Abstract
Background
3',4'-Dihydroxyflavonol (DiOHF) is an effective antioxidant that acutely preserves nitric oxide (NO) activity in the presence of elevated reactive oxygen species (ROS). We hypothesized that DiOHF treatment (7 days, 1 mg/kg per day s.c.) would improve relaxation in mesenteric arteries from diabetic rats where endothelial dysfunction is associated with elevated oxidant stress.
Methodology/Principal Findings
In mesenteric arteries from diabetic rats there was an increase in ROS, measured by L-012 and 2',7'-dichlorodihydrofluorescein diacetate fluorescence. NADPH oxidase-derived superoxide levels, assayed by lucigenin chemiluminescence, were also significantly increased in diabetic mesenteric arteries (diabetes, 4892±946 counts/mg versus normal 2486±344 counts/mg, n = 7–10, p<0.01) associated with an increase in Nox2 expression but DiOHF (2094±300 counts/mg, n = 10, p<0.001) reversed that effect. Acetylcholine (ACh)-induced relaxation of mesenteric arteries was assessed using wire myography (pEC50 = 7.94±0.13 n = 12). Diabetes significantly reduced the sensitivity to ACh and treatment with DiOHF prevented endothelial dysfunction (pEC50, diabetic 6.86±0.12 versus diabetic+DiOHF, 7.49±0.13, n = 11, p<0.01). The contribution of NO versus endothelium-derived hyperpolarizing factor (EDHF) to ACh-induced relaxation was assessed by evaluating responses in the presence of TRAM-34+apamin+iberiotoxin or N-nitro-L-arginine+ODQ respectively. Diabetes impaired the contribution of both NO (maximum relaxation, Rmax diabetic 24±7 versus normal, 68±10, n = 9–10, p<0.01) and EDHF (pEC50, diabetic 6.63±0.15 versus normal, 7.14±0.12, n = 10–11, p<0.01) to endothelium-dependent relaxation. DiOHF treatment did not significantly affect the EDHF contribution but enhanced NO-mediated relaxation (Rmax 69±6, n = 11, p<0.01). Western blotting demonstrated that diabetes also decreased expression and increased uncoupling of endothelial NO synthase (eNOS). Treatment of the diabetic rats with DiOHF significantly reduced vascular ROS and restored NO-mediated endothelium-dependent relaxation. Treatment of the diabetic rats with DiOHF also increased eNOS expression, both in total and as a dimer.
Conclusions/Significance
DiOHF improves NO activity in diabetes by reducing Nox2-dependent superoxide production and preventing eNOS uncoupling to improve endothelial function.
Introduction
Endothelial dysfunction, characterized by the impairment of endothelium-dependent relaxation, is recognised as a critical and initiating factor in the development of diabetes-induced vascular complications [1], [2]. Oral hypoglycaemic agents and insulin have been used to treat diabetes but they do not prevent the development of diabetic vascular complications [3], [4], [5]. In diabetes, hyperglycaemia-induced oxygen radical generation, mainly superoxide anion radicals, play a key role in the pathogenesis of vascular complications [6], [7], [8]. Despite this, clinical trials with antioxidants have failed to clearly demonstrate any beneficial effect on vascular function [9], [10]. More effective antioxidants, acting perhaps by targeting the specific sources of reactive oxygen species (ROS) might prove more beneficial than direct scavenging strategies [8], [11], [12]. Potential targets for pharmacological therapies include NADPH oxidase, endothelial nitric oxide synthase (eNOS) and mitochondria all of which have been reported to be sources of increased ROS in diabetes [2], [13].
Flavonols are one class of a large family of plant-derived polyphenolic compounds known as flavonoids. They exhibit a variety of biological actions such as antithrombotic, anti-inflammatory, antioxidant and vasorelaxant effects [14], [15]. There is growing evidence that consumption of a flavonoid-rich diet reduces the risk of cardiovascular disease states associated with overproduction of ROS [16], [17]. In addition, a number of studies have shown that, in animal models of diabetes, chronic treatment with the flavonol quercetin preserves endothelial function [18], reduces pancreatic beta-cell death [19] and protects against diabetic nephropathy [20].
Structure-activity relationship studies have demonstrated that a synthetic flavonol, 3',4'-dihydroxyflavonol (DiOHF) is significantly more potent than a number of natural flavones and flavonols in its antioxidant ability [21], [22]. In addition, the antioxidant ability of DiOHF has been shown to preserve endothelial function in the aorta in the presence of oxidative stress [23], [24]. Furthermore, DiOHF effectively reduces oxidative stress related impairment of cardiovascular function after ischaemia/reperfusion in rats [23] and sheep [25], [26] and in diabetic aorta [27]. Recently, we have shown the acute antioxidant activity of DiOHF is able to restore endothelial function in the diabetic microvasculature [28] but it is not known whether treatment with DiOHF is able to inhibit the sources of ROS production[29] in the diabetic microvasculature to improve endothelial function.
Diabetes-induced endothelial dysfunction is due to the impairment of both NO-mediated and endothelium-dependent hyperpolarizing factor (EDHF)-type relaxation, which is accompanied by an increase in Nox2-derived superoxide production and eNOS uncoupling in the mesenteric artery [30]. Therefore, the aim of the present study was to investigate whether short term in vivo treatment with DiOHF preserves microvascular endothelial function in mesenteric artery from type 1 diabetic rats and, if so, whether it acts via direct scavenging of ROS and/or by inhibiting the sources of ROS production in the diabetic microvasculature. In addition, we also sought to verify whether the antioxidant activity of DiOHF treatment would have any effect on NO-mediated relaxation and/or EDHF-type relaxation in the diabetic arteries.
Results
Body weights and blood glucose
The body weight gained, blood glucose and HbA1c levels of the rats are shown in Table 1. 8 weeks after treatment with streptozotocin or vehicle, the body weight gained in normal rats was significantly greater than in diabetic rats (Table 1). The blood glucose and HbA1c level of diabetic rats were significantly greater than normal rats. Treatment with DiOHF had no significant effect on body weight gain, blood glucose or HbA1c levels in normal rats, but significantly increased the body weight gain in diabetic rats. In comparison to DiOHF-treated normal rats, the diabetic rats that were treated with DiOHF had significantly lower body weight gain and significantly greater blood glucose and HbA1c level (Table 1).
Table 1. Mean body weight gained, blood glucose and glycated haemoglobin levels at the end of the experiment of normal and diabetic rats with or without 3′, 4′-dihydroxyflavonol (DiOHF, 1 mg/kg s.c. daily for 7 days) treatment.
| 8 weeks after vehicle or STZ treatment | ||||||||
| n | Normal | n | Normal+DiOHF | n | Diabetic | n | Diabetic+DiOHF | |
| Body weight gained (g) | 22 | 307±12 | 19 | 286±5 | 19 | 126±10* | 20 | 177±10† ‡ |
| Blood glucose (mM) | 22 | 8.3±0.6 | 19 | 7.3±0.6 | 19 | 32.4±0.3* | 20 | 30.2±0.9‡ |
| HbA1c (%) | 8 | 6.1±0.2 | 7 | 5.8±0.2 | 10 | 13.5±0.5* | 10 | 12.6±0.6‡ |
n = the number of rats. * Significantly different from normal group (Bonferroni's test, p<0.05), † Significantly different to diabetic group, p<0.05, Bonferroni's test. ‡ Significantly different to Normal+DiOHF group, p<0.05, Bonferroni's test. Results are shown as mean±SEM.
