Abstract
Lung hypoplasia (LH) is a life-threatening congenital abnormality with various causes. It involves vascular bed underdevelopment with abnormal arterial muscularisation leading to pulmonary hypertension. Because underlying molecular changes are imperfectly known and sometimes controversial, we determined key factors of angiogenesis along intrauterine development, focusing at the angiopoietin (ANG)/TIE2 system. Lung specimens from medical terminations of pregnancy (9 to 37wk) were used, including LH due to congenital diaphragmatic hernia (CDH) or other causes, and non-pulmonary disease samples used as controls. ELISA determination indicated little ANG1 change during pregnancy and no effect of LH whereas TIE2 declined similarly between 9 and 37 wk in LH and controls. By contrast, ANG2 markedly increased in LH from 24 wk whereas it remained stable in controls. Because VEGF increased also, this was interpreted as an attempt to overcome vascular underdevelopment. Hypothesizing that its inefficiency might be due to impaired downstream mechanism, eNOS was determined by semi-quantitative western blot and found to be reduced about 75%, mostly in the instance of CDH. In conclusion, angiogenesis remains defective in hypoplastic lungs despite reactive enhancement of VEGF and ANG2 production, which could be due, at least in part, to insufficient eNOS expression.
Keywords: Congenital Diaphragmatic Hernia, Lung Development, Pulmonary Hypertension, eNOS
Keywords: Angiopoietin-1; metabolism; Angiopoietin-2; metabolism; Female; Humans; Hypertension, Pulmonary; Lung; abnormalities; blood supply; embryology; Neovascularization, Pathologic; physiopathology; Nitric Oxide Synthase Type III; deficiency; Pregnancy; Receptor, TIE-2; metabolism; Vascular Endothelial Growth Factor A; metabolism
Lung hypoplasia (LH) commonly occurs in association with congenital anomalies, especially congenital diaphragmatic hernia (CDH) and kidney or urinary tract disorders (33, 43). Hypoplastic lungs present marked reduction in the number of bronchial divisions, as well as in the development of distal airways. Because vascular development parallels that of airways, underdevelopment of the pulmonary vascular bed, which is associated with abnormal muscularisation of the arterial walls, is a feature of hypoplastic lungs (20, 28). This, along with excessive vasoconstrictor mechanisms (9), leads to pulmonary hypertension at birth (6). Despite progress in the care of newborn infants with LH and pulmonary hypertension, the mortality rate remains elevated. Although vascular abnormalities with decreased number of vessels per lung volume-unit and increased muscularisation of pulmonary arteries emerged as a determinant of survival in LH, little information exists with regard to underlying mechanisms. Better understanding the pathophysiology of LH may help defining novel therapeutic approaches and achieving better outcome.
Only few factors involved in lung vasculogenesis/angiogenesis have been documented in human fetuses or neonates with LH. Indeed, abnormal expression of sonic hedgehog, hypoxia-inducible factor 1, and vascular endothelial growth factor (VEGF)-A, all known to direct airway branching and/or distal pulmonary vascular development (45) was reported in human fetuses with CDH (10, 34, 44). Factors that regulate pulmonary vascular tone during transition of pulmonary circulation at birth have been also partially explored. Higher concentrations of endothelin-1, considered as a powerful vasoconstrictive mediator, and increased expression of its receptors ETA and ETB have been found in plasma and pulmonary arteries of newborns with CDH, respectively (9, 21). Lastly, previous attempts to demonstrate the contribution of nitric oxide synthases (NOS) in LH-induced hypertension have yielded mixed results, with some studies showing that NOS were decreased at birth (35, 38), whereas others showed unaffected expression level of these enzymes (10, 35).
In the present study, we focused on the angiopoietin (ANG)/TIE2 system as potential candidate regulators of vascular remodeling in LH. Vasculogenesis and angiogenesis are mainly regulated by the interplay between VEGF and angiopoietins. ANG1 and 2 bind the endothelial receptor tyrosine kinase TIE-2. During normal development, ANG1 and TIE2 are required for correct organization and maturation of newly formed vessels. Mice deficient for these factors die at mid gestation and display dilated vessels, diminished branching, and reduced number of small vessels (13, 42). A similar phenotype was observed in mice overexpressing the Ang2 gene, indicating that ANG2 is able to counteract ANG1 activity (25). A number of studies, however, suggested that ANG2 displays pro-angiogenic properties at high concentration (26) or in combination with VEGF-A (24, 48), revealing the complexity of ANG2 functions. In addition to its role in vascular development, growing evidence suggests that the ANG/TIE2 pathway influences pulmonary smooth muscle hyperplasia (11, 12).
Given these considerations, we hypothesized that the hypoplastic lung might present unbalanced expression of angiopoietins, TIE2, and VEGF, which might in turn contribute to vascular underdevelopment and lung hypertension at birth. We determined the pulmonary content of these factors in human fetuses with LH of various causes at different developmental stages, comparatively to normal fetal lungs. This represents the first study of this pathway in human lung in the course of gestational development.
MATERIALS AND METHODS
Human lung tissue
Post mortem lung tissue samples were obtained at autopsy after medical termination of pregnancy or neonatal death Lung tissues were immediately frozen and stored at −80°C. Terminations were performed according to the July 1994 French legislation, and the study was undertaken with the agreement of the institutional Ethical Committee. Parents were informed about the procedure and issues of post mortem study, and signed consent was obtained for all included samples. The prenatal diagnosis of CDH, lung hypoplasia, and other congenital abnormalities was made by ultrasound and was confirmed by post mortem examination. Reduced lung weight and consistent histological appearance confirmed the diagnosis of pulmonary hypoplasia (lung to body weight ratio <0.015 before 28 wk of pregnancy, and <0.012 thereafter) [Table 1]. Fetuses were checked for other malformations and for the presence or absence of associated chromosomal abnormality. In the instance of CDH, the lung ipsilateral to the hernia was used. Lungs from fetuses with nonpulmonary malformations were used as controls; they appeared to be histologically normal by post mortem examination and were not hypoplastic. No cardiovascular disease was included in the control group. Clinical data are depicted in Table 2. Previous studies using those human lung tissue samples had shown preservation of proteins and feasibility of their analysis (5). Preservation of RNAs was by contrast insufficient and inconsistent, and therefore neither quantitative study at the pre-translational level nor in situ hybridization could be performed. Fetal age (post conceptional) is used throughout the paper.
