Abstract
Non-small-cell lung cancer (nsclc) has the highest prevalence of all types of lung cancer, which is the second most common cancer and the leading cause of cancer-related mortality in Canada. The need for more effective and less toxic treatment options for nsclc has led to the development of agents targeting the epidermal growth factor receptor (egfr)–mediated signalling pathway, such as egfr tyrosine kinase inhibitors (egfr-tkis). Although egfr-tkis are less toxic than traditional anti-neoplastic agents, they are commonly associated with acneiform-like rash and diarrhea. This review summarizes the clinical presentation and causes of egfr-tki–induced rash and diarrhea, and presents strategies for effective assessment, monitoring, and treatment of these adverse effects. Strategies to improve the management of egfr-tki–related adverse events should improve clinical outcomes, compliance, and quality of life in patients with advanced nsclc.
Keywords: Epidermal growth factor receptor, adverse drug reaction, adverse drug event, skin rash, diarrhea
1. INTRODUCTION
Lung cancer remains the second most common cancer and the leading cause of cancer-related mortality in Canada. In 2010, 24,400 new lung cancer cases and 20,600 related deaths were predicted, representing a significant health care burden 1. The most common form of lung cancer, non-small-cell lung cancer (nsclc), consists of a heterogeneous group of histologies, of which adenocarcinoma, squamous cell carcinoma, and large-cell anaplastic carcinoma are the most frequent 2. Despite recent advances in treatment, prognosis is generally poor in patients with advanced nsclc, and the median survival rate is only 10–12 months with treatment 3.
First-line treatment for nsclc is usually platinum-based two-drug combination chemotherapy, with or without bevacizumab for non-squamous pathology. For patients with poor performance status, single-agent chemotherapy is often recommended, but little evidence exists to guide recommendations in this patient group 4–6. Despite the success of platinum doublets in first-line treatment, the introduction of a third chemotherapeutic agent increases toxicity without improving efficacy 7. The standard approach is to give a chemotherapy agent as second-line treatment or an epidermal growth factor receptor (egfr) tyrosine kinase inhibitor (tki). The egfr-tkis are better tolerated, have a more convenient method of administration, and can be given for longer periods of time 7. In addition, patients with late-stage nsclc are often symptomatic and experience comorbidities that affect quality of life 8. Effective treatment options of lower toxicity are therefore needed for patients with advanced nsclc.
The need for more effective and less toxic treatment options for nsclc has led to the development of targeted agents such as egfr inhibitors. A number of solid tumours, including 40%–80% of nsclc tumours, express or overexpress egfr. Several studies showed that high egfr expression is associated with poor prognosis in lung cancer patients. Consequently, egfr is an attractive target for the treatment of nsclc 8,9.
Various clinical trials have demonstrated that egfr inhibitors are clinically efficacious in the management of several solid tumour types, such as those of breast, colon, pancreas, head and neck, kidney, gastrointestinal stroma, and lung 10. The egfr-tkis impede phosphorylation of the intracellular tyrosine kinase component of egfr and thus block signal transduction pathways associated with the proliferation and survival of cancer cells 8. Given as second- or third-line therapy in advanced nsclc, the egfr-tkis are clinically efficacious compared with supportive care or chemotherapy 8,11–14.
Erlotinib is an egfr-tki that has been approved in the United States, Canada, and many other countries around the world for use in nsclc, based on clinical trial results showing it to be safe and efficacious. Another egfr-tki, gefitinib, was recently granted marketing authorization by the European Medicines Agency, the United States, and Canada for the treatment of egfr mutation–positive nsclc 7. Newer egfr-tkis such as afatinib (BIBW 2992) and PF-00299804 are currently in development 15–21,a.
Although targeted agents are generally less toxic than traditional anti-neoplastic agents, egfr-tkis are associated with a number of bothersome adverse effects that need to be managed in most patients. Because egfr is expressed mainly on cells of epithelial origin, such as those of the skin and gastrointestinal tract, the most common adverse events of the egfr inhibitors are rash and diarrhea, which are the focus of the present paper. Strategies to improve the assessment and management of egfr-tki–related adverse events such as rash and diarrhea should result in superior clinical outcomes, better compliance, and improved quality of life for patients with advanced nsclc 10.
2. RASH INDUCED BY EGFR-TKIs
2.1. Patient Monitoring
Before initiating treatment with an egfr-tki, physicians should educate their patients about the associated potential side effects so that such reactions can be managed early and effectively. Because symptoms of rash generally appear as early as 2 weeks into treatment, early monitoring is essential 10.
Patients should be advised that rash is a common complication of egfr inhibitors and an indication of treatment efficacy 22. To prevent dose reduction or discontinuation of therapy, it is also important to inform patients that early treatment of rash can prevent symptoms from worsening.
Although not recommended in current guidelines, prophylactic treatments to prevent egfr-tki–induced rash have been studied in a number of trials. One randomized double-blind trial compared prophylactic oral minocycline with placebo in patients treated with cetuximab for metastatic colorectal cancer (n = 48) 23. Patients were also randomized to receive topical tazarotene applied either to the left or the right side of the face. After 4 weeks of treatment with cetuximab, the minocycline group had a significantly lower total facial lesion count (p = 0.005). A trend was also observed suggesting that a lower proportion of treated patients were experiencing moderate-to-severe itching (20% vs. 50% receiving placebo, p = 0.05). The use of topical tazarotene provided no clinical benefit and was associated with significant skin irritation.
A second randomized double-blind trial in patients (n = 61) receiving egfr-tkis compared prophylactic tetracycline treatment (500 mg twice daily) with placebo over 4 weeks 24. Although tetracycline did not prevent rash, a reduction in the severity of rash was observed. At week 4, grade 2 rash was reported in 17% of the tetracycline group and in 55% of the placebo group (p = 0.04). Treatment also improved certain quality-of-life measures, including skin burning or stinging and skin irritation.
The stepp (Skin Toxicity Evaluation Protocol with Panitumumab) study compared primary pre-emptive skin treatment with reactive skin treatment in patients receiving panitumumab in a randomized prospective study 25. Patients on the pre-emptive arm received daily skin treatment for a total of 6 weeks, starting 24 hours before the first dose of panitumumab. Pre-emptive treatment included skin moisturizer, sunscreen, 1% hydrocortisone cream, and doxycycline 100 mg twice daily. Patients on the reactive arm received treatment after the development of rash. Compared with reactive treatment, pre-emptive treatment reduced the incidence of grade 2 or greater rash by more than 50%, without additional side effects. Time to first occurrence of grade 2 or greater rash was also significantly delayed in the pre-emptive arm. Additional research is needed to determine the benefit of prophylactic treatment for the prevention of egfr-tki–induced rash.
During the first 6 weeks of treatment, patients should be assessed weekly for any signs of rash. When symptoms of rash are apparent, early intervention is of key importance to prevent more serious complications. After 6 weeks of treatment, assessment of skin toxicities can be performed less frequently—for example, every 6–8 weeks. Rash assessment can be performed by any member of the health care team who is able to reliably evaluate it.
2.2. Causes and Incidence
Epidermal growth factor plays an integral role in the growth and keratinization of skin epithelium, and egfrs are expressed within the follicular epithelium, sebaceous glands, and dermal capillaries. It is therefore not surprising that egfr inhibition leads to a number of skin reactions 10,26.
