Abstract
Background
The evidence that red and processed meat influences colorectal carcinogenesis was judged convincing in the 2007 World Cancer Research Fund/American Institute of Cancer Research report. Since then, ten prospective studies have published new results. Here we update the evidence from prospective studies and explore whether there is a non-linear association of red and processed meats with colorectal cancer risk.
Methods and Findings
Relevant prospective studies were identified in PubMed until March 2011. For each study, relative risks and 95% confidence intervals (CI) were extracted and pooled with a random-effects model, weighting for the inverse of the variance, in highest versus lowest intake comparison, and dose-response meta-analyses. Red and processed meats intake was associated with increased colorectal cancer risk. The summary relative risk (RR) of colorectal cancer for the highest versus the lowest intake was 1.22 (95% CI = 1.11−1.34) and the RR for every 100 g/day increase was 1.14 (95% CI = 1.04−1.24). Non-linear dose-response meta-analyses revealed that colorectal cancer risk increases approximately linearly with increasing intake of red and processed meats up to approximately 140 g/day, where the curve approaches its plateau. The associations were similar for colon and rectal cancer risk. When analyzed separately, colorectal cancer risk was related to intake of fresh red meat (RR for 100 g/day increase = 1.17, 95% CI = 1.05−1.31) and processed meat (RR for 50 g/day increase = 1.18, 95% CI = 1.10−1.28). Similar results were observed for colon cancer, but for rectal cancer, no significant associations were observed.
Conclusions
High intake of red and processed meat is associated with significant increased risk of colorectal, colon and rectal cancers. The overall evidence of prospective studies supports limiting red and processed meat consumption as one of the dietary recommendations for the prevention of colorectal cancer.
Introduction
Colorectal cancer is the third most frequently diagnosed cancer worldwide, accounting for more than one million cases and 600 000 deaths every year. Incidence rates are highest in North America, Western Europe, Australia/New Zealand, and in Asian countries that have experienced nutrition transition, such as Japan, Singapore, and North-Korea [1]. Incidence rates are stable or decreasing in long-standing economically developed countries, while they continue to increase in economically transitioning countries. Recent declines in mortality from colorectal cancer have been observed in North America and Japan, possibly due to primary prevention (surveillance and screening) and improved treatment [2]. Decreasing trends in colorectal cancer mortality have also been observed in most Western European countries [3].
The role of environmental and lifestyle factors on colorectal carcinogenesis is indicated by the increase in colorectal cancer incidence in parallel with economic development and adoption of a western lifestyle [4], as well as by the results of migration studies that demonstrate a greater lifetime incidence of colorectal cancer among immigrants to high-incidence, industrialized countries compared to residents remaining in low-incidence countries [5]. Screening and surveillance of adenomatous polyps, a precursor of colorectal cancer, is currently the cornerstone for primary prevention of colorectal cancer [6]. However, understanding the role of environmental factors in colorectal carcinogenesis may inform additional primary prevention strategies that can further reduce risk.
Several plausible biological mechanisms have been suggested to explain the association of red and processed meats with colorectal cancer [7]–[9]. These include the potential mutagenic effect of heterocyclic amines (HCA) contained in meat cooked at high temperature [10], but this is not specific of red and processed meats since HCA's are also formed in poultry. A second mechanism involves endogenous formation in the gastrointestinal tract of N-nitroso compounds, many of which are carcinogenic. Red meat but not white meat intake shows a dose–response relation with the endogenous formation of nitroso compounds in humans [11]. This has been explained by the abundant presence of heme in red meat that can readily become nitrosylated and act as a nitrosating agent [12], [13]. Nitrites or nitrates added to meat for preservation could increase exogenous exposure to nitrosamines, N-nitroso compounds, and their precursors; meats cured with nitrite have the same effect as fresh red meat on endogenous nitrosation [14].
In the 2007 World Cancer Research Fund and American Institute of Cancer Research (WCRF/AICR) report “Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective”, an international panel of experts based on an extensive review of the existing evidence concluded that high intake of red and processed meat convincingly increases the risk of colorectal cancer [15]. However two recent reviews of prospective studies concluded that the available epidemiologic evidence is not sufficient to support an independent positive association between red meat or processed meat consumption and colorectal cancer, because the likely influence of confounding by other dietary and lifestyle factors, the weak magnitude of the observed association, and its variability by gender and cancer subsite [16], [17]. Indeed, a positive association has been suggested in most but not all epidemiologic studies [15], and in some well conducted prospective studies, the association between red and processed meat and colorectal cancer was attenuated after better adjustment for potential confounders [18].
Since then, new results from ten prospective studies [19]–[28] have been published. This included studies in Asian populations [20], [25], [27], [28], a Canadian breast cancer screening cohort [24], a US multi-ethnic cohort [26], and four American cohorts [19], [21]–[23].We have focused our review on prospective studies, because case-control studies are more liable to recall and selection bias, and randomized controlled trials on red and processed meats and colorectal cancer are considered not feasible. The data on the relation of red and processed meats and colorectal cancers are summarized in highest versus lowest meta-analyses. Because stronger causal inference can be drawn from dose-response associations, we also conduct linear dose-response analyses. None of the previous meta-analyses have examined the shape of the dose-response relationship; we further explore whether there is a non-linear dose-response relationship between red and processed meats intake and colorectal cancer risk.
Methods
Data sources and search
We performed a systematic search for publications on red and processed meat and colorectal cancer in Pubmed, without any language restriction from 1966 to 31 March 2011, using the search strategy implemented for the WCRF/AICR report [15] (Text S1). The medical subject headings and text words covered a broad range of factors on foods and foods components, physical activity, and anthropometry. We also hand-searched reference lists from retrieved articles, reviews, and meta-analysis papers. The complete protocol and full search strategy used is available at http://www.dietandcancerreport.org/cu/ [29].
Inclusion criteria and data extraction
Studies were included if they reported estimates of the association of red meats, processed meats, or both with colorectal, colon, or rectal cancer risk. “Red meat” was described in most studies as the intake of beef, veal, pork, mutton and lamb. “Processed meat” was defined as the total intake of ham, bacon, sausages, cured or preserved meats. Here, “red and processed meats” is used to denote the food item that includes both “red meats” and “processed meats” into a single item in the studies identified in the search.
To be included in the dose-response meta-analyses, the numbers of cases and the denominators in the cohort studies or the information required to derive them using standard methods [30] had to be reported. Other data extracted were study characteristics, cancer outcome, description of meat item, method of dietary assessment, and adjustment factors. When multiple articles on the same study were found, the selection of results for the meta-analysis was based on longer follow-up, more cases identified, and completeness of the information required to do the meta-analysis.
The search, study selection, and data extraction was conducted by several reviewers (led by EK) at Wageningen University, The Netherlands up to June 2006, and by two reviewers (DSMC and RL, led by TN) at Imperial College London from June 2006 to March 2011.
