This article describes the distribution of major cytoskeleton components in the retinal nerve fiber layer (RNFL) during the development of glaucoma. The study found that distortion of axonal cytoskeleton can occur before the thinning of the RNFL.
Abstract
Purpose.
Glaucoma damages the retinal nerve fiber layer (RNFL). The purpose of this study was to investigate the distribution of major cytoskeleton components, F-actin, microtubules (MTs), and neurofilaments (NFs), in the RNFL during the development of glaucoma.
Methods.
Intraocular hypertension was induced in a rat model by laser photocoagulation of the trabecular meshwork. Retinas were obtained after 2 to 3.5 weeks of treatment. Multiple fluorescent stains were used to identify F-actin, MTs, NFs, and nuclei simultaneously in the same tissue. Distribution of these components in a whole-mounted retina was examined by confocal microscopy. Fluorescent stain was quantitatively described.
Results.
In normal RNFL F-actin, MTs, and NFs were intensely stained. Along the bundles, F-actin and MTs were strongly colocalized, but alternating strands of F-actin and NFs were apparent. Normal RNFL lacked nuclei. In glaucomatous retinas, irregular staining of F-actin, MTs, and NFs was found within the bundles. A strong network of F-actin appeared on the RNFL surface and between the bundles. In severely damaged retinal regions total loss of F-actin and MTs was found, whereas residual strands of NFs were evident. Before the decrease in RNFL thickness, irregularity of F-actin stain and density of nuclei in the RNFL significantly increased.
Conclusions.
The results suggest that F-actin, MTs, and NFs are rich and approximately uniformly distributed in the normal RNFL. Glaucoma causes alteration of the cytoskeleton in the RNFL. F-actin is the most sensitive component in its response to stress on the retina. An increase in the number of nuclei in the RNFL may be an early sign of glaucomatous damage
Glaucoma damage to the retinal nerve fiber layer (RNFL) usually precedes detectable visual field loss, and direct assessment of RNFL is therefore more sensitive in predicting disease progression. Newly developed optical methods have provided quantitative and objective assessment of the RNFL. For instance, optical coherence tomography (OCT) measures RNFL thickness by detecting the reflectance of the RNFL, and scanning laser polarimetry (SLP) evaluates axonal ultrastructure by measuring the birefringence of the RNFL, an optical property associated with microtubules. The optical properties of the RNFL depend on the cylindrical structure of axons, including cytoskeleton components.1–5 Elucidation of the precise subcellular components of axons preferentially affected by glaucoma could lead to the design of diagnostic methods that are sensitive to the earliest structural changes, methods that may open a therapeutic window, during which damage might be prevented.
Actin filaments (F-actin), microtubules (MTs), and neurofilaments (NFs), the most abundant intermediate filament in neurons are the major protein filaments of the axonal cytoskeleton.6 These components, individually, and in association with one another, play an important role in axonal function and architecture.7–14 F-actin, a major component maintaining cytoarchitecture, is assembled in two general types, bundles and networks. Bundled F-actin in axons is concentrated in the region of and runs parallel to MTs.8 It provides a substrate for MT transport and affects the structural organization of MTs.9,11 MTs closely coordinate with F-actin to maintain cell shape and position cellular organelles. NFs are major determinants of the diameter of myelinated axons, which in turn controls how fast electrical signals travel down the axon.15,16 NFs also play a role in controlling MT polymerization and, in turn, MTs are required for NF transport in growing axons.12–14 It is known that axonal cytoarchitecture is shaped by external compressive forces,17 and therefore cytoskeleton components are expected to change in states of elevated neural tissue pressure.
Numerous studies have demonstrated a change in the axonal cytoskeleton in glaucoma and other optic nerve diseases. Loss of NFs, the most frequently studied component, is evident as shown in various animal models of optic nerve diseases.16,18–22 A recent study has found both degenerative changes and aberrant growth of NFs in a mouse glaucoma model.23 Changes in MTs and MT-associated proteins are observed in the RNFL and at the optic nerve head (ONH), with elevation of intraocular pressure (IOP).22–24 Alteration of F-actin distribution often appears in a fan-shaped region and occurs first in the dorsal retina with a rat model of glaucoma.25 Studies also show that structural change precedes axonal functional and macroscopic change. Balaratnasingam et al.22 have demonstrated that a change in NF distribution at the optic nerve precedes axonal transport alteration during acute IOP elevation. Fortune et al. (IOVS 2008;49:ARVO E-Abstract 3761) have shown that a decrease in RNFL birefringence, hence loss of MTs, precedes measurable RNFL thinning after onset of experimental glaucoma. We have recently suggested that RNFL reflectance, which arises from light scattering by the axonal cytostructure, changes before the decrease in RNFL thickness (Huang X-R, et al. IOVS 2010;51:ARVO E-Abstract 4808).
Although changes in the axonal cytoskeleton and loss of retinal ganglion cells (RGCs) are known to occur in retinas with optic nerve injury, previous reports are limited to the study of damage mechanisms in one or two cytoskeleton components. In this study, we used simultaneous immunohistochemical staining of F-actin, MTs, NFs, and nuclei to study features of the structural change in each component and their interrelationship in the context of glaucoma in a rat model.
Material and Methods
Rat Model of Glaucoma
Female Wistar rats weighing 250 to 350 g were used. Animals were housed under a 12-hour light–12-hour dark cycle, with standard food and water provided ad libitum. Experimental glaucoma was induced by translimbal laser photocoagulation of the trabecular meshwork.26 Animals were anesthetized with intraperitoneal ketamine (50 mg/kg) and xylazine (5 mg/kg) and topical proparacaine 1% eye drops. The laser treatment (a diode laser with wavelength of 532 nm, 500-mW power, 0.6-second duration, 50-μm-diameter spot size) was administered in the left eye of each rat. Around 55 to 60 trabecular burns were evenly distributed. A second treatment after a week was applied to those eyes that did not maintain elevated IOP. The contralateral eye was untreated and served as the control.
A rebound tonometer (Tonolab; Tonolab, Espoo, Finland) was used to monitor the IOP after the animals were deeply anesthetized.27 The IOP in both eyes was measured just before treatment and 1, 3, 5, and 7 days after treatment and then once a week until enucleation or the IOP returned to its baseline level. For each rat, graphs of IOP (IOP in mm Hg versus days after treatment) for treated and fellow eyes were constructed. Cumulative IOP (cIOP), the area between the two curves in units of mm Hg-days, was calculated.26
All experiments adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The protocol for the use of animals was approved by the Animal Care and Use Committee of the University of Miami.
