High extracellular osmotic pressure causes stress to corneal epithelial wound healing. The present study demonstrates a novel signaling mechanism in addition to stress-induced MAPK pathways in human corneal epithelial cells to regulate the AP-1 transcription complex through the activation of Plk3, resulting in changes of cell fate.
Abstract
Purpose.
Hyperosmotic stress causes cell shrinkage, perturbs cell function, and damages DNA, resulting in cell cycle arrest and apoptosis. In the present study, the authors explore the mechanism involving hyperosmotic stress-induced activation of c-Jun/AP-1 through a novel Plk3 pathway in human corneal epithelial cells.
Methods.
Human primary corneal epithelial cells and cell line were cultured in a serum-free keratinocyte medium and DMEM/F12 medium containing 10% FBS in a 37°C incubator supplied with 5% CO2, respectively. Western blot analysis was used to determine protein expression and phosphorylation levels. Protein kinase activities were measured by immunocomplex kinase assay. Cell viability and apoptosis were determined by MTT assay and caspase-3 (DEVDase) activity.
Results.
It was found that hyperosmotic stress-induced increases in the phosphorylation of c-Jun, resulting in apoptosis through the activation of Plk3 in human corneal epithelial cells. Plk3 was activated by extracellular hyperosmotic stress to directly phosphorylate c-Jun in the serine 63 and 73 residues. Hyperosmotic stress-induced c-Jun phosphorylation was enhanced by overexpression of constitutively positive Plk3 mutants and suppressed by the knockdown of Plk3 mRNA with Plk3-specific siRNA. Further studies indicated that the phosphorylation of c-Jun by Plk3 was responsible for hyperosmotic stress-induced apoptosis, which was independent from activation of the JNK signaling pathway in human corneal epithelial cells.
Conclusions.
These results, for the first time, provide a novel and alternative signaling mechanism that involves hyperosmotic stress-induced activation of the Plk3 pathway in addition to JNK/p38 MAPK pathways to regulate the c-Jun/AP-1 transcriptional complex and human corneal epithelial cell fate.
The corneal epithelial layer in the front of the eye forms the first line of defense to protect the structures behind the cornea from the insults and injuries of environmental hazards, including hyperosmotic stress.1 Changes in extracellular osmotic pressure are often found in some pathologic conditions, such as diabetes mellitus, uremia, dehydration after exercise, heat shock, fatal burns, and inflammation sites in various tissues, including the cornea.2–5 In the cornea, hyperosmotic stress from environmental and pathologic conditions strikes the epithelial cells and alters the fluid balance, resulting in cell shrinkage, which may contribute to delayed wound healing and dry eye diseases.6 Hyperosmotic stress extracts water from the cell, inducing cell shrinkage. To restore the volume, cells undergo a process of regulated volume increase within several minutes by the uptake of inorganic ions and water.7–9 Cell shrinkage and increased ionic strength alternate cell architecture compartments, denature proteins, and disturb cell function.10–12 It has been shown that persistent hyperosmotic stress can induce DNA damage, cell cycle arrest, and apoptosis.11,13
Hyperosmotic stress induces a series of changes in intracellular kinase cascades that include the activation of JNK, p38, and other signaling pathways to activate the important AP-1 (activating protein-1) transcription factor complex.12,14–16 AP-1 acts as a central switch, in addition to the NF-κB and NFAT5 pathway, which has been known as a primary pathway in response to osmotic stress, to convert extracellular signals into genetic responses by regulating stress-related gene expression to determine cell proliferation, differentiation, and apoptosis.17,18 One of the important hyperosmotic stress-activated transcription factors is c-Jun, which participates in the formation of AP-1 dimers with a group of the other transcription factors, such as members of the Jun, Fos, and ATF families.19,20 In response to stress stimulation, c-Jun is activated mainly through the JNK signaling pathway in various cell types, including corneal epithelial cells. In addition, activation of the JNK signaling pathway by stress stimulation results in increased cell mobility and apoptosis.21–24 In addition, hyperosmotic stress can induce the production of certain corneal epithelial proteins, resulting in decreased cell viability through JNK MAPK-mediated pathways.25 In our previous studies, we found that c-Jun can also be activated by UV irradiation and hypoxia/reoxygenation stress through activation of the Polo-like kinase 3 (Plk3) signaling pathway in corneal epithelial cells.26,27