Statistical analysis
Relative risk estimates were pooled using fixed-effects and random-effects models. We present the results from the random-effects meta-analysis that accounts for between-study heterogeneity [31] unless otherwise specified. We conducted meta-analyses for red and processed meats, combined and separately, using the description of the meat items given in the articles. In highest versus lowest meta-analyses (the comparison of the highest intake level to the lowest intake level), the relative risk (RR) estimate from each study was weighted by the inverse of the variance to calculate summary relative risks (RR) and 95% confidence intervals (CI). In linear dose-response meta-analyses, we pooled the relative risk estimates per unit of intake increase (with its standard error) reported in the studies, or computed by us from the categorical data using generalized least-squares for trend estimation [32]. When intake was expressed in “times” or “servings of intake”, we converted it into grams (g) using 120 g as a standard portion size for red and processed meat combined and for red meat, and 50 g was assumed as standard portion size for processed meat, as in the WCRF/AICR report [15]. Means or medians of the intake categories were used when reported in the articles; if not reported, midpoints were assigned to the relative risk of the corresponding category. Zero consumption was used as boundary when the lowest category was open-ended and when the highest category was open-ended, we used the amplitude of the lower nearest category. For studies reporting intakes in grams/1000 kcal/day [22], [26], [33], the intake in grams/day was estimated using the average energy intake reported in the article. When a study provided results by gender, we first pooled these estimates using a fixed-effects model and included the pooled value in the meta-analysis. One study provided results for distal and proximal colon cancer [34] and we derived the relative risk for colon cancer using the same procedure. We also conducted meta-analyses stratified by cancer sub-site, gender, and geographic area. Dose-response relationships were expressed per increment of intake of 100 grams per day for red and processed meat, and 50 grams per day for processed meat as in previous meta-analyses [15], [35].
To assess heterogeneity, we computed the Cochran Q test and I2 statistic [36]. Sources of heterogeneity were explored in stratified analysis and by linear meta-regression, with gender, geographic area, year of publication, length of follow-up, and adjustment for confounders as potential explanatory factors. We also explored if heterogeneity of results was explained by the studies in which a standard portion size was used to convert times/servings per day to grams per day, and by method of dietary assessment. Small study and publication bias were examined visually in funnel plots for asymmetry and by Egger's test [37]. The influence of each individual study on the summary RR was examined by excluding each study in turn from the pooled estimate [38].
We further examined the potential non-linear dose-response relationship between red and processed meats and colorectal cancer using fractional polynomial models [39]. We determined the best fitting second order fractional polynomial regression model, defined as the one with the lowest deviance. Non-linearity was tested using the likelihood ratio test [40]. All analyses were conducted using Stata version 9.2 (StataCorp. 2005. Stata Statistical Software: Release 9. College Station, TX: StataCorp LP). P<0.05 was considered statistically significant.
Results
Results of search and study selection
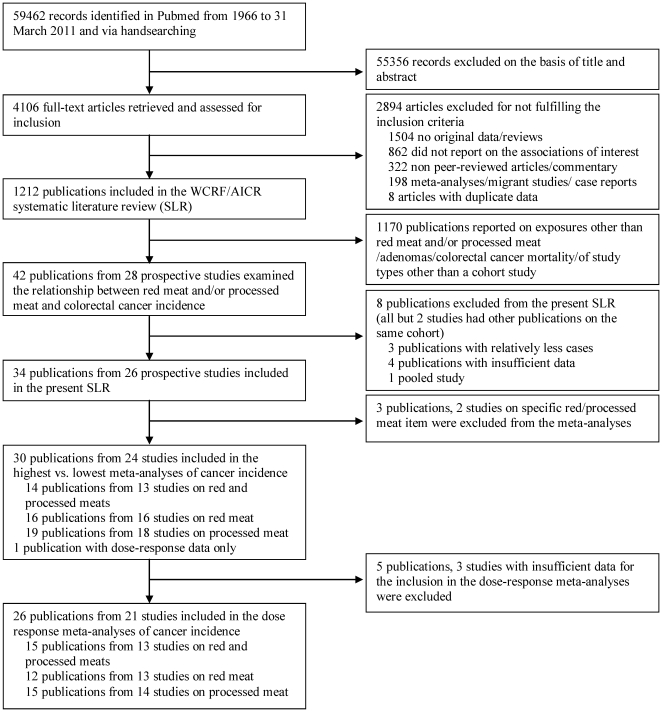
Forty-two articles from 28 prospective studies that examined the relationship of red and/or processed meat intakes and colorectal, colon, and rectal cancer incidence were identified (Figure 1). Eight articles were excluded [41]–[48] because other articles of the same cohort studies with more cases [49]–[51] or with information required in the meta-analysis were already included [18], [52], [53]. We could not include the UK Dietary Cohort Consortium [42], as data from two of the seven component cohorts were in other cohort consortium that was included in the meta-analysis because it had more cancer cases [50]. Hence, 24 prospective studies (2 case-cohort, 3 nested case-control and 19 cohort studies) were included in the highest versus lowest meta-analyses, of which 21 studies provided enough information to be included in the dose-response meta-analyses.
Figure 1. Flow diagram of systematic literature search on red and processed meat and the risk of colorectal cancer.
Characteristics of the study cohorts
There were 13 cohorts of men and women, three male cohorts, and eight female cohorts. Twelve studies were from North-America, including a multiethnic cohort. The European Prospective Investigation into Cancer and Nutrition (EPIC) study involved ten European countries. The remaining were two studies each from Finland, the Netherlands, and Japan, and one study each from Australia, Canada, Sweden, China, and a Singaporean study with Chinese participants.
In all studies, relative risk estimates were adjusted for age and sex, and all except two adjusted for total energy intake. More than half of the study results were adjusted for body mass index (BMI), smoking, alcohol consumption, or physical activity, close to half controlled for dairy food or calcium intake, social economic status, family history of colorectal cancer, or plant food or folate intake. In some studies, the estimates were controlled for use of non-steroidal anti-inflammatory drugs, fish or white meat intake. The main characteristics of studies included in the dose-response meta-analysis are shown in table s1. Study results not included in the dose-response meta-analysis are detailed in table S2.
