Abstract
Rationale
The synthetic nonpeptide NOP (nociceptin/orphanin FQ peptide) receptor agonist Ro 64-6198 produces antinociception in rhesus monkeys. In rodents, it has much more variable effects on pain responses, but has response-rate increasing effects on punished operant behavior and decreases drug reward.
Objectives
The aim of this study was to compare Ro 64-6198 with the benzodiazepine diazepam in tests of analgesia, drug self-administration, and response-increasing effects in rhesus monkeys.
Results
Ro 64-6198 (0.001 – 0.01 mg/kg, i.v.) produced antinociception against an acute noxious stimulus (50° C water) in the absence of sedation, whereas diazepam (0.32 – 3.2 mg/kg, i.v.) did not have analgesic effects without sedation. Diazepam (1.0 – 5.6 mg/kg, i.v.) and the largest dose of Ro 64-6198 (0.32 mg/kg, i.v.) decreased lever pressing maintained by intravenous self-administration of the mu-opioid agonist, remifentanil, but neither effect could be distinguished from sedative effects. Although neither drug consistently increased responding during nonreinforcement, such effects were observed more frequently following diazepam administration. The effects of Ro 64-6198 on lever pressing were blocked by the NOP-receptor antagonist, J-113397, but not by the benzodiazepine antagonist, flumazenil.
Conclusions
These findings suggest the effects of Ro 64-6198 on operant lever pressing are mediated by NOP receptors and that larger doses are required to impact operant behavior when compared directly with those that produce antinociception. Therefore, the present findings support previous literature suggesting NOP receptors are a viable target for pain management.
Keywords: antinociception, self-administration, NOP receptor, Ro 64-6198, J-113397, benzodiazepine, diazepam, flumazenil, remifentanil, rhesus monkey
Introduction
The endogenous nociceptin/orphanin FQ peptide (NOP) binds to receptors located throughout the central and peripheral nervous systems (see Lambert 2008). The NOP receptor is considered a member of the opioid receptor family (Mollereau et al. 1994; Foord et al. 2005) in that it shares structural features with mu (MOP), delta (DOP), and kappa (KOP) opioid receptors. In addition, NOP receptor agonists produce actions similar to other opioid receptor agonists at the cellular level (Meunier et al. 1995; Rizzi et al. 2007). However, the effects of NOP receptor agonists are not blocked by administration of naltrexone, a drug that traditionally antagonizes opioid agonist effects (Ko et al. 2009; Varty et al. 2005). NOP receptors are implicated in numerous biological and behavioral processes, including immunity, pain, stress, anxiety, and drug abuse/addiction (see Lambert 2008). Investigation of integrated behavioral responses to NOP receptor activation in animal models has been facilitated by the development of the selective nonpeptidic NOP receptor agonist, Ro 64-6198, and antagonist, J-113397 (see Shoblock 2007).
There has been particular focus on the role of NOP receptors in mediating pain responses. The effect of NOP receptor agonists on pain measures appears to be influenced by a number of experimental variables, including species, dose of NOP receptor agonist, route of administration, and the particular test conditions (Heinricher 2005). In rodents, systemically administered Ro 64-6198 produced antinociceptive effects in some studies (e.g., Reiss et al. 2008) but no antinociceptive effect in others (e.g., Jenck et al. 2000). In primates, however, systemic Ro 64-6198 and the MOP receptor agonist alfentanil produced antinociception using both warm water and capsaicin as nociceptive stimuli in rhesus monkeys (Ko et al. 2009). Given that monkeys’ opioid receptor systems are similar to humans (Mansour et al. 1988), monkeys might be a more appropriate species in which to study the behavioral effects of NOP receptor agonists, including nociception. These effects of Ro 64-6198 were blocked by J-113397, but not by naltrexone. Furthermore, unlike the MOP receptor agonist alfentanil, Ro 64-6198 did not produce scratching, or respiratory depression, and did not maintain intravenous self-administration. These findings in monkeys suggest that Ro 64-6198 might produce analgesic effects at the NOP receptor without some of the undesirable effects typical of MOP receptor agonists.
Ro 64-6198 has shown promise as a potential therapeutic agent for treatment of addiction using rodents as experimental subjects. For instance, Ro 64-6198 has been shown to diminish the rewarding effects of drugs of abuse, including alcohol self-administration/reinstatement (Kuzmin et al. 2007) and place conditioning with alcohol (Kuzmin et al. 2003) and morphine (Shoblock et al. 2005). The effects of Ro 64-6198 on intravenous drug self-administration, however, have yet to be assessed in rhesus monkeys.
The aim of the present study was to characterize further the behavioral effects of Ro 64-6198 with rhesus monkeys as experimental subjects. Specifically, analgesic effects investigated by Ko et al. (2009) were explored further by examining the duration of action of intravenous Ro 64-6198 on acute-thermal antinociception. In addition, the effect of Ro 64-6198 on self-administration of the short-acting MOP receptor agonist, remifentanil, was assessed in the presence of one stimulus context. The receptor mechanisms mediating the effects of Ro 64-6198 on remifentanil self-administration were assessed using the selective NOP receptor antagonist, J-113397. Finally, the effects of Ro 64-6198 on responding in a context associated with nonreinforcement provided an assessment of potential response-rate increasing effects and effects on stimulus control (see Hanson et al., 1967; Miczek, 1973).
