Abstract
With the recent approval by the U.S. Food and Drug Administration of the first therapeutic vaccine for cancer, the long-awaited goal of harnessing a patient’s immune system to attack cancer through this modality is finally realized. However, as researchers in the field of cancer immunotherapy continue to perform randomized definitive studies, much remains to be learned about potential surrogate endpoints and appropriate patient populations for therapeutic vaccines. The present review addresses available data from clinical trials of immunotherapeutic agents relevant to the selection of appropriate patient populations. We believe that the weight of evidence supports the use of immunotherapy earlier in the disease course and in patients with less aggressive disease, and that the relevant findings have important implications for the design of clinical trials with therapeutic vaccines.
Keywords: Cancer vaccine, immunotherapy, overall survival, Prostvac, TroVax, Stimuvax
THERAPEUTIC VACCINES IN PROSTATE CANCER
In April 2010, sipuleucel-T (Provenge: Dendreon Corporation, Seattle, WA, U.S.A.) became the first therapeutic cancer vaccine to be approved by the U.S. Food and Drug Administration. Approval was based on the results of two randomized placebo-controlled phase iii clinical trials that demonstrated a consistent overall survival advantage with the use of sipuleucel-T 1–3.
Sipuleucel-T is an autologous antigen-presenting-cell-based therapeutic vaccine derived from a leucapheresis pack from a prostate cancer patient. This cellular product is then pulsed with a fusion protein (granulocyte–macrophage colony–stimulating factor/prostatic acid phosphatase) and re-infused into the patient within 48 hours. The pivotal impact (Immunotherapy for Prostate Adenocarcinoma Treatment) trial, whose endpoint was overall survival, enrolled 512 patients with either minimal or no symptoms from metastatic castration-resistant prostate cancer (mcrpc). Predicted median overall survival, as analyzed using a validated nomogram 4, was 21 months. Patients treated with sipuleucel-T had a 22% decrease in risk of death [hazard ratio (hr): 0.78; p = 0.03] and a 4.1-month improvement in median overall survival (25.8 months vs. 21.7 months with placebo). No difference in time to objective disease progression was observed between the treatment arms. Those findings led to approval of the vaccine in the United States.
Another therapeutic vaccine to recently report an overall survival advantage in prostate cancer is psa-tricom (Prostvac: Bavarian Nordic Immunotherapeutics, Mountain View, CA, U.S.A.) 5. This vaccine consists of an “off-the-shelf” poxvirus vector encoding prostate-specific antigen (psa) and 3 T-cell co-stimulatory molecules (tricom) 6,7. It can be manufactured in large quantities, stored frozen for many years, and then thawed and injected into patients. In a placebo-controlled double-blind multicentric randomized phase iib study (n = 125), patients treated with psa-tricom had a 44% reduction in death rate and an 8.5-month improvement in median overall survival (p = 0.006) when compared with patients treated with wild-type poxvirus vectors. As in the impact trial, no improvement in time to progression was observed. The psa-tricom trial also enrolled patients with mcrpc who had minimal or no symptoms and a median Halabi-predicted survival (hps) of 21 months. A smaller study (n = 32) of psa-tricom reported an overall survival of 26.6 months, with a median improvement in survival of 9.2 months compared with predicted survival 8.
The consistent improvement in overall survival noted in the foregoing studies has led to the design of a global randomized phase iii study in 1200 patients with asymptomatic or minimally symptomatic mcrpc (prospect study), scheduled to open in 2011.
KINETICS OF CLINICAL RESPONSE AFTER THERAPEUTIC VACCINATION
Previous pivotal studies with the chemotherapeutic agent docetaxel were the first to demonstrate any improvement in overall survival (2–3 months) in men with mcrpc. Although the randomized studies of vaccines showed clinically meaningful improvements in overall survival (4.1–8.5 months), no improved time to progression was noted (Table i). The same phenomenon of improved overall survival without improved median time to progression was also noted in another recently published phase iii immunotherapy clinical trial of an anti–cytotoxic T-lymphocyte antigen 4 antibody (ipilimumab) in patients with metastatic melanoma 9.
TABLE I.