Total red and processed meats
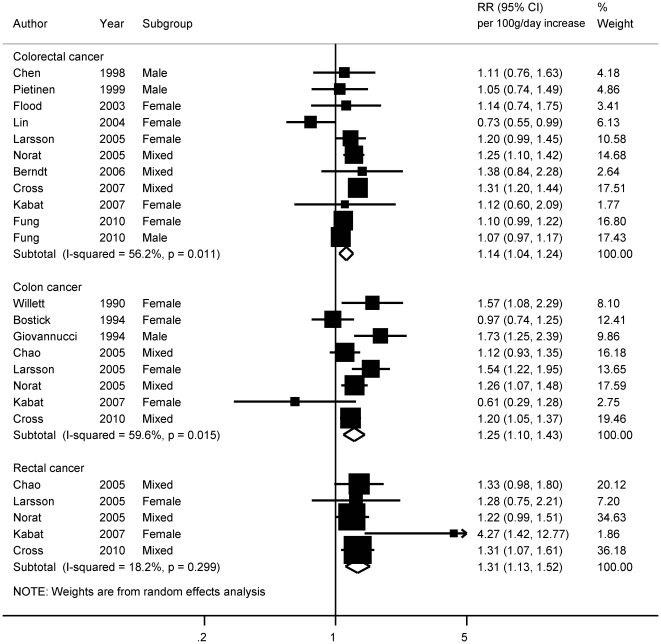
Thirteen prospective studies[19], [21]–[24], [33], [34], [50]–[52], [54]–[58] on total red and processed meats and colorectal cancer incidence were included in the highest versus lowest and dose-response meta-analyses. In highest versus lowest meta-analyses, red and processed meats intake was significantly related to an increased risk of colorectal (RR highest vs lowest = 1.22, 95% CI = 1.11−1.34), colon (RR highest vs lowest = 1.19, 95% CI = 1.06−1.34), and rectal cancer (RR highest vs lowest = 1.51, 95% CI = 1.31−1.75) (Table1). The mean values of the highest category of red and processed meats intake in the studies ranged from 46 to 211 grams per day. In dose-response meta-analysis, red and processed meats intake was positively related to colorectal cancer risk (RR for 100 g/day increase = 1.14, 95% CI = 1.04−1.24) (11 studies, 11358 cases) (Table1) (Figure 2). There was evidence of moderate heterogeneity between studies (I2 = 56%, P = 0.01), that was significantly explained by intake unit conversion in the meta-regression (P = 0.00). Studies that required conversion from times or servings to grams per day [23], [51], [56] were significantly associated with a lower summary estimate than studies that did not require the conversion [19], [22], [24], [33], [34], [50], [57].
Table 1. Summary relative risks of meta-analyses of red and processed meats, red meat and processed meat, and colorectal cancer for all studies and by subgroups.
| Red and processed meats | Red meat | Processed meat | |||||||
| Pooled RR (95% CI)*,P value | n | Heterogeneity I2, P value | Pooled RR (95% CI)*,P value | n | HeterogeneityI2, P value | Pooled RR (95% CI)*,P value | n | Heterogeneity I2, P value | |
| Dose-response meta-analysis Per 100 g/day | Per 100 g/day | Per 50 g/day | |||||||
| All studies | |||||||||
| Colorectal cancer | 1.14 (1.04–1.24), 0.00 | 11 | 56%, 0.01 | 1.17 (1.05–1.31), 0.01 | 8 | 0%, 0.48 | 1.18 (1.10–1.28), 0.00 | 9 | 12%, 0.33 |
| Colon cancer | 1.25 (1.10–1.43), 0.00 | 8 | 60%, 0.02 | 1.17 (1.02–1.33), 0.02 | 10 | 0%, 0.65 | 1.24 (1.13–1.35), 0.00 | 10 | 0%, 0.65 |
| Proximal colon cancer | 1.11 (0.88–1.40), 0.37 | 2 | 0%, 0.67 | – | 1 | – | 1.12 (0.81–1.56), 0.49 | 2 | 0%, 0.64 |
| Distal colon cancer | 1.22 (0.62–2.38), 0.57 | 2 | 90%, 0.00 | – | 1 | – | 1.41 (0.93–2.14), 0.10 | 2 | 0%, 0.69 |
| Rectal cancer | 1.31 (1.13–1.52), 0.00 | 5 | 18%, 0.30 | 1.18 (0.98–1.42), 0.08 | 7 | 0%, 0.67 | 1.12 (0.99–1.28), 0.08 | 8 | 0%, 0.56 |
| By gender | |||||||||
| Men† | |||||||||
| Colorectal cancer | 1.07 (0.98–1.16), 0.14 | 3 | 0%, 0.97 | 1.28 (0.49–3.35), 0.61 | 2 | 64%, 0.09 | 1.11 (0.86–1.44), 0.42 | 2 | 35%, 0.22 |
| Colon cancer | 1.41 (0.98–2.03), 0.07 | 2 | 71%, 0.06 | 1.06 (0.75–1.50), 0.73 | 2 | 0%, 0.98 | 1.64 (0.94–2.84), 0.08 | 3 | 72%, 0.03 |
| Women | |||||||||
| Colorectal cancer | 1.05 (0.90–1.23), 0.51 | 5 | 49%, 0.10 | 1.05 (0.78–1.42), 0.73 | 3 | 22%, 0.28 | 1.09 (0.89–1.33), 0.43 | 4 | 0%, 0.48 |
| Colon cancer | 1.15 (0.87–1.52), 0.33 | 5 | 70%, 0.01 | 1.16 (0.84–1.61), 0.37 | 5 | 28%, 0.23 | 1.33 (1.07–1.66), 0.01 | 5 | 0%, 0.75 |
| Rectal cancer | 2.12 (0.66– 6.77), 0.21 | 2 | 73%, 0.05 | 0.90 (0.60–1.35), 0.60 | 3 | 0%, 0.86 | 0.94 (0.62–1.44), 0.79 | 2 | 0%, 0.89 |
| By geographic area | |||||||||
| Europe | |||||||||
| Colorectal cancer | 1.22 (1.10–1.35), 0.00 | 3 | 0%, 0.65 | 1.23 (1.08–1.40), 0.00 | 5 | 0%, 0.63 | 1.13 (1.04–1.24), 0.01 | 4 | 0%, 0.73 |
| Colon cancer | 1.37 (1.13–1.66), 0.00 | 2 | 47%, 0.17 | 1.29 (1.08–1.54), 0.01 | 3 | 0%, 0.37 | 1.18 (1.05–1.33), 0.01 | 3 | 0%, 0.99 |
| Rectal cancer | 1.23 (1.01–1.50), 0.04 | 2 | 0%, 0.87 | 1.20 (0.95–1.50), 0.13 | 3 | 0%, 0.74 | 1.07 (0.92–1.25), 0.37 | 3 | 0%, 0.70 |
| North America | |||||||||
| Colorectal cancer | 1.11 (0.98–1.25), 0.09 | 8 | 66%, 0.00 | – | 1 | – | 1.21 (1.04–1.42), 0.01 | 4 | 11%, 0.34 |
| Colon cancer | 1.20 (1.01–1.43), 0.04 | 6 | 62%, 0.02 | 1.11 (0.86–1.44), 0.43 | 4 | 0%, 0.75 | 1.27 (1.10–1.47), 0.00 | 5 | 0%, 0.74 |
| Rectal cancer | 1.44 (1.05–1.96), 0.02 | 3 | 54%, 0.12 | 0.93 (0.54–1.60), 0.80 | 2 | 0%, 0.95 | 1.19 (0.92–1.55), 0.18 | 4 | 0%, 0.44 |
| Asia-Pacific | |||||||||
| Colorectal cancer | – | 0 | – | 1.01 (0.69–1.48), 0.96 | 2 | 56%, 0.13 | – | 1 | – |
| Colon cancer | – | 0 | – | 0.94 (0.69–1.27), 0.67 | 3 | 0%, 0.81 | 1.91 (1.05–3.48), 0.04 | 2 | 27%, 0.24 |
| Rectal cancer | – | 0 | – | 1.16 (0.57–2.39), 0.68 | 2 | 60%, 0.11 | – | 1 | – |
| Highest versus lowest meta-analysis | |||||||||
| Colorectal cancer | 1.22 (1.11–1.34), 0.00 | 10 | 14%, 0.31 | 1.10 (1.00–1.21), 0.04 | 12 | 22%, 0.22 | 1.17 (1.09–1.25), 0.00 | 13 | 6%, 0.39 |
| Colon cancer | 1.19 (1.06–1.34), 0.00 | 8 | 20%, 0.27 | 1.18 (1.04–1.35), 0.01 | 10 | 0%, 0.70 | 1.19 (1.11–1.29), 0.00 | 11 | 0%, 0.88 |
| Proximal colon cancer | 1.13 (0.97–1.32), 0.11 | 5 | 0%, 0.85 | 1.13 (0.83–1.54), 0.43 | 2 | 0%, 0.83 | 1.04 (0.90–1.20), 0.59 | 5 | 0%, 0.78 |
| Distal colon cancer | 1.36 (0.93–1.98), 0.11 | 5 | 71%, 0.01 | 1.57 (0.98–2.49), 0.06 | 2 | 53%, 0.15 | 1.20 (1.01–1.44), 0.04 | 5 | 0%, 0.41 |
| Rectal cancer | 1.51 (1.31–1.75), 0.00 | 6 | 0%, 0.76 | 1.14 (0.83–1.56), 0.43 | 7 | 38%, 0.14 | 1.19 (1.02–1.39), 0.03 | 9 | 20%, 0.27 |
*RR – relative risk; CI – confidence interval; n – number of studies †There is only one male cohort reported results on rectal cancer.