Tissue Preparation and Immunohistochemistry
After the treated eyes were exposed to elevated IOP for a certain period, both eyes of each animal were removed and prepared for immunohistochemical study. Tissue preparation followed previously developed procedures.28,29 Briefly, both eyes of an anesthetized animal were removed, and the animal was euthanatized. For each eye, an eye cup of 5 mm diameter that included the optic nerve was excised and placed in a dish of warm (33–35°C) oxygenated physiologic solution. After it was dissected from the retinal pigment epithelium and choroid, the retina was placed on a membrane with the photoreceptor side against the membrane, fixed in 4% paraformaldehyde for 30 minutes at room temperature, and rinsed thoroughly in phosphate-buffered saline (PBS). The tissue was then removed from the membrane for further immunohistochemical staining.
Orientation of retinas was documented by first marking the caudal side of the eye in situ with a skin marker and then, after dissection, cutting a notch into the eye cup and retinal edge at the marked position.
Three major cytoskeleton components of axons were studied by multiply labeling a whole-mounted retina with phalloidin to stain F-actin, anti-β-tubulin monoclonal antibody to mark the MTs, and anti-neurofilament antibody to label the NFs. The nuclei in the inner retina were also identified by 4′,6-diamidino-2-phenylindole (DAPI) fluorescent counterstain. The tissue was permeabilized in PBS containing 0.8% TritonX-100 for 1 hour followed by incubation in blocking serum (5% goat serum and 0.8% TritonX-100) for 1 hour at room temperature. The tissue was transferred into a primary antibody solution (1:500, rabbit anti-neurofilament 200 kDa Sigma) at 4°C overnight. After the tissue was washed in PBS (three changes of 10 minutes each), it was incubated in a mixed solution of the secondary antibody (1:250, AlexaFluor 647 goat anti-rabbit IgG; Invitrogen, Carlsbad, CA) and anti-β-tubulin antibody (1:100, Cy3 conjugated; Sigma-Aldrich, St. Louis, MO) overnight at 4°C. After it was again washed in PBS, the tissue was transferred into a solution of phalloidin (1:100, AlexaFluor 488 phalloidin; Invitrogen) for 1 hour at room temperature. After a rinse in PBS, it was incubated in DAPI (FluoroPure grade; Invitrogen-Molecular Probes, Eugene, OR) for 30 minutes in subdued lighting. The stained retina was rinsed again and mounted on a glass slide with an antifade mounting medium (Vectashield; Vector Laboratories, Inc., Burlingame, CA). The retina was stored at 4°C for confocal microscopy imaging.
A series of control experiments were performed and confirmed that the retinas had weak autofluorescence, the secondary antibody specifically bound to the primary antibody for NF labeling, and there were unnoticeable spectral bleed-through signals between fluorescence detectors.
Confocal Laser Scanning Imaging
A confocal laser scanning microscope (Leica TCS SP5, Leica Microsystems, Bannockburn, IL) was used to provide both en face and cross-sectional (CS) images of whole-mounted fluorescently stained retinas. A 40× oil objective provided en face images of the tissue with a full field of view of 389 μm2 and a resolution limited to the sampling density of 0.76 μm/pixel. To cover all bundles merging from the ONH, at least a 3 × 3 tiled array of images was taken that covered a retinal area of 1.2 mm2 with the ONH at the center. For each array position, en face images were collected at evenly spaced positions in depth (1 μm apart in tissue) starting from the RNFL surface through the retina to a depth at least including the ganglion cell layer. The retina was then reconstructed in 3-D, and CS images were synthesized from the reconstruction with customized software. The depth of an en face image was defined as the distance between it and an image with the first recognizable RNFL located at the image center. The depth of individual bundles could vary across an image due to tilt of the tissue. To view cytoskeleton detail in regions of interest, we also collected images with a 63× oil objective. If not otherwise stated, images displayed and used for data analysis were taken with the 40× oil objective.
Except for detector gain, which was adjusted for each detector to display nerve fiber bundles at approximately full dynamic range without inducing spectral bleed-through between channels, for each objective the same confocal parameters were applied to all tissues. Scans of different fluorescent channels were collected sequentially. The image resolution and z-scaling (Z-step size with an assumed refractive index of 1.52 for microscope immersion oil) were read from the system with no additional calibration. No deconvolution was applied to the images.
Quantitative Analysis of RNFL Texture
An earlier study of F-actin distribution in glaucomatous eyes revealed that elevation of IOP altered the qualitatively perceived texture of the fluorescence labeled cytoskeleton.25 In this study, we used a quantitative approach for texture analysis based on statistical properties of the intensity histogram.30,31 Letting Z be a variable indicating intensity in a region, p(Z) the histogram of the intensity levels within the region, and L the number of possible intensity levels, then U = p2 (Zi) is a measure of the uniformity of intensity in the region. Further, if the intensity in a region has variance σ2, R = 1 − [1/(1 + σ2)] measures the relative smoothness of the intensity. U is the maximum, and R is 0 in regions of constant intensity. In this study, U and R were used to quantify the cytoskeletal distribution at early stages of glaucomatous damage.
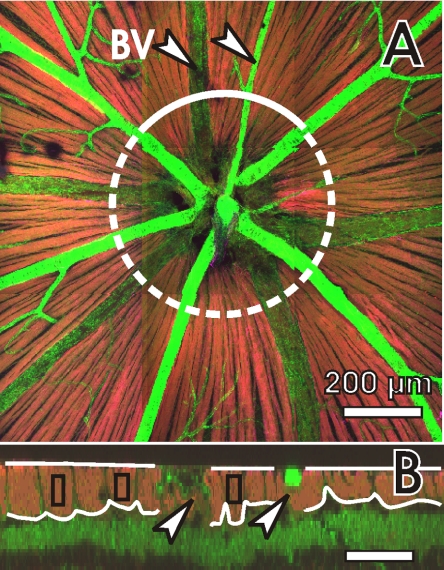
CS images of the RNFL were obtained along a circle around the ONH from reconstructed 3-D images of the retina (Fig. 1). The circle radius was 300 μm. Boundaries of the RNFL were manually defined without inclusion of large blood vessels (Fig. 1B). The average thickness of each defined RNFL sector along the whole CS image was calculated. For normal retinas, the mean and range of RNFL thicknesses of all the normal retinas were then determined. For treated retinas, those RNFL sectors with an average RNFL thickness within the range of the normal thickness were selected and used in texture analysis.