Adverse skin reactions occur in more than 50% of patients given egfr inhibitors; they are the most common treatment-related adverse events. Skin reactions associated with egfr inhibitors include xerosis (dry skin), pruritus, hair changes and alopecia, nail alterations, and hand and foot reactions 10,26,27. The most common skin reaction reported in patients treated with egfr-tkis is a follicular acneiform eruption also known as “acne-like rash” or “folliculitis.” Inhibition of egfr is thought to alter keratinocyte proliferation, differentiation, migration, and attachment, which may explain the papulopustular reaction and xerosis seen with egfr-tkis30.
Incidence of rash with egfr-tkis varies from 37%–78% in phase iii clinical trials and appears to be dose-dependent (Table i). Table ii presents a detailed description of the clinical trials that have examined the incidence of rash with egfr-tkis in nsclc.
TABLE I.
Incidence of acneiform rash with epidermal growth factor receptor (egfr) tyrosine kinase inhibitors (tkis) in non-small-cell lung cancer trials
| egfr-tki | Description | Grades (%) | |
|---|---|---|---|
| All | ≥3 | ||
| Erlotinib 150 mg | All studies | 33–79 | 3–10 |
| Phase iii studies | 62–76 | 3–10 | |
| Gefitinib 250 mg and 500 mg | All studies | 34–75 | 0–13 |
| 250 mg | 34–66 | 0–4 | |
| 500 mg | 57–75 | 4–13 | |
| Phase iii studies | 37–67 | 2–13 | |
| 250 mg | 37–66 | 2–4 | |
| 500 mg | 57–67 | 12–13 | |
| Afatinib 40 mg and 50 mg | All studies | 67–100 | 0–25 |
| 40 mg | 90–100 | 0–7 | |
| 50 mg | 67–92 | 0–25 | |
| Phase iii studies | 78 | 14 | |
| 50 mg | |||
| PF-00299804 30 mg and 45 mg | All studies (phase ii)a | 68–100 | 0–15 |
| 30 mg | 69 | 0 | |
| 45 mg | 68–85 | 15 | |
No phase iii study results using PF-00299804 are available to date.
TABLE II.
Incidence of rash and diarrhea with epidermal growth factor receptor (egfr) tyrosine kinase inhibitors (tkis) in non-small-cell lung cancer (nsclc) clinical trials
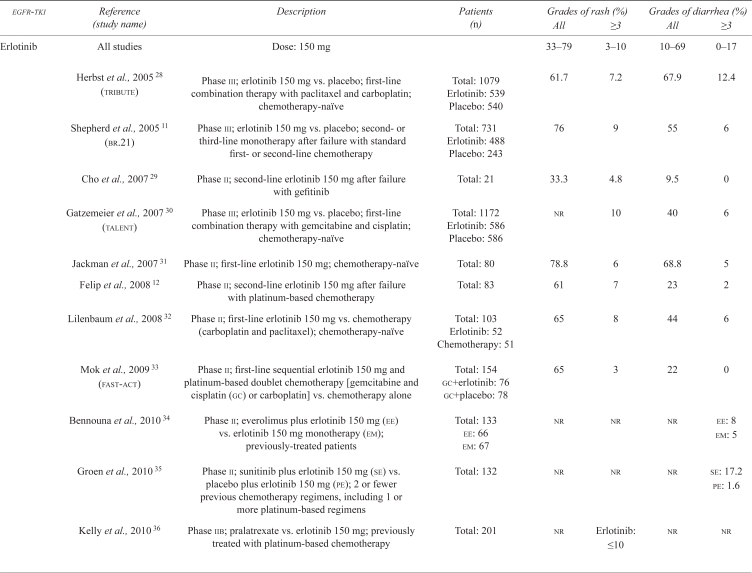
| egfr-tki | Reference (study name) | Description | Patients (n) | Grades of rash (%) | Grades of diarrhea (%) | ||
|---|---|---|---|---|---|---|---|
| All | ≥3 | All | ≥3 | ||||
| Erlotinib | All studies | Dose: 150 mg | 33–79 | 3–10 | 10–69 | 0–17 | |
| Herbst et al., 2005 28 (tribute) | Phase iii; erlotinib 150 mg vs. placebo; first-line combination therapy with paclitaxel and carboplatin; chemotherapy-naïve | Total: 1079 Erlotinib: 539 Placebo: 540 |
61.7 | 7.2 | 67.9 | 12.4 | |
| Shepherd et al., 2005 11 (br.21) | Phase iii; erlotinib 150 mg vs. placebo; second- or third-line monotherapy after failure with standard first- or second-line chemotherapy | Total: 731 Erlotinib: 488 Placebo: 243 |
76 | 9 | 55 | 6 | |
| Cho et al., 2007 29 | Phase ii; second-line erlotinib 150 mg after failure with gefitinib | Total: 21 | 33.3 | 4.8 | 9.5 | 0 | |
| Gatzemeier et al., 2007 30 (talent) | Phase iii; erlotinib 150 mg vs. placebo; first-line combination therapy with gemcitabine and cisplatin; chemotherapy-naïve | Total: 1172 Erlotinib: 586 Placebo: 586 |
nr | 10 | 40 | 6 | |
| Jackman et al., 2007 31 | Phase ii; first-line erlotinib 150 mg; chemotherapy-naïve | Total: 80 | 78.8 | 6 | 68.8 | 5 | |
| Felip et al., 2008 12 | Phase ii; second-line erlotinib 150 mg after failure with platinum-based chemotherapy | Total: 83 | 61 | 7 | 23 | 2 | |
| Lilenbaum et al., 2008 32 | Phase ii; first-line erlotinib 150 mg vs. chemotherapy (carboplatin and paclitaxel); chemotherapy-naïve | Total: 103 Erlotinib: 52 Chemotherapy: 51 |
65 | 8 | 44 | 6 | |
| Mok et al., 2009 33 (fast-act) | Phase ii; first-line sequential erlotinib 150 mg and platinum-based doublet chemotherapy [gemcitabine and cisplatin (gc) or carboplatin] vs. chemotherapy alone | Total: 154 gc+erlotinib: 76 gc+placebo: 78 |
65 | 3 | 22 | 0 | |
| Bennouna et al., 2010 34 | Phase ii; everolimus plus erlotinib 150 mg (ee) vs. erlotinib 150 mg monotherapy (em); previously-treated patients | Total: 133 ee: 66 em: 67 |
nr | nr | nr |
ee: 8 em: 5 |
|
| Groen et al., 2010 35 | Phase ii; sunitinib plus erlotinib 150 mg (se) vs. placebo plus erlotinib 150 mg (pe); 2 or fewer previous chemotherapy regimens, including 1 or more platinum-based regimens | Total: 132 | nr | nr | nr |
se: 17.2 pe: 1.6 |
|
| Kelly et al., 2010 36 | Phase iib; pralatrexate vs. erlotinib 150 mg; previously treated with platinum-based chemotherapy | Total: 201 | nr | Erlotinib: ≤10 | nr | nr | |
| Scagliotti et al., 2010 37 | Phase iii; sunitinib plus erlotinib 150 mg (se) vs. placebo plus erlotinib 150 mg (pe); 2 or fewer previous chemotherapy regimens | Total: 960 | nr |
se: 8.2 pe: 2.5 |
nr |
se: 10.4 pe: 1.7 |
|
| Zhao et al., 2010 38 (ML 22206) | Phase ii; erlotinib 150 mg plus capecitabine; first-line in elderly patients with advanced disease | Total: 62 | nr | nr | nr | nr | |