Table 1.
Characteristics of Fetuses with Pulmonary Hypoplasia
| Number | Fetal Age (weeks) | Sex | Syndrome | Body Weight (g) | Lung Weight-to-Body Weight Ratio |
|---|---|---|---|---|---|
| 1 | 13 | M | CDH, left | 62 | 0.015 |
| 2 | 21 | F | CDH, right | 550 | 0.01 |
| 3 | 22 | F | CDH, right | 610 | 0.008 |
| 4 | 22 | F | CDH, right | 780 | 0.008 |
| 5 | 22 | M | CDH | 500 | 0.005 |
| 6 | 22 | F | CDH, left | 660 | 0.008 |
| 7 | 22 | ND | CDH | ND | ND |
| 8 | 23 | F | CDH, left | 850 | 0.015 |
| 9 | 23 | M | CDH | 730 | 0.009 |
| 10 | 23 | M | CDH | 780 | 0.006 |
| 11 | 23 | M | CDH, left | 970 | 0.011 |
| 12 | 25 | M | CDH, left | 1270 | 0.008 |
| 13 | 29 | M | CDH, left | 1480 | 0.0117 |
| 14 | 31 | F | CDH, left | 1900 | 0.004 |
| 15 | 32 | F | CDH, left | 1960 | 0.006 |
| 16 | 33 | F | CDH, left | 2200 | 0.012 |
| 17 | 36 | F | CDH, left | 2340 | 0.0043 |
| 18 | 36 | F | CDH, left | 2800 | 0.018 |
| 19 | 37 | F | CDH, right | 3350 | 0.005 |
| 20 | 37 | ND | CDH | 3852 | 0.012 |
| 21 | 17 | M | Hydrothorax | 408 | 0.008 |
| 22 | 18 | M | Renal anomaly | 225 | 0.019 |
| 23 | 19 | M | Anamnios | 320 | 0.013 |
| 24 | 23 | M | Anamnios, multicystic kidney | 580 | 0.012 |
| 25 | 23 | M | Premature rupture of membranes | 890 | 0.012 |
| 26 | 23 | M | Multicystic kidney | 1160 | 0.011 |
| 27 | 24 | M | Multicystic kidney | 930 | 0.0113 |
| 28 | 24 | M | Posterior urethral valves | 880 | 0.013 |
| 29 | 25 | M | Anamnios | 800 | 0.015 |
| 30 | 26 | M | Renal dysplasia | 1070 | 0.014 |
| 31 | 31 | F | Polymalformation | 1580 | 0.006 |
| 32 | 32 | M | Anamnios, Multicystic kidney | 2460 | 0.01 |
| 33 | 32 | F | Akinesia | 1620 | 0.009 |
| 34 | 33 | M | Hydrothorax | 3080 | 0.007 |
ND: not determined.
Table 2.
Characteristics of Fetuses with non-pulmonary Diseases Used as Controls
| Number | Fetal Age (weeks) | Sex | Syndrome | Body Weight (g) | Lung Weight-to-Body Weight Ratio |
|---|---|---|---|---|---|
| 35 | 9 | M | Brachio-oto-renal syndrome | 3.3 | ND |
| 36 | 11 | F | Cystic Hygroma | 19.2 | ND |
| 37 | 12 | F | Inencephaly | 16 | 0.037 |
| 38 | 14 | M | Cloacal defect | 150 | 0.024 |
| 39 | 15 | M | Hydrops | 87 | 0.013 |
| 40 | 16 | F | Brain anomaly | 173 | 0.046 |
| 41 | 16 | F | Thanatophoric dwarfism | 143 | 0.026 |
| 42 | 18 | M | Spina bifida | 253 | 0.031 |
| 43 | 21 | M | Omphalocele | 530 | 0.022 |
| 44 | 21 | M | Osteogenesis imperfecta | 510 | 0.023 |
| 45 | 23 | M | Holoprosencephaly | 800 | 0.025 |
| 46 | 23 | F | Brain anomaly | 795 | 0.017 |
| 47 | 23 | M | Hydrops | 1100 | 0.021 |
| 48 | 23 | M | In utero death | 810 | 0.019 |
| 49 | 24 | M | Limb | 940 | 0.027 |
| 50 | 24 | M | Ectrodactily | 760 | 0.0255 |
| 51 | 24 | M | Brain anomaly | 790 | 0.024 |
| 52 | 25 | M | Limb | 1050 | 0.027 |
| 53 | 25 | M | Brain anomaly | 900 | 0.024 |
| 54 | 26 | F | Arthrogriposis | 1100 | 0.017 |
| 55 | 27 | M | Brain anomaly | 420 | 0.072 |
| 56 | 28 | M | Brain anomaly | 1430 | 0.023 |
| 57 | 29 | F | Brain anomaly | 1400 | 0.026 |
| 58 | 30 | F | Bourneville disease | 1700 | 0.0205 |
| 59 | 30 | M | Brain anomaly | 1780 | 0.023 |
| 60 | 31 | M | Osteochondrodysplasia | 1400 | 0.016 |
| 61 | 31 | F | Brain anomaly | 2300 | 0.016 |
| 62 | 33 | M | Distal arthrogryposis | 2560 | 0.016 |
| 63 | 33 | M | Trisomy 21 | 2450 | 0.026 |
| 64 | 34 | M | Brain anomaly | 2450 | 0.023 |
| 65 | 35 | F | Hydrocephaly | 2500 | 0.018 |
| 66 | 35 | M | Chondrodysplasia | 3170 | 0.0157 |
| 67 | 36 | F | Holoprosencephaly | 1600 | 0.021 |
| 68 | 36 | F | Holoprosencephaly | 1800 | 0.022 |
| 69 | 37 | F | Spina Bifida | 3140 | 0.017 |
Lung histology and vascular immunohistochemistry
Because of restrictions in human tissue sampling conditions, it was not possible to proceed to lung fixation at constant pressure, and therefore to perform morphometry. Pieces of human lung tissue were fixed in formalin 24h after death. Fixed tissues were paraffin-embedded. Five-μm sections were dehydrated in xylene and graded ethanol, and then stained with orcein-picroindigo-carmine.