The effects of Ro 64-6198 were compared on each endpoint to those produced by the benzodiazepine diazepam, a drug that has mixed analgesic effects (Morichi and Pepeu 1979; Zambotti et al. 1991), produces some suppression of drug self-administration (Hedlund and Wahlstrom 1998), diminishes stimulus control (Cole 1990; Hanson et al. 1967), and has strong response-rate increasing effects on punished operant behavior (Rowlett et al., 2006).
Materials and Methods
Subjects
Six adult (3 males and 3 females) rhesus monkeys (Macaca mulatta) with body weights ranging from 7.9 to 11.9 kg participated in the nociception experiment. Three adult (2 males and 1 female) rhesus monkeys with body weights ranging from 11.7 to 14.1 kg participated in the remifentanil self-administration experiment. All monkeys were housed individually with free access to water in stainless steel cages (83.3 cm high × 76.2 cm wide × 91.4 cm deep). Diets consisted of 25 to 30 Purina Monkey Chow biscuits (Ralston Purina Co., St. Louis, MO) and fresh fruit daily. Housing was accredited by the American Association for the Accreditation of Laboratory Animal Care. Methods were in accordance with the University Committee on the Use and Care of Animals at the University of Michigan (Ann Arbor, MI) and the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health (Bethesda, MD).
In the monkeys participating in the remifentanil self-administration study, silicone rubber indwelling i.v. catheters were implanted in a jugular, femoral, external jugular, or brachial vein and were routed subcutaneously to the midscapular area of each monkey. Flexible tethers protecting the catheters were held in place by a Teflon mesh jacket (Lomir, Quebec, Canada) and connected behind the cages to infusion pumps. Catheters were implanted under ketamine (10 mg/kg, i.m.) and xylazine (2 mg/kg, i.m.) anesthesia.
Experimental Procedures
Acute thermal nociception
The warm water tail-withdrawal assay was used to measure nociceptive responses to thermal stimuli and the duration of action of the antinociceptive effects of test compounds (Ko et al. 1999). Monkeys were seated in primate-restraining chairs, which allowed access to their shaved tails (approximately 15 cm). Nociception evaluation was performed by placing the tail in a thermal flask containing water maintained at 50° C. The time required for the monkey to remove its tail from the warm water was recorded. If a monkey did not remove its tail within 20 sec, the flask was removed and a maximum time of 20 sec was recorded. Test sessions began with a determination of baseline response (a control latency) to 50° C water prior to intravenous administration of the test compound. Test compounds (i.e., vehicle or different doses) were administered intravenously over a 30 sec period through a temporary catheter (Angiocath, 24G/0.75") that was placed into the saphenous vein, and removed immediately after the infusion was given. Tail-withdrawal latencies were determined every 30 min for 3 hours after administration of the test compound. In addition, 5 min before determination of tail-withdrawal latency at each time point, drug-induced sedation was scored based on a scale used previously (Ko et al. 1999).
Remifentanil self-administration
The three monkeys had prior exposure to remifentanil self-administration under similar reinforcement schedules as those used in the present experiment and therefore did not require preliminary training. A panel was mounted on one side of the cages containing three depressible levers (Model 121-07, BRS-LVE) requiring 0.10 to 0.15 N to operate. Levers were separated by 0.3-cm dividers that extended 8 cm from the panel. Stimulus lights with a diameter of 2.5 cm were located directly above each lever. Only the left lever and left and center stimulus lights were used in the present experiment. Computers located in an adjacent room operating MED-PC IV interfacing and software (Med-Associates, Georgia, VT, USA) controlled all experimental events.
Sessions were approximately 2 hrs long and conducted twice daily (6:00 AM and 12:15 PM), seven days per week. Each of three components of a multiple schedule of reinforcement was signaled by a different colored stimulus light over the left lever. Each component was 5 min long and was presented eight times per session. Sessions were divided into 3-component blocks in which the order of component presentations was randomized. Pressing the lever in two of the components, signaled by red and green stimulus lights over the left lever, resulted in an injection of remifentanil on random-ratio (RR) 30 schedules of reinforcement. Thus, each response had a 3% chance in resulting in reinforcement. Reinforcement consisted of a 5-sec infusion of 1 ml of a solution containing 0.0001 mg/kg/inj remifentanil. During drug delivery, the stimulus light over the left lever was turned off and the green stimulus light over the center lever was illuminated. This was followed by a return to the component stimulus on the left lever. Given that the reinforcement contingencies were identical and performance was similar across these two components, response rates were averaged across these components and hereafter is referred to as the RR component. These two components were arranged to answer a different experimental question unrelated to this study. The third component was signaled by a white keylight and no remifentanil was presented for lever pressing in that component. In addition, responses made within the last 10 sec of that component delayed the onset of the following component by an additional 10 sec using a differential-reinforcement-of-other-behavior schedule (i.e., DRO 10-sec schedule). This component hereafter is referred to as the DRO component.
Presession treatments with drugs occurred following three sessions of stable responding in all components, determined by visual inspection that indicated no increasing or decreasing trends and with at least three days between pretreatments. When the agonists diazepam or Ro 64-6198 were administered, they were injected i.v. through the catheter 10 min prior to the experimental session. The dose-effect curve was completed for diazepam followed by Ro 64-6198. When the antagonists flumazenil or J-113397 were administered, they were injected i.m. 20 min prior to the session alone or followed by the agonists. Finally, the 0.1 mg/kg dose of Ro 64-6198 and the 3.2 mg/kg dose of diazepam were retested following evaluation of the antagonists.