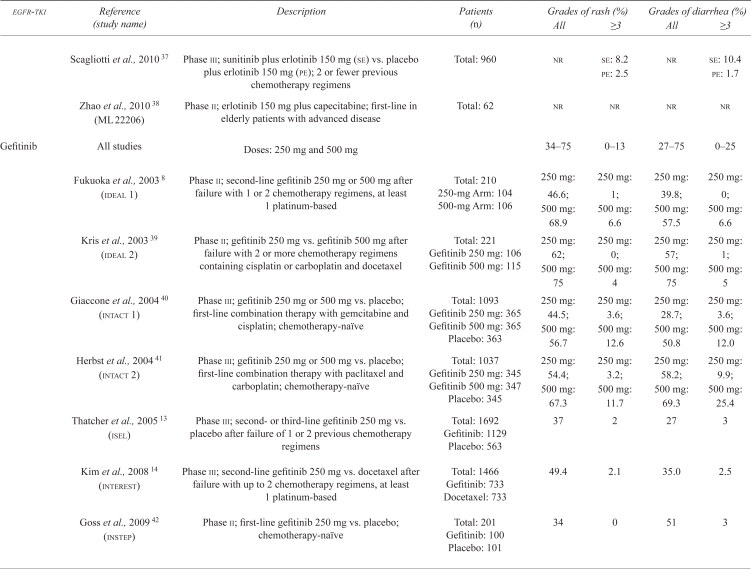
| Gefitinib | All studies | Doses: 250 mg and 500 mg | 34–75 | 0–13 | 27–75 | 0–25 | |
| Fukuoka et al., 2003 8 (ideal 1) | Phase ii; second-line gefitinib 250 mg or 500 mg after failure with 1 or 2 chemotherapy regimens, at least 1 platinum-based | Total: 210 250-mg Arm: 104 500-mg Arm: 106 |
250 mg: 46.6; 500 mg: 68.9 |
250 mg: 1; 500 mg: 6.6 |
250 mg: 39.8; 500 mg: 57.5 |
250 mg: 0; 500 mg: 6.6 |
|
| Kris et al., 2003 39 (ideal 2) | Phase ii; gefitinib 250 mg vs. gefitinib 500 mg after failure with 2 or more chemotherapy regimens containing cisplatin or carboplatin and docetaxel | Total: 221 Gefitinib 250 mg: 106 Gefitinib 500 mg: 115 |
250 mg: 62; 500 mg: 75 |
250 mg: 0; 500 mg: 4 |
250 mg: 57; 500 mg: 75 |
250 mg: 1; 500 mg: 5 |
|
| Giaccone et al., 2004 40 (intact 1) | Phase iii; gefitinib 250 mg or 500 mg vs. placebo; first-line combination therapy with gemcitabine and cisplatin; chemotherapy-naïve | Total: 1093 Gefitinib 250 mg: 365 Gefitinib 500 mg: 365 Placebo: 363 |
250 mg: 44.5; 500 mg: 56.7 |
250 mg: 3.6; 500 mg: 12.6 |
250 mg: 28.7; 500 mg: 50.8 |
250 mg: 3.6; 500 mg: 12.0 |
|
| Herbst et al., 2004 41 (intact 2) | Phase iii; gefitinib 250 mg or 500 mg vs. placebo; first-line combination therapy with paclitaxel and carboplatin; chemotherapy-naïve | Total: 1037 Gefitinib 250 mg: 345 Gefitinib 500 mg: 347 Placebo: 345 |
250 mg: 54.4; 500 mg: 67.3 |
250 mg: 3.2; 500 mg: 11.7 |
250 mg: 58.2; 500 mg: 69.3 |
250 mg: 9.9; 500 mg: 25.4 |
|
| Thatcher et al., 2005 13 (isel) | Phase iii; second- or third-line gefitinib 250 mg vs. placebo after failure of 1 or 2 previous chemotherapy regimens | Total: 1692 Gefitinib: 1129 Placebo: 563 |
37 | 2 | 27 | 3 | |
| Kim et al., 2008 14 (interest) | Phase iii; second-line gefitinib 250 mg vs. docetaxel after failure with up to 2 chemotherapy regimens, at least 1 platinum-based | Total: 1466 Gefitinib: 733 Docetaxel: 733 |
49.4 | 2.1 | 35.0 | 2.5 | |
| Goss et al., 2009 42 (instep) | Phase ii; first-line gefitinib 250 mg vs. placebo; chemotherapy-naïve | Total: 201 Gefitinib: 100 Placebo: 101 |
34 | 0 | 51 | 3 | |
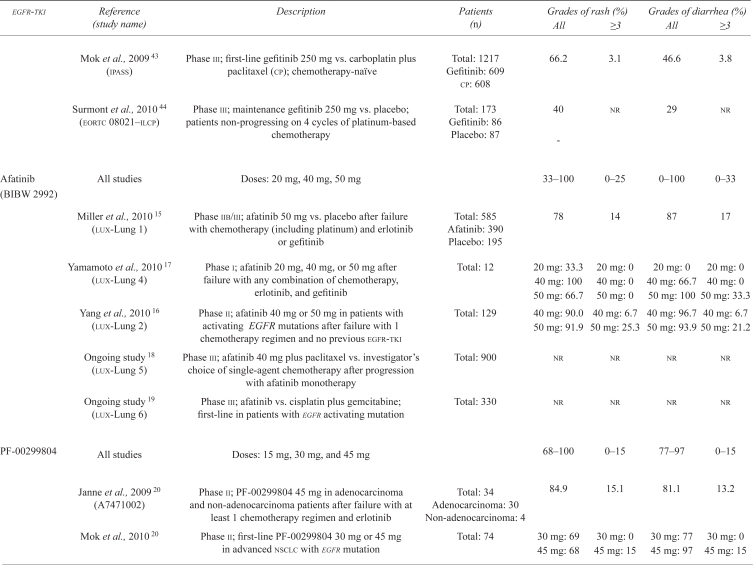
| Mok et al., 2009 43 (ipass) | Phase iii; first-line gefitinib 250 mg vs. carboplatin plus paclitaxel (cp); chemotherapy-naïve | Total: 1217 Gefitinib: 609 cp: 608 |
66.2 | 3.1 | 46.6 | 3.8 | |
| Surmont et al., 2010 44 (eortc 08021–ilcp) | Phase iii; maintenance gefitinib 250 mg vs. placebo; patients non-progressing on 4 cycles of platinum-based chemotherapy | Total: 173 Gefitinib: 86 Placebo: 87 |
40 - |
nr | 29 | nr | |
| Afatinib (BIBW 2992) | All studies | Doses: 20 mg, 40 mg, 50 mg | 33–100 | 0–25 | 0–100 | 0–33 | |
| Miller et al., 2010 15 (lux-Lung 1) | Phase iib/iii; afatinib 50 mg vs. placebo after failure with chemotherapy (including platinum) and erlotinib or gefitinib | Total: 585 Afatinib: 390 Placebo: 195 |
78 | 14 | 87 | 17 | |
| Yamamoto et al., 2010 17 (lux-Lung 4) | Phase i; afatinib 20 mg, 40 mg, or 50 mg after failure with any combination of chemotherapy, erlotinib, and gefitinib | Total: 12 | 20 mg: 33.3 40 mg: 100 50 mg: 66.7 |
20 mg: 0 40 mg: 0 50 mg: 0 |
20 mg: 0 40 mg: 66.7 50 mg: 100 |
20 mg: 0 40 mg: 0 50 mg: 33.3 |
|
| Yang et al., 2010 16 (lux-Lung 2) | Phase ii; afatinib 40 mg or 50 mg in patients with activating egfr mutations after failure with 1 chemotherapy regimen and no previous egfr-tki | Total: 129 | 40 mg: 90.0 50 mg: 91.9 |
40 mg: 6.7 50 mg: 25.3 |
40 mg: 96.7 50 mg: 93.9 |
40 mg: 6.7 50 mg: 21.2 |
|
| Ongoing study 18 (lux-Lung 5) | Phase iii; afatinib 40 mg plus paclitaxel vs. investigator’s choice of single-agent chemotherapy after progression with afatinib monotherapy | Total: 900 | nr | nr | nr | nr | |
| Ongoing study 19 (lux-Lung 6) | Phase iii; afatinib vs. cisplatin plus gemcitabine; first-line in patients with egfr activating mutation | Total: 330 | nr | nr | nr | nr | |
| PF-00299804 | All studies | Doses: 15 mg, 30 mg, and 45 mg | 68–100 | 0–15 | 77–97 | 0–15 | |
| Janne et al., 2009 20 (A7471002) | Phase ii; PF-00299804 45 mg in adenocarcinoma and non-adenocarcinoma patients after failure with at least 1 chemotherapy regimen and erlotinib | Total: 34 Adenocarcinoma: 30 Non-adenocarcinoma: 4 |
84.9 | 15.1 | 81.1 | 13.2 | |
| Mok et al., 2010 20 | Phase ii; first-line PF-00299804 30 mg or 45 mg in advanced nsclc with egfr mutation | Total: 74 | 30 mg: 69 45 mg: 68 |
30 mg: 0 45 mg: 15 |
30 mg: 77 45 mg: 97 |
30 mg: 0 45 mg: 15 |
|
| Ramalingam et al., 2010 19 | Phase ii; PF-00299804 45 mg vs. erlotinib 150 mg in advanced nsclc patients who were erlotinib-naïve and had failed at least 1 chemotherapy regimen | Total: 188 Erlotinib: 94 PF-00299804: 94 |