Figure 2. Dose-response meta-analyses of total red and processed meats consumption and the risk of colorectal, colon and rectal cancers.
References: Chen, 1998 [51]; Pietinen, 1999 [57]; Flood, 2003 [33]; Lin, 2004 [56]; Larsson, 2005 [34]; Norat, 2005 [50]; Berndt, 2006 [19]; Cross, 2007 [22]; Kabat, 2007 [24]; Fung, 2010 [23]; Willett, 1990 [58]; Bostick, 1994 [52]; Giovannuccci, 1994 [55]; Chao, 2005 [54]; Cross, 2010 [21].
Intake of red and processed meats was significantly associated with an increased risk of colon cancer (RR for 100 g/day increase = 1.25, 95% CI = 1.10−1.43) (8 studies, 5426 cases), with significant heterogeneity between studies (I2 = 60%, P = 0.02). Meta-regression analysis showed that studies adjusted for age and energy only [55], [58] reported stronger associations than the more adjusted studies [9], [21], [24], [34], [52], [54] (P = 0.03). Red and processed meats intake was significantly associated with rectal cancer (RR for 100 g/day increase = 1.31, 95% CI = 1.13−1.52) (5 studies, 2091 cases). In influence analysis, the statistical significance of the associations with colorectal, colon, and rectal cancers remained when each study was excluded in turn.
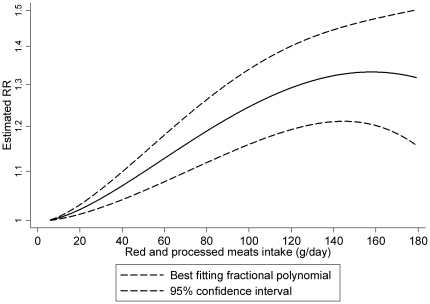
There was evidence of a non-linear association of red and processed meats and colorectal cancer (P = 0.03). Visual inspection of the curve (Figure 3) suggests that the risk increases linearly up to approximately 140 g/day of intake. Above that intake level, the risk increase is less pronounced.
Figure 3. Non-linear dose-response meta-analysis of red and processed meats consumption and the risk of colorectal cancer.
No significant associations were observed for proximal and distal colon cancers in the meta-analysis of the two [34], [54] out of the five studies [21], [34], [50], [54], [55] identified in the search (Table 1).
Red meat
Sixteen prospective studies on red meat and colorectal cancer could be included in the highest versus lowest meta-analyses [18], [20], [21], [25]–[27], [34], [50], [52], [53], [57], [59]–[63]. From these, four articles could not be included in the dose-response meta-analyses because they did not provide sufficient data [20], [21], [59], [62].
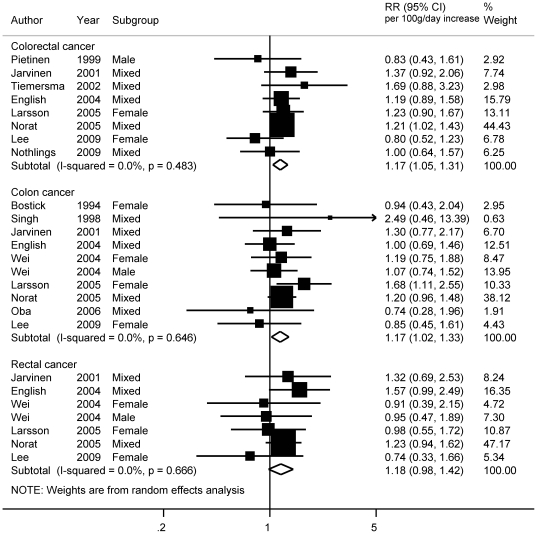
The summary RRs for the highest versus lowest red meat intake comparison were 1.10 (95% CI = 1.00−1.21), 1.18 (95% CI = 1.04−1.35), and 1.14 (95% CI = 0.83−1.56) for colorectal, colon, and rectal cancer respectively (Table 1). The mean of the highest category of red meat intake ranged from 26 to 197 grams per day in the studies. In dose-response meta-analyses, red meat was statistically significantly associated with increased risk of colorectal (RR for 100 g/day increase = 1.17, 95% CI = 1.05−1.31) (8 studies, 4314 cases) and colon cancer (RR for 100 g/day increase = 1.17, 95% CI = 1.02−1.33) (10 studies, 3561 cases) (Table 1) (Figure 4). No significant association was observed with rectal cancer (RR for 100 g/day increase = 1.18, 95% CI = 0.98−1.42) (7 studies, 1477 cases). Influence analyses did not suggest strong influence from any of the individual studies on the summary estimates.
Figure 4. Dose-response meta-analyses of red meat consumption and the risk of colorectal, colon and rectal cancers.
References: Pietinen, 1999 [57]; Jarvinen, 2001 [61]; Tiemersma, 2002 [63]; English, 2004 [60]; Larsson, 2005 [34]; Norat, 2005 [50]; Lee, 2009 [25]; Nothlings, 2009 [26]; Bostick, 1994 [52]; Singh, 1998 [53]; Wei, 2004 [18]; Oba, 2006 [27]
For proximal and distal colon cancers, no association was observed when combining the two studies identified [34], [50] (Table 1).