Comparison of sipuleucel-T and psa-tricom (poxvirus vector encoding prostate-specific antigen, plus 3 T-cell co-stimulatory molecules)
| Reference (study name) | Study arm | Pts (n) | pfs | os (months) | hr | p value |
|---|---|---|---|---|---|---|
| Small et al., 2006 1(D9901, phase iii rct) | Total: 127 | |||||
| Placebo | 45 | 10.0 weeks | 21.4 | 0.59 | 0.01 | |
| Sipuleucel-T | 82 | 11.7 weeks | 25.9 | |||
| Kantoff et al., 2010 3 (impact, phase iii rct) | Total: 512 | |||||
| Placebo | 171 | 14.6 weeks | 21.7 | 0.778 | 0.032 | |
| Sipuleucel-T | 341 | 14.4 weeks | 25.8 | |||
| Kantoff et al., 2010 5 (psa-tricom, phase ii rct) | Total: 125a | |||||
| Vector control | 40 | 3.7 months | 16.6 | 0.54 | 0.0061 | |
| psa-tricom | 82 | 3.8 months | 25.1 |
Three patients were not evaluable.
Pts = patients; pfs = progression-free survival; os = overall survival; hr = hazard ratio; impact = Immunotherapy for Prostate Adenocarcinoma Treatment; rct = randomized clinical trial.
With traditional tumour-targeted cytotoxic agents, it is widely believed that improved time to progression is a prerequisite for improved overall survival. That connection is certainly intuitive because, on the whole, cytoxic agents affect the tumour only during the period of administration. Soon after the drug is discontinued, its antitumour activity ends. By contrast, with active immunotherapies, the mechanism of action and kinetics of clinical response appear to be quite different. Therapeutic vaccines do not directly target the tumour, but rather the immune system, which in turn targets the tumour. That immune response can be enhanced extrinsically by continued booster vaccinations and intrinsically by immune-mediated killing, leading to cross-priming and cross-presentation and to a resultant broadening of the immune repertoire as antigen cascade or epitope spreading.
This broader, perhaps more relevant, immune response may take some time to develop; however, an antitumour immune response can induce memory cells that may provide ongoing antitumour activity long after the vaccine is given. Although they may not bring about a significant reduction in tumour burden, therapeutic vaccines can apply continuous antitumour activity over a long period of time, resulting in a slower tumour growth rate. (A more detailed review of the foregoing concepts has recently been published 10,11.) This deceleration in growth rate may take weeks or months to commence, but may continue for months or years and even through subsequent therapies. This process can lead to clinically significant improved overall survival, often with no difference in time to progression and a low rate of objective responses.
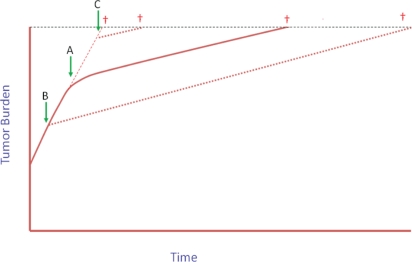
This concept of slowing the rate of tumour growth suggests that treating patients with immunotherapy earlier rather than later in the course of disease may result in far better outcomes (Figure 1). Thus, patients treated with vaccines within the last 6–12 months of life (a typical phase i patient population) may not experience any clinically relevant improvement in survival from a slightly altered tumour growth rate, but those with a lower tumour burden treated earlier in their disease course may eventually experience a significant improvement in overall survival, even with the same modest decrease in the rate of tumour growth. Recently published data suggest that this supposition may indeed be true. In the phase ii trial of psa-tricom described earlier, patients with more aggressive or more advanced disease, as indicated by a hps of less than 18 months (n = 17), had a 2.3-month improvement over predicted median overall survival; those with less advanced or less aggressive disease (hps ≥ 18 months) had an improvement in overall survival of 16.5 months or more 8.
FIGURE 1.
Tumour growth, a dynamic process, is the result of new cells dividing and other cells dying. Growth rate is the change in tumour size over time. Intrinsic tumour biology and extrinsic factors such as therapies affect tumour growth rate. Immunotherapy may not cause dramatic changes in tumour burden over a short period of time, as is often seen with cytotoxic therapy, but even slight decreases in growth rate maintained over time by a memory response, especially if started early in the disease course, may lead to substantial improvements in overall survival (see Stein et al., 2011 11 for more details). (A) Immunotherapy initiated when tumour burden is intermediate. If the change in the slope of the growth rate is maintained, it is evident that (B) starting immunotherapy earlier in the disease course may lead to much greater improvements in overall survival than (C) starting immunotherapy later.
