional anisotropy in humans. Hippocampus. 2009;18:335–9. doi: 10.1002/hipo.20400. [DOI] [PubMed] [Google Scholar]
- 39.Moffat SD, Elkins W, Resnik SM. Age differences in the neural systems supporting allocentric spatial navigation. Neurobiol Aging. 2006;27:965–72. doi: 10.1016/j.neurobiolaging.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Pine DS, Grun J, Maguire EA, Burgess EA, Burgess N, Zarahn E, et al. Neurodevelopmental aspects of spatial navigation: A virtual reality fMRI study. Neuroimage. 2002;15:396–406. doi: 10.1006/nimg.2001.0988. [DOI] [PubMed] [Google Scholar]
- 41.Grön G, Wunderlich AP, Spitzer M, Tomczak R, Riepe MW. Brain activation during human navigation: Gender-different neural networks as substrate of performance. Nat Neurosci. 2000;3:404–8. doi: 10.1038/73980. [DOI] [PubMed] [Google Scholar]
- 42.Muir GM, Taube JS. The neural correlates of navigation: Do head direction and place cells guide spatial behavior? Behav Cogn Neurosci Rev. 2002;1:297–317. doi: 10.1177/1534582302238339. [DOI] [PubMed] [Google Scholar]
- 43.Shinder ME, Taube JS. Differentiating ascending vestibular pathways to the cortex involved in spatial cognition. J Vestib Res. 2010;20:3–23. doi: 10.3233/VES-2010-0344. [DOI] [PubMed] [Google Scholar]
- 44.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 45.Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8 2008. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- 46.Piper BJ, Fraiman JB, Owens CB, Ali SF, Meyer JS. Dissociation of the neurochemical and behavioral toxicology of MDMA (‘Ecstasy’) by citalopram. Neuropsychopharmacology. 2008;33:1192–205. doi: 10.1038/sj.npp.1301491. [DOI] [PubMed] [Google Scholar]
- 47.Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16:248–66. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- 48.Kessels RPC, Ruis EBC, Brands AMA. The backward span of the Corsi Block-Tapping task and its association with the WAIS-III Digit span. Assessment. 2008;15:426–34. doi: 10.1177/1073191108315611. [DOI] [PubMed] [Google Scholar]
- 49.Zeamer A, Heuer E, Bachevalier J. Developmental trajectory of object recognition in infant rhesus macaques with and without hippocampal lesions. J Neurosci. 2010;30:9157–65. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reger ML, Hovda DA, Giza CC. Ontogeny of rat recognition memory measured by the novel object recognition task. Dev Psychobiol. 2009;51:672–8. doi: 10.1002/dev.20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutcliffe JS, Marshall KM, Neill JC. Influence of gender on working and spatial memory in the novel object recognition task in the rat. Behav Brain Res. 2007;177:117–25. doi: 10.1016/j.bbr.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 52.Wright L, Hardie S, Rodway P. Pause before you respond: Handedness influences response style on the Tower of Hanoi task. Laterality. 2004;9:133–47. doi: 10.1080/13576500244000265. [DOI] [PubMed] [Google Scholar]
- 53.Luders E, Cherbuin N, Thompson PM, Gutman B, Anstey KJ, Sachdev P, et al. When more is less: associations between corpus callosum size and handedness lateralization. Neuroimage. 2010;52:43–9. doi: 10.1016/j.neuroimage.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tzourio-Mazoyer N, Simon G, Crivello F, Jobard G, Zago L, Perchey G, et al. Effect of familial sinistrality on planum temporale surface and brain tissue asymmetries. Cereb Cortex. 2010;20:1476–85. doi: 10.1093/cercor/bhp209. [DOI] [PubMed] [Google Scholar]
- 55.Strauss E, Sherman EMS, Spreen OA. Compendium of Neuropsychological Test Administration, Norms, and Commentary. 3. New York: Oxford; 2006. [Google Scholar]
- 56.Driscoll I, Sutherland RJ. The aging hippocampus: Navigating between rat and human experiments. Rev Neurosci. 2005;16:87–121. doi: 10.1515/revneuro.2005.16.2.87. [DOI] [PubMed] [Google Scholar]
- 57.Fray PJ, Robbins TW. CANTAB battery: proposed utility in neurotoxicology. Neurotoxicol Teratol. 1996;18:499–504. doi: 10.1016/0892-0362(96)00027-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.