In mammalian cells, Plk3 belongs to a family consisting of four members (Plk1, Plk2, Plk3, Plk4) that share highly conserved homologies to Drosophila Polo kinase.28–31 All Plk family members contain a kinase domain (KD) at the N terminus that phosphorylates downstream proteins at serine/threonine residues and a Polo-box domain (PBD) at the C terminus that binds to interactive motifs in the target proteins. As a multifunctional protein, Plk3 is involved in regulating a variety of molecular and intracellular events, including DNA damage response, cell cycle control, and apoptosis.32,33 Plk3 undergoes substantial changes in its kinase activity and subcellular distribution after cell cycle progression. Plk3 is rapidly activated on stress stimulation by ionizing radiation, reactive oxygen species, methylmethane sulfonate, UV irradiation, and hypoxia.26,27,34 In addition, hypoxic stress-induced delay of corneal epithelial wound healing is significantly improved in Plk3-deficient mice, indicating that Plk3 plays an important role in the wound healing process.26 However, it is still unclear in hyperosmotic stress-stimulated cells whether Plk3 is activated and how active Plk3 phosphorylates c-Jun to regulate cell function. In the present study, we report that Plk3 is involved in hyperosmotic stress-induced cell death through the phosphorylation of c-Jun protein to activate c-Jun in corneal epithelial cells. Our results reveal that Plk3 plays an important role in the signaling cascades to transmit extracellular hyperosmotic stress signals to the regulation of c-Jun in the AP-1 transcription complex in addition to the known JNK signaling pathway, which has been recognized to connect hyperosmotic stress stimulation to cell fate.
Materials and Methods
Culture of Human Corneal Epithelial Cells
Human corneal epithelial (HCE) cells used in this study included the primary human corneal epithelial (PHCE) cell, human telomerase-immortalized corneal epithelial (HTCE) cell, and human corneal epithelial (HCE) cell lines. PHCE and HTCE cells were cultured in a serum-free keratinocyte medium (Defined Keratinocyte-SFM; Invitrogen, Carlsbad, CA), and HCE cells were grown in Dulbecco's modified Eagle's medium/F-12 (1:1) containing 10% fetal bovine serum and 5 μg/mL insulin. Cells were cultured in an incubator supplied with 95% air and 5% CO2 at 37°C. The medium was replaced every 2 days, and cells were subcultured by treatment of cells with 0.05% trypsin-EDTA. Hyperosmotic stress stimulation was performed by adding various concentrations of sorbitol, sucrose, NaCl, and glucose to the culture media after a time course. Osmotic pressures of hyperosmotic media were measured by using a vapor pressure osmometer (Vapro 5520; Wescor, Inc., Logan, UT).
Transfection of HCE Cells
Plasmid constructs of cDNA encoding the full-length Plk3 and its mutants, including constitutively active pEGFP-Plk3-Polo box domain (amino acids 312–652, termed pEGFP-Plk3-PBD), Plk3-KD (a mutant containing the kinase domain only), and kinase-defective Plk3K52R (a mutant that had a substitution of lysine 52 with arginine), were transfected into corneal epithelial cells by a reagent (Lipofectamine; Invitrogen)–mediated method and a transfection reagent (FuGENE HD Transfection System; Roche, Basel, Switzerland). The transfected cells were subjected to immunoblot analysis, cell survival, and Plk3 activity assays. Transfection mixtures of JNK1-specfic siRNA (catalog no. SI02758637; Qiagen, Valencia, CA) or Plk3-specific siRNA (catalog no. SI02223473; Qiagen) were made by adding 25 nM siRNA and 12 μL transfection reagent (HiPerFect; H301705; Qiagen) in 100 μL culture medium without serum. Transfection complexes in the mixture were formed after 10 minutes of incubation at 22°C. The mixture was evenly and slowly dropped into cultured cells. Transfected cells were cultured under normal growth conditions for 48 to 84 hours before the experiments. Control cells were transfected with nonsilencing siRNA using the method described.