Figure 1.
RNFL assessment by confocal microscopy: the images are composed of the images with F-actin (green), MT (red) and NF (magenta) stain. (A) A 3 × 3 tiled en face image at a depth of 6 μm (see definition in the Methods section) with the ONH placed at the center. Retinal nerve bundles are identified as bright stripes (red-orange). The circle indicates a path for reconstructing a cross-sectional (CS) image at a radius of 300 μm. (B) Partial CS image along the solid curve in (A). The RNFL appears as a bright layer (red-orange) near the retinal surface. Curved lines define the boundaries of the RNFL. Separate RNFL sectors are defined to avoid inclusion of blood vessels. Rectangles: bundles selected for texture analysis. To illustrate details in the RNFL, the CS image is stretched vertically by a factor of 4. BV and arrow: blood vessel. Scale bar: (B) horizontal, 100 μm; vertical, 25 μm.
To analyze textural change of the RNFL, areas on the nerve fiber bundles were selected by drawing rectangular boxes (Fig. 1B) that were located 2 to 3 pixels below the RNFL surface to avoid stain artifacts on the tissue surface that occasionally occurred, especially in the NF images. The thickness of the selected bundles was calculated as the average RNFL thickness within the width of the defined box. Bundles with capillaries, identified by strong phalloidin staining, were not selected. For each normal retina, 11 to 13 bundles approximately evenly distributed around the ONH were selected, and the texture parameters U and R of each bundle were calculated. In the treated retinas, any bundle with a thickness within the normal range was identified. Six retinas had seven to nine selected bundles, which were all used for texture analysis. Four retinas had at least 12 bundles identified, and eight bundles were randomly chosen. The average of these seven to nine bundles for each of the 10 treated retinas was used for data and statistical analysis.
Quantification of Nucleus Density
Nucleus density was calculated for the RNFL and the nucleus layer just below the RNFL (the retinal ganglion cell layer [RGCL]) for the RNFL sectors that were used in texture analysis. The numbers of nuclei in the RNFL and RGCL, respectively, were manually counted and the horizontal length of the RNFL sectors was measured. Linear density of nuclei (nuclei per mm) in each layer was then calculated as the ratio of the number of nuclei and the sector length. Total linear density in both layers was also calculated.
Statistical Analysis
Bundle thickness, texture parameters, and nuclear density were studied by using an analysis of variance approach (two-way unbalanced ANOVA). The significance level was set at P < 0.05.
Statistical tests were performed in commercial software (MatLab Statistics Toolbox; Matlab Version 2009b; The MathWorks, Inc., Natick, MA). Image reconstruction and data analysis were implemented with customized programs written in the software.
Results
Twelve Wistar rats were treated unilaterally. Two eyes developed severe loss of RNFL and were excluded from the study. The baseline IOP before treatment was not significantly different, with 10.2 ± 0.6 and 10.0 ± 0.6 mm Hg for control and treated eyes, respectively. The IOP of all treated eyes remained elevated for at least 2 weeks. Table 1 summarizes the IOP of treated eyes and the duration of animal survival after the first laser treatment. The baseline IOP value reported here was lower than that reported in our previous publication.25 The discrepancy may be due to the use of different tonometers (Tonolab versus Tonopen XL; produced by Tonolab and Reichert Technologies, DePew, NY, respectively). The value reported herein is comparable with the values measured in similar conditions by other investigators.32,33
Table 1.
Summary of the Treated Retinas
| Rat | IOPbaseline (mm Hg) | cIOP (mm Hg-days) | IOPpeak (mm Hg) | Duration (wk) |
|---|---|---|---|---|
| 1 | 10 | 68 | 40 | 2.5 |
| 2 | 10 | 76 | 39 | 3.5 |
| 3 | 10 | 97 | 31 | 2.5 |
| 4 | 9 | 110 | 42 | 3 |
| 5 | 9 | 117 | 33 | 3 |
| 6 | 10 | 120 | 46 | 3 |
| 7 | 10 | 121 | 43 | 3.5 |
| 8 | 11 | 142 | 38 | 3 |
| 9 | 11 | 201 | 54 | 2 |
| 10 | 10 | 261 | 52 | 3 |
| Mean ± SD | 10.0 ± 0.6 | 131 ± 59 | 42 ± 7 | 3.0 ± 0.3 |
Axonal Cytoskeleton in the Normal Retinas
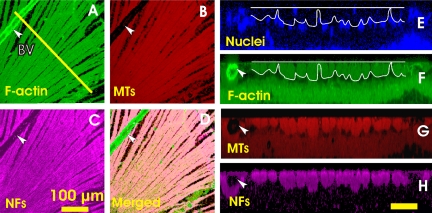
Nerve fiber bundles were identified as bright stripes in en face images by fluorescence-labeled F-actin, MTs, and NFs (Figs. 2A–C). Each labeled structure demonstrated tightly packed axonal bundles that converged into the ONH. With the 40× objective the stain of the cytoskeleton components appeared strong and uniform within the bundles. Blood vessels were distinguished from bundles by strong phalloidin staining or as hollow structures. Although CS images showed that phalloidin and anti-β-tubulin staining also labeled deep retinal layers (Figs. 2F, 2G), bundles were specifically stained, lying just under the retinal surface, and separated from deeper layers by a single layer of nuclei with DAPI counterstain (Fig. 2E). The RNFL lacked nuclei, except for areas around blood vessels.
Figure 2.
Cytoskeleton components in normal RNFL with simultaneous fluorescence staining. (A–C) En face images of axonal F-actin, MTs and NFs of the same nerve fiber bundles, at a depth of 6 μm. (D) A composite of images (A), (B), and (C), showing colocalization of the cytoskeleton components. (E–H) CS images of the retina along the line in (A). The line is at a distance of 550 μm from the ONH center. (E) DAPI counterstain shows a single layer of nuclei lying under the RNFL. White lines: RNFL boundaries. (F–H) Normal RNFL appears as a bright, uniformly stained layer near the retinal surface. Arrow and BV: blood vessel. Scale bar: (H) horizontal, 50 μm; and vertical, 25 μm.
