High-resolution MRI shows heterogeneous extraocular muscle features in strabismus, typical of superior oblique palsy. Rectus pulley counterrotations in response to head tilt may be paradoxical and depend on the presence of superior oblique atrophy, which is detected in half of patients.
Abstract
Purpose.
Although alteration in hypertropia induced by head tilt is considered a clinical criterion for diagnosis of superior oblique (SO) palsy, the mechanism of this head-tilt–dependent hypertropia (HTDHT) is unclear. In this study, magnetic resonance imaging (MRI) was used to study extraocular muscle (EOM) responses to head tilt in HTDHT.
Methods.
Orbital MRI was used to study 16 normal subjects and 22 subjects with HTDT, of whom 12 had unilateral SO atrophy and 10 had “masquerading” SO palsy with normal SO size. Sizes and paths of all EOMs were compared in 90° roll tilts.
Results.
Normal subjects exhibited the expected 3° to 7° physiologic extorsion of all four rectus pulleys in the orbit up-versus-down roll positions, corresponding to ocular counterrolling. In orbits with SO atrophy, the lateral (LR) and inferior rectus (IR) pulleys paradoxically intorted by approximately 2°. Subjects with HTDHT but normal SO size exhibited reduced or reversed extorsion of the medial, superior, and LR pulleys, whereas pulley shift was normal in nonhypertropic fellow orbits in HTDHT. In normal subjects and in SO atrophy, the inferior oblique (IO) muscle contracted in the orbit up-versus-down roll position, but paradoxically relaxed in HTDHT without SO atrophy.
Conclusions.
The ipsilesional IR and LR pulleys shift abnormally during head tilt in HTDHT with SO atrophy. In HTDHT without SO atrophy, the ipsilesional MR, SO, and LR pulleys shift abnormally, and the IO relaxes paradoxically during head tilt. These widespread alterations in EOM pulling directions suggest that complex neural adjustments to the otolith–ocular reflexes mediate HTDHT.
Abundant evidence supports the idea that acute, unilateral SO palsy produces a small ipsilateral hypertropia that increases with contralateral gaze and with roll tilt of the head toward the ipsilateral shoulder.1 The basis of this three-step test has traditionally been attributed to otolith-mediated ocular counterrolling, so that the eye ipsilateral to roll head tilt is normally intorted by the SO and superior rectus (SR) muscles whose vertical actions cancel.2 However, ipsilateral to a palsied SO, unopposed SR elevating action is supposed to create hypertropia. The three-step test has been the cornerstone of diagnosis and classification of cyclovertical strabismus for generations of clinicians.3 When the three-step test is positive, clinicians infer SO weakness and attribute the large amount of interindividual alignment variability to secondary changes4 such as inferior oblique (IO) overaction and SR contracture. Much evidence, however, indicates that the three-step test's mechanism is misunderstood. Kushner5 pointed out that, were traditional teaching true, IO weakening should increase the head-tilt–dependent hypertropia (HTDHT) in SO palsy; however, IO weakening reliably decreases the HTDHT. Instead of creating a very large HTDHT, maximally aggressive simultaneous surgical ablation of both the SO and IO typically creates little or no HTDHT at all.6 Among numerous inconsistencies with common clinical observations,5 bilateral SO palsy should cause a greater head-tilt–dependent change in hypertropia than does unilateral SO palsy; however, the opposite has been found.7 Computer simulation suggests that SO weakness alone cannot account for typical three-step test findings.8,9 Multiple conditions can simulate the SO palsy pattern of incomitant hypertropia.10 Vestibular lesions produce HTDHT. A central lesion of the otolith–ocular reflex pathways causing skew deviation11 can mimic SO palsy in the three-step test.12 Heterotopy of rectus EOM pulleys can simulate SO palsy.13 Although the three-step test has long been a lynchpin of clinical strabismology, neither the test's mechanism nor its implications in EOM disease are understood.
Advances in imaging now offer additional insights into the mystery of HTDHT. Magnetic resonance imaging (MRI) has quantified normal changes in SO cross-sectional area with vertical gaze, as well as SO atrophy and loss of gaze-related contractility typical of SO palsy.14–16 A monkey model of acquired SO palsy due to intracranial trochlear neurectomy has demonstrated the reliable finding of rapid and substantial neurogenic atrophy and elongation of the SO belly, readily demonstrated by MRI.17 A striking and consistent MRI finding has been the nonspecificity of the three-step test for structural abnormalities of the SO belly, tendon, and trochlea, found in only in ∼50% of patients.18 There is wide variation in SO size in unilateral congenital SO palsy, ranging from aplasia to size exceeding the putatively normal fellow SO.19 Even in patients selected because MRI demonstrated profound SO atrophy, there was no correlation between clinical motility and IO size or contractility.16
Although until now, MRI has not provided insight into the head-tilt test in SO palsy, it can be performed in lateral decubitus positions equivalent to 90° head tilts.20 In normal subjects positioned to 90° tilt, MRI shows counterpositioning of the array of rectus EOM pulleys, along with contractile changes in the oblique EOMs that are consistent with their roles in ocular counterrolling.20 This finding has motivated the proposition that the orbital connective tissue apparatus normally comprises a “nested gimbal” arrangement: the rectus EOM pulley array forms the inner gimbal that is rotated torsionally by the “outer gimbal” composed of the oblique EOMs.21 The outer gimbal is activated by binocular convergence,22 and by vestibular input, such as from the otoliths during roll head tilt.20 The experimental primate observation that the direction of eye movements evoked by electrical stimulation of the abducens nerve counterrolls with head tilt supports the outer gimbal concept.23 Known mechanical couplings of the oblique EOMs' orbital layers to the rectus pulleys suggest that SO palsy should alter otolith–mediated shifts of rectus pulleys during head tilting.24,25
The foregoing suggests that the traditional explanation for the three-step test in SO palsy is both physiologically implausible and inconsistent with common clinical observations. We alternatively suppose that abnormal rectus pulley shifts during head tilting may underlie the clinical pattern of hypertropia in SO palsy and similar forms of HTDHT. Locations of the rectus pulleys determine the pulling directions of the rectus EOMs. Depending on its instantaneous pulley location, any rectus EOM can develop horizontal, vertical, and torsional actions. Abnormal vertical and torsional actions of rectus EOMs induced by abnormal location changes of their pulleys may now be considered a potential explanation for HTDHT. A correct understanding of the mechanism of the three-step test would better inform clinical evaluation and therapeutic decision-making in cyclovertical strabismus.
