Abstract
We have previously reported a novel polymeric delivery vehicle that is assembled via interaction between heparin and the vascular endothelial growth factor (VEGF). Here, the cell-responsiveness of this hydrogel — including the delivery of VEGF in response to VEGFR-2 overexpressing PAE/KDR cells (porcine aortic endothelial cells (PAE) equipped with the transcript for the kinase insert domain receptor (KDR)), consequent erosion of the hydrogel matrix, and cellular response — are highlighted. The release of VEGF and hydrogel erosion reached 100% only in the presence of PAE/KDR. The [PEG-LMWH/VEGF] hydrogel (PEG = poly(ethylene glycol), LMWH = low molecular weight heparin) correspondingly prompted increases in VEGFR-2 phosphorylation and proliferation of PAE/KDR cells. This study proves that growth factor-crosslinked hydrogels can liberate VEGF in response to specific receptors, causing gel erosion and desired cell responses. The promise of these approaches in therapeutic applications, including targeted delivery, is suggested.
Keywords: dendrimers, heparin, hydrogel, responsive hydrogels, targeted delivery, VEGF, VEGFR-2
Introduction
Hydrogels of many compositions have served as highly functional delivery vehicles for biological molecules, because of their high water content, readily tunable mechanical properties, and controllable degradation rates.[1–4] Engineered delivery systems for bioactive signaling molecules, such as growth factors (GFs), have been increasingly studied, as the vehicles provide stabilization of GF activity and prolonged delivery, which increases the effectiveness of the GF in regulation of cell migration, proliferation, and gene expression.[5–8] Affinity-based sequestration of growth factors has emerged as a popular and useful approach in the development of GF-delivering systems, with heparin functionalization of matrices widely employed.[6,9–13] Specific binding, between such extracellular matrix (ECM)-mimetic materials and proteins of therapeutic interest, has been a useful tool for delivery, permitting sequestration and stabilization of the proteins.[14] Our group and others have reported heparinized hydrogel systems, equipped with bioactive signaling molecules, for modulating cellular responses.[6,15–30]
Vascular endothelial growth factor (VEGF), an endothelial-cell-specific mitogen that binds to heparin-like moieties and stimulates endothelial cell functions via receptor-mediated endocytosis,[31–33] has been widely investigated in such studies owing to its role in both beneficial and pathological angiogenesis.[34,35] Hydrogel systems designed to be selectively responsive to VEGF-based signaling have been reported for manipulation of cell migration in materials,[35] and VEGF-conjugated drugs have been shown to be selectively targeted to solid tumors in vivo.[36] These studies, coupled with the increasing use of biomolecular interactions as a strategy for manipulating hydrogel assembly and responsiveness,[21,22,37–43] suggest the enormous potential for directing targeted disassembly of hydrogels via their selective interaction with cell surface receptors in a pathological environment.
We have previously demonstrated that the noncovalent interactions of heparin and heparin-binding peptides support hydrogel formation and that GF delivery from such hydrogels can be regulated based on the erosion profile of the hydrogel.[15,16,18] Importantly, we have also demonstrated that heparin-binding VEGF can crosslink such materials, conferring to the gels erosion behavior that is responsive to the presence of the VEGFR-2 receptor (KDR).[17] Although our initial results were reported for VEGFR-2 responsive delivery in a model microparticle system, this approach should be advantageous for the targeted delivery of VEGF to its relevant receptors on cells and subsequent erosion without the need for external stimuli such as enzymatic degradation.
Here, we report the important observation that these materials are indeed selectively responsive to cells in culture that overexpressVEGFR-2, that the selective erosion observed is directly linked to VEGFR-2 concentration, and that the consequent activation of the VEGFR-2 receptor is correlated with the concentration of released VEGF. The impact of endothelial cells on hydrogel erosion, indicated by the cumulative release of VEGF and PEG-LMWH, was studied in order to confirm the cell-responsive nature of the hydrogel. These studies were conducted with two different cell types — porcine aortic endothelial (PAE) cells overexpressing 2.5 × 105 VEGFR-2/cell (PAE/(KDR)) and PAE cells that are not equipped with the transcript for VEGFR-2. The phosphorylation of VEGFR-2 was also investigated, via immunochemical methods, in the presence and absence of the VEGF-containing hydrogels to determine whether the release of the VEGF activated the target receptor. The effect of the hydrogels on the proliferation of endothelial cells was also studied in cultures of the PAE/KDR and PAE cells. The results indicate the selective, cell-mediated erosion of the hydrogel networks, as well as the activation of the target receptor by the released VEGF. These studies thus suggest the potential utility of these materials in directing the delivery of drugs to VEGFR-2-overexpressing cells in vivo, and in directing the VEGFR-2-mediated migration of cells in ECM-mimetic materials.
Experimental Part
Materials
Four-arm star PEG-thiol was purchased from Polymer Source Inc. (Montreal, QC, Canada). Low molecular weight heparin (LMWH), bovine serum albumin (BSA), NP-40 (nonyl phenoxylpolyethoxylethanol, a non-ionic detergent), sodium orthovanadate, and aprotinin were purchased from Sigma–Aldrich (Allentown, PA). Leupeptin and 4-(N-maleimidomethyl)cyclohexanecarboxylic acid N-hydroxysuccinimide ester (SMCC) were purchased from Sigma (Saint Louis, MO). o-toluidine blue was purchased from Sigma–Aldrich (Milwaukee, WI). Dulbecco’s phosphate buffered saline (D-PBS) was purchased from Mediatech, Inc. (Manassas, VA). The lysis buffer for mammalian cells (RIPA buffer) was purchased from Pierce Technology (Rockford, IL). The human recombinant VEGF was a kind gift from Genentech (San Francisco, CA). The 125I-labeled VEGF was purchased from PerkinElmer (Waltham, MA). Other chemicals were purchased from Sigma–Aldrich, unless otherwise noted. All materials were used as received.
Synthesis of the PEG-LMWH Conjugate
The PEG-LMWH conjugate was kindly provided by Dr. Nori Yamaguchi (currently at SABIC Innovative Plastics). The general procedure for the synthesis of PEG-LMWH has been previously described.[16]
Assembly of Hydrogels
The non-covalently assembled hydrogel was prepared at a concentration of 8 wt.% in PBS. The PEG-LMWH (0.4mg per sample) was placed in 1.5 mL-vials and sterilized under germicidal UV overnight. To each was added 3 µL sterile PBS followed by sonication in a water bath sonicator for 1 h. To make the PEG-LMWH/VEGF hydrogel, 1 µL VEGF (2 µg · µL−1 in PBS, sterilized via centrifugation through a 0.22 µm filter (Spin-X)) and 1 µL 125I-VEGF (0.417 ng · µL−1, PerkinElmer, Boston, MA; also sterilized via centrifugal filtration) were added to the PEG-LMWH-containing vial. To make the PEG-LMWH control, 2 µL sterile PBS was added into the PEG-LMWH-containing vial. The hydrogels were thoroughly mixed by vigorous pipetting. Hydrogels were supported on a transwell-permeable insert in following studies. The transwell permeable support (Costar 3422, 8 µm pore, 6.5 mm dia., Corning, NY) was blocked with 10% BSA (pre-filtered through a 0.22 µm filter) at room temperature for 2 h, washed with PBS five times, and sterilized under germicidal UV overnight. The hydrogels were placed on this BSA-coated transwell support prior to use. Hydrogels are indicated by the use of square brackets in the text.
General Cell Culture and Release Studies
The PAE cells, expressing 2.5 × 105 VEGFR-2 (Flk-1/KDR) per cell (PAE/KDR, hereafter), were purchased from Sibtech, Inc. (Brookfield, CT).[44–46] The porcine aortic endothelial (PAE, hereafter) cells were a kind gift from Sibtech, Inc. The cells were cultured in DMEM medium(Mediatech, Manassas, VA) supplied with 10% fetal bovine serum (heat inactivated), 2mm L-glutamine, and penicillin/streptomycin at 37 °C with 5% CO2. The cells for each data point (day 1, 2, 3, 4, and 5) were initially seeded on 24-well plate at a density of 3 000 cm−2 and allowed to attach overnight.