Effect of DiOHF on ROS production
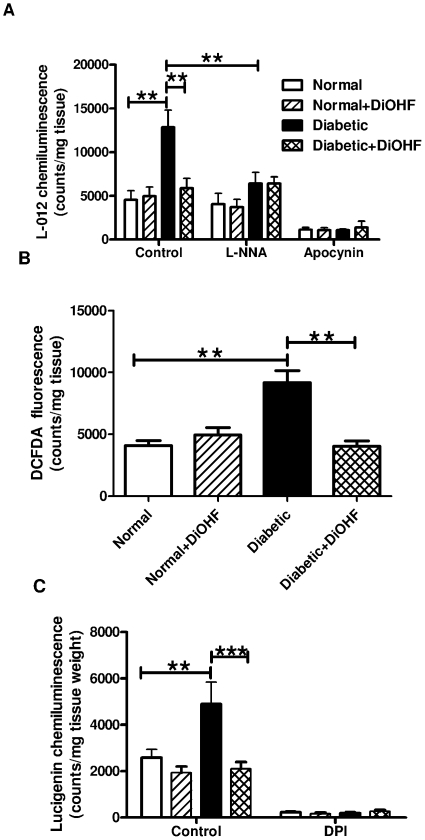
The ROS level in mesenteric arteries was measured by L-012 chemiluminescence and 2',7'-dichlorodihydrofluorescein diacetate (DCFDA) fluorescence. The superoxide and DCFDA-induced fluorescence levels from diabetic rats were significantly higher than in normal rats (Figure 1). 7 days treatment with DiOHF attenuated the generation of superoxide (Figure 1A) and DCFDA-induced fluorescence levels (Figure 1B) in diabetic rats, but had no effect in normal arteries. The presence of L-NNA attenuated the generation of superoxide in diabetic arteries, but had no effect in either normal or DiOHF-treated arteries. Apocynin, a ROS scavenger, attenuated the production of superoxide in arteries from all groups (Figure 1A).
Figure 1. ROS measurement in intact mesenteric arteries.
A, Superoxide, B, DCFDA-induced flurorescence levels and C, NADPH-oxidase activity was increased in diabetic arteries which was reduced with DiOHF treatment. A, Superoxide levels in diabetic arteries were attenuated by the presence of L-NNA (100 µM), indicating eNOS uncoupling. n = 9–10 experiments. * p<0.05, ** p<0.01, *** p<0.001.
The level of NADPH oxidase-driven superoxide production detected by L-012-enhanced chemiluminescence in mesenteric arteries from diabetic rats was significantly increased in comparison to normal rats (Figure 1C). DiOHF treatment of the rats did not affect superoxide production by mesenteric arteries from normal rats but significantly reduced levels generated by diabetic mesenteric arteries to levels observed from normal rats. In all groups, NADPH oxidase-driven superoxide production from mesenteric arteries could be inhibited by diphenyl iodonium, a flavoprotein inhibitor that inhibits NADPH oxidase (Figure 1C).
Effect of DiOHF on vascular function
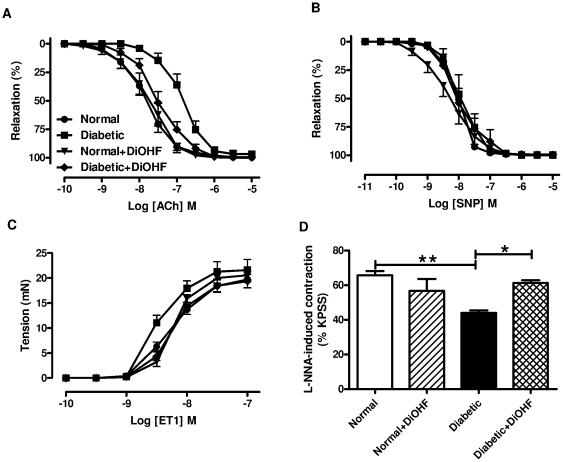
The level of the reference contraction, high K+ physiological saline solution (KPSS, 123 mmol/l), was not affected by diabetes or DiOHF treatment (Figure S1). Diabetes significantly reduced the sensitivity, but not the maximum relaxation, to ACh in mesenteric arteries (Figure 2A, Table 2), whereas the sensitivity (diabetic, 7.96±0.20 vs. normal, 7.89±0.16, n = 5–6, p>0.05) and maximum relaxation (diabetic, 100±0% vs. normal, 99±1%, n = 5–6, p>0.05) to sodium nitroprusside (SNP) were not affected (Figure 2B). Treatment of normal rats with DiOHF (1 mg/kg s.c.) for 7 days did not affect responses to ACh, however in mesenteric arteries from diabetic rats treated with DiOHF, the sensitivity to ACh was significantly increased in comparison to the response in mesenteric arteries from untreated diabetic rats (Figure 2, Table 2). DiOHF treatment did not affect responses to SNP in normal rats (pEC50 8.18±0.18, Rmax 100±0%, n = 9) or diabetic rats (pEC50 7.84±0.18, Rmax 99±1%, n = 6).
Figure 2. Vascular function in mesenteric arteries.
Cumulative concentration-response curves to A, ACh, B, SNP, C, ET1 and D, basal NO release in endothelium-intact mesenteric arteries. In each group of experiments (A, B), mesenteric arteries were precontracted with phenylephrine to similar level: (A) normal 59±3, normal+DiOHF 59±3, diabetic 62±2, diabeticDiOHF 60±3, (B) normal 58±2, normal+DiOHF 57±3, diabetic 61±3, diabeticDiOHF 59±4%KPSS, n = 5–12 experiments. Results are shown as mean±SEM. * p<0.05, ** p<0.01. See Table 2 or results section for pEC50 and Rmax values.
Table 2. Effect of L-NNA, ODQ and potassium channel blockers on ACh-induced relaxation of mesenteric arteries from normal and diabetic rats with or without 3′, 4′-dihydroxyflavonol (DiOHF, 1 mg/kg s.c. daily for 7 days) treatment in the presence of indomethacin.
