Abstract
Background
Cancer pain is highly prevalent, and existing treatments are often insufficient to provide adequate relief.
Objectives
We assessed the long-term safety and efficacy of subcutaneous tetrodotoxin treatment in reducing the intensity of chronic cancer-related pain.
Methods
In this multicentre open-label longitudinal trial, 30 μg tetrodotoxin was administered subcutaneously twice daily for 4 days in a heterogeneous cohort of patients with persistent pain despite opioids and other analgesics. “Responder” was defined as a mean reduction of 30% or more in pain intensity from baseline; and “clinical responder” as some pain reduction, but less than 30%, plus agreement on the part of both the patient and the physician that a meaningful analgesic response to treatment had occurred.
Results
Of 45 patients who entered the longitudinal trial, 41 had sufficient data for analysis. Of all 45 patients, 21 (47%) met the criteria for “responder” [16 patients (36%)] or “clinical responder” [5 patients (11%)]. Onset of pain relief was typically cumulative over days, and after administration ended, the analgesic effect subsided over the course of a few weeks. No evidence of loss of analgesic effect was observed during subsequent treatments (2526 patient–days in total and a maximum of 400 days in 1 patient). One patient withdrew from the study because of adverse events. Toxicity was usually mild (82%) or moderate (13%), and remained so through subsequent treatment cycles, with no evidence of cumulative toxicity or tolerance.
Conclusions
Long-term treatment with tetrodotoxin is associated with acceptable toxicity and, in a substantial minority of patients, resulted in a sustained analgesic effect. Further study of tetrodotoxin for moderate-to-severe cancer pain is warranted.
Keywords: Clinical trial, cancer pain, analgesic, tetrodotoxin, long-term, safety, efficacy, open-label
1. INTRODUCTION
The World Health Organization predicts that cancer incidence worldwide will soar by 50% over the next 20 years, to reach 15 million new cancer cases every year by 2020 1. A recent survey reported that 82% of patients with cancer experience pain and that at least 61% experience “very distressing” pain 2. In patients with cancer, pain results directly from the tumour in about 80% of cases, primarily from anticancer treatments in 17%, and from causes unrelated to cancer or its treatments in 3% 3.
World Health Organization guidelines for the treatment of cancer pain are based on a 3-step ladder, and they remain the clinical model for pain management 4. Opioid analgesics are the mainstay of therapy for mild to severe cancer-related pain 5; however, in 10%–20% of patients, opioids and adjuvant therapy will be insufficient to relieve pain 6,7. Moreover, 65% of patients on opioids will experience adverse effects such as nausea, vomiting, constipation, somnolence, and dizziness, and 12% of patients will experience more severe adverse effects such as cognitive impairment and hallucinations, respiratory depression, and on rare occasions, death 8. These data indicate that, despite the existing analgesic arsenal, cancer-related pain is a prevalent and serious public health issue, and that new approaches are urgently required to effectively control pain.
Tetrodotoxin (ttx) is a naturally occurring potent sodium channel blocker found in several species of tetraodon pufferfish, notably the Fugu genus. Animal studies have shown that ttx exhibits a strong analgesic effect 9–11. The mechanism by which ttx exerts its analgesic effect is thought to be related to its sodium channel blocking properties, because voltage-gated sodium channels play a critical role in many chronic pain syndromes and are found on most nociceptive pain fibres 12,13.
In an open-label multicentre dose escalation study of ttx in severe cancer-related pain, 24 patients underwent 31 courses of treatment. The ttx was administered 2 or 3 times daily for a total of 4 days. The study showed that 17 of the 31 treatments resulted in a clinically meaningful reduction in pain intensity and that relief of pain persisted for up to 2 weeks or longer 14. This dose escalation study found that 30 μg twice daily for 4 days provided an acceptable toxicity and analgesic profile.
The dose escalation study was followed by a randomized double-blind placebo-controlled parallel-design multicentric study. It included patients with moderate or severe unrelieved cancer pain; they received 30 μg ttx or placebo subcutaneously over 4 days and were observed through treatment and to day 15 or longer. At the end of the study, all patients were permitted to enrol into a formal open-label safety and efficacy extension trial at the same dose 15. The blinded study failed to meet its primary endpoint (decline in pain of at least 30%). However, a strong analgesic signal appeared to be present within the data, and further testing of ttx is ongoing.