The contrast between predicted and actual overall survival results in the psa-tricom vaccine trial compared with that from a trial of docetaxel with or without thalidomide in a similar patient population during a similar time frame at the same institution is interesting 12–15 (Table ii). Particularly interesting is the observation that patients who received docetaxel alone had a median overall survival of 15.5 months, compared with a predicted survival of 16.5 months, adding weight to the use of hps as an estimate of survival in patients treated with standard therapies at that particular institution. Notably, patients on the docetaxel combination arm (plus thalidomide) had an overall median survival that was better than predicted by about 10 months, whether their hps was or was not less than 18 months. To the best of our knowledge, of all the published randomized studies in mcrpc, the docetaxel–thalidomide combination showed the best improvement in overall survival over docetaxel alone. For patients with a HPS of 18 months or more, the median predicted survival values are almost identical for patients from both trials, suggesting a similar patient population. However, the difference between the predicted and the actual overall survival was 5.7 months for the chemotherapy trial compared with 16.4 months or more for the vaccine trial (see Table ii). That comparison is consistent with our hypothesis that, although chemotherapy delivers an effect during or immediately after treatment, therapeutic vaccines can induce subtle but long-lived changes in tumour growth rates, and those changes can be more apparent clinically if the vaccine is given earlier in the disease course.
TABLE II.
Survival predicted by Halabi nomogram compared with actual survival in patients treated with chemotherapy or with vaccine therapy
| Study and variable |
Patient groups |
||
|---|---|---|---|
| Overall | hps<18 months | hps≥18 months | |
| Vaccine (psa-tricom)—Gulley et al., 2010 8 | |||
| Patients (n) | 32 | 17 | 15 |
| Median survival (months) | |||
| By Halabi nomogram | 17.4 | 12.3 | 20.9 |
| Actual overall | 26.6 | 14.6 | Not reached (8 patients alive at 37.3 months)a |
| Patients surviving | 22 | 10 | 12 |
| longer than predicted | (69) | (59) | (80) |
| by Halabi nomogram [n (%)] | p=0.035b | ||
| Differencec (months) | 9.2 | 2.3 | ≥16.4 |
| Chemotherapy (docetaxel ± thalidomide)—Dahut et al., 2004 12, Figg et al., 2005 13 | |||
| Patients (n) | 71 | 46 | 25 |
| Median survival (months) | |||
| By Halabi nomogram | 15.0 | 13.0 | 20.0 |
| Actual overall | 22.8 | 16.8 | 25.7 |
| Patients surviving | 50 | 34 | 16 |
| longer than predicted | (70) | (74) | (64) |
| by Halabi nomogram [n (%)] | |||
| Differencec (months) | 7.8 | 3.8 | 5.7 |
The overall survival curve plateaus at 51% after 38 months, with 8 of 14 patients alive at a median potential follow-up of 44 months.
Two-tailed and based on an exact binomial test, with p = 0.5 as the fraction living longer than expected if this were a random occurrence.
The number of months by which the actual median overall survival (actuarial) exceeds the arithmetic median survival predicted by Halabi score.
hps = Halabi-predicted survival; psa-tricom = poxvirus vector encoding prostate-specific antigen, plus 3 T-cell co-stimulatory molecules.
It is interesting to note that the randomized trials of psa-tricom and sipuleucel-T enrolled patients with a median hps of 21 months, but that the clinical trial of the cancer vaccine Gvax (BioSante Pharmaceuticals, Lincolnshire, IL, U.S.A.), which showed no improvement in overall survival (hr: 1.01) in a comparison with docetaxel, enrolled patients with a median hps of 16 months 16. Additionally, multiple randomized controlled trials of immunotherapy, including the randomized studies of sipuleucel-T and psa-tricom, showed a delayed separation in their overall survival curves, with no evidence of benefit from vaccine for patients who died within the first 6–12 months after vaccine treatment was initiated. That observation provides further evidence that patients with more advanced disease (those destined to die sooner after treatment with vaccine) do not benefit from cancer immunotherapy, and that most, if not all, of the overall survival benefit is seen in patients with less advanced disease.
DATA FROM NON–PROSTATE CANCER IMMUNOTHERAPY CLINICAL TRIALS
Some of the best data on the effect of tumour burden and disease aggression on a therapeutic immune response come from the transplant literature. Among the earliest compelling evidence of the effect of the immune system on cancer is the graft-versus-leukemia effect seen after transplantation. For patients with chronic myeloid leukemia who relapse after allogeneic hematopoietic stem-cell transplant, infusion of lymphocytes from the original donor (“donor lymphocyte infusion”) is generally the treatment of choice, because it re-induces complete remission in a high percentage of patients. The likelihood of response to donor lymphocyte infusion is much higher in patients with a lower tumour burden (indicated by fewer blasts) than in patients with higher blast counts (Table iii) 17.