Drugs
Ro 64-6198 was provided by F. Hoffmann-LaRoche AG (Basel, Switzerland). (+) J-113397 was obtained from the National Institute on Drug Abuse (Bethesda, MD, USA). Diazepam and flumazenil were purchased from Henry Schein Medical Supplies (henryschein.com). Ro 64-6198, flumazenil, and (+) J-113397 were dissolved in a solution of DMSO/Tween 80/sterile water in a ratio of 1:1:8. Diazepam was provided in solution containing 40% propylene glycol, 10% alcohol, 5% sodium benzoate and benzoic acid and 1.5% benzyl alcohol. In the nociception study, all drugs were administered in a volume of 0.1 ml/kg.
Data Analysis
In the antinociceptive assay, mean values (mean ± S.E.M.) were calculated from individual values for tail-withdrawal latencies. Individual tail-withdrawal latencies were converted to percentage of maximum possible effect, which was defined as ((test latency – control latency)/(cutoff latency, 20 sec – control latency))×100. This is a standard approach to normalize data to determine monkeys’ thermal nociceptive responses (Butelman et al. 1999; Ko et al. 1999). Measurement differences were compared across all test sessions in the same experiment. Data were analyzed by using two-way repeated measures (dose × time) analysis of variance followed by the Newman-Keuls test for multiple comparisons. The criterion for significance for all tests was set at p<0.05. In the self-administration assays, data were collected as responses made in the presence of each of the stimulus lights divided by the sec that the light was illuminated. Data from the remifentanil self-administration study were examined on an individual-subject basis assessing responding on a session-by-session basis for visual stability and effects resulting from dosing conditions.
Results
Acute thermal nociception
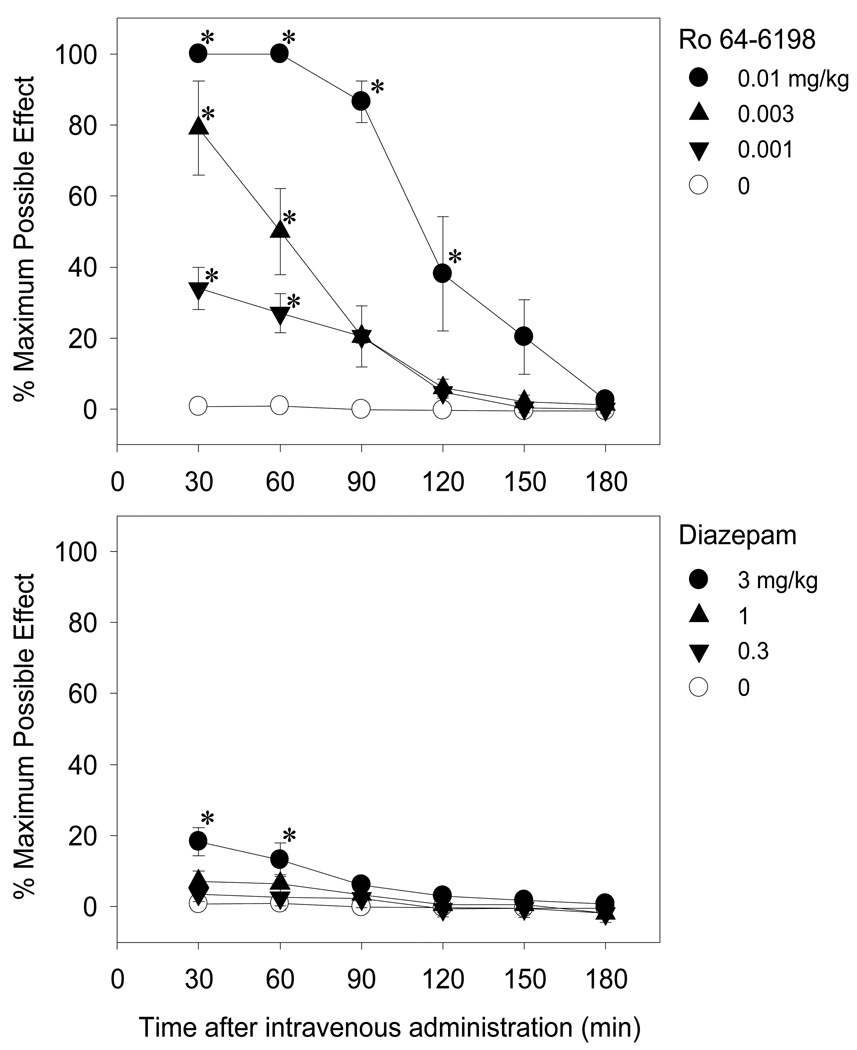
Figure 1 shows the antinociceptive effects of Ro 64-6198 (top panel) and diazepam (bottom panel) as a function of time after intravenous administration. As shown in the top panel, Ro 64-6198 produced dose- [F(3,15)=54.4; p<0.05] and time- [F(5,25)=83.8; p<0.05] dependent increases in tail withdrawal latency. Post hoc comparisons indicated differences from vehicle at 30 and 60 min for all doses and up to 120 min for the largest dose (0.01 mg/kg). For all doses, peak effects occurred during the first observation period of 30 min, as well at 60 min for the 0.01 mg/kg dose. At the 30- and 60-min time points, the 0.01 mg/kg dose induced the maximal effect of a 20-sec latency. Although Ro 64-6198 produced antinociception at doses between 0.001 and 0.01 mg/kg, at these doses this compound did not cause sedation, according to the sedation rating scale described by Ko et al. (1999). As shown in the bottom panel of Figure 1, diazepam also produced dose- [F(3,15)=3.3; p<0.05] and time- [F(5,25)=14.8; p<0.05] dependent increases in latency to withdraw the tail from 50° C water. Post hoc comparisons indicated that the largest dose of 3 mg/kg of intravenous diazepam produced slight but significant antinociception during the first hour. However, this mild antinociceptive effect was associated with sedation as monkeys showed heavy eyelids but responded to noises in the procedure room (i.e., scores of 2–3 in the sedation rating scale of Ko et al. 1999). These scores are midway between no sedation observed and completely alert (score of 0) to completely sedated and unresponsive to touch (score of 6).