Here, we describe the analgesic effect and safety of ttx, with multiple cycles of treatment, in patients who enrolled in the open-label safety and efficacy extension trial.
2. METHODS
2.1. Trial Design
This multicentric open-label trial of ttx in inadequately controlled moderate-to-severe cancer-related pain evaluated long-term ttx safety and efficacy in patients who had previously participated in a double-blind placebo-controlled trial 15. The primary objective was to assess the long-term safety and efficacy of subcutaneous ttx treatment in reducing the intensity of cancer-related pain. The secondary objective was to assess the duration of analgesia after repeated cycles of ttx treatment.
The primary endpoints were
the proportion of patients who showed a clinically meaningful analgesic response for each treatment cycle.
the number of consecutive treatment cycles in which patients met the criteria for “responder” or “clinical responder.”
safety, as assessed through adverse events and abnormal laboratory results.
The secondary endpoints were
the duration in days from the first day that patients met the criteria for “responder” after the first ttx injection of a treatment cycle to the day that the patient was no longer a “responder.”
the total cumulative time that the patient satisfied the criteria for “clinical responder.”
Patients received ttx 30 μg by subcutaneous injection twice daily for 4 consecutive days and were thereafter observed at least to day 15 or until pain returned, at which time patients were re-evaluated for re-treatment in the open-label study. For subsequent treatment cycles, the patients had to meet the eligibility criteria for a repeated treatment cycle: that is, they were classified as “responders” or “clinical responders,” with a clinically meaningful response to treatment as judged by both the patient and the physician.
Predetermined definitions included “responder,” “clinical responder,” and “non-responder”:
“Responder” was defined as a patient having a mean reduction in pain intensity of 30% or more from baseline. The decline of pain could occur during the early post-injection period (days 5–8) or the late post-injection period (days 9–15) and was assessed by the patient’s worst or average pain as measured using the Brief Pain Inventory (bpi). Some patients had more than one discrete pain; if measures for any one of their pains fell by 30% or more from baseline, the patient was deemed a responder. These individual, discrete pains were, by convention, described within the trial’s pain assessment documents as “component specific pains.” Mean opioid analgesic dose (morphine equivalents) during the same period had to be less than 125% of the mean baseline period use.
“Clinical responder” was defined a patient who, during the first treatment cycle, had experienced a reduction in pain intensity of less than 30% from baseline (measured by the criteria already defined) and who, with the physician’s concurrence, indicated that the analgesic response to treatment was meaningful.
“Non-responder” was defined as a person who, after treatment, had not fulfilled either of the foregoing criteria.
Average baseline pain intensity as measured by bpi question 5 or worst pain intensity as measured by bpi question 3, for pain overall or for a specific measurable pain, had to be 4 or greater to be eligible for the longitudinal study. Measurements were taken over a 5-day screening period. For study purposes, a score of 4 or 5 was deemed to be “moderate pain” and a score of 6 or more (out of 10) was defined as “severe pain.” Patients had a life expectancy greater than 3 months in the opinion of the site investigator. They also had to have the ability to communicate with the investigator and to comply with the requirements of the study, and they had to be willing to provide written informed consent.
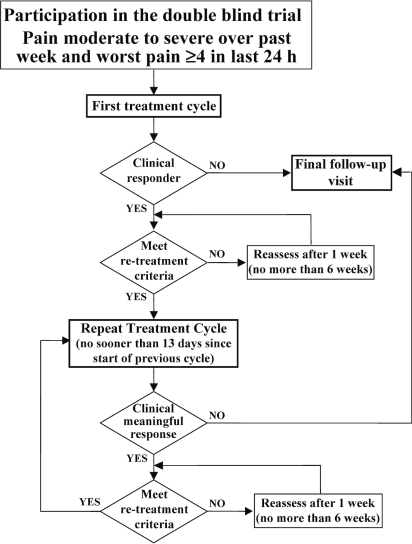
After screening and baseline data collection, patients were admitted to the hospital (inpatients) or made daily visits to the site’s outpatient care facility (outpatients) to receive ttx for 4 days (Figure 1). Daily patient assessments (daily patient diary) were completed during the first 3 weeks of a treatment cycle. Weekly patient assessments (weekly patient diary) were completed in subsequent weeks. Additional safety assessments were made during the clinic visits on treatment days (clinical laboratory assessments on day 4, vital signs, brief physical examination, and electrocardiogram if indicated). Study staff reviewed the results of the assessments with the patients weekly by telephone. A patient who fulfilled responder or clinical responder criteria was eligible to enter a new treatment cycle when pain intensity, as measured by any one of the patient’s numeric rating scales, increased to 4 or more, or when global or component-specific pain was no longer very much improved or much improved. Initiation of the next treatment cycle occurred at least 15 days after the start of the previous treatment cycle. Patients who were not eligible for the next treatment cycle or who elected not to continue in the trial were assessed at a final follow-up visit.