The present study was conducted to clarify the three-step test's mechanism by using MRI to study EOM contractility and pulling directions during head tilting in both well-characterized unilateral SO palsy confirmed by SO atrophy, as well as in HTDHT without reduced SO size “masquerading” as SO palsy.18 A further purpose was to determine whether features evident on clinical motility examination could identify patients with SO atrophy and so obtain insight into specificity of the three-step test.
Materials and Methods
Subjects
Subjects gave written, informed consent according to a protocol adherent to the Declaration of Helsinki. Institutional Review Board approval was obtained before the study began. Control data were reanalyzed from scans of subjects whose data had been published20 and were obtained from 16 adult volunteers with a mean ± SD age of 25.7 ± 4.6 years. Reanalysis was identical with the method performed for newly collected MRIs, to assure comparable results. Control subjects underwent complete examinations verifying normal corrected vision, normal ocular versions, orthotropia in all gaze positions, and normal stereopsis of 40 arc sec by Titmus testing. Twenty-two subjects with cyclovertical strabismus volunteered from a clinical population after clinical diagnosis of HTDHT; all underwent complete sensorimotor evaluation, including Hess screen testing and prism-cover testing in upright and roll head tilt positions. In the Hess screen test, one eye views a sequence of red targets on a 50-cm distant tangent screen through a red filter, while the subject, viewing with the other eye through a green filter, directs a green laser spot into apparent superimposition with the red targets. Strabismus can be quantified by measuring the separation of the red and green lights on the tangent screen that directly indicates the angular disparity in the foveal directions of the two eyes. Cyclotropia was determined by using double Maddox rods.
All subjects with HTDHT had clinical findings compatible with unilateral SO palsy1 and had normal corrected vision in each eye. Subjects with HTDHT were excluded if they had sustained direct orbital trauma, had EOM myopathy, had undergone prior strabismus surgery, or had evidence at subsequent surgery of an abnormality of the SO tendon or its insertion. Ocular motility findings on subjects with HTDHT are summarized in Table 1. Question marks in Table 1 indicate presumptive etiologies. All subjects with HTDHT had corrected visual acuities in each eye of 0.1 logarithm of the minimum angle resolvable (logMAR, 20/25) or better.
Table 1.
Characteristics of Subjects with Head Tilt Dependent Hypertropia
| Subject | Sex | Age (y) | Duration (y) | Etiology | Vertical Imbalance In Adduction |
Upright Hypertropia (Δ) |
Head Tilt Hypertropia (Δ) |
Excyclo. (deg) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infraduct. | Supraduct. | Central | Ipsiversion | Contraversion | Ipsi-Tilt | Contra-Tilt | Tilt Difference | ||||||
| SO Palsy | |||||||||||||
| 1 | M | 76 | 10 | Idiopathic | −1 | +2 | 6 | 6 | 6 | 8 | 2 | 6 | 7 |
| 2 | M | 41 | 41 | Congenital? | −2 | +3 | 25 | 16 | 30 | 40 | 1 | 39 | 0 |
| 3 | F | 60 | 60 | Congenital? | 0 | 0 | 8 | 8 | 8 | 15 | 6 | 9 | ND |
| 4 | F | 48 | 9 | Traumatic | −1 | +1 | 8 | 4 | 20 | 30 | 1 | 29 | 10 |
| 5 | M | 46 | 0.25 | Traumatic | −1 | 0 | 10 | 2 | 12 | 12 | 2 | 10 | 9 |
| 6 | M | 41 | 3 | Idiopathic | −2 | +4 | 20 | 1 | 35 | 35 | 4 | 31 | 4.5 |
| 7 | F | 9 | 9 | Congenital | −2 | +3 | 30 | 40 | 45 | 70 | 0 | 70 | 6 |
| 8 | M | 43 | 43 | Congenital | −4 | +2 | 10 | 12 | 20 | 25 | 3 | 22 | 15 |
| 9 | M | 48 | 48 | Congenital? | −2 | +2 | 4 | 10 | 12 | 16 | 2 | 14 | 7 |
| 10 | M | 27 | 14 | Traumatic | 0 | +3 | 22 | 2 | 30 | 30 | 0 | 30 | 10 |
| 11 | F | 46 | 46 | Congenital? | −4 | +4 | 20 | 25 | 25 | 25 | 10 | 15 | 9 |
| 12 | F | 14 | 1 | Idiopathic | −3 | +3 | 25 | 20 | 30 | 30 | 15 | 15 | ND |
| Mean | 41.6 | 23.5 | −1.8 | 2.2 | 17.4 | 12.9 | 24.2 | 29.7 | 4.7 | 25.0 | 7.8 | ||
| SEM | 5.3 | 6.4 | 0.4 | 0.4 | 2.4 | 3.8 | 3.8 | 4.9 | 1.8 | 5.1 | 1.3 | ||
| Masquerade | |||||||||||||
| 13 | F | 28 | 3 | Idiopathic | −1 | 2 | 3 | 0 | 5 | 5 | 2 | 3 | 0 |
| 14 | M | 24 | 8 | Idiopathic | −3 | 3 | 14 | 0 | 20 | 22 | 4 | 18 | ND |
| 15 | M | 24 | 3 | Idiopathic | −2 | 3 | 0 | 0 | 8 | 6 | 0 | 6 | 4 |
| 16 | M | 55 | 3 | Idiopathic | −2 | 2 | 22 | 8 | 30 | 30 | 3 | 27 | 8 |
| 17 | F | 13 | 3 | Idiopathic | −2 | 2 | 6 | 10 | 20 | 20 | 0 | 20 | 10 |
| 18 | M | 79 | 21 | Idiopathic | 0 | 0 | 6 | 6 | 10 | 5 | 5 | 0 | 0 |
| 19 | F | 32 | 6 | Idiopathic | −2 | 2 | 27 | 15 | 36 | 40 | 6 | 34 | 5 |
| 20 | F | 49 | 49 | Congenital? | 0 | 4 | 25 | 12 | 35 | 37 | 0 | 37 | 4 |
| 21 | M | 49 | 25 | Idiopathic | −2 | 3 | 3 | 0 | 16 | 8 | 2 | 6 | 9 |
| 22 | M | 28 | 28 | Congenital? | −2 | 3 | 18 | 18 | 18 | 30 | 2 | 28 | 5 |
| Mean | 38.1 | 12.4 | −1.6 | 2.4 | 12.4 | 6.9 | 19.8 | 20.3 | 2.4 | 17.9 | 5.0 | ||
| SEM | 6.2 | 5.0 | 0.3 | 0.3 | 3.2 | 2.2 | 3.4 | 4.3 | 0.7 | 4.3 | 1.2 | ||
| P | 0.672 | 0.202 | 0.756 | 0.650 | 0.218 | 0.288 | 0.284 | 0.177 | 0.292 | 0.307 | 0.132 | ||
Question marks indicate presumptive etiology.
