Metastatic disease of the liver is the most common cause of death in patients with uveal melanoma. This study showed that a unique population of lymphocytes, natural killer T cells, suppress the immune response in the liver and promote metastasis of intraocular melanomas in mice.
Abstract
Purpose.
To explore the role of natural killer T (NKT) cells in the development of liver metastases in mice harboring intraocular melanomas.
Methods.
Cells derived from the cutaneous B16 melanoma cell line (B16LS9) were transplanted either into the vitreous body or under the spleen capsules of wild-type C57BL/6 mice and NKT-cell–deficient Jα18−/− and CD1d−/− mice. The development of liver metastases was evaluated by histopathology. The effect of NK cells on liver metastases was determined by selective depletion with anti-asialo-GM1 antiserum in vivo and NK-cell–mediated cytolysis of B16LS9 melanoma cells in vitro. The role of IL-10 and transforming growth factor (TGF)-β in the inhibition of liver NK resistance to liver metastases was determined by in vivo and in vitro neutralization with monoclonal antibodies.
Results.
Liver NKT cells, especially type I NKT cells, enhanced liver metastases arising from intraocular melanomas. NKT-cell–deficient mice developed significantly fewer liver metastases that were NK-cell dependent. Tumor-induced liver NKT cells, especially type I NKT cells, inhibited liver NK-cell cytotoxicity by an IL-10-dependent process.
Conclusions.
NKT cells exert protective effects in many murine tumor models. However, the present results reveal that NKT cells exacerbate liver metastases arising from intraocular melanomas. To the authors' knowledge, this is the first report that liver NKT cells, especially type I NKT cells, inhibit liver NK-cell antimetastatic activity by the production of IL-10. These results suggest that hepatic NKT cell activity can have an important effect in the immune surveillance of liver metastases.
Uveal melanoma is the most common intraocular tumor in adults. Liver metastasis is the leading cause of death in uveal melanoma patients and it has been reported that approximately 95% of patients who die of uveal melanoma have liver metastases.1 At the present time, there are no therapeutic modalities that significantly control liver metastases or extend the 5-year survival of patients harboring liver metastases arising from uveal melanomas.2 Although immunotherapy has been touted as a promising therapeutic modality, the results to date have been disappointing.3,4 A possible explanation is the observation that tumors employ a wide array of strategies for evading immune surveillance. These mechanisms include downregulation of antitumor immune responses by CD4+CD25+ regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), M2 macrophages, and natural killer T (NKT) cells.3,5,6
In recent years, it has become clear that innate T cells, such as NKT cells, play an important role in modulating the adaptive immune response.7 NKT cells express both T-cell and NK-cell receptors, but unlike conventional T cells that respond to peptides presented by conventional major histocompatibility (MHC) molecules, NKT cells recognize lipid antigens presented by CD1d, a nonclassic MHC molecule. Despite being a small proportion of the total T lymphocyte population (1%–3% of circulating T cells in mice and 0.02%–0.2% in humans),8,9 NKT cells are involved in a broad range of immunologic phenomena, including autoimmune diseases, such as type 1 diabetes, graft-versus-host disease, graft rejection, airway hypersensitivity, and cancer.7,10,11 CD1d-restricted NKT cells can function as either effector or regulatory cells. In cancer, type I NKT cells exert antitumor effects by producing IFN-γ, which activates NK cells and CD8+ T cells and by activating dendritic cells. By contrast, type II NKT cells, which recognize a more diverse array of glycolipids presented by CD1d, inhibit tumor immunity by inducing regulatory cytokines, such as TGF-β, or by recruitment of Tregs.11,12 NKT cells also function differently, depending on their anatomic location. Murine liver–derived NKT cells are protective and control tumor growth, unlike thymic and splenic NKT cells, which have far less antitumor effects but have immunoregulatory properties.13
The liver is the target organ for metastases arising from uveal melanoma. It also has the highest NKT-cell/T-cell ratio in the body. Up to 50% of the lymphocytes in the liver are NKT cells.14–16 Given the wide range of activities mediated by NKT cells, we sought to determine the role that liver NKT cells have in the development of liver metastases arising from intraocular melanomas.
Materials and Methods
Cells
The B16LS9 cutaneous murine melanoma cell line was kindly provided by Hans E. Grossniklaus (Emory University School of Medicine, Atlanta, GA). B16LS9 cells were derived from hepatic metastases originating from posterior chamber inoculation of B16-F1 cutaneous melanoma cells in C57BL/6 mice.17 The cells were maintained in complete DMEM. B16LS9 cells were tested for the expression of CD1d by flow cytometry with anti-CD1d monoclonal antibody (clone 20H2) and were found to be negative (data not shown). Cells were also validated by flow cytometry for H-2b expression as confirmation of their C57BL/6 origin and tested by ELISA for mycoplasma contamination during the course of this study and were found to be negative (data not shown).
Mice
C57BL/6 mice were purchased from the Wakeland Animal Colony at the University of Texas Southwestern Medical Center (Dallas, TX). Breeding pairs of Jα18−/− mice (C57BL/6 background), which lack invariant NKT cells, were kindly provided by Joan Stein-Streilein (Schepens Eye Research Institute, Boston, MA), with permission from the originator of the knockout mouse strain (Masaru Taniguchi; RIKEN Research Center for Allergy and Immunology, Yokohama, Japan). These mice have been backcrossed to C57BL/6 mice for nine generations.18 Breeding pairs of CD1d−/− mice (C57BL/6 background), which lack both invariant NKT cells and NKT cells expressing diverse T-cell receptors, were kindly provided by Mark Exley (Beth Israel Deaconess Medical Center, Boston, MA) and had been backcrossed to C57BL/6 mice for 12 generations (Exley M, personal communication August 26, 2010). C57BL/6 severe combined immunodeficient (SCID) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All the mice were maintained in a dedicated pathogen-free environment and used between 6 and 14 weeks of age. All the animals were housed and cared for in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of Texas Southwestern Medical Center and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Tumor Models and Treatment Approaches
Intraocular tumor inoculation was performed as described previously.19 This technique produces intraocular melanoma that invades the retina and choroid and causes liver metastasis.20,21 B16LS9 tumor cells (1 × 105) were injected into the vitreous cavity (VC). Tumor-bearing eyes were enucleated when the eyes reached 4 mm in diameter. The mice were euthanatized 17 days after enucleation or when moribund, and the livers were collected for histology.