FIGURE 1.
Study plan and patient flow.
Patients were encouraged to remain on their current analgesic and cancer treatments. Analgesics could be tapered or discontinued according to clinical need and the presence of toxicity. Previously administered cancer treatments such as chemotherapy and bisphosphonates could be continued, but patients were not eligible to receive ttx if their cancer treatments had been found to be associated with an analgesic response.
2.2. Assessment Methods
The trial used several instruments to measure outcome. Spontaneously reported adverse events and open-ended questionnaires were recorded by the nursing staff before and during treatment and also during telephone follow-up on days 8 and 15, and at subsequent contacts. Efficacy measures included worst pain in the preceding 24 hours (bpi question 3), average pain in the preceding 24 hours (bpi question 5), component-specific pain in the preceding 24 hours over time, and global impression of change on the part of the subject and the physician (7-point categorical scale, 1 being very much improved and 7 being very much worse). Duration of the analgesic response was measured based on change in pain scores over time. All data are presented as mean ± standard deviation.
2.3. Safety Analysis
Safety assessments included adverse event reporting and measures of blood pressure, heart rate, clinical chemistry, and hematology. Adverse events were spontaneously reported by patients during the period of direct nursing observation (typically, patients were directly observed for about 90 minutes after each subcutaneous injection of ttx) and by inquiry of the nursing staff (“Have you noticed any side effects to this treatment since you were last in the clinic?”). In each instance, the event was coded as “mild,” “moderate,” “severe,” or “serious,” and “not related,” possibly related,” “probably related,” and “definitely related” to treatment.
Adverse events (incidence, emergent adverse events on treatment, incidence of serious adverse events, and incidence of adverse events leading to discontinuation) were summarized for the patients overall.
3. RESULTS
This open-label continuation trial enrolled 45 patients (24 men, 21 women; mean age: 58 ± 12 years). The primary cancer diagnoses included lung cancer (n = 12), gastrointestinal cancer (n = 8), breast cancer (n = 6), prostate cancer (n = 4), and others (n = 15). In 23 patients, pain was judged to be caused directly by cancer; in 16, it was caused by cancer treatment; and in 6 patients, the origin of the pain was both cancer and its treatment. In 28 patients, the pain was categorized as having a major neuropathic component; in 16, as having no neuropathic component; and in 1 patient, no categorization could be reached. Patients were receiving a wide range of opioid, non-opioid, and adjuvant analgesics. Pain scores within the cohort were high: at baseline, the mean score for worst pain in the preceding 24 hours (bpi question 3) was 7.4 ± 1.7, and mean score for average pain in the preceding 24 hours (bpi question 5) was 5.6 ± 1.9.
Of the 45 patients enrolled, 4 patients lacked available data for further analysis of drug safety and efficacy: 2 patients withdrew consent; 1 was ineligible (pain was mild, with a bpi question 3 score below 4), and 1 discontinued because of an adverse event (nausea, dizziness). Hence, 41 patients were evaluable for the ttx safety and efficacy analysis.
3.1. Analgesic Effect
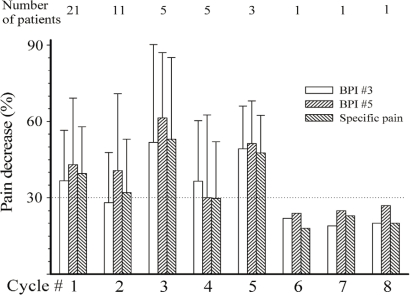
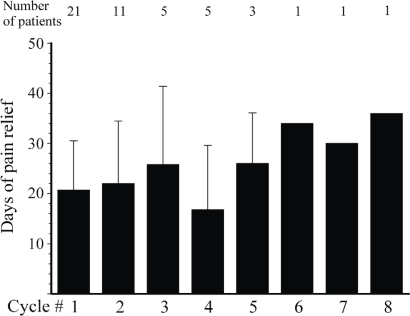
Of the 41 evaluable patients, 16 were responders, 5 were clinical responders, and 20 were non-responders to ttx at completion of treatment cycle 1. Table i summarizes the response to ttx according to pain pathophysiology and causative cancer. After the first cycle of ttx, the average global decrease in pain in the overall cohort was more than 35% for bpi question 3, bpi question 5, and individual pains (Figure 2). At 2.19 ± 0.75, the impression of the patients about the pain was, on average, close to “much improved” (1 = “very much improved”; 2 = “much improved”; 3 = “minimally improved”; 4 = “no change”). After the first cycle of ttx 30 μg bid for 4 days, pain relief lasted, on average, 21 days (Figure 3).