Immunohistochemistry of platelet endothelial cell adhesion molecule-1 (PECAM-1) was performed using the biotin/streptavidin RTU Vectastain Universal Quick kit (Vector laboratories, Burlingame, CA), according to the manufacturer’s instructions. Briefly, the slides were dewaxed and rehydrated in graded ethanol solutions. Endogenous peroxidases were quenched by incubating the slides in methanol/0.3% H2O2 at room temperature for 30 min. Antigen retrieval was performed by microwave heating in citrate buffer solution (pH 6.0). Sections were then blocked in PBS containing 1.5% normal horse serum for 1 hour at room temperature, probed with monoclonal mouse anti-human PECAM-1 antibody (diluted 1:50, clone: JC/70A, DAKO, Carpintera, CA) overnight at 4°C, and incubated with prediluted biotinylated panspecific IgG for 10 minutes. Lung sections serving as negative controls received the same dilution of non-immune mouse IgG. The slides were incubated with HRP-conjugated streptavidin for 5 minutes. NovaRED (Vector Laboratories) was used as substrate. After counterstaining with hematoxylin, tissue sections were dehydrated and mounted. Light microscopy images were captured with a digital camera.
Western blot
Human lung tissue was homogenized in RIPA buffer containing protease inhibitors (Roche Diagnostics, Meylan, France). Protein content was assessed with Bradford assay. Sixty micrograms of total proteins were electrophoresed on 7.5% SDS-poplyacrylamide gels then transferred onto polyvinylidene-fluoride membrane (Millipore, Velizy, France). To document equivalent protein loading, membranes were stained with Ponceau S dye (Sigma, Saint-Quentin Fallavier, France) and photographed prior to antibody incubations. After blocking with 5% nonfat dry milk in Tris-buffer saline containing 0.05% Tween-20 (TTBS) at room temperature for 2h, membranes were exposed to rabbit anti-eNOS (Santa Cruz Biotechnology, Santa Cruz, CA) antibody, washed in TTBS, and exposed to horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (Santa Cruz Biotechnology) and incubated in enhanced chemiluminescence reagent (GE Healthcare Life Sciences, Saclay, France) before exposure to Kodak BioMax MS film. Signals were quantified by densitometry (NIH Image, Betehesda, MD).
Quantitative assays for VEGF, ANG1, aANG2, and TIE2
VEGF-A165, Angiopoietin-1 and -2, and TIE2 concentrations in human fetal lung tissue were assessed with commercially sensitive and specific ELISA (R&D Systems, Lille, France), following the manufacturer’s instructions, and normalized to total proteins. The intra and inter-assay coefficients of variance were less than 5% for each essay.
Stastistical analysis
Relationships between ANG1, ANG2, TIE2, and VEGF-A165 concentrations and fetal age were analyzed using linear regression models, including main effects and interaction terms to compare concentrations between groups. Sensitivity analyses were performed when outliers were present. Analyses were performed using R statistical software. For eNOS data, multiple group comparisons were made using Kruskal-Wallis test, and two-group comparisons were made using the Mann-Whitney U-test. A p-value of 0.05 was considered to be the limit of statistical significance.
Results
Comparative histology and PECAM-1 immunohistochemistry
Control lungs aged 32–33 weeks (fetal age, late saccular stage) displayed large-sized, simple airspaces, relatively wide septa, and focal early secondary crest formation, characteristic of late saccular stage of lung development. Although displaying variable morphology, hypolastic lungs of same age appeared immature with thicker septa, denser tissue, and characteristic thickening of blood vessel walls (Fig. 1A–C). In accordance with histological findings, immunostaining of the endothelial-cell marker PECAM-1 (CD31) in control lungs demonstrated abundant microvascular structures within the septa, both subepithelially and centrally (Fig. 2A). By contrast, lungs of fetuses with LH displayed defective vascular development with fewer capillaries less evenly distributed and embedded in a thicker mesenchyme (Fig. 2B,C).
Figure 1.
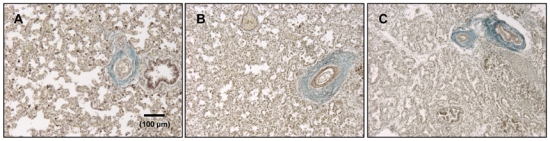
Representative histological sections of fetal human lungs aged 32–33 weeks (fetal age) without (A, control lung) or with (B, CDH, and C, urogenital defect) lung hypoplasia (LH). Tissue was stained by orcein-picroindigo-carmine. All pictures are at same magnification. Structure of hypoplastic lungs appears immature with dense parenchyma, thicker septa, and reduced presumptive airspaces. Note thickening of blood-vessel walls in B and C.
Figure 2.
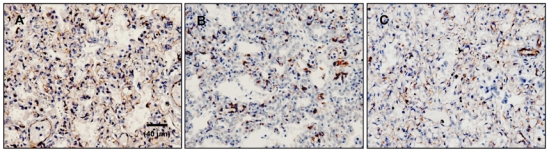
Immunostaining of platelet endothelial cell adhesion molecule-1 (PECAM-1). Lung sections from fetuses aged 32–33 weeks (fetal age) without (A, control lung) or with (B, CDH, and C, urogenital defect) LH were immunostained with mouse monoclonal anti-human PECAM-1 antibody and counterstained with hematoxylin. All pictures are at same magnification. Control fetuses had extensive staining of endothelial cells within septa, whereas immunostaining appeared less intense in the hypoplastic lungs that displayed reduced complex intertwining of alveolar capillaries.
