This study shows that wall teichoic acids, which are major polyanionic polymer components of the cell wall of Staphylococcus aureus, are necessary for growth and survival of S. aureus in the eye.
Abstract
Purpose.
Wall teichoic acids (WTAs) are major polyanionic polymer components of the cell wall of Staphylococcus aureus. However, little is known about their role at the host–pathogen interface, especially in endophthalmitis. This study was designed to investigate the extent to which WTAs contribute to the pathogenicity of S. aureus in models of endophthalmitis and to determine whether there would be value in targeting their biosynthesis as a new therapeutic approach.
Methods.
S. aureus RN6390 and its isogenic WTA-null mutant (RN6390ΔtarO) were used to evaluate the role of WTAs in endophthalmitis. RN6390 and RN6390ΔtarO were cultured in bovine vitreous humor (VH) in vitro or inoculated into the vitreous chamber of C57B6 mice. Changes in the number of bacteria, organ function as determined by electroretinography (ERG), and histopathologic changes were assessed throughout the course of infection. In addition, the efficacy of WTA biosynthesis inhibitors in VH in vitro was examined.
Results.
It was observed that a component of VH synergized with WTA biosynthesis inhibitors in vitro and killed the S. aureus. This effect was also seen when mutants incapable of expressing WTA were exposed to VH. The killing activity of VH was lost on treatment with a protease inhibitor. RN6390ΔtarO could not survive in mouse eyes and did not affect organ function, nor was it able to establish endophthalmitis.
Conclusions.
WTAs are essential cellular constituents for the manifestation of virulence by S. aureus in endophthalmitis, and appears to be a viable target for treating the endophthalmitis caused by S. aureus strains.
Bacterial endophthalmitis is a severe and sight-threatening ocular infection.1 It is characterized by massive inflammation and tissue damage caused both by bacterial infection and subsequent immune response. Bacterial endophthalmitis usually occurs in the context of ocular surgery, trauma, microbial keratitis, or the hematogenous spread of the organism to the eye. Postoperative endophthalmitis is a severe complication of ocular surgery, such as cataract surgery, glaucoma surgery, or vitrectomy.1 Although the technical procedures used in surgery are improving, the incidence of endophthalmitis has not changed and may be increasing.2
Staphylococcus aureus is a commensal species that colonizes the mucosa and skin adjacent to the eye, and as a result, often contaminates surgical sites and leads to endophthalmitis.1,3 Methicillin resistance in S. aureus is on the rise in the community, which in the United States has been associated with the proliferation of the USA300 MRSA (methicillin-resistant S. aureus) lineage,4,5 which likely has contributed to the increased incidence of endophthalmitis caused by MRSA.6,7 Although fluoroquinolones and cephalosporins are widely used for the treatment and prevention of S. aureus endophthalmitis, they are less efficacious against MRSA.6,7 Moreover, S. aureus recently acquired resistance to vancomycin, a drug of last resort.8–10 Because of the rapid emergence of resistant strains, new antibacterial targets that lack cross-resistance with drugs currently in use to treat S. aureus infection are urgently needed.
A possible therapeutic target that is now being explored is S. aureus wall teichoic acid (WTA) biosynthesis. WTAs are anionic polymers composed of repeating units of ribitol-phosphate that are covalently linked to the bacterial cell wall in many Gram-positive pathogens.11 They influence critical properties of the cell envelope, including charge, cation binding, tensile strength, rigidity, and permeability.11 WTAs are not required for the growth of S. aureus in vitro, but are needed to establish infection in most animal models.12–15
S. aureus WTA polymer biosynthesis is performed by the Tar enzymes.11,16 Of interest, this biosynthetic pathway contains two classes of targets: antivirulence targets and antibacterial targets. The first two genes in the pathway (tarO or tarA) can be knocked out, and the resulting mutants are viable in vitro, making the products of these genes potential antivirulence targets. However, genes downstream of tarA in the WTA pathway cannot be deleted in a wild-type background,16 making them antibacterial targets in wild-type strains. However, if flux through the pathway is diverted, either through genetic mutation (of tarO or tarA) or pharmacologic inhibition of TarO by the natural product tunicamycin, these downstream enzymes become expendable, suggesting that the essentiality of these downstream functions results from the accumulation of WTA biosynthetic intermediates in the pathway. The characterization of the first small-molecule inhibitor of an antibacterial target within the WTA biosynthetic pathway was recently reported by one of our groups (SW).17 The compound, 1835F03, inhibits TarG, the transmembrane component of the ABC transporter that exports WTAs to the cell surface.17 A structure–activity relationship study of 1835F03 led to the discovery of a second-generation analogue, targocil, which is 10 times more potent.18 Because blocking polymer synthesis at the beginning of the pathway renders TarG nonessential, these WTA inhibitors generate mutants in which WTAs are no longer expressed. Thus, they can be regarded as both antibiotics and antivirulence factor agents.17
Although the roles of WTAs have been investigated in S. aureus colonization of epithelial and endothelial cells,12–15 the role of WTAs in vivo in eye infection and the extent to which this pathway could represent a viable treatment target have not yet been explored. In the present study, we assessed the necessity for WTA biosynthesis during S. aureus endophthalmitis, both in vitro and in vivo. The WTA transport inhibitor, targocil, was previously shown to halt S. aureus growth through a bacteriostatic mechanism.17,18 In this study, we showed the surprising finding that targocil treatment killed S. aureus in vitro in vitreous humor (VH). Further, we found that a WTA-deficient mutant cannot survive in VH in vitro or in vivo, and as a result WTA-deficient mutants are highly attenuated, with little capacity to cause endophthalmitis.