Processed meat
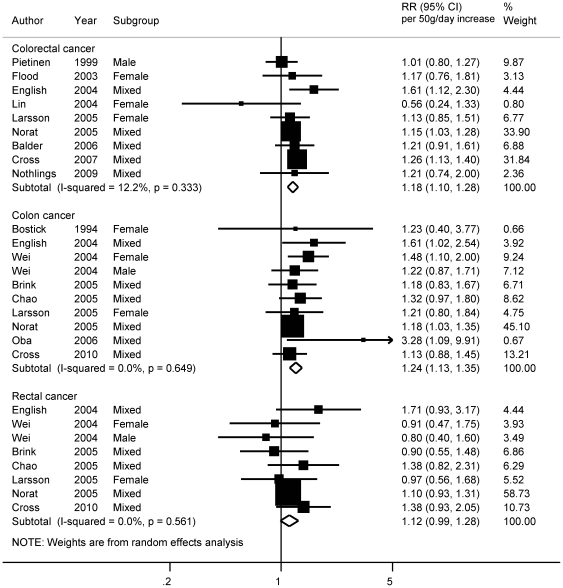
Eighteen studies were included in the highest versus lowest meta-analyses [18], [20]–[22], [26]–[28], [33], [34], [49], [50], [52], [54], [56], [57], [60], [62], [64], [65]. Processed meat intake was significantly related to the risk of colorectal (RR highest vs lowest = 1.17, 95% CI = 1.09−1.25), colon (RR highest vs lowest = 1.19, 95% CI = 1.11−1.29), and rectal cancer (RR highest vs lowest = 1.19, 95% CI = 1.02−1.39) (Table 1). The mean of the highest category of processed meat intake ranged from 16 to 122 grams per day in the studies. Four studies could not be used to derive dose-response estimates [20], [28], [62], [66]. The summary RR for every 50 g/day increase in processed meat was 1.18 (95% CI = 1.10−1.28) (9 studies, 10863 cases) for colorectal cancer and 1.24 (95% CI = 1.13−1.35) (10 studies, 6727 cases) for colon cancer (Table 1) (Figure 5). For rectal cancer, no significant dose-response association was observed (RR for 50 g/day increase = 1.12, 95% CI = 0.99−1.28) (8 studies, 2565 cases). Influence analyses did not suggest strong influence from any of the individual studies on the summary estimates.
Figure 5. Dose-response meta-analyses of processed meat consumption and the risk of colorectal, colon and rectal cancers.
References: Pietinen, 1999 [57]; Flood, 2003 [33]; English, 2004 [60]; Lin, 2004 [56]; Larsson, 2005 [34]; Norat, 2005 [50]; Balder, 2006 [64]; Cross, 2007 [22]; Nothlings, 2009 [26]; Bostick, 1994 [52]; Wei, 2004 [18]; Brink, 2005 [49]; Chao, 2005 [54]; Oba, 2006 [27]; Cross, 2010 [21].
No significant associations were observed for proximal and distal colon cancers in the meta-analysis of the two [34], [54] out of five studies [21], [28], [34], [50], [54] identified in the search (Table 1).
Small study or publication bias
In the analyses, no evidence of small study or publication bias was detected by visual inspection of the funnel plots. P for Egger's test ranged from 0.13 to 0.98 in the different analyses. The only evidence of publication bias was in the studies on processed meat and colon cancer, which suggested small studies with inverse association are missing (Egger's test P = 0.06).
Subgroup analyses
Table 1 shows the results of the dose-response meta-analyses by gender and geographic area. In most strata the number of studies was low and in some there was significant evidence of heterogeneity. Stratified analysis did not suggest any difference across gender. The association between red meat and colon cancer tended to be stronger in European studies (RR for 100 g/day increase = 1.29, 95% CI = 1.08−1.54) (3 studies, 1307 cases) compared to the North American (RR for 100 g/day increase = 1.11, 95% CI = 0.86−1.44) (4 studies, 1476 cases) and Asia-Pacific studies (RR for100 g/day increase = 0.94, 95% CI = 0.69−1.27, P = 0.67) (3 studies, 732 cases).
Discussion
Principal findings
The accumulated evidence from prospective studies supports that red and processed meats intake is associated with increased risk of colorectal, colon, and rectal cancers. The risk increase in colorectal cancer estimated in linear dose-response models was 14% for every 100 g/day increase of total red and processed meats, 25% in colon cancer, and 31% in rectal cancer. These results are consistent with those of the highest versus lowest meta-analyses. In non-linear models, colorectal cancer risk appears to increase almost linearly with increasing intake of red and processed meats up to approximately 140 g/day. Above this level, the risk increase is less pronounced.
Red meat intake (assessed separately from processed meat) was associated with increased risk of colorectal and colon cancers, but the association with rectal cancer was not statistically significant. Similarly, processed meat intake was related with risk of colorectal and colon cancers, but not with rectal cancer. The lack of association with rectal cancer is in contrast with the results observed when red and processed meats were combined into a single food item, where similar associations with colon and rectal cancers were observed. This may be due to a lower number of studies in the analyses of rectal cancer than in those of colorectal and colon cancers.
Our estimates are consistent with those reported in the 2007 WCRF/AICR expert report [15], where the risk increase of colon cancer was 37% for every 100 g/day increase in red and processed meats, and the risk increase of colorectal cancer was 29% for every 100 g/day increase in red meat, and 21% for every 50 g/day increase in processed meat.
Selective reporting or publication bias
Some articles [20], [28], [59], [62], [66] could not be included in the dose-response meta-analysis because of insufficient information, but the dose-response meta-analyses were consistent with the highest versus lowest meta-analyses that included these studies that suggests that the exclusions from the dose-response meta-analyses did not bias our results. Two cohort studies could not be included in the meta-analysis. These studies reported positive but non-significant associations between fried sausage [67] and pork [68] and colon cancer. We could not include the results of the UK Dietary Cohort Consortium [42] that reported no association of red and/or processed meat and colorectal cancer in a pooled analysis of seven prospective studies with 579 colorectal cancer cases. The two largest cohorts – EPIC-Norfolk and EPIC-Oxford, participating in this consortium were included in our meta-analyses (Norat et al. EPIC); whereas individual results from the remaining five studies were not available. Our results are not in agreement with a preliminary analysis of 14 prospective studies with 7743 colorectal cancer cases from the Pooling Project of Prospective Studies of Diet and Cancer, that reported no association between red meat and processed meat and colorectal cancer (Cho and Smith-Warner. Proceedings of the American Association for Cancer Research. 2004; volume 45; abstract #491).
No evidence of publication bias emerged from visual inspection of funnel plots and Egger's tests in the analyses conducted, except for processed meat and colon cancer where there was a suggestion of small studies with inverse association missing. Since larger studies in the analysis have produced consistent results, it is unlikely for the missing studies to affect the association observed.
Exploration of heterogeneity
There was evidence of heterogeneity between studies on red and processed meats and colorectal cancer, that was significantly explained by intake unit conversion in the meta-regression analysis. The summary risk estimate was lower in the studies for which we used a standard portion size in the unit conversion, compared to other studies. The approximation may have attenuated the association, and the real association may be stronger than showed in our estimates.
