Abstract
Gene therapy based on delivery of viral and nonviral vectors has shown great promise for the treatment of human ocular diseases; however, limitations have consistently prevented its widespread clinical application. Viral vectors have generally been better in terms of efficiency but have safety concerns. Nonviral vectors, on the other hand, offer safety but have often been disappointing in terms of efficiency of nuclear delivery and gene expression. Extensive animal studies have reported significant progress using both systems, but thus far only a few studies have shown promise in human clinical trials. This article reviews both viral and nonviral work with focus on two candidates for clinical ocular application—AAV and nanoparticles. Of particular interest are various requirements for successful clinical application of these technologies including vector trafficking, delivery, specific gene expression, and treatment safety, and tolerance.
Gene therapy for the eye can be split into two categories: gene replacement and gene regulation (such as knocking down a gene or turning it on or off). Because of the monogenic nature of many of the inherited forms of retinal degeneration and the relative immune privilege of the eye, ocular gene therapy is a promising research avenue for treatment of blinding disorders. Gene therapy requires a vector to deliver the therapeutic gene to the target cells. The most challenging aspect of gene therapy is delivering precisely the right quantity of properly regulated gene to yield optimal levels of expression in the right cell type and at the right time without stimulating host immunity.
Thus far, delivery methods can be broadly divided into viral and nonviral approaches (Tables 1, 2). Since the first gene therapy clinical trials began in 1989, employing a retroviral vector,1,2 over 1537 trials have been approved, initiated, and reviewed (http://clinicaltrials.gov/ and http://www.wiley.co.uk/genmed/clinical/). Despite the difficulties encountered in some of the early trials, progress in the field is evident. As one example, three AAV-based ocular clinical trials for Leber's congenital amaurosis (LCA), begun in 2007 to 2008, have all reported promising initial results (see later discussion).3–6
Table 1.
Types of Viral Vectors Used in Clinical Trials
| Vector | Characteristics | Disadvantages | Examples of Clinical Trials* |
|---|---|---|---|
| Retrovirus | Single stranded RNA virus, infects only dividing cells, up to 7.5 kb packaging capacity; high transduction efficiency | Insertional mutagenesis; requires cell division | SCID-X1, HIV, glioblastoma, ADA deficiency |
| Lentivirus | A subclass of retroviruses, infects both dividing and nondividing cells; up to 7 kb packaging capacity; high transduction efficiency | Insertional mutagenesis; risk of replication competent HIV | Mucopolysaccharidosis type VII, HIV-1 |
| Adenovirus | Double stranded DNA virus; up to 30 kb packaging capacity; high transduction efficiency | Immune response; transient gene expression | HIV, prostate cancer, melanoma, lung cancer, colon carcinoma |
| AAV | Single-stranded DNA virus; infects both dividing and nondividing cells; up to 5 kb capacity; good for small scale only | Small packaging capacity | Age related macular degeneration, malignant melanoma, cystic fibrosis, Alzheimer's disease, Duchen ne muscular dystrophy, LCA |
| Herpesvirus | Double stranded DNA virus; infects neurons; up to 50 kb packaging capacity; high efficiency | Inflammatory and immune response | Malignant melanoma, glioma, neuroblastoma |
Clinical trial data from http://clinicaltrials.gov/ and http://www.wiley.co.uk/genmed/clinical/.
Table 2.
Types of Nonviral Vectors Used in Clinical Trials
| Vector | Characteristics | Disadvantages | Examples of Clinical Trials* |
|---|---|---|---|
| Naked DNA | Any DNA, simplicity, cheap | Low efficiency, transient expression | HIV, critical limb ischaemia, renal cell carcinoma, coronary heart disease |
| Oligonucleotides | Including antisense oligonucleotide, siRNA and double stranded oligodeoxynucleotide | Transient expression | Age-related macular degeneration |
| Lipolexes | Complexes of liposome with DNA, high efficiency; may protecting DNA from enzymatic degradation and tissue specific | Possible toxicity, transient expression | Melanoma, chronic renal insufficiency, glioblastoma, cystic fibrosis |
| Nanoparticles | Small size (<500 nm), large capacity, high loading densities, efficient, biocompatible, biodegradable | Costly, quality control difficulties | Cystic fibrosis |
| Sleeping beauty | Reconstitution of an ancient transposon; chromosomal integration | Very low efficiency, potential mutagenesis | T lymphocytes cancer (CD 19+ B-lymphoid malignancies) |
Clinical trial data from http://clinicaltrials.gov/ and http://www.wiley.co.uk/genmed/clinical/.
Successful gene replacement therapy relies on, among other things, successful delivery of the vector to the tissue of interest. Historically, viral vectors have been the most efficient (in terms of cell delivery) and the most popular delivery choice. To date, 70% of gene therapy clinical trials have used viral vectors.1,7 The largest obstacles to the use of viral vectors for human gene therapy have been their potential for infection, insertional mutagenesis, and induction of an innate and/or acquired immune response. For example, in September 1999, an 18-year-old participant in an adenoviral trial for ornithine transcarboxylase deficiency died as a result of multiple organ failure 4 days after the onset of treatment.8 His death was attributed to a severe immune response to the adenoviral carrier and drastically limited future work using unmodified adenoviral vectors. Another unfortunate outcome in a gene therapy trial resulted from activation of an oncogene by an integrating retroviral vector. In 2000, Cavazzana-Calvo and her colleagues in France9–12 first reported successful treatment of children suffering from X-linked severe combined immunodeficiency (SCID-X1). However, by the end of the trial, 4 of the 10 treated children developed leukemia-like symptoms and one died. The disease resulted from activation of the LM02 oncogene after integration of the vector, and the results highlighted the need for more basic science research into the mechanisms underlying exogenous gene delivery and expression. These two severe setbacks highlighted a downside to the use of poorly understood viral vectors. Fortunately, these setbacks have prompted extensive research into safer viral delivery strategies including the use of adeno-associated virus (AAV). Although AAV is inherently much safer than the other viruses, it has been most successful in two organs—the eye and the brain—which are both relatively immune privileged.