Measurements of Cell Viability
Cell Survival Index Determined by MTT Assays.
A tetrazolium component (MTT) assay was performed in the laboratory in accordance with an established protocol.35 Briefly, a colorimetric assay system was used to measure the reduction of MTT to an insoluble formazan product by the mitochondria of viable cells. The culture medium was replaced with 1 mL serum-free Dulbecco's modified Eagle's medium/F-12 (1:1), and 100 μL MTT solution (5 mg/mL in PBS) was added to each well and incubated for 1 hour in a moisturized CO2 incubator. Acidic isopropyl alcohol (0.4 mL of 0.04 M HCl in absolute isopropyl alcohol) was added to dissolve the colored crystals. All samples were placed into an ELISA plate reader (DU-600 Spectrophotometer; Beckman, Fullerton, CA) at a wavelength of 595 nm with background subtraction. The amount of color produced, normalized with the background, was directly proportional to the number of viable cells.
Measurement of Caspase 3 Activity.
Apoptotic responses of cells were determined by measuring caspase 3 activity with a system (CaspACE; Promega, Madison, WI) that provides a highly sensitive and quantitative measurement of caspase 3 activity.
Immunochemistry Experiments
Western Blot Analysis.
Western blot analysis was performed as described previously.36 In brief, 5 × 105 cells were harvested in 0.5 mL lysate buffer containing 137 mM NaCl, 1.5 mM MgCl2, 2 mM EDTA, 10 mM sodium pyrophosphate, 25 mM β-glycerophosphate, 10% mM glycerol, 1% mM Triton X-100, 1 mM Na-orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 mg/mL leupeptin, 10 μg/mL aprotinin, and 20 mM Tris, pH 7.5. Cell lysates were rinsed by PBS and precleared by centrifugation at 13,000g for 20 minutes. Samples were denatured by adding an equal volume of 2× Laemmli buffer and boiling for 5 minutes and then were loaded onto a 12% SDS-PAGE gel. After fractionation by electrophoresis, proteins were electrotransferred to a polyvinylidene membrane. The membrane was incubated with blocking buffer (5% nonfat milk in TBS-0.1% Tween 20 [TBS-T]) for 1 hour at 22°C and then with respective antibodies overnight at 4°C. After three washes with TBS-T buffer, the membrane was incubated with horseradish peroxidase (HRP)-linked secondary antibody for 1 hour at 22°C. Protein expression was detected with a Western blot detection kit (Santa Cruz Biotechnology, Santa Cruz, CA).
Immunocomplex Kinase Assay.
Immunoprecipitation experiments were performed by rinsing cells with ice-cold PBS and incubating them with 1 mL lysis buffer (20 mM Tris, pH 7.5, 137 mM NaCl, 1.5 mM MgCl2, 2 mM EDTA, 10 mM sodium pyrophosphate, 25 mM glycerophosphate, 10% glycerol, 1% Triton X-100, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 250 μM 4 NPP, 10 μg/mL leupeptin, and 10 μg/mL aprotinin) on ice for 30 minutes. The cell lysates were spun at 13,000g for 10 minutes at 4°C and incubated with antibodies against Plk3, JNK, or p38 (Santa Cruz Biotechnology) at 4°C overnight. The immunocomplexes were recovered by incubation with 50 μL of 10% protein A/G-Sepharose (Santa Cruz Biotechnology). The immunocomplex beads were washed twice with lysis buffer and once with kinase buffer. The resultant protein was subjected to immunoblotting and kinase assay. For the Plk3 kinase assay, the experiment was carried out by incubation of the immunocomplex of Plk3 with GST-c-Jun fusion protein in 30 μL kinase buffer (20 mM HEPES pH 7.6, 5 mM MgCl2, 10 μM MnCl2, 25mM glycerophosphate, 1 mM sodium orthovanadate, 2 mM dithiothreitol, 20 μM ATP, and 10 μCi γ-[32P]ATP) for 30 minutes at 37°C. Samples with an equal volume were displayed on 12% SDS-PAGE and visualized by exposure on x-ray film. For the cold kinase assay, γ-[32P]ATP was omitted from the reaction kinase buffer. Kinase reactions were determined by Western blot analysis with site-specific phosphorylation antibodies.