Lung contents in ANG1, ANG2, and TIE2
The concentration of ANG1, ANG2 and TIE2 proteins was determined in lung tissue samples from 69 fetuses with or without LH ranging from 9 to 37 wk of pregnancy. As already observed for other parameters in similar samples, (5) the values were largely dispersed with some overlap between groups. This seems to be inherent of this type of approach with human samples, but did not prevent from evidencing strongly significant differences between groups. In control and LH lungs, ANG1 concentration did not change significantly during pregnancy and displayed a mean level of 200–250 pg/mg protein (LH slope vs Control slope: p= 0.154, Fig. 3A). Whereas ANG2 concentration remained similarly unchanged throughout pregnancy in control lungs with a mean level of 750–1000 pg/mg, a marked increase was observed in LH lungs (LH slope vs Control slope: p= 0.00154, Fig. 3B). In the latter group, ANG2 increased linearly to reach approximately 3000–4000 pg/mg protein at the later stages. Over the studied period, TIE2 concentration displayed similar profiles in control and LH fetuses, slightly decreasing from 9 to 37 wk; no statistical difference was observed between groups, either considering CDH and other causes separately or altogether (LH slope vs Control slope: p=0.79, Fig. 3C).
Figure 3.
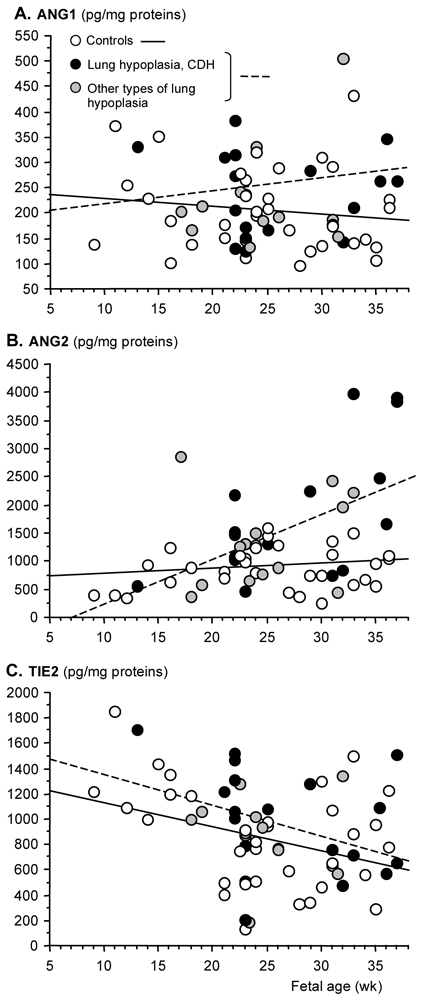
Scatter plots of ANG1, ANG2, and TIE2 concentrations (pg/mg protein) in human lung fetuses as a function of fetal age (wk). Individual values are shown: open, black, and grey points correspond to control fetuses, fetuses with pulmonary hypoplasia due to CDH, and fetuses with pulmonary hypoplasia due to other causes, respectively. Solid line represents the regression line of control fetuses, hatched line represents the regression line of fetuses with lung hypoplasia (either cause). Two extreme ANG1, ANG2, and TIE2 outliers were discarded for calculating scatters plots. Difference between control and LH fetuses (CDH + other causes) was significant only for Ang2 that was increased in late gestation (LH slope vs Control slope: p= 0.00154). Clinical data of fetuses are depicted in Tables 1 and 2.
Lung contents in VEGF
Given the fact that ANG2 has been shown to have a dual role in angiogenesis depending on the availability of VEGF-A, VEGF-A concentration was determined during pregnancy in human fetuses with or without pulmonary hypoplasia. Various VEGF-A isoforms are present in the developing lung. Because (i) temporal and spatial regulation of VEGF-A controls vascular patterning in the embryonic lung (2) whereas VEGF-C and D are mainly considered to be lymphangiogenic, (ii) defective pulmonary vascular development in mice was consecutive to the loss of both VEGF164 (termed VEGF165 in humans) and 188 isoforms (14), and (iii) transgenic mice expressing only the VEGF164 isoform are indistinguishable from the wild type (39), the 164/165 isoform appears to have an irreplaceable role and was therefore studied herein. Similar to ANG2, we found elevated VEGF-A165 expression level in fetuses with lung hypoplasia (LH slope vs Control slope: p=0.02, Fig 4). Considering separately CDH and other causes, the difference between fetuses with LH due to other causes (designated LHo) and controls remained statistically significant (p=0.0235), whereas that for the CDH group did not (p=0.077), although very high VEGF-A165 level was present in some CDH specimens.
Figure 4.
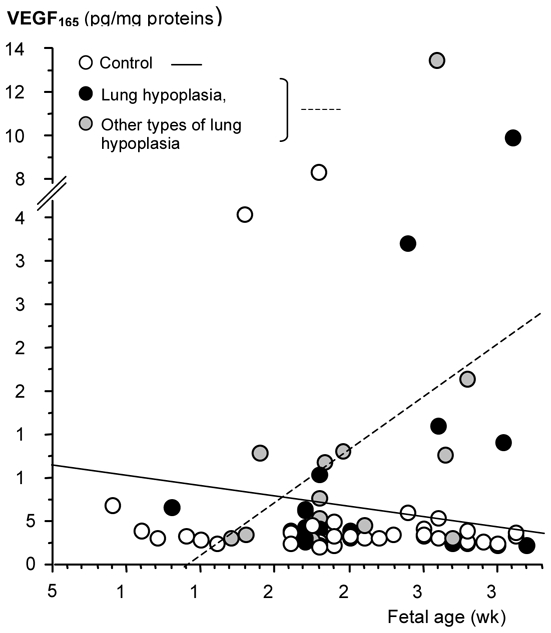
Scatter plot of VEGF165 concentration (pg/mg protein) in human lung fetuses as a function of fetal age (wk). Individual values are shown: open, black, and grey points correspond to control fetuses, fetuses with pulmonary hypoplasia due to CDH, and fetuses with pulmonary hypoplasia due to other causes, respectively. Solid line represents the regression line of control fetuses, hatched line represents the regression line of fetuses with lung hypoplasia (either cause). VEGF165 was markedly increased in hypoplastic lungs in late gestation (LH vs controls slope: p=0.02).