TABLE I.
Response to tetrodotoxin treatment based on pain pathophysiology and cancer cause
| Cancer origin |
Responder |
Non-responder |
|||
|---|---|---|---|---|---|
| np | Non-np | np | Non-np | ||
| Abdominal cavity | Intestine | ||||
| Small intestine | 1 | 1 | |||
| Large intestine | 1 | 1 | |||
| Rectum | 2 | ||||
| Pancreas | 1 | ||||
| Gynecologic | |||||
| Ovary | 1 | ||||
| Uterus | 1 | ||||
| Cervix | 1 | ||||
| Kidney | 1 | ||||
| Prostate | 2 | ||||
| Testis | 1 | ||||
| Thorax | Lung | 4 | 1 | 3 | 3 |
| Breast | 1 | 1 | 3 | 1 | |
| Bone marrow | 2 | 1 | |||
| Other | Oropharynx | 1 | |||
| Spinal | 1 | 4 | |||
| Cervical ependymoma | 1 | ||||
| TOTAL | 9 | 7 | 18 | 7 | |
np = neuropathic.
FIGURE 2.
Extent of pain relief in successive cycles. bpi = Brief Pain Inventory.
FIGURE 3.
Duration of pain relief in responders and clinical responders in successive cycles.
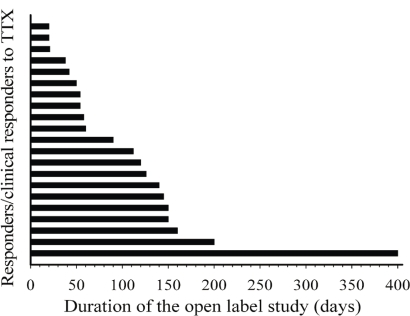
For patients enrolled in successive cycles, the decrease in pain remained similar to that seen after the first cycle of ttx: that is, 30% or more (Figure 2). The impression of the patients with regard to pain improvement remained fairly constant: for example, 1.73 ± 0.75, 1.60 ± 0.55, 2.40 ± 0.55, and 1.67 ± 0.58 in cycles 2, 3, 4, and 5 respectively. Similar observations were seen with the Physician’s Global Impression of Change (data available on request). Finally, the average duration of pain relief remained at about 20 days for all subsequent cycles (Figure 3). The mean total duration of the open-label study was 105 ± 86 days, but durations varied greatly between the patients (Figure 4), ranging from 20 days to 400 days.
FIGURE 4.
Duration of participation in the open-label study for each responder and clinical responder. ttx = tetrodotoxin.
In the patients who remained on the trial, the medication remained tolerable, and pain relief was seen through additional treatment cycles. There were no clinical features of the pain that clearly predicted a positive analgesic outcome: patients with somatic, visceral, or neuropathic pain were identified among the non-responders and the responders alike.
3.2. Adverse Events
Spontaneous adverse events were recorded in most participants, with most events being described as mild (82%) or moderate (13%) in severity; all were well tolerated and were of short duration, lasting from 20 minutes to several hours (Table ii). Adverse events were most commonly associated with the nervous and gastrointestinal systems: close to half the patients described mild peri-oral tingling or numbness, which generally began about half an hour after injection of ttx and resolved about an hour later. Transient nausea was also reported by approximately one third of patients. A range of other symptoms, including irritation at the administration site, were also reported. The adverse events presented during successive cycles were similar to those described during the first cycle, and the range and prevalence of events reported in the present study were similar in nature to those reported in previous trials 14,15.
TABLE II.