Results
Hyperosmotic Stress-Induced c-Jun Phosphorylation and Plk3 Activation
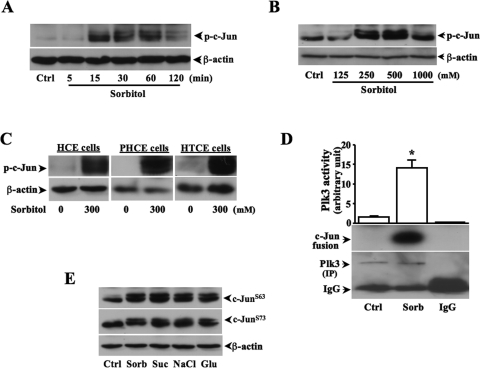
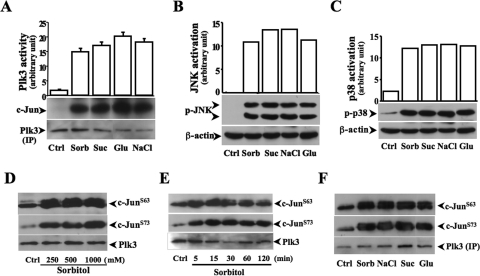
Previous studies demonstrate that increases in extracellular hyperosmotic pressure induce activation of MAPK cascades, including both JNK and p38 pathways.37–39 Hyperosmotic stress-induced JNK activation results in increased phosphorylation of both c-Jun and ATF-2. However, in many cell types hyperosmotic stress-induced p38 activation induces only the phosphorylation of ATF-2, not of c-Jun. We found in HCE cells that the phosphorylation level of c-Jun was increased in 15 minutes and maintained a high level to 60 minutes after exposure of the cells to hyperosmotic stress (Fig. 1A). Hyperosmotic stress-induced c-Jun phosphorylation in HCE cells was sorbitol concentration-dependent and reached peak level at the concentration of 500 mM sorbitol (Fig. 1B). Hyperosmotic stress-induced increases in the phosphorylation of c-Jun were also found in various human corneal epithelial cells (Fig. 1C). Plk3 activity was significantly increased, and phosphorylated c-Jun fusion proteins were detected by immunocomplex kinase assay in hyperosmotic stress-induced HCE cells (Fig. 1D). In addition, hyperosmotic stress-induced effects by increasing concentrations of various extracellular solutes, including sorbitol, sucrose, NaCl, and glucose, were examined in HCE cells. On hyperosmotic stimulation, there were phosphorylations occurring in two serine sites (S63 and S73) of c-Jun detected by Western blot analysis (Fig. 1E). It has been shown that hyperosmotic stress activates JNK and p38 pathways in many cell types. In HCE cells, we measured hyperosmotic stress-induced Plk3, JNK, and p38 activities by increases in concentrations of various extracellular solutes. We found that increased extracellular osmotic pressures significantly activated Plk3 activity and increased JNK and p38 phosphorylation (Figs. 2A–C). To further verify the effect of hyperosmotic stress on c-Jun phosphorylation through the activation of Plk3, antibodies against c-Jun at sites of S63 and S73 were used in Western blot analysis. Hyperosmotic stress-activated Plk3 directly phosphorylated c-Jun at residue sites of S63 and S73 in concentration- and time-dependent fashions determined by immunocomplex assay experiments (Figs. 2D–F). The results demonstrate that there are significant increases in Plk3 activity and phosphorylation of c-Jun at residue sites of S63 and S73 in hyperosmotic stress-induced human corneal epithelial cells.
Figure 1.