Tolerable in both agents | Tolerable in both agents | |||
| Takahashi et al., 2010 21 | Phase i; PF-00299804 15 mg, 30 mg, or 45 mg in advanced solid-tumour patients who had failed all standards of care | Total: 13 | 100a | 45 mg: 15.4 | 92a | nr | |
| Ongoing study 24 (br.26) | Phase iii; PF-00299804 45 mg vs. placebo in advanced nsclc patients after failure with at least 1 chemotherapy regimen and erlotinib or gefitinib (or both) | Total: 720 | nr | nr | nr | nr | |
| Ongoing study 25 (A7471028) | Phase ii; PF-00299804 45 mg vs. erlotinib 150 mg in advanced nsclc patients after failure with at least 1 chemotherapy regimen | Total: 160 | nr | nr | nr | nr | |
Adverse events by dose subgroup not given.
nr = not reported; eortc = European Organisation for Research and Treatment of Cancer.
When rash is not adequately managed, treatment compliance may be negatively affected, leading to dose modifications or treatment discontinuation, and ultimately reducing the overall clinical benefits of treatment. Appropriate strategies are therefore needed to assess and manage egfr-tki–induced rash 10,45.
2.3. Assessment and Grading
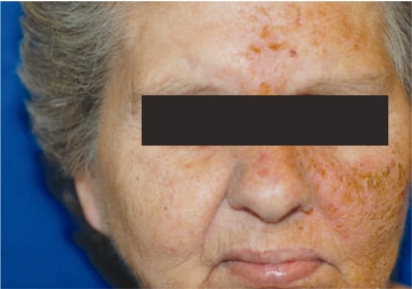
Acneiform rash is defined as an eruption of papules and pustules, typically appearing on the face, scalp, upper chest, and back 46. Although egfr-tki–induced rash most closely resembles acneiform rash in presentation, no comedones or blackheads are visible 10 (Figure 1). Rash induced by egfr-tki usually appears within 2 weeks of treatment start and generally presents on the face, shoulders, and upper part of the back and chest. The rash tends to improve over time with continued use of the medication and resolves fully after discontinuation of treatment 10,26. However, in about 35% of patients, dry itchy skin of the arms and legs may occur, which can potentially become secondarily infected with a Staphylococcus aureus or Herpes simplex infection 10.
FIGURE 1.
Rash induced by epidermal growth factor receptor.
The U.S. National Cancer Institute (nci) Common Terminology Criteria for Adverse Events (ctcae) are typically used to grade symptoms for clinical trials, but those criteria have some limitations for describing egfr-tki–induced rash 46 (Table iii). The ctcae grading uses the affected percentage of body surface area to assess rash severity, but egfr-tki–induced rash is typically restricted to the face, scalp, and upper torso. The ctcae grading also does not take into account the severity of rash complications, which may include oozing, burning, crusting, or disfigurement 47. Given that no other standard exists for grading egfr-tki–induced rash, the ctcae grading remains the standard for assessment.
TABLE III.
U.S. National Cancer Institute grading for acneiform rasha
| Grade 1 | Papules or pustules, or both, covering less than 10% of body surface area, which may or may not be associated with symptoms of pruritus or tenderness |
| Grade 2 | Papules or pustules, or both, covering 10%–30% body surface area, which may or may not be associated with symptoms of pruritus or tenderness Associated with psychosocial impact Limits instrumental activities of daily living |
| Grade 3 | Papules or pustules, or both, covering more than 30% body surface area, which may or may not be associated with symptoms of pruritus or tenderness Limits self-care activities of daily living Associated with local superinfection, with oral antibiotics indicated |
| Grade 4 | Papules or pustules, or both, covering any percentage of body surface area, which may or may not be associated with symptoms of pruritus or tenderness and which are associated with extensive superinfection, with intravenous antibiotics indicated Life-threatening consequences |
| Grade 5 | Death |
Adapted from the Common Terminology Criteria for Adverse Events, 2010 46.
2.4. Management
Before initiating egfr-tki therapy, physicians should counsel their patients about preventive measures to reduce the risk of skin rash. The educational messages that need to be communicated to patients being prescribed egfr-tkis are these10,22,27,45:
Any areas of dry skin should be moisturized twice daily using a thick, alcohol-free emollient.
Sun exposure should be minimized. Where sun exposure is unavoidable, a broad-spectrum sunscreen with a sun protection factor of 15 or higher should be applied 1–2 hours before exposure, especially on the face and upper body. Physical sunscreens that contain zinc oxide or titanium dioxide are preferable to chemical sunscreens.
Products that dry the skin—such as soaps, alcohol-based or perfumed products, and over-the-counter acne products—should be avoided. Because long hot showers can also dry the skin, shower time should be limited, and lukewarm water should be used.