Materials and Methods
Bacteria and Growth Conditions
The S. aureus strains used in this study are listed in Table 1. The WTA-deficient tarO mutant (RN6390ΔtarO) was generated from RN6390 by allelic exchange with a tetracycline resistance marker.22,23 RN6390ΔtarO is defective in WTA production because of deletion of the first essential enzyme in the pathway. For reasons yet unknown, the mutant was subtly affected in toxin production. Supernatants from overnight cultures of RN6390ΔtarO were found to be one half to one fourth that of RN6390. In addition RN4220 PspactarO, a strain that expresses tarO under the control of an isopropyl β-d-1-thiogalactopyranoside (IPTG)-inducible promoter, was used to verify the role of TarO.17 S. aureus strains were grown in tryptic soy broth (TSB) at 37°C, unless otherwise noted. Tetracycline (Tc; 2.5 μg/mL) and erythromycin (Em; 10 μg/mL) were used for selection where appropriate. To inhibit various steps in WTA biosynthesis, targocil and tunicamycin were dissolved in dimethyl sulfoxide (DMSO) and stored at −20°C. In controls and experimental groups, final concentrations of DMSO were adjusted to 1% in all assays, unless otherwise stated.
Table 1.
Bacterial Strains
| Strain | Genotype and/or Phenotype | Reference |
|---|---|---|
| RN6390 | Prophage-cured derivative of NCTC 8325 | 19 |
| RN4220 | A mutant of NCTC 8325–4 that accepts foreign DNA partial agr defect | 20 |
| RN6390ΔtarO | RN6390 ΔtarO::tetL | This study |
| RN4220ΔtarO | RN4220 ΔtarO | 21 |
| PspactarO | RN4220 ΔtarO::tetL [geh:: (pCL25int-PspactarO) Emr] | 17 |
Assessment of Activity in Vitreous Humor
Bovine eyes obtained from Sierra for Medical Science (Whittier, CA) were used within 24 hours of enucleation and stored at 4°C before use. VH was aspirated with a syringe fitted with an 18-gauge needle. Liquefied portions of the vitreous were carefully aspirated, taking care to avoid areas of adhesion to the retina and other tissues. The VH was filtered through a GD/X sterile 0.45 μm PES syringe filter (Whatman, Clifton, NJ) and stored at −20°C. Each experiment used vitreous from an individual eye.
Animal Care and Use
Female C57BL/6J mice were obtained from the Charles River Laboratory (Boston, MA). All animals were humanely treated according to the guidelines of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All procedures involving mice were approved by the Schepens Eye Research Institute Institutional Animal Care and Use Committee (IACUC). When appropriate, the mice were anesthetized by intraperitoneal injection of ketamine (62.5 mg/kg) and xylazine (12.5 mg/kg). The animals were euthanatized at the appropriate time points by CO2 asphyxiation after anesthesia.
Bacterial Growth in Vitreous Humor In Vitro
Targocil and tunicamycin were used as WTA biosynthesis inhibitors. Targocil inhibits a late step in WTA biosynthesis and stops S. aureus growth bacteriostatically.18 The minimum inhibitory concentration (MIC) of targocil against RN6390 is 1 μg/mL. Tunicamycin is an inhibitor of a large class of enzymes that couple sugar phosphates to membrane-embedded lipid phosphates,24 and 1 μg/mL tunicamycin inhibits WTA production by S. aureus in vitro without affecting bacterial growth rates.17 An overnight culture of S. aureus RN6390 was diluted to approximately 104 CFU in 100 μL of VH or brain–heart infusion (BHI) medium containing 1% DMSO, 5× MIC (5 μg/mL) targocil, or 1 μg/mL tunicamycin and cultured at 37°C statically for 24 hours. Bacteria were enumerated by plating a serial 10-fold dilution. To examine rates of growth of the RN6390 and RN6390ΔtarO strains, we diluted overnight cultures to approximately 105 CFU in 1 mL of VH or BHI medium and cultured the bacteria at 37°C statically. The bacteria were enumerated by serial dilution at each time point. In addition RN4220 PspactarO was tested for bacterial growth in VH. Dilutions of an overnight culture of S. aureus RN4220 wild-type or PspactarO were inoculated into VH, with or without 1 mM IPTG, and bacterial growth was monitored over a 24-hour period. Experiments were performed three times independently.