| Normal | Normal + DiOHF | Diabetic | Diabetic + DiOHF | |||||||||
| n | pEC50 | Rmax (%) | n | pEC50 | Rmax (%) | n | pEC50 | Rmax (%) | n | pEC50 | Rmax (%) | |
| Control | 12 | 7.94±0.13 | 100±0 | 10 | 7.78±0.16 | 100±0 | 11 | 6.86±0.12† | 97±3 | 11 | 7.49±0.13§ | 100±0 |
| TRAM-34 + apamin | 12 | 7.26±0.19* | 91±3 | 9 | 6.93±0.22* | 92±2 | 11 | 6.86±0.16 | 66±8* † | 11 | 7.04±0.11* | 86±3* § |
| TRAM-34 + apamin+Ibtx | 10 | 6.83±0.15* | 68±10* | 9 | 6.84±0.19* | 71±11* | 10 | ND | 31±9* † | 11 | 6.98±0.12* | 69±6* § |
| 30 mM K+ | 8 | 6.91±0.10* | 78±4* | 9 | 7.28±0.11* † | 73±4* | 8 | 6.35±0.07* † | 52±5* † | 7 | 6.81±0.13* ‡ § | 70±2* § |
| L-NNA + ODQ | 12 | 7.14±0.12* | 100±0 | 10 | 7.07±0.15* | 98±1 | 10 | 6.63±0.15† | 97±1 | 11 | 6.85±0.12* | 99±1 |
| L-NNA + ODQ + TRAM-34 + apamin | 10 | 5.48±0.23* | 59±10* | 10 | 6.10±0.30* | 70±9* | 6 | ND | 1±1* † | 7 | ND | 2±2* ‡ |
| L-NNA + ODQ + TRAM-34 + apamin+Ibtx | 7 | ND | 3±3* | 5 | ND | 2±2* | ||||||
A comparison of the sensitivity (pEC50) and maximum relaxation (Rmax) to ACh in the absence (control), or in the presence of TRAM-34 (1 µM)+apamin (1 µM), TRAM-34 (1 µM)+apamin (1 µM) +Ibtx (100 nM), 30 mM K+, L-NNA (100 µM)+ODQ (10 µM), L-NNA (100 µM)+ODQ (10 µM)+TRAM-34 (1 µM)+apamin (1 µM) or L-NNA (100 µM)+ODQ (1 µM)+TRAM-34 (1 µM)+apamin (1 µM)+Ibtx (100 nM) in endothelium intact mesenteric arteries. All experiments were conducted in the presence of indomethacin (10 µM). n = the number of experiments.
*Significantly different to control within each group, p<0.05, Dunnet's test,
Significantly different to normal within inhibitor group, p<0.05, Bonferroni's test.
Significantly different to normal+DiOHF within inhibitor group, p<0.05, Bonferroni's test.
Significantly different to diabetic within inhibitor group, p<0.05, Bonferroni's test. Results are shown as mean±SEM, ND = Not determined.
The diabetic arteries also showed a significant increase in sensitivity to endothelin-1 (ET-1, diabetic, 8.46±0.07 vs. normal, 8.19±0.06, n = 7–8, p<0.05), without affecting the maximum contraction. Treatment with DiOHF had no effect in normal arteries (normal+DiOHF, 8.16±0.03, n = 7), but significantly reduced the sensitivity to ET-1 in diabetic arteries in comparison to untreated diabetic arteries (diabetic, 8.46±0.07 vs. diabetic+DiOHF, 8.20±0.07, n = 7–8, p<0.05), (Figure 2C).
Relative contribution of NO and EDHF to endothelium-dependent relaxation
In normal mesenteric arteries, ACh-induced relaxation could be partly inhibited by either the combination of a NO synthase inhibitor, N-nitro-L-arginine (L-NNA) and a soluble guanylate cyclase inhibitor, 1H-(1,2,4)-oxadiazolo(4,2-a)quinoxalin-1-one (ODQ), or KCa channels inhibitors, 1-[(2-chlorophenyl)(diphenyl)methyl]-1H-pyrazole (TRAM-34), apamin and iberiotoxin (Ibtx), to block the intermediate-conductance calcium-activated K+ channel (IKCa),small-conductance calcium-activated K+ channel (SKCa) and large-conductance calcium-activated K+ channel (maxi KCa) respectively, indicating that both NO and EDHF contributed to endothelium-dependent relaxation. However, in diabetic arteries, ACh-induced relaxation could only be inhibited by the KCa channels inhibitors, suggesting that EDHF was the predominant contributor to endothelium-dependent relaxation in diabetes. In the presence of both NO and EDHF inhibitors, endothelium-dependent relaxation were abolished in all groups of rats (Table 2).
Effect of DiOHF on NO-mediated relaxation
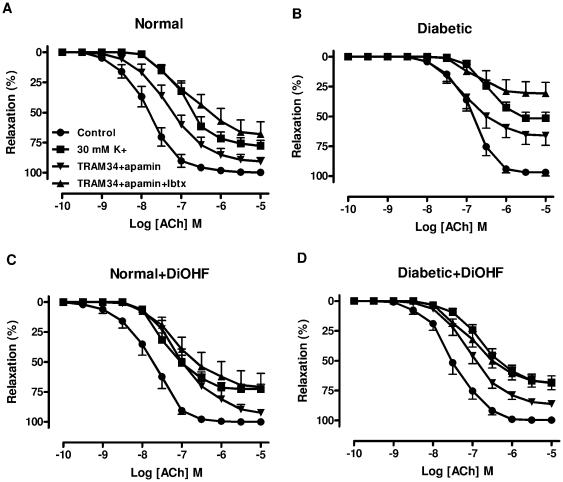
To determine the NO-mediated component of the relaxation, responses to ACh were evaluated in the presence of TRAM-34+apamin or TRAM-34+apamin+Ibtx. When these responses were compared between arteries from normal (Figure 3A, Table 2) and diabetic (Figure 3B, Table 2) rats, it is apparent that diabetes decreased the maximum relaxation to ACh in the presence of TRAM-34+apamin (diabetic 66±8% vs. normal, 91±3%, n = 11–12, p<0.05). A similar difference was also apparent in the presence of TRAM-34+apamin+Ibtx (diabetic 31±9% vs. normal, 68±10%, n = 10, p<0.05), indicating that diabetes impaired the contribution of NO to endothelium-dependent relaxation. Treatment with DiOHF (1 mg/kg s.c.) for 7 days had no effect in normal rats but significantly increased the maximum relaxation to ACh (diabetic 66±8% vs. diabetic+DiOHF, 86±3%, n = 11, p<0.05) in the presence of TRAM-34+apamin (Figure 3B, D, Table 2). A similar finding was observed in DiOHF-treated diabetic arteries (Figure 3B, D, Table 2) in the presence of TRAM-34+apamin+Ibtx (diabetic 31±9% vs. diabetic+DiOHF, 69±6%, n = 11, p<0.05), indicating that DiOHF treatment improved the contribution of NO to endothelium-dependent relaxation in diabetic arteries. In addition, in arteries pre-contracted with a depolarizing solution of 30 mM K+, to eliminate any contribution of the opening of potassium channels, ACh-induced relaxation was significantly attenuated in diabetic arteries in comparison to normal arteries, but this response was significantly improved by 7 days treatment with DiOHF in diabetic rats (Figure 3, Table 2).
Figure 3. Contribution of NO to endothelium-dependent relaxation in mesenteric arteries.
NO-mediated relaxation in mesenteric arteries isolated from A, normal, B, diabetic, C, normal+DiOHF, D, diabetic+DiOHF rats. In each group of experiments, arteries were precontracted to similar level: A, 57±3, B, 58±2, C, 61±4, D, 59±2%KPSS, n = 9–15 experiments. Results are shown as mean±SEM. See Table 2 for values.
The basal level of NO release was assessed by measuring the contraction induced by L-NNA in arteries with PE-induced tone (i.e. 20% KPSS) (Figure 2D). The L-NNA-induced contraction was significantly greater in arteries from normal rats compared with diabetic rats (normal, 66±2% vs. diabetic, 44±2%, n = 6–7, p<0.05), indicating that diabetes impaired the basal release of NO. Treatment with DiOHF had no effect on L-NNA-induced contraction in normal arteries (normal+DiOHF, 57±7%), but significantly increased the L-NNA-induced contraction in diabetic arteries in comparison to untreated diabetic arteries (diabetic+DiOHF, 61±2% vs. diabetic, 44±2%, n = 5–6, p<0.05).