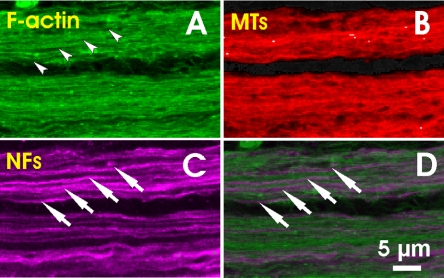
Within nerve fiber bundles, the cytoskeleton ran parallel to the axonal direction, which was better when viewed at higher power, to overcome the resolution and sampling limits of the 40× objective. The images of two bundles in Figure 3 were taken with a 63× oil-immersion objective at a depth of 4 μm. They show dense, striated staining of F-actin, MTs, and NFs within bundles. The gap between bundles was devoid of MT and NF staining, but showed faint F-actin staining. Linear regions of lighter F-actin stain were discernible within bundles (Fig. 3A, arrowheads). The appearance of MT staining within the bundles was similar to that of F-actin. Staining for NFs, however, revealed several discrete strands that were easily identifiable (Fig. 3C, arrows). Moreover, these densely stained strands of NFs were located within the lighter-stained F-actin regions, presenting a complementary pattern of striations in a merged F-actin and NF image (Fig. 3D). The width of these NF strands ranged from 0.3 to 0.9 μm.
Figure 3.
Distribution of cytoskeleton components in normal retina viewed with a 63× oil objective (0.24 μm/pixel lateral resolution). (A, B) Within the bundles, F-actin showed linear regions of lighter stain (A, arrowheads) between strands of intensely stained F-actin. (C) Discrete strands of NFs were easily discernible along bundles. (D) A merged image of (A) and (C) shows that NFs and F-actin appear as a complementary pattern of striations. Images were taken at 4 μm below the RNFL surface.
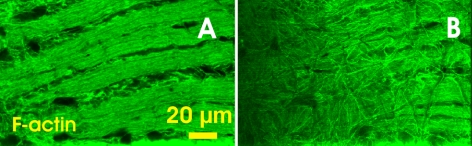
The lack of MT and NF stain between nerve fiber bundles made the bundle boundaries sharp and clear. In the F-actin images (Figs. 3A, 4A), however, diffusely distributed F-actin was found between the bundles. Moreover, a disoriented F-actin network appeared on the RNFL surface, as shown in the left side of Figure 4A where the bundles were nearer the RNFL surface because of tissue tilt. This network was well demonstrated in the en face image taken 1 μm below the surface (Fig. 4B). No such network was found in the corresponding MT- and NF-labeled images (not shown).
Figure 4.
F-actin distribution at different depths near the surface of the normal RNFL. (A) En face image of F-actin stain at 2 μm below the RNFL surface. Diffused strands of F-actin appear between bundles. (B) En face image of the same bundles at 1 μm below the RNFL surface. A network of F-actin appears on the surface of the RNFL.
Alteration of Cytoskeleton in the Hypertensive Retinas
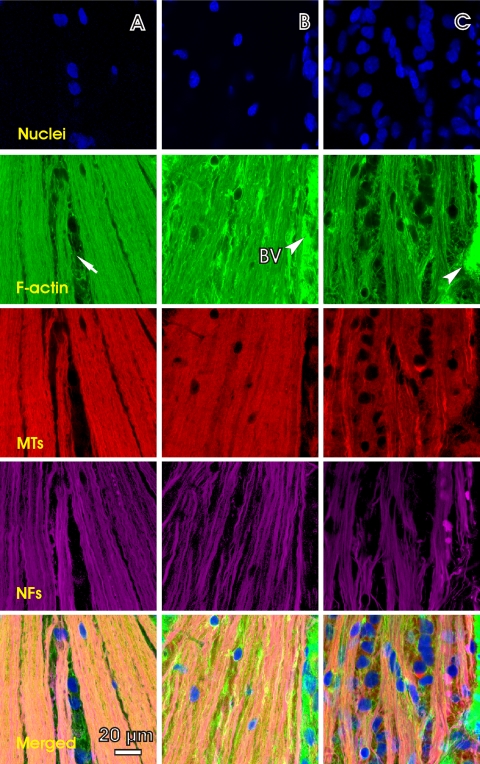
Elevated IOP caused alteration of the axonal cytoskeleton. Figure 5 shows en face images of a control retina and mildly damaged retinal regions in two treated retinas obtained with the 40× objective. In the control retina, each cytoskeletal component was uniformly distributed within the bundles and ran along the axons. Bundle boundaries were well defined. The few nuclei present were located mostly in the gap areas between the bundles. In contrast, sporadic staining of F-actin was found in the treated retinas (Figs. 5B, 5C; F-actin). Bundle boundaries were hardly perceptible (Fig. 5B) but cytoskeleton components were still abundant and ran along the bundle courses. A less oriented structural network developed across the images (Figs. 5B, 5C, merged). Nuclei became abundant within the RNFL (Fig. 5C).
Figure 5.
Distortion of the axonal cytoskeleton in retinas with IOP elevation. All images were at a depth of 5 μm. (A) En face images of a control retina with brightly stained and uniformly distributed cytoskeleton components within the bundles. (B, C) En face images of the retinas with elevated IOP (B, rat 9; C, rat 3 in Table 1). (B) Irregular phalloidin stain appears; bundle boundaries become indiscernible. Strands of NFs are more apparent. (C) More severe irregularity of staining shows in all three cytoskeleton components. Nuclei are abundant and embedded within the bundles. The top of each image is 450 μm from the ONH center. The average RNFL thickness along a horizontal central line (not shown) were 11.5 ± 2.3, 11.8 ± 2.1, and 10.8 ± 3.2 μm for (A), (B), and (C) respectively. Arrow: gap between bundles. Arrowhead and BV: blood vessel.
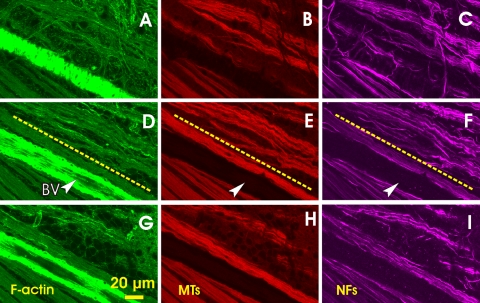
The pattern of structural distortion varied through the depth of the RNFL, as shown in Figure 6, which also clearly demonstrates localized damage of nerve fiber bundles. The bundles in the lower left region were uniformly stained and appeared smooth and normal looking (Figs. 6D–F). However, the bundles in the upper right were wavy, and strands of cytoskeleton components were apparent within the bundles. The images in Figures 6A–C were taken at 1 μm below the RNFL surface. Aberrant strands across the bundles were developed both in the F-actin and NF images. At a depth of 2 μm (Figs. 6D–F), the bundle courses were clear, running approximately parallel to the normal ones. Except for the diffusely oriented F-actin between bundles, the crossover strands of F-actin and NF networks disappeared at this depth. At a depth of 10 μm from the RNFL surface (Figs. 6G–F), the distorted bundles started to disappear. The average bundle thickness in the upper right was ∼12 μm, whereas the neighboring normal bundles were 28 μm thick.