Meta-regression analysis indicated that level of adjustment partially explained the heterogeneity between studies on colon cancer. Studies adjusted for age and energy only (Nurses' Health Study - NHS [58] and Health Professional Follow-up Study - HPFS [55] showed a stronger association than studies with higher level of adjustments. However, after the exclusion of the studies adjusted only for age and energy intake from the analysis, moderate unexplained heterogeneity persisted. In a more recent article on the NHS and the HPFS, the associations of red meat and processed meat and colon cancer were attenuated after better adjustment for confounders and longer follow-up [18]. Nevertheless, in another recent article on the NHS, women who consumed one serving of red or processed meat daily for 40 years had a 20% increased risk of colon cancer compared with women who did not eat any red or processed meat [48]. This estimate is consistent with the results of our meta-analysis.
Although we cannot rule out residual confounding, most studies included in the meta-analyses adjusted results by smoking, alcohol consumption, BMI and physical activity [18], [22]–[24], [26], [27], [50], [51], [54], [56], [57], [64] in addition to age, sex and energy; in several cohort studies the multivariate adjusted models also included folate intake [18], [24], [34], [50], and other studies additionally adjusted for aspirin or other anti-inflammatory drug use [23], [25], [53], [54]. Several potential confounders were not included in the final statistical models in some studies because, as the authors reported, their inclusion in the model did not substantially modified the relative risk estimates [19], [33], [49], [52], [60], [63].
Implications
The remaining question is whether there is substantial potential for primary prevention of colorectal cancer through limiting the intake of red meat and processed meat in high meat consumers.
At a population level, the preventability estimates for red meat intake and colorectal cancer were 5% in the US, and the UK; and 7% in Brazil, and China; where 26%, 25%, 45% and 37% of the respective populations were estimated to consume more than 80 g of red meat per day [69]. Dietary and lifestyle factors are usually interrelated and it is likely that a change in a habit that is considered detrimental, such as high intake of red meat, will be accompanied by other healthful changes.
In the large prospective cohort of American Nurses (NHS), it was estimated that women who consumed high amounts of red and processed meat, did not exercise, had a low folate intake, and had a consistently excess in body weight experienced over 3.5 times' the cumulative incidence of colon cancer, by age 70 years, than women who maintained a low-risk lifestyle and diet (defined as consuming low amounts of red and processed meat, exercising regularly, consuming 400 µg/day of folate, and maintaining a low relative body weight) [48]. Under different scenarios for red meat consumption, reduction of physical inactivity, obesity, alcohol consumption, early adulthood cigarette smoking, and low intake of folic acid from supplements, the population attributable risk of colon cancer for the combined modifiable risk factors ranged from 39% to 55% of cancers in an American cohort of middle age men [70]. The preventability of colorectal cancer in United Kingdom through reduced consumption of red meat, increased fruit and vegetables, increased physical activity, limited alcohol consumption and weight control was estimated to be 31.5% of colorectal cancer in men and 18.4% in women [15], [71]. The preventability estimates of colorectal cancer through increasing intake of foods containing fiber, reducing intake of red and processed meat, alcohol, physical inactivity and body fatness were estimated to be close to 40% in USA, UK and Brazil, and 17% in China [69]. Measurement error might have attenuated the relative risk estimates in the individual studies in which the estimates are based, as well as in our meta-analyses.
Conclusions
The current evidence from prospective studies supports limiting the amount of red and processed meat in the high consumers for colorectal cancer prevention. Primary prevention of colorectal cancer should emphasize modification of multiple diet and lifestyle factors.
Supporting Information
Main characteristics of the prospective studies included in the dose-response meta-analyses.
(DOC)
Studies or results not included in the dose-response meta-analyses and reasons for exclusion.
(DOC)
Search strategy for the WCRF/AICR Continuous Update Project.
(DOC)
Acknowledgments
We thank the systematic literature review team at the Wageningen University for their contribution to the data extraction.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by the World Cancer Research Fund International (Grant number: 2007/SP01) (http://www.wcrf-uk.org/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The views expressed in this review are the opinions of the authors. They may not represent the views of WCRF International/AICR and may differ from those in their future updates of the evidence related to food, nutrition, physical activity and cancer risk.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. IntJCancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. JNatl Cancer Inst. 2008;100:1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.La Vecchia C, Bosetti C, Lucchini F, Bertuccio P, Negri E, et al. Cancer mortality in Europe, 2000-2004, and an overview of trends since 1975. Ann Oncol. 2010;21:1323–1360. doi: 10.1093/annonc/mdp530. [DOI] [PubMed] [Google Scholar]
- 4.Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA CancerJClin. 2009;59:366–378. doi: 10.3322/caac.20038. [DOI] [PubMed] [Google Scholar]
- 5.Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138:2029–2043. doi: 10.1053/j.gastro.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666–689. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 7.Bingham SA. High-meat diets and cancer risk. Proc Nutr Soc. 1999;58:243–248. doi: 10.1017/s0029665199000336. [DOI] [PubMed] [Google Scholar]
- 8.Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44:44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- 9.Norat T, Riboli E. Meat consumption and colorectal cancer: a review of epidemiologic evidence. Nutr Rev. 2001;59:37–47. doi: 10.1111/j.1753-4887.2001.tb06974.x. [DOI] [PubMed] [Google Scholar]
- 10.Sinha R, Rothman N, Brown ED, Mark SD, Hoover RN, et al. Pan-fried meat containing high levels of heterocyclic aromatic amines but low levels of polycyclic aromatic hydrocarbons induces cytochrome P4501A2 activity in humans. Cancer Res. 1994;54:6154–6159. [PubMed] [Google Scholar]
- 11.Bingham SA, Hughes R, Cross AJ. Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response. JNutr. 2002;132:3522S–3525S. doi: 10.1093/jn/132.11.3522S. [DOI] [PubMed] [Google Scholar]
- 12.Cross AJ, Pollock JR, Bingham SA. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003;63:2358–2360. [PubMed] [Google Scholar]