The release studies were conducted in a 24-well plate equipped with a transwell insert (pre-blocked with BSA) in the presence and absence of PAE/KDR or PAE cells. (Each type of material (i.e., [PEG-LMWH/VEGF] or [PEG-LMWH] hydrogel) was employed with each type of cell.) Control experiments for the release studies were conducted by inserting hydrogel-loaded transwell supports in a 24-well plate that did not contain any cells. Control experiments for the cell proliferation studies were conducted by culturing cells with 100 ng · mL−1 VEGF-containing medium (without hydrogels or transwells). The samples were incubated in 1 ml of medium at 37 °C with 5% CO2. At each data point (1, 2, 3, 4, and 5 days), 0.5 mL of medium was collected and replaced with fresh 0.5 mL medium (100 ng · mL−1 VEGF-containing medium for controls without hydrogels). Collected samples were stored in 1.5 mL centrifuge tubes (Eppendorf Protein LoBind, Fisher Scientific, Pittsburgh, PA) at −80 °C until the analysis of PEG-LMWH and VEGF content.
Analysis of Released PEG-LMWH and VEGF
The released amount of PEG-LMWH in each sample was determined via toluidine blue assay, as previously described, with slight modification.[47] The toluidine blue solution (0.005%) was prepared by mixing 25 mg o-toluidine blue, 0.49 mL 37% HCl, 1 g NaCl in 500 mL distilled water. Each collected sample (0.125 mL) was placed in a 1.5 mL centrifuge vial. To each were added 0.15 mL NaCl solution (0.1%) and 0.0625 mL toluidine blue solution (0.005%). The vials were vortexed vigorously and centrifuged at 4 000 rpm for 5 min. To each was added 0.5 mL hexane and this was vortexed for 30 s and centrifuged at 13 000 rpm for 10 min. The aqueous phase of each sample, 100 µL, was transferred to a 96-well plate (Maxisorp, Nunc Brand, Denmark). The absorbance at 620 nm was obtained on a Wallac Victor3V Multilabel Counter (PerkinElmer, Waltham, MA), with Wallac 1420 Workstation software. The concentration of PEG-LMWH was estimated by extrapolating from a linear standard calibration curve consisting of 0, 0.019, 0.032, 0.049, 0.061, 0.076, 0.116, 0.204mg · mL−1 PEG-LMWH.
In the [PEG-LMWH/VEGF] hydrogels, 125I-labeled VEGF (125I-VEGF) was added as a tracer. The released amount of VEGF was assessed by monitoring the radioactivity of collected samples. Each collected sample, in 50 µL aliquots, was mixed with 5 mL liquid scintillation cocktail (CytoScint, Fisher Scientific, Pittsburgh, PA). The radioactivity in cpm was monitored on an LS 6500 Multi-Purpose Scintillation Counter (Beckman Instruments, Inc., Columbia, MD). The VEGF concentration of each collected sample was extrapolated from a linear standard calibration curve, composed by the analysis of standard samples of 0, 0.013, 0.026, 0.052, 0.104, 0.208, and 0.417 ng · mL−1 125I-VEGF in DMEM. In order to take the decay of the 125I isotope (half-life = 60 days) into consideration in the determination of VEGF concentration, the standards were analyzed at the start of the experiment, as well as at all subsequent time points. All experiments were conducted on six replicates.
Proliferation of PAE Cells in the Presence of Hydrogels
The effects of hydrogels on proliferation of PAE/KDR and PAE cells were assessed in a 24-well plate assay format with hydrogel-loaded transwells. The 8 wt.% hydrogels were prepared by mixing PEG-LMWH (0.4 mg in 3 µL PBS) with 2 µL VEGF solution (in PBS, containing 2 µg VEGF + 0.417 ng 125I-VEGF) for the [PEG-LMWH/VEGF] hydrogel, or with 2 µL PBS for the [PEG-LMWH] hydrogel, respectively. The hydrogel was loaded on the transwell and this was inserted into the 24-well plate seeded with PAE/KDR or PAE cells. Control experiments for the cell proliferation studies were conducted by culturing cells with 100 ng · mL−1 VEGF-containing medium (without hydrogels or transwells). The number of cells after 4 days incubation were quantified by monitoring the amount of resorufin converted from resazurin by metabolically active cells,[48,49] using the CellTiter-Blue reagent (Promega, Madison, WI). The CellTiter-Blue reagent was added to the cultured cell medium at 10 v/v% and incubated for 2 h. The supernatant medium was transferred to a 96-well plate and the absorbance at 570 nm was analyzed on a Wallac Victor3V Multilabel Counter (PerkinElmer, Waltham, MA), with Wallac 1420 Workstation software. The absorbance at 620 nm was subtracted from the absorbance at 570 nm. The cell growth data are presented as the % of control, in which the PAE/KDR and PAE were cultured in medium containing 100 ng · mL−1 VEGF, but lacking any hydrogels. All experiments were conducted on six replicates.
Phosphorylation of VEGFR-2
The cells were lysed to investigate the phosphorylation of VEGFR-2 in each sample at each data point. The cells were lysed in 100 µL lysis buffer consisting of 10% glycerol, 2 mM EDTA, 1 mM activated sodium orthovanadate, 10 µg · mL−1 aprotinin, and 10 µg · mL−1 leupeptin in RIPA buffer (25 mM Tris-HCl, pH 7.6, 150 mM NaCl, 1% NP-40, 0.1% SDS, 1% sodium deoxycholate) on ice for 30 min. The lysate was collected by pipetting several times and then was placed in a 1.5-mL centrifuge vial (Eppendorf Protein LoBind, Fisher Scientific, Pittsburgh, PA). The lysate was centrifuged at 14 000 rpm and 4 °C for 10 min. The supernatant was carefully collected, put in a clean 1.5-ml centrifuge vial, and stored at −80 °C until the assay.
The total amount of VEGFR-2 and phosphorylated VEGFR-2 in each sample was immunochemically measured using ELISA-type kits (DuoSet IC Total VEGF R2/KDR ELISA and DuoSet IC Phospho-VEGFR2/KDRELISA, R&D Systems, Inc., Minneapolis, MN).Briefly, the capture antibody at recommended concentrations was incubated in a 96-well plate (Maxisorp, Nunc Brand, Denmark) overnight. The solution was removed and the plate was washed thoroughly with 0.05%Tween 20 (in PBS) and aspirated for a total of five washes (referred to as the wash step). The plate was blocked with buffer containing 1% BSA and 0.05% NaN3 for 2 h and the solution was removed. The sample (or standard) was added and incubated for 1 h. The wash step was repeated. For total VEGFR-2, the biotinylated detection antibody was added and incubated for 2 h, and the wash step was repeated. The streptavidin-HRP (horseradish peroxidase) solution was added and incubated for 20min; direct light was avoided. For phosphorylated VEGFR-2 quantitation, the detection antibody (conjugated with HRP) solution was added and incubated for 2 h; again avoiding direct light. The wash was repeated as described above. The 1-Step Ultra TMB solution (Pierce-Thermo Fisher Scientific, Inc., Rockford, IL) was added and incubated for 20 min, avoiding direct light. To this was added 2N H2SO4 to halt the colorimetric reaction. The absorbance at 450 nm was measured on a Fusion Universal Microplate Analyzer (PerkinElmer, Boston, MA). The absorbance at 570nm was subtracted from the absorbance at 450 nm, in order to correct for the optical imperfection of the plate. The concentrations of total VEGFR-2 and phosphorylated VEGFR-2 were determined via extrapolation from a linear standard curve consisting of 0, 0.030, 0.063, 0.125, 0.25, 0.50, 1.0 and 2.0 ng · mL−1 total VEGFR-2 or phosphorylated VEGFR-2, respectively. All experiments were conducted with six replicates.
Statistical Analysis
The statistical analysis between different sample sets (each consisting of six replicates as mentioned above) was performed using the Students’ t-test. Determined p-values less than 0.05 are considered statistically significant.