Effect of DiOHF on EDHF-type relaxation
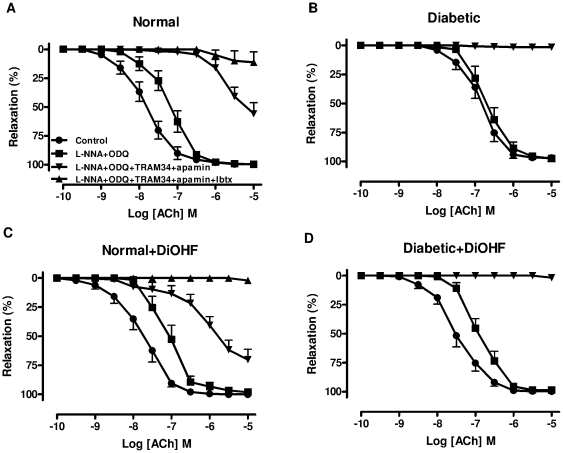
To characterise EDHF-type relaxation, the contribution of NO was eliminated by the combination of L-NNA and ODQ. In the presence of L-NNA+ODQ, the sensitivity to ACh was significantly lower in the diabetic compared to the normal arteries (diabetic, 6.63±0.15 vs. normal, 7.14±0.12, n = 10–12, p<0.05), indicating that diabetes impairs the contribution of EDHF to endothelium-dependent relaxation. Treatment of normal and diabetic rats with DiOHF (1 mg/kg s.c.) for 7 days had no significant effect on ACh-mediated EDHF-type relaxation (Table 2).
The addition of TRAM-34 and apamin to the presence of L-NNA+ODQ, abolished ACh-induced relaxation in either untreated diabetic or DiOHF-treated diabetic arteries. In normal or DiOHF-treated normal arteries, however, ACh continued to cause a maximum relaxation of almost 60% (Figure 4, Table 2). The residual relaxation observed in normal and DiOHF-treated normal arteries could be abolished by the additional presence of Ibtx (Figure 4A, C, Table 2), suggesting an additional role of other endothelium-derived factors to cause relaxation in normal arteries, but not in diabetes.
Figure 4. Contribution of EDHF to endothelium-dependent relaxation in mesenteric arteries.
EDHF-type relaxation in mesenteric arteries isolated from A, normal, B, diabetic, C, normal+DiOHF, D, diabetic+DiOHF rats. In each group of experiments, arteries were precontracted with phenylephrine to similar level: A, 62±3, B, 63±4, C, 63±4, D, 61±2%KPSS, n = 5–12 experiments. Results are shown as mean±SEM. See Table 2 for values.
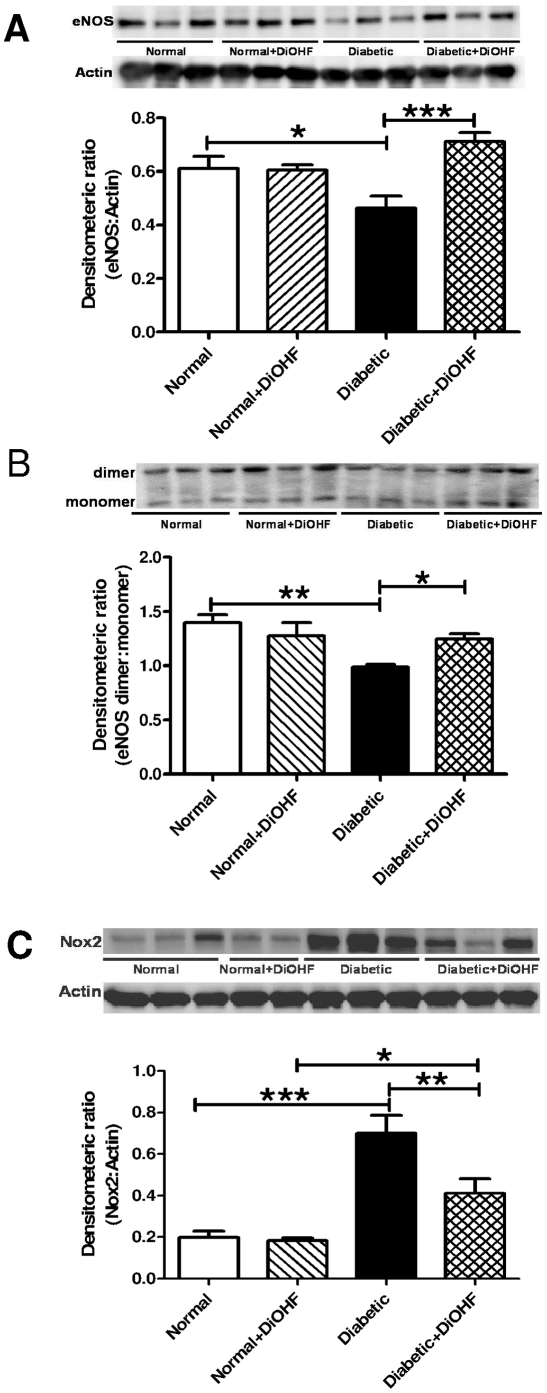
Effect of DiOHF on Nox2 and NOS expression, and eNOS uncoupling
Diabetes significantly decreased the expression of total eNOS in the mesenteric arteries but this was reversed by treatment with DiOHF (Figure 5A). In addition, diabetes significantly decreased the proportion of eNOS expressed as a dimer. Treatment of diabetic rats with DiOHF significantly increased the proportion of eNOS expressed as a dimer (Figure 5B). The expression of Nox2 was also significantly increased in diabetic arteries compared to normal arteries. In DiOHF-treated diabetic arteries, Nox2 expression was significantly reduced, but it remained significantly higher in comparison to DiOHF-normal arteries (Figure 5C). Treatment with DiOHF in normal rats had no effect on eNOS and Nox2 expression and inducible-NOS expression was not detected in any group (data not shown).
Figure 5. Western blot analysis of protein expression.
Western blot of A, eNOS (130 kDa), B, eNOS dimers and monomers (260 kDa) and C, Nox2 (58 kDa) in the normal and diabetic mesenteric arteries with or without DiOHF treatment. Diabetes significantly reduced the expression of eNOS and decreased the proportion of eNOS expressed as the dimer, and increased the expression of Nox2. Treatment with DiOHF significant increased the expression of eNOS, decreased the expression of Nox2 and increased the proportion of eNOS expressed as the dimer. Representative blots were shown on each corresponding graphs. n = 5–6 experiments. Results are shown as mean±SEM. * p<0.05, ** p<0.01, *** p<0.001.
Discussion
This study demonstrates that treatment of type 1 diabetic rats with the synthetic flavonol DiOHF (1mg/kg, per day) for 7 days reduces the levels of vascular oxidative stress and improves endothelium-dependent relaxation in mesenteric arteries. Endothelial dysfunction in the diabetic rats is associated with a decreased contribution of both NO-mediated and EDHF-type relaxation to endothelium-dependent relaxation. Treatment with DiOHF improves NO-mediated relaxation in diabetic rats accompanied by an increased expression of eNOS, reduced eNOS uncoupling and downregulation of Nox2 expression.