Magnetic Resonance Imaging
High-resolution, T1 or T2 fast spin-echo (T2FSE)–weighted MRI was performed with a 1.5-T scanner (Signa; GE Healthcare; Milwaukee, WI). Crucial aspects of this technique, described in detail elsewhere, include use of the dual-phased surface coil array (Medical Advances, Milwaukee, WI).13,26–28 For T1 imaging, gadodiamide contrast was injected intravenously in doses of 0.1 millimole/kg in each of the two head-tilt positions; no contrast was injected for T2FSE imaging. The two scanning protocols provide equivalent measurements.
During MRI, subjects, at a distance of 2 cm, fixated with the upper part of the eye the illuminated end of a fine optical fiber. Initially, a triplanar (axial, coronal, and sagittal) scan was obtained in 5-mm thickness image planes at 940-μm resolution using a 256 × 256 matrix over a 24-cm2 field of view (FOV). These images, axial examples of which are shown in the insets in Figure 1, were used to verify correct head positioning and absence of convergence. We then obtained sets of 18 to 20 contiguous, 2-mm-thick, quasi-coronal images in a plane perpendicular to the long axis of each orbit, using a 256 × 256 matrix over an 8-cm FOV (resolution 312 μm, Fig. 1), and quasi-sagittal images in planes parallel to the long axis of each orbit (Fig. 2). Scanning was repeated in both the right side down and left side down positions.
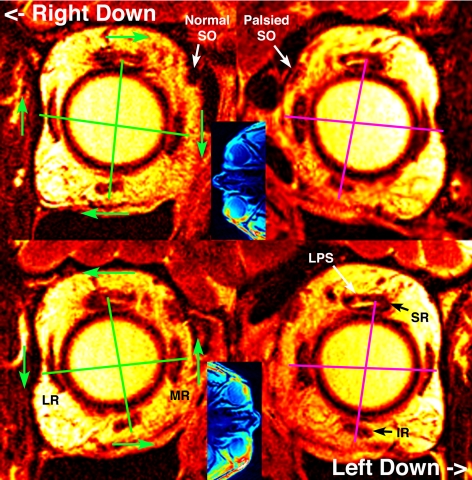
Figure 1.
Quasi-coronal T2FSE MRI in planes close to the rectus pulleys, in a subject with left SO palsy. Blue insets: are axial views indicating actual head orientation relative to gravity: top panels tilted right down and bottom panels tilted left down. The right orbit appears on the left. Green lines connect corresponding horizontal and vertical rectus pulleys in the normal right orbit, and show with green arrows normal pulley array rotation counter to head tilt. If it had been normal, the left orbit would have shown clockwise rotation with the same sense as the left orbit in right down and counterclockwise with the left down. Instead, magenta lines in the left orbit with SO palsy show reduction of the pulley array rotation. IR, inferior rectus muscle; LPS, levator palpebrae superioris muscle; LR, lateral rectus muscle; MR, medial rectus muscle.
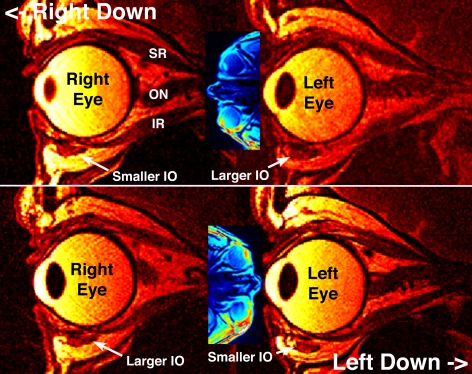
Figure 2.
Quasi-sagittal MRI in plane of the inferior rectus (IR) center, in subject with left SO palsy depicted in Figure 1. Note that for both inferior oblique (IO) muscles, the cross section (white dotted outlines) is larger in the upper than in the lower orbit during 90° roll tilt. ON, optic nerve.
Analysis
Digital MRI images were quantified using the programs NIH Image or ImageJ (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/). Analyses were performed by investigators masked to the diagnoses and laterality of patients with HTDHT.
In MRI images, the cross section of each EOM was digitally outlined, and its area automatically determined. The location of each rectus EOM was described by a single point, using the area centroid function,29 which represents the best estimate of the position of the EOM.30 Next, approximating the globe as spherical, we determined its 3-D center, as previously described.30 Rectus EOM positions were then translated to place the 3-D coordinate origin at the computed globe center. The three Cartesian coordinates were positive lateral, positive superior, and positive anterior.30 All EOM path data were rotated into a standard orientation, with the interhemispheric fissure of the brain and the junction of the superior ethmoid air sinus and the orbit used as orientation references.30 After the data were transformed, coordinates were scaled to normalize each globe to the measured average diameter of 24.3 mm, permitting averaging of 3-D rectus EOM paths over the group of subjects.30
The volumes of the rectus EOMs and the SO were analyzed in the five contiguous quasi-coronal image planes immediately posterior to the globe–optic nerve junction in central gaze, beginning with the image plane containing the globe–optic nerve junction. For classification purposes, SO volumes in individual subjects were averaged in the tilt-up and tilt-down positions.
Torsion of EOM paths and pulleys was defined as rotation about the long axis of the orbit, analogous to the definition of ocular torsion as globe rotation around the line of sight. Intorsion was regarded as the medial shift of the SR, the inferior shift of the medial rectus (MR), the lateral shift of the IR, and the superior shift of the lateral rectus (LR).
For evaluation of IO contractility, sets of quasi-sagittal image planes were aligned on the center of the IR crossing. When two image planes were equally close to the center of the IR crossing, IO cross sections in both of these planes were averaged. Contractility was taken to be the change in cross section from the right-ear-down to left-ear-down positions in the image plane of the IR crossing.22,25
Subjects with HTDHT were divided into two groups based on SO size determined by quasi-coronal MRI: Twelve subjects had significant unilateral SO atrophy (Fig. 3), and 10 had bilaterally normal SO size. The former group was considered to represent “true” SO palsy, with the latter group representing “masquerading” SO palsy. No subject with HTDHT had evidence of abnormality of the SO tendon or its scleral insertion, either on MRI, or at surgical exploration, if surgery was performed later. Two additional subjects with HTDHT were studied, but have been excluded from all analyses, because at strabismus surgery, they were found to have abnormal SO tendons at the insertions.