Hyperosmotic stress-induced c-Jun phosphorylation and Plk3 activation. (A) Effect of high sorbitol concentrations on c-Jun phosphorylation after a time course. (B) Extracellular sorbitol concentration-dependent increases in phosphorylation of c-Jun. (C) Hyperosmotic stress-induced c-Jun activation in HCE, PHCE, and HTCE cells. (D) Hyperosmotic stress-induced increases in Plk3 activity. Hyperosmotic stress-induced Plk3 activation was determined by immunocomplex kinase assay, and c-Jun fusion protein was used as the substrate. (E) Effect of increased concentrations of various extracellular solutes on c-Jun phosphorylation detected by Western blot analysis. All experiments were repeated four times. *P < 0.05.
Figure 2.
Effect of hyperosmotic stress-induced activation of signaling pathways. (A) Effect of hyperosmotic stress generated by various extracellular solutes on Plk3 activation. Plk3 activity was determined by immunocomplex kinase assay. (B) Effect of hyperosmotic stress on JNK activity. (C) Effect of hyperosmotic stress on p38 activity. (D) Determination of site-specific phosphorylation of c-Jun catalyzed by activated Plk3 in a sorbitol concentration-dependent manner. (E) Time course of hyperosmotic stress-activated Plk3 to phosphorylate c-Jun at sites of S63 and S73. (F) Determination of site-specific phosphorylation of c-Jun catalyzed by activated Plk3 in cells induced by high concentrations of different extracellular solutes. Hyperosmotic stress was generated by extracellular sorbitol (300 mM), glucose (300 mM), sucrose (300 mM), and NaCl (300 mM), respectively.
Comparison of Hyperosmotic Stress-Activated c-Jun through Plk3, JNK, and p38
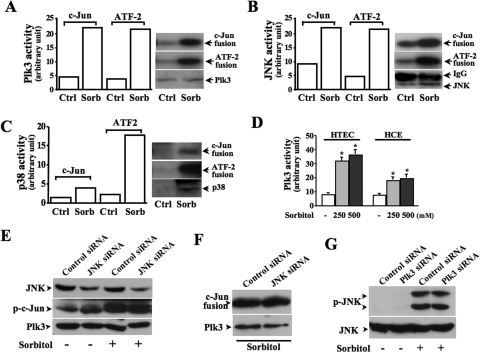
It has been shown that hyperosmotic stress activates p38 and JNK kinases, resulting in AP-1 activation in many cell types, including human corneal epithelial cells. In this study, we found that Plk3 also played a role in hyperosmotic stress-induced AP-1 activation in addition to the effects of JNK and p38. To understand the interactions of these kinases, the effects of hyperosmotic stress-activated Plk3, JNK, and p38 on the phosphorylation of c-Jun and ATF-2 were evaluated and compared by using immunocomplex kinase assays. In hyperosmotic stress-induced HCE cells, stress-activated Plk3 and JNK were able to phosphorylate both c-Jun and ATF-2 fusion proteins (Figs. 3A, 3B). However, the activated p38 in these cells only phosphorylated the ATF-2 fusion protein, but not the c-Jun fusion protein, indicating that p38 may have a different function in hyperosmotic stress-induced HCE cells (Fig. 3C). Sensitivities of Plk3 activation in response to different concentrations of sorbitol were studied by comparing hyperosmotic stress-induced Plk3 activity in HCE and HTCE cells (Fig. 3D). Inhibition of JNK expression using JNK-specific siRNA resulted in a weak effect on hyperosmotic stress-induced c-Jun phosphorylation and had no effect on Plk3 activity (Figs. 3E, 3F). In addition, the knockdown of Plk3 mRNA had no effect on hyperosmotic stress-induced JNK phosphorylation (Fig. 3G). The results suggest that Plk3, in parallel to the effects of JNK and p38 signaling pathways, responds to hyperosmotic stimulation and activates c-Jun and ATF-2, which can form the heterodimer AP-1 complex in human corneal epithelial cells.
Figure 3.