Results and Discussion
Hydrogel Design
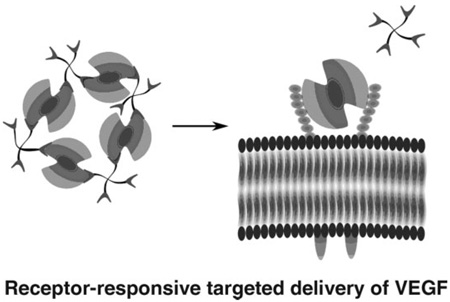
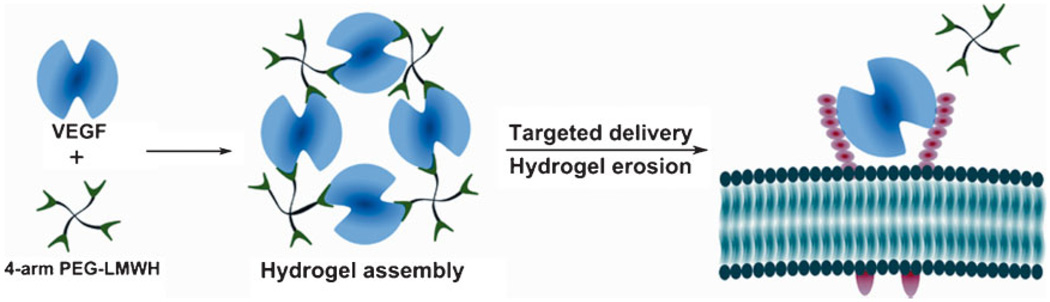
A novel reservoir for VEGF that can simultaneously release VEGF and erode in response to the VEGFR-2 cell receptor is illustrated in Scheme 1. The hydrogel is assembled via interaction between LMWH and VEGF. Heparin has been shown to protect GFs from inactivation, increase their binding to receptors, and sustain the release of GF over prolonged periods.[6,11,50,51] LMWH was employed in this study in order to minimize the intramolecular interaction of VEGF with heparin, which would reduce crosslinking density.[52] The LMWH was chemically immobilized to the termini of 4-arm star poly(ethylene glycol) (10 kDa) to yield PEG-LMWH. Three out of four arms were functionalized on average (f = 3), which permits network formation upon the addition of the dimeric VEGF cross-linker (f = 2). Upon mixing of the two components, a viscoelastic hydrogel was instantly formed as indicated by a distinct increase in the storage modulus over that of the PEG-LMWH alone. (The PEG-LMWH conjugate itself also forms very weak hydrogels due to the self-association of heparin.[52]) The addition of a control protein, BSA, did not result in such an increase in the storage modulus, confirming that dimeric VEGF acts as an active crosslinker of PEG to form a [PEG-LMWH/VEGF] hydrogel. The elastic moduli of hydrogels of this composition, as previously reported and as assessed via optical probe microrheology, was on the order of 10 Pa.[17] The immediate formation of the hydrogel suggests the potential for the use of these materials via injection protocols that are less invasive than implantation. Initial studies employing VEGFR-2-modified poly(styrene) particles indicated that the release of VEGF from the [PEG-LMWH/VEGF]was dependent on the presence of VEGFR-2, a relevant VEGF receptor[17] and a useful target owing to the importance of VEGFR-2 as the primary mediator of the proliferation of endothelial cells during development, homeostasis, vasculogenesis, and angiogenesis.[31–33,53–55] Our initial studies clearly demonstrated the responsiveness of the hydrogels to VEGFR-2.
Scheme 1.
Non-covalently assembled hydrogel for targeted delivery of VEGF.
Successful implementation of these hydrogels in biological environments, however, requires that these materials also be selectively responsive to VEGFR-2 in the presence of the glycosaminoglycans and proteins present on cell surfaces. To test the potential of these hydrogels to selectively release VEGF (and thus erode) in response to cells that overexpress VEGFR-2, two engineered endothelial cell types were employed. Waltenberger et al. reported that PAE cells lacking endogeneous VEGF receptors can show a mitogenic response to VEGF when transfected with a plasmid encoding the VEGFR-2 receptor.[56] VEGF induces endothelial cell proliferation through VEGF-dependent tyrosine phosphorylation initiated by binding to Flk-1/KDR (VEGFR-2); binding through Flt-1 (VEGFR-1) results in less phosphorylation although Flt-1 binds VEGF with strong affinity.[31,53,56] Thus, in our studies, [PEG-LMWH/VEGF] was incubated in the presence of porcine aortic endothelial cells (PAE) engineered to overexpress (2.5 × 105 VEGFR-2 per cell; PAE/KDR) or in the presence of cells lacking the transcript forVEGFR-2(PAE).[45,46] The cumulative release of VEGF and PEG-LMWH was monitored in a transwell assay cell culture format in which hydrogels were placed in the transwell insert and PAE cells were cultured on the PS surface of the well of a 24-well plate.[17] A well lacking any cells was employed as a control. This transwell assay format optimized cell proliferation and was necessary given that the hydrogels lack any cell-adhesive domains; this format is also representative of situations in which the [PEG-LMWH/VEGF] would be employed as a drug delivery vehicle. The two types of PAE cells were also used to evaluate the impact, on endothelial cell proliferation, of the released VEGF from the non-covalently assembled [PEG-LMWH/VEGF]. The phosphorylation of VEGFR-2 was also investigated in the presence and absence of the VEGF-containing hydrogel to determine whether the release of the VEGF activated the target receptor.
Release of PEG-LMWH
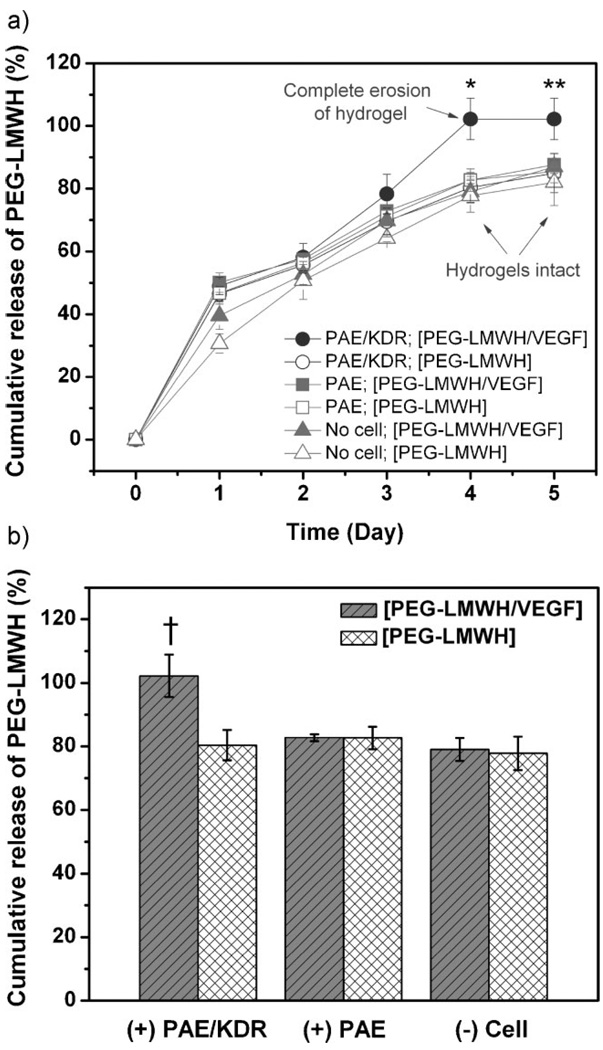
The impact of VEGFR-2, the main mitogenic VEGF receptor, on hydrogel erosion was investigated by monitoring the release of the PEG-LMWH carrier from the gels in the presence of PAE cells equipped with or without the VEGFR-2 (i.e., PAE/KDR or PAE, respectively). PAE/KDR or PAE were seeded in 24-well plates for each data point (1, 2, 3, 4, and 5 days; data were not collected after day 5 owing to the confluence of the cells.). The [PEG-LMWH/VEGF] or [PEG-LMWH] hydrogels were placed on a transwell support and these were inserted in each of the cell-containing wells, respectively. The amount of PEG-LMWH in the removed aliquots of medium was evaluated via toluidine blue assay. The cumulative release of PEG-LMWH from hydrogels is shown in Figure 1, and indicates the degree of hydrogel erosion. Specifically, Figure 1(a) shows the release profiles of PEG-LMWH from the [PEG-LMWH/VEGF] and [PEG-LMWH] in the presence of PAE/KDR cells, PAE cells, and in the absence of cells. Burst release was observed in all sets of gels, with 49.0 ± 2.8% release of PEG-LMWH from [PEG-LMWH/VEGF] in the presence of PAE/KDR and an average of 43.6 ± 6.8% release in other samples. The burst release of PEG-LMWH from all of the non-covalently assembled hydrogels (both in the absence and presence of cells) is consistent with our observations in previous studies,[18,57] and is likely a result of gel heterogeneity. Similarly, the fact that the burst release (in the absence of cells) was very slightly lower for the [PEG-LMWH] gel than for the [PEG-LMWH/VEGF] gel (p ≤ 0.17, despite the greater crosslinking expected for the [PEG-LMWH/VEGF]) most likely also results from heterogeneity introduced during gel preparation. The cumulative release of PEG-LMWH at day 4 under all different experimental conditions is shown in Figure 1(b) to facilitate comparison.