Transmission Electron Microscopy
RN6390ΔtarO or RN6390 were inoculated to approximately 109 CFU per milliliter of VH and cultured at 37°C for 6 hours. The cells were collected at 0 or 6 hours, fixed in Karnovsky's fixative (2% paraformaldehyde and 2.5% glutaraldehyde in cacodylate buffer [pH 7.4]), and processed for transmission electron microscopy (TEM) by using standard procedures described elsewhere.25 For TEM, 60- to 90-Å sections were obtained, viewed, and photographed (model 410 microscope; Philips Electronics NV, Eindhoven, The Netherlands). Diameters of all cells within three microscopic fields (50–80 cells per strain) were measured with ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html).
Effect of Enzyme or Chemical Treatment on the Killing Ability of VH
Bacterial growth was examined in VH or VH treated in various ways to illuminate the basis for killing. In one case, VH was pretreated with 100 μg/mL protease K (New England Biolabs Inc., Beverly, MA) at 37°C overnight and then boiled for 5 minutes to inactivate the added protease. VH was also pretreated with protease inhibitors for 1 hour at 37°C at the following concentrations: 104 mM 4-(2-aminoethyl)benzenesulfonylfluoride (AEBSF), 1.4 mM trans-epoxysuccinyl-l-leucylamido(4-guanidino)butane (E-64), 4 mM bestatin, and 1× protease inhibitor (PI) cocktail, EDTA-free (Sigma-Aldrich, Poole, UK). DMSO, the diluent for all inhibitors, was adjusted to 1% in VH and used as control. Growth of RN6390ΔtarO in variously treated VH was examined for 24 hours.
Murine Model of Endophthalmitis
The vitreous of eyes of 6- to 8-week-old mice were inoculated by insertion of a borosilicate microcapillary, pulled to a tip size of 50 μm, immediately behind the limbus-parallel conjunctival vessels, corresponding to the narrow murine pars plana, essentially as described.26,27 The right eyes of the mice were injected with 0.5 μL of a bacterial suspension containing 5000 CFU of either S. aureus RN6390 or RN6390ΔtarO diluted in physiologic saline. The left eye of each mouse was left untreated and served as an internal control for electroretinography (ERG) studies. Experiments were performed with a minimum of four animals per experimental group and repeated at least twice.
Quantification of Bacterial Growth
Eyes were enucleated after euthanatization, and the residual adnexal tissue was trimmed before the eyes were rinsed and placed in cold phosphate-buffered saline (PBS). The enucleated eyes were disrupted and homogenized to release bacteria for enumeration by bead-beating with 1.0-mm glass beads (FastPrep; Thermo Scientific, Waltham, MA) in 1 mL PBS for 1 minute at maximum speed. The homogenates were serially diluted, plated onto BHI agar plates, and incubated overnight at 37°C.
Electroretinography
ERG was performed on the mice at 24 and 48 hours after inoculation, using a protocol modified slightly from those previously published.26,27 Briefly, the mice were dark adapted for at least 4 hours and then anesthetized. The pupils were dilated using 1% tropicamide ophthalmic solution (Bausch & Lomb, Tampa, FL). After anesthesia, the body temperature was maintained at 37°C with a microwave heating pad. Gold wire electrodes (0.25 mm; Alfa Aesar, Ward Hill, MA) were placed on the cornea after application of a hypromellose ophthalmic prism solution (Akorn, Inc. Buffalo Grove, IL) and connected to a visual electrodiagnostic system (UTAS-E 3000; LKC Technologies, Gaithersburg, MD). Needle electrodes placed in the anterior scalp and the tail served as reference and ground leads, respectively. The b-wave amplitude (measured from the trough of the a-wave to the peak of the b-wave) in response to a bright flash in a Ganzfeld illumination sphere was recorded for the injected right eye and the contralateral (internal-control) left eye simultaneously. A total of 30 readings at 0.6 cd-s/m2 flash intensity with a 1-second interval between flashes were taken and averaged. The retinal function was defined as the ratio of the b-wave amplitude (from the trough of the a-wave to the peak of the b-wave) of the experimentally treated eye divided by the value for the contralateral untreated eye.
Histologic Analysis
The mice were euthanatized 48 hours after infection, and the eyes were enucleated with Stevens curved, sharp-tip scissors. The eyes were fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (HE).
Statistical Analysis
Data were analyzed by Student's t-test for significance. Values of P < 0.05 were considered significant.