TABLE III.
Response after donor lymphocyte infusion (dli) based on percentage of blasts in patients with relapsed chronic myelogenous leukemia 17
| Disease stage at relapse | Blasts (%) | Response to dli (%) |
|---|---|---|
| Cytogenetic (chronic) phase | <10 | 75 |
| Accelerated phase | 10–20 | 33 |
| Blast crisis | >20 | 17 |
Data from other hematologic malignancies also indirectly support using vaccine in a patient population with a lower tumour burden. Three large randomized controlled clinical trials of anti-idiotypic therapeutic vaccines for follicular lymphoma (Table iv) used the patients’ own tumours to derive the vaccine. Only one of these three studies showed an improvement in the primary endpoint of progression-free survival. Interestingly, that study was the only one to enrol just patients with a complete response, suggesting that tumour burden may be a significant factor in the success of therapeutic vaccines in that setting also.
TABLE IV.
Phase iii trials of idiotypic vaccines in follicular lymphoma
| Reference | Vaccine | Enrolment criteria | Improved pfs? |
|---|---|---|---|
| Schuster et al., 2009 18 | BiovaxIDa | cr to previous therapy | Yes (p=0.045) |
| Freedman et al., 2009 19 | Mitumprotimut-Tb | cr, pr, or sd to previous therapy | No Worse in refractory disease |
| Genitope Corporation, 2007 20 | MyVaxc | cr or pr to previous therapy | No Trends better in responders |
Biovest International, Tampa, FL, U.S.A.
Favrille, San Diego, CA, U.S.A.
Genitope Corporation, Fremont, CA, U.S.A.
pfs = progression-free survival; cr = complete response; pr = partial response; sd = stable disease.
The BLP25 liposome vaccine [L-BLP25 (Stimuvax: Oncothyreon, Seattle, WA, U.S.A.)] targets the exposed core peptide of the muc1 (cell surface–associated mucin 1) tumour-associated antigen 21. The vaccine is designed to induce a cellular immune response that may lead to immune rejection of tumour tissues that express the muc1 antigen. A randomized phase iib trial of L-BLP25 in patients with stage iiib– iv non-small-cell lung cancer after stable disease or response to primary chemotherapy has been completed 21. The vaccination arm included 88 patients, and the best supportive care (bsc) arm, 83. The most common adverse events were grade 1 ’flu-like symptoms and mild injection-site reactions. The median overall survival was 17.4 months for the vaccination arm compared with 13.0 months for bsc, a difference that did not reach statistical significance (p = 0.066, unadjusted Cox). The 2-year survival rate was 43.2% in the L-BLP25 arm compared with 28.9% in the bsc arm. The greatest difference in survival was observed in patients with stage iiib locoregional disease (adjusted hr: 0.524; 95% confidence interval: 0.261 to 1.052; p = 0.069). In a recently reported update of the study, with a median follow-up of 53 months, the updated observed 2-year survival rate for patients with stage iiib locoregional disease was 60% (median survival: 30.6 months) in the L-BLP25 arm compared with 36.7% in the bsc arm (median survival: 13.3 months; Table v) 22. Those findings have led to an ongoing confirmatory global randomized controlled phase iii study in more than 1300 patients with unresectable stage iiib non-small-cell lung cancer (see clinicaltrials.gov/show/NCT00409188).
TABLE V.
Subpopulations from randomized clinical trials suggest improved outcomes after immunotherapy for patients with less advanced or more indolent disease
| Reference | Cancer diagnosis | Therapy |
Patient groups |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Overall |
Less advanced |
|||||||||
| (n) | os (months) | hr | p value | (n) | os(months) | hr | p value | |||
| Butts et al., 2005 and 2007 21,22 | Lung | Stimuvaxa | 88 | 17.4 | 0.74 | 0.11 | 35 | 30.6 | 0.52 | 0.069 |
| bsc | 83 | 13.0 | 30 | 13.3 | ||||||
| Hanna et al., 2006 23 | Colorectal | OncoVaxb | 128 | 75.0%c | nr | 0.10 | 80 | 82.5%c | nr | 0.014 |
| Observation | 126 | 71.4%c | 77 | 72.7%c | ||||||
| Kantoff et al., 2010 24 | Renal cell | TroVaxd | 365 | 19.2 | 1.07 | 0.55 | 50 | Not reached | 0.54 | 0.04 |
| Placebo | 368 | 20.1 | 50 | 19.5 | ||||||
Oncothyreon, Seattle, WA, U.S.A.