Fig. 1.
Antinociceptive effects of intravenously administered Ro 64-6198 (top panel) and diazepam (bottom panel) against an acute noxious stimulus, 50°C water. Each point represents mean and error bars represent S.E.M. (n=6). Symbols represent different dosing conditions in the same monkeys. Asterisks represents a significant difference from the vehicle condition at the corresponding time point (*, p<0.05)
Remifentanil self-administration
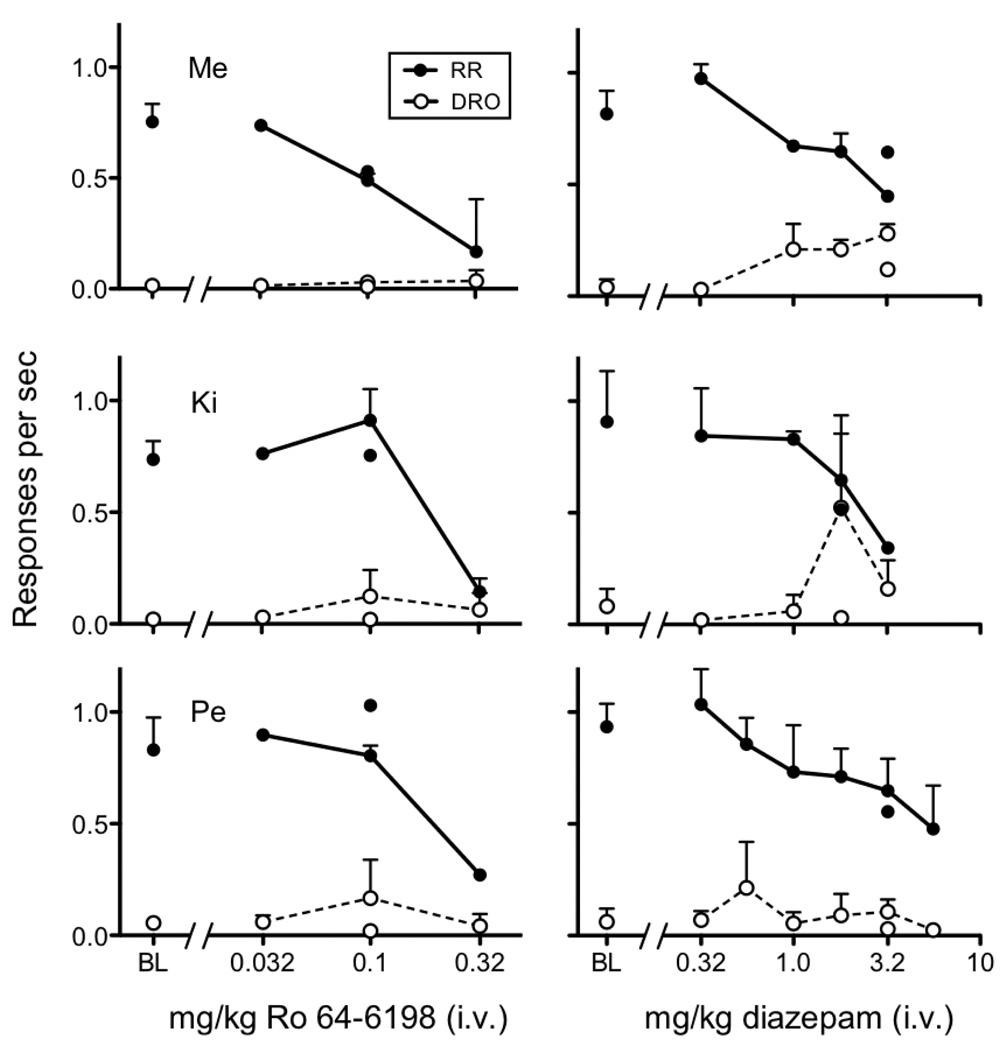
Figure 2 shows response rates for individual monkeys averaged across the two RR components and in the DRO component during baseline and following intravenous injections of Ro 64-6198 (0.03 – 0.3 mg/kg) in the left column and diazepam (0.3 – 5.6 mg/kg) in the right column. In all monkeys, mean baseline (BL) response rates in the RR component were between 0.7 and 1.0 responses per sec and below 0.1 responses per sec in the DRO component. The left column reveals a response rate decreasing effect on RR component responding with increasing dose of Ro 64-6198 and little to no change in response rates in the DRO component at any dose. Small response rate increases in the DRO component occurred for monkeys Ki and Pe with the 0.1 mg/kg dose, but those effects were not consistent. Informal observations indicated that Ro 64-6198 produced sedative effects at the 0.3 mg/kg dose.