eNOS protein evaluation in human fetal lungs
eNOS is known to mediate biological effects of both VEGF-A and Angiopoietins (1, 3, 47). Comparatively to mice devoid of the other isoforms (i.e. inducible NOS and neuronal NOS), the lung phenotype of eNOS−/− mice is the most severe with abnormal lung development as well as elevated pulmonary arterial pressure in adulthood (16, 38). For these reasons, eNOS expression was evaluated semi-quantitatively by western blot in lung tissue samples from fetuses with LH either induced by CDH or other causes and compared to age-matched controls. In lungs at the canalicular stage (i.e. 22–25 wk), eNOS protein level was significantly diminished to 25 (p<0.01) and 45% (p<0.05) of control level in CDH and LHo groups respectively (Figure 5). During saccular stage (i.e. 31–33 wk), eNOS content was reduced to 30% of control level in the CDH group (p<0.05), but not significantly changed in the LHo group. Because of the restricted number of specimen available at the alveolar stage, no statistical comparison could be made. However, as shown in Figure 5C, pulmonary eNOS level was markedly reduced in one fetus with CDH compared to two age-matched controls.
Figure 5.
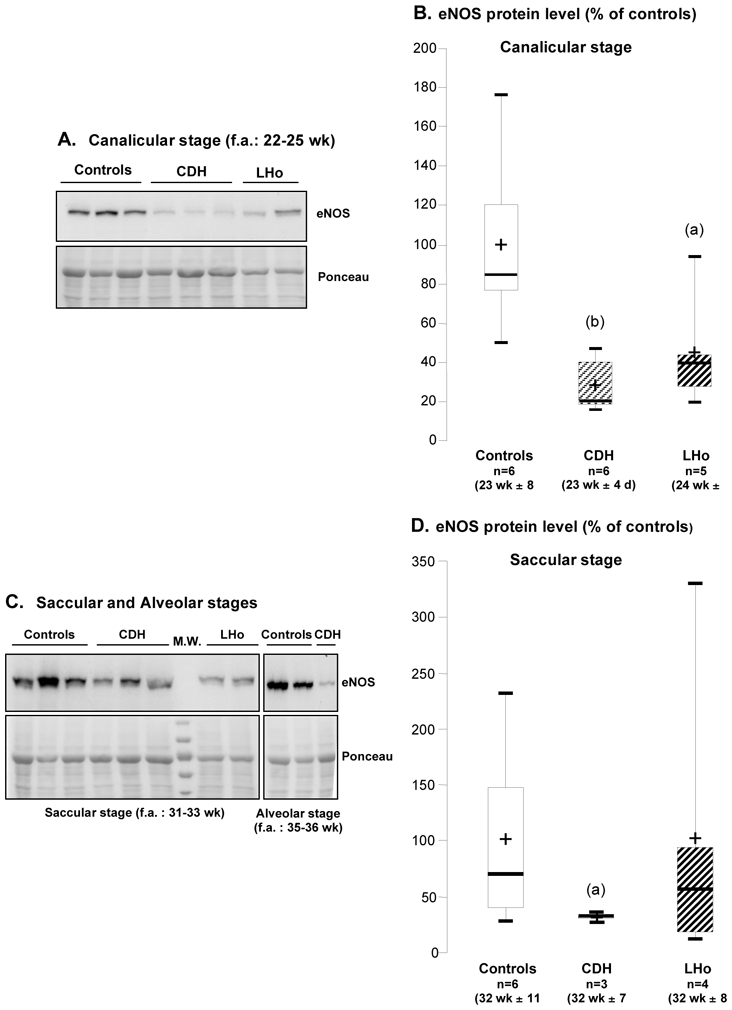
eNOS protein level as a function of developmental stages in human lung fetuses with or without pulmonary hypoplasia. Canalicular stage: (A) representative western blots in CDH, lung hypoplasia due to other causes (LHo), and control lungs; (B) corresponding densitometric analysis normalized by Ponceau. Saccular and alveolar stages: (C) representative western blots; (D) densitometric analysis for saccular stage normalized by Ponceau S. Signal for eNOS protein was evidenced at 135 kDa (B and D). Results are expressed as percentage of control-group mean value. Box plot: the boundary of the box indicates the 25th and 75th percentiles, the bold line and the cross within the box mark the median and mean values, respectively, and whiskers represent minimum and maximum values. eNOS protein was significantly diminished in CDH and LHo groups at the canalicular stage, and in CDH group at the saccular stage (significant difference with respective control group for: (a) p<0.05; (b) p<0.01).
Discussion
Successful adaptation of the newborn to extrauterine life depends upon the ability of the pulmonary circulation to undergo rapid fall in vascular resistance within the first minutes after birth. This critical step follows a lengthy series of highly orchestrated events that characterize normal growth and maturation of the fetal pulmonary circulation. Since the ANG/Tie2 system plays a crucial role in the control of vascular development in normal angiogenesis and has been found to be disordered in pathological conditions, we speculated that abnormal balance in the ANG/Tie2 system might account for the structural vascular abnormalities seen in human fetuses with lung hypoplasia. We found that the latter is associated with elevated concentration of pulmonary ANG2 during the second half of pregnancy.