Vaccinogen, Frederick, MD, U.S.A.
Proportion of patients alive at 5 years.
Oxford BioMedica, Oxford, U.K.
os = overall survival; hr = hazard ratio; nr = not reported; bsc = best supportive care; hps = Halabi-predicted survival.
The therapeutic cancer vaccine mva-5T4 (TroVax: Oxford BioMedica, Oxford, U.K.) consists of a modified Vaccinia Ankara (mva) vector expressing the tumour-associated antigen 5T4. A large randomized double-blind placebo-controlled phase iii trial of mva-5T4 was recently reported 24. The trial was designed to determine whether mva-5T4, added to first-line standard-of-care therapy (sunitinib, low-dose interleukin 2, or interferon alfa), could improve overall survival for patients with clear cell renal cancer with good or intermediate prognosis. Prognostic criteria were based on standard clinical parameters associated with high tumour burden (low hemoglobin, decreased performance status, high calcium, high lactate dehydrogenase) and more aggressive disease (shorter time from diagnosis to treatment for relapsed disease) 25. Patients (n = 733) were randomized 1:1 to vaccine or to placebo in addition to standard of care (Table v). Although no improvement in overall survival between the arms was observed, a prospectively planned subset analysis demonstrated a 46% improvement in the death rate for patients with good prognosis (no risk factors) who were treated with vaccine and interleukin 2 (p = 0.04). Thus, patients with a lower tumour burden appeared to derive benefit when vaccine was added to treatment with interleukin 2, but patients with a higher tumour burden did not.
The autologous whole-tumour-cell vaccine OncoVax (Vaccinogen, Frederick, MD, U.S.A.) is delivered with bacille Calmette–Guérin. In a randomized controlled study of 254 patients with stage ii and iii colorectal cancer who all received standard therapy, a trend toward improved survival (p = 0.073) was observed in the patients who, in addition, received 3 weekly vaccinations, followed by a booster at 6 months (n = 128) 23. The randomization was stratified by disease stage, and a prospective planned analysis by disease stage was performed. Subjects with stage ii disease had a clinically meaningful and statistically significant improvement in 5-year recurrence-free survival (78.7% vs. 62.3%, p = 0.008). Furthermore, improved 5-year overall survival was observed in vaccine-treated patients (82.5%) compared with patients in the control group (72.7%, p = 0.014). The findings from this study led to approval of OncoVax in Europe and to a confirmatory global phase iii randomized study in patients with stage ii colorectal cancer (n = 550) 26.
LESSONS FOR THE FIELD
Traditionally, experimental cancer therapies are tested first in patients for whom all available agents of known benefit have failed. In the case of experimental chemotherapeutic agents, substantial ethical considerations are involved in exposing patients to therapies with significant toxicities and no known benefit when therapies with known benefit are available. For experimental cancer immunotherapies, this traditional approach has meant that early testing has been done in patients who have failed multiple regimens of chemotherapy—exactly the wrong patient population for testing therapeutic cancer vaccines. In one study, the ability to mount an immune response to therapeutic vaccine was directly correlated with fewer prior chemotherapy regimens and longer time since last chemotherapy 27.
It is interesting to note that until 2010, only 1 chemotherapy regimen showed a survival benefit in prostate cancer. In the randomized clinical trials of therapeutic prostate cancer vaccines that showed a survival benefit, only a small minority of patients had received prior chemotherapy. Because (a) the average life expectancy of patients with mcrpc is 3–4 years from initial progression on androgen-deprivation therapy 28, (b) the median time to progression on docetaxel-based therapy for mcrpc is about 6 months 29, and (c) there is no accepted standard timing for the initiation of chemotherapy in the course of mcrpc (early vs. late), it is reasonable to offer second-line androgen-deprivation therapy (which has not been shown to improve survival) or therapeutic vaccines (experimental or not) before chemotherapy. Furthermore, the suggestion has been made that, if treated with vaccine first, patients may experience improved outcomes with subsequent therapies 14,15,30–34.