Figure 6.
Different distortion patterns at different depths of the RNFL (rat 2 in Table 1). (A–C) En face images at 1 μm below the RNFL surface. Strands of MTs and NFs within the bundles are apparent. Aberrant strands of F-actin and NFs cross the bundles. (D–F) En face images of the same bundles in (A–C) at 2 μm below the RNFL surface. The images show normal-looking bundles in the bottom left (below the dashed lines) and distorted bundles in the top right. Wavy strands of cytoskeleton components run along the bundles. (G–I) En face images of the same bundles at 10 μm below the RNFL surface. Bundles have started to disappear. Arrowhead and BV: blood vessel.
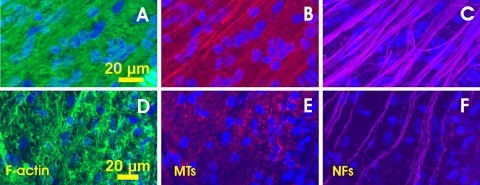
The pattern of structural alteration could be different for different cytoskeleton components as well. Figures 7A–C show a severely damaged retinal region. Strands of all components along the bundle's direction were much less dense. Although the traces of bundles identified by F-actin, MT and NF staining were similar, the details of the patterns of each component along the bundle courses were different. More NFs were retained, as shown in Figure 7C. Figures 7D–F show a retinal region without visible RNFL. Strong network and irregular staining of F-actin occurred across the entire image, but there was no apparent bundle trace identified by F-actin staining. Also no strands of MTs were observed (Fig. 7E). However, clear strands of NFs still existed and were found in four consecutive en face images, suggesting ∼3 μm of thickness.
Figure 7.
Distortion pattern of cytoskeleton in severely damaged retinal regions (A–C: rat 2 and D–F: rat 10 in Table 1). (A–C) Bundle traces were still perceptible with the F-actin and MT staining but the strand patterns of the F-actin and MTs appear different from that in the NF image. Images were 2 μm below the RNFL surface. The average thickness of the RNFL was 4 μm. (D–F) A retinal region with total loss of the RNFL. There were no apparent strands of F-actin and MTs. A few strands of NFs ran along the bundle direction, inferred from the position of the ONH.
Figures 5, 6, and 7 also demonstrate that the alteration in the pattern of each component was different at different stages of tissue damage.
Structural Disorganization before RNFL Thickness Change
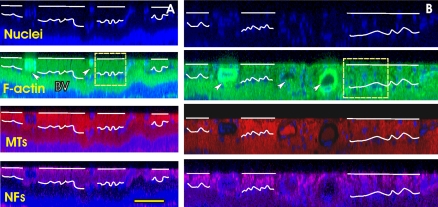
Distortion of the axonal cytoskeleton could occur before the change in RNFL thickness. Figure 8 shows CS images in the caudal regions of a treated retina and its fellow control retina. The average RNFL thickness in the defined regions was 16 ± 4 and 17 ± 5 μm in the normal and treated eyes, respectively. The normal retina showed uniform staining of the cytoskeleton components across the RNFL, which was well separated by a single layer of nuclei from the deep layers. However, the treated retina demonstrated irregular staining of the cytostructures, especially in the F-actin and NF images, without a reduction in RNFL thickness. Similar to the features demonstrated in the en face images in Figures 5B and 5C, bundle boundaries were hardly perceptible in the CS images. Nuclei, which were hardly seen in the normal RNFL (Fig. 8A), were found embedded within the bundles and a single layer of nuclei still lay under the RNFL (Fig. 8B).
Figure 8.
Distortion of cytoskeleton before the change in RNFL thickness (rat 6 in Table 1). (A) CS images of the control retina showing uniformly stained bundles in all three cytoskeletal images. White curves: the boundaries of the RNFL lacking of nuclei. (B) CS images of the fellow treated retina without thinning of the RNFL. The stain looks nonuniform. Both sections were taken in the caudal–ventral regions at a distance of 250 μm from the ONH center. Arrow and BV: blood vessel. The scale bars: (A, B) horizontal, 100 μm; vertical, 50 μm.
To quantify early change in the cytostructural distribution within the cross section of bundles, we performed texture analysis on bundles selected in RNFL regions with no apparent thinning. A two-way unbalanced ANOVA analysis was performed to characterize the contributions to the total variance of treatment and biological variability between animals. Table 2 summarizes the global means and standard deviations for the texture parameters U and R, as well as the P-value associated with the treatment effect. The average thickness of the selected bundles in the control and treated retinas was similar. For F-actin stain, there was a significant treatment effect for both uniformity and smoothness. For MT stain, there was a significant difference associated with treatment for uniformity but not for smoothness. In contrast, both texture parameters of the NF stain were not different in the control and treated eyes. It should be pointed out that in all studied rats we found considerable variability between animals. The ANOVA model consistently showed statistically significant differences across the different animals.
Table 2.
Summary of Texture Analysis of Cytoskeletal Components
| Bundles | Thickness (μm) | Uniformity (U) |
Smoothness (R) (scaled by 1000) |
||||
|---|---|---|---|---|---|---|---|
| F-actin | MTs | NFs | F-actin | MTs | NFs | ||
| Control (n = 120)* | 16 ± 4 | 0.14 ± 0.05 | 0.12 ± 0.06 | 0.28 ± 0.11 | 0.15 ± 0.12 | 0.22 ± 0.22 | 0.20 ± 0.18 |
| Treated (n = 80) | 17 ± 3 | 0.12 ± 0.05 | 0.11 ± 0.05 | 0.23 ± 0.12 | 0.34 ± 0.37 | 0.24 ± 0.32 | 0.21 ± 0.25 |
| P | 0.8 | 0.003 | 0.03 | 0.38 | <0.001 | 0.47 | 0.10 |
n, number of bundles analyzed.