Although controversial, the implication of the ANG1-Tie2 pathway in the pathogenesis of pulmonary arterial hypertension has been the object of numerous investigations (11, 12, 22). By contrast, the role of the ANG/Tie2 system during lung development has been only partially explored. Hato T et al. (17) recently reported that mice expressing a potent Ang1 variant under the control of a lung-specific promoter displayed blood vessel enlargement and 50% lethality at birth due to respiratory failure, indicating that a precise regulation of Tie2 signaling through angiopoietin control is required for harmonious lung vascular network formation. Previous descriptive studies suggested that ANG2 is a complex regulator of vascular remodeling that may play antagonistic roles in both vessel sprouting and vessel regression, depending on the availability of VEGF-A. Supporting such roles, expression analyses revealed that ANG2 is rapidly induced in the absence of VEGF-A in settings of vascular regression (15), whereas ANG2 is induced together with VEGF-A in settings of angiogenic sprouting (18, 41). In the latter instance, it has been proposed that ANG2 makes mature vessels unstable by blocking the effects of ANG1. This ANG2-mediated destabilization is thought to facilitate access to other classes of angiogenic factors such as VEGF-A. To try clarifying the pathophysiological significance of ANG2 elevation in fetuses with LH, we therefore explored VEGF-A165 protein level. In agreement with a previous report that VEGF immunoreactivity was increased in the endothelium and medial smooth muscles cells of pulmonary arteries in newborns with CDH and pulmonary hypertension (34), we found enhanced VEGF-A165 expression in lung tissue from fetuses with LH compared to controls during the second half of pregnancy. Consequently, we speculate that concomitant increases in ANG2 and VEGF-A165 may reflect an attempt of the hypoplastic lung to overcome vascular underdevelopment. Considering the persistence of impaired angiogenesis illustrated by decreased lung-vessel density observed in previous post mortem studies (10, 20) as well as in the present study, this appears to be inefficient, which suggests abnormal function of effectors. To test this hypothesis, attention was paid to eNOS that we found to be decreased in fetuses with LH, especially in the instance of the one associated with CDH. Using immunohistochemistry, a previous study had evidenced no difference in pulmonary eNOS expression between fetuses with LH and age-matched controls (10). A possible explanation for the discrepancy between this and our investigation is the lack of sensitivity of immunohistochemistry to quantitatively appreciate expression changes, whereas western blotting that we used allows semi-quantitative comparison to be performed. Our finding of reduced eNOS expression in human fetuses with LH is by contrast consistent with that from another investigation conducted on arteries of newborns with CDH complicated by persistent pulmonary hypertension in which eNOS expression level was found to be decreased (38). Moreover, in the present study, we demonstrated that eNOS reduction occurs early in the course of pathological lung development. A marked reduction in hypoplastic lungs was thus observed at the canalicular stage during which intracinar capillaries develop, and was likely to be still present at saccular and alveolar stages. Interestingly, mice devoid of eNOS exhibit major defects in lung morphogenesis with a lung phenotype that closely mimics histological features of alveolar capillary dysplasia (ACD) (16). CDH and ACD are both characterized by decreased number of alveolar units, medial thickening of small pulmonary arteries with muscularization of arterioles, and thickened interalveolar septa (37). Newborns with PPHN secondary to CDH or ACD are often refractory to inhaled NO (19). In addition treatment of lung explants with nitro-L-arginine, a NOS inhibitor, led to a significant loss in branching morphogenesis (27) and a failure of compensatory lung growth following pneumonectomy has been demonstrated in eNOS knockout mice (23). Taken together, these data provide direct evidence that substantial alterations in NO production not only contribute to dysregulation of vascular tone at birth, but also disturb the molecular and cellular mechanisms that mediate the cross talk between epithelium and mesenchyme to coordinate airway and vessel development during lung development.
The mechanism leading to eNOS decrease is not clear. Since physical forces regulate eNOS mRNA expression and play a crucial role in lung growth, prolonged mechanical restriction due to inadequate amniotic fluid production and/or lung compression by viscera could be responsible for the low levels of eNOS observed in fetuses with lung hypoplasia.
Rodent and lamb models have been developed and extensively used to explore the physiopathology of human CDH and to test the safety and efficacy of new therapies. Numerous opposite results have been reported in both inter- and intra-animal models, especially regarding surfactant status and vascular development, underlying the inherent limitations of animal models. For instance, Oue et al. (31) showed that VEGF mRNA as well as protein levels were significantly increased in lungs of nitrofen-treated rats, whereas two other studies (7, 8) reported diminished VEGF expression in the same model. Similar discrepancies were found for eNOS (29, 30).
This study however presents some limitations that must be considered. Firstly, and despite the fact that specimens were processed in the same way, histological investigations were performed on small lung tissue samples obtained at autopsy and fixed after sample removal, a procedure known to influence histological interpretation. Next, the relatively small number of fetuses examined, especially at the alveolar stage, may have contributed to reduce the power of the study. Investigation on a larger population is therefore desirable to confirm the present findings. Finally, the mechanism leading to, and the biological significance of ANG2 and VEGF upregulation in fetuses with LH are uncertain. It must be underlined that the increase of ANG2 expression during pregnancy may counteract the specific vessel-stabilizing effects of ANG1, or mediate deleterious effects, as reported in the instances of bronchopulmonary dysplasia (4) or of acute septic lung injury (32).
Collectively, our results nonetheless document further the abnormal vascular development associated with LH. Enhancing pulmonary eNOS appears as a therapeutic objective in this condition. This is further supported by the finding that NO-donor promoted branching morphogenesis in control and nitrofen-exposed fetal lung explants (27, 36, 46).
Acknowledgments
OB was supported by a postdoctoral fellowship grant from the Fondation pour la Recherche Médicale and from the INSERM/FRSQ research exchanges.