Figure 1.
The release of PEG-LMWH from non-covalently assembled hydrogels as indicative of hydrogel erosion. (a) The release profiles of [PEG-LMWH/VEGF] (closed) or [PEG-LMWH] (open) hydrogels, in the presence of PAE/KDR (●;○) and PAE (■;□) or in the absence of cells (▲;△), respectively. *p < 0.001; **p < 0.007. (b) The cumulative release of PEG-LMWH at day 4. †p < 0.001.
The release profiles of PEG-LMWH in all samples were similar during this experiment, except for that of [PEG-LMWH/VEGF] in the presence of PAE/KDR; this exception was statistically significant from day 3 onward (p < 0.001 at day 4 and p < 0.007 day 5), with an approximate 20% difference in release between the samples after day 4. Despite the fact that the passive release observed in these experiments is greater here than we have previously reported in model studies or have observed in similar cell culture experiments (see Figures S1 and S2 in the Supporting Information),[17] the impact of the VEGFR-2 on increasing hydrogel erosion was unambiguous: the [PEG-LMWH/VEGF] hydrogel was completely eroded by day 4 in the presence of PAE/KDR, whereas the hydrogels in all other samples remained intact until the end of the release experiment (day 5; experiments could not be run longer owing to the confluence of the PAE cell cultures). Comparison of PEG-LMWH release from [PEG-LMWH/VEGF] versus [PEG-LMWH] hydrogels in the presence of PAE/KDR indicated the greater rate of erosion of the VEGF-crosslinked hydrogel (Figure 1(b) (p < 0.001)), confirming that the binding of VEGF to VEGFR-2 accelerates the erosion of [PEG-LMWH/VEGF]. The fact that the release of PEG-LMWH for all other samples is essentially identical confirms not only that both VEGF and VEGFR-2 are necessary for the accelerated erosion and but also that other cell-surface molecules of the PAE cells do not accelerate erosion of these gels. Otherwise, the release of PEG-LMWH from the gels in the presence of PAE would be greater than that observed in the absence of cells. In addition, given the similarity in release profiles of the [PEG-LMWH] gels in the presence and absence of PAE cells, erosion of [PEG-LMWH] is suggested to occur via passive diffusion. Although reliable minimization of passive release (to levels we observe in other studies, Figures S1 and S2) will be necessary prior to practical implementation of these approaches, this result clearly indicates that the presence of VEGFR-2 on cell surfaces accelerates the erosion of [PEG-LMWH/VEGF] hydrogels.
Release of VEGF
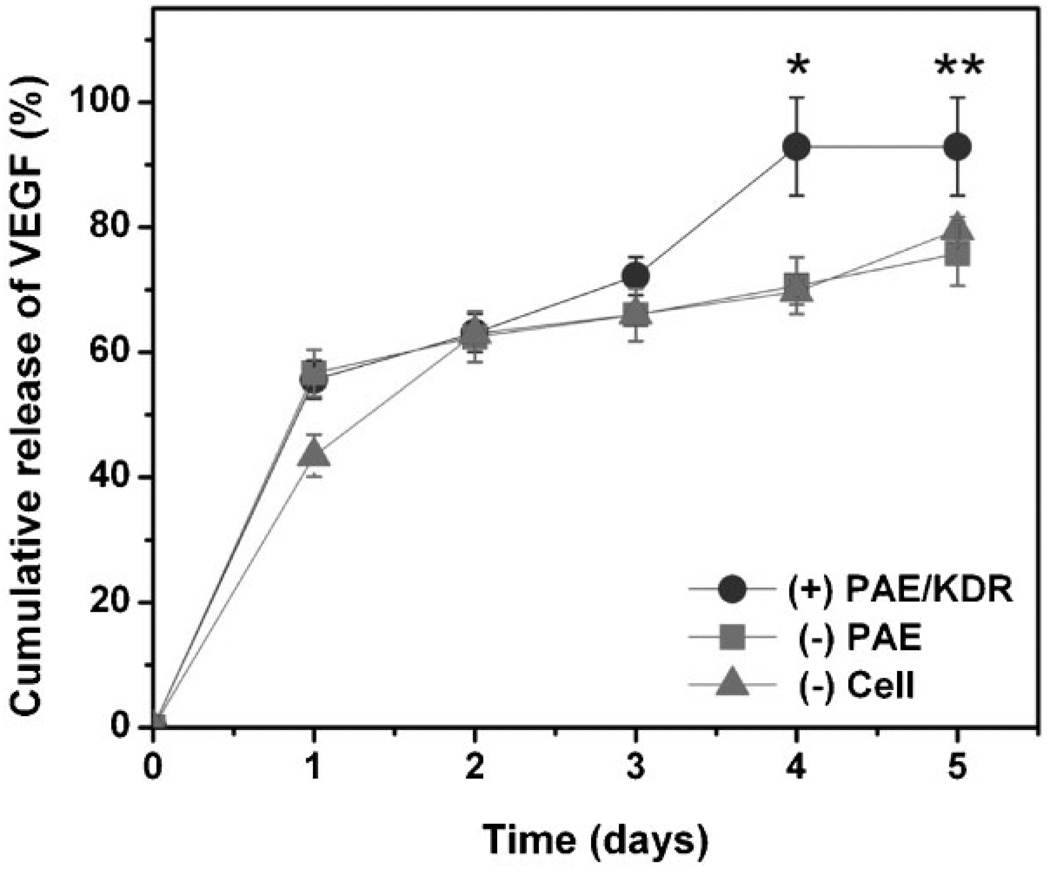
Release of VEGF in response to the VEGFR-2 presenting cells was also investigated for comparison with PEG-LMWH release. The release of VEGF from [PEG-LMWH/VEGF] hydrogels in the presence and absence of cells was monitored via measuring the radioactivity of 125I-labeled VEGF[58,59] in the collected medium. The release profiles are shown in Figure 2. In general, the release profiles of VEGF exhibited very similar trends to those observed for PEG-LMWH release from [PEG-LMWH/VEGF] hydrogels, with the release of VEGF being significantly different in the presence of PAE/KDR versus in the presence of PAE or in the absence of cells (p < 0.094 at day 3, p < 0.002 at day 4, and p < 0.0035 at day 5). The VEGF release in the presence of PAE/KDR showed a burst release of 55.6 ± 3.0% at day 1, and reached a maximum of 92.9 ± 7.8% at day 4. In contrast, burst release of 56.7 ± 3.8% was observed at day 1 in the presence of PAE, and the cumulativereleasewas75.7 ± 5.1% at day 5.The initial release of VEGF from [PEG-LMWH/VEGF] hydrogels in the absence of cells was 43.4 ± 3.4% with a cumulative release of 79.6 ± 2.0% at day 5. VEGF release from [PEG-LMWH/VEGF] reached the maximum at day 4 in the presence of PAE/KDR, concurrent with the complete erosion of the gel.
Figure 2.
The release profile of VEGF from non-covalently assembled [PEG-LMWH/VEGF] hydrogels. Release profiles of VEGF in the presence of PAE/KDR (●) or PAE (■) cells, and in the absence of cells (▲), respectively. *p < 0.002; **p < 0.004.