Intrasplenic tumor cell injection is an effective method of producing liver metastases, as it facilitates tumor cell dissemination to the liver via the splenic portal–circulatory route.22 Melanoma cells (5 × 104 cells in 100 μL PBS) were injected beneath the spleen capsule, and the mice were euthanatized 14 days later or when moribund.
NK cells were depleted by giving 50 μL IP anti-asialo GM1(asGM1; Wako Pure Chemical, Osaka, Japan) on days −3, +1, and +5 relative to tumor cell implantation. To inactivate NKT cells, we injected the mice IP with 50 μg of anti-CD1d (clone 20H2) three times a week, starting on day −7. In vivo inactivation of CD25+ Tregs was achieved by IP injection of anti-CD25 (clone PC61 5.3) twice a week beginning 7 days before tumor cell injection.23,24 Anti-TGF-β (clone 1D11.16.8) was administered IP (1.0 mg/injection) three times a week starting on day −7, to neutralize TGF-β1 and -β2.25 To neutralize IL-10, we treated the mice with 200 μg anti-IL-10 (clone Jes5.2A5), given daily from day −7 through day −1 and once a week thereafter. Cyclophosphamide (Bristol-Myers, Princeton, NJ) was administered IP (100 mg/kg) every 14 days beginning on day −1.
Splenectomy
The mice underwent splenectomy 7 days before intraocular tumor injection, as described previously.26 Briefly, the mice were anesthetized with ketamine hydrochloride (0.6 mg given IP), and their spleens were exteriorized through an incision in the abdominal wall. The splenic pedicle was severed with scissors. Hemorrhage was arrested by tamponade of the splenic blood vessels. Wounds were closed with stainless-steel wound clips.
Assessment of Liver Metastases
Liver metastases of B16LS9 melanomas were readily demonstrable by histologic examination, as previously described.21,27 The livers were sectioned at 100-μm intervals, stained with hematoxylin and eosin (H&E), and examined in a masked fashion by three independent observers. The results were reported as the mean number of metastases per 10 random low-power fields (×100 magnification).
Liver metastases arising from intrasplenic B16LS9 melanoma injection were assessed by counting surface tumor nodules on the liver with a dissecting microscope.28,29 When the liver had more than 200 surface metastatic nodules, the liver metastases were assessed by measuring the area of metastatic tumors from three H&E-stained slides with an ocular micrometer. The results were reported as the mean area of metastasis per 10 random low-power fields (×100).
In Vitro NK Cell-Mediated Cytotoxicity Assay
Liver mononuclear cells were isolated as described elsewhere29 and were enriched for NK cells with a kit (EasySep Mouse NK Cell Enrichment Kit; Stemcell, Vancouver, BC, Canada). Purified liver NK cells were co-cultured with B16LS9 cells at different E:T ratios for 18 hours, and cytotoxicity was assessed (CytoTox 96 NonRadioactive Cytotoxicity Assay Kit; Promega, Madison, WI). Liver NK cells isolated from Poly I:C-treated mice or anti-asialo GM1-treated mice served as positive and negative controls, respectively.
Flow Cytometric Analysis and Cell Sorting
The number of liver NK cells, MDSCs, and Tregs was assessed by flow cytometry. NK cells were defined as TCR-β–NK1.1+, or TCR-β–DX5+ cells. Tregs were identified as CD4+CD25+FoxP3+ cells, and MDSCs were identified as CD11b+Gr-1+ cells.
Liver NKT cells were enriched from isolated liver mononuclear cells by cell sorting. A sample of sorted cells was always analyzed for purity. Sorted NKT cell (NK1.1+ TCR-β+) populations were always ≥92% pure. Liver NKT cell production of IL-10 was assessed by treating purified liver NKT cells with Brefeldin A (8 μg/mL) for 90 minutes at 37°C (Sigma-Aldrich, St. Louis, MO). The samples were resuspended in fixation–permeabilization solution containing 1 μg rat IgG2b anti-mouse CD16/CD32 monoclonal antibody (BD Fc Block; BD Biosciences, San Diego, CA) for 20 minutes at 4°C, washed, and resuspended in 50 μL permeabilization– washing buffer (BD Perm/Wash; BD Biosciences). The cells were incubated with either 0.5 μg of anti-IL-10-FITC or isotype control antibody for 30 minutes at 4°C, washed, and resuspended in 250 μL 2% formalin solution. Fluorescence was detected with a flow cytometer (FACScan; BD Biosciences). The results were analyzed with allied software (CellQuest ver. 3.1f; BD Biosciences).
Real-Time RT-PCR
Primers used in real-time RT-PCR that are specific for mouse YM-1 (PPM25130A), mouse Arg-1 (PPM31770A), mouse interleukin-10 (PPM03017B-200), and mouse GAPDH (PPM02946E-200) were purchased from Qiagen (Valencia, CA). Real-time RT-PCR amplifications were performed on an RT-PCR detection system (iCycler IQ; Bio-Rad, Hercules, CA) according to a standard protocol (95°C, 10 minutes and 40 cycles: 95°C, 15 seconds and 60°C, 1 minute).
Statistical Analysis
Results were expressed as the mean ± SD. Differences between groups were analyzed by Student's t-test. P < 0.05 was considered statistically significant.