Fig. 2.
Response rates in the RR and DRO component during baseline (BL) and as a function of dose of intravenous Ro 64-6198 (left column) and diazepam (right column). Symbols not connected by line are replications following determinations with antagonists.
The right column of Figure 2 shows that response rates in the RR component decreased with increasing doses of diazepam in all monkeys. Diazepam dose dependently increased monkey Me’s responding in the DRO component across a range of doses (1.0 – 3.0 mg/kg). Monkey Ki’s response rates increased at the 1.8 mg/kg dose and to a lesser extent at the 3.0 mg/kg dose, although these increases were not consistent across determinations, as indicated by no increase at the 1.8 mg/kg dose replication. Finally, there were no clear or consistent increases in responding in the DRO component for monkey Pe.
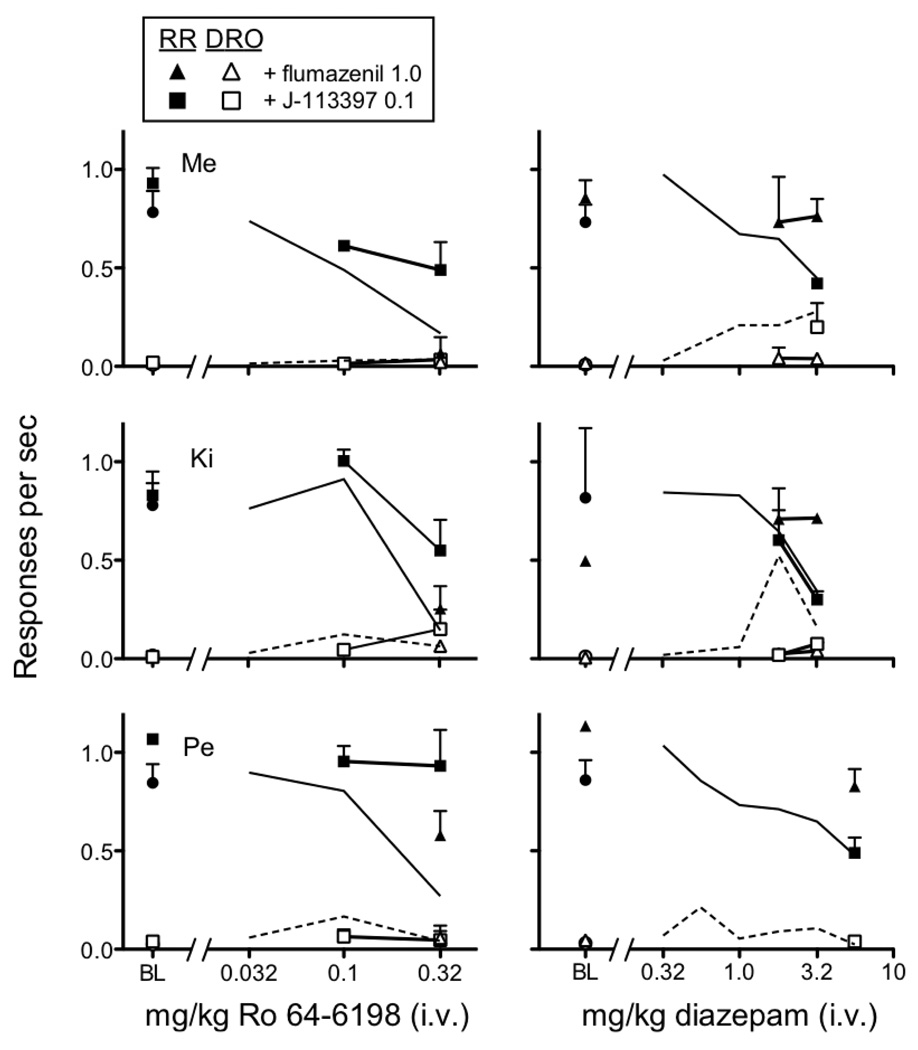
Figure 3 shows the effects of the NOP receptor antagonist, J-113397 (0.1 mg/kg, i.v.), and the benzodiazepine antagonist, flumazenil (1.0 mg/kg, i.v.) on remifentanil self-administration. When administered in the absence of Ro 64-6198 and diazepam, neither J-113397 nor flumazenil produced systematic changes in response rates in the RR or DRO components (labeled as BL in figure). In the left column, J-113397 diminished the response rate decreasing effect of the 0.3 mg/kg dose of Ro 64-6198 in the RR component in all monkeys. Conversely, the effects of flumazenil followed by Ro 64-6198 were not different from the effects of Ro 64-6198 given alone. Neither flumazenil nor J-113397 consistently altered the effects of any dose of Ro 64-6198 in the DRO component. The right column shows that flumazenil blocked the response rate decreasing effects of the largest diazepam doses examined in the three monkeys. Conversely, J-113397 did not block the rate-decreasing effects of diazepam in the RR component for any monkey. For monkey Me, flumazenil blocked increases in response rates produced by 3.2 mg/kg diazepam in the DRO component, but J-113397 did not. For monkeys Ki and Pe, response rates in the DRO component were not altered consistently by flumazenil or J-113397 under any dosing condition. Given the lack of systematic effects of diazepam on DRO component responding for monkeys Ki and Pe, it is difficult to make any general statements about how flumazenil or J-113397 impacted the effects of diazepam on responding in the DRO component.