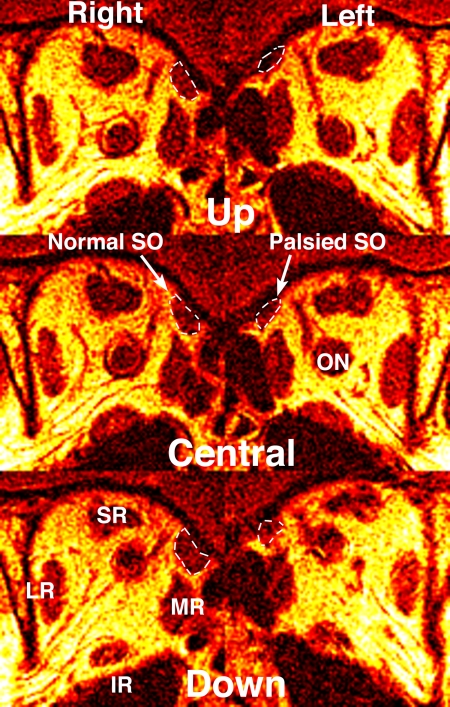
Figure 3.
Quasi-coronal MRI in midorbit of the subject with left SO palsy illustrated in Figures 1 and 2. The cross section of the normal right SO is larger than that of the palsied left SO in central gaze, and the cross section of the right but not the left SO increases from up to down gaze. This represents neurogenic atrophy of the left SO. Abbreviations as in Figures 1 and 2.
Results
Clinical Findings
Subjects with true versus masquerading SO palsy did not differ significantly in any demographic or clinical features (P > 0.25, Table 1). Vertical imbalances in adduction, central gaze hypertropia, lateral incomitance, head-tilt difference, and excyclotropia by double Maddox rod testing all were similar in subjects with SO palsy versus those with masquerading SO palsy. In both groups, there was a central gaze hypertropia averaging 12 to 17 Δ that averaged 13 to 14 Δ greater in contraversion than in ipsiversion, and 18 to 25 Δ greater in ipsilateral than in contralateral head tilt. Subjective excyclotropia by double Maddox rod testing averaged 7.8° ± 1.3° (SEM) in SO palsy, not significantly different from the value of 5.0° ± 1.2° in masquerading SO palsy. A Hess screen test pattern typical of SO palsy was present in six of nine subjects tested who had true SO palsy, and in four of eight tested with masquerading SO palsy. The remaining subjects who underwent Hess screen testing in both groups either had fully concomitant hypertropia or hypertropia that was horizontally comitant but increased in downward gaze (deorsumversion). Most subjects in both groups adopted a contraversive compensatory head tilt. Facial abnormalities were present in three of 12 subjects with true SO palsy, consisting of one case each of bilateral malar hypoplasia, symmetrical mandibular hypoplasia, and hemifacial microsomia. All other subjects in both groups had normal and symmetric faces.
The etiology and duration of strabismus in both groups were difficult to ascertain due to gradually progressive onset. The mean duration of diplopia was 23.5 ± 6.4 years in subjects with SO palsy and 12.4 ± 5.0 years in subjects with masquerading SO palsy (P > 0.25). Subjects were carefully questioned about the onset and duration of diplopia and head tilt, and causal relationships to prior events were inferred only when these events were plausible and immediately before the onset of symptomatic diplopia. Three subjects had SO-palsy–related acute onset of diplopia after credible episodes of severe, acute head trauma in automobile or bicycle traffic accidents. Two subjects with SO palsy had clear photographic evidence of the congenital duration of compensatory head tilt. In the remaining subjects with SO palsy and all subjects with masquerading SO palsy, the disorder had an idiopathic origin that could not be distinguished on clinical grounds from a decompensated congenital condition or unknown progressive condition.
SO Size
Mean SO volume for control subjects was 183.5 ± 5.4 mm3, not significantly different from volumes of the hypertropic (203.5 ± 9.2 mm3) and hypotropic (193.7 ± 9.1 mm3) SO in masquerade subjects and not significantly different from the hypotropic SO in true SO palsy (176.3 ± 13.3 mm3; Table 2). However, the volume of the hypertropic SO in subjects with true SO palsy was 96.1 mm3, significantly smaller than in the other groups (P < 0.00001; Table 2).
Table 2.
SO Volume*
| Group | Hypertropic SO Volume (mm3) |
Hypotropic SO Volume (mm3) |
||
|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |
| SO Palsy (n = 12 subjects) | 96.1† | 7.2 | 176.3 | 13.3 |
| Masquerade (n = 10 subjects) | 203.5 | 9.23 | 193.7 | 9.1 |
| Group | Normal SO Volume (mm3) |
Hypotropic SO Volume (mm3) |
||
|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |
| Control (n = 39 orbits) | 183.5 | 5.4 | — | — |
Averaged for ipsilateral and contralateral tilt.
Different from normal and SO palsy hypotropic side at P < 0.00001.
Rectus Pulley Positions
Shifts in positions of the pulleys during head tilt were demonstrable by MRI, indicating that head tilting changes the pulling directions of the rectus EOMs to impart oblique and torsional actions. In both control subjects and in subjects with HTDHT, the coronal plane coordinates of rectus EOMs were generally more excyclorotated in the 90° head tilt-up than tilt-down position. This effect is illustrated in a subject with SO palsy in Figure 1, showing quasi-coronal MRI views near the rectus pulleys. Since arbitrary quasi-coronal planes are unlikely to exactly include the planes containing all four rectus pulleys simultaneously, a published analytical approach was used to improve precision of pulley localization.20 Coordinates for all rectus pulleys were averaged across both orbits of all subjects for the ipsi- and contralateral ear-downward positions. Mean Cartesian coordinates of the rectus pulleys are plotted in Figure 4.