Effect of DiOHF on endothelial function
In the present study, diabetes increases the level of vascular ROS production, associated with an increase in Nox2 expression and a selective impairment of endothelium-dependent relaxation, as we and others have previously described [1], [30], [31], [32]. Treatment with the synthetic flavonol DiOHF (1mg/kg, per day) for 7 days in vivo reduces the levels of oxidative stress, at least in part by inhibiting superoxide production by Nox2 and uncoupled eNOS in the diabetic mesenteric arteries, and improves endothelium-dependent relaxation in mesenteric arteries from diabetic rats. These observations are consistent with other studies that have demonstrated that treatment with flavonols in diabetic rats reduces vascular oxidant stress and preserves endothelial function in conduit arteries such as the aorta [18], [27] or renal vasculature [20]. Whilst there are several reports that flavonols attenuate diabetes-induced endothelial dysfunction, the mechanism of the vasoprotective effect of flavonols remain poorly understood, in particular the effect of flavonols on the relative contribution of NO and EDHF to endothelium-dependent relaxation.
Effects of DiOHF on NO-mediated relaxation
In order to evaluate NO-mediated relaxation, the EDHF-type relaxation was inhibited either by endothelial KCa channel blockers or by the presence of a depolarizing K+ solution. This confirmed an impaired release of NO, as under those conditions the maximum relaxation to ACh was decreased in the diabetic mesenteric arteries in comparison to normal arteries. Treatment with DiOHF in vivo for 7 days significantly increased the maximum relaxation to ACh in diabetic arteries in comparison to untreated diabetic rats. In addition to stimulated NO release, basal release of NO was also decreased in diabetic mesenteric arteries as demonstrated by impaired contraction in response to NOS inhibition, and this was also reversed in DiOHF treated diabetic arteries. Hence, treatment with DiOHF preserves NO-mediated relaxation in diabetic small mesenteric arteries.
Consistent with impairment of NO-mediated relaxation, we found that the expression of total eNOS was decreased and uncoupling of eNOS was indicated by the decreased proportion of eNOS expressed as a dimer in the diabetic mesenteric vasculature. This suggests that in the diabetic rats, in addition to producing NO, eNOS is producing superoxide, further demonstrated by a reduction of superoxide levels in the diabetic mesenteric arteries in the presence of NOS inhibition [13], [33]. However, in DiOHF-treated diabetic rats, the expression of eNOS was increased to levels that were comparable to normal rats. In addition, treatment with DiOHF promoted the re-coupling of eNOS, which was shown by the increased proportion of eNOS expressed as a dimer compared to untreated diabetic rats and the lack of inhibitory effect of L-NNA on superoxide production in DiOHF-treated arteries.
A further contributing factor to the restoration of NO activity by DiOHF treatment could be the reduction of vascular superoxide production in diabetic mesenteric arteries, which could then increase the bioavailability of NO by preventing the degradation of NO by superoxide to form peroxynitrite. The reduction of vascular ROS activity in the DiOHF-treated diabetic vasculature could be either due to the rapid free radical scavenging effect of DiOHF to preserve NO bioavailability [23] or by decreasing the expression and/or activity of the enzymatic sources of superoxide production in the vasculature such as the catalytic subunit of NADPH oxidases, Nox2 and its regulatory subunits [34]. Taken together, DiOHF treatment protects the beneficial activity of NO by increasing eNOS expression, preventing eNOS uncoupling and decreasing Nox2-dependent superoxide production in the diabetic mesenteric arteries.
Effect of DiOHF on EDHF-type relaxation
In the rat mesenteric artery, endothelium-dependent relaxation is mediated by NO, the classical EDHF pathway and there is also a role for the non-classical EDHF pathway [35]. To investigate the role of EDHF, we assessed endothelium-dependent relaxation in the presence of L-NNA and ODQ to inhibit NO synthesis and sGC activity respectively. The guanylate cyclase inhibitor was used in addition to the NOS inhibitor to ensure the inhibition of the actions of NO derived from non-NOS sources such as nitrosothiols, which we have previously reported to act as a NO source in diabetes [33]. In the presence of L-NNA+ODQ, the sensitivity to ACh was decreased in diabetic arteries when compared to normal arteries, indicating that diabetes impaired of the contribution of EDHF-type relaxation, which is consistent with several other studies [36], [37], [38]. Treatment with DiOHF caused a modest improvement in EDHF-type relaxation in diabetic mesenteric arteries, but the change was not statistically significant. It should be noted however that whereas ACh-induced relaxation in the presence of L-NNA+ODQ was impaired in diabetes compared to normal arteries, under the same conditions there was no significant difference in the responses to ACh in arteries from normal and diabetic treated with DiOHF. Thus, the evidence is equivocal as to whether DiOHF improves EDHF-type relaxation in the diabetic mesenteric arteries.
The cause of the impairment of EDHF-type relaxation in diabetes remains controversial [30], [37], [39], [40], [41], [42] but is partly attributed to an overproduction of ROS. Indeed, Ma et al., (2008) demonstrated that superoxide anions generated by auto-oxidation of pyrogallol could impair EDHF responses in rat mesenteric arteries, an effect which could be reversed by the acute presence of the flavones, apigenin and/or luteolin, in the tissue bath [43]. In the present study, it is important to note that the final administration of the DiOHF was more than 24 hours before the conduct of all experiments. Although there appears to be no information available regarding the pharmacokinetics of flavonols in rats, in humans it is reported that quercetin reaches peak plasma concentrations within 2–5 hours after oral ingestion and is predominantly excreted in the urine within 5–12 hours [44], [45]. Given the structural similarities between quercetin and DiOHF, it is unlikely that there will be any acute effect of DiOHF during experimentation. Therefore, the in vivo effect of DiOHF on EDHF-type relaxation remains to be elucidated.
Conclusion
We have demonstrated that the synthetic flavonol DiOHF increases NO activity to improve endothelial function in diabetic microvasculature. It was less certain as to whether DiOHF treatment had a beneficial effect on the EDHF-type component of endothelium-dependent relaxation. The protective actions of DiOHF occur through at least two mechanisms. The flavonol is able to rapidly scavenge ROS and/or inhibit the enzymatic source for superoxide production to reduce inactivation of NO. In addition, treatment with DiOHF in vivo for 7 days prevents eNOS uncoupling and thus helps to maintain endothelium-dependent relaxation. The beneficial effect of DiOHF to protect NO activity found in this study indicates that it has potential as a therapeutic agent for use in the prevention of the microvascular complications of diabetes.
Methods
Ethics statement: All procedures were approved by the Animal Experimentation Ethics Committee of RMIT University (approval number 0822) and conformed to the National Health and Medical Research Council of Australia code of practice for the care and use of animals for scientific purposes.
Induction of diabetes
Male 6–8 week old Wistar rats weighing approximately 200 g (Animal Resource Centre, Perth, WA, Australia) were randomly divided into two groups: normal and diabetic. Type 1 diabetes was induced by a single injection of streptozotocin (STZ, 50 mg/kg) into the tail vein after the rats were fasted overnight. The control groups received an equivalent volume of the vehicle (0.1 mol/l citrate buffer, pH 4.5) alone. Once the rats were rendered diabetic (blood glucose >25 mmol/l) the following week after STZ injection, all diabetic rats were maintained on a low insulin dose (4–5 IU, s.c., 3 injections per week, Protaphane, Novo Nordisk, NSW, Australia) to promote weight gain and reduce mortality [46]. At the end of the experimental period, blood samples were obtained from the left ventricle and the glucose concentration and glycated haemogloblin (HbA1c) were measured using a one touch glucometer (Roche, Sydney, NSW, Australia) and Micromat HbA1c analyser (Biorad, Sydney, NSW, Australia) respectively.