AAV is a nonpathogenic human parvovirus that can infect both nondividing and dividing cells. Studies suggest that AAV can either integrate into the genome or remain stably episomal. However, in either case, it generally has a favorable safety profile, and does not induce insertional mutagenesis.13–20 Over 120 capsid variants are known to exist and vary widely in tissue tropism, enabling them to be directed with specificity to certain tissues.21,22 Over 71 AAV-based phase I/II/III clinical trials (http://www.wiley.co.uk/genmed/clinical/) have now approved, initiated, and/or reviewed targeting diseases such as LCA, Parkinson's, and Alzheimer's.1,4–6,23,24 AAV vectors have a substantially improved safety record compared with previous retroviral or adenoviral vectors, and so far no major adverse events have been reported. The most exciting developments in AAV gene therapy for genetic ocular diseases come from the trials treating LCA, a heretofore incurable congenital ocular disease. Two critical milestones should be mentioned. First, in 2001, a team composed of researchers from Cornell University, the University of Pennsylvania, and the University of Florida reported that AAV carrying wild-type RPE65 had successfully restored vision in young Briard dogs that were blind due to a mutation in RPE65.25 This study and its subsequent companion publications represented a critical advancement that paved the way for the onset of clinical trials for LCA patients with mutations in RPE65s. In 2007 to 2008, three independent phase I AAV2 gene therapy trials for LCA were highlighted in the news. In the three studies, AAV carrying the human RPE65 cDNA was delivered to the subretinal space of participating LCA patients. In an effort to design optimum treatments, each group chose a different promoter to drive RPE65 gene expression; Maguire et al.4 used a chicken beta actin (CBA) promoter, Bainbridge et al.5 used the human RPE65 promoter, and Cideciyan et al.6,26 used a modified CBA promoter combined with a modified CMV immediate early enhancer. Unfortunately, the small number of patients in each initial trial, coupled with significant variations in outcome measures from trial to trial, makes definitive conclusions about the efficacy of these treatments impossible at this time. However, all three trials reported encouraging preliminary results. In Maguire et al.,4 all three patients (19–26 years of age) showed improvements in visual acuity and pupillometry starting at 6 weeks after the injection. However, one patient also showed visual improvement in the untreated eye. Similarly, in Bainbridge et al.,5 one of the three patients (17–23 years of age) showed visual improvement in both the treated and untreated eye, although the other two participants did not exhibit improvements. Interestingly, improvements in microperimetry and dark-adapted perimetry did not correlate with the area of retina exposed to vector. In Cideciyan et al.,6 all three patients (21–24 years of age) showed improvement in visual sensitivity responses and visual acuity starting at 30 days after the injection. Their follow-up publication reported the exciting news that these improvements persisted to 1 year posttreatment.27 Most important, all the phase I trials reported that the treatments were safely delivered, were well tolerated, and resulted in no significant accumulation of antibodies against the vector or other signs of immune response and no serious adverse events.4–6,27 As a result of these initial results, at least three AAV-RPE65 long-term phase II/III trials for LCA have been initiated (http://clinicaltrials.gov/ct2/results?term=lca+and+aav) as well as an additional trial in Israel.28
Nonviral vectors are typically composed of plasmid DNA packaged in some sort of coating, often with the addition of a targeting peptide. The composition of the packaging varies greatly, but recent advances in the formulation of nonviral vectors have greatly improved their efficiency. Consequently, nonviral gene therapy research has been growing in popularity. Clinical trials for various diseases have also been undertaken using nonviral vectors for gene delivery.1 To date, nonviral delivery methods, including naked plasmid DNA, lipofection, sleeping beauty transposon (which represents an ancient element recovered from salmonid fish genomes capable of invading host genomes through horizontal transmission) and various types of nanoparticles (NPs) have all been successfully used in human clinical trials (http://clinicaltrials.gov/ and http://www.wiley.co.uk/genmed/clinical/). Although nonviral gene delivery has typically been less successful than viral gene therapy in terms of efficiency of uptake and persistence of transgene expression, recent progress has begun to overcome many of these limitations. One approach for more successful nonviral gene therapy is to use a specific type of polylysine-based DNA NPs (called CK30-NPs, discussed further below) to deliver therapeutic genes. Results using this method demonstrate that it does not suffer from the safety concerns of some viral vectors, or the low transfection efficiency and transient gene expression that have historically plagued other nonviral methods.29–32 Development of CK30-NPs has relied on the multidisciplinary collaboration of basic fields (such as chemistry, physics, mathematics, and biology) and applied fields (such as materials science and the various areas of engineering) and has led to novel application (gene therapy) of a well-studied phenomenon (DNA compaction, see below). To date, CK30-NPs have been successfully used to transfect dividing and nondividing cells and have been used in a clinical trial for cystic fibrosis.33–35
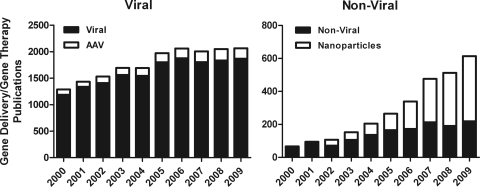
Of numerous vector delivery options, two stand out as particularly promising for the development of broadly applicable human therapeutics: AAV and CK30-NPs. AAV (along with viral gene therapy in general) has been popular for quite some time (Fig. 1, left), and the field is quite well-developed, as demonstrated by the stability in the number of related publications over the past decade. In contrast, nonviral and NP research has been steadily growing as the field develops, and exciting new results have been obtained (although the total number of publications is significantly less than that seen for viral or AAV-mediated gene therapy). Certainly, in cases of ocular gene therapy, both options yield good transfectivity, robust gene expression, and favorable safety profiles. For the remainder of the review, we will focus on these two methodologies and their application to the treatment of blinding diseases.
Figure 1.
Results of a PubMed search (number of “hits”) with the key words “viral and AAV” and “nonviral and NPs” from 2000 to 2009. The AAV and NPs hits are part of the total “viral” and “nonviral” hits, respectively.
AAV and NPs: Challenges and Opportunities
AAV is a single-stranded DNA virus with a genome ∼4.7 kb in size and was first described in 1965 as a contaminant of adenovirus.36 The genome contains two open reading frames and alternative splicing signals and is flanked by two inverted terminal repeats (ITRs). The first gene encodes four proteins for replication, whereas the second encodes three structural proteins that form the capsid. Thus far, more than 120 capsid variants are known to exist that vary widely in tissue tropism. AAV is one of the smallest known viruses (∼22 nm) and cannot usually replicate without co-infection of another virus, such as HSV or adenovirus, and is thus classified as genus Dependovirus, part of the Parvoviridae family.17,37 Consistent with the observation that up to 90% of adults are seropositive for AAV2, but have no signs of disease, AAV2 vectors have exhibited an excellent safety record in clinical trials. Although the small size of this virus may be beneficial from an infection standpoint, it also means that the gene delivery payload has historically been quite limited (∼5 kb). Some retinal disease-causing cDNAs are, alone, too large to fit in a traditional AAV vector. For example, Stargardt's macular dystrophy (MD) can be caused by mutations in the ABCA4 gene, which has a 6.8-kb cDNA transcript. An Italian group has recently identified a way to significantly enlarge the carrying capacity of AAV up to 8.9 kb.38 This report was quite controversial39 and has not been replicated. A subsequent study has demonstrated that AAV2 and AAV5 do not package more than 5.2 kb of DNA, but that larger vectors are broken up into smaller pieces and packaged separately.40 It has been reported that genes spread out across multiple viral particles can drive gene expression, possibly by annealing of overlapping regions or recombination after entry in the host cell. Efficiency of gene expression from the fragmented vectors was much lower than that driven by comparable intact vectors,40 but with optimization, this phenomenon may be useful for future AAV-mediated delivery of large genes.
There are many different types of DNA NPs used for gene therapy. These NPs usually range between 25 and 500 nm in diameter.41,42 NPs usually consist of two parts: DNA and one or more polymer coatings.42 Various coating and compacting agents have been intensively investigated, including poly (lactic acid) (PLA), poly(lactide-co-glycolide) (PLGA), and polylysine (the compacting agent for CK30-NPs).41–43 The polymer coatings are generally cationic and bind negatively charged DNA.44 By modifying NPs, efficiency, specificity, and temporal control of mediated gene expression can be further enhanced. For example, coating NPs with polyethylene glycol (PEG) prevents adsorption of proteins to the surfaces of the NPs, thereby increasing their circulation time after intravenous administration.45 Researchers have demonstrated that coating with a biodegradable polymer such as PLGA can lead to long-term gene expression, theoretically as a result of sustained cytosolic release of the plasmid.29,46 However, PLGA-based DNA NPs (usually less than 500 nm)41 are much larger than polylysine-based DNA NPs, such as CK30-NPs (usually less than 25 nm),42 which can affect particle uptake and translocation to the nucleus. NPs can be further targeted by using peptide linkages to target-specific tissues or trafficking pathways.47 In addition to modification of the particles, extensive effort has been invested in modifying the vector DNA to maximize gene expression. Promoter choice (to influence expression and tissue specificity), inclusion of regions leading to DNA self-replication, nuclear localization (to enhance stability and expression), and use of linear or minicircle DNA (to minimize vector related toxicity or silencing) have all been adopted to improve gene delivery vectors.48–53 Application of these systems to ocular gene therapy is ongoing.