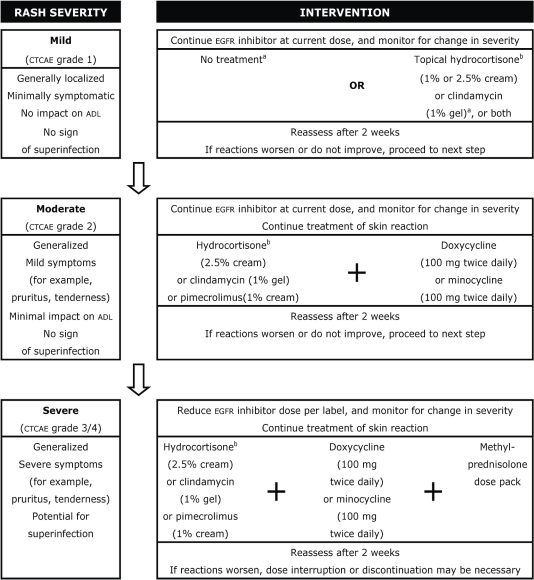
A number of treatment algorithms have been proposed for the management of skin rash induced by egfr inhibitors; the algorithm presented in Figure 2 represents an amalgam of existing frameworks 10,27,48–50.
FIGURE 2.
Management of rash induced by epidermal growth factor receptor (egfr) tyrosine kinase inhibitor (tki). ctcae = Common Terminology Criteria for Adverse Events; adl = activities of daily living. Adapted from Eaby et al., 2008 48; Harandi et al., 2009 27; Lynch et al., 2007 10.
a Guidelines presented here recommend treatment with minocycline for moderate-to-severe rash only. However, early treatment of mild rash can prevent the development of more severe forms and the need for higher, more toxic doses of minocycline. Early treatment of mild rash with 50 mg minocycline plus 1% hydrocortisone is therefore a reasonable option.
b Topical steroids should be pulsed according to institution guidelines.
The egfr-tki dosage should remain unchanged for all patients, except those with severe rash (grade 3 or higher). Patients with mild toxicities (grade 1) may need no intervention; however, treatment with topical hydrocortisone (1% or 2.5% cream) or clindamycin (1% gel) is reasonable. For patients with moderate toxicities (grade 2), treatment with hydrocortisone (2.5% cream), clindamycin (1% gel), or pimecrolimus (1% cream) is recommended, with the addition of either oral doxycycline (100 mg twice daily) or minocycline (100 mg twice daily). For patients with severe toxicities (grade 3 or higher), concomitant intervention is the same as for moderate toxicities, with the addition of a methylprednisolone dose pack (a package containing a specific number of pills to be taken at designated times over a period of a few days). If the rash does not dissipate sufficiently within 2–4 weeks, interruption of egfr inhibitor therapy is recommended in accordance with the prescribing information 10,27.
Novel treatments for egfr-tki–induced rash, such as menadione lotion, retinoids, and alpha-hydroxy acids, are under investigation in preliminary studies. However, until controlled studies are performed, their efficacy remains unknown, because rash often resolves spontaneously in patients taking egfr-tkis 47.
Because the half-life of egfr-tkis is long, management of adverse skin reactions should continue until those reactions have sufficiently diminished or resolved, even if treatment is discontinued or reduced. Once adverse reactions have sufficiently resolved, egfr-tki therapy may be restarted or escalated to the original dosing scheme, with reasonable confidence that toxicities will be well managed 27.
2.5. Rash As an Indicator of Treatment Response
Numerous studies have shown a significant correlation between skin rash severity and response to egfr inhibitor treatment 10,26,47. In nsclc, several gefitinib and erlotinib trials have supported that finding 22,39,51.
A phase ii study by Kris et al. 39 (ideal) examined the efficacy and safety of gefitinib (250 mg vs. 500 mg daily) in patients with pre-treated advanced nsclc. Although efficacy outcomes were similar across dose groups, skin toxicity was reported in 86% of patients (72 of 84) with symptom improvement [a 2-point increase in score on the Functional Assessment of Cancer Therapy–Lung (fact-L) scale], but in only 58% of patients (76 of 132) with no symptom improvement (observed difference: 28%; 95% confidence interval: 17% to 39%). A retrospective study by Mohamed et al. 51 analyzed the clinical characteristics of patients who were associated with survival after treatment with gefitinib as part of the Expanded Access Program. Median survival was longer in patients who developed skin rash than in those who did not (10.8 months vs. 4.0 months, p < 0.0001) 26,51.
A phase iii study by Shepherd et al. 11 (br.21) examined the efficacy and safety of erlotinib in patients with pre-treated advanced nsclc. Rash occurred in 76% of patients given erlotinib (368 of 485), with 9% experiencing severe (grade 3 or higher) rash. A retrospective study by Wacker et al. 22 analyzed the association between rash and clinical outcome using data from the study by Shepherd and colleagues and also data from a phase iii study comparing single-agent gemcitabine with gemcitabine plus erlotinib as first-line therapy for advanced pancreatic cancer (pa.3) 52. The response rate was 1% among patients who did not develop rash compared with 10% among those who developed grade 1 rash and 13% among those who developed grade 2 or higher rash (grade 1 vs. grade 0, p = 0.048; grade 2 vs. grade 0, p = 0.017). After controlling for baseline factors in multivariate analyses, the presence of rash correlated strongly with overall and progression-free survival; the correlations increased with rash severity (p < 0.05) 22,26.
The positive relationship between skin rash and response or survival (or both) suggests that rash could be a potential surrogate marker for egfr-tki efficacy. Whether rash reflects the local effects of egfr inhibition in skin or indicates a systemic inflammatory reaction, it may serve as a useful pharmacodynamic marker of target inhibition 22. The relationship between rash and survival is currently being evaluated in ongoing studies, and results should help guide the use of egfr-tkis.
3. DIARRHEA INDUCED BY EGFR-TKIs
3.1. Patient Monitoring and Diarrhea Causes and Incidence
Patients should be advised to immediately discuss any symptoms of diarrhea with their health care team. The diarrhea can then be managed early and effectively, preventing dose reductions or treatment discontinuation.
Because diarrhea is a common side effect of many cancer treatment regimens, management of diarrhea in the oncology setting is well established 53–55. Diarrhea induced by egfr-tkis is thought to be a result of excess chloride secretion, causing a secretory form of diarrhea 10. Severe diarrhea can result in fluid and electrolyte losses, which may lead to dehydration, electrolyte imbalances, and renal insufficiency. Nutritional deficiencies can also develop from alterations in gastrointestinal transit and digestion, negatively affecting a patient’s quality of life 55.
The incidence of diarrhea with egfr-tkis varies from 27% to 87% in phase iii clinical trials, with up to 25% of patients experiencing severe reactions (grade 3 or higher; Table iv). Table ii gives a detailed description of the clinical trials that have examined the incidence of diarrhea with egfr-tkis in nsclc.
TABLE IV.