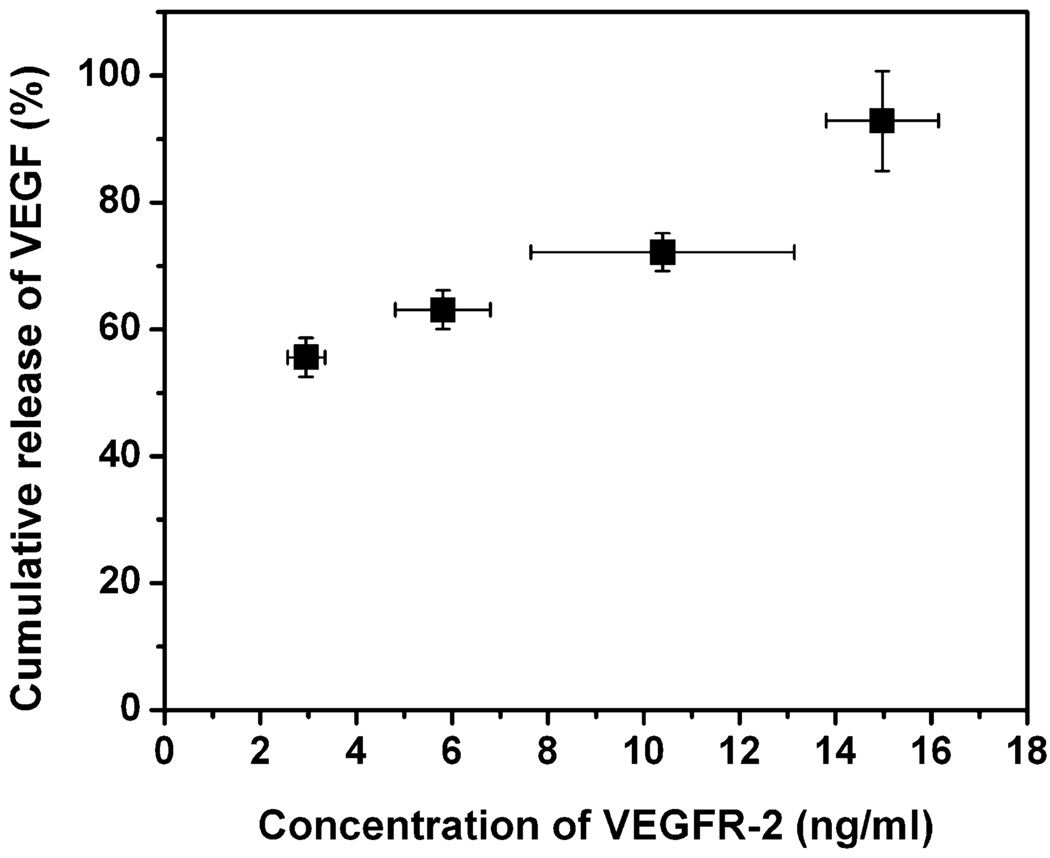
The release of VEGF from the [PEG-LMWH/VEGF] was in factmediatedbyVEGFR-2, rather than by other select surface molecules present on PAE/KDR, was also confirmed by the correlation of the amount of liberated VEGF with the concentration of the VEGFR-2 present in the PAE/KDR cultures, as determined by immunochemical methods. The concentration of VEGFR-2 from the PAE/KDR cells in the presence of the [PEG-LMWH/VEGF] was measured daily using an ELISA kit for total VEGFR-2. The cumulative release of VEGF is graphically presented versus the measured concentration of VEGFR-2 in Figure 3. The data presented in Figure 3 clearly indicate the correlation of the release of VEGF from [PEG-LMWH/VEGF] with the total amount of VEGFR-2 present on PAE/KDR, indicating that the VEGF release and consequent [PEG-LMWH/VEGF] erosion was accelerated due to the demand for VEGF by VEGFR-2. This observation is consistent with the reported selectivity of cytotoxicity of VEGF-toxin conjugates to PAE cells, dependent on the KDR expression level, with IC50 values of 0.19 nm for PAE/KDR cells versus 2.85 nm for PAE/0.1KDR cells.[45] Considering that normal VEGFR-2 expressing cells (such as human umbilical vein endothelial cells, HUVEC) express 3–5 × 104 VEGFR-2/cell (commensurate with the level of expression of PAE/0.1KDR), it is anticipated that this VEGFR-2 responsive hydrogel system might therefore release some VEGF in response to normal endothelial cells, although at a slower rate than in response to pathological cells. Notably, the VEGF-toxin conjugates, despite their ability to bind to both PAE/KDR and PAE/0.1KDR cells, were shown to be selectively toxic toVEGFR-2-overexpressing PAE/KDR while not affecting the other endothelial cells,[45,46] suggesting that appropriately engineered [PEG-LMWH/VEGF] would show similar selectivity. Overall, our results suggest that the non-covalently assembled [PEG-LMWH/VEGF] is similarly responsive to cell-surface receptors and, in light of recent reports of the in vivo selectivity of VEGF-modified vehicles,[36] represents a viable strategy for targeted hydrogel erosion and drug delivery.
Figure 3.
Release of VEGF with respect to the concentration of VEGFR-2 in PAE/KDR cell cultures.
Phosphorylation of VEGFR-2
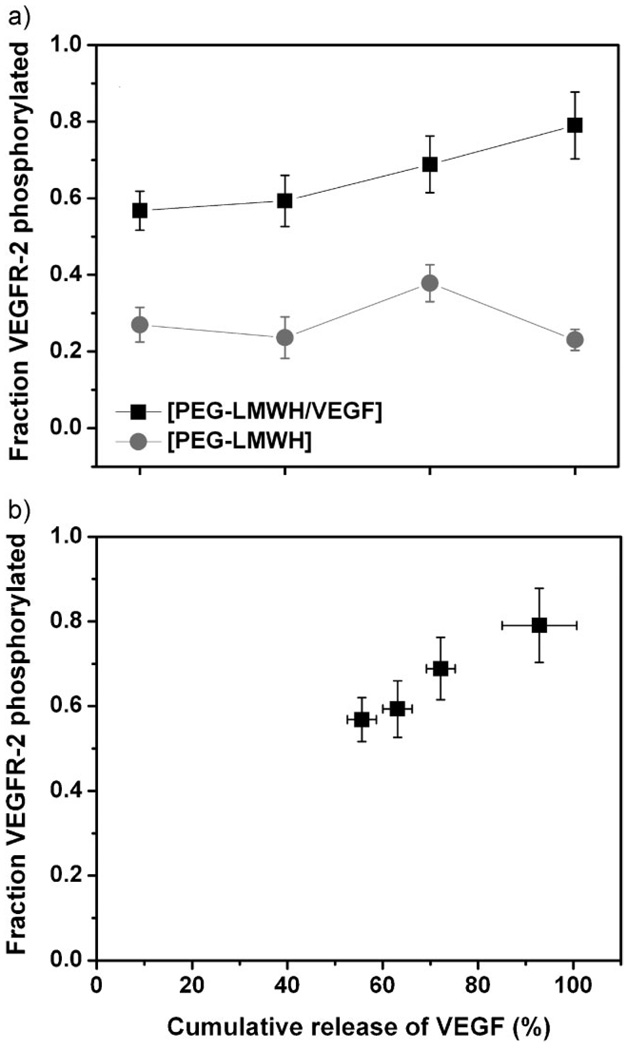
The effect of released VEGF, from the [PEG-LMWH/VEGF] hydrogel, on the activation of VEGFR-2 was monitored via assessment of the degree of tyrosine phosphorylation of VEGFR-2 observed in response to released VEGF. Upon binding with VEGF, the VEGFR-2 undergoes dimerization and tyrosine phosphorylation, resulting in mitogenic, chemotactic, and prosurvival signals.[54] The efficacy of delivered VEGF, and the responsiveness of the endothelial cells to the gels, can therefore be indicated by the level of VEGFR-2 phosphorylation,[5] which was determined immunochemically. Figure 4 presents the fraction of phosphorylated VEGFR-2 (relative to the total VEGFR-2 isolated from PAE/KDR cells), as a function of time. The cells were lysed at each data point in the presence of kinase inhibitors to maintain the level of phophorylation at the time of cell collection. The concentration of total VEGFR-2 and concentration of phosphorylated VEGFR-2 in lysates were measured using ELISA kits. As shown in the data, the phosphorylated fraction of VEGFR-2 in PAE/KDR cultures incubated with the VEGF-releasing hydrogel [PEG-LMWH/VEGF] increased from 0.57 ± 0.05 at day 1 to 0.79 ± 0.09 (p < 0.003) at day 4. This level was significantly higher than that observed in control experiments in which PAE/KDR were cultured in the presence of the [PEG-LMWH] hydrogel (0.28 ± 0.07, Figure 4(a)), as well as that of a control in which cultures of PAE/KDR were incubated without hydrogel (0.22 ± 0.04, not shown); in addition, the level of phosphorylation in the control did not show a clear increase with time.