DiOHF treatment
After seven weeks of STZ-induced diabetes, the 2 groups of rats were further divided into 2 groups (Normal, Normal+DiOHF, Diabetic, Diabetic+DiOHF) receiving either vehicle (10% DMSO+90% peanut oil) or (DiOHF, 1mg/kg s.c. per day) for a period of 7 days. The last dose of DiOHF was administered at least 24 hours prior to the start of experimentation.
Isolation of mesenteric arteries
Eight weeks after STZ treatment, the rats were killed with pentobarbitone sodium (325 mg/kg, i.p, Virbac, Australia). The mesenteric arcade was isolated and immediately placed in ice cold Krebs bicarbonate solution (118 mmol/l NaCl, 4.7 mmol/l KCl, 1.18 mmol/l MgSO4, 1.2 mmol/l KH2PO4, 25 mmol/l NaHCO3, 11.1 mmol/l D-glucose, and 1.6 mmol/l CaCl2) containing indomethacin (10 µmol/l), a non-selective cyclo-oxygenase (COX) inhibitor, to inhibit the synthesis of prostanoids. Our preliminary data suggested that there is no significant contribution of prostanoids to endothelium-dependent in mesenteric arteries from all groups of rats (data not shown). Small mesenteric arteries (third-order branch of the superior mesenteric artery, internal diameter ∼300 µm) were isolated, cleared of fat and connective tissue, cut into 2 mm long rings and mounted on a Mulvany-Halpern myograph (model 610M, Danish Myo Technology, Aarhus, Denmark). After the arteries were mounted, the vessels were allowed to stabilize at zero tension for 15 min before normalisation. The passive tension-internal circumference was determined by stretching to achieve an internal circumference equivalent to 90% of that of the blood vessel under a transmural pressure of 100 mmHg [47], [48]. All experiments were performed at 37°C and the baths were bubbled with carbogen (95% O2 and 5% CO2).
Assessment of vascular reactivity
Thirty minutes after normalization, vessels were maximally contracted with KPSS (123 mmol/l). After several washouts using normal Krebs solution, basal tension was regained. To assess the integrity of the endothelium, mesenteric arteries were precontracted to ∼50–60% of the KPSS response with phenylephrine (0.1–3 µmol/l) and a high concentration of acetylcholine (ACh, 10 µmol/l) was used to relax the artery rings. ACh-induced relaxation was greater than 80% of the precontracted tone in all cases, indicating that the endothelium was functionally intact. After further washouts, arteries were again precontracted to a similar level using phenylephrine (0.1–3 µmol/l) or in some cases 30 mmol/l K+, and cumulative concentration-response curves to ACh (0.1 nmol/l–10 µmol/l) and SNP (0.01 nmol/l–10 µmol/l) were determined. In addition, responses to ACh and SNP were examined after 20 minutes incubation with different combinations of L-NNA (100 µmol/l), a non-selective nitric oxide synthase (NOS) inhibitor, ODQ (10 µmol/l), a soluble guanylate cyclase (sGC) inhibitor, TRAM-34 (1 µmol/l), a selective blocker of the intermediate-conductance calcium-activated K+ channel (IKCa or KCa3.1), ibtx (100 nmol/l), a selective blocker of maxi KCa and apamin (1 µmol/l), a SKCa inhibitor.
To evaluate the constrictor reactivity, cumulative concentration-response curves to ET-1 (0.1 nmol/l–0.1 µmol/l) were constructed in the absence of indomethacin.
Assessment of basal release of NO in mesenteric arteries
In another separate set of experiments, the effect of diabetes on basal levels of NO release was also examined in the absence of indomethacin through the addition of L-NNA (100 µmol/l) in endothelium-intact rings precontracted with phenylephrine (10–100 nmol/l) to approximately ∼20% KPSS. Under those conditions a contractile response to L-NNA was considered to reflect the level of the basal release of NO [30].
Western Blot
Western blots were performed as described previously [30] with the following modifications. Endothelium-intact mesenteric arteries from 2 animals from the same treatment group were pooled and considered as n = 1. Equal amounts of protein homogenate were subjected to SDS-PAGE and western blot analysis with mouse/rabbit primary antibodies (all 1∶1000, overnight, 4°C) against endothelial NO synthase (eNOS), inducible-NOS, Nox2 (all BD Transduction Laboratories, Lexington, KY, USA). To normalize for the amount of protein, membranes were reprobed with a loading control antibody (actin). All proteins were detected by enhanced chemiluminescence (Amersham, GE Healthcare, Sydney, NSW, Australia) after incubation with anti-mouse/rabbit secondary antibody (Millipore, Billerica, MA, USA) for 1 hour at room temperature (1∶2000). All protein bands were quantified by densitometry (Biorad Chemidoc, Sydney, NSW, Australia) and expressed as a ratio of the loading control. To investigate eNOS homodimer formation in the tissue, a non-boiled sample was resolved by 6% SDS-PAGE at 4°C [49], and the membranes were probed and visualized as described above.
Measurement of ROS in mesenteric artery
Two different methods of ROS measurement were employed. Superoxide production in the mesenteric artery was measured using L-012 as previously described [30]. Mesenteric arteries were incubated at 37°C for 30 minutes in Krebs-HEPES buffer either alone, in the presence of apocynin, a ROS scavenger [50] (300 µmol/l) or L-NNA (100 µmol/l), which determines NOS-derived superoxide production. 300 µL of Krebs-HEPES buffer, containing L-012 (100 µmol/l, Wako Pure Chemicals, Osaka, Japan) and the appropriate treatments were placed into a 96-well Optiplate, which was loaded into a Polarstar Optima photon counter (BMG Labtech, Melbourne, VIC, Australia) to measure background photon emission at 37°C. After background counting was completed, a single ring segment of mesenteric artery was added to each well and photon emission was re-counted. Reactive oxygen species (H2O2) were measured with DCFDA [51] with the following modification. Rings of mesenteric artery were loaded with DCFDA solution (10 µmol/l) for 60 min, followed by 3 washes in Krebs-HEPES buffer. Background fluorescence was measured after excitation at 485 nm and emission at 520 nm. After being rinsed three times with Krebs buffer to remove excess probe, a single segment of the mesenteric artery was added to each well, and fluorescence intensity was recounted. The luminescence and fluorescence counts were normalized with dry tissue weight.
NADPH oxidase activity
NADPH oxidase-driven superoxide production in the mesenteric artery was measured using lucigenin-enhanced chemiluminescence. Mesenteric arteries were preincubated for 45 min at 37°C in Krebs–HEPES buffer containing diethylthiocarbamic acid (1 mmol/l), to inactivate superoxide dismutase, and NADPH (100 µmol/l) as a substrate for NADPH oxidase, and either alone or in the presence of diphenylene iodonium (5 µmol/l), as a flavoprotein inhibitor that inhibits NADPH oxidase. 300 µL of Krebs-HEPES buffer containing lucigenin (5 µmol/l) and the appropriate treatments were placed into a 96-well Optiplate, and superoxide production was measured and quantified as previously described [27].