Results
NKT-Cell–Deficient Mice Develop Fewer Liver Metastases Arising from Intraocular or Intrasplenic Melanomas Than Do Mice with Intact NKT Cell Repertoires
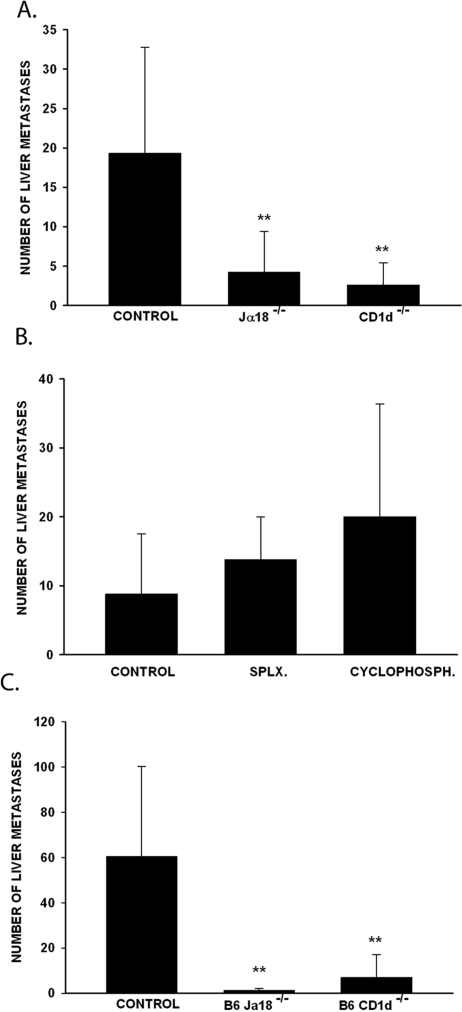
To test whether NKT cells play a role in the development of liver metastases arising from intraocular tumors, we injected B16LS9 tumor cells into the VC of C57BL/6 wild-type mice; Jα18−/− mice, which lack type I NKT cells; and CD1d−/− mice, which lack both type I and II NKT cells. Tumor-bearing eyes were enucleated when the eyes reached 4 mm in diameter. The mice were euthanatized 17 days after enucleation, and the livers were examined for metastases. The results of three separate experiments demonstrated that the NKT-cell–deficient Jα18−/− and CD1d−/− mice developed significantly fewer liver metastases than did the wild-type mice (Fig. 1A).
Figure 1.
NKT-cell–deficient mice developed fewer liver metastases arising from intraocular tumor and intrasplenic tumor injection. (A) Liver metastases in mice harboring intraocular B16LS9 melanomas. (B) Liver metastases in wild-type mice treated by either splenectomy or low-dose cyclophosphamide before injecting melanoma cells into the VC. (C) Liver metastases after intrasplenic injection of B16LS9 melanoma cells. **P < 0.01. All three experiments were performed three times with similar results. n = 10 mice/each group. Results are expressed as the mean ± SD.
Antigens introduced into the eye elicit a systemic downregulation of T-cell immunity termed anterior chamber–associated immune deviation (ACAID),30–32 which may have affected immune surveillance of intraocular tumors or their metastases. NKT cells are essential in the development of ACAID.33 Therefore, NKT-cell–deficient mice would be expected to have elevated T-cell immunity compared with normal mice, which theoretically may develop ACAID in response to the intraocular melanomas. Accordingly, experiments were performed to test whether maneuvers that abrogate ACAID would affect the development of liver metastases. ACAID can be ablated by either splenectomy or low-dose cyclophosphamide treatment before intraocular injection of antigens.34 Accordingly, B16LS9 melanoma cells were injected into the eyes of splenectomized and cyclophosphamide-treated mice, and the development of liver metastases was assessed. The results clearly demonstrate that ablation of ACAID by either splenectomy or cyclophosphamide treatment did not affect the development of metastases (Fig. 1B).
To further rule out the possibility that the unique immunologic properties of the eye contribute to the reduction of liver metastases in NKT-cell–deficient mice, we induced liver metastases via the splenic portal system. Wild-type, Jα18−/−, and CD1d−/− mice were injected intrasplenically with B16LS9 tumor cells and assessed for liver metastases 14 days later. The results of these experiments paralleled those found when B16LS9 tumor cells were placed into the eye. That is, the NKT-cell–deficient Jα18−/− and CD1d−/− mice developed significantly reduced liver metastases than did the wild-type mice (Fig. 1C). Thus, we conclude that NKT cells promote the development of liver metastases arising from intraocular melanomas or from blood-borne melanoma cells.
Resistance to Liver Metastases in NKT-Cell–Deficient Mice Is NK-Cell Dependent
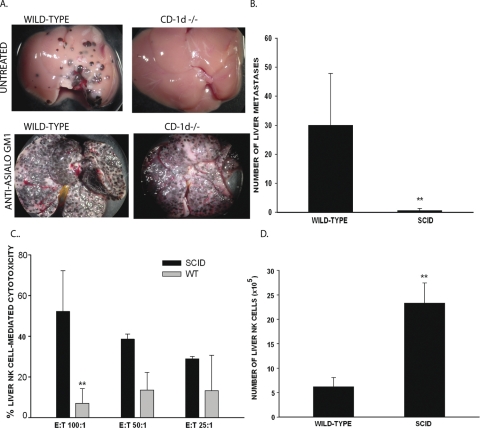
Since NK cells play a critical role in the resistance to liver metastases arising from intraocular melanomas in mice and humans,32,35 experiments were performed to determine whether the resistance to liver metastases in NKT-cell–deficient mice was NK-cell dependent. Wild-type mice, CD1d−/− mice, and anti-CD1d-treated wild-type mice were depleted of NK cells with anti-asialo GM1 antiserum. The mice were then injected intrasplenically with B16LS9 melanoma cells, and the number of liver metastases was determined 14 days later. NK-cell depletion ablated the resistance to liver metastases in the NKT-cell–deficient mice (Fig. 2A). The microscopic liver metastasis area in the wild-type mice was 45.08 ± 16.3 mm2/30 fields, compared with 61.51 ± 9.62 mm2/30 fields in the anti-CD1d–treated mice or 33.27 ± 11.1 mm2/30 fields in CD1d−/− mice. An important finding that the differences in the liver metastasis areas in the NKT-cell–deficient mice and wild-type mice were not significantly different when the hosts' NK-cell repertoire was disabled with anti-asialo GM1 (P > 0.05) thus provides further evidence that NK cells play a key role in controlling the liver metastases of B16LS9 tumors. However, NK-cell–dependent resistance to liver metastasis is profoundly downregulated by NKT cells. Although B16 melanomas are notoriously weak immunogens, it was still important to rule out a possible role of T-cell–dependent resistance. Therefore, B16LS9 melanoma cells were injected intrasplenically in SCID mice that are devoid of T cells, but have an intact NK-cell repertoire. The results indicate that T-cell–dependent immunity did not protect against liver metastases; in fact, the SCID mice displayed a remarkable resistance to liver metastases (Fig. 2B). One explanation for the reduced liver metastases in the SCID mice lies in their elevated liver NK-cell activity, which at an E:T ratio of 100:1 was five times higher than that in the wild-type mice (Fig. 2C). Moreover, the SCID mice had a fivefold higher number of liver NK cells compared with the wild-type mice (Fig. 2D).