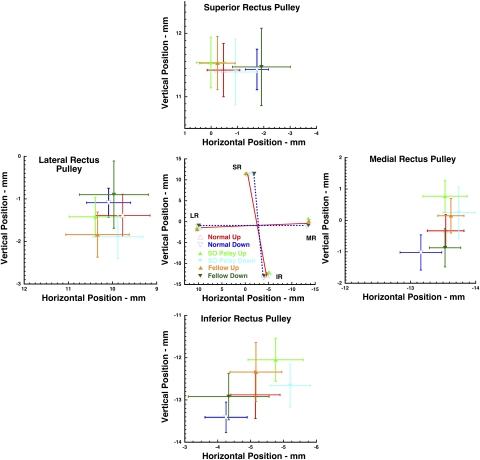
Figure 4.
The effect of 90° upward and downward roll tilt on coronal plane coordinates of the four rectus pulleys, referenced to the globe center, in 31 normal control orbits and 12 affected and 12 fellow orbits in subjects with SO palsy. Error bars, 95% confidence intervals. Solid and broken lines between coordinates in the center panel connect the normal pulley array. The four side panels expand data for individual pulleys. Note the torsional shifts of normal rectus pulleys (center graph), but the complex pattern of different shifts in palsied and fellow orbits in SO palsy.
Figure 4 confirms that the normal rectus pulley array counterrotates during head tilt, so that when the orbit is tilted 90° upward, the SR shifts temporally, the LR inferiorly, the IR nasally, and the MR superiorly, relative to their positions when the orbit is tilted 90° downward. The red and blue lines connecting the corresponding rectus pulleys in the two roll tilt positions in Figure 4 highlight this counterrotation in normal subjects. The four flanking graphs in Figure 4 illustrate 95% confidence intervals for the corresponding rectus pulley coordinates for hypertropic and hypotropic orbits in each subject group, in comparison with normal subjects. These confidence regions may be seen to vary in a complex way, both by roll position and by subject group. A more informative presentation requires angular conversion.
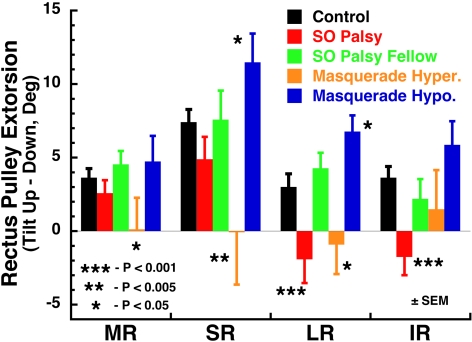
Since the rectus pulleys are arrayed around the circumference of the globe, shifts in their Cartesian coordinates correspond to angular rotations of the pulleys in the array measured in the 31 normal orbits, for both right and left head tilts, to have a mean diameter of the array of 24.17 mm. Torsional pulley shifts are plotted in Figure 5, for normal subjects and subjects with true or masquerading SO palsy. Torsional shifts of all pulleys of normal subjects were significantly different from 0 at the 0.005 level. For orbits with SO palsy, shifts of the MR and SR pulleys were significant only at the 0.05 level; LR and IR shifts were not significantly different from 0 and were different from normal at the 0.01 level. For fellow orbits of SO palsy, shifts of all pulleys were significant at the 0.05 level and were not significantly different from normal. For hypertropic orbits in masquerading SO palsy, none of the pulleys exhibited shifts significantly different from 0, although all pulleys in the fellow hypotropic orbits exhibited extorsional shifts significant at the 0.005 level.
Figure 5.
Torsional shift of rectus pulleys in the 90° roll tilt up-versus-down positions in 31 normal control orbits and 12 affected and 12 fellow orbits in subjects with SO palsy and in 10 hypertropic and 10 hypotropic orbits of subjects with masquerading SO palsy. Note the reversed shift of LR and IR pulleys in the orbits with SO palsy, but different patterns of pulley shift in both orbits in masquerading SO palsy. Deg, degrees; Hyper, hypertropic; Hypo, hypotropic.
Abnormal rectus pulley shifts and, by direct implication, abnormal rectus pulling directions were associated with head tilt in HTDHT (Fig. 5). The LR and IR pulleys in orbits with SO palsy intorted, rather than extorted, during upward head tilt. This movement was significantly different from normal at the 0.01 level and significantly different from the fellow orbit at the 0.05 level. All pulley shifts were not significantly different from normal in the fellow orbits in SO palsy and in the hypotropic orbits in masquerading SO palsy. In hypertropic orbits in masquerading SO palsy, the attenuated shifts of the MR, SR, and LR pulleys were significantly different from normal at the 0.05 level, but were not intorted by head tilt, as was the case for the LR and IR pulleys in SO palsy.
SO Cross Section
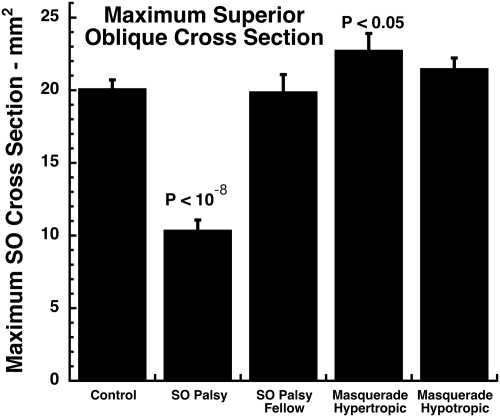
When the cross-sectional areas in the tilt-right with tilt-left positions were averaged, the mean maximum SO was approximately 50% smaller than normal in the hypertropic orbit in SO palsy (P < 10−8), but the maximum SO cross section did not differ significantly among controls, the hypotropic orbit in SO palsy, or the hypotropic orbit in masquerading SO palsy (Fig. 6). However, the SO cross section in the hypertropic orbit in masquerading SO palsy was approximately 15% larger than normal (P < 0.05).
Figure 6.
Maximum SO cross section, averaged for right and left head tilts. The SO cross section was highly significantly reduced from normal in the hypertropic orbit in SO palsy, but increased from normal in the hypertropic orbit in masquerading SO palsy. SO cross sections in hypotropic orbits of both SO true and masquerading palsies did not differ from normal. Error bars, SEM.