Results
Bacterial Growth in Vitreous Humor In Vitro
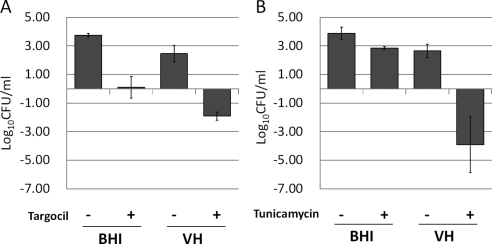
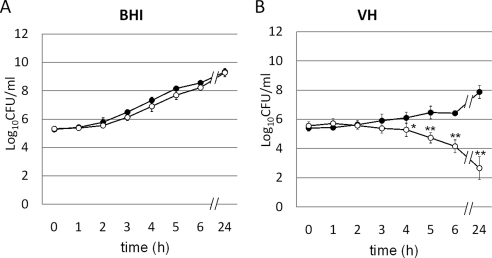
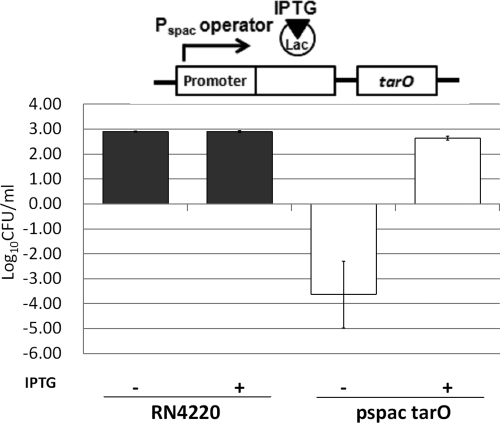
The antibacterial activity of targocil in VH was examined initially. As expected, targocil readily inhibited the growth of RN6390 at levels five times the in vitro MIC in BHI at 24 hours (Fig. 1A). Unexpectedly, however, targocil at identical levels reduced the number of viable bacteria in VH (Fig. 1A). The TarO inhibitor, tunicamycin, which does not inhibit bacterial growth at 1 μg/mL in BHI, also was capable of killing S. aureus in VH (Fig. 1B). Thus, a component of VH synergized with compounds that inhibit steps in the WTA biosynthetic pathway, one bacteriostatic and the other permissive for growth in vitro, killed the S. aureus. Similar behavior was observed for strains in which specific deletions were engineered into the tarO gene, providing independent verification of the mechanism. Growth patterns for RN6390 and RN6390ΔtarO in BHI broth were similar, whereas the number of viable bacteria for only RN6390ΔtarO was reduced in VH (Fig. 2). To prove the role of WTAs in this effect, we used an S. aureus strain expressing tarO from an IPTG-inducible promoter, confirming that WTAs are required for bacterial growth and survival in VH. The growth of the wild-type strain was not influenced by the presence of IPTG. However, the IPTG-inducible Pspac strain was killed in VH without IPTG induction (WTA−), but was rescued by IPTG induction of WTA synthesis (Fig. 3).
Figure 1.
Efficacy of WTA inhibitors in VH. (A) Growth change (−Δlog CFU/mL) of RN6390 bacteria after a 24-hour incubation with 1% DMSO or 5 μg/mL targocil in BHI or VH is shown. Targocil inhibited bacterial growth in BHI, but killed S. aureus in VH. (B) Growth change (−Δlog CFU/mL) of RN6390 bacteria, which were treated with 1% DMSO or 1 μg/mL tunicamycin for 24 hours in BHI or VH, is shown. At the concentration tested, tunicamycin did not inhibit bacterial growth in BHI. The number of tunicamycin-treated bacteria present in VH decreased. Data are expressed as the mean ± SEM (n = 3).
Figure 2.
Growth curve of RN6390 (●) and RN6390ΔtarO (○) in BHI (A) or VH (B). Although RN6390ΔtarO grew as well as the parental strain in BHI, it was killed in VH. Data are expressed as the mean ± SEM (n = 3). *P < 0.05, **P < 0.001 (in comparison with RN6390ΔtarO at 0 hours).
Figure 3.
The regulation of tarO by the lac repressor binding to the Pspac operator. Induction of tarO occurred on addition of IPTG. Growth of RN4220 and the IPTG-inducible tarO strain in the absence and presence of IPTG in VH showed that WTA expression (through IPTG-mediated TarO induction) protected S. aureus from the VH killing effect.