Incidence of diarrhea with epidermal growth factor receptor (egfr) tyrosine kinase inhibitors (tkis) in non-small-cell lung cancer trials
| egfr-tki | Description | Grade (%) | |
|---|---|---|---|
| All | ≥3 | ||
| Erlotinib | All studies | 10–69 | 0–17 |
| 150 mg | Phase iii studies | 40–68 | 2–12 |
| Gefitinib | All studies | 27–75 | 0–25 |
| 250 mg and 500 mg | 250 mg | 27–58 | 0–10 |
| 500 mg | 51–75 | 5–25 | |
| Phase iii studies | 27–69 | 3–25 | |
| 250 mg | 27–58 | 3–10 | |
| 500 mg | 51–69 | 12–25 | |
| Afatinib | All studies | 67–100 | 0–33 |
| 40 mg and 50 mg | 40 mg | 67–97 | 0–7 |
| 50 mg | 87–100 | 17–33 | |
| Phase iii studies | |||
| 50 mg | 87 | 17 | |
| PF-00299804 | All studies (phase ii)a | 77–97 | 0–15 |
| 15 mg, 30 mg, and 45 mg | 30 mg | 77 | 0 |
| 45 mg | 81–97 | 13–15 | |
No phase iii study results using PF-00299804 are available to date.
3.2. Assessment and Grading
The first step in the assessment of egfr-tki–induced diarrhea is to rule out other potential causes. Possible causes of diarrhea include medications such as laxatives, stool softeners, antacids, or antibiotics; dietary factors, such as excess consumption of fibre or lactose; comorbid infections; intestinal obstruction; fecal impaction; surgeries such as short-bowel or gastrectomy; and radiation toxicity.
Laboratory investigations are also useful to rule out other causes of diarrhea. Recommended laboratory investigations include a complete blood count and differential to rule out neutropenia, blood tests to assess renal function and to determine the presence of electrolyte abnormalities, and stool culture or a Clostridium difficile toxin screen to identify whether bacterial pathogens are present. Other investigations may include abdominal radiography, endoscopy, or biopsy to rule out co-existing disorders such as bowel obstruction or perforation. Additional information—such as the duration of the episode, stool characteristics, and co-existing symptoms—should also be obtained during the patient evaluation 54,55.
The nci ctcae are generally used to grade diarrhea severity (Table v). However, because the nci criteria do not provide a complete assessment, the additional information already described should be obtained during the patient evaluation 54,55.
TABLE V.
U.S. National Cancer Institute grading for diarrheaa
| Grade 1 | Increase of fewer than 4 stools per day over baseline |
| Grade 2 | Increase of 4–6 stools per day over baseline |
| Grade 3 | Increase of 7 or more stools per day over baseline |
| Incontinence | |
| Hospitalization indicated | |
| Limits self-care activities of daily living | |
| Grade 4 | Life-threatening consequences |
| Urgent intervention indicated | |
| Grade 5 | Death |
Adapted from the Common Terminology Criteria for Adverse Events, v4.03, 2010 46.
3.3. Management
Although egfr-tki–induced diarrhea is usually mild to moderate, early management is essential to prevent dose reduction or discontinuation of anticancer therapies. The management of egfr-tki–induced diarrhea is identical to that of chemotherapy-induced diarrhea and can generally be handled using dietary changes and antidiarrheal medications 53–55.
Dietary modifications are not recommended in anticipation of diarrhea, and patients without symptoms may eat an unrestricted diet. Patients experiencing diarrhea should avoid foods that exacerbate symptoms, such as greasy, spicy, and fried items. An initial “brat” diet of bananas, rice, apple sauce, and toast is often helpful until symptoms begin to resolve. Foods that are difficult to digest—such as cabbage, Brussels sprouts, and broccoli—should be avoided, because those foods may increase abdominal cramping and bloating. Once diarrhea begins to improve, other foods such as pasta, chicken without skin, and eggs may be added as tolerated 53–55.
Daily fluid intake of approximately 3–4 L is recommended to avoid dehydration from volume loss attributable to diarrhea. At least some of the fluids should contain sugar or salt to avoid hyponatremia and hypokalemia caused by electrolyte loss. Good options include non-caffeinated beverages, gelatine products, and clear broths. Milk products should be avoided for about a week after a diarrhea episode because lactase activity may be diminished during prolonged diarrhea, resulting in temporary lactose intolerance 53–55.
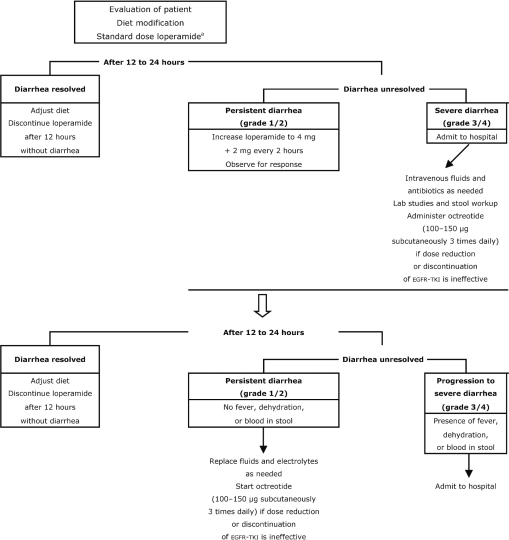
The pharmacologic management of diarrhea is usually limited to treatment with over-the-counter loperamide (Figure 3). Patients should begin taking loperamide at the first sign of diarrhea, starting with 4 mg (2 tablets), followed by 2 mg (1 tablet) every 4 hours or after each loose stool to a maximum dose of 20 mg (10 tablets) daily. If diarrhea persists for more than 24 hours, the dose of loperamide may be increased to 4 mg, followed by 2 mg every 2 hours. After 12 hours have passed with no episodes of diarrhea, pharmacologic treatment can be stopped, and the patient’s diet can be expanded as tolerated 53–56.
FIGURE 3.
Management of diarrhea induced by epidermal growth factor receptor (egfr) tyrosine kinase inhibitor (tki). Adapted from BC Cancer Agency, 2004 54; Moore et al., 2007 52; and Saltz, 2003 53. a Starting dose is 4 mg, followed by 2 mg for a maximum of 20 mg daily.
If patients present with grade 3 or 4 diarrhea, dose reduction or discontinuation of egfr-tkis may be necessary. If diarrhea fails to resolve after dose reduction or discontinuation, use of octreotide may be considered. It is very unlikely that octreotide would be needed to combat egfr-tki–induced diarrhea as it is for chemotherapy-induced diarrhea; no evidence supports its use in the egfr-tki setting. In general, most physicians would therefore discontinue egfr-tki therapy rather than start treatment with octreotide. Once severe diarrhea has subsided, egfr-tkis may be restarted at a lower dose.
4. SUMMARY
Despite the success of platinum doublets, the need for more effective agents in the treatment of advanced nsclc remains. Because nsclc patients are often symptomatic and may have a number of comorbidities, effective, low-toxicity treatment options are also required. The advent of targeted therapies has led to the development of a number of less toxic anticancer agents 8,10.
Targeting the egfr pathway has proved to be an effective strategy in a number of cancers, including nsclc. Clinical trials using egfr-tkis in nsclc have demonstrated promising anticancer activity, resulting in improved tumour control and patient survival 8,10.
Although egfr-tkis have been successful in improving clinical outcomes, they often result in a number of bothersome side effects, such as rash and diarrhea. Strategies to manage those adverse effects are essential to increase patient compliance, quality of life, and overall treatment outcome. With proper and early management, egfr-tkis may provide a less toxic treatment option for patients with advanced nsclc 8,10.