Figure 2.
Resistance to liver metastases in NKT-cell–deficient mice was NK-cell dependent and T-cell independent. (A) Liver metastases in mice before and after NK cell depletion. This experiment was performed twice with similar results. n = 5 mice/group/experiment. (B) Liver metastases in SCID mice and in wild-type mice that received an intrasplenic injection of B16LS9 melanoma cells. Results represent pooled results from three independent experiments. (C) Liver NK cell-mediated cytotoxicity in SCID mice and wild-type mice bearing B16LS9 liver metastases induced by intrasplenic melanoma cell injection. The results are pooled from two independent experiments. (D) Number of liver NK cells (DX5+TCR-β −) detected by flow cytometry in mice bearing liver metastases produced by intrasplenic injection of B16LS9 melanoma cells. This experiment was performed twice with similar results (n = 5 mice/group/experiment). **P < 0.01. Results are expressed as the mean ± SD.
Melanoma-Induced Liver NKT Cells Inhibit Liver NK-Cell–Mediated Cytotoxicity
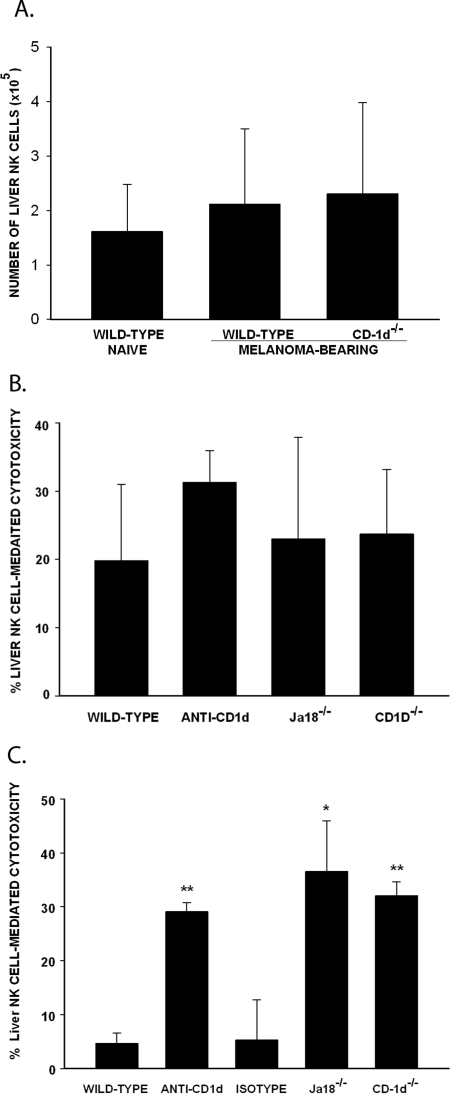
Since the remarkable resistance of SCID mice to liver metastases correlated with an increased number of liver NK cells, we considered the hypothesis that a similar condition was present in the NKT-cell–deficient mice and accounted for their resistance to liver metastases. Wild-type and CD1d−/− mice were injected intrasplenically with B16LS9 cells. Liver lymphocytes were isolated at day 14 and the number of liver NK cells was evaluated by flow cytometry. The number of liver NK in the melanoma-bearing CD1d−/− mice was similar to that in the normal and melanoma-bearing wild-type mice (Fig. 3A).
Figure 3.
Liver metastasis–induced NKT cells inhibited NK-cell–mediated cytotoxicity against B16LS9 melanoma cells. (A) Number of liver NK cells (NK1.1+TCR-β−) detected by flow cytometry in mice harboring liver metastases induced by intrasplenic injection of B16LS9 melanoma cells (n = 5 mice/group/experiment). (B) Cytotoxicity of liver NK cells against B16LS9 tumor cells in naïve mice. (C) Liver NK cell cytotoxicity against B16LS9 tumor cells in tumor-bearing mice. All experiments were repeated twice. *P < 0.05; **P < 0.01. Results are expressed as the mean ± SD.
Another explanation for the reduced liver metastases in NKT-cell–deficient mice is that NKT cells in wild-type mice suppress the cytotoxicity of the liver NK cell. This notion was tested by comparing the in vitro NK-cell–mediated killing of B16LS9 melanoma cells by liver lymphocytes isolated from non–tumor-bearing naïve wild-type mice, anti-CD-1d–treated mice, Jα18−/− mice, and CD1d−/− mice. Liver lymphocytes isolated from each of these groups of naïve mice demonstrated similar cytotoxicity against B16LS9 melanoma cells, which suggested that normal mice with liver NKT cells do not constitutively express higher NK-cell–mediated cytotoxicity in the liver (Fig. 3B). Additional experiments were performed to test whether the presence of melanoma induces NKT cells to exert a suppressive effect on liver NK cells. The mice were injected intrasplenically with B16LS9 tumor cells, and 14 days later, liver NK cells from different groups of mice were isolated and tested in an in vitro NK-induced killing assay. Liver NK cells from Jα18−/−, CD1d−/−, and anti-CD1d–treated mice caused significantly higher cytotoxicity in the B16LS9 cells than in the wild-type mice (Fig. 3C), which indicates that melanoma liver metastases induce liver NKT cells to downregulate NK-cell–mediated cytotoxicity in the liver.