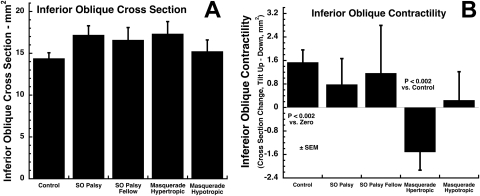
IO Contractility
This effect was quantified by its change in cross section from ipsi- to contralateral tilt (Fig. 7). The cross-sectional area of the IO in the hyper- or hypotropic orbits of subjects with true or masquerading SO palsies did not vary significantly from the control value (Fig. 7A). Control subjects exhibited significantly greater IO cross section in the tilt-up versus tilt-down position, which is consistent with physiological extorsion during ocular counterrolling (P < 0.002; Fig. 7B). The change in IO cross section was not significantly different from normal in either orbit of subjects with SO palsy. However, the IO exhibited a significant paradoxical relaxation in the hypertropic orbit of subjects with masquerading SO palsy (P < 0.002; Fig. 7B). There was no significant change in IO cross section associated with head tilt in the hypotropic orbit of subjects with masquerading SO palsy.
Figure 7.
Inferior oblique (IO) cross section in the image plane of the IR crossing. (A) There was no significant variation from normal in mean cross-sectional area in the orbit of subjects with either true or masquerading SO palsy. (B) Normal subjects exhibited significantly larger IO cross section in tilt-up minus tilt-down position, as appropriate to extorsional ocular counterrolling. IO muscles in hypertropic orbits of subjects with masquerading palsy, but no other orbits, exhibited significant reduction in IO cross section in tilt-up minus tilt-down positions, interpreted as paradoxical IO relaxation.
Discussion
This study confirmed and extended an earlier report that the classic clinical three-step test is not specific for deficiency of SO size demonstrable by high-resolution MRI.18 The group of 12 subjects with significant SO atrophy had ipsilateral HTDHT, and the magnitude and variation with gaze and head tilt did not differ significantly from that of the group of 10 subjects who had normal SO size by MRI. Although both groups had similar versions and strabismus patterns typical of SO palsy, we used a novel technique of MRI during head tilt to reveal major differences in behavior of the rectus and inferior oblique EOMs during head tilt. Rather than exhibiting physiological extorsion of the rectus pulley array during upward tilt, in orbits ipsilesional to SO atrophy, the LR and IR pulleys shifted in the opposite direction, changing the pulling directions of these EOMs. Subjects with HTDHT but normal SO size exhibited reduced or reversed extorsion of the MR, SR, and LR pulleys, whereas pulley shift was normal in hypotropic fellow orbits. In normal subjects and in subjects with SO atrophy, the IO contracted in the orbit up-versus-down roll position, but paradoxically relaxed in HTDHT without SO atrophy. These significant differences in EOM behavior support a fundamental difference in pathophysiology of HTDHT in the setting of SO atrophy versus in the absence of SO atrophy.
This study confirms a prior a report that the IO is neither hypertrophic nor hypercontractile ipsilateral to SO palsy.16 IO cross section and contractility did not differ significantly from normal in either orbit of subjects with HTDHT, consistent with another MRI report that oblique EOM size does not correlate with head-tilt difference in hypertropia in SO palsy.31 No subject with HTDHT in this study exhibited supernormal IO contractile thickening during head tilt. Rather than overacting during head tilt to the hypertropic side, the IO of subjects with HTDHT and no SO atrophy exhibited relaxational thinning of the hypertropic IO. Thus, functional imaging demonstrated that some subjects with HTDHT actually exhibited paradoxical IO underaction!
Since it has recently been demonstrated that experimental trochlear neurectomy in monkeys produces rapid SO atrophy17 of approximately 50% that is similar to that observed in the subjects with SO atrophy studied herein (Fig. 6), it is reasonable to regard such subjects as having “true” SO palsy. Reduced SO cross section correlates strongly with reduced change in cross-sectional area from supraduction to infraduction and so appears to correlate with reduced contractility.14,16 In monkey, the denervated SO muscle elongates in a way that makes it appear floppy,17 consistent with the report that intraoperative mechanical assessment of SO tendon laxity correlates with atrophy of the SO belly.32 Evidence now clearly indicates that a previously normal SO subjected to acute denervation will become thin, elongated, and consequently deliver weak contractile force to the globe. Nevertheless, we have questioned the clinical reliability of the intraoperative SO traction test,33,34 because in our hands, it has been insensitive even to profound SO atrophy demonstrated by MRI. A prospective study of SO traction testing showed only fair agreement by masked examiners, little correlation between observed SO laxity and diagnosed SO palsy, and frequent observation of SO laxity in clinical situations where SO palsy was not a consideration.35
There should be little controversy about considering subjects with MRI evidence of SO atrophy to have true SO palsy. Extrapolating from the sample in this study, perhaps half (10/22) of subjects with clinical examinations consistent with SO palsy would not exhibit MRI evidence of SO atrophy or reduced SO contractility. Was it reasonable for us to consider this latter group of subjects with HTDHT to have masquerading SO palsy? The masquerade group does not have a deficiency of SO size or contractility, and so the only way the HTDHT could be related to SO function would be if the SO generates force under appropriate conditions that could not be transmitted to rotate the globe.
Plausible abnormalities of SO force transmission to the globe seem to be limited to the pathology of the SO's scleral insertion, its path through the trochlea, or its tendon. Wide anatomic variation in the size and location of the SO insertion on the sclera has been described.36 In the absence of significant trauma, however, reduced mechanical advantage due to malposition of the SO insertion would have to be a congenital anomaly. This study excluded two subjects found at surgery to have anomalous SO tendon insertions, and only two other subjects with masquerading SO palsy (subjects 20 and 22) had lifelong evidence of strabismus. In fact, most subjects with masquerading palsy had symptoms of diplopia for only a few years, about half as long on average as subjects with true SO palsy (Table 1). Although a cause of strabismus was never identified in any subject with masquerading SO palsy, the evolution of symptoms was so late and rapid as to make a congenital SO tendon or insertion anomaly implausible for all but two.
A limitation of this study is the inability to assess the mechanical properties of the SO tendon or the location of the SO insertion. With this consideration, it may be concluded that patients considered to have masquerading SO palsy do not have trochlear neuropathy and do have normal SO muscle size and contractility. When the clinical history further indicates progressive, acquired diplopia as was generally the case here, patients with masquerading palsy probably have no abnormality of the SO at all, unless there is a currently unknown progressive degeneration of the SO tendon and insertion. Progressive, idiopathic degeneration of rectus and IO insertions is not recognized to occur, making the possibility of SO tendon degeneration unlikely.