Fig. 3.
Effects of J-113397 and flumazenil on response rates in the RR and DRO component during baseline (BL) and as a function of dose of intravenous Ro 64-6198 (left column) and diazepam (right column). Note that mean effects of Ro 64-6198 and diazepam alone from Figure 2 are presented as solid and dashed lines, respectively
Discussion
Intravenous Ro 64-6198 induced antinociceptive effects in rhesus monkeys; diazepam had no antinociceptive effects at doses less than those producing sedation. Ro 64-6198 and diazepam both decreased lever pressing maintained by remifentanil self-administration; the dose of Ro 64-6198 required to produce these decreases were substantially larger than those producing antinociception. Importantly, NOP receptors and benzodiazepine-receptor sites mediated decreases in remifentanil self-administration with Ro 64-6198 and diazepam, respectively: the NOP antagonist, J-113397, but not the benzodiazepine antagonist, flumazenil, attenuated the effects Ro 64-6198; flumazenil but not J-113397 attenuated the effects of diazepam. Finally, increases in nonreinforced responding were inconsistent for both drugs but more apparent with diazepam than Ro 64-6198. These findings demonstrate that Ro 64-6198 and diazepam are behaviorally and pharmacologically distinct in rhesus monkeys.
The antinociceptive effects of intravenous Ro 64-6198 in the present study were consistent with the effects of NOP receptor agonists in other studies in rhesus monkeys. NOP receptor agonists appear to have clear and consistent antinociceptive effects in the monkey following systemic (Ko et al. 2009) and intrathecal (Hu et al. 2010; Ko and Naughton 2009; Ko et al. 2006) administration. In rodents, however, both anti- and pro-nociceptive effects of NOP receptor agonists have been reported across a range of experimental conditions (Heinricher 2005). Differences between monkeys and rodents in pain responses to NOP receptor agonists might be a result of differences in NOP receptor localization (Berthele et al. 2003; Bridge et al. 2003). The extent to which species differences are responsible for these effects is unclear at this time because the effect of NOP receptor systems in responses to pain have not been examined nearly as extensively in monkeys as in rodents. Given that the analgesic effects of Ro 64-6198 in the present study were large and occurred in the absence of sedation, the present findings add to the existing literature suggesting NOP receptors as a potential target as therapeutics for pain management in humans.
In addition to pain management, NOP receptor agonists have been implicated as a target for treating drug abuse and addiction (Lambert 2008; Shoblock 2007). In the present study, Ro 64-6198 decreased remifentanil self-administration, consistent with studies suggesting NOP receptor agonists attenuate the rewarding effects of some drugs of abuse. For instance, studies in rodents have found Ro 64-6198 disrupts acquisition and reinstatement of morphine conditioned place preference (Shoblock et al. 2005). Moreover, NOP receptor agonists decreased morphine-induced dopamine release in the nucleus accumbens, part of the brain reward pathway (Di Giannuario and Pieretti 2000). It is unclear, however, whether purported attenuation of drug reward by Ro 64-6198 can be demonstrated in the absence of its sedative effects (Shoblock 2007). In the present study, while Ro 64-6198 consistently decreased remifentanil self-administration in all monkeys, it did so only at the largest dose tested (0.32 mg/kg, i.v.), and only under conditions of general sedation. Decreases in motor activity are a primary effect of Ro 64-6198 in rodents (Higgins et al. 2001; Jenck et al. 2000; Varty et al. 2005) and provide an index of doses with limited therapeutic potential. Because Ro 64-6198-induced decreases in rates of remifentanil self-administration were not distinguished from a general disruption in operant behavior in the present study, it is difficult to suggest that this drug might have a selective effect in the treatment of drug abuse. Moreover, potential interactions between remifentanil, a MOP receptor agonist and Ro 64-6198, a NOP receptor agonist, reflect the need for additional studies assessing the effects of Ro 64-6198 on responding maintained by other reinforcer types (e.g., food, water, other drugs) in rhesus monkeys to substantiate this claim.
Anxiolytic-like effects have been one of the most promising endpoints of Ro 64-6198 when studied in rodents (Shoblock 2007). Ro 64-6198 produces anxiolytic-like effects in rodents both in ethological tests (e.g., vocalizations) and response-rate increases in conflict procedures (e.g., Varty et al. 2005). Unlike in those studies, the present study assessed response-rate increasing effects during a context of nonreinforcement (i.e., the DRO component), procedures that likely are more well-suited for assessing disruptions in stimulus control than anxiolytic-like effects (Cole 1990; Hanson et al. 1967). Neither Ro 64-6198 nor diazepam produced consistent increases in responding during the DRO component, although diazepam tended to increase responding during the DRO component more frequently. These findings suggest Ro 64-6198 produces little response-rate increases or disruption in control by discriminative stimuli. Nonetheless, it would be useful to compare anxiolytic-like effects of diazepam and Ro 64-6198 in traditional conflict procedures with rhesus monkeys (e.g., Rowlett et al. 2006).