Footnotes
5. CONFLICT OF INTEREST DISCLOSURES
The author acknowledges medical writing support from Anna Christofides msc rd of SAGE Medica; her support was funded by Boehringer Ingelheim Canada.
See also NCT01085136, NCT01121393, NCT01000025, and NCT00769067 at clinicaltrials.gov/ct2/search.
6. REFERENCES
- 1.Canadian Cancer Society’s Steering Committee . Canadian Cancer Statistics 2010. Toronto, ON: Canadian Cancer Society; 2010. [Available online at: www.cancer.ca/canada-wide/aboutcancer/cancerstatistics/~/media/CCS/Canadawide/FilesList/Englishfilesheading/pdfnotinpublicationssection/CanadianCancerStatistics2020102020English.ashx; cited September 14, 2010] [Google Scholar]
- 2.United States, National Institutes of Health, National Cancer Institute (nci) Bethesda, MD: NIH; Non-small cell lung cancer treatment (PDQ) [Web page]. Health professional version. [Available at: www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/healthprofessional; cited September 12, 2010] [Google Scholar]
- 3.Sandler A, Yi J, Dahlberg S, et al. Treatment outcomes by tumour histology in Eastern Cooperative Group study E4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:1416–23. doi: 10.1097/JTO.0b013e3181da36f4. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. Ver. 2.2010. Fort Washington, PA: NCCN; 2010. [Google Scholar]
- 5.Azzoli CG, Baker S, Jr, Temin S, et al. American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage iv non-small-cell lung cancer. J Clin Oncol. 2009;27:6251–66. doi: 10.1200/JCO.2009.23.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pisters K, Evans WK, Azzoli CG, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages i–iiia resectable non-small-cell lung cancer guideline. J Clin Oncol. 2007;25:5506–18. doi: 10.1200/JCO.2007.14.1226. [DOI] [PubMed] [Google Scholar]
- 7.Hirsh V. Systemic therapies in metastatic non-small-cell lung cancer with emphasis on targeted therapies: the rational approach. Curr Oncol. 2010;17:13–23. doi: 10.3747/co.v17i2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase ii trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol. 2003;21:2237–46. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 9.Hodkinson PS, MacKinnon A, Sethi T. Targeting growth factors in lung cancer. Chest. 2008;133:1209–16. doi: 10.1378/chest.07-2680. [DOI] [PubMed] [Google Scholar]
- 10.Harandi A, Zaidi AS, Stocker AM, Laber DA. Clinical efficacy and toxicity of anti-egfr therapy in common cancers. J Oncol. 2009;2009:567486. doi: 10.1155/2009/567486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 12.Felip E, Rojo F, Reck M, et al. A phase ii pharmacodynamic study of erlotinib in patients with advanced non-small-cell lung cancer previously treated with platinum-based chemotherapy. Clin Cancer Res. 2008;14:3867–74. doi: 10.1158/1078-0432.CCR-07-5186. [DOI] [PubMed] [Google Scholar]
- 13.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–37. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 14.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non–small-cell lung cancer (interest): a randomised phase iii trial. Lancet. 2008;372:1809–18. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 15.Miller VA, Hirsh V, Cadranel J, et al. Phase iib/iii double-blind randomized trial of afatinib (BIBW 2992, an irreversible inhibitor of egfr/her1 and her2) plus best supportive care (bsc) versus placebo plus bsc in patients with nsclc failing 1–2 lines of chemotherapy and erlotinib or gefitinib (lux-Lung 1) [abstract LBA1] Ann Oncol. 2010;21(suppl 8):viii1–12. [Google Scholar]
- 16.Yang C, Shih J, Su W, et al. A phase ii study of BIBW 2992 in patients with adenocarcinoma of the lung and activating egfr/her1 mutations (lux-Lung 2) [abstract 367PD] Ann Oncol. 2010;21(suppl 8):viii123. doi: 10.1093/annonc/mdp303. [DOI] [Google Scholar]
- 17.Yamamoto N, Tamura T, Takahashi T, et al. Phase i open-label trial of continuous dose of BIBW 2992 in patients with advanced non-small cell lung cancer failing chemotherapy and/or erlotinib and/or gefitinib (lux-Lung 4) [abstract 230] J Thorac Oncol. 2010;5:91. [Google Scholar]
- 18.Janne PA, Reckamp K, Koczyway M, et al. Efficacy and safety of PF-00299804 (PF299) in patients (pt) with advanced nsclc after failure of at least one prior chemotherapy regimen and prior treatment with erlotinib (e): a two-arm, phase ii trial [abstract 8063] J Clin Oncol. 2009;27 [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=32872; cited April 14, 2011] [Google Scholar]
- 19.Ramalingam SS, Boyer MJ, Park K, et al. Randomized phase 2 study of PF299804, an irreversible human epidermal growth factor receptor (egfr) inhibitor versus erlotinib (e) in patients (pts) with advanced non-small cell lung cancer (nsclc) after chemotherapy (ct) failure: quantitative and qualitative benefits [abstract 365PD] Ann Oncol. 2010;21(suppl 8):122. [Google Scholar]
- 20.Mok T, Spigel DR, Park K. Efficacy and safety of PF299804 as first-line treatment (tx) of patients (pts) with advanced (adv) nsclc selected for activating mutation (mu) of epidermal growth factor receptor (egfr) [abstract LBA18] Ann Oncol. 2010;21(suppl 8):viii8. [Google Scholar]
- 21.Takahashi T, Boku N, Murakami H, et al. First report of the safety, pharmacokinetics, and preliminary activity of PF299804 in Japanese patients (pts) with advanced solid tumors [abstract 533P] Ann Oncol. 2010;21(suppl 8):174. [Google Scholar]
- 22.Wacker B, Nagrani T, Weinberg J, Witt K, Clark G, Cagnoni PJ. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase iii studies. Clin Cancer Res. 2007;13:3913–21. doi: 10.1158/1078-0432.CCR-06-2610. [DOI] [PubMed] [Google Scholar]
- 23.Scope A, Agero AL, Dusza SW, et al. Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol. 2007;25:5390–6. doi: 10.1200/JCO.2007.12.6987. [DOI] [PubMed] [Google Scholar]
- 24.Jatoi A, Rowland K, Sloan JA, et al. Tetracycline to prevent epidermal growth factor receptor inhibitor–induced skin rashes: results of a placebo-controlled trial from the North Central Cancer Treatment Group (N03CB) Cancer. 2008;113:847–53. doi: 10.1002/cncr.23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacouture ME, Mitchell EP, Piperdi B, et al. Skin toxicity evaluation protocol with panitumumab (stepp), a phase ii, open-label, randomized trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:1351–7. doi: 10.1200/JCO.2008.21.7828. [DOI] [PubMed] [Google Scholar]
- 26.Giovannini M, Gregorc V, Belli C, et al. Clinical significance of skin toxicity due to egfr-targeted therapies. J Oncol. 2009;2009:849051. doi: 10.1155/2009/849051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch TJ, Jr, Kim ES, Eaby B, Garey J, West DP, Lacouture ME. Epidermal growth factor receptor inhibitor–associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007;12:610–21. doi: 10.1634/theoncologist.12-5-610. [DOI] [PubMed] [Google Scholar]