Although multiple different types of NPs have been used to deliver therapeutic genes, the CK30-NPs have been among the most successful in driving long-term, safe gene expression and improving disease phenotypes. These particles contain a single molecule of plasmid DNA compacted into NPs by 10-kDa PEG-substituted lysine 30-mers (CK30PEG).31,34,35,50 The resulting particles are very small (8–20 nm in diameter) and are either rod or ellipsoid in shape, depending on the counterion present at the time of compaction. CK30-NPs have no theoretical limitation on plasmid size and have been tested with plasmids up to 20 kb. Furthermore, they do not provoke an immune response in the systems tested (lung, brain, and eye), and can be highly concentrated.29–31,35,54–56 The compacted particle is efficiently taken up into dividing and nondividing cells and remains episomal.31,50,57 These NPs have been shown to be safe and effective in a human clinical trial for cystic fibrosis and are currently being used in multiple organ systems.30–32,34,35,50,57,58
Intracellular Trafficking of AAV and CK30-NPs
Although gene therapy holds great promise for correcting genetic eye diseases, one of the biggest challenges that we face today is directing gene expression to the specific tissue where it is needed. Accomplishing tissue-specific targeting requires both physical delivery by a method that will enable access to the tissue of interest and a delivery vector that can be efficiently taken up and expressed in that tissue.
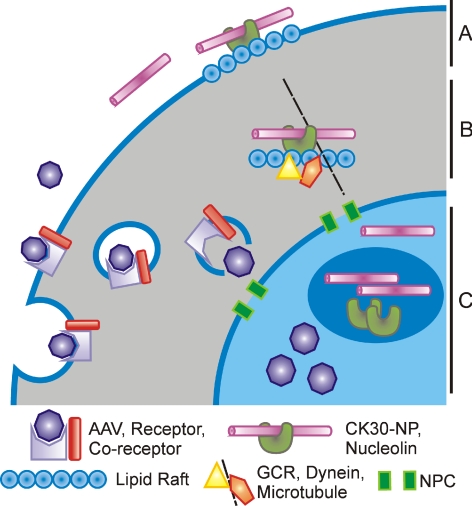
Viruses are usually taken up into cells as a result of specific interaction with cell surface receptors.59 This step has been one of the major rate-limiting steps in viral transduction for many cell types. As shown in Figure 2A, the early steps of AAV infection involve attachment to a variety of cell surface receptors followed by clathrin-dependent or -independent internalization.60 For AAV2, receptors include heparin sulfate proteoglycan (HSPG), integrins, and fibroblast growth factor receptor, and in many cases, endocytosis is facilitated by a co-receptor.59,61 AAV1, -4, -5, and -6 have been shown to bind to sialic acid and demonstrate serotype specificity for either O-linked (AAV4) or N-linked (AAV1, -5, and -6) forms.62,63 The second stage of infection involves receptor-mediated endocytosis of the virus, including activation of cellular pathways that trigger endocytosis, whereas the final steps involve AAV movement to the nucleus, including escape from endosomes (Fig. 2B) and nuclear transport (Fig. 2C). This step also includes viral uncoating and conversion of the single-stranded viral genome to a transcriptionally active, double-stranded intermediate.60,64,65 Currently, little is known about the processes by which AAV crosses nuclear pores. Since the size of an AAV virion (22 nm) is comparable to the size of the nuclear pore (20–25 nm),54,66,67 translocation could happen by diffusion. However, active transport by nuclear receptors cannot be ruled out. Currently, our knowledge of AAV intracellular trafficking is still relatively limited and is not fully understood. The reason for the lack of development in this area is twofold: First, AAV has more than 120 different serotypes, and each may use a different receptor and co-receptors, and second, there are so many different target cell types, each of which may have different trafficking pathways.19 AAV2, as an example, attaches HSPG and its co-receptors, traffics through three different endosomal pathways (early endosome, late endosomes, and perinuclear recycling endosomes),60,65,68,69 to enter the nucleus for its transduction. Investigators also found that acidification and phospholipase A2 activity of AAV VP1 is essential for AAV2 endosomal escape,70 and the ubiquitin-proteasome pathway may also play an important role in the disassembling of the AAV2 capsid and transport to the nucleus. Meanwhile, nucleolin has also been shown to associate with AAV2 virions from the cytoplasm to the nucleus.71
Figure 2.
Diagram of AAV versus CK30PEG NP trafficking. (A) First trafficking steps involve AAV and CK30PEG NP binding to cognate receptors such as HSPG for AAV and nucleolin for NPs. (B) AAV undergoes endocytosis, usually by a clathrin-mediated pathway and is processed through the endosomal system before being released. CK30PEG NPs are internalized via raft-mediated endocytosis and traffic to the nucleus, using the cellular microtubule system. (C) The mechanisms for AAV and CK30PEG NP entry in the nucleus are not clear but may rely on diffusion through the nuclear pore complex (NPC) or processing by nuclear receptors.
The intracellular trafficking mechanism for CK30-NPs is incompletely understood. However, their high transfectivity suggests an efficient trafficking mechanism. Two hypotheses are currently prevalent for general NP trafficking: First, if their size is sufficiently small, they may diffuse through the cell membrane, and second, NPs may interact with cell surface receptors.72 Studies on the CK30-NPs showed that they do not use traditional clathrin-mediated endocytosis to enter the cell.58,73 They are taken up into the cell and transported directly to the nucleus in less than an hour via the cell surface receptor nucleolin (Fig. 2A).58 Nucleolin is a protein associated with ribosome biogenesis and nucleocytoplasmic transport.74 Using surface plasmon resonance, a recently published study demonstrated that nucleolin binds to CK30-NPs directly.58 Downregulation of cell surface nucleolin has been shown to significantly reduce transgene expression (by 54.7%), whereas overexpression increases expression by 47.7%.58 Recent data suggest that trafficking of CK30-NP-nucleolin complexes proceeds via lipid-raft–mediated endocytosis. During trafficking to the nucleus, nucleolin has been shown to interact with a glucocorticoid receptor (GCR) and dynein, suggesting that microtubule-based transport is involved (Fig. 2B).75 Transport of CK30-NPs across the nuclear membrane has been suggested to occur, either by diffusion through nuclear pores, or as a result of nucleolin-based receptor interactions (Fig. 2C). Thus far, it has not been demonstrated that other NPs use this pathway. Other groups have shown that PEGylated gelatin-based NP uptake reaches a plateau within 6 to 12 hours of incubation in cell cultures.76 Studies have shown that the polymeric coatings often used in NP formulation can protect both large77 (100 nm) and small (8–20 nm, in the case of CK30PEG) NPs from degradation by intracellular DNases.34,35,58 Several studies have also used sustained-release polymers—for example, PLGA—which can enhance the duration of transgene expression by stabilizing the vector once it is inside the cell.77–79
Therapeutic Delivery of AAV and CK30-NPs to Retinal Tissues
The retina and the vitreous are generally inaccessible by systemic administration, because the BRB separates the subretinal space from the blood supply, thus protecting it from most immune-mediated damage. Subretinal and intravitreal injections have therefore been the most common delivery methods for targeting the retina. In comparison to subretinal delivery, intravitreal delivery is less invasive; however, this technique requires that the delivered therapeutics diffuse through the vitreous and, depending on the targeted tissue, several layers of inner retinal cells, before it reaches the photoreceptor cells and the retinal pigment epithelium.