Adverse events by system
| Body system |
Events |
|
|---|---|---|
| (n) | (%) | |
| Cardiac disorders | 0 | 0 |
| Ear and labyrinth disorders | 1 | 2.2 |
| Eye disorders | 1 | 2.2 |
| Gastrointestinal disorders [oral hypoesthesia (23%), nausea (8%), vomiting (3%), oral pain (1%)] | 16 | 35.6 |
| General disorders and administration-site conditions (for example, local injection pain) | 20 | 44.4 |
| Infections and infestations | 2 | 4.4 |
| Investigations | 3 | 6.7 |
| Metabolism and nutrition disorders | 1 | 2.2 |
| Musculoskeletal and connective tissue disorders | 7 | 15.6 |
| Nervous system disorders [oral paresthesia (18%), hypoesthesia (15%), dizziness (3%), dysgeusia (4%), somnolence (8%), vertigo (4%)] | 21 | 46.7 |
| Psychiatric disorders | 4 | 8.9 |
| Respiratory, thoracic and mediastinal disorders | 6 | 13.3 |
| Skin and subcutaneous tissue disorders | 11 | 24.4 |
| Vascular disorders | 4 | 8.9 |
| TOTAL | 45 | 100 |
3.3. Serious Adverse Events
Four serious adverse events were reported in the trial. Three were judged to be unrelated to the study drug: 1 was an episode of cholelithiasis that resolved in 6 days; 1 was sudden onset of severe abdominal pain that resolved spontaneously in 24 hours; and 1 was a pulmonary infection that resolved in 5 days. In the 4th patient, a serious adverse event developed that was judged to probably be related to the study drug. This 64-year-old woman with a history of mild chronic obstructive lung disease had colon cancer with metastases to lung and liver. She started the study drug at a dose of 30 μg twice daily, and an hour after the second dose on day 1, she experienced hypertension and dizziness. Her blood pressure was 176/96; her respiration rate, 16; and her oxygen saturation, 97%. Hypertension continued, with a high at one point of 204/107. An intravenous line was inserted, and she was admitted to hospital. Results of a 12-lead electrocardiogram, a complete blood count, and electrolyte tests were all within normal limits. The investigator assessed both events—dizziness and hypertension—as moderate in severity. Both events resolved the same day, and the patient was discharged the following day. She completed the final 2 days of dosing, with a dose reduced to 15 μg twice daily. No further episodes of severe hypertension or dizziness were observed.
3.4. Death
No deaths occurred while patients were on study.
4. DISCUSSION
Two key findings emerged from this open-label long-term efficacy study. First, ttx has acceptable tolerability, even with long-term administration. Second, in patients who describe an analgesic effect, relief of chronic cancer pain is persistent within successive treatment cycles up to and beyond 1 year, without evidence of tolerance: the anti-nociceptive effect of ttx is maintained for an average of 3 weeks, and this pain relief does not diminish with repeated cycles of the drug.
Previous studies have reported the range of acute toxicity with administration of ttx at the dose used in the present work 14,15. Typically, toxicity has been mild and primarily sensory, with transient peri-oral numbness or tingling as the predominant experience. Toxicity was similar in the present study. With repeated cycles over a period of more than 1 year, toxicity in subsequent cycles was essentially identical; neither cumulative toxicity nor tolerance to adverse events was observed.
The data suggest that about half the patients experienced an analgesic effect, but efficacy data in open-label trials must be interpreted cautiously. In uncontrolled studies with an analgesically active compound, the analgesic signal generally includes some true analgesic effect plus some placebo effect. Further, in a cohort of patients with substantial burden of underlying illness, a challenging trial protocol involving twice-daily subcutaneous injections for 4 days, in cycles occurring every few or several weeks, can be difficult to tolerate; dropouts can be predicted to occur, and there is a bias in favour of patients with less toxicity and a better analgesic response. Further, compared with controlled trials, long-term open-label studies use fewer patient assessments so as to keep the demands on patients as modest as is practical; thus, toxicity and analgesic outcomes data are more focused and less comprehensive. However, longitudinal open-label analgesic trials do allow preliminary conclusions to be drawn regarding long-term tolerability and continuing analgesic effect.
The present trial also illustrates challenges in capturing an analgesic signal in a heterogeneous cohort of patients with complex pain and advanced illness. Consistent with clinical trials in other areas of pain research 16,17, we found that a decline in pain as a single dimension is often insufficient to fully characterize the analgesic effect.