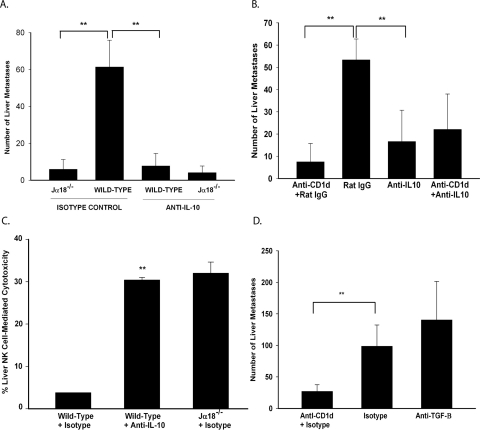
Downregulation of Liver NK Cell Activity by NKT Cells Is IL-10 Dependent but TGF-β Independent
IL-10 and TGF-β are two important cytokines that regulate NK-cell function.36–39 Experiments were performed to determine whether the suppression of liver NK cytotoxicity by tumor-induced NKT cells was IL-10 or TGF-β dependent. Wild-type mice, anti-CD1d–treated mice, and Jα18−/− mice were treated with either anti- IL-10 or anti-TGF-β and injected intrasplenically with B16LS9 cells. Liver metastases were assessed as before. Anti-IL-10–treated wild-type mice had a six-fold reduction in liver metastases than did the rat IgG isotype control–treated wild-type mice (Fig. 4A). The wild-type mice treated with anti-IL-10 and the Jα18−/− mice developed a similar number of liver metastases, demonstrating that the enhanced development of liver metastases in NKT-competent mice was IL-10 dependent. These results were confirmed by disabling NKT cells in wild-type mice by administering an anti-CD1d antibody (Fig. 4B). To confirm that the reduced liver NK-cell activity in tumor-bearing mice was due to IL-10, wild-type mice were treated with anti-IL-10 and injected intrasplenically with B16LS9 cells. At day 14, liver NK cells were isolated and used as effector cells in an in vitro assay of NK-killed B16LS9 cells. Anti-IL-10 treatment restored liver NK-cell cytotoxicity in the wild-type mice to a level similar to that in the NKT-deficient Jα18−/− mice (Fig. 4C). However, when the melanoma-bearing wild-type mice were treated with anti-TGF-β or isotype control IgG, they developed a similar number of liver metastases, indicating that TGF-β was not involved in the inhibition of liver NK-cell activity in this model (Fig. 4D).
Figure 4.
Reduced liver metastasis in NKT-cell–deficient mice was IL-10 dependent and TGF-β independent. (A) Liver metastases in mice treated with anti-IL-10 or rat IgG isotype. n = 15 for all groups. (B) Liver metastases in anti-CD1d-treated wild-type mice after anti-IL-10 treatment. n = 15 for all groups. (C) Liver NK cell cytotoxicity in mice bearing melanoma metastases induced by intrasplenic injection of B16LS9 cells and treated with anti-IL-10 or rat IgG isotype control. The experiment was performed three times with similar results. (D) Liver metastases in mice treated with anti-TGF-β or mouse IgG isotype control. n = 15 for all groups. The experiment was performed twice with similar results. **P < 0.01. Results are expressed as the mean ± SD.
Tumor-Induced NKT Cells Produce IL-10 to Suppress the Antimetastatic Effects of Liver NK Cells
It is known that IL-10 can be produced by macrophages, dendritic cells (DCs), MDSCs, B-cells, NKT cells, and Tregs.40–44 Experiments tested the hypothesis that tumor-induced liver NKT cells recruit Tregs, MDSCs, and M2 macrophages into the liver to produce IL-10, which subsequently suppresses liver NK-cell activity. Livers from naïve mice and wild-type mice with liver metastases were assessed for the presence of CD4+ Tregs and MDSCs by flow cytometry and for gene expression of FoxP3, YM-1, and arginase-1 (Arg-1) by qPCR. The expression of FoxP3 reflects the functional status of Tregs,45 whereas the expression of YM-1 and Arg-1 correlates with M2 macrophages.
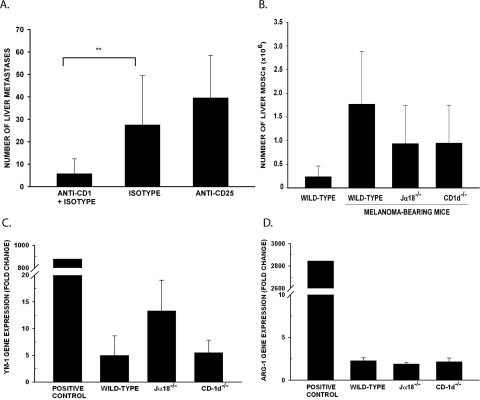
The number of liver CD4+CD25+FoxP3+ Tregs, mean fluorescence intensity (MFI) of FoxP3 expression, and the FoxP3 gene expression in liver mononuclear cells were similar in the naïve mice, tumor-bearing wild-type mice, and NKT-cell–deficient mice (data not shown; P > 0.05). To further rule out the involvement of Treg IL-10 production, mice were treated with anti-CD25 to disable Tregs.46,47 The anti-CD25–treated wild-type mice had several liver metastases, similar to the number in the rat IgG isotype controls, which demonstrated that Tregs are not involved in the development of liver metastases (Fig. 5A). The number of MDSCs was also similar in the naïve mice, tumor-bearing wild-type mice, and NKT-cell–deficient mice (P > 0.05; Fig. 5B). Gene expression of YM-1 or Arg-1 was not significantly different between the tumor-bearing wild-type mice and NKT cell–deficient mice, which indicates that the tumor-bearing wild-type mice and NKT-cell–deficient mice have a similar number of M2 liver macrophages (Figs. 5C, 5D). Thus, Tregs, MDSCs, and M2 macrophages are not the source of IL-10 in the livers of tumor-bearing mice.
Figure 5.
Role of Tregs, MDSCs, and M2 macrophages in IL-10 production in liver metastases produced by intrasplenic injection of B16LS9 melanoma cells. (A) Liver metastases in mice treated with either anti-CD25 or rat IgG isotype control. n = 15 for all groups. (B) Number of liver MDSCs (CD11b+Gr-1+) detected by flow cytometry. (C) Expression of YM-1 and (D) Arg-1 genes detected by qPCR using liver mononuclear cells of mice bearing melanoma metastases. Raw 264.7 cells treated with IL-4, -10, and -13 served as the positive control. **P < 0.01. Results are expressed as the mean ± SD.