- 28.Herbst RS, Prager D, Hermann R, et al. tribute: a phase iii trial of erlotinib hydrochloride (osi-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–9. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 29.Cho BC, Im CK, Park MS, et al. Phase ii study of erlotinib in advanced non-small-cell lung cancer after failure of gefitinib. J Clin Oncol. 2007;25:2528–33. doi: 10.1200/JCO.2006.10.4166. [DOI] [PubMed] [Google Scholar]
- 30.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase iii study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Traceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–52. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 31.Jackman DM, Yeap BY, Lindeman NI, et al. Phase ii clinical trial of chemotherapy-naïve patients ≥70 years of age treated with erlotinib for advanced non-small-cell lung cancer. J Clin Oncol. 2007;25:760–6. doi: 10.1200/JCO.2006.07.5754. [DOI] [PubMed] [Google Scholar]
- 32.Lilenbaum R, Axelrod R, Thomas S, et al. Randomized phase ii trial of erlotinib or standard chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. J Clin Oncol. 2008;26:863–9. doi: 10.1200/JCO.2007.13.2720. [DOI] [PubMed] [Google Scholar]
- 33.Mok T, Wu YL, Yu CJ, et al. Randomized, placebo-controlled, phase ii study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:5080–7. doi: 10.1200/JCO.2008.21.5541. [DOI] [PubMed] [Google Scholar]
- 34.Bennouna J, Besse B, Leighl NB, et al. Everolimus plus erlotinib versus erlotinib alone in previously treated patients with advanced non-small cell lung cancer (nsclc) [abstract 419P] Ann Oncol. 2010;21(suppl 8):140. doi: 10.1093/annonc/mdp505. [DOI] [PubMed] [Google Scholar]
- 35.Groen HJM, Socinski M, Grossi F, et al. Randomized phase ii study of sunitinib (su) plus erlotinib (e) vs. placebo (p) plus e for the treatment of metastatic non-small cell lung cancer (nsclc) [abstract 417P] Ann Oncol. 2010;21(suppl 8):139. [Google Scholar]
- 36.Kelly K, Azzoli CG, Patel JC, et al. Randomized phase iib study of pralatrexate vs. erlotinib in patients with stage iiib/iv non-small cell lung cancer (nsclc) after failure of prior platinum-based therapy [abstract LBA17] Ann Oncol. 2010;21(suppl 8):viii8. doi: 10.1097/JTO.0b013e31824cc66c. [DOI] [PubMed] [Google Scholar]
- 37.Scagliotti GV, Krzakowski M, Szczesna A, et al. Sunitinib (su) in combination with erotinib (e) for the treatment of advanced/metastatic nonsmall cell lung cancer (nsclc): a phase iii study [abstract LBA6] Ann Oncol. 2010;21(suppl 8):viii3. doi: 10.1093/annonc/mdq482. [DOI] [Google Scholar]
- 38.Zhao HY, Gongyan C, Feng J, et al. A phase ii study of erlotinib plus capecitabine (xel) as first line treatment for Asian elderly patients (pts) with advanced adenocarcinoma of lung (ML 22206 study) [abstract 420P] Ann Oncol. 2010;21(suppl 8):140. doi: 10.1093/annonc/mdp505. [DOI] [PubMed] [Google Scholar]
- 39.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–58. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 40.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase iii trial—intact 1. J Clin Oncol. 2004;22:777–84. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase iii trial—intact 2. J Clin Oncol. 2004;22:785–94. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 42.Goss G, Ferry D, Wierzbicki R, et al. Randomized phase ii study of gefitinib compared with placebo in chemotherapy-naïve patients with advanced non-small-cell lung cancer and poor performance status. J Clin Oncol. 2009;27:2253–60. doi: 10.1200/JCO.2008.18.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 44.Surmont VF, Gaafar RM, Scagliotti GV, et al. A double-blind, randomized, placebo-controlled phase iii study of gefitinib (g) versus placebo (p) in patients (pts) with advanced nsclc, non-progressing after first-line platinum-based chemotherapy (eortc 08021–ilcp) [abstract 368PD] Ann Oncol. 2010;21(suppl 8):124. doi: 10.1016/j.ejca.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 45.Melosky B, Burkes R, Rayson D, Alcindor T, Shear N, Lacouture M. Management of skin rash during egfr-targeted monoclonal antibody treatment for gastrointestinal malignancies: Canadian recommendations. Curr Oncol. 2009;16:14–24. doi: 10.3747/co.v16i1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.United States, Department of Health and Human Services, National Institutes of Health, National Cancer Institute(nci) Common Terminology Criteria for Adverse Events (CTCAE) Bethesda, MD: NCI; 2010. Ver. 4.03. [Available online at: evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf; cited September 16, 2010] [Google Scholar]
- 47.Pérez–Soler R, Delord JP, Halpern A, et al. her1/egfr inhibitor–associated rash: future directions for management and investigation outcomes from the HER1/EGFR Inhibitor Rash Management Forum. Oncologist. 2005;10:345–56. doi: 10.1634/theoncologist.10-5-345. [DOI] [PubMed] [Google Scholar]
- 48.Eaby B, Culkin A, Lacouture ME. An interdisciplinary consensus on managing skin reactions associated with human epidermal growth factor receptor inhibitors. Clin J Oncol Nurs. 2008;12:283–90. doi: 10.1188/08.CJON.283-290. [DOI] [PubMed] [Google Scholar]
- 49.Segaert S, Tabernero J, Chosidow O, et al. The management of skin reactions in cancer patients receiving epidermal growth factor receptor targeted therapies. J Dtsch Dermatol Ges. 2005;3:599–606. doi: 10.1111/j.1610-0387.2005.05058.x. [DOI] [PubMed] [Google Scholar]
- 50.Lacouture ME, Basti S, Patel J, Benson A., 3rd The series clinic: an interdisciplinary approach to the management of toxicities of egfr inhibitors. J Support Oncol. 2006;4:236–8. [PubMed] [Google Scholar]
- 51.Mohamed MK, Ramalingam S, Lin Y, Gooding W, Belani CP. Skin rash and good performance status predict improved survival with gefitinib in patients with advanced non-small cell lung cancer. Ann Oncol. 2005;16:780–5. doi: 10.1093/annonc/mdi157. [DOI] [PubMed] [Google Scholar]
- 52.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase iii trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 53.Saltz LB. Understanding and managing chemotherapy-induced diarrhea. J Support Oncol. 2003;1:35–46. [PubMed] [Google Scholar]
- 54.BC Cancer Agency (bcca) BCCA Guidelines for Management of Chemotherapy-induced Diarrhea. Vancouver, BC: BCCA; 2004. [Available online at: www.bccancer.bc.ca/NR/rdonlyres/4E7EF86A-EAA5-4F3C-B147-B2512799F6B3/7371/GuidelinesforManagementofCID.pdf; cited September 15, 2010]. [Google Scholar]
- 55.Maroun JA, Anthony LB, Blais N, et al. Prevention and management of chemotherapy-induced diarrhea in patients with colorectal cancer: a consensus statement by the Canadian Working Group on Chemotherapy-Induced Diarrhea. Curr Oncol. 2007;14:13–20. doi: 10.3747/co.2007.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McNeil Consumer Healthcare . Imodium Product Monograph. Guelph, ON: McNeil Consumer Healthcare; 2008. [Google Scholar]