References
- 1.Ahmed A, Fujisawa T, Niu XL, Ahmad S, Al-Ani B, Chudasama K, Abbas A, Potluri R, Bhandari V, Findley CM, Lam GK, Huang J, Hewett PW, Cudmore M, Kontos CD. Angiopoietin-2 confers Atheroprotection in apoE−/− mice by inhibiting LDL oxidation via nitric oxide. Circ Res. 2009;104:1333–1336. doi: 10.1161/CIRCRESAHA.109.196154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akeson AL, Greenberg JM, Cameron JE, Thompson FY, Brooks SK, Wiginton D, Whitsett JA. Temporal and spatial regulation of VEGF-A controls vascular patterning in the embryonic lung. Dev Biol. 2003;264:443–455. doi: 10.1016/j.ydbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Babaei S, Teichert-Kuliszewska K, Zhang Q, Jones N, Dumont DJ, Stewart DJ. Angiogenic actions of angiopoietin-1 require endothelium-derived nitric oxide. Am J Pathol. 2003;162:1927–1936. doi: 10.1016/S0002-9440(10)64326-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, Zhang X, Matthay MA, Ware LB, Homer RJ, Lee PJ, Geick A, de Fougerolles AR, Elias JA. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med. 2006;12:1286–1293. doi: 10.1038/nm1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucherat O, Benachi A, Chailley-Heu B, Franco-Montoya ML, Elie C, Martinovic J, Bourbon JR. Surfactant maturation is not delayed in human fetuses with diaphragmatic hernia. PLoS Med. 2007;4:e237. doi: 10.1371/journal.pmed.0040237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boucherat O, Benachi A, Storme L, Bourbon JR. New Insights in Congenital Diaphragmatic Hernia. Curr Respir Med Rev. 2008;4:157–173. [Google Scholar]
- 7.Chang R, Andreoli S, Ng YS, Truong T, Smith SR, Wilson J, D’Amore PA. VEGF expression is downregulated in nitrofen-induced congenital diaphragmatic hernia. J Pediatr Surg. 2004;39:825–828. doi: 10.1016/j.jpedsurg.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Chinoy MR, Graybill MM, Miller SA, Lang CM, Kauffman GL. Angiopoietin-1 and VEGF in vascular development and angiogenesis in hypoplastic lungs. Am J Physiol Lung Cell Mol Physiol. 2002;283:L60–L66. doi: 10.1152/ajplung.00317.2001. [DOI] [PubMed] [Google Scholar]
- 9.de Lagausie P, de Buys-Roessingh A, Ferkdadji L, Saada J, Aisenfisz S, Martinez-Vinson C, Fund X, Cayuela JM, Peuchmaur M, Mercier JC, Berrebi D. Endothelin receptor expression in human lungs of newborns with congenital diaphragmatic hernia. J Pathol. 2005;205:112–118. doi: 10.1002/path.1677. [DOI] [PubMed] [Google Scholar]
- 10.de Rooij JD, Hosgor M, Ijzendoorn Y, Rottier R, Groenman FA, Tibboel D, de Krijger RR. Expression of angiogenesis-related factors in lungs of patients with congenital diaphragmatic hernia and pulmonary hypoplasia of other causes. Pediatr Dev Pathol. 2004;7:468–477. doi: 10.1007/s10024-003-0109-2. [DOI] [PubMed] [Google Scholar]
- 11.Dewachter L, Adnot S, Fadel E, Humbert M, Maitre B, Barlier-Mur AM, Simonneau G, Hamon M, Naeije R, Eddahibi S. Angiopoietin/Tie2 pathway influences smooth muscle hyperplasia in idiopathic pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1025–1033. doi: 10.1164/rccm.200602-304OC. [DOI] [PubMed] [Google Scholar]
- 12.Du L, Sullivan CC, Chu D, Cho AJ, Kido M, Wolf PL, Yuan JX, Deutsch R, Jamieson SW, Thistlethwaite PA. Signaling molecules in nonfamilial pulmonary hypertension. N Engl J Med. 2003;348:500–509. doi: 10.1056/NEJMoa021650. [DOI] [PubMed] [Google Scholar]
- 13.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 14.Galambos C, Ng YS, Ali A, Noguchi A, Lovejoy S, D’Amore PA, DeMello DE. Defective pulmonary development in the absence of heparin-binding vascular endothelial growth factor isoforms. Am J Respir Cell Mol Biol. 2002;27:194–203. doi: 10.1165/ajrcmb.27.2.4703. [DOI] [PubMed] [Google Scholar]
- 15.Goede V, Schmidt T, Kimmina S, Kozian D, Augustin HG. Analysis of blood vessel maturation processes during cyclic ovarian angiogenesis. Lab Invest. 1998;78:1385–1394. [PubMed] [Google Scholar]
- 16.Han RN, Babaei S, Robb M, Lee T, Ridsdale R, Ackerley C, Post M, Stewart DJ. Defective lung vascular development and fatal respiratory distress in endothelial NO synthase-deficient mice: a model of alveolar capillary dysplasia? Circ Res. 2004;94:1115–1123. doi: 10.1161/01.RES.0000125624.85852.1E. [DOI] [PubMed] [Google Scholar]
- 17.Hato T, Kimura Y, Morisada T, Koh GY, Miyata K, Tabata M, Kadomatsu T, Endo M, Urano T, Arai F, Araki K, Suda T, Kobayashi K, Oike Y. Angiopoietins contribute to lung development by regulating pulmonary vascular network formation. Biochem Biophys Res Commun. 2009;381:218–223. doi: 10.1016/j.bbrc.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 18.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 19.Karamanoukian HL, Glick PL, Zayek M, Steinhorn RH, Zwass MS, Fineman JR, Morin FC., 3rd Inhaled nitric oxide in congenital hypoplasia of the lungs due to diaphragmatic hernia or oligohydramnios. Pediatrics. 1994;94:715–718. [PubMed] [Google Scholar]
- 20.Kitagawa M, Hislop A, Boyden EA, Reid L. Lung hypoplasia in congenital diaphragmatic hernia. A quantitative study of airway, artery, and alveolar development. Br J Surg. 1971;58:342–346. doi: 10.1002/bjs.1800580507. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi H, Puri P. Plasma endothelin levels in congenital diaphragmatic hernia. J Pediatr Surg. 1994;29:1258–1261. doi: 10.1016/0022-3468(94)90818-4. [DOI] [PubMed] [Google Scholar]
- 22.Kugathasan L, Ray JB, Deng Y, Rezaei E, Dumont DJ, Stewart DJ. The angiopietin-1-Tie2 pathway prevents rather than promotes pulmonary arterial hypertension in transgenic mice. J Exp Med. 2009;206:2221–2234. doi: 10.1084/jem.20090389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leuwerke SM, Kaza AK, Tribble CG, Kron IL, Laubach VE. Inhibition of compensatory lung growth in endothelial nitric oxide synthase-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1272–L1278. doi: 10.1152/ajplung.00490.2001. [DOI] [PubMed] [Google Scholar]