- 13.Bonnett R, Charalambides AA, Martin RA, Sales KD, Fitzsimmons BW. Reactions of nitrous acid and nitric oxide with porphyrins and haems. Nitrosylhaems as nitrosating agents. JChem Soc Chem Commun. 1975;884-885 [Google Scholar]
- 14.Joosen AM, Kuhnle GG, Aspinall SM, Barrow TM, Lecommandeur E, et al. Effect of processed and red meat on endogenous nitrosation and DNA damage. Carcinogenesis. 2009;30:1402–1407. doi: 10.1093/carcin/bgp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Cancer Research Fund/American Institute for Cancer Research. . AICR; 2007. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. [Google Scholar]
- 16.Alexander DD, Miller AJ, Cushing CA, Lowe KA. Processed meat and colorectal cancer: a quantitative review of prospective epidemiologic studies. EurJCancer Prev. 2010;19:328–341. doi: 10.1097/CEJ.0b013e32833b48fa. [DOI] [PubMed] [Google Scholar]
- 17.Alexander DD, Cushing CA. Red meat and colorectal cancer: a critical summary of prospective epidemiologic studies. Obes Rev. 2010. no. doi: 10.1111/j.1467-789X.2010.00785.x. In press. [DOI] [PubMed]
- 18.Wei EK, Giovannucci E, Wu K, Rosner B, Fuchs CS, et al. Comparison of risk factors for colon and rectal cancer. IntJCancer. 2004;108:433–442. doi: 10.1002/ijc.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berndt SI, Platz EA, Fallin MD, Thuita LW, Hoffman SC, et al. Genetic variation in the nucleotide excision repair pathway and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:2263–2269. doi: 10.1158/1055-9965.EPI-06-0449. [DOI] [PubMed] [Google Scholar]
- 20.Butler LM, Wang R, Koh WP, Yu MC. Prospective study of dietary patterns and colorectal cancer among Singapore Chinese. BrJCancer. 2008;99:1511–1516. doi: 10.1038/sj.bjc.6604678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cross AJ, Ferrucci LM, Risch A, Graubard BI, Ward MH, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 2010;70:2406–2414. doi: 10.1158/0008-5472.CAN-09-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cross AJ, Leitzmann MF, Gail MH, Hollenbeck AR, Schatzkin A, et al. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med. 2007;4:e325. doi: 10.1371/journal.pmed.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fung TT, Hu FB, Wu K, Chiuve SE, Fuchs CS, et al. The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer. AmJClin Nutr. 2010;92:1429–1435. doi: 10.3945/ajcn.2010.29242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabat GC, Miller AB, Jain M, Rohan TE. A cohort study of dietary iron and heme iron intake and risk of colorectal cancer in women. BrJCancer. 2007;97:118–122. doi: 10.1038/sj.bjc.6603837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SA, Shu XO, Yang G, Li H, Gao YT, et al. Animal origin foods and colorectal cancer risk: a report from the Shanghai Women's Health Study. Nutr Cancer. 2009;61:194–205. doi: 10.1080/01635580802419780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nothlings U, Yamamoto JF, Wilkens LR, Murphy SP, Park SY, et al. Meat and heterocyclic amine intake, smoking, NAT1 and NAT2 polymorphisms, and colorectal cancer risk in the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18:2098–2106. doi: 10.1158/1055-9965.EPI-08-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oba S, Shimizu N, Nagata C, Shimizu H, Kametani M, et al. The relationship between the consumption of meat, fat, and coffee and the risk of colon cancer: a prospective study in Japan. Cancer Lett. 2006;244:260–267. doi: 10.1016/j.canlet.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 28.Sato Y, Nakaya N, Kuriyama S, Nishino Y, Tsubono Y, et al. Meat consumption and risk of colorectal cancer in Japan: the Miyagi Cohort Study. EurJCancer Prev. 2006;15:211–218. doi: 10.1097/01.cej.0000197455.87356.05. [DOI] [PubMed] [Google Scholar]
- 29.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Systematic Literature Review Specification Manual 2003 [Google Scholar]
- 30.Bekkering GE, Harris RJ, Thomas S, Mayer AM, Beynon R, et al. How much of the data published in observational studies of the association between diet and prostate or bladder cancer is usable for meta-analysis? AmJEpidemiol. 2008;167:1017–1026. doi: 10.1093/aje/kwn005. [DOI] [PubMed] [Google Scholar]
- 31.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 32.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. StataJ. 2006;6:40–57. [Google Scholar]
- 33.Flood A, Velie EM, Sinha R, Chaterjee N, Lacey JV, et al. Meat, fat, and their subtypes as risk factors for colorectal cancer in a prospective cohort of women. AmJEpidemiol. 2003;158:59–68. doi: 10.1093/aje/kwg099. [DOI] [PubMed] [Google Scholar]
- 34.Larsson SC, Rafter J, Holmberg L, Bergkvist L, Wolk A. Red meat consumption and risk of cancers of the proximal colon, distal colon and rectum: the Swedish Mammography Cohort. IntJCancer. 2005;113:829–834. doi: 10.1002/ijc.20658. [DOI] [PubMed] [Google Scholar]
- 35.Sandhu MS, White IR, McPherson K. Systematic review of the prospective cohort studies on meat consumption and colorectal cancer risk: a meta-analytical approach. Cancer Epidemiol Biomarkers Prev. 2001;10:439–446. [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 37.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. JClin Epidemiol. 2000;53:1119–1129. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 38.Tobias A. Assessing the influence of a single study in meta-analysis. Stata Tech Bull. 1999;47:15–17. [Google Scholar]
- 39.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. IntJEpidemiol. 1999;28:964–974. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 40.Bagnardi V, Zambon A, Quatto P, Corrao G. Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality. AmJEpidemiol. 2004;159:1077–1086. doi: 10.1093/aje/kwh142. [DOI] [PubMed] [Google Scholar]
- 41.Luchtenborg M, Weijenberg MP, de Goeij AF, Wark PA, Brink M, et al. Meat and fish consumption, APC gene mutations and hMLH1 expression in colon and rectal cancer: a prospective cohort study (The Netherlands). Cancer Causes Control. 2005;16:1041–1054. doi: 10.1007/s10552-005-0239-0. [DOI] [PubMed] [Google Scholar]
- 42.Spencer EA, Key TJ, Appleby PN, Dahm CC, Keogh RH, et al. Meat, poultry and fish and risk of colorectal cancer: pooled analysis of data from the UK dietary cohort consortium. Cancer Causes Control. 2010;21:1417–1425. doi: 10.1007/s10552-010-9569-7. [DOI] [PubMed] [Google Scholar]
- 43.Sorensen M, Autrup H, Olsen A, Tjonneland A, Overvad K, et al. Prospective study of NAT1 and NAT2 polymorphisms, tobacco smoking and meat consumption and risk of colorectal cancer. Cancer Lett. 2008;266:186–193. doi: 10.1016/j.canlet.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 44.Sellers TA, Bazyk AE, Bostick RM, Kushi LH, Olson JE, et al. Diet and risk of colon cancer in a large prospective study of older women: an analysis stratified on family history (Iowa, United States). Cancer Causes Control. 1998;9:357–367. doi: 10.1023/a:1008886715597. [DOI] [PubMed] [Google Scholar]