Change of Nucleus Distribution in the RNFL
Elevated IOP could also cause increase of nuclei in the RNFL. In normal retinas, nuclei with DAPI counterstain in the RNFL were mostly distributed between bundles and around or within blood vessels. However, in the treated retinas, abundant nuclei were found in the RNFL and embedded within the bundles, as revealed in Figures 5 and 8D. Moreover, increased nuclei were found in the regions without apparent thinning of the RNFL thickness. Table 3 summarizes the total nuclear density and the nuclear densities in the RNFL and RGCL calculated for the same RNFL sectors as were used in the texture analysis. In both normal and treated retinas, the nuclear density was significantly lower in the RNFL than in the RGCL. However, the density in the RNFL of the treated retinas greatly increased compared with that in the normal RNFL. Meanwhile, the density in the RGCL of the treated retinas significantly decreased. Interestingly, the total nuclear density of the treated retinas was found to be significantly higher than that in the control.
Table 3.
Linear Density of Nuclei in the RNFL and RGCL
| Linear Density | RNFL+RGCL | RNFL | RGCL | P (RNFL vs. RGCL) |
|---|---|---|---|---|
| Control | 46 ± 5 | 5 ± 2 | 41 ± 5 | <0.001 |
| Treated | 53 ± 5 | 22 ± 4 | 31 ± 3 | 0.01 |
| P (control vs. treated) | 0.01 | <0.001 | <0.001 |
Data are expressed as the number of nuclei per millimeter.
Discussion
Axonal F-actin, MTs and NFs closely associate with each other to maintain the rigid architecture of axons. In this study, we used simultaneous immunohistologic labeling and confocal imaging, to investigate the distribution of these cytoskeleton components and nuclei in retinas exposed to elevated IOP.
In normal retina, F-actin, MTs, and NFs were all intensely stained with immunofluorescence, indicating that normal RNFL is rich in each of these cytoskeleton components. In a lower-power view of the structure, the components appeared uniformly stained along and across nerve fiber bundles, suggesting an approximately uniform distribution of these cytostructures within bundles. F-actin and MTs were well colocalized, whereas colocalization of F-actin and NFs was not perfect, as revealed in images with a higher-power view (Fig. 3). Because the width of alternating NF and F-actin strands was of the magnitude of axonal diameters, the strands may represent different types of axons, axons rich in NFs, and axons with denser F-actin, but staining artifact could not be ruled out without more advanced analysis, such as electron microscopy. Colocalization and alternation of the structures most likely reflect the interdependence of these components in both functional and structural organization.8–12
Many studies have been conducted to investigate topographic change of the cytoskeleton in the RNFL and demonstrated patterns of structural change around the ONH in response to various insults (Villegas-Perez MP, et al. IOVS 2005;46:ARVO E-Abstract 1234).18,19,22,23,34 In the present study, we found that elevated IOP altered the distribution of axonal cytoskeleton along bundles and across bundle cross-sections. Features of these cytoskeletal changes include (1) different distortion patterns for different cytoskeleton components; (2) for each component the pattern of alteration varied through the depth of the RNFL and also depended on the stage of tissue damage; (3) alteration of the axonal cytoskeleton could occur before thinning of the RNFL; and (4) F-actin may be the most sensitive and vulnerable structure in its response to elevated IOP.
Striking features of F-actin distribution in the RNFL were found in this study. In addition to bundled F-actin along the course of axons, diffusely distributed actin filaments between bundles and an actin network on the RNFL surface (Fig. 4) were found in normal retina. No corresponding MTs and/or NFs were found. Because these F-actin structures do not run along axons, one speculation is that these F-actin filaments are associated with nonneural structures, such as Müller cells, to maintain the architecture of nerve fiber bundles within the RNFL. In early stages of axonal damage, the amount of diffusely distributed F-actin between bundles was increased and bright staining of this F-actin, indicating condensation of F-actin filaments (Wheat JL, et al. IOVS 2010;51:ARVO E-Abstract 2104), was often found (Fig. 5B). F-actin is the key component responsible for maintaining cell shape. The increase in F-actin between bundles may significantly strengthen the axonal architecture and could be an early response to stress on the retina. The result also is consistent with increased activity of microglia observed in glaucomatous retinas (Wheat JL, et al. IOVS 2010;51:ARVO E-Abstract 2104).35 Irregular staining of F-actin also appeared within the bundles and probably along the same strands of F-actin (Figs. 6D, 6G). Interestingly, the intensely stained strands were often located near bundle boundaries. Formation of condensed F-actin near the bundle boundaries during exposure to elevated IOP may suggest a differential response to stress; alternatively, it could be an early indication of apoptotic death of ganglion cell axons, as disorganization of F-actin has been related to cell apoptosis.36,37
Detailed features of structural change of each component are different, which may provide an insight into their damage mechanisms during the development of glaucoma. In mildly damaged retina, the strands of cytoskeleton within the bundles became apparent for all three components (Figs. 5C, 6), which may represent some remaining axons and also suggests a reduction in F-actin, MTs, and NFs in the RNFL. Irregular staining of MTs and NFs along strands was also found within the bundles, similar to irregular staining of F-actin. However, aberrant NFs developed near the RNFL surface (Fig. 6C). In severely damaged nerve fiber bundles, bundled F-actin and MTs were hardly identifiable or did not exist at all, while clear strands of NFs still ran along the bundle direction (Fig. 7). Consistent with the earlier discussion of normal bundles, a possible explanation is that the remaining strands of NFs represent a type of axon with denser NFs, the axons with denser F-actin being more vulnerable to glaucomatous damage. This result may suggest that NFs are the cytoskeletal component most tolerant of glaucomatous damage, at least at late stages of the disease. The result may also indicate that each cytoskeletal component plays a different role in maintenance of axonal architecture and function38 and therefore responds differently to axonal injury.