Although one of the earliest serotypes (AAV2) was inefficient at infecting many cell types; the variety of currently available serotypes has enabled researchers to find optimally transfecting options for their tissue of interest. So far, 10 of more than 120 different AAV serotypes have been tested in animal models.22,80 In the eye, AAV serotypes 1, 2, 3, 4, 5, 6, 7, 8, and 9 have been evaluated after intravitreal and subretinal injection.80–82 Of these, AAV2 appears to be the only vector able to efficiently transduce retinal cells after intravitreal injection; inner retinal cells, specifically retinal ganglion cells and optic nerve fibers, expressed the reporter gene.83 Results on AAV expression after subretinal injection vary by serotype. After subretinal injection into the murine eyes, Müller cells are efficiently transduced by AAV9.84 AAV serotypes 2, 5, and 8 on the other hand, mediate expression in RPE and rod and cone photoreceptors. However, serotypes 1, 4, and 6 mainly target RPE,81,83,85,86 whereas AAV3 does not transduce retinal cells.87 Some forms of AAV5 also appear to transduce some inner retinal cells.80,83 AAV2-mediated expression in both rod and cone photoreceptors and in RPE is typically of late onset after subretinal delivery, reaching maximum levels at 2 to 4 weeks after delivery.82,83,88,89 In contrast, AAV1, 5, 7, 8, and 9 tend to drive early-onset gene expression, with eGFP observed in the retina and RPE cells as early as 5 to 7 days after subretinal delivery to the murine eyes (Table 3).80 Subretinal delivery of AAV5 to nonhuman primate eyes led to efficient reporter gene expression, specifically in rod photoreceptors, suggesting that species may also have a bearing on tissue tropism.90 Meanwhile, it should be noted that the purification method and the promoter (see later description) used will also affect transduction efficiency. For example, Lotery et al.90 reported that there was no successful primate cone transduction using AAV5 with a CMV promoter; in contrast to Mancuso et al.91 who showed viral transduction and functional cone color vision improvement using AAV5 with a cone-specific promoter.
Table 3.
Characteristics of AAV1–9 and CK30PEG in Ocular Cells after Subretinal Injection
| Vector | Target Retinal Cells | Capacity | Onset of Expression |
|---|---|---|---|
| AAV1 | RPE83 | Less than 5 kb | 5–7 days80 |
| AAV2 | RPE, PRs and GCs83,86 | Less than 5 kb | 2–4 weeks80,83 |
| AAV3 | Rare87 | Less than 5 kb | Undetermined |
| AAV4 | RPE86 | Less than 5 kb | Undetermined |
| AAV5 | RPE and PRs86 | Less than 5 kb | 5–7 days80 |
| AAV6 | RPE87 | Less than 5 kb | 5 weeks87 |
| AAV7 | RPE and PRs80,84 | Less than 5 kb | 5–7 days80 |
| AAV8 | RPE, PRs and GCs80,84 | Less than 5 kb | 5–7 days80 |
| AAV9 | RPE and Müller cells80,84 | Less than 5 kb | 5–7 days80 |
| CK30PEG | RPE, PRs and GC31 | 20 kb55 | 2 days31 |
RPE, retinal pigment epithelium; PRs, photoreceptor cells; GCs, ganglion cells.
NP gene delivery to the back of the eye has been studied for an extended period.31,92–94 Studies conducted by Bourges et al.95 in rabbits has shown that NPs of different sizes and electric charges, when injected into the vitreous, migrate through the retinal layers and tend to accumulate in RPE cells. A study also showed that small molecules can diffuse rapidly in the vitreous, but the mobility of larger molecules, particularly if positively charged, is restricted.96 Nanospheres made of polystyrene and polylactide were also shown to deliver their payload to the retina after intravitreal injection.95,97 The polystyrene particles were detected in the RPE by 24 hours after treatment and remained there for an extended period (up to 4 months).95 CK30-NPs have a well-characterized ocular expression profile. After intravitreal administration, they are expressed in retinal ganglion cells and optic nerve cells, whereas subretinal delivery yields expression in photoreceptors and RPE cells.31 Robust gene expression was observed 2 days after injection (Table 3). Furthermore, our results suggest that the uptake of CK30-NPs is not limited to the site of injection, since reporter gene expression is observed throughout the retina.31 Lack of long-term gene expression has always been one of the major limitations of nonviral vectors. For example, clinical trials using both CK30-NPs and lipid-mediated vectors to deliver therapeutic genes to the nasal mucosa of patients with cystic fibrosis reported no detectable gene expression, although vector DNA was detectable for at least 2 weeks, and improvements in therapeutic outcomes (nasal potential difference) were noted.35 This finding is probably due to the rapid silencing mediated by the presence of the CMV promoter. Studies using similar NPs (containing CMV-eGFP) in the eye showed robust gene expression 2 days after injection, but it disappeared after 2 weeks31 because of the immunologic reactivity to lipid-DNA conjugates.34,35,98 Encouragingly, the CK30-NPs have been shown to drive long-term gene expression when a different promoter is used. Recently, we observed that delivery of CK30-NPs containing the human interphotoreceptor retinoid-binding protein (IRBP) promoter and the wild-type retinal degeneration slow gene (RDS) to the neonatal mouse eye promotes rescue of the autosomal dominant retinitis pigmentosa disease phenotype in the rds+/− mouse for up to 4 months, with transgene expression and protein levels remaining significantly elevated through that time point.50 These results suggest that some formulations of NPs coupled with the judicious promoter choice may be effective in treating chronic ocular diseases.
Promoter-Specific Gene Expression
Although injection site and inherent vector tropisms can help target therapeutics to the proper location, additional specificity can be conferred by the choice of promoter and regulatory elements. The same gene driven by different promoters may have different therapeutic effects and safety profiles.31,99–101 Depending on the gene or tissue of interest, improper promoter choice can lead to potentially harmful ectopic expression, incorrect temporal expression patterns, and gene expression levels either too high or too low to cause the desired effect. For example, the CMV promoter is one of the most commonly used promoters for gene transfer therapy because of its high initial transgene expression; however, its tendency to be quickly silenced makes it fairly inefficient in terms of treating chronic diseases such as retinitis pigmentosa (RP).102,103 The inclusion of additional regulatory elements, such as enhancers or nuclear targeting and stability elements, may help stabilize CMV-driven gene expression.102 The effective use of various cell-type–specific promoters has been well studied with AAV and NP vectors: for example, the rhodopsin promoter is used to drive expression in rod photoreceptors,30,103 the human red opsin promoter for cone-specific expression,104 the CRX and IRBP promoters for rod and cone expression,105 and the vitelliform macular dystrophy (VMD2) promoter for RPE expression.106 Despite the utility of cell-type–specific promoters, there is some evidence that they are less effective as the targeted tissue degenerates. For example, Le Meur et al.107 reported that gene expression driven by the hRPE65 promoter was expressed in both young (8–11 months) and old (30 months) dogs, but functional rescue or vision restoration was observed only in the young animals. Although promoter choice for NP vectors is dictated strictly by therapeutic concerns, selection for inclusion in AAV vectors has had to take into account the traditional cargo limitations (<4.7 kb): the larger the promoter/regulatory region, the smaller the space for the therapeutic gene. As larger capacity vectors are developed, however, these issues may subside. Finally, although promoter choice is a key regulator of tissue specificity, it should be noted that other factors affect the choice. For example, the transduction profile can often be modified by micro-RNAs, which can in turn be regulated by intrinsic and extrinsic factors (such as light).108 Consideration of these issues will enable optimal vector design and transduction profiles.