A remarkable observation is that the SO cross section in the hypertropic orbit of subjects with masquerading SO palsy was significantly larger than normal (Fig. 6). This finding is the opposite of what might be expected after SO denervation. Such a paradoxical finding might even be considered a compensatory SO hypertrophy or hypercontractility to reduce, rather than increase, the hypertropia. Compensatory changes in EOM size are increasingly recognized as complicating hypertropia.37 Hypercontractility of the contralateral IR has been reported as perhaps representing a perverse adaptive mechanism that increases hypertropia in SO palsy,37,38 but hypertrophy of the contralateral SR may be a compensatory mechanism.37
Although prospectively collected, the sample size of subjects with HTDHT was limited to 22 by rigorous inclusion criteria. These criteria included: clinical findings generally considered typical of unilateral SO palsy; absence of prior strabismus surgery, orbital trauma, or myopathy; and performance of high-resolution MRI of the orbits. Subjects were also excluded if subsequent surgery demonstrated abnormality of the SO insertion, although the SO insertion was not examined in every patient, and SO traction testing was not performed as a part of the study. The operational definition of true SO palsy was rigorous, requiring significant unilateral hypoplasia of the SO belly without imaging evidence of abnormality of the SO tendon or trochlea. Although these criteria are unequivocally specific for highly significant unilateral SO hypoplasia consistent with trochlear denervation in the 12 subjects with HTDHT due to SO palsy, they may have excluded milder cases of SO paresis. On the other hand, the group of 10 subjects with HTDHT classified as masquerading SO palsy had SO muscles that were quantitatively not subnormal in size. In fact, the SO muscles in the hypertropic orbits of subjects with masquerading SO palsy had maximum cross sections significantly larger than normal. Rather than overacting as conventionally assumed to occur in SO palsy, the IOs of subjects with masquerading SO palsy paradoxically relaxed during head tilt. The present study thus provides evidence that a significant subset of subjects fulfilling traditional clinical criteria for SO palsy have SO muscles that are not atrophic, and IO muscles that do not overact by MRI criteria. Pulley shifts during head tilt in such masquerading palsy subjects are significantly different from those in clinically identical subjects who have highly significant SO atrophy.
This study protocol cannot address some factors postulated to influence EOM action. MRI cannot detect hypothesized intrinsic changes within the EOM that may affect contractility, such as fiber-type changes, sarcomere addition, or fibrosis and contracture. Changes in sarcomere size have been shown to occur in EOMs39 and have been speculated to affect EOM mechanical behavior in a fashion similar to overaction.40 Such changes have not been demonstrated in association with cyclovertical strabismus in humans or animals, however. It might be plausible for transient, resolved SO denervation to induce SO lengthening during a period of temporary paralysis. However, subjects in the current series with masquerading SO palsy did not have histories compatible with this scenario.
Clinicians may be uncomfortable with our proposition that the positive three-step test nonspecifically includes patients who do not have an abnormality of SO size reflective of denervation and therefore are unlikely to have a deficit in SO contractility. Historically, it has become customary for many clinicians to consider a positive three-step test to be the operational definition of SO palsy itself.41 Even in light of all limitations, the present study demonstrates that no set of clinical examination findings, including the three-step test, is specific for denervation atrophy of the SO muscle belly. A positive three-step test does not necessarily imply the existence of a trochlear denervating lesion, an implication that may be clinically useful in guiding possible further neurologic investigations. Routine use of the three-step test should perhaps be limited to clinical situations where acutely acquired, unilateral SO palsy is plausible. It should be recognized that approxiamtely half of patients with HTDHT and positive three-step tests may have normal or even supernormal SO size and contractility. These patients with masquerading SO palsy may be particularly good candidates for surgery to plicate or advance the SO tendon. On the other hand, SO tendon surgery in patients with true SO palsy leading to an atrophic, noncontractile SO muscle may not be expected to have a good, long-term response to SO tendon surgery. Prior surgical outcome studies have not assessed EOM anatomy in SO palsy. It would therefore be valuable if future clinical studies of outcomes of surgery for SO palsy included imaging assessment of the status of the SO belly and assessment of the locations and dynamic behavior of pulley positions that control the cyclovertical actions of the rectus EOMs.
Footnotes
Supported by National Eye Institute Grant EY-08313 and Research to Prevent Blindness. JLD is the Leonard Apt Professor of Ophthalmology.
Disclosure: J.L. Demer, None; J. Kung, None; R.A. Clark, None
References
- 1. von Noorden GK, Murray E, Wong SY. Superior oblique paralysis: a review of 270 cases. Arch Ophthalmol. 1986;104:1771–1776 [DOI] [PubMed] [Google Scholar]
- 2. Adler FE. Physiologic factors in differential diagnosis of paralysis of superior rectus and superior oblique muscles. Arch Ophthalmol. 1946;36:661–673 [DOI] [PubMed] [Google Scholar]
- 3. Scott WE, Kraft SP. Classification and treatment of superior oblique palsies, II: bilateral superior oblique palsies. In: Caldwell D. ed. Pediatric Ophthalmology and Strabismus: Transactions of the New Orleans Academy of Ophthalmology. New York: Raven Press; 1986:265–291 [PubMed] [Google Scholar]
- 4. Straumann D, Steffen H, Landau K, et al. Primary position and Listing's law in acquired and congenital trochlear nerve palsy. Invest Ophthalmol Vis Sci. 2003;44:4282–4292 [DOI] [PubMed] [Google Scholar]
- 5. Kushner BJ. Ocular torsion: rotations around the “WHY” axis. J AAPOS. 2004;8:1–12 [DOI] [PubMed] [Google Scholar]