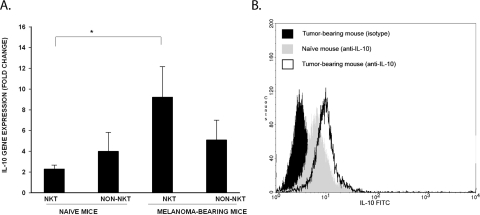
NKT cells can also produce immunoregulatory cytokines, such as IL-10, after activation. This phenomenon was examined by isolating NKT cells from the livers of naïve mice and wild-type mice bearing liver melanoma metastases and probing for IL-10 gene expression by qPCR and assessing IL-10 protein expression. Liver NKT cells from the melanoma-bearing mice displayed significantly higher levels of IL-10 gene expression (Fig. 6A) and IL-10 protein expression (Fig. 6B) compared with that in the naïve mice. By contrast, IL-10 gene expression was similar in non-NKT cells isolated from the livers of the naïve mice and those bearing liver melanoma metastases.
Figure 6.
IL-10 production by liver NKT cells in mice bearing melanoma liver metastases induced by intrasplenic injection of B16LS9 melanoma cells. Liver mononuclear cells were isolated from mice harboring melanoma liver metastases. Mononuclear cells were sorted for NKT cells (NK1.1+TCR-β+) and non-NKT cells (single-positive cells or double-negative cells). (A) Assessment of IL-10 gene expression by qPCR. *P < 0.05. Results represent mean ± SD. (B) Production of IL-10 by liver NKT cells. The black-shaded histogram represents liver NKT cells from tumor-bearing mice stained with the isotype control. Liver NKT cells from naive mice stained with the isotype control show similar results (data not shown). The gray-shaded histogram represents IL-10 production by naïve liver NKT cells. The open histogram represents IL-10 production by liver NKT cells from tumor-bearing mice. All results are representative of two independent experiments.
Discussion
It is widely believed that NKT cells can influence the immune response to tumors. Type I NKT cells exert antitumor effects, whereas type II NKT cells generally promote malignancy and impair antitumor immunity.11 However, in the present study, type I NKT cells played a role in enhancing the development of liver metastases arising from either intraocular melanomas or via intrasplenic tumor injection. That is, the Jα18 KO mice, which lack type I NKT cells, but have an intact type II NKT cell repertoire, developed significantly fewer liver metastases than did the wild-type mice, which have an intact type I NKT-cell population. If type I NKT cells play a role in restricting the development of liver metastases, one would expect results the opposite of those reported here. That is, mice deficient in type I NKT cells would have more, not fewer, liver metastases. On first blush, the reduced number of liver metastases in the CD1d−/− mice, which lack both type I and II NKT cells, suggests that the deficiency in type II NKT cells in these mice accounted for the increased resistance to liver metastases, as type II NKT cells are known to have an inhibitory effect on tumor immunity. However, the resistance to liver metastases was also observed in Jα18 KO mice, which lack type I NKT cells, but have an intact type II NKT cell repertoire. Thus, the resistance to liver metastases persisted, even in the presence of type II NKT cells and occurred only when type I NKT cells were absent.
The mechanism whereby type I NKT cells inhibit hepatic NK-cell activity and promote liver metastases remains to be resolved. However, several explanations come to mind. Type I NKT cells can produce IFN-γ, which could inhibit NK activity by (1) upregulating indoleamine dioxygenase (IDO), which is known to inhibit NK activity.48; (2) upregulating MHC class I molecules on melanoma cells, which send “off” signals that silence the cytolytic machinery of the NK cells13; and (3) disabling the perforin-mediated cytolytic pathway used by the NK cells.49
The results also indicate that the enhanced resistance to liver metastasis in mice deficient or depleted of NKT cells was not due to the ablation of ACAID, which requires the presence of an intact NKT cell repertoire.33 However, the resistance to liver metastasis is NK-cell dependent and T-cell independent. NKT-cell–competent mice and NKT-cell–deficient mice have a similar number of liver NK cells, which express comparable levels of NK-cell–mediated cytotoxicity against B16LS9 melanoma cells. The data indicate that the liver NKT cell repertoire is altered by the presence of melanoma metastases in the liver and as a result, suppresses NK-cell cytotoxicity through the production of IL-10.
Other studies have shown that NKT cells can have dual roles in tumor immunity by either exerting antitumor effects or by suppressing antitumor immune responses.7,10,48 NKT cells derived from different organs also function differently. Those residing in the liver typically express antitumor properties.13 However, the present findings indicate that the presence of melanoma liver metastases converts NKT cells to a suppressive phenotype. This curious departure from other tumor models may be related to the origin of the B16LS9 melanoma cell line, which was derived from liver metastases of B16-F1 cells injected into the ocular posterior chamber of C57BL/6 mice.17 The effect of B16LS9 on the development of liver metastases appears to be organ-specific. That is, there were no differences in the growth of B16LS9 in the VC of the eye or the spleens in the Jα18 KO mice, CD1d KO mice, and wild-type C57BL/6 mice. Experiments are in progress to determine whether tumors that successfully colonize the liver undergo epigenetic changes that influence their interactions with NKT cells.
In the present study, we found that the Jα18−/− mice and CD1d−/− mice had fewer liver metastases arising from intraocular B16LS9 melanoma that was not related to the ablation of ACAID, which is a form of systemic immune tolerance that is induced when antigens enter the eye. However, the resistance to liver metastases that emerged when NKT cells were eliminated, either by gene deletion or by anti-CD1d treatment, correlated with elevated liver NK-cell activity. This result is in keeping with those from animal studies and findings in uveal melanoma patients. NK cells are known to play a major role in controlling liver metastases from intraocular melanomas in mice.21 Moreover, there is a strong correlation between liver NK-cell activity and resistance to liver metastases in uveal melanoma patients.49 The results reported here lend further support to the notion that NK cells play a critical role in controlling melanoma metastases in the liver. In particular, our findings indicate that NKT cells can profoundly suppress liver NK-cell activity and create a local environment that favors the emergence of melanoma liver metastases. Liver NKT cells appear to produce their suppression of liver NK cells through the elaboration of IL-10, but not via TGF-β. The reversal of suppressed NK activity and the restoration of resistance to liver metastases that follows in vivo treatment with anti-IL-10 antibody further support the hypothesis that liver NKT cells enhance liver metastases by a local suppression of NK cells via IL-10. By contrast, administration of exceptionally high doses of anti-TGF-β antibody (1 mg, three times per week) did not relieve suppressed liver NK-cell activity.