The present findings also provide additional pharmacological evidence that the behavioral effects of Ro 64-6198 in rhesus monkeys are mediated by NOP receptors. Specifically, the suppression of remifentanil self-administration by Ro 64-6198 was attenuated by pretreatment with the NOP receptor antagonist, J-113397, but not by the benzodiazepine-receptor-site antagonist, flumazenil. Likewise, the effects of diazepam were reduced by flumazenil, but not by J-113397. These findings join those demonstrating thermal antinociceptive effects of Ro 64-6198 that were blocked dose dependently by J-113397 but not by naltrexone (e.g., Ko et al. 2009). Therefore, the present findings do suggest that behavioral effects of Ro 64-6198 are mediated by NOP receptors in rhesus monkeys, even at large doses that sedate and disrupt operant performance.
Doses of Ro 64-6198 producing therapeutic-like effects and those producing negative side effects are different across rodent species (Shoblock 2007). For instance, the therapeutic window between anxiolytic-like effects and those producing motor disturbances appears larger for rats than for mice (Varty et al. 2005). The present findings in primates suggest a fairly large therapeutic window between doses producing antinociceptive effects (0.001 – 0.01 mg/kg) and those producing sedation (0.32 mg/kg). In addition, because both effects were antagonized by J-113397, the present findings suggest these behavioral effects of Ro 64-6198 are mediated by NOP receptors and that Ro 64-6198 could be a useful analgesic at doses producing few motor disturbances. Overall, these findings add to the literature suggesting NOP receptors are promising targets for pain management.
Acknowledgments
We thank Eric Hu and Kelly Tuzi for technical assistance of data collection in the nociception study and Kathy Carey, Amy Hall, and Susan Pouliot for technical assistance in the remifentanil self-administration study. We also thank Corina Jimenez-Gomez for helpful comments on a previous version of this manuscript. This study was supported by U.S. Department of Defense, Peer Reviewed Medical Research Program, Grant W81XWH-07-1-0162 to MCK and U.S. Public Health Service Grants DA-015449 and DA-023992 to GW. CAP was supported by the National Institutes of Health under Ruth L. Kirschstein National Service Research Service Award T32 DA007268. The author MCK received research support from Grunenthal GmbH, Ingenium Pharmaceuticals, Elan Pharmaceuticals, and Purdue Pharma. The author JHW received research support from Grunenthal GmbH, Alkermes, Roche, Adolor Corporation, and Reckitt Benckiser Pharmaceuticals.
Footnotes
The authors declare that, except for income received from their primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service except as listed below, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Berthele A, Platzer S, Dworzak D, Schadrack J, Mahal B, Büttner A, Assmus HP, Wurster K, Ziegigänsberger W, Conrad B, Tölle TR. [3H]-Nociceptin ligand-bindings and nociception opioid receptor mRNA expression in the human brain. Neurosci. 2003;121:629–640. doi: 10.1016/s0306-4522(03)00484-6. [DOI] [PubMed] [Google Scholar]
- Bridge KE, Wainwright A, Reilly K, Oliver KR. Autoradiographic localization of 125I[Tyr14]nociception/orphanin FQ binding sites in macaque primate CNS. Neurosci. 2003;118:513–523. doi: 10.1016/s0306-4522(02)00927-2. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, Perez A, Kreek MJ. Effects of systemically administered dynorphin A(1–17) in rhesus monkeys. J Pharmacol Exp Ther. 1999;290:678–686. [PubMed] [Google Scholar]
- Cole SO. Diazepam-induced impairment of a go-no go successive discrimination. Behav Neural Bio. 1990;53:371–377. doi: 10.1016/0163-1047(90)90240-7. [DOI] [PubMed] [Google Scholar]
- Di Giannuario A, Pieretti S. Nociceptin differentially affects morphine-induced dopamine release from the nucleus accumbens and nucleus caudate in rats. Peptides. 2000;21:1125–1130. doi: 10.1016/s0196-9781(00)00250-3. [DOI] [PubMed] [Google Scholar]
- Foord SM, Bonner TI, Neubig RR, Rosser EM, Pin J-P, Davenport AP, Spedding M, Harmar AJ. International Union of Pharmacology. XLVI. G Protein-Coupled Receptor List. Pharmacol Rev. 2005;57:279–288. doi: 10.1124/pr.57.2.5. [DOI] [PubMed] [Google Scholar]
- Hanson HM, Witoslawski JJ, Campbell EH. Drug effects in squirrel monkeys trained on a multiple schedule with a punishment contingency. J Exp Anal Behav. 1967;10:565–569. doi: 10.1901/jeab.1967.10-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund L, Wahlstrom G. The effect of diazepam on voluntary ethanol intake in a rat model of alcoholism. Alcohol Alcohol. 1998;33:207–219. doi: 10.1093/oxfordjournals.alcalc.a008384. [DOI] [PubMed] [Google Scholar]
- Heinricher MM. Nociceptin/orphanin FQ: Pain, stress and neural circuits. Life Sci. 2005;77:3127–3132. doi: 10.1016/j.lfs.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Grottick AJ, Ballard TM, Richards JG, Messer J, Takeshima H, Pauly-Evers M, Jenck F, Adam G, Wichmann J. Influence of the selective ORL1 receptor agonist, Ro 64-6198, on rodent neurological function. Neuropharm. 2001;41:97–107. doi: 10.1016/s0028-3908(01)00048-x. [DOI] [PubMed] [Google Scholar]