Besides bearing the potentially negative impact of prior chemotherapy on the immune system, patients who progress after chemotherapy often have a larger tumour burden—a fact that has several implications for the use of therapeutic vaccines. Tumours often secrete cytokines such as transforming growth factor β and interleukin 10 that have negative effects on the immune system. Another immune-relevant molecule found in tumours is indoleamine 2,3-dioxygenase, an inducible enzyme involved in tryptophan catabolism. The depletion of tryptophan in tumours by this enzyme decreases the functionality of effector T cells and causes dendritic cells to become immunosuppressive 35. In addition, tumours often harbour immunosuppressive cells such as regulatory T cells, myeloid-derived suppressor cells, and immature dendritic cells, so that a larger tumour mass may have an even greater inhibitory effect on the immune system. Taking all of those factors into account, therapeutic vaccination as a single modality for patients with rapidly growing tumours or extensive symptomatic metastatic disease may be not only unreasonable, but also biologically futile.
SUMMARY
Emerging data from randomized clinical trials support the intuitive hypothesis that therapeutic vaccines may be most effective in patients with early-stage disease and a lower tumour burden. Future clinical studies of therapeutic vaccines should enrol patients with more indolent disease characteristics, lower tumour burden, or both. Where possible, therapeutic vaccines should be tested before the patients receiving the vaccines undergo long courses of systemic chemotherapy. Deciding how early in the disease course to test therapeutic vaccines should arise from an understanding of the effects of currently available standard therapeutic options, together with expected overall survival.
To date, overall survival is the only consistent indicator of clinical benefit for therapeutic cancer vaccines. Efforts are being made to identify earlier markers of clinical benefit that correlate with survival. Such metrics include relapse-free survival or metastasis-free survival in patients with no evidence of disease, and immunologic parameters. Until such endpoints are validated, overall survival should be the primary endpoint of definitive efficacy studies of therapeutic cancer vaccines as single agents.
ACKNOWLEDGMENTS
Grant support was provided by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, National Institutes of Health. The authors thank Bonnie L. Casey for her editorial assistance in the preparation of this manuscript.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest.
REFERENCES
- 1.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase iii trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–94. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 2.Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–9. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 3.Kantoff P, Higano C, Shore N, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 4.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–7. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 5.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase ii randomized controlled trial of a poxviral-based psa-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madan RA, Arlen PM, Mohebtash M, Hodge JW, Gulley JL. Prostvac-VF: a vector-based vaccine targeting psa in prostate cancer. Expert Opin Investig Drugs. 2009;18:1001–11. doi: 10.1517/13543780902997928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arlen PM, Skarupa L, Pazdur M, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol. 2007;178:1515–20. doi: 10.1016/j.juro.2007.05.117. [DOI] [PubMed] [Google Scholar]
- 8.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based psa vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59:663–74. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madan R, Gulley J, Fojo T, Dahut W. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15:969–75. doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein WD, Gulley JL, Schlom J, et al. Tumor regression and growth rates determined in five intramural nci prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011;17:907–17. doi: 10.1158/1078-0432.CCR-10-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahut WL, Gulley JL, Arlen PM, et al. Randomized phase ii trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J Clin Oncol. 2004;22:2532–9. doi: 10.1200/JCO.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 13.Figg WD, Retter AS, Steinberg SM, Dahut W. In reply to: inhibition of angiogenesis: thalidomide or low-molecular-weight heparin? J Clin Oncol. 2005;23:2113–14. doi: 10.1200/JCO.2005.05.296. [DOI] [PubMed] [Google Scholar]