IL-10 can be produced by a wide variety of cells, including those with immunoregulatory properties (e.g., Tregs, MDSCs, M2 macrophages, and NKT cells). The present findings indicate that a deficiency in NKT cells did not affect the number of Tregs, MDSCs, or M2 macrophages in the livers of tumor-bearing mice. IL-10 gene expression in liver non-NKT cells, which included Tregs, MDSCs, and M2 macrophages, from the NKT-cell–deficient mice was the same as that found in the non-NKT cells isolated from the livers of wild-type, tumor-bearing mice, a finding that further suggests that the upregulation of IL-10 was restricted to the liver NKT cells. By contrast, IL-10 gene expression was significantly increased in liver NKT cells isolated from the tumor-bearing mice compared with the naïve mice, which is consistent with the proposition that liver NKT cells from naïve mice do not constitutively suppress liver NK activity, whereas liver NKT cells from tumor-bearing mice inhibit liver NK-cell activity in an IL-10-dependent fashion. It has been shown that NKT cells can downregulate tumor immune surveillance through the IL-4-STAT 6 pathway, and in a CD8-dependent manner by decreasing CTL activity.10,11 To our knowledge, this is the first report that liver NKT cells, especially type I NKT cells, inhibit the antimetastatic activity of liver NK cells via the production of IL-10.
NKT cells, especially type I NKT cells, have been administered to patients with lung cancer, melanoma, glioma, breast cancer, colorectal cancer, liver cancer, kidney cancer, and prostate cancer in several phase I clinical trials.10 Extrapolating the present finding to human uveal melanoma should be viewed with caution. B16LS9 was derived from the cutaneous B16 murine melanoma and thus, it is not a uveal melanoma. However, some similarities between human uveal melanomas and B16LS9 cutaneous melanomas transplanted into the poster compartment of the eye bear noting. Human uveal melanomas and B16LS9 melanomas both display a strong predilection to form liver metastases, and both are susceptible to NK-cell–mediated cytolysis. It is also important to note that both humans and mice have NKT cells in their livers.13,50,51
A growing body of evidence indicates that type I NKT cells can play both effector and regulatory roles in antitumor immunity. Therefore, it is important to examine the role of NKT cells in different types of tumor models to gain a better understanding of their function. Based on our study, modulation liver NKT cells may be an important consideration when designing immunotherapy in the management of liver metastases in uveal melanoma patients.
Acknowledgments
The authors thank Joseph Brown for excellent assistance.
Footnotes
Supported by National Institutes of Health Grants CA030276 and EY020799 and Research to Prevent Blindness, New York, NY.
Disclosure: W. Yang, None; H. Li, None; E. Mayhew, None; J. Mellon, None; P.W. Chen, None
References
- 1. Albert DM. The ocular melanoma story: LIII Edward Jackson Memorial Lecture. Part II. Am J Ophthalmol. 1997;123:729–741 [DOI] [PubMed] [Google Scholar]
- 2. Bedikian AY. Metastatic uveal melanoma therapy: current options. Int Ophthalmol Clin. 2006;46:151–166 [DOI] [PubMed] [Google Scholar]
- 3. Terabe M, Berzofsky JA. Immunoregulatory T cells in tumor immunity. Curr Opin Immunol. 2004;16:157–162 [DOI] [PubMed] [Google Scholar]
- 4. Valmori D, Dutoit V, Ayyoub M, et al. Simultaneous CD8+ T cell responses to multiple tumor antigen epitopes in a multipeptide melanoma vaccine. Cancer Immun. 2003;3:15. [PubMed] [Google Scholar]
- 5. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998 [DOI] [PubMed] [Google Scholar]
- 6. Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13:5243–5248 [DOI] [PubMed] [Google Scholar]
- 7. Seino K, Taniguchi M. Functionally distinct NKT cell subsets and subtypes. J Exp Med. 2005;202:1623–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davodeau F, Peyrat MA, Necker A, et al. Close phenotypic and functional similarities between human and murine alphabeta T cells expressing invariant TCR alpha-chains. J Immunol. 1997;158:5603–5611 [PubMed] [Google Scholar]
- 9. van Der Vliet HJ, Nishi N, de Gruijl TD, et al. Human natural killer T cells acquire a memory-activated phenotype before birth. Blood. 2000;95:2440–2442 [PubMed] [Google Scholar]
- 10. Ambrosino E, Berzofsky JA, Terabe M. Regulation of tumor immunity: the role of NKT cells. Expert Opin Biol Ther. 2008;8:725–734 [DOI] [PubMed] [Google Scholar]
- 11. Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santodomingo-Garzon T, Han J, Le T, Yang Y, Swain MG. Natural killer T cells regulate the homing of chemokine CXC receptor 3-positive regulatory T cells to the liver in mice. Hepatology. 2009;49:1267–1276 [DOI] [PubMed] [Google Scholar]
- 13. Crowe NY, Coquet JM, Berzins SP, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eberl G, Lees R, Smiley ST, Taniguchi M, Grusby MJ, MacDonald HR. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J Immunol. 1999;162:6410–6419 [PubMed] [Google Scholar]
- 15. Hammond EH, Compton CC. Protocols for the examination of tumors of diverse sites: introduction. Cancer Committee, College of American Pathologists. Arch Pathol Lab Med. 1999;123:11–13 [DOI] [PubMed] [Google Scholar]
- 16. Winau F, Hegasy G, Weiskirchen R, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129 [DOI] [PubMed] [Google Scholar]
- 17. Diaz CE, Rusciano D, Dithmar S, Grossniklaus HE. B16LS9 melanoma cells spread to the liver from the murine ocular posterior compartment (PC). Curr Eye Res. 1999;18:125–129 [DOI] [PubMed] [Google Scholar]
- 18. Cui J, Shin T, Kawano T, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626 [DOI] [PubMed] [Google Scholar]