Figure 4.
The phosphorylated fraction of VEGFR-2 in PAE/KDR cultures. (a) Phosphorylation of VEGFR-2 of PAE/KDR cultured in the presence of [PEG-LMWH/VEGF] (■) and [PEG-LMWH] (●) hydrogels. (b) Phosphorylation of VEGFR-2 as a function of cumulative release of VEGF from the [PEG-LMWH/VEGF] hydrogel.
The fraction of phosphorylated VEGFR-2 was plotted against the cumulative release of VEGF from the [PEG-LMWH/VEGF] hydrogels, to confirm if the phosphorylation of the receptor was correlated with VEGF release. As shown in Figure 4(b), the fraction of VEGFR-2 phosphorylated was correlated to the cumulative release of VEGF, confirming that the released VEGF is interacting with and stimulating the phosphorylation of VEGFR-2, and corroborating this interaction as the relevant one in modulating hydrogel behavior (release and erosion). It is also of note that although the phosphorylation of VEGFR-2 increased with released VEGF, the total VEGFR-2 expression level per cell did not change ((8.7 ± 1.2) × 10−5 ng · mL−1 per cell at day 1 versus (8.5 ± 0.7) × 10−5 ng · mL−1 at day 4). This suggests that while the [PEG-LMWH/VEGF] hydrogels are responsive to VEGFR-2 and induce greater signaling by the cell, that they do not alter VEGFR-2 expression that could present a potentially pathological response.
Proliferation of PAE Cells
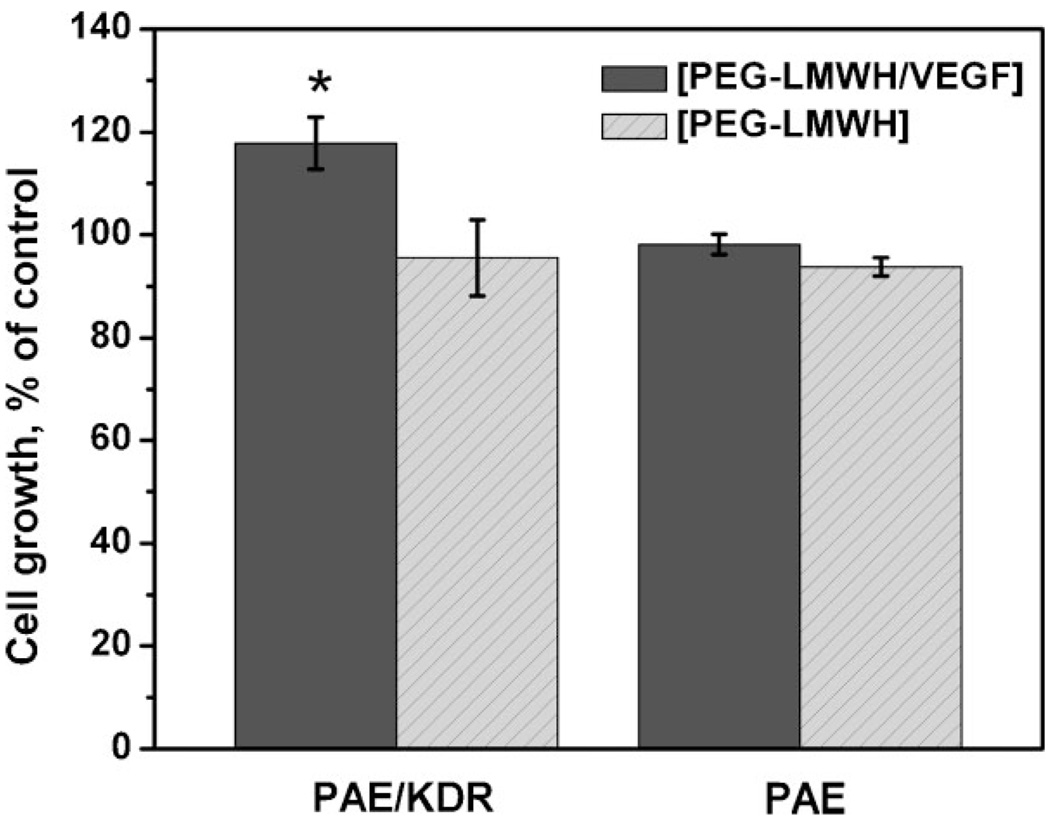
The mitogenic effects of VEGF released from the hydrogels, suggested by the phosphorylation data, were corroborated in cell culture. Figure 5 presents the percentage of cell growth (at day 4, relative to a control) of PAE/KDR and PAE in the presence of [PEG-LMWH/VEGF] and [PEG-LMWH], as colorimetrically monitored via CellTiter-Blue assay. Each cell type was cultured separately in the presence of 100 ng · mL−1 (2.63 nm) soluble VEGF as the control. The number of cells was quantified at day 4 owing both to the complete erosion of the [PEG-LMWH/VEGF] at day 4 and the confluence of the PAE/KDR cells after day 5. As shown in the data, the PAE/KDR cultures in the presence of [PEG-LMWH/VEGF] show cell numbers approximately 120% of those observed for the control (PAE/KDR in the presence of soluble VEGF; p < 0.003). Importantly, the number of PAE/KDR cells was ca. 30% higher in the presence of [PEG-LMWH/VEGF] than in the presence of [PEG-LMWH] (p < 0.001). This result is completely consistent both with the increased phosphorylation of VEGFR-2 as a result of VEGF release from [PEG-LMWH/VEGF] and with the known responsiveness of VEGFR-2 to VEGF as a key signaling event in endothelial cell proliferation.[31,54,56] In addition, the effect of [PEG-LMWH/VEGF] hydrogels on the proliferation of PAE, which lacks the VEGFR-2 transcript, was no different than the effect of the [PEG-LWMH], indicating the key role of the VEGF/VEGFR-2 interactions on the observed cell proliferation. This is consistent with our observed experimental differences in phosphorylation efficiency, as well as with differences in the mitogenic effects of VEGFR-2 over VEGFR-1.[56] That the increased proliferation is not quite as high as we have previously observed[17] is likely due to the differences in the amount of VEGF loaded in the gels; in these studies the estimated amount of VEGF released (52 ± 4 nM at maximum release) is greater than the optimal VEGF concentration range for PAE/KDR (1–10 nM).[44] Sufficiently high VEGF concentrations can inhibit the dimerization of the receptors and the subsequent receptor-mediated endocytosis that triggers proliferation. At this VEGF concentration, the suppression of receptor dimerization would be significant in normal endothelial cells (such as HUVECs). Due to the high expression of VEGFR-2 in PAE/KDR, coupled with our observation of VEGFR-2 phosphorylation, it is clear that the dimerization was not completely inhibited in our study, and our results indicate that the released VEGF from the hydrogel selectively increases proliferation of VEGFR-2-expressing endothelial cells. Gel crosslinking and erosion rates could be manipulated to modulate the amount of VEGF released and its effect on consequent receptor dimerization, depending on the target application.
Figure 5.
The proliferation of PAE/KDR and PAE cells after 4 days incubation, in the presence of non-covalently assembled [PEG-LMWH/VEGF] or [PEG-LMWH] hydrogels, respectively. The data are presented as % of control in which PAEs were cultured in the presence of 2.63 nm (100 ng · ml−1) VEGF in medium. *p < 0.003.