Reagents
All drugs were purchased from Sigma-Aldrich (St Louis, MO, USA), except for acetylcholine perchlorate (BDH Chemicals, Poole, Dorset, UK), DiOHF (Indofine Chemicals, Hillsborough, NJ, USA) and ODQ (Cayman Chemical, Ann Arbor, MI, USA). All drugs were all dissolved in distilled water, with the exception of indomethacin, which was dissolved in 0.1 mol/l sodium carbonate, L-NNA, which was dissolved in 0.1 mol/l sodium bicarbonate, ODQ and TRAM-34, which were dissolved in dimethyl sulfoxide (DMSO).
Statistical analyses
All results are expressed as the mean±s.e.m., n represents the number of animals per group or the number of assays when tissue from animals was pooled. Concentration-response curves from rat isolated mesenteric arteries were computer fitted to a sigmoidal curve using nonlinear regression (Prism version 5.0, GraphPad Software, San Diego, CA, USA) to calculate the sensitivity of each agonist (pEC50). Maximum relaxation (Rmax) to ACh or SNP was measured as a percentage of precontraction to phenylephrine. Group pEC50 and Rmax values were compared by one-way ANOVA with post-hoc analysis using Dunnett's test or Bonferroni's selected comparison test (normal vs. normal+DiOHF, normal vs. diabetic, normal+DiOHF vs. diabetic+DiOHF and diabetic vs. diabetic+DiOHF) as appropriate. P<0.05 was considered statistically significant.
Supporting Information
KPSS induced maximum contraction in mesenteric arteries. Exposure of mesenteric arteries from normal and diabetic rats with or without 3′, 4′-dihydroxyflavonol (DiOHF, 1 mg/kg s.c. daily for 7 days) treatment to high K+ physiological saline solution (KPSS, 123 mmol/l). The contraction to KPSS was not affected by diabetes or DiOHF treatment. Results are shown as mean±SEM. NS = not significant.
(TIF)
Acknowledgments
C.H. Leo is a recipient of RMIT University International Research Scholarship. The authors thank Ms Priya Sivakumaran and Mr Indrajeetsinh Rana for their assistance in the induction of diabetes in rats.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: C.H. Leo is a recipient of RMIT University International Research Scholarship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.De Vriese AS, Verbeuren TJ, Van de Voorde J, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fatehi-Hassanabad Z, Chan CB, Furman BL. Reactive oxygen species and endothelial function in diabetes. Eur J Pharmacol. 2010;636:8–17. doi: 10.1016/j.ejphar.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 3.Holman RR, Paul SK, Bethel A, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in tpe 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown A, Reynolds R, Bruemmer D. Intensive glycemic control and cardiovascular disease: an update. Nat Rev Cardiol. 2010;7:369–375. doi: 10.1038/nrcardio.2010.35. [DOI] [PubMed] [Google Scholar]
- 6.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 7.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 8.Forstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med. 2008;5:338–349. doi: 10.1038/ncpcardio1211. [DOI] [PubMed] [Google Scholar]
- 9.Lonn E, Yusuf S, Hoogwerf B, Pogue J, Yi Q, et al. Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: Results of the HOPE study and MICRO-HOPE substudy. Diabetes Care: 2002;25:1919–1927. doi: 10.2337/diacare.25.11.1919. [DOI] [PubMed] [Google Scholar]
- 10.Song Y, Cook NR, Albert CM, Denburgh MV, Manson JE. Effects of vitamin C and E and β-carotene on the risk of type 2 diabetes in woman at high risk of cardiovascular disease: a randomized controlled trial. Am J Clin Nutr. 2009;90:429–437. doi: 10.3945/ajcn.2009.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansen J, Harris A, Rychly D, Ergul A. Oxidative stress and the use of antioxidants in diabetes: Linking basic science to clinical practice. Cardiovasc Diabetology. 2005;4:1–11. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willcox BJ, Curb JD, Rodriguez BL. Antioxidants in cardiovascular health and disease: key lessons from epidemiologic studies. Am J Cardiol. 2008;101:75D–86D. doi: 10.1016/j.amjcard.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Hink U, Li H, Mollnau H, Oelze M, Matheis E, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:14–22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 14.Woodman OL, Chan ECH. Vascular and anti-oxidant actions of flavonols and flavones. Clin Exp Pharmacol Physiol. 2004;31:786–790. doi: 10.1111/j.1440-1681.2004.04072.x. [DOI] [PubMed] [Google Scholar]
- 15.Yap S, Qin CX, Woodman OL. Effects of resveratrol and flavonols on cardiovascular function: Physiological mechanisms. Biofactors. 2010;36:350–359. doi: 10.1002/biof.111. [DOI] [PubMed] [Google Scholar]
- 16.Engler MB, Engler MM. The emerging role of flavonoid-rich cocoa and chocolate in cardiovascular health and disease. Nutr Rev. 2006;64:109–118. doi: 10.1111/j.1753-4887.2006.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 17.Geleijnse JM, Launer LJ, Van der Kuip DA, Hofman A, Witteman JC. Inverse association of tea and flavonoid intakes with incident myocardial infarction: the Rotterdam Study. Am J Clin Nutr. 2002;75:880–886. doi: 10.1093/ajcn/75.5.880. [DOI] [PubMed] [Google Scholar]
- 18.Machha A, Achike FI, Mustafa AM, Mustafa RM. Quercetin, a flavonoid, modulates endothelium-derived nitric oxide bioavailability in diabetic rat aortas. Nitric Oxide. 2007;16:441–447. doi: 10.1016/j.niox.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Coskun O, Kanter M, Korkmaz A, Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol Res. 2005;51:117–123. doi: 10.1016/j.phrs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Anjaneyulu M, Chopra K. Quercetin, an anti-oxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2004;31:244–248. doi: 10.1111/j.1440-1681.2004.03982.x. [DOI] [PubMed] [Google Scholar]
- 21.Woodman OL, Meeker WF, Boujaoude M. Vasorelaxant and antioxidant activity of flavonols and flavones: Structure-activity relationships. J Cardiovasc Pharmacol. 2005;46:302–309. doi: 10.1097/01.fjc.0000175431.62626.07. [DOI] [PubMed] [Google Scholar]
- 22.Chan EC, Pannangpetch P, Woodman OL. Relaxation to flavones and flavonols in rat isolated thoracic aorta: mechanism of action and structure-activity relationships. J Cardiovasc Pharmacol. 2000;35:326–333. doi: 10.1097/00005344-200002000-00023. [DOI] [PubMed] [Google Scholar]