There are two striking features of the action of ttx. First, it is a highly selective sodium channel blocker, and second, administered over a 4-day period, it results in pain relief that lasts for weeks. It is noteworthy that the intravenous administration of lidocaine, another sodium channel blocker, can produce long-lasting analgesia. In cancer patients with opioid-refractory pain, mean duration of analgesia was 9.3 ± 2.6 days after a single infusion 18. For intractably painful diabetic neuropathy, intensity of pain was diminished for up to 28 days after an infusion 19. Trigeminal nerve block with a high dose of lidocaine in patients with trigeminal neuralgia produced complete pain relief in 11 patients 1 day after the block, which lasted for 3–172 weeks 20.
We wondered if particular pain syndromes would emerge that would predictably be responsive to ttx as an analgesic. Although a large proportion of patients experienced an analgesic effect, the response to ttx did not appear to be associated with the type or location of the cancer, the presence or absence of metastases, the source of the pain (the cancer, the treatment, or both), the nature of the pain (neuropathic or non-neuropathic), or the age or sex of the patient.
The mechanism of the analgesic effect of ttx is not fully understood. Ectopic activity in damaged and dysfunctional sensory afferents has a role in the generation and maintenance of pain, and one of the mechanisms underlying this ectopic firing involves abnormal modulation of voltage-gated sodium channels 21,22. Moreover, there is evidence that damaged sensory afferents such as those found in neuromas secondary to trauma, amputation, compression, or surgery show overexpression of Nav1.3, Nav1.7, and Nav1.8 23. We postulate that the response to ttx is associated with the expression of voltage-gated sodium channels (Nav) and not with the characteristics or causes of the pain.
The fact that adverse effects are rapidly reversible (for example, 20–60 minutes for peri-oral paresthesias, and 24–48 hours for ataxia) suggests that the long anti-nociceptive effect of ttx is modulated by a mechanism or mechanisms that are different from those that mediate toxicity. That the analgesic effect of ttx lasts weeks in cancer patients is a remarkable phenomenon, but the mechanism of this effect is not fully known.
5. CONCLUSIONS
This open-label multicentric trial of ttx for moderate-to-severe cancer pain demonstrated that toxicity was almost always mild and well tolerated. A cumulative analgesic effect over the course of several days was seen in no more than half the patients, and the effect then wore off over the course of weeks. There was no evidence of tolerance to the analgesic effect in repeated cycles administered over a period of more than 1 year. Responses were achieved for neuropathic and non-neuropathic pain, and for pain from cancer and from its treatment. Further study of ttx for moderate-to-severe cancer pain is warranted.
6. ACKNOWLEDGMENT
This study was funded by Wex Pharmaceuticals (Vancouver, BC). The authors gratefully acknowledge the support of co-investigators Drs. Stakiw, Clein, Girard, and Moulin; the study nurses; and other members of the Canadian Tetrodotoxin Study Group.
Footnotes
7. CONFLICT OF INTEREST DISCLOSURES
NAH, BL, MOL, and PdS have received honoraria or consulting fees for certain activities related to this trial.
8. REFERENCES
- 1.Frankish H. 15 Million new cancer cases per year by 2020, says who. Lancet. 2003;361:1278. doi: 10.1016/S0140-6736(03)13038-3. [DOI] [PubMed] [Google Scholar]
- 2.Costantini M, Ripamonti C, Beccaro M, et al. Prevalence, distress, management, and relief of pain during the last 3 months of cancer patients’ life. Results of an Italian mortality follow-back survey. Ann Oncol. 2009;20:729–35. doi: 10.1093/annonc/mdn700. [DOI] [PubMed] [Google Scholar]
- 3.Foley KM. Acute and chronic cancer pain syndromes. In: Doyle D, Hanks G, Cherny N, Calman K, editors. Oxford Textbook of Palliative Medicine. 3rd ed. Oxford, U.K: Oxford University Press; 2004. pp. 299–316. [Google Scholar]