Patterns of cytoskeletal alteration varied through the depth of the RNFL (Fig. 6). A possible explanation of this feature is that stress, induced by elevated IOP and experienced by the cytoskeleton, varies with depth in the RNFL, resulting in differential responses.17
A goal of our study was to look for early structural change before irreversible damage of axons. In this study, we developed the texture analysis method to quantify structural change in bundle cross sections and found that axonal cytostructure was altered before thinning of the RNFL. Many studies show microglia responding to elevation of IOP by increasing expression in the RNFL (Wheat JL, et al. IOVS 2010;51:ARVO E-Abstract 2104).35 Invasion of microglia may contribute to the retention of RNFL thickness. In bundles without apparent thickness change, both of the textural parameters, uniformity and smoothness of F-actin staining were significantly different from that in the fellow control eyes, suggesting alteration of F-actin distribution within bundles of treated retina. Uniformity of MT staining was also changed significantly, whereas none of the parameters was shown to be changed with the NF stain. Based on the current analysis method axonal F-actin is the most sensitive cytoskeletal component responding to elevation of IOP. Putting together the characteristic changes in F-actin distribution found in this study, we may speculate that under states of high IOP, axonal F-actin tries to strengthen cytoarchitecture by developing actin networks between bundles, on bundle surfaces, and even within bundles, and on failure of mechanical protection of axons a cascade of structural degeneration affects all cytoskeleton components due to their interdependent nature.8–12
Morphologic and topographic change of RGCs is most often studied in optic nerve diseases. RGC apoptosis and loss of RGCs are evident as shown by many studies with different glaucoma models.21,35,39–41 In this study, we used DAPI counterstain to identify nuclei in retinal regions where the RNFL thickness did not apparently change. We found that nuclear density in the RGCL significantly decreased in treated retinas. This result could be interpreted as loss of nuclei in the RGCL. However, the nuclear density in the corresponding RNFL, just above the RGCL, greatly increased. Furthermore, total nuclear density in the RNFL and RGCL was significantly higher in the treated retinas. Approximately 50% of cells in the RGCL are estimated to be displaced amacrine cells. Because we did not directly quantify RGCs, we cannot determine how many, if any, RGCs were displaced to the RNFL. Microglia are highly reactive and mobile cells that respond to glaucomatous damage.35 One explanation for the increase in nuclei could be that the microglia participate in phagocytosis of degenerating RGCs. Further study must overcome the staining limitations and identify change in RGCs and other nuclei in response to glaucomatous damage.
In this study whole-mounted retinas were used to provide a full view of nerve fiber bundle distribution around the ONH, which allows detection of local changes in axonal cytoskeleton across the retina. Degrees of RNFL change varied from distortion of axonal cytostructures without change in the RNFL thickness (Figs. 5, 8) to thinning of the RNFL (Fig. 6) and total loss of the nerve fiber bundles (Fig. 7). Furthermore, as Figure 6 demonstrates, these characteristic changes could occur in the same retina. The result is consistent with our previous study of F-actin change.25 The multiple fluorescence staining method revealed distributions of different structural components simultaneously, making it a powerful tool for studying responses of different cytoskeleton components to axonal injury under the same circumstance. The confocal imaging method provided an in-depth view of structural distribution and enhanced the assessment of cytoskeletal change at different depths of RNFL, which, to our best knowledge, has not been shown previously.
In the present study, we investigated glaucomatous damage to the axonal cytoskeleton. We found that elevated IOP altered the distribution of axonal F-actin, MTs, and NFs and detailed patterns of change are different for each component and during the development of the disease. The findings in this study will provide a map to guide future studies of cytoskeletal change at the ultrastructural level with more focused methods such as immunoelectron microscopy.
Acknowledgments
The authors thank Robert W. Knighton for his timely review of the manuscript and George McNamara and Gabriel Gaidosh for assistance in confocal imaging.
Footnotes
Supported by National Institutes of Health Grant R01-EY019084; American Health Assistance Foundation Grant G2008-033; National Institutes of Health Core Grant P30-EY014801; a Department of Defense Career Development Award; and an unrestricted grant from Research to Prevent Blindness.
Disclosure: X.-R.Huang, None; W. Kong, None; Y. Zhou, None; G. Gregori, None
References
- 1. Zhou Q, Knighton RW. Light scattering and form birefringence of parallel cylindrical arrays that represent cellular organelles of the retinal nerve fiber layer. Appl Opt. 1997;36:2273–2285 [DOI] [PubMed] [Google Scholar]
- 2. Knighton RW, Huang X-R, Zhou Q. Microtubule contribution to the reflectance of the retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 1998;39:189–193 [PubMed] [Google Scholar]
- 3. Knighton RW, Huang X-R. Directional and spectral reflectance of the rat retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 1999;40:639–647 [PubMed] [Google Scholar]
- 4. Huang X-R, Knighton RW. Microtubules contribute to the birefringence of the retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 2005;46:4588–4593 [DOI] [PubMed] [Google Scholar]
- 5. Huang X-R, Knighton RW, Cavuoto LN. Microtubule contribution to the reflectance of the retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 2006;47:5363–5367 [DOI] [PubMed] [Google Scholar]
- 6. Darnell J, Lodish H, Baltimore D. Molecular Cell Biology. New York: Scientific American Books; 1990:820–822 [Google Scholar]
- 7. Sato M, Leimbach G, Schwarz WH, Pollard TD. Mechanical properties of actin. 1985:8585–8592 [PubMed] [Google Scholar]
- 8. Fath KR, Lasek RJ. Two classes of actin microfilaments are associated with the inner cytoskeleton of axons. J Cell Biol. 1988;107:613–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goode BL, Drubin DG, Bames G. Functional cooperation between the microtubule and actin cytoskeletons. Curr Opin Cell Biol. 2000;12:63–71 [DOI] [PubMed] [Google Scholar]