- 19. Alizadeh H, Howard K, Mellon J, Mayhew E, Rusciano D, Niederkorn JY. Reduction of liver metastasis of intraocular melanoma by interferon-beta gene transfer. Invest Ophthalmol Vis Sci. 2003;44:3042–3051 [DOI] [PubMed] [Google Scholar]
- 20. Dithmar S, Rusciano D, Grossniklaus HE. A new technique for implantation of tissue culture melanoma cells in a murine model of metastatic ocular melanoma. Melanoma Res. 2000;10:2–8 [PubMed] [Google Scholar]
- 21. Dithmar SA, Rusciano DA, Armstrong CA, Lynn MJ, Grossniklaus HE. Depletion of NK cell activity results in growth of hepatic micrometastases in a murine ocular melanoma model. Curr Eye Res. 1999;19:426–431 [DOI] [PubMed] [Google Scholar]
- 22. Reinmuth N, Liu W, Ahmad SA, et al. Alphavbeta3 integrin antagonist S247 decreases colon cancer metastasis and angiogenesis and improves survival in mice. Cancer Res. 2003;63:2079–2087 [PubMed] [Google Scholar]
- 23. Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjogren's Syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006;176:3950–3957 [DOI] [PubMed] [Google Scholar]
- 24. Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218 [PubMed] [Google Scholar]
- 25. Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240 [DOI] [PubMed] [Google Scholar]
- 26. Streilein JW, Niederkorn JY. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J Exp Med. 1981;153:1058–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dithmar S, Rusciano D, Lynn MJ, Lawson DH, Armstrong CA, Grossniklaus HE. Neoadjuvant interferon alfa-2b treatment in a murine model for metastatic ocular melanoma: a preliminary study. Arch Ophthalmol. 2000;118:1085–1089 [DOI] [PubMed] [Google Scholar]
- 28. Beck AW, Luster TA, Miller AF, et al. Combination of a monoclonal anti-phosphatidylserine antibody with gemcitabine strongly inhibits the growth and metastasis of orthotopic pancreatic tumors in mice. Int J Cancer. 2006;118:2639–2643 [DOI] [PubMed] [Google Scholar]
- 29. Subleski JJ, Hall VL, Back TC, Ortaldo JR, Wiltrout RH. Enhanced antitumor response by divergent modulation of natural killer and natural killer T cells in the liver. Cancer Res. 2006;66:11005–11012 [DOI] [PubMed] [Google Scholar]
- 30. Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. 2006;7:354–359 [DOI] [PubMed] [Google Scholar]
- 31. Niederkorn JY. The induction of anterior chamber-associated immune deviation. Chem Immunol Allergy. 2007;92:27–35 [DOI] [PubMed] [Google Scholar]
- 32. Niederkorn JY. Immune escape mechanisms of intraocular tumors. Prog Retin Eye Res. 2009;28:329–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sonoda KH, Exley M, Snapper S, Balk SP, Stein-Streilein J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J Exp Med. 1999;190:1215–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cunnusamy K, Paunicka K, Reyes N, Yang W, Chen PW, Niederkorn JY. Two different regulatory T cell populations that promote corneal allograft survival. Invest Ophthalmol Vis Sci. 2010;51:6566–6574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Niederkorn JY, Wang S. Immunology of intraocular tumors. Ocul Immunol Inflamm. 2005;13:105–110 [DOI] [PubMed] [Google Scholar]
- 36. Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–5777 [DOI] [PubMed] [Google Scholar]
- 37. Hsu DH, Moore KW, Spits H. Differential effects of IL-4 and IL-10 on IL-2-induced IFN-gamma synthesis and lymphokine-activated killer activity. Int Immunol. 1992;4:563–569 [DOI] [PubMed] [Google Scholar]
- 38. Kriegel MA, Li MO, Sanjabi S, Wan YY, Flavell RA. Transforming growth factor-beta: recent advances on its role in immune tolerance. Curr Rheumatol Rep. 2006;8:138–144 [DOI] [PubMed] [Google Scholar]
- 39. Wahl SM. Transforming growth factor-beta: innately bipolar. Curr Opin Immunol. 2007;19:55–62 [DOI] [PubMed] [Google Scholar]
- 40. Bunt SK, Clements VK, Hanson EM, Sinha P, Ostrand-Rosenberg S. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J Leukoc Biol. 2009;85:996–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kamanaka M, Kim ST, Wan YY, et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952 [DOI] [PubMed] [Google Scholar]
- 42. Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765 [DOI] [PubMed] [Google Scholar]
- 43. Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983 [DOI] [PubMed] [Google Scholar]
- 44. Watte CM, Nakamura T, Lau CH, Ortaldo JR, Stein-Streilein J. Ly49 C/I-dependent NKT cell-derived IL-10 is required for corneal graft survival and peripheral tolerance. J Leukoc Biol. 2008;83:928–935 [DOI] [PubMed] [Google Scholar]
- 45. Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10:689–695 [DOI] [PubMed] [Google Scholar]
- 46. Kohm AP, McMahon JS, Podojil JR, et al. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol. 2006;176:3301–3305 [DOI] [PubMed] [Google Scholar]
- 47. Tanaka H, Tanaka J, Kjaergaard J, Shu S. Depletion of CD4+ CD25+ regulatory cells augments the generation of specific immune T cells in tumor-draining lymph nodes. J Immunother. 2002;25:207–217 [DOI] [PubMed] [Google Scholar]
- 48. Terabe M, Berzofsky JA. NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol. 2007;28:491–496 [DOI] [PubMed] [Google Scholar]
- 49. Jager MJ, Hurks HM, Levitskaya J, Kiessling R. HLA expression in uveal melanoma: there is no rule without some exception. Hum Immunol. 2002;63:444–451 [DOI] [PubMed] [Google Scholar]
- 50. Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Norris S, Doherty DG, Collins C, et al. Natural T cells in the human liver: cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogenous and include Valpha24-JalphaQ and gammadelta T cell receptor bearing cells. Hum Immunol. 1999;60:20–31 [DOI] [PubMed] [Google Scholar]