- Hu E, Calò G, Guerrini R, Ko MC. Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112. Pain. 2010;148:107–113. doi: 10.1016/j.pain.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenck F, Wichmann J, Dautzenberg FM, Moreau JL, Ouagazzal AM, Martin JR, Lundstrom K, Cesura AM, Poli SM, Roever S, Kolczewski S, Adam G, Kilpatrick G. A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: Anxiolytic profile in the rat. Proc Natl Acad Sci USA. 2000;97:4938–4943. doi: 10.1073/pnas.090514397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MCH, Johnson MD, Butelman ER, Willmont KJ, Mosberg HI, Woods JH. Intracisternal nor-binaltorphimine distinguishes central and peripheral kappa-opioid antinociception in rhesus monkeys. J Pharmacol Exp Ther. 1999;291:1113–1120. [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Woods JH, Fantegrossi WE, Galuska CM, Wichmann J, Prinssen EP. Behavioral effects of a synthetic agonist slective for Nociceptin/Orphanin FQ peptide receptors in monkeys. Neurpsychopharm. 2009;318:1257–1264. doi: 10.1038/npp.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Naughton NN. Antinociceptive effects of nociceptin/orphanin FQ administered intrathecally in monkeys. J Pain. 2009;10:509–516. doi: 10.1016/j.jpain.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MCH, Wei H, Woods JH, Kennedy RT. Effects of intrathecally administered nociceptin/orphanin FQ in monkeys: Behavioral and mass spectrometric studies. The J Pharm Exp Ther. 2006;318:1257–1264. doi: 10.1124/jpet.106.106120. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Kreek MJ, Bakalkin G, Liljequist S. The nociceptin/orphanin FQ receptor agonist Ro 64-6198 reduces alcohol self-administration and prevents relapse-like alcohol drinking. Neuropsychopharm. 2007;32:902–910. doi: 10.1038/sj.npp.1301169. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: Effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharm Exp Ther. 2003;304:310–318. doi: 10.1124/jpet.102.041350. [DOI] [PubMed] [Google Scholar]
- Lambert DG. The nociceptin/orphanin FQ receptor: A target with broad therapeutic potential. Nat Rev Drug Dis. 2008;7:694–710. doi: 10.1038/nrd2572. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Tr Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour J-L, Guillemot J-C, Ferrara P, Monsarrat B, Mazargull H, Vassart G, Parmentier M, Costentin J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Miczek KA. Effects of scopolamine, amphetamine and chlordiazepoxide on punishment. Psychopharmacol. 1973;28:373–389. doi: 10.1007/BF00422757. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- Morichi R, Pepeu G. A study of the influence of hydroxyzine and diazepam on morphine antinociception in the rat. Pain. 1979;7:173–180. doi: 10.1016/0304-3959(79)90008-3. [DOI] [PubMed] [Google Scholar]
- Reiss D, Wichmann J, Tekeshima H, Kieffer BL, Ouagazzal AM. Effects of nociceptin/orphanin FQ receptor (NOP) agonist, Ro64-6198, on reactivity to acute pain in mice: Comparison to morphine. Eur J Pharm. 2008;579:141–148. doi: 10.1016/j.ejphar.2007.10.031. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Spagnolo B, Wainford RD, Fischetti C, Guerrini R, Marzola G, Baldisserotto A, Salvadori S, Regoli D, Kapusta DR, Calo G. In vitro and in vivo studies on UFP-112, a novel potent and long lasting agonist selective for the nociceptin/orphanin FQ receptor. Peptides. 2007;28:1240–1251. doi: 10.1016/j.peptides.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Lelas S, Tornatzky W, Licata SC. Anti-conflict effects of benzodiazepines in rhesus monkeys: relationships with therapeutic doses in humans and role of GABAA receptors. Psychopharm. 2006;184:201–211. doi: 10.1007/s00213-005-0228-8. [DOI] [PubMed] [Google Scholar]
- Shoblock JR. The pharmacology of Ro 64-6198, a systemically active, nonpeptide NOP receptor (opiate receptor-like 1, ORL1) agonist with diverse preclinical therapeutic activity. CNS Drug Rev. 2007;13:107–136. doi: 10.1111/j.1527-3458.2007.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoblock JR, Wichmann J, Maidment NT. The effect of a systemically active ORL-1 agonist, Ro 64-6198, on the acquisition, expression, extinction, and reinstatement of morphine conditioned place preference. Neuropharm. 2005;49:439–446. doi: 10.1016/j.neuropharm.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Varty GB, Hyde LA, Hodgson RA, Lu SX, McCool MF, Kazdoba TM, Del Vecchio RA, Guthrie DH, Pond AJ, Grzelak ME, et al. Characterization of the nociceptin receptor (ORL-1) agonist, Ro64-6198, in tests of anxiety across multiple species. Psychopharm. 2005;182:132–143. doi: 10.1007/s00213-005-0041-4. [DOI] [PubMed] [Google Scholar]
- Zambotti F, Zonta N, Tammiso R, Conci F, Hafner B, Zecca L, Ferrario P, Mantegazza P. Effects of diazepam on nociception in rats. Naunyn-Schmiedeberg’s Arch Pharmacol. 1991;344:84–89. doi: 10.1007/BF00167386. [DOI] [PubMed] [Google Scholar]