- 23.Chan EC, Drummond GR, Woodman OL. 3′, 4'-Dihydroxyflavonol enhances nitric oxide bioavailability and improves vascular function after ischemia and reperfusion injury in the rat. J Cardiovasc Pharmacol. 2003;42:727–735. doi: 10.1097/00005344-200312000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Qin CX, Chen XQ, Hughes RA, Williams SJ, Woodman OL. Understanding the cardioprotective effects of flavonols: Discovery of relaxant flavonols without antioxidant activity. J Med Chem. 2008;51:1847–1884. doi: 10.1021/jm070352h. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Dusting GJ, May CN, Woodman OL. 3',4'-Dihydroxyflavonol reduces infarct size and injury associated with myocardial ischaemia and reperfusion in sheep. Br J Pharmacol. 2004;142:443–452. doi: 10.1038/sj.bjp.0705815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang S, Thomas CJ, Dusting GJ, Woodman OL, May CN. 3′,4′-Dihydroxyflavonol improves post-ischaemic coronary endothelial function following 7 days reperfusion in sheep. Eur J Pharmacol. 2009;624:31–37. doi: 10.1016/j.ejphar.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Woodman OL, Malakul W. 3′,4′-Dihydroxyflavonol prevents diabetes-induced endothelial dysfunction in rat aorta. Life Sci. 2009;85:54–59. doi: 10.1016/j.lfs.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Leo CH, Hart JL, Woodman OL. 3′,4′-dihydroxyflavonol restores endothelium dependent relaxation in small mesenteric artery from rats with type 1 and type 2 diabetes. Eur J Pharmacol In Press. 2011 doi: 10.1016/j.ejphar.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Mladenka P, Zatloukalova L, Filipsky T, Hrdina R. Cardiovascular effects of flavonoids are not caused only by direct antioxidant activity. Free Rad Biol Med. 2010;49:963–975. doi: 10.1016/j.freeradbiomed.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Leo CH, Hart JL, Woodman OL. Impairment of both nitric oxide-mediated and EDHF-type relaxation in small mesenteric arteries from rats with streptozotocin-induced diabetes. Br J Pharmacol. 2011;162:365–377. doi: 10.1111/j.1476-5381.2010.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding H, Hashem M, Wiehler WB, Lau W, Martin J, et al. Endothelial dysfunction in the streptozotocin-induced diabetic apoE-deficient mouse. Br J Pharmacol. 2005;146:1110–1118. doi: 10.1038/sj.bjp.0706417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malakul W, Thirawarapan S, Suvitayavat W, Woodman OL. Type 1 diabetes and hypercholesterolaemia reveal the contribution of endothelium-derived hyperpolarizing factor to endothelium-dependent relaxation of the rat aorta. Clin Exp Pharmacol Physiol. 2007;35:192–200. doi: 10.1111/j.1440-1681.2007.04811.x. [DOI] [PubMed] [Google Scholar]
- 33.Leo CH, Joshi A, Woodman OL. Short term type 1 diabetes alters the mechanism of endothelium-dependent relaxation in the rat carotid artery. Am J Physiol Heart Circ Physiol. 2010;299:H502–H511. doi: 10.1152/ajpheart.01197.2009. [DOI] [PubMed] [Google Scholar]
- 34.Jiang F, Guo N, Dusting GJ. Modulation of nicotinamide adenine dinucleotide phosphate oxidase expression and function by 3',4'-dihydroxyflavonol in phagocytic and vascular cells. J Pharmacol Exp Ther. 2008;324:261–269. doi: 10.1124/jpet.107.131433. [DOI] [PubMed] [Google Scholar]
- 35.Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 36.Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A. Alteration in endothelium-dependent hyperpolarization and relaxation in mesenteric arteries from streptozotocin-induced diabetic rats. Br J Pharmacol. 1997;121:1383–1391. doi: 10.1038/sj.bjp.0701258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumoto T, Kobayashi T, Kamata K. Alteration in EDHF-type relaxation and phosphodiesterase activity in mesenteric arteries from diabetic rats. Am J Physiol Heart Circ Physiol. 2003;285:H283–H291. doi: 10.1152/ajpheart.00954.2002. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto T, Kobayashi T, Wakabayashi K, Kamata K. Cilostazol improves endothelium-derived hyperpolarizing factor type relaxation in mesenteric arteries from diabetic rats. Am J Physiol Heart Circ Physiol. 2005;289:H1933–H1940. doi: 10.1152/ajpheart.00303.2005. [DOI] [PubMed] [Google Scholar]
- 39.Burnham MP, Johnson IT, Weston AH. Impaired small-conductance Ca2+-activated K+ channel-dependent EDHF responses in type II ZDF rats. Br J Pharmacol. 2006;148:434–441. doi: 10.1038/sj.bjp.0706748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weston AH, Absi M, Harno E, Geraghty AR, Ward DT, et al. The expression and function of Ca2+-sensing receptors in rat mesenteric artery; comparative studies using a model of type II diabetes. Br J Pharmacol. 2008;154:652–662. doi: 10.1038/bjp.2008.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsumoto T, Miyamori K, Kobayashi T, Kamata K. Specific impairment of endothelium-derived hyperpolarizing factor-type relaxation in mesenteric arteries from streptozotocin-induced diabetic mice. Vasc Pharmacol. 2006;44:450–460. doi: 10.1016/j.vph.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Makino A, Ohuchi K, Kamata K. Mechanisms underlying the attenuation of endothelium-dependent vasodilatation in the mesenteric arterial bed of the streptozotocin-induced diabetic rat. Br J Pharmacol. 2000;130:549–556. doi: 10.1038/sj.bjp.0703354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma X, Li YF, Gao Q, Ye ZG, Lu XJ, et al. Inhibition of superoxide anion-mediated impairment of endothelium by treatment with luteolin and apigenin in rat mesenteric artery. Life Sci. 2008:110–117. doi: 10.1016/j.lfs.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Erlund I, Kosonen T, Alfthan G, Mäenpää J, Perttunen K, et al. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur J Clin Pharmacol. 2000;56:545–553. doi: 10.1007/s002280000197. [DOI] [PubMed] [Google Scholar]
- 45.Loke WM, Hodgson JM, Proudfoot JM, McKinley AJ, Puddey IB, et al. Pure dietary flavonoids quercetin and (-)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am J Clin Nutr. 2008;88:1018–1025. doi: 10.1093/ajcn/88.4.1018. [DOI] [PubMed] [Google Scholar]
- 46.Qi W, Chen X, Holian J, Tan CY, Kelly DJ, et al. Transcription factors Krüppel-like factor 6 and peroxisome proliferator-activated receptor γ mediate high glucose-induced thioredoxin-interacting protein. Am J Pathol. 2009;175:1858–1867. doi: 10.2353/ajpath.2009.090263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McPherson GA. Assessing vascular reactivity of arteries in the small vessel myograph. Clin Exp Pharmacol Physiol. 1992;19:815–825. doi: 10.1111/j.1440-1681.1992.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 48.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 49.Klatt P, Schmidt K, Lehner D, Glatter O, Bachinger HP, et al. Structural analysis of porcine brain nitric oxide synthase reveals a role for tetrahydrobiopterin and L-arginine in the formation of an SDS-resistant dimer. EMBO J. 1995;14:3687–3695. doi: 10.1002/j.1460-2075.1995.tb00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, et al. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 51.Shi Y, Vanhoutte PM. Oxidative stress and COX cause hyper-responsiveness in vascular smooth muscle of the femoral artery from diabetic rats. Br J Pharmacol. 2008;154:639–651. doi: 10.1038/bjp.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
KPSS induced maximum contraction in mesenteric arteries. Exposure of mesenteric arteries from normal and diabetic rats with or without 3′, 4′-dihydroxyflavonol (DiOHF, 1 mg/kg s.c. daily for 7 days) treatment to high K+ physiological saline solution (KPSS, 123 mmol/l). The contraction to KPSS was not affected by diabetes or DiOHF treatment. Results are shown as mean±SEM. NS = not significant.
(TIF)