Effects of suppressing JNK on c-Jun and Plk3 activation. (A) Effect of hyperosmotic stress-induced Plk3 activation on c-Jun and ATF-2 phosphorylation. (B) Effect of hyperosmotic stress-induced JNK activation on c-Jun and ATF-2 phosphorylation. (C) Effect of hyperosmotic stress-induced p38 activation on c-Jun and ATF-2 phosphorylation. (D) Comparison of sensitivity of hyperosmotic stress-induced Plk3 activation in HCE and HTCE cells. (E) Effect of knocking down JNK mRNA on hyperosmotic stress-induced c-Jun phosphorylation. (F) Effect of knocking down JNK mRNA on hyperosmotic stress-induced Plk3 activity. (G) Effect of knocking down Plk3 mRNA on hyperosmotic stress-induced JNK activity. Phosphorylation of JNK and c-Jun was measured by using anti-phospho-JNK and anti-phospho-c-Jun antibodies, respectively. Plk3 activity was determined by immunocomplex kinase assay. Asterisk: significant differences in four independent sets of experiments.
Effect of Altered Plk3 Activity on Hyperosmotic Stress-Induced c-Jun Phosphorylation
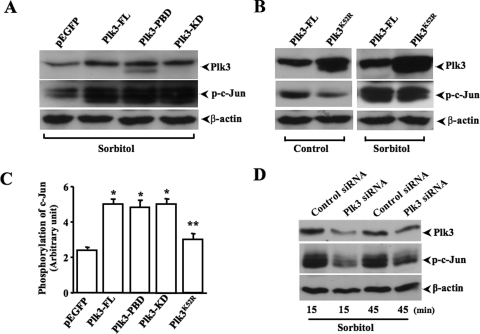
The effect of altered Plk3 activity on hyperosmotic stress-induced c-Jun phosphorylation was determined by transfection of HCE cells with cDNAs encoding full-length Plk3 and several Plk3 mutants. Transfections of cells with cDNA encoding the full-length Plk3 (Plk3-FL), constitutively active Plk3-Polo box domain (Plk3-PBD), and a mutant containing the active kinase domain (Plk3-KD) enhanced Plk3 activity in these cells, resulting in increased hyperosmotic stress-induced c-Jun phosphorylation (Fig. 4A). Overexpression of a kinase-defective mutant of Plk3K52R weakened the effect of hyperosmotic stress on the phosphorylation of c-Jun in transfected cells (Fig. 3B). The statistical significance of these transfection experiments were analyzed and presented as the mean ± SE (Fig. 4C). Finally, Plk3 activity was suppressed by knocking down Plk3 mRNA using Plk3-specific siRNAs. Partial knockdown of Plk3 mRNA resulted in strong suppression of hyperosmotic stress-induced c-Jun phosphorylation in 15 and 45 minutes (Fig. 4D). The data collected from hyperosmotic stress-stimulated and Plk3-transfected cells provide further evidence that there is a close correlation between Plk3 activity and hyperosmotic stress-induced c-Jun phosphorylation in vivo.
Figure 4.
Effects of altered Plk3 activity on hyperosmotic stress-induced c-Jun phosphorylation. (A) Effects of overexpressing wild-type Plk3 (Plk3-FL), constitutively active Plk3-Polo box domain (Plk3-PBD), and active Plk3 kinase domain (Plk3-KD) on c-Jun phosphorylation. (B) Effects of overexpressing kinase-defective Plk3 mutant (Plk3K52R) on hyperosmotic stress-induced c-Jun phosphorylation. (C) Comparison of altered Plk3 effects on hyperosmotic stress-induced changes of c-Jun phosphorylation. (D) Effect of knocking down Plk3 mRNA on hyperosmotic stress-induced c-Jun phosphorylation at 15 and 45 minutes. Control experiments were performed by transfecting HCE cells with pEGFP-tagged Plk3 or the vector only. Significant differences between (asterisk) control and transfected cells and (double asterisks) cells transfected with active and negative mutants, respectively.