Ultrastructural Comparison
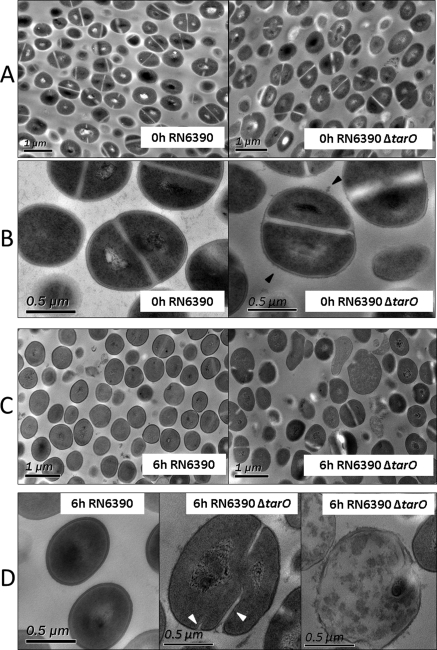
The loss of WTA had a profound effect on cell morphology in VH, as observed by TEM (Fig. 4). The WTA-deficient mutant cultured in BHI showed a rough surface with many surface protrusions, as previously reported (Fig. 4B, right).28 The average longitudinal diameter of RN6390 and RN6390ΔtarO at 0 hours was 0.74 ± 0.10 and 0.75 ± 0.14 μm, respectively. The WTA-deficient mutant exposed to VH for 6 hours, however, contained a subpopulation of strikingly enlarged or irregularly shaped cells, compared with the wild-type (Fig. 4C). The average longitudinal diameter of RN6390 in VH at 6 hours was 0.69 ± 0.06 μm compared with 0.91 ± 0.19 μm for RN6390ΔtarO, corresponding to an average increase of 32% (P < 0.001, t-test). Some WTA-null mutant cells show aberrant septal positioning, additional septal initiation parallel to already existing septa (Fig. 4D, middle), and disruption and lysis (Fig. 4D, right). These aberrations were rare or absent in wild-type cells (Fig. 4D, left).
Figure 4.
Cell morphology in VH. TEM images of RN6390 and RN6390ΔtarO in VH at 0 (A, B) and 6 (C, D) hours at a lower magnification (A, C) and a higher magnification (B, D). Black arrowheads: rough surface with many surface protrusions. White arrowheads: initiation of parallel septa observed in the WTA-null mutant, RN6390ΔtarO, in VH at 6 hours.
Killing of WTA-Deficient S. aureus Relates to Endogenous Protease Activity
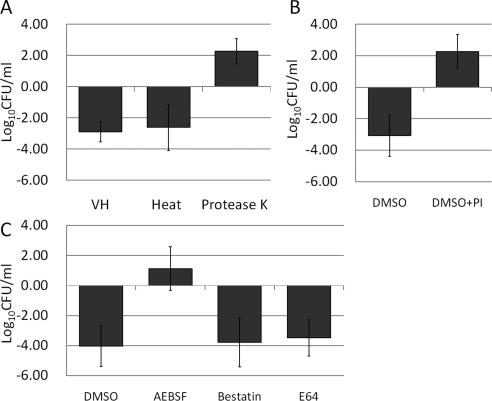
Bovine vitreous was treated in various ways, to collect evidence to explain the unexpected synergistic killing of WTA-deficient S. aureus. Boiling for 5 minutes did not eliminate the synergistic killing activity. However, treatment with protease K abolished the activity, implicating a heat-stable, protein-based activity (Fig. 5A). Proteases in VH were inhibited using a PI cocktail. When VH was preincubated with this mixture for 1 hour, synergistic killing of the WTA-deficient mutant was significantly inhibited (Fig. 5B). Although other inhibitors such as a broad-spectrum cysteine protease inhibitor (E64) and the aminopeptidase inhibitor (bestatin) had no effect on synergistic killing in VH, addition of a serine protease inhibitor (AEBSF) eliminated the killing and permitted growth of WTA-null mutants in VH (Fig. 5C). This is prima facie evidence that WTA provide S. aureus with protection from lethal activity that relates to an endogenous protease. The nature of that lethal activity is the subject of ongoing study.
Figure 5.
Effect of enzyme or chemical pretreatment on the killing ability of VH. (A) Growth change (−Δlog CFU/mL) of RN6390ΔtarO after a 24-hour incubation in VH; heated VH and VH treated with protease K are shown. Protease K treatment diminished the VH killing effect. (B) Growth change (−Δlog CFU/mL) of RN6390ΔtarO after a 24-hour incubation in VH treated with 1% DMSO or a protease inhibitor (PI) cocktail are shown. PI-treated VH lost its ability to kill RN6390ΔtarO. (C) Growth change (−Δlog CFU/mL) of RN6390ΔtarO after a 24-hour incubation in VH treated with 1% DMSO or protease inhibitors is shown. Only VH treated with the serine protease AEBSF lost its ability to kill RN6390ΔtarO. Data are expressed as the mean ± SEM (n = 3).
Virulence in Endophthalmitis
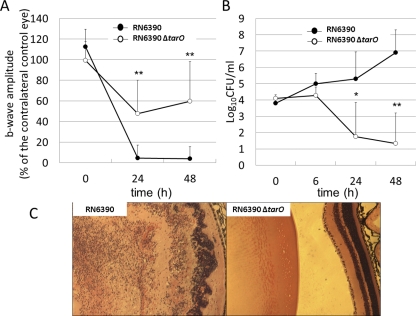
Ocular infections established with RN6390 resulted in a significantly greater reduction of b-wave amplitude than did infection with RN6390ΔtarO, from 24 to 48 hours after inoculation (P ≤ 0.001; Fig. 6A). All eyes infected with RN6390 became unresponsive to ERG 24 to 48 hours after inoculation. In contrast, the WTA-deficient mutant RN6390ΔtarO did not establish endophthalmitis and did not result in loss of organ function, as measured by ERG. The number of recovered RN6390 increased over the course of the infection, whereas that of RN6390ΔtarO decreased from 24 to 48 hours after inoculation (Fig. 6B). Histologically, eyes infected with RN6390 showed signs of disruption of the retinal layers with massive infiltration of the vitreous cavity by inflammatory cells. In contrast, the eyes infected with RN6390ΔtarO showed only sparsely distributed inflammatory cells in the vitreous body. The retinas of these eyes appeared normal (Fig. 6C).