Immune Response and Safety Considerations
Gene therapies can induce immune responses (e.g., cytokines, hormones, or antibodies), the consequences of which may include partial or complete loss of gene expression, decrease in therapeutic benefit, cross-reactivity with the host's endogenous proteins, or systemic reactions that may be severe or life-threatening. It is also clear that the immune responses seen in many mammalian model systems are quite different from those in humans.109 In many cases, gene therapy vectors have been successful in animal models, but the goal of successfully reversing inherited disease in humans has been more elusive.109 The issue of a systemic immune response may be less of a concern with intraocular administration than with other routes of administration, but such a contention would still have to be proven in clinical trials. In the normal retina, the neural tissues are protected by the BRB, thus allowing the eye to enjoy a relatively immune-privileged status. However, when a preexisting disease is present, such as retinitis pigmentosa or age-related macular degeneration, disruption in the BRB can occur, thus increasing the likelihood of a later, gene therapy–related immune response.110
The immune profile of AAV is generally considered to be comparatively benign; however, during childhood many humans are infected with AAV2, which can lead to the development of neutralizing antibodies (NAbs) directed against the vector capsid, which in turn will prevent effective readministration of AAV vectors of the same serotype. This issue is also a concern when considering the efficacy of multiple dosage regimens. For example, in clinical studies of AAV gene transfer for hemophilia B, anti-AAV2 antibodies were generated in these patients,109 thus potentially limiting its utility for this disease class. Fortunately, the three ocular AAV clinical trials previously mentioned have not reported significant adverse events.
Safety and toxicity studies using NPs both in the eye and in other systems have reported little immune response.31,111–114 Biodegradable PLGA NPs, offer a nontoxic and nonimmunogenic gene delivery system,115 and previous studies in our laboratory using the CK30-NPs in the eye showed no treatment-associated signs of toxicity, inflammation, or disruption of retinal structure or function after subretinal or intravitreal injection.30–32 When administered to airway epithelia of mice, it was reported that the CK30-NPs elicited only minimal signs of toxicity at the highest dose of 100 μg DNA.56 CK30-NPs have been repetitively administered to the lungs of mice, without any decrement of transgene activity, indicating that prolonged treatment may be feasible without induction of NAbs.34 Moreover, a recent phase I clinical trial of CK30-NPs in cystic fibrosis reported no significant adverse events.35
Future Directions
Further improvements in AAV vector design include the cloning of self-complementary genomes, which can bypass the secondary-strand DNA synthesis, the use of specific promoter sequences to restrict expression to the cell-type of interest, and discovery of multiple capsid serotypes for gene therapy. The major future development issues for NPs will include additional improvements in the duration and levels of gene expression and enhancements in stability, biocompatibility, systemic bioavailability, and sustained release. These issues may be particularly important for nonocular routes of administration.
There are more than 200 different retinal disease-causing genes, many of which can contain several different disease-causing mutations.29 An ultimate ocular gene therapy goal may be the design of vectors that can target multiple mutations and genes with one therapeutic instead of having to develop a new treatment for each one. Examples of these treatments may be co-delivery of a shRNA against the endogenous (mutant gene) and delivery of an RNA-resistant normal gene (to enable elimination of multiple different types of mutant alleles). A second approach in this vein is the delivery of a neuroprotective gene designed not to target the disease-causing gene, but to stabilize or enhance the health of the degenerating retina. Although some of these approaches are eminently suited to delivery by AAV, others may be more amenable to NP-mediated delivery; particularly, if multiple expression cassettes must be delivered in the same vector or if large regulatory elements are necessary for regulated expression of the transgenes.
Once again, some genes are too large for AAV, such as the 6.8-kb ABCA4 cDNA. In addition, although most current gene therapies use the cDNA form, studies have shown that, in some cases, the noncoding elements are also required for optimal expression of the transgene in vivo.116 Addition of these noncoding regions would make even more genes unsuitable for AAV using current technology. These large-sized genes are examples that highlight the importance of using high-payload vectors such as NPs as a vehicle and of having multiple vector options.
Summary
Viral vectors have been successfully used for ocular delivery of nucleic acids. They have yielded high levels of therapeutic gene expression in ocular cells and improvement in disease phenotypes.83,95,117,118 Thus far, AAV is the most advanced and safest form of viral gene therapy, taking into account data gathered from both animal studies and clinical trials. Traditional limitations of AAV include the small packaging capacity and the potential for adverse interactions with other viruses preexisting in the target tissue14,109,119,120; however, gene expression and safety profiles are generally favorable. Nonviral vectors have traditionally been safer and less limited in carrying capacity than their viral counterparts, but have suffered low gene expression levels and short duration. Newer NP formulations, especially CK30-NPs, may overcome these barriers. As the field progresses, NPs may emerge as a clinically vital complementary method of safe, proper, and precise delivery of therapeutic genes to ocular tissues.
Researchers have repeatedly observed that development of gene therapy strategies that are both safe and therapeutically effective is much more difficult than had been imagined. The eye, in contrast to other organs, is easy to access for treatment, and it is possible to readily chart the efficacy of treatment using OCT, ERG, and/or other psychophysical testing, such as measurement of optomotor responses. It is also relatively immune privileged, and ocular gene therapy is offering new hope for the treatment of inherited and acquired blinding diseases. Data indicate that both AAV and some NP vectors are efficient and well-tolerated methods of delivering genes to the postmitotic cells of the retina. The field of ocular gene delivery using AAV is currently more advanced than that of NPs; however, the demands of each therapeutic situation are distinct, and the development of multiple types of clinically viable gene delivery strategies is wise. Careful and critical evaluation of the benefits and risks must occur as new AAV and NP vectors are developed for treating human ocular diseases.
Footnotes
Supported by the financial support of the National Eye Institute (EY10609 [MIN], EY018656 [MIN], and EY018512 [SMC]), the Foundation Fighting Blindness (MIN), the Oklahoma Center for the Advancement of Science and Technology (ZH, MIN), and Dr. William “Bill” W. Talley II Research Award (ZH).