Hyperosmotic Stress-Induced Apoptosis through Plk3 Activation
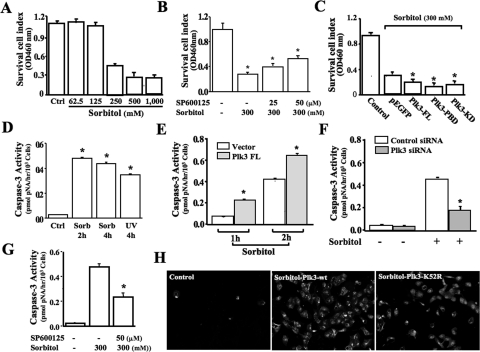
Previous studies indicate that Plk3 is activated by UV irradiation and hypoxic stress to regulate cell fate. We hypothesize that Plk3 is also involved in hyperosmotic stress-induced apoptosis in human corneal epithelial cells. First, the HCE cell survival index was determined by MTT assay after exposure of human corneal epithelial cells to various concentrations of sorbitol (Fig. 5A). Inhibition of JNK using a JNK-specific blocker (SP600125 at concentrations of 25 to 50 μM) suppressed the effect of hyperosmotic sorbitol on the cell survival index (Fig. 5B). Enhanced Plk3 activity by transfections of cDNA encoding full-length Plk3 and active Plk3 mutants significantly reduced cell viability compared with vector plasmid-transfected control cells in response to hyperosmotic stimulation (Fig. 5C). Second, hyperosmotic stress-induced apoptosis was markedly increased by measuring caspase 3 activity, which indicates activation of the apoptotic pathways. Treatment of HCE cells with high concentrations of sorbitol (300 mM) resulted in significant increases in caspase 3 activity in 2 and 4 hours (Fig. 5D). Enhanced Plk3 activity by transfection of cDNA encoding the full-length Plk3 resulted in significant increases in hyperosmotic stress-induced apoptosis (Fig. 5E). In contrast, suppressed Plk3 activity by the knockdown of Plk3 mRNA protected cells from the hyperosmotic stress-induced apoptotic response (Fig. 5F). Inhibition of JNK with SP600125 (50 μM) could not totally suppress hyperosmotic sorbitol-induced apoptosis determined by measuring caspase 3 activity (Fig. 5G). Finally, annexin V immunostaining experiments were performed to detect cells that express phosphatidylserine on the cell surface, a feature found in apoptotic cells. In HCE cells transfected with wild-type Plk3-wt and kinase-defective mutant, Plk3K52R resulted in enhanced and suppressed annexin V levels in sorbitol hyperosmotic stress-induced cells, respectively. These results support the notion that Plk3 plays a functional role in hyperosmotic stress-activated signaling pathways to regulate cell fate.
Figure 5.
Effect of Plk3 activation on hyperosmotic stress-induced cell death. (A) Effect of different sorbitol concentrations on survival cell index. (B) Effect of inhibiting JNK with SP600125 on hyperosmotic sorbitol-induced decreases in survival cell index. (C) Effect of overexpression of wild-type Plk3 and active Plk3 mutants on survival cell index in hyperosmotic stress-induced cells. (D) Comparison of hyperosmotic stress- and UV irradiation-induced apoptosis. (E). Effect of overexpressing wild-type Plk3 on hyperosmotic stress-induced apoptosis at 1 hour and 2 hours. (F) Effect of knocking down Plk3 mRNA on hyperosmotic stress-induced apoptosis. (G) Effect of inhibiting JNK with SP600125 on hyperosmotic sorbitol-induced apoptosis. (H) Hyperosmotic stress-induced increases of annexin V level in Plk3-wt and Plk3K52R transfected cells. Survival cell index and apoptosis were evaluated by MTT assays and by measuring caspase 3 activity and annexin V levels, respectively. Significant differences in data collected from four independent experiments (*P < 0.05; n = 4).