Figure 6.
Role of WTA in a mouse endophthalmitis model. (A) ERG. Retinal responsiveness of eyes infected with RN6390 (●) or RN6390ΔtarO (○). RN6390ΔtarO is significantly less able to establish endophthalmitis and cause vision loss. Data represent the mean ± SEM (n = 8). **P < 0.001 (in comparison to eyes infected with RN6390). (B) Number of viable RN6390 (●) or RN6390ΔtarO (○) recovered from mouse eyes. The numbers of recovered RN6390ΔtarO reduced significantly from 24 to 48 hours after inoculation. Data are expressed as the mean ± SEM (n = 4–8). *P < 0.05, **P < 0.001 (in comparison to the number of RN6390ΔtarO at 0 hours). (C) Histologic analysis. At 48 hours, eyes infected with RN6390 showed disruption of the retinal layers (left). In contrast, the retinas of eyes infected with RN6390ΔtarO had a normal appearance (right). Hematoxylin and eosin; magnification, ×4.
Discussion
Recent work has shown that WTAs are dispensable for growth under laboratory conditions.12,16,29 However, little is known about the contribution of WTAs to growth at sites of infection. Moreover, the efficacy of WTA inhibitors on bacterial growth in biological conditions has not been reported.
In this study, tunicamycin, which inhibits TarO (the first enzyme in WTA biosynthesis) and shuts off WTA production, was found to reduce the number of bacteria in vitreous humor (VH). Furthermore, targocil, which is a bacteriostatic agent in growth media, can kill S. aureus in VH. One explanation of the observed decrease in S. aureus viability may be that bacteria treated with tunicamycin or targocil, thereby having inhibited WTA production, are weakened against an unidentified killing mechanism in VH. To confirm this hypothesis, we used a genetic mutant deficient in tarO expression. VH can kill this mutant, which lacks WTA. In addition, another tarO mutant that has been engineered to express only tarO when IPTG was added was tested. Using this strain, we were able to reverse this effect by inclusion of IPTG, confirming that WTAs are not just required for growth but also survival in VH. These findings show that WTAs play an essential role in protecting S. aureus in this in vivo environment.
Microscopic examination of WTA-null mutants treated in VH revealed an increase in cell size, irregularly shaped cells, and aberrant placement of cell division sites. These morphologic changes were not observed in WTA mutants grown in laboratory media or in the wild-type strain grown in either VH or BHI. Previous studies have demonstrated that WTA mutants show rough surfaces, but changes in cell size were not reported.28 The precise mechanism for the observed defects in the cell envelope and cell division functions cannot yet be deduced from these data. As several reports have demonstrated that mutants of cell wall components such as MsrR- or penicillin-binding proteins show enlarged cells and aberrant placement of cell division sites,30,31 VH may influence the cell wall components of WTA-deficient mutants.
Biochemical investigation has shown that vitreous humor contains a variety of substances vital for bacterial growth.32,33 However, antimicrobial agents such as lysozyme and cationic antimicrobial peptides (CAMPs), which are important components of the innate response, are also present in the vitreous body.34–36 It is possible that these antimicrobial agents in VH act more effectively against WTA-null mutants than against wild-type S. aureus. However, others have reported that the antimicrobial activity of lysozyme, lactoferrin, and some CAMPs are not affected by the lack of WTAs.12,28,37 Furthermore, the absence of WTAs in S. aureus causes a selective increase in bacterial resistance to CAMPs, such as group IIA phospholipase A2 and human β-defensin 3, potentially because WTAs, which are anionic polymers, may participate in the binding of CAMPs.28 Other potential VH antimicrobial agents are proteases. A recent report demonstrated that some proteases could exhibit antimicrobial activity.38–40 In our study, a heat-stable, protease-K–sensitive component in VH was directly or indirectly responsible for VH killing activity. Serine protease inhibitors selectively rescued WTA-deficient mutants from the killing action of VH. Serine proteases contain an activated serine residue at the active site, and some serine proteases could have antimicrobial activity.39,40 Although CAMPs function by disrupting anionic bacterial surfaces, the serine proteases may degrade bacterial proteins including virulence factors.40 Since WTAs are located outside of the cell wall and are covalently linked to peptidoglycan, they could protect the cell wall or membrane from protease cleavage. We re-examined possible direct antimicrobial activity of common serine proteases that could contribute to VH antimicrobial activity for S. aureus. Plasmin, thrombin, or neutrophil elastase did not show antimicrobial activity against either wild-type or WTA mutants (data not shown). Further investigation is needed to determine precisely what synergizes with the inhibitors of WTA in VH.