- 24.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 26.Mochizuki Y, Nakamura T, Kanetake H, Kanda S. Angiopoietin 2 stimulates migration and tube-like structure formation of murine brain capillary endothelial cells through c-Fes and c-Fyn. J Cell Sci. 2002;115:175–183. doi: 10.1242/jcs.115.1.175. [DOI] [PubMed] [Google Scholar]
- 27.Muehlethaler V, Kunig AM, Seedorf G, Balasubramaniam V, Abman SH. Impaired VEGF and nitric oxide signaling after nitrofen exposure in rat fetal lung explants. Am J Physiol Lung Cell Mol Physiol. 2008;294:L110–L120. doi: 10.1152/ajplung.00407.2007. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura Y, Yamamoto I, Fukuda S, Hashimoto T. Pulmonary acinar development in diaphragmatic hernia. Arch Pathol Lab Med. 1991;115:372–376. [PubMed] [Google Scholar]
- 29.North AJ, Moya FR, Mysore MR, Thomas VL, Wells LB, Wu LC, Shaul PW. Pulmonary endothelial nitric oxide synthase gene expression is decreased in a rat model of congenital diaphragmatic hernia. Am J Respir Cell Mol Biol. 1995;13:676–682. doi: 10.1165/ajrcmb.13.6.7576705. [DOI] [PubMed] [Google Scholar]
- 30.Okoye BO, Losty PD, Fisher MJ, Wilmott I, Lloyd DA. Effect of dexamethasone on endothelial nitric oxide synthase in experimental congenital diaphragmatic hernia. Arch Dis Child Fetal Neonatal Ed. 1998;78:F204–F208. doi: 10.1136/fn.78.3.f204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oue T, Yoneda A, Shima H, Taira Y, Puri P. Increased vascular endothelial growth factor peptide and gene expression in hypoplastic lung in nitrofen induced congenital diaphragmatic hernia in rats. Pediatr Surg Int. 2002;18:221–226. doi: 10.1007/s003830100625. [DOI] [PubMed] [Google Scholar]
- 32.Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3:e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reale FR, Esterly JR. Pulmonary hypoplasia: a morphometric study of the lungs of infants with diaphragmatic hernia, anencephaly, and renal malformations. Pediatrics. 1973;51:91–96. [PubMed] [Google Scholar]
- 34.Shehata SM, Mooi WJ, Okazaki T, El-Banna I, Sharma HS, Tibboel D. Enhanced expression of vascular endothelial growth factor in lungs of newborn infants with congenital diaphragmatic hernia and pulmonary hypertension. Thorax. 1999;54:427–431. doi: 10.1136/thx.54.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shehata SM, Sharma HS, Mooi WJ, Tibboel D. Pulmonary hypertension in human newborns with congenital diaphragmatic hernia is associated with decreased vascular expression of nitric-oxide synthase. Cell Biochem Biophys. 2006;44:147–155. doi: 10.1385/CBB:44:1:147. [DOI] [PubMed] [Google Scholar]
- 36.Shinkai M, Shinkai T, Pirker ME, Montedonico S, Puri P. Effect of nitric oxide on the development of nitrofen-induced fetal hypoplastic lung explants. J Pediatr Surg. 2005;40:17–21. doi: 10.1016/j.jpedsurg.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Sirkin W, O’Hare BP, Cox PN, Perrin D, Cutz E, Silver MM. Alveolar capillary dysplasia: lung biopsy diagnosis, nitric oxide responsiveness, and bronchial generation count. Pediatr Pathol Lab Med. 1997;17:125–132. [PubMed] [Google Scholar]
- 38.Solari V, Piotrowska AP, Puri P. Expression of heme oxygenase-1 and endothelial nitric oxide synthase in the lung of newborns with congenital diaphragmatic hernia and persistent pulmonary hypertension. J Pediatr Surg. 2003;38:808–813. doi: 10.1016/jpsu.2003.50172. [DOI] [PubMed] [Google Scholar]
- 39.Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouché A, Yuce A, Fujisawa H, Hermans B, Shani M, Jansen S, Hicklin D, Anderson DJ, Gardiner T, Hammes HP, Moons L, Dewerchin M, Collen D, Carmeliet P, D’Amore PA. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002;109:327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steudel W, Ichinose F, Huang PL, Hurford WE, Jones RC, Bevan JA, Fishman MC, Zapol WM. Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (NOS 3) gene. Circ Res. 1997;81:34–41. doi: 10.1161/01.res.81.1.34. [DOI] [PubMed] [Google Scholar]
- 41.Stratmann A, Risau W, Plate KH. Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. Am J Pathol. 1998;153:1459–1466. doi: 10.1016/S0002-9440(10)65733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 43.Thibeault DW, Beatty EC, Jr, Hall RT, Bowen SK, O’Neill DH. Neonatal pulmonary hypoplasia with premature rupture of fetal membranes and oligohydramnios. J Pediatr. 1985;107:273–277. doi: 10.1016/s0022-3476(85)80148-7. [DOI] [PubMed] [Google Scholar]
- 44.Unger S, Copland I, Tibboel D, Post M. Down-regulation of sonic hedgehog expression in pulmonary hypoplasia is associated with congenital diaphragmatic hernia. Am J Pathol. 2003;162:547–5557. doi: 10.1016/S0002-9440(10)63848-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White AC, Lavine KJ, Ornitz DM. FGF9 and SHH regulate mesenchymal Vegfa expression and development of the pulmonary capillary network. Development. 2007;134:3743–3752. doi: 10.1242/dev.004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young SL, Evans K, Eu JP. Nitric oxide modulates branching morphogenesis in fetal rat lung explants. Am J Physiol Lung Cell Mol Physiol. 2002;282:L379–L385. doi: 10.1152/ajplung.00462.2000. [DOI] [PubMed] [Google Scholar]
- 47.Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest. 1997;99:2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu Y, Lee C, Shen F, Du R, Young WL, Yang GY. Angiopoietin-2 facilitates vascular endothelial growth factor-induced angiogenesis in the mature mouse brain. Stroke. 2005;36:1533–1537. doi: 10.1161/01.STR.0000170712.46106.2e. [DOI] [PubMed] [Google Scholar]