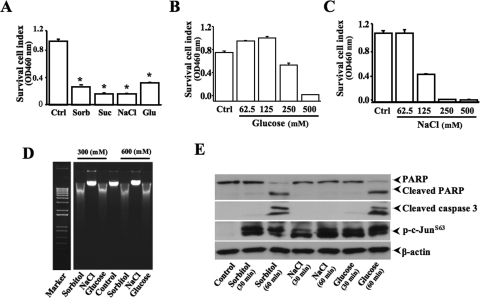
Comparison of Various Extracellular Solute Effects on Cell Viability and Apoptosis
The different effects of extracellular solute-dependent and hyperosmotic stress-induced apoptosis were analyzed by MTT assay, measurement of DNA fragmentation, and detection of caspase 3 and PARP cleavages. HCE cell viabilities were significantly decreased on treatments of hyperosmotic stress generated by 300 mM sorbitol, sucrose, glucose, or NaCl (Fig. 6A). The concentration and response relationship of hyperosmotic stress (glucose and NaCl) and the cell survival index were examined by MTT assay (Figs. 6B, 6C). DNA fragmentation that is a fingerprint of cell apoptosis induced by hyperosmotic stress with various extracellular solutes was detected to determine apoptotic responses (Fig. 6D). Interestingly, hyperosmotic NaCl stress induced a suppression of cell viability, but it did not evoke apoptotic responses in HCE cells. To further verify the effect of hyperosmotic stress on apoptosis in HTCE cells, caspase 3 and PARP cleavages were detected in 60 minutes after hyperosmotic stimulation by using specific antibodies (Fig. 6E). The results of detecting caspase 3 and PARP cleavages were consistent with the findings of DNA fragmentation experiments, suggesting that hyperosmotic stress was able to induce apoptosis; however, hyperosmotic NaCl stress, which induced a suppression of cell survival index, had no effects on apoptosis in HCE and HTCE cells.
Figure 6.
Effects of different solutes on hyperosmotic stress-induced cell death. (A) Effects of different hyperosmotic solutes on survival cell index. (B) Concentration-dependent effect of hyperosmotic glucose on survival cell index. (C) Concentration-dependent effect of hyperosmotic NaCl on survival cell index. (D) Effects of different hyperosmotic solutes on DNA condensation and fragmentation. (E) Effects of different hyperosmotic solutes on caspase 3 and PARP cleavages in HTCE cells. Survival cell index and apoptosis were evaluated by MTT assays and by measuring caspase 3 and PARP cleavages, respectively. Significant differences in data collected from four independent experiments (*P < 0.05; n = 4).
Discussion
Corneal epithelial cells play important roles in maintaining the normal function of vision. Corneal epithelial cells form the first line of defense in the front of the eye to protect eye structures behind the cornea from strikes of environmental stress, such as UV irradiation, hypoxia/reoxygenation, osmotic pressure, and infection. Extracellular hyperosmotic stress is caused by differences in concentrations of salts and macromolecules across the plasma membrane. In the present study, the effect of hyperosmotic stress on corneal epithelial cell fate was investigated by eliciting a novel signaling mechanism that hyperosmotic stress induces corneal epithelial cell apoptosis through the activation of Plk3. It has been shown that Plk3 is an important mediator of the cellular response to genotoxic stress.26,27,34 Recent studies demonstrate that Plk3 plays an important role in hypoxic stress-induced delay of corneal epithelial wound healing.40 In fact, Plk3 is a key component in an alternative-signaling pathway, in addition to the JNK pathway, to mediate UV irradiation and hypoxic stress-induced c-Jun activation in corneal epithelial cells.26,27,34 In the present study, Plk3 activity was significantly increased in response to stimulation of hyperosmotic stress generated by elevated extracellular concentrations of sorbitol, sucrose, NaCl, and glucose. Hyperosmotic stress-activated Plk3 directly phosphorylated c-Jun at residues of S63 and S73, which is similar to the effect of JNK activation on the c-Jun protein in hyperosmotic stress-induced cells. However, the inhibition of JNK with a specific inhibitor incompletely suppressed hyperosmotic stress-induced apoptosis, suggesting that there is an alternative pathway in addition to the JNK effects (Fig 5). We have also confirmed in human corneal epithelial cells that hyperosmotic stress-induced c-Jun phosphorylation did not occur through activation of the p38 signaling pathway (Fig. 3).
Hyperosmotic stress activates a series of defense mechanisms in mammalian cells. A quick response to hyperosmotic stress is the shrinkage of the cell volume to activate the Na-K cotransporter, ion channels, and water channels, leading to a regulatory volume increase.7–9 Increased ion strength can also harm cells and induces DNA double-strand breaks resulting in growth cessation and apoptosis. In corneal epithelial cells, hyperosmotic stress also affected the stability of DNA double strands by detecting the increase in the phosphorylation level of H2AX (data not shown). It has been shown that hyperosmotic stress also occurs in