- 10. Zhou F-Q, Waterman-Storer CM, Cohan CS. Focal loss of actin bundles causes microtubule redistribution and growth cone turning. J Cell Biol. 2002;157:839–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hasaka TP, Myers KA, Baas PW. Role of actin filaments in the axonal transport of microtubules. J Neurosci. 2004;24:11291–11301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Francis F, Roy S, Brady ST, Black MM. Transport of neurofilaments in growing axons requires microtubules but not actin filaments. J Neurosci Res. 2005;79:442–450 [DOI] [PubMed] [Google Scholar]
- 13. Minami Y, Murofushi H, Sakai H. Interaction of tubulin with neurofilaments: formation of networks by neurofilament-dependent tubulin polymerization. J Biochem. 1982;92:889–898 [DOI] [PubMed] [Google Scholar]
- 14. Minami Y, Sakai H. Effects of microtubule-associated proteins on network formation by neurofilament-induced polymerization of tubulin. FEBS Lett. 1986;195:68–72 [DOI] [PubMed] [Google Scholar]
- 15. Hoffman PN. Review: the synthesis, axonal transport, and phosphorylation of neurofilaments determine axonal caliber in myelinated nerve fibers. Neuroscientist. 1995;1:76–83 [Google Scholar]
- 16. Hoffman PN, Cleveland DW, Griffin JW, Landes PW, Cowan NJ, Price DL. Neurofilament gene expression: a major determinant of axonal caliber. Proc Natl Acad Sci. 1987;84:3472–3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Price RL, Lasek RJ, Katz MJ. Internal axonal cytoarchitecture is shaped locally by external compressive forces. Brain Res. 1990;530:205–214 [DOI] [PubMed] [Google Scholar]
- 18. McKerracher L, Essagian C, Aguayo AJ. Temporal changes in beta-tubulin and neurofilament mRNA levels after transection of adult rat retinal ganglion cell axons in the optic nerve. J Neurosci. 1993;13:2617–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vickers JC, Schumer RA, Podos SM, Wang RF, Riederer BM, Morrison JH. Differential vulnerability of neurochemically identified subpopulations of retinal neurons in a monkey model of glaucoma. Brain Res. 1995;680:23–35 [DOI] [PubMed] [Google Scholar]
- 20. Villegas-Perez M, Vidal-Sanz M, Bray G, Aguayo A. Influences of peripheral nerve grafts on the survival and regrowth of axotomized retinal ganglion cells in adult rats. J Neurosci. 1988;8:265–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jakobs TC, Libby RT, Ben Y, John SW, Masland RH. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol. 2005;171:313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Balaratnasingam C, Morgan WH, Bass L, Cringle SJ, Yu D-Y. Time-dependent effects of elevated intraocular pressure on optic nerve head axonal transport and cytoskeleton proteins. Invest Ophthalmol Vis Sci. 2008;49:986–999 [DOI] [PubMed] [Google Scholar]
- 23. Fu CT, Sretavan D. Laser-induced ocular hypertension in albino CD-1 mice. Invest Ophthalmol Vis Sci. 2010;51:980–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ou B, Ohno S, Tsukahara S. Ultrastructural changes and immunocytochemical localization of microtubule-associated protein 1 in guinea pig optic nerves after acute increase in intraocular pressure. Invest Ophthalmol Vis Sci. 1998;39:963–971 [PubMed] [Google Scholar]
- 25. Huang X-R, Knighton RW. Altered F-actin distribution in retinal nerve fiber layer of a rat model of glaucoma. Exp Eye Res. 2009;88:1107–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levkovitch-Verbin H, Quigley HA, Martin KRG, Valenta D, Baumrind LA, Pease ME. Translimbal laser photocoagulation to the trabecular meshwork as a model of glaucoma in rats. Invest Ophthalmol Vis Sci. 2002;43:402–410 [PubMed] [Google Scholar]
- 27. Moore CG, Milne ST, Morrison JC. Noninvasive measurement of rat intraocular pressure with the Tono-Pen. Invest Ophthalmol Vis Sci. 1993;34:363–369 [PubMed] [Google Scholar]
- 28. Knighton RW, Huang X-R. Visible and near-infrared imaging of the nerve fiber layer of the isolated rat retina. J Glaucoma. 1999;8:31–37 [PubMed] [Google Scholar]
- 29. Huang X-R, Knighton RW, Shestopalov V. Quantifying retinal nerve fiber layer thickness in whole-mounted retina. Exp Eye Res. 2006;83:1096–1101 [DOI] [PubMed] [Google Scholar]
- 30. Gonzalez RC, Woods RE, Eddins SL. Digital Image Processing Using MatLab. 2nd ed. Knoxville, TN: Gatesmark Publishing; 2009 [Google Scholar]
- 31. Materka A, Strzelecki M. Texture Analysis Methods: A Review. COST B11 Report. Lodz-Brussels: Technical University of Lodz; 1998. PDF file available at http://scholar.google.com/scholar?q=Texture+Analysis+Methods:+A+Review.&hl=en&as_sdt=0&as_vis=1&oi=scholart [Google Scholar]
- 32. Pease M, Hammond JC, Quigley HA. Manometric calibration and comparison of Tonolab and Tonopen tonometers in rats with experimental glaucoma and in normal mice. J Glaucoma. 2006;15:512–519 [DOI] [PubMed] [Google Scholar]
- 33. Lee EJ, Park KH, Kim DM, Yoo YC, Kang SH, Kim YJ. Assessing intraocular pressure by rebound tonometer in rats with an air-filled anterior chamber. Jpn J Ophthalmol. 2008;52:500–503 [DOI] [PubMed] [Google Scholar]
- 34. Balaratnasingam C, Morgan WH, Johnstone V, Cringle SJ, Yu D-Y. Heterogeneous distribution of axonal cytoskeleton proteins in the human optic nerve. 2009;50:2824–2838 [DOI] [PubMed] [Google Scholar]
- 35. Naskar R, Wissing M, Thanos S. Detection of early neuron degeneration and accompanying microglial responses in the retina of a rat model of glaucoma. Invest Ophthalmol Vis Sci. 2002;43:2962–2968 [PubMed] [Google Scholar]
- 36. Van de Water B, Kruidering M, Nagelkerke JF. F-actin disorganization in apoptotic cell death of cultured rat renal proximal tubular cells. Am J Physiol. 1996;270:F593–F603 [DOI] [PubMed] [Google Scholar]
- 37. Okada T, Otani H, Wu Y, et al. Role of F-actin organization in p38 MAP kinase-mediated apoptosis and necrosis in neonatal rat cardiomyocytes subjected to simulated ischemia and reoxygenation. Am J Physiol Heart Circ Physiol. 2005;289:H2310–H2318 [DOI] [PubMed] [Google Scholar]
- 38. Schliwa M. The Cytoskeleton. Vienna: Springer-Verlag; 1986 [Google Scholar]
- 39. Schlamp CL, Li Y, Dietz JA, Janssen KT, Nickells RW. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neuroscience. 2006;7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Filippopoulos T, Danias J, Chen B, Podos SM, Mittag TW. Topographic and morphologic analyses of retinal ganglion cell loss in old DBA/2NNia mice. Invest Ophthalmol Vis Sci. 2006;47:1968–1974 [DOI] [PubMed] [Google Scholar]
- 41. Reichstein D, Ren L, Filippopoulos T, Mittag T, Danias J. Apoptotic retinal ganglion cell death in the DBA/2 mouse model of glaucoma. Exp Eye Res. 2007;84:13–21 [DOI] [PubMed] [Google Scholar]