Responsive hydrogels such as these, as well as those reported by others, provide potential opportunities for controlled/targeted drug delivery and tissue engineering by exploiting biomolecular interactions at the cellular level. Receptor-responsiveness has been employed previously in the assembly of hydrogels, as reported by Lee et al.[37] Addition of RGD-modified alginate to MC3T3-E1 mouse calvarial preosteoblast resulted in formation of a hydrogel with G′ values of approximately 10 Pa, suggesting its potential application as a tissue engineering scaffold. Lutolf et al. reported enzyme-responsive hydrogel degradation via the action of matrix metalloproteinases (MMPs), in which human primary fibroblast cells invaded hydrogels by cleaving the susceptible peptide sequence.[60] The formation of the PEG-LMWH/VEGF hydrogel, while sharing similarities with the noncovalently assembled hydrogels of Lee et al., illustrates that these principles can indeed be employed for hydrogel formation between partners of significantly lower functionality (f = 3 and f = 2). Such flexibility suggests the broad range of types, architectures, and compositions of polymeric systems and biomolecules that can be employed in materials assembly. In contrast to the previously reported studies of Lutolf et al., the PEG-LMWH/VEGF hydrogel erodes in response to a relevant cell-surface receptor upon the liberation of cross-linker; the combination of both enzymatically induced and cell-surface receptor-induced degradation/erosion thus suggests an equally broad range of strategies and selectivities that may be engineered to manipulate degradation of hydrogels.
The previously reported, targeted delivery of VEGF-containing molecules also illustrates the potential applicability of the PEG-LMWH/VEGF hydrogels. Backer et al. demonstrated the receptor-targeted delivery of multiple VEGFs (e.g., Shiga-like toxin-VEGF fusions (SLT-VEGF) or modified single-chained VEGFs (scVEGF)), and demonstrated that VEGFR-2-specific receptor binding was crucial for their delivery.[36,45,46] This observation is similar to our results in which VEGF release and hydrogel erosion were notably promoted in the presence of overexpressed VEGFR-2. While others have also reported receptor-specific targeted delivery systems, including those responsive to the epidermal growth factor receptor (EGFR),[61] folate receptor (FR),[62] and fibroblast growth factor receptor (FGFR),[63] in these previously reported studies the targeting molecules were conjugated to vehicles such as liposomes, nanogels, and dendrimers, and the drug (doxorubicin, for example) was incorporated into the vehicles. The PEG-LMWH/VEGF hydrogel presented here is easily assembled non-covalently via the interaction between VEGF and heparinized PEG to constitute a stabilized drug carrier; VEGF serves not only as a targeting molecule, but also as a crosslinker and potential therapeutic. The hydrogel delivers VEGF in a receptor-responsive manner and induces the expected cellular response. While that response is an increase in cell proliferation in the studies here, VEGF-antagonist crosslinkers could also be employed to produce drug delivery vehicles with the opposite impact. This novel concept thus demonstrates a robust approach for targeted delivery of receptor-binding bioactive molecules in numerous therapeutic applications.
Conclusions
A novel targeted VEGF delivery system that is sensitive to cells on the basis of cell-surface receptor-ligand interactions has been demonstrated. The hydrogel was assembled via non-covalent interactions between four-arm star PEG-LMWH and dimeric, heparin-binding VEGF. Upon application to the targeted cells, the hydrogel released VEGF in response to the relevant VEGF receptor, i.e., VEGFR-2, and the hydrogel consequently eroded. The fact that hydrogel erosion and VEGF release was only greater in the presence of VEGFR-2-expressing cells, coupled with the observation that erosion and release were identical for samples incubated in the presence of non-VEGFR-2-expressing cells and in the absence of any cells, indicates the selectivity of the erosion to VEGFR-2 on the cell surface. The receptor-responsiveness of the hydrogel and the efficacy of delivered VEGF were also confirmed via the selective phosphorylation of VEGFR-2 and the increased proliferation of VEGFR-2-expressing cells in the presence of the [PEG-LMWH/VEGF]. Hydrogels of this type could be utilized in multiple applications such as therapeutic angiogenesis where a sustained and targeted delivery of VEGF to the relative receptor could accelerate a selective angiogenic response, and in which the hydrogels would be completely eroded and eliminated. The identification and use of heparin-binding, VEGF antagonist crosslinks would offer opportunities for targeted antiangiogenic therapies. Successful targeted delivery in vivo is suggested by previous studies of VEGFR-2 targeted delivery of VEGF-modified drugs in vivo;[46] in vivo studies of targeted delivery by these hydrogels are planned.
Supplementary Material
Acknowledgments
This work was financially supported by the National Institutes of Health (1 RO1 EB003172-01) and the Arnold and Mabel Beckman Foundation. The authors thank Dr. Nori Yamaguchi for providing PEG-LMWH, and Ann Daugherty (Genentech, Inc.) is thanked for providing the human recombinant VEGF.
Biographies

Sung Hye Kim received her Ph.D. in Materials Science and Engineering at University of Delaware under the direction of Professor Kristi L. Kiick. Her dissertation topic was about developing novel polymeric hydrogel systems for controlled delivery of therapeutic proteins. She is currently a postdoctoral fellow in the Department of Chemistry and Biochemistry at University of California-Los Angeles, under the supervision of Professor Heather D. Maynard. Her research interests are on conjugation of proteins and polymers for therapeutic applications and on manipulation of cellular responses on nanopatterened surfaces.

Kristi Kiick is an Associate Professor of Materials Science and Engineering at the University of Delaware. She received a BS in Chemistry from the University of Delaware in 1989, an MS in Chemistry as an NSF Predoctoral Fellow from the University of Georgia in 1991. She began doctoral studies in 1996 under the direction of David Tirrell, graduating with a PhD in Polymer Science and Engineering from the University of Masschusetts Amherst in 2001 after completing her research as an NDSEG Fellow at the California Institute of Technology. Her current research programs are focused on combining biosynthetic techniques, chemical methods, and bioinspired assembly strategies in the production of polymeric materials with novel multifunctional behavior.
Footnotes
Supporting information for this article is available at the bottom of the article’s abstract page, which can be accessed from the journal’s homepage at http://www.mrc-journal.de, or from the author.