- 6. Agarwal S, Kushner BJ. Results of extraocular muscle surgery for superior oblique myokymia. J AAPOS. 2010;13:472–476 [DOI] [PubMed] [Google Scholar]
- 7. Kushner BJ. The diagnosis and treatment of bilateral masked superior oblique palsy. Am J Ophthalmol. 1988;105:186–194 [DOI] [PubMed] [Google Scholar]
- 8. Robinson DA. Bielschowsky head-tilt test, II: Quantitative mechanics of the Bielschowsky head-tilt test. Vision Res. 1985;25:1983–1988 [DOI] [PubMed] [Google Scholar]
- 9. Simonsz HJ, Crone RA, van der Meer J, Merckel-Timmer CF, van Mourik-Noordenbos AM. Bielschowsky head-tilt test, I: ocular counterrolling and Bielschowsky head-tilt test in 23 cases of superior oblique palsy. Vision Res. 1985;25:1977–1982 [DOI] [PubMed] [Google Scholar]
- 10. Kushner BJ. Errors in the three-step test in the diagnosis of vertical strabismus. Ophthalmology. 1987;96:127–132 [DOI] [PubMed] [Google Scholar]
- 11. Brodsky ME. Three dimensions of skew deviation. Br J Ophthalmol. 2003;87:1440–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donahue SP, Lavin PJ, Hamed LM. Tonic ocular tilt reaction simulating a superior oblique palsy: diagnostic confusion with the three-step test. Arch Ophthalmol. 1999;117:347–352 [DOI] [PubMed] [Google Scholar]
- 13. Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2:17–25 [DOI] [PubMed] [Google Scholar]
- 14. Demer JL, Miller JM. Magnetic resonance imaging of the functional anatomy of the superior oblique muscle. Invest Ophthalmol Vis Sci. 1995;36:906–913 [PubMed] [Google Scholar]
- 15. Chan TK, Demer JL. Clinical features of congenital absence of the superior oblique muscle as demonstrated by orbital imaging. J AAPOS. 1999;3:143–150 [DOI] [PubMed] [Google Scholar]
- 16. Kono R, Demer JL. Magnetic resonance imaging of the functional anatomy of the inferior oblique muscle in superior oblique palsy. Ophthalmology. 2003;110:1219–1229 [DOI] [PubMed] [Google Scholar]
- 17. Demer JL, Poukens V, Ying H, Shan X, Tian J, Zee DS. Effects of intracranial trochlear neurectomy on the structure of the primate superior oblique muscle. Invest Ophthalmol Vis Sci. 2010;51:3485–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Demer JL, Miller MJ, Koo EY, Rosenbaum AL, Bateman JB. True versus masquerading superior oblique palsies: muscle mechanisms revealed by magnetic resonance imaging. In: Lennerstrand G. ed. Update on Strabismus and Pediatric Ophthalmology. Boca Raton, FL: CRC Press; 1995;303–306 [Google Scholar]
- 19. Uchiyama E, Matsuo T, Imai S, Itoshima E. Paretic side/normal side ratios of cross-sectional areas of the superior oblique muscle vary largely in idiopathic superior oblique palsy. Am J Ophthalmol. 2010;149:508–512 [DOI] [PubMed] [Google Scholar]
- 20. Demer JL, Clark RA. Magnetic resonance imaging of human extraocular muscles during static ocular counter-rolling. J Neurophysiol. 2005;94:3292–3302 [DOI] [PubMed] [Google Scholar]
- 21. Demer JL. Mechanics of the orbita. Dev Ophthalmol. 2007;40:132–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol. 2003;89:2072–2085 [DOI] [PubMed] [Google Scholar]
- 23. Klier EM, Meng H, Angelaki D. Revealing the kinematics of the oculomotor plant with tertiary eye positions and ocular counterroll. J Neurophysiol. 2011;105:640–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kono R, Poukens V, Demer JL. Superior oblique muscle layers in monkeys and humans. Invest Ophthalmol Vis Sci. 2005;46:2790–2799 [DOI] [PubMed] [Google Scholar]
- 25. Demer JL, Oh SY, Clark RA, Poukens V. Evidence for a pulley of the inferior oblique muscle. Invest Ophthalmol Vis Sci. 2003;44:3856–3865 [DOI] [PubMed] [Google Scholar]
- 26. Demer JL, Miller JM. Orbital imaging in strabismus surgery. In: Rosenbaum AL, Santiago AP. eds. Clinical Strabismus Management: Principles and Techniques. Philadelphia: WB Saunders; 1999;84–98 [Google Scholar]
- 27. Clark RA, Miller JM, Demer JL. Displacement of the medial rectus pulley in superior oblique palsy. Invest Ophthalmol Vis Sci. 1998;39:207–212 [PubMed] [Google Scholar]
- 28. Clark RA, Rosenbaum AL, Demer JL. Magnetic resonance imaging after surgical transposition defines the anteroposterior location of the rectus muscle pulleys. J AAPOS. 1999;3:9–14 [DOI] [PubMed] [Google Scholar]
- 29. Miller JM. Functional anatomy of normal human rectus muscles. Vision Res. 1989;29:223–240 [DOI] [PubMed] [Google Scholar]
- 30. Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–3797 [PubMed] [Google Scholar]
- 31. Kono R, Okanobu H, Ohtsuki H, Demer JL. Absence of relationship between oblique muscle size and Bielschowsky head tilt phenomenon in clinically diagnosed superior oblique palsy. Invest Ophthalmol Vis Sci. 2009;50:175–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sato M. Magnetic resonance imaging and tendon anomaly associated with congenital superior oblique palsy. Am J Ophthalmol. 1999;127:379–387 [DOI] [PubMed] [Google Scholar]
- 33. Plager DA. Tendon laxity in superior oblique palsy. Ophthalmology. 1992;99:1032–1038 [DOI] [PubMed] [Google Scholar]
- 34. Plager DA, Helveston EM, Fahad B. Superior oblique muscle atrophy/hypodevelopment in superior oblique palsy. Abstracts of the 22nd Annual AAPOS Meering 1996;10 [Google Scholar]
- 35. Sato M, Amano E, Okamoto Y, Ota Y, Hirai T. Interexaminer differences in the traction test of the superior oblique tendon. Jpn J Ophthalmol. 2005;49:216–219 [DOI] [PubMed] [Google Scholar]
- 36. Fink WH. Surgery of the Vertical Muscles of the Eye. Springfield, IL: Thomas; 1962;37–121 [Google Scholar]
- 37. Clark RA, Demer JL. Magnetic resonance imaging demonstrates enhanced vertical rectus contractility in superior oblique palsy. Arch Ophthalmol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jiang L, Demer JL. Magnetic resonance imaging of the functional anatomy of the inferior rectus muscle in superior oblique muscle palsy. Ophthalmology. 2008;115:2079–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scott AB. Change of eye muscle sarcomeres according to eye position. J Pediar Ophthalmol Strabismus. 1994;31:85–88 [DOI] [PubMed] [Google Scholar]
- 40. Kushner BJ. Multiple mechanisms of extraocular muscle “overaction”. Arch Ophthalmol. 2006;124:680–688 [DOI] [PubMed] [Google Scholar]
- 41. Demer JL. Clarity of words and thoughts about strabismus. Am J Ophthalmol. 2001;132:757–759 [DOI] [PubMed] [Google Scholar]