- 14.Schlom J, Arlen PM, Gulley JL. Cancer vaccines: moving beyond current paradigms. Clin Cancer Res. 2007;13:3776–82. doi: 10.1158/1078-0432.CCR-07-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulley JL, Madan RA, Arlen PM. Enhancing efficacy of therapeutic vaccinations by combination with other modalities. Vaccine. 2007;25(suppl 2):B89–96. doi: 10.1016/j.vaccine.2007.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madan RA, Mohebtash M, Schlom J, Gulley JL. Therapeutic vaccines in metastatic castration-resistant prostate cancer: principles in clinical trial design. Expert Opin Biol Ther. 2010;10:19–28. doi: 10.1517/14712590903321421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins RH, Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15:433–44. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 18.Schuster S, Neelapu S, Gause B. Idiotype vaccine therapy (BioVaxID) in follicular lymphoma in first complete remission: phase iii clinical trial results [abstract 2] J Clin Oncol. 2009;27 [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=33572; cited April 11, 2011] [Google Scholar]
- 19.Freedman A, Neelapu SS, Nichols C, et al. Placebo-controlled phase iii trial of patient-specific immunotherapy with mitumprotimut-T and granulocyte–macrophage colony– stimulating factor after rituximab in patients with follicular lymphoma. J Clin Oncol. 2009;27:3036–43. doi: 10.1200/JCO.2008.19.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auckland, NZ: Drugs.com; Genitope Corporation reports initial results of phase 3 clinical trial of MyVax personalized immunotherapy [press release, Web page] [Available at: www.drugs.com/clinical_trials/genitope-corporation-reports-initial-results-phase-3-clinical-trial-myvax-r-personalized-3087.html; cited September 3, 2010] [Google Scholar]
- 21.Butts C, Murray N, Maksymiuk A, et al. Randomized phase iib trial of BLP25 liposome vaccine in stage iiib and iv non-small-cell lung cancer. J Clin Oncol. 2005;23:6674–81. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 22.Butts C, Maksymiuk A, Goss G. A multicentre phase iib randomized controlled study of BLP25 liposome vaccine (L-BLP25 or Stimuvax) for active specific immunotherapy of non-small cell lung cancer (nsclc): updated survival analysis [abstract B1-01] J Thorac Oncol. 2007;2(suppl 4):S332–3. doi: 10.1097/01.JTO.0000283139.22682.5e. [DOI] [Google Scholar]
- 23.Hanna MG, Jr, Hoover HC, Jr, Pinedo HM, Finer M. Active specific immunotherapy with autologous tumor cell vaccines for stage ii colon cancer: logistics, efficacy, safety and immunological pharmacodynamics. Hum Vaccin. 2006;2:185–91. doi: 10.4161/hv.2.4.3196. [DOI] [PubMed] [Google Scholar]
- 24.Kantoff P, Higano C, Shore N, et al. Sipuleucel-T for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 25.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–96. doi: 10.1200/JCO.20.1.289. [DOI] [PubMed] [Google Scholar]
- 26.Frederick, MD: Vaccinogen; 2010. Vaccinogen selects Clinipace Worldwide to manage global phase 3B clinical trial [press release, Web page] [Available at: www.vaccinogeninc.com/vaccinogen/news/vaccinogen-selects-clinipace-worldwide-to-manage-global-phase-3b-clinical-trial/; cited September 20, 2010] [Google Scholar]
- 27.von Mehren M, Arlen P, Gulley J, et al. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (alvac-cea B7.1) in patients with metastatic carcinoma. Clin Cancer Res. 2001;7:1181–91. [PubMed] [Google Scholar]
- 28.Sharifi N, Dahut WL, Steinberg SM, et al. A retrospective study of the time to clinical endpoints for advanced prostate cancer. BJU Int. 2005;96:985–9. doi: 10.1111/j.1464-410X.2005.05798.x. [DOI] [PubMed] [Google Scholar]
- 29.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 30.Arlen PM, Gulley JL, Todd N, et al. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;174:539–46. doi: 10.1097/01.ju.0000165159.33772.5b. [DOI] [PubMed] [Google Scholar]
- 31.Arlen PM, Gulley JL, Parker C, et al. A randomized phase ii study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–9. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulley JL, Arlen PM, Tsang KY, et al. Pilot study of vaccination with recombinant cea-muc-1-tricom poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14:3060–9. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gribben JG, Ryan DP, Boyajian R, et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin Cancer Res. 2005;11:4430–6. doi: 10.1158/1078-0432.CCR-04-2111. [DOI] [PubMed] [Google Scholar]
- 34.Chiappori AA, Soliman H, Janssen WE, Antonia SJ, Gabrilovich DI. INGN-225: a dendritic cell-based p53 vaccine (Ad.p53-DC) in small cell lung cancer: observed association between immune response and enhanced chemotherapy effect. Expert Opin Biol Ther. 2010;10:983–91. doi: 10.1517/14712598.2010.484801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soliman H, Mediavilla–Varela M, Antonia S. Indoleamine 2,3-dioxygenase: is it an immune suppressor? Cancer J. 2010;16:354–9. doi: 10.1097/PPO.0b013e3181eb3343. [DOI] [PMC free article] [PubMed] [Google Scholar]