References
- 1.Hoffman AS. Adv. Drug Delivery Rev. 2002;54:3. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 2.Kopeček J. Biomaterials. 2007;28:5185. doi: 10.1016/j.biomaterials.2007.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin C-C, Metters AT. Adv. Drug Delivery Rev. 2006;58:1379. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Park K, Shalaby WSW, Park H. Biodegradable Hydrogels for Drug Delivery. 1st edition. Lancaster: CRC; 1993. [Google Scholar]
- 5.Babensee JE, McIntire LV, Mikos AG. Pharm. Res. 2000;17:497. doi: 10.1023/a:1007502828372. [DOI] [PubMed] [Google Scholar]
- 6.Lee AC, Yu VM, Lowe JB, III, Brenner MJ, Hunter DA, Mackinnon SE, Sakiyama-Elbert SE. Exp. Neurol. 2003;184:295. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 7.Nimni ME. Biomaterials. 1997;18:1201. doi: 10.1016/s0142-9612(97)00050-1. [DOI] [PubMed] [Google Scholar]
- 8.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Nat. Biotechnol. 2001;19:1029. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 9.Edelman ER, Nugent MA, Smith LT, Karnovsky MJ. J. Clin. Invest. 1992;89:465. doi: 10.1172/JCI115607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishihara M, Sato M, Hattori H, Saito Y, Yura H, Ono K, Masuoka K, Kikuchi M, Fujikawa K, Kurita A. J. Biomed. Mater. Res. 2001;56:536. doi: 10.1002/1097-4636(20010915)56:4<536::aid-jbm1125>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Liao IC, Wan ACA, Yim EKF, Leong KW. J. Controlled Release. 2005;104:347. doi: 10.1016/j.jconrel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Wissink MJB, Beernink R, Poot AA, Engbers GHM, Beugeling T, van Aken WG, Feijen J. J. Controlled Release. 2000;64:103. doi: 10.1016/s0168-3659(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 13.Linhardt RJ, Murugesan S, Jin X. Curr. Top. Med. Chem. 2008;8:80. doi: 10.2174/156802608783378891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KY, Peters MC, Anderson KW, Mooney DJ. Nature. 2000;408:998. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi N, Chae B-S, Zhang L, Kiick KL, Furst EM. Biomacromolecules. 2005;6:1931. doi: 10.1021/bm0500042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaguchi N, Kiick KL. Biomacromolecules. 2005;6:1921. doi: 10.1021/bm050003+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi N, Zhang L, Chae B-S, Palla CS, M. Furst E, Kiick KL. J. Am. Chem. Soc. 2007;129:3040. doi: 10.1021/ja0680358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Furst EM, Kiick KL. J. Controlled Release. 2006;114:130. doi: 10.1016/j.jconrel.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nie T, Baldwin A, Yamaguchi N, Kiick KL. J. Controlled Release. 2007;122:287. doi: 10.1016/j.jconrel.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nie T, Akins RE, Jr, Kiick KL. Acta Biomater. 2009;5:865. doi: 10.1016/j.actbio.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seal BL, Panitch A. Biomacromolecules. 2003;4:1572. doi: 10.1021/bm0342032. [DOI] [PubMed] [Google Scholar]
- 22.Seal BL, Panitch A. Macromolecules. 2006;39:2268. [Google Scholar]
- 23.Benoit DSW, Anseth KS. Acta Biomater. 2005;1:461. doi: 10.1016/j.actbio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Benoit DSW, Durney AR, Anseth KS. Biomaterials. 2007;28:66. doi: 10.1016/j.biomaterials.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 25.Tae G, Scatena M, Stayton PS, Hoffman AS. J. Biomat. Sci., Polym. Ed. 2006;17:187. doi: 10.1163/156856206774879090. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Cai S, Shu XZ, Shelby J, Prestwich G, D Wound Repair Regen. 2007;15:245. doi: 10.1111/j.1524-475X.2007.00211.x. [DOI] [PubMed] [Google Scholar]
- 27.Go DH, Joung YK, Lee SY, Lee MC, Park KD. Macromol. Biosci. 2008;8:1152. doi: 10.1002/mabi.200800098. [DOI] [PubMed] [Google Scholar]
- 28.Fujita M, Ishihara M, Simizu M, Obara K, Ishizuka T, Saito Y, Yura H, Morimoto Y, Takase B, Matsui T, Kikuchi M, Maehara T. Biomaterials. 2004;25:699. doi: 10.1016/s0142-9612(03)00557-x. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura S, Ishihara M, Obara K, Masuoka K, Ishizuka T, Kanatani Y, Takase B, Matsui T, Hattori H, Sato T, Kariya Y, Maehara T. J. Biomed. Mater. Res. A. 2006;78A:364. doi: 10.1002/jbm.a.30688. [DOI] [PubMed] [Google Scholar]
- 30.Fujita M, Ishihara M, Shimizu M, Obara K, Nakamura S, Kanatani Y, Morimoto Y, Takase B, Matsui T, Kikuchi M, Maehara T. Wound Repair Regen. 2007;15:58. doi: 10.1111/j.1524-475X.2006.00185.x. [DOI] [PubMed] [Google Scholar]
- 31.Ferrara N, Davis-Smyth T. Endocr. Rev. 1997;18:4. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 32.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. J. Biol. Chem. 1992;267:26031. [PubMed] [Google Scholar]
- 33.Park JE, Keller GA, Ferrara N. Mol. Biol. Cell. 1993;4:1317. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pike DB, Cai S, Pomraning KR, Firpo MA, Fisher RJ, Shu XZ, Prestwich GD, Peattie RA. Biomaterials. 2006;27:5242. doi: 10.1016/j.biomaterials.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 35.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, Schmokel H, Bezuidenhout D, Djonov V, Zilla P, Hubbell JA. FASEB J. 2003;17:2260. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 36.Backer MV, Levashova Z, Patel V, Jehning BT, Claffey K, Blankenberg FG, Backer JM. Nat. Med. 2007;13:504. doi: 10.1038/nm1522. [DOI] [PubMed] [Google Scholar]
- 37.Lee KY, Kong HJ, Larson RG, Mooney DJ. Adv. Mater. 2003;15:1828. [Google Scholar]
- 38.Miyata T, Asami N, Uragami T. Nature. 1999;399:766. doi: 10.1038/21619. [DOI] [PubMed] [Google Scholar]
- 39.Wang D-A, Williams CG, Yang F, Cher N, Lee H, Elisseeff JH. Tissue Eng. 2005;11:201. doi: 10.1089/ten.2005.11.201. [DOI] [PubMed] [Google Scholar]
- 40.Xu C, Breedveld V, Kopecĕk J. Biomacromolecules. 2005;6:1739. doi: 10.1021/bm050017f. [DOI] [PubMed] [Google Scholar]
- 41.Yang J, Xu C, Wang C, Kopeček J. Biomacromolecules. 2006;7:1187. doi: 10.1021/bm051002k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen W, Zhang K, Kornfield JA, Tirrell DA. Nat. Mater. 2006;5:153. doi: 10.1038/nmat1573. [DOI] [PubMed] [Google Scholar]
- 43.Qiu Z, Yu H, Li J, Wang Y, Zhang Y. Chem. Commun. 2009:3342. doi: 10.1039/b822840j. [DOI] [PubMed] [Google Scholar]
- 44.Backer JM. PAE/KDR product information. Brookfield, CT: Sibtech, Inc., PAE/KDR product information; 2001. [Google Scholar]
- 45.Backer MV, Backer JM. Bioconjugate. Chem. 2001;12:1066. doi: 10.1021/bc015534j. [DOI] [PubMed] [Google Scholar]
- 46.Backer MV, Budker VG, Backer JM. J. Controlled Release. 2001;74:349. doi: 10.1016/s0168-3659(01)00346-7. [DOI] [PubMed] [Google Scholar]
- 47.Duncan AC, Boughner D, Campbell G, Wan WK. Eur. Polym. J. 2001;37:1821. [Google Scholar]
- 48.Gloeckner H, Jonuleit T, Lemke H-D. J. Immunol. Methods. 2001;252:131. doi: 10.1016/s0022-1759(01)00347-7. [DOI] [PubMed] [Google Scholar]
- 49.O’Brien J, Wilson I, Orton T, Pognan F. Eur. J. Biochem. 2000;267:5421. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 50.Gospodarowicz D. J. Cheng, J. Cell. Physiol. 1986;128:475. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- 51.Roghani M, Mansukhani A, Dell’Era P, Bellosta P, Basilico C, Rifkin D, Moscatelli D. J. Biol. Chem. 1994;269:3976. [PubMed] [Google Scholar]
- 52.Spinelli FJ, Kiick KL, Furst EM. Biomaterials. 2008;29:1299. doi: 10.1016/j.biomaterials.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. Science. 1992;255:989. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 54.Ferrara N, Gerber H-P. J. LeCouter, Nat. Med. 2003;9:669. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 55.Jakeman LB, Armanini M, Phillips HS, Ferrara N. Endocrinology. 1993;133:848. doi: 10.1210/endo.133.2.7688292. [DOI] [PubMed] [Google Scholar]
- 56.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. J. Biol. Chem. 1994;269:26988. [PubMed] [Google Scholar]
- 57.Kiick KL. Soft Matter. 2008;4:29. doi: 10.1039/b711319f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li S, Peck-Radosavljevic M, Koller E, Koller F, Kaserer K, Kreil A, Kapiotis S, Hamwi A, Weich HA, Valent P, Angelberger P, Dudczak R, Virgolini I. Int. J. Cancer. 2001;91:789. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1126>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 59.Yoshimoto M, Kinuya S, Kawashima A, Nishii R, Yokoyama K, Kawai K. Nucl. Med. Biol. 2006;33:963. doi: 10.1016/j.nucmedbio.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 60.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Proc. Natl. Acad. Sci. USA. 2003;100:5413. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hwang SY, Cho DY, Kim HK, Cho SH, Choo J, Yoon WJ, Lee EK. Bioconjugate Chem. 21:345. doi: 10.1021/bc9004409. [DOI] [PubMed] [Google Scholar]
- 62.Lee ES, Kim D, Youn YS, Oh KT, Bae YH. Angew. Chem., Int. Ed. 2008;47:2418. doi: 10.1002/anie.200704121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomas TP, Shukla R, Kotlyar A, Kukowska-Latallo J, Baker JR., Jr Bioorg. Med. Chem. Lett. 2009;20:700. doi: 10.1016/j.bmcl.2009.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.