- 4.Mercadante S, Fulfaro F. World Health Organization guidelines for cancer pain: a reappraisal. Ann Oncol. 2005;16(suppl 4):iv132–5. doi: 10.1093/annonc/mdi922. [DOI] [PubMed] [Google Scholar]
- 5.Ripamonti C, Bandieri E. Pain therapy. Crit Rev Oncol Hematol. 2009;70:145–59. doi: 10.1016/j.critrevonc.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Laird B, Colvin L, Fallon M. Management of cancer pain: basic principles and neuropathic cancer pain. Eur J Cancer. 2008;44:1078–82. doi: 10.1016/j.ejca.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Fallon M. When morphine does not work. Support Care Cancer. 2008;16:771–5. doi: 10.1007/s00520-008-0402-8. [DOI] [PubMed] [Google Scholar]
- 8.Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11(suppl 2):S105–20. [PubMed] [Google Scholar]
- 9.Omana–Zapata I, Khabbaz MA, Hunter JC, Clarke DE, Bley KR. Tetrodotoxin inhibits neuropathic ectopic activity in neuromas, dorsal root ganglia and dorsal horn neurons. Pain. 1997;72:41–9. doi: 10.1016/S0304-3959(97)00012-2. [DOI] [PubMed] [Google Scholar]
- 10.Lyu YS, Park SK, Chung K, Chung JM. Low dose of tetrodotoxin reduces neuropathic pain behaviors in an animal model. Brain Res. 2000;871:98–103. doi: 10.1016/S0006-8993(00)02451-3. [DOI] [PubMed] [Google Scholar]
- 11.Marcil J, Walczak JS, Guindon J, Ngoc AH, Lu S, Beaulieu P. Antinociceptive effects of tetrodotoxin (ttx) in rodents. Br J Anaesth. 2006;96:761–8. doi: 10.1093/bja/ael096. [DOI] [PubMed] [Google Scholar]
- 12.Lai J, Porreca F, Hunter JC, Gold MS. Voltage-gated sodium channels and hyperalgesia. Annu Rev Pharmacol Toxicol. 2004;44:371–97. doi: 10.1146/annurev.pharmtox.44.101802.121627. [DOI] [PubMed] [Google Scholar]
- 13.Amir R, Argoff CE, Bennett GJ, et al. The role of sodium channels in chronic inflammatory and neuropathic pain. J Pain. 2006;7(suppl 3):S1–29. doi: 10.1016/j.jpain.2006.01.444. [DOI] [PubMed] [Google Scholar]
- 14.Hagen NA, Fisher KM, Lapointe B, et al. An open-label, multi-dose efficacy and safety study of intramuscular tetrodotoxin in patients with severe cancer-related pain. J Pain Symptom Manage. 2007;34:171–82. doi: 10.1016/j.jpainsymman.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Hagen NA, du Souich P, Lapointe B, et al. Tetrodotoxin for moderate to severe cancer pain: a randomized, double blind, parallel design multicenter study. J Pain Symptom Manage. 2008;35:420–9. doi: 10.1016/j.jpainsymman.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 17.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: immpact recommendations. J Pain. 2008;9:105–21. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S, Rajagopal MR, Palat G, Singh C, Haji AG, Jain D. A phase ii pilot study to evaluate use of intravenous lidocaine for opioid-refractory pain in cancer patients. J Pain Symptom Manage. 2009;37:85–93. doi: 10.1016/j.jpainsymman.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Viola V, Newnham HH, Simpson RW. Treatment of intractable painful diabetic neuropathy with intravenous lignocaine. J Diabetes Complications. 2006;20:34–9. doi: 10.1016/j.jdiacomp.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Han KR, Kim C, Chae YJ, Kim DW. Efficacy and safety of high concentration lidocaine for trigeminal nerve block in patients with trigeminal neuralgia. Int J Clin Pract. 2008;62:248–54. doi: 10.1111/j.1742-1241.2007.01568.x. [DOI] [PubMed] [Google Scholar]
- 21.Matzner O, Devor M. Hyperexcitability at sites of nerve injury depends on voltage-sensitive Na+ channels. J Neurophysiol. 1994;72:349–59. doi: 10.1152/jn.1994.72.1.349. [DOI] [PubMed] [Google Scholar]
- 22.Silos–Santiago I. The role of tetrodotoxin-resistant sodium channels in pain states: are they the next target for analgesic drugs? Curr Opin Investig Drugs. 2008;9:83–9. [PubMed] [Google Scholar]
- 23.Black JA, Nikolajsen L, Kroner K, Jensen TS, Waxman SG. Multiple sodium channel isoforms and mitogen-activated protein kinases are present in painful human neuromas. Ann Neurol. 2008;64:644–53. doi: 10.1002/ana.21527. [DOI] [PubMed] [Google Scholar]