In conclusion, we have found that WTA-deficient mutants cannot establish endophthalmitis and that these mutants are incapable of growth in the eye. This observation is consistent with our in vitro data using VH, which collectively show that WTAs are necessary for growth and survival of S. aureus in the eye. Previous animal experiments have shown that WTAs play critical roles in tissue adhesion to establish infections.12,13,15 Collectively, these observations implicate the WTA biosynthetic pathway in S. aureus as a useful target to treat or prevent endophthalmitis and other infections.
Acknowledgments
The authors thank Timothy Meredith for the PspactarO strain and helpful discussions and Daisuke Todokoro and Patricia Pearson for technical expertise.
Footnotes
Supported by Public Health and Human Services (PHHS) Grants EY008289 (MSG), Harvard-wide project AI083214, and by Grant GM078477 (SW), Fellowship Grants F3178727 (JGS) and F32AI084316 (JC), the Uehara Memorial Foundation (TS), and the Japanese Eye Bank Society (TS).
Disclosure: T. Suzuki, None; J. Campbell, None; J.G. Swoboda, None; S. Walker, None; M.S. Gilmore, None
References
- 1. Callegan MC, Engelbert M, Parke DW, 2nd, Jett BD, Gilmore MS. Bacterial endophthalmitis: epidemiology, therapeutics, and bacterium-host interactions. Clin Microbiol Rev. 2002;15:111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. West ES, Behrens A, McDonnell PJ, Tielsch JM, Schein OD. The incidence of endophthalmitis after cataract surgery among the U.S. Medicare population increased between 1994 and 2001. Ophthalmology. 2005;112:1388–1394 [DOI] [PubMed] [Google Scholar]
- 3. Speaker MG, Milch FA, Shah MK, Eisner W, Kreiswirth BN. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology. 98:639–649, 1991; discussion 650 [DOI] [PubMed] [Google Scholar]
- 4. Tenover FC, Goering RV. Methicillin-resistant Staphylococcus aureus strain USA300: origin and epidemiology. J Antimicrob Chemother. 2009;64:441–446 [DOI] [PubMed] [Google Scholar]
- 5. Rutar T, Chambers HF, Crawford JB, et al. Ophthalmic manifestations of infections caused by the USA300 clone of community-associated methicillin-resistant Staphylococcus aureus. Ophthalmology. 2006;113:1455–1462 [DOI] [PubMed] [Google Scholar]
- 6. Deramo VA, Lai JC, Winokur J, Luchs J, Udell IJ. Visual outcome and bacterial sensitivity after methicillin-resistant Staphylococcus aureus-associated acute endophthalmitis. Am J Ophthalmol. 2008;145:413–417 [DOI] [PubMed] [Google Scholar]
- 7. Major JC, Jr, Engelbert M, Flynn HW, Jr, Miller D, Smiddy WE, Davis JL. Staphylococcus aureus endophthalmitis: antibiotic susceptibilities, methicillin resistance, and clinical outcomes. Am J Ophthalmol. 2010;149:278–283 [DOI] [PubMed] [Google Scholar]
- 8. Chang S, Sievert DM, Hageman JC, et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med. 2003;348:1342–1347 [DOI] [PubMed] [Google Scholar]
- 9. Peterson DL. Vancomycin-resistant Staphylococcus aureus. Infect Med. 1999;16:235–238 [Google Scholar]
- 10. Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC. Vancomycin-resistant Staphylococcus aureus in the United States. 2002–2006. Clin Infect Dis. 2008;46:668–674 [DOI] [PubMed] [Google Scholar]
- 11. Swoboda JG, Campbell J, Meredith TC, Walker S. Wall teichoic acid function, biosynthesis, and inhibition. Chembiochem. 2010:11:35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weidenmaier C, Kokai-Kun JF, Kristian SA, et al. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med. 2004;10:243–245 [DOI] [PubMed] [Google Scholar]
- 13. Weidenmaier C, Kokai-Kun JF, Kulauzovic E, et al. Differential roles of sortase-anchored surface proteins and wall teichoic acid in Staphylococcus aureus nasal colonization. Int J Med Microbiol. 2008;298:505–513 [DOI] [PubMed] [Google Scholar]
- 14. Weidenmaier C, Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat Rev Microbiol. 2008;6:276–287 [DOI] [PubMed] [Google Scholar]
- 15. Weidenmaier C, Peschel A, Xiong YQ, et al. Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. J Infect Dis. 2005;191:1771–1777 [DOI] [PubMed] [Google Scholar]