Disclosure: Z. Han, None; S.M. Conley, None; M.I. Naash, None
References
- 1. Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2007: an update. J Gene Med. 2007;9:833–842 [DOI] [PubMed] [Google Scholar]
- 2. Rosenberg SA, Aebersold P, Cornetta K, et al. Gene transfer into human: immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323:570–578 [DOI] [PubMed] [Google Scholar]
- 3. Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239 [DOI] [PubMed] [Google Scholar]
- 6. Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A. 2008;105:15112–15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cotrim AP, Baum BJ. Gene therapy: some history, applications, problems, and prospects. Toxicol Pathol. 2008;36:97–103 [DOI] [PubMed] [Google Scholar]
- 8. Raper SE, Chirmule N, Lee FS, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158 [DOI] [PubMed] [Google Scholar]
- 9. Cavazzana-Calvo M, Fischer A. Gene therapy for severe combined immunodeficiency: are we there yet? J Clin Invest. 2007;117:1456–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672 [DOI] [PubMed] [Google Scholar]
- 11. Fischer A, Abina SH, Thrasher A, von Kalle C, Cavazzana-Calvo M. LMO2 and gene therapy for severe combined immunodeficiency. N Engl J Med. 2004;350:2526–2527; author reply 2526–2527 [DOI] [PubMed] [Google Scholar]
- 12. Thrasher AJ, Gaspar HB, Baum C, et al. Gene therapy: X-SCID transgene leukaemogenicity. Nature. 2006;443:E5–6; discussion E6–7 [DOI] [PubMed] [Google Scholar]
- 13. Deyle DR, Russell DW. Adeno-associated virus vector integration. Curr Opin Mol Ther. 2009;11:442–447 [PMC free article] [PubMed] [Google Scholar]
- 14. Han Z, Zhong L, Maina N, et al. Stable integration of recombinant adeno-associated virus vector genomes after transduction of murine hematopoietic stem cells. Hum Gene Ther. 2008;19:267–278 [DOI] [PubMed] [Google Scholar]
- 15. Flotte T, Carter B, Conrad C, et al. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum Gene Ther. 1996;7:1145–1159 [DOI] [PubMed] [Google Scholar]
- 16. Kay MA, Manno CS, Ragni MV, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261 [DOI] [PubMed] [Google Scholar]
- 17. Muzyczka N, Samulski RJ, Hermonat P, Srivastava A, Berns KI. The genetics of adeno-associated virus. Adv Exp Med Biol. 1984;179:151–161 [DOI] [PubMed] [Google Scholar]
- 18. Srivastava A, Lusby EW, Berns KI. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983;45:555–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhong L, Zhao W, Wu J, Maina N, Han Z, Srivastava A. Adeno-associated virus-mediated gene transfer in hematopoietic stem/ progenitor cells as a therapeutic tool. Curr Gene Ther. 2006;6:683–698 [DOI] [PubMed] [Google Scholar]
- 20. Bell P, Wang L, Lebherz C, et al. No evidence for tumorigenesis of AAV vectors in a large-scale study in mice. Mol Ther. 2005;12:299–306 [DOI] [PubMed] [Google Scholar]
- 21. Gao G, Vandenberghe LH, Alvira MR, et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bartus RT, Herzog CD, Bishop K, et al. Issues regarding gene therapy products for Parkinson's disease: the development of CERE-120 (AAV-NTN) as one reference point. Parkinsonism Relat Disord. 2007;13(suppl 3):S469–S477 [DOI] [PubMed] [Google Scholar]
- 24. Carter BJ. Adeno-associated virus vectors in clinical trials. Hum Gene Ther. 2005;16:541–550 [DOI] [PubMed] [Google Scholar]
- 25. Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95 [DOI] [PubMed] [Google Scholar]
- 26. Cideciyan AV, Hauswirth WW, Aleman TS, et al. Vision 1 year after gene therapy for Leber's congenital amaurosis. N Engl J Med. 2009;361:725–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cideciyan AV, Hauswirth WW, Aleman TS, et al. Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther. 2009;20:999–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Banin E, Bandah-Rosenfeld D, Obolensky A, et al. Molecular anthropology meets genetic medicine to treat blindness in the North African Jewish population: human gene therapy initiated in Israel. Hum Gene Ther. 2010;21:1749–1757 [DOI] [PubMed] [Google Scholar]
- 29. Conley SM, Cai X, Naash MI. Nonviral ocular gene therapy: assessment and future directions. Curr Opin Mol Ther. 2008;10:456–463 [PMC free article] [PubMed] [Google Scholar]
- 30. Cai X, Conley SM, Nash Z, Fliesler SJ, Cooper MJ, Naash MI. Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J. 2010;24:1178–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Farjo R, Skaggs J, Quiambao AB, Cooper MJ, Naash MI. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS ONE. 2006;1:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ding XQ, Quiambao AB, Fitzgerald JB, Cooper MJ, Conley SM, Naash MI. Ocular delivery of compacted DNA-nanoparticles does not elicit toxicity in the mouse retina. PLoS ONE. 2009;4:e7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yurek DM, Fletcher AM, Smith GM, et al. Long-term transgene expression in the central nervous system using DNA nanoparticles. Mol Ther. 2009;17:641–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davis PB, Cooper MJ. Vectors for airway gene delivery. AAPS J. 2007;9:E11–E17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Konstan MW, Davis PB, Wagener JS, et al. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Hum Gene Ther. 2004;15:1255–1269 [DOI] [PubMed] [Google Scholar]
- 36. Atchison RW, Casto BC, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149:754–756 [DOI] [PubMed] [Google Scholar]
- 37. Kotin RM, Siniscalco M, Samulski RJ, et al. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci U S A. 1990;87:2211–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Allocca M, Doria M, Petrillo M, et al. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest. 2008;118:1955–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hirsch ML, Agbandje-McKenna M, Samulski RJ. Little vector, big gene transduction: fragmented genome reassembly of adeno-associated virus. Mol Ther. 2010;18:6–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu Z, Yang H, Colosi P. Effect of genome size on AAV vector packaging. Mol Ther. 2010;18:80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ravi Kumar MN, Bakowsky U, Lehr CM. Preparation and characterization of cationic PLGA nanospheres as DNA carriers. Biomaterials. 2004;25:1771–1777 [DOI] [PubMed] [Google Scholar]
- 42. Sun W, Ziady AG. Real-time imaging of gene delivery and expression with DNA nanoparticle technologies. Methods Mol Biol. 2009;544:525–546 [DOI] [PubMed] [Google Scholar]
- 43. Bhardwaj V, Ankola DD, Gupta SC, Schneider M, Lehr CM, Kumar MN. PLGA nanoparticles stabilized with cationic surfactant: safety studies and application in oral delivery of paclitaxel to treat chemical-induced breast cancer in rat. Pharm Res. 2009;26:2495–2503 [DOI] [PubMed] [Google Scholar]
- 44. Conley SM, Naash MI. Nanoparticles for retinal gene therapy. Prog Retin Eye Res. 2010;29:376–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Redhead HM, Davis SS, Illum L. Drug delivery in poly(lactide-co-glycolide) nanoparticles surface modified with poloxamer 407 and poloxamine 908: in vitro characterisation and in vivo evaluation. J Control Release. 2001;70:353–363 [DOI] [PubMed] [Google Scholar]