- 45.Fraser GE. Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California Seventh-day Adventists. AmJClin Nutr. 1999;70:532S–538S. doi: 10.1093/ajcn/70.3.532s. [DOI] [PubMed] [Google Scholar]
- 46.Ma J, Giovannucci E, Pollak M, Chan JM, Gaziano JM, et al. Milk intake, circulating levels of insulin-like growth factor-I, and risk of colorectal cancer in men. JNatl Cancer Inst. 2001;93:1330–1336. doi: 10.1093/jnci/93.17.1330. [DOI] [PubMed] [Google Scholar]
- 47.Wu K, Hu FB, Fuchs C, Rimm EB, Willett WC, et al. Dietary patterns and risk of colon cancer and adenoma in a cohort of men (United States). Cancer Causes Control. 2004;15:853–862. doi: 10.1007/s10552-004-1809-2. [DOI] [PubMed] [Google Scholar]
- 48.Wei EK, Colditz GA, Giovannucci EL, Fuchs CS, Rosner BA. Cumulative risk of colon cancer up to age 70 years by risk factor status using data from the Nurses' Health Study. AmJEpidemiol. 2009;170:863–872. doi: 10.1093/aje/kwp210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brink M, Weijenberg MP, de Goeij AF, Roemen GM, Lentjes MH, et al. Meat consumption and K-ras mutations in sporadic colon and rectal cancer in The Netherlands Cohort Study. BrJCancer. 2005;92:1310–1320. doi: 10.1038/sj.bjc.6602491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norat T, Bingham S, Ferrari P, Slimani N, Jenab M, et al. Meat, fish, and colorectal cancer risk: the European Prospective Investigation into cancer and nutrition. JNatl Cancer Inst. 2005;97:906–916. doi: 10.1093/jnci/dji164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J, Stampfer MJ, Hough HL, Garcia-Closas M, Willett WC, et al. A prospective study of N-acetyltransferase genotype, red meat intake, and risk of colorectal cancer. Cancer Res. 1998;58:3307–3311. [PubMed] [Google Scholar]
- 52.Bostick RM, Potter JD, Kushi LH, Sellers TA, Steinmetz KA, et al. Sugar, meat, and fat intake, and non-dietary risk factors for colon cancer incidence in Iowa women (United States). Cancer Causes Control. 1994;5:38–52. doi: 10.1007/BF01830725. [DOI] [PubMed] [Google Scholar]
- 53.Singh PN, Fraser GE. Dietary risk factors for colon cancer in a low-risk population. AmJEpidemiol. 1998;148:761–774. doi: 10.1093/oxfordjournals.aje.a009697. [DOI] [PubMed] [Google Scholar]
- 54.Chao A, Thun MJ, Connell CJ, McCullough ML, Jacobs EJ, et al. Meat consumption and risk of colorectal cancer. JAMA. 2005;293:172–182. doi: 10.1001/jama.293.2.172. [DOI] [PubMed] [Google Scholar]
- 55.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, et al. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994;54:2390–2397. [PubMed] [Google Scholar]
- 56.Lin J, Zhang SM, Cook NR, Lee IM, Buring JE. Dietary fat and fatty acids and risk of colorectal cancer in women. AmJEpidemiol. 2004;160:1011–1022. doi: 10.1093/aje/kwh319. [DOI] [PubMed] [Google Scholar]
- 57.Pietinen P, Malila N, Virtanen M, Hartman TJ, Tangrea JA, et al. Diet and risk of colorectal cancer in a cohort of Finnish men. Cancer Causes Control. 1999;10:387–396. doi: 10.1023/a:1008962219408. [DOI] [PubMed] [Google Scholar]
- 58.Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Speizer FE. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. NEnglJMed. 1990;323:1664–1672. doi: 10.1056/NEJM199012133232404. [DOI] [PubMed] [Google Scholar]
- 59.Chan AT, Tranah GJ, Giovannucci EL, Willett WC, Hunter DJ, et al. Prospective study of N-acetyltransferase-2 genotypes, meat intake, smoking and risk of colorectal cancer. IntJCancer. 2005;115:648–652. doi: 10.1002/ijc.20890. [DOI] [PubMed] [Google Scholar]
- 60.English DR, MacInnis RJ, Hodge AM, Hopper JL, Haydon AM, et al. Red meat, chicken, and fish consumption and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1509–1514. [PubMed] [Google Scholar]
- 61.Jarvinen R, Knekt P, Hakulinen T, Rissanen H, Heliovaara M. Dietary fat, cholesterol and colorectal cancer in a prospective study. BrJCancer. 2001;85:357–361. doi: 10.1054/bjoc.2001.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kato I, Akhmedkhanov A, Koenig K, Toniolo PG, Shore RE, et al. Prospective study of diet and female colorectal cancer: the New York University Women's Health Study. Nutr Cancer. 1997;28:276–281. doi: 10.1080/01635589709514588. [DOI] [PubMed] [Google Scholar]
- 63.Tiemersma EW, Kampman E, Bueno de Mesquita HB, Bunschoten A, van Schothorst EM, et al. Meat consumption, cigarette smoking, and genetic susceptibility in the etiology of colorectal cancer: results from a Dutch prospective study. Cancer Causes Control. 2002;13:383–393. doi: 10.1023/a:1015236701054. [DOI] [PubMed] [Google Scholar]
- 64.Balder HF, Vogel J, Jansen MC, Weijenberg MP, van den Brandt PA, et al. Heme and chlorophyll intake and risk of colorectal cancer in the Netherlands cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15:717–725. doi: 10.1158/1055-9965.EPI-05-0772. [DOI] [PubMed] [Google Scholar]
- 65.Knekt P, Steineck G, Jarvinen R, Hakulinen T, Aromaa A. Intake of fried meat and risk of cancer: a follow-up study in Finland. IntJCancer. 1994;59:756–760. doi: 10.1002/ijc.2910590608. [DOI] [PubMed] [Google Scholar]
- 66.Knekt P, Jarvinen R, Dich J, Hakulinen T. Risk of colorectal and other gastro-intestinal cancers after exposure to nitrate, nitrite and N-nitroso compounds: a follow-up study. IntJCancer. 1999;80:852–856. doi: 10.1002/(sici)1097-0215(19990315)80:6<852::aid-ijc9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 67.Gaard M, Tretli S, Loken EB. Dietary factors and risk of colon cancer: a prospective study of 50,535 young Norwegian men and women. EurJCancer Prev. 1996;5:445–454. [PubMed] [Google Scholar]
- 68.Chen K, Cai J, Liu XY, Ma XY, Yao KY, et al. Nested case-control study on the risk factors of colorectal cancer. WorldJGastroenterol. 2003;9:99–103. doi: 10.3748/wjg.v9.i1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, and Physical Activity: a Global Perspective. Washington DC: AICR; 2009. Policy and Action for Cancer Prevention. [Google Scholar]
- 70.Platz EA, Willett WC, Colditz GA, Rimm EB, Spiegelman D, et al. Proportion of colon cancer risk that might be preventable in a cohort of middle-aged US men. Cancer Causes Control. 2000;11:579–588. doi: 10.1023/a:1008999232442. [DOI] [PubMed] [Google Scholar]
- 71.Parkin DM, Olsen AH, Sasieni P. The potential for prevention of colorectal cancer in the UK. EurJCancer Prev. 2009;18:179–190. doi: 10.1097/CEJ.0b013e32830c8d83. [DOI] [PubMed] [Google Scholar]
- 72.Goldbohm RA, van den Brandt PA, van ', V, Brants HA, Dorant E, et al. A prospective cohort study on the relation between meat consumption and the risk of colon cancer. Cancer Res. 1994;54:718–723. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Main characteristics of the prospective studies included in the dose-response meta-analyses.
(DOC)
Studies or results not included in the dose-response meta-analyses and reasons for exclusion.
(DOC)
Search strategy for the WCRF/AICR Continuous Update Project.
(DOC)