- 16. D'Elia MA, Pereira MP, Chung YS, et al. Lesions in teichoic acid biosynthesis in Staphylococcus aureus lead to a lethal gain of function in the otherwise dispensable pathway. J Bacteriol. 2006;188:4183–4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swoboda JG, Meredith TC, Campbell J, et al. Discovery of a small molecule that blocks wall teichoic acid biosynthesis in Staphylococcus aureus. ACS Chem Biol. 2009;4:875–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee K, Campbell J, Swoboda JG, Cuny GD, Walker S. Development of improved inhibitors of wall teichoic acid biosynthesis with potent activity against Staphylococcus aureus. Bioorg Med Chem Lett. 20:1767–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peng HL, Novick RP, Kreiswirth B, Kornblum J, Schlievert P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1988;170:4365–4372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kreiswirth BN, Lofdahl S, Betley MJ, et al. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305:709–712 [DOI] [PubMed] [Google Scholar]
- 21. Grundling A, Schneewind O. Cross-linked peptidoglycan mediates lysostaphin binding to the cell wall envelope of Staphylococcus aureus. J Bacteriol. 2006;188:2463–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bae T, Schneewind O. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid. 2006;55:58–63 [DOI] [PubMed] [Google Scholar]
- 23. Meredith TC, Swoboda JG, Walker S. Late-stage polyribitol phosphate wall teichoic acid biosynthesis in Staphylococcus aureus. J Bacteriol. 2008;190:3046–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Price NP, Tsvetanova B. Biosynthesis of the tunicamycins: a review. J Antibiot (Tokyo). 2007;60:485–491 [DOI] [PubMed] [Google Scholar]
- 25. Gipson IK, Grill SM, Spurr SJ, Brennan SJ. Hemidesmosome formation in vitro. J Cell Biol. 1983;97:849–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Engelbert M, Gilmore MS. Fas ligand but not complement is critical for control of experimental Staphylococcus aureus Endophthalmitis. Invest Ophthalmol Vis Sci. 2005;46:2479–2486 [DOI] [PubMed] [Google Scholar]
- 27. Whiston EA, Sugi N, Kamradt MC, et al. alphaB-crystallin protects retinal tissue during Staphylococcus aureus-induced endophthalmitis. Infect Immun. 2008;76:1781–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koprivnjak T, Weidenmaier C, Peschel A, Weiss JP. Wall teichoic acid deficiency in Staphylococcus aureus confers selective resistance to mammalian group IIA phospholipase A(2) and human beta-defensin 3. Infect Immun. 2008;76:2169–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. D'Elia MA, Millar KE, Beveridge TJ, Brown ED. Wall teichoic acid polymers are dispensable for cell viability in Bacillus subtilis. J Bacteriol. 2006;188:8313–8316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hubscher J, McCallum N, Sifri CD, et al. MsrR contributes to cell surface characteristics and virulence in Staphylococcus aureus. FEMS Microbiol Lett. 2009;295:251–260 [DOI] [PubMed] [Google Scholar]
- 31. Pereira SF, Henriques AO, Pinho MG, de Lencastre H, Tomasz A. Role of PBP1 in cell division of Staphylococcus aureus. J Bacteriol. 2007;189:3525–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coutselinis A, Boukis D, Kalofoutis A. Concentrations of some trace elements in vitreous humor after death. Clin Chem. 1977;23:915–916 [PubMed] [Google Scholar]
- 33. Davey P, Barza M, Peckman C. Spontaneous inhibition of bacterial growth in experimental gram-negative endophthalmitis. Invest Ophthalmol Vis Sci. 1987;28:867–873 [PubMed] [Google Scholar]
- 34. Haynes RJ, McElveen JE, Dua HS, Tighe PJ, Liversidge J. Expression of human beta-defensins in intraocular tissues. Invest Ophthalmol Vis Sci. 2000;41:3026–3031 [PubMed] [Google Scholar]
- 35. Yamagata M, Rook SL, Sassa Y, et al. Bactericidal/permeability-increasing protein's signaling pathways and its retinal trophic and anti-angiogenic effects. FASEB J. 2006;20:2058–2067 [DOI] [PubMed] [Google Scholar]
- 36. Stainer GA, Peyman GA, Berkowitz R, Tessler HH. Intraocular lysozyme in experimental uveitis in rabbits: aqueous and vitreous assay. Invest Ophthalmol. 1976;15:312–315 [PubMed] [Google Scholar]
- 37. Bera A, Biswas R, Herbert S, et al. Influence of wall teichoic acid on lysozyme resistance in Staphylococcus aureus. J Bacteriol. 2007;189:280–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Houghton AM, Hartzell WO, Robbins CS, Gomis-Ruth FX, Shapiro SD. Macrophage elastase kills bacteria within murine macrophages. Nature. 2009;460:637–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Standish AJ, Weiser JN. Human neutrophils kill Streptococcus pneumoniae via serine proteases. J Immunol. 2009;183:2602–2609 [DOI] [PubMed] [Google Scholar]
- 40. Belaaouaj A, Kim KS, Shapiro SD. Degradation of outer membrane protein A in Escherichia coli killing by neutrophil elastase. Science. 2000;289:1185–1188 [DOI] [PubMed] [Google Scholar]