- 46. Prabha S, Labhasetwar V. Nanoparticle-mediated wild-type p53 gene delivery results in sustained antiproliferative activity in breast cancer cells. Mol Pharm. 2004;1:211–219 [DOI] [PubMed] [Google Scholar]
- 47. Rhee M, Davis P. Mechanism of uptake of C105Y, a novel cell-penetrating peptide. J Biol Chem. 2006;281:1233–1240 [DOI] [PubMed] [Google Scholar]
- 48. Chen ZY, He CY, Meuse L, Kay MA. Silencing of episomal transgene expression by plasmid bacterial DNA elements in vivo. Gene Ther. 2004;11:856–864 [DOI] [PubMed] [Google Scholar]
- 49. Chen ZY, Yant SR, He CY, Meuse L, Shen S, Kay MA. Linear DNAs concatemerize in vivo and result in sustained transgene expression in mouse liver. Mol Ther. 2001;3:403–410 [DOI] [PubMed] [Google Scholar]
- 50. Cai X, Nash Z, Conley SM, Fliesler SJ, Cooper MJ, Naash MI. A partial structural and functional rescue of a retinitis pigmentosa model with compacted DNA nanoparticles. PLoS ONE. 2009;4:e5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Riu E, Chen ZY, Xu H, He CY, Kay MA. Histone modifications are associated with the persistence or silencing of vector-mediated transgene expression in vivo. Mol Ther. 2007;15:1348–1355 [DOI] [PubMed] [Google Scholar]
- 52. Baiker A, Maercker C, Piechaczek C, et al. Mitotic stability of an episomal vector containing a human scaffold/matrix-attached region is provided by association with nuclear matrix. Nat Cell Biol. 2000;2:182–184 [DOI] [PubMed] [Google Scholar]
- 53. Piechaczek C, Fetzer C, Baiker A, Bode J, Lipps HJ. A vector based on the SV40 origin of replication and chromosomal S/MARs replicates episomally in CHO cells. Nucleic Acids Res. 1999;27:426–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cai X, Conley S, Naash M. Nanoparticle applications in ocular gene therapy. Vision Res. 2008;48:319–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fink TL, Klepcyk PJ, Oette SM, et al. Plasmid size up to 20 kbp does not limit effective in vivo lung gene transfer using compacted DNA nanoparticles. Gene Ther. 2006;13:1048–1051 [DOI] [PubMed] [Google Scholar]
- 56. Ziady AG, Gedeon CR, Muhammad O, et al. Minimal toxicity of stabilized compacted DNA nanoparticles in the murine lung. Mol Ther. 2003;8:948–956 [DOI] [PubMed] [Google Scholar]
- 57. Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47:65–81 [DOI] [PubMed] [Google Scholar]
- 58. Chen X, Kube DM, Cooper MJ, Davis PB. Cell surface nucleolin serves as receptor for DNA nanoparticles composed of pegylated polylysine and DNA. Mol Ther. 2008;16:333–342 [DOI] [PubMed] [Google Scholar]
- 59. Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77 [DOI] [PubMed] [Google Scholar]
- 60. Douar AM, Poulard K, Stockholm D, Danos O. Intracellular trafficking of adeno-associated virus vectors: routing to the late endosomal compartment and proteasome degradation. J Virol. 2001;75:1824–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wu Z, Miller E, Agbandje-McKenna M, Samulski RJ. Alpha2,3 and alpha2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J Virol. 2006;80:9093–9103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kaludov N, Brown KE, Walters RW, Zabner J, Chiorini JA. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J Virol. 2001;75:6884–6893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hansen J, Qing K, Kwon HJ, Mah C, Srivastava A. Impaired intracellular trafficking of adeno-associated virus type 2 vectors limits efficient transduction of murine fibroblasts. J Virol. 2000;74:992–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ding W, Zhang L, Yan Z, Engelhardt JF. Intracellular trafficking of adeno-associated viral vectors. Gene Ther. 2005;12:873–880 [DOI] [PubMed] [Google Scholar]
- 66. Kronenberg S, Kleinschmidt JA, Bottcher B. Electron cryo-microscopy and image reconstruction of adeno-associated virus type 2 empty capsids. EMBO Rep. 2001;2:997–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van der Aa MA, Mastrobattista E, Oosting RS, Hennink WE, Koning GA, Crommelin DJ. The nuclear pore complex: the gateway to successful nonviral gene delivery. Pharm Res. 2006;23:447–459 [DOI] [PubMed] [Google Scholar]
- 68. Duan D, Yue Y, Yan Z, Yang J, Engelhardt JF. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest. 2000;105:1573–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ding W, Zhang LN, Yeaman C, Engelhardt JF. rAAV2 traffics through both the late and the recycling endosomes in a dose-dependent fashion. Mol Ther. 2006;13:671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Suikkanen S, Antila M, Jaatinen A, Vihinen-Ranta M, Vuento M. Release of canine parvovirus from endocytic vesicles. Virology. 2003;316:267–280 [DOI] [PubMed] [Google Scholar]
- 71. Qiu J, Brown KE. A 110-kDa nuclear shuttle protein, nucleolin, specifically binds to adeno-associated virus type 2 (AAV-2) capsid. Virology. 1999;257:373–382 [DOI] [PubMed] [Google Scholar]
- 72. Jin S, Ye K. Nanoparticle-mediated drug delivery and gene therapy. Biotechnol Prog. 2007;23:32–41 [DOI] [PubMed] [Google Scholar]
- 73. Walsh M, Tangney M, O'Neill MJ, et al. Evaluation of cellular uptake and gene transfer efficiency of pegylated poly-L-lysine compacted DNA: implications for cancer gene therapy. Mol Pharm. 2006;3:644–653 [DOI] [PubMed] [Google Scholar]
- 74. Ginisty H, Sicard H, Roger B, Bouvet P. Structure and functions of nucleolin. J Cell Sci. 1999;112:761–772 [DOI] [PubMed] [Google Scholar]
- 75. Chen X, Shank S, Davis PB, Ziady AG. Nucleolin-mediated cellular trafficking of DNA nanoparticle is lipid raft and microtubule dependent and can be modulated by glucocorticoid. Mol Ther. 2011;19:93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kaul G, Amiji M. Cellular interactions and in vitro DNA transfection studies with poly(ethylene glycol)-modified gelatin nanoparticles. J Pharm Sci. 2005;94:184–198 [DOI] [PubMed] [Google Scholar]
- 77. Prabha S, Zhou WZ, Panyam J, Labhasetwar V. Size-dependency of nanoparticle-mediated gene transfection: studies with fractionated nanoparticles. Int J Pharm. 2002;244:105–115 [DOI] [PubMed] [Google Scholar]
- 78. Cohen H, Levy RJ, Gao J, et al. Sustained delivery and expression of DNA encapsulated in polymeric nanoparticles. Gene Ther. 2000;7:1896–1905 [DOI] [PubMed] [Google Scholar]
- 79. Vasir JK, Labhasetwar V. Polymeric nanoparticles for gene delivery. Expert Opin Drug Deliv. 2006;3:325–344 [DOI] [PubMed] [Google Scholar]
- 80. Lebherz C, Maguire A, Tang W, Bennett J, Wilson JM. Novel AAV serotypes for improved ocular gene transfer. J Gene Med. 2008;10:375–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Surace EM, Auricchio A. Versatility of AAV vectors for retinal gene transfer. Vision Res. 2008;48:353–359 [DOI] [PubMed] [Google Scholar]
- 82. Bainbridge JW, Tan MH, Ali RR. Gene therapy progress and prospects: the eye. Gene Ther. 2006;13:1191–1197 [DOI] [PubMed] [Google Scholar]
- 83. Auricchio A, Kobinger G, Anand V, et al. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum Mol Genet. 2001;10:3075–3081 [DOI] [PubMed] [Google Scholar]
- 84. Allocca M, Mussolino C, Garcia-Hoyos M, et al. Novel adeno-associated virus serotypes efficiently transduce murine photoreceptors. J Virol. 2007;81:11372–11380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rabinowitz JE, Samulski RJ. The adeno-associated virus crystal: impact inversely proportional to size. Mol Ther. 2002;6:443–445 [DOI] [PubMed] [Google Scholar]
- 86. Weber M, Rabinowitz J, Provost N, et al. Recombinant adeno-associated virus serotype 4 mediates unique and exclusive long-term transduction of retinal pigmented epithelium in rat, dog, and nonhuman primate after subretinal delivery. Mol Ther. 2003;7:774–781 [DOI] [PubMed] [Google Scholar]
- 87. Yang GS, Schmidt M, Yan Z, et al. Virus-mediated transduction of murine retina with adeno-associated virus: effects of viral capsid and genome size. J Virol. 2002;76:7651–7660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sarra GM, Stephens C, Schlichtenbrede FC, et al. Kinetics of transgene expression in mouse retina following sub-retinal injection of recombinant adeno-associated virus. Vision Res. 2002;42:541–549 [DOI] [PubMed] [Google Scholar]
- 89. Voutetakis A, Kok MR, Zheng C, et al. Reengineered salivary glands are stable endogenous bioreactors for systemic gene therapeutics. Proc Natl Acad Sci U S A. 2004;101:3053–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lotery AJ, Yang GS, Mullins RF, et al. Adeno-associated virus type 5: transduction efficiency and cell-type specificity in the primate retina. Hum Gene Ther. 2003;14:1663–1671 [DOI] [PubMed] [Google Scholar]
- 91. Mancuso K, Hauswirth WW, Li Q, et al. Gene therapy for red-green colour blindness in adult primates. Nature. 2009;461:784–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kim H, Robinson SB, Csaky KG. Investigating the movement of intravitreal human serum albumin nanoparticles in the vitreous and retina. Pharm Res. 2009:26;329–337 [DOI] [PubMed] [Google Scholar]
- 93. Silva GA. Nanomedicine: seeing the benefits of ceria. Nat Nanotechnol. 2006;1:92–94 [DOI] [PubMed] [Google Scholar]
- 94. Cai X, Conley SM, Nash Z, Fliesler SJ, Cooper MJ, Naash MI. Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J. 2010;24:1178–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bourges JL, Gautier SE, Delie F, et al. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Invest Ophthalmol Vis Sci. 2003;44:3562–3569 [DOI] [PubMed] [Google Scholar]
- 96. Pitkanen L, Ruponen M, Nieminen J, Urtti A. Vitreous is a barrier in nonviral gene transfer by cationic lipids and polymers. Pharm Res. 2003;20:576–583 [DOI] [PubMed] [Google Scholar]
- 97. Sakurai E, Ozeki H, Kunou N, Ogura Y. Effect of particle size of polymeric nanospheres on intravitreal kinetics. Ophthalmic Res. 2001;33:31–36 [DOI] [PubMed] [Google Scholar]
- 98. Alton EW, Stern M, Farley R, et al. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: a double-blind placebo-controlled trial. Lancet. 1999;353:947–954 [DOI] [PubMed] [Google Scholar]
- 99. Glushakova LG, Timmers AM, Pang J, Teusner JT, Hauswirth WW. Human blue-opsin promoter preferentially targets reporter gene expression to rat s-cone photoreceptors. Invest Ophthalmol Vis Sci. 2006;47:3505–3513 [DOI] [PubMed] [Google Scholar]
- 100. Buch PK, Bainbridge JW, Ali RR. AAV-mediated gene therapy for retinal disorders: from mouse to man. Gene Ther. 2008;15:849–857 [DOI] [PubMed] [Google Scholar]
- 101. Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872 [DOI] [PubMed] [Google Scholar]
- 102. Deuschle U, Meyer WK, Thiesen HJ. Tetracycline-reversible silencing of eukaryotic promoters. Mol Cell Biol. 1995;15:1907–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Flannery JG, Zolotukhin S, Vaquero MI, LaVail MM, Muzyczka N, Hauswirth WW. Efficient photoreceptor-targeted gene expression in vivo by recombinant adeno-associated virus. Proc Natl Acad Sci U S A. 1997;94:6916–6921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li Q, Timmers AM, Guy J, Pang J, Hauswirth WW. Cone-specific expression using a human red opsin promoter in recombinant AAV. Vision Res. 2008;48:332–338 [DOI] [PubMed] [Google Scholar]
- 105. Porrello K, Bhat SP, Bok D. Detection of interphotoreceptor retinoid binding protein (IRBP) mRNA in human and cone-dominant squirrel retinas by in situ hybridization. J Histochem Cytochem. 1991;39:171–176 [DOI] [PubMed] [Google Scholar]
- 106. Esumi N, Oshima Y, Li Y, Campochiaro PA, Zack DJ. Analysis of the VMD2 promoter and implication of E-box binding factors in its regulation. J Biol Chem. 2004;279:19064–19073 [DOI] [PubMed] [Google Scholar]
- 107. Le Meur G, Stieger K, Smith AJ, et al. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther. 2007;14:292–303 [DOI] [PubMed] [Google Scholar]
- 108. Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610 [DOI] [PubMed] [Google Scholar]
- 109. Hasbrouck NC, High KA. AAV-mediated gene transfer for the treatment of hemophilia B: problems and prospects. Gene Ther. 2008;15:870–875 [DOI] [PubMed] [Google Scholar]
- 110. Grisanti S, Ishioka M, Kosiewicz M, Jiang LQ. Immunity and immune privilege elicited by cultured retinal pigment epithelial cell transplants. Invest Ophthalmol Vis Sci. 1997;38:1619–1626 [PubMed] [Google Scholar]
- 111. Bejjani RA, BenEzra D, Cohen H, et al. Nanoparticles for gene delivery to retinal pigment epithelial cells. Mol Vis. 2005;11:124–132 [PubMed] [Google Scholar]
- 112. Halberstadt C, Emerich DF, Gonsalves K. Combining cell therapy and nanotechnology. Expert Opin Biol Ther. 2006;6:971–981 [DOI] [PubMed] [Google Scholar]
- 113. Borlongan CV, Masuda T, Walker TA, et al. Nanotechnology as an adjunct tool for transplanting engineered cells and tissues. Curr Mol Med. 2007;7:609–618 [DOI] [PubMed] [Google Scholar]
- 114. Prow T, Smith JN, Grebe R, et al. Construction, gene delivery, and expression of DNA tethered nanoparticles. Mol Vis. 2006;12:606–615 [PubMed] [Google Scholar]
- 115. Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 2002;16:1217–1226 [DOI] [PubMed] [Google Scholar]
- 116. Xia CF, Chu C, Li J, et al. Comparison of cDNA and genomic forms of tyrosine hydroxylase gene therapy of the brain with Trojan horse liposomes. J Gene Med. 2007;9:605–612 [DOI] [PubMed] [Google Scholar]
- 117. Bennett J, Tanabe T, Sun D, et al. Photoreceptor cell rescue in retinal degeneration (rd) mice by in vivo gene therapy. Nat Med. 1996;2:649–654 [DOI] [PubMed] [Google Scholar]
- 118. Tschernutter M, Schlichtenbrede FC, Howe S, et al. Long-term preservation of retinal function in the RCS rat model of retinitis pigmentosa following lentivirus-mediated gene therapy. Gene Ther. 2005;12:694–701 [DOI] [PubMed] [Google Scholar]
- 119. Donsante A, Vogler C, Muzyczka N, et al. Observed incidence of tumorigenesis in long-term rodent studies of rAAV vectors. Gene Ther. 2001;8:1343–1346 [DOI] [PubMed] [Google Scholar]
- 120. Donsante A, Miller DG, Li Y, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. [DOI] [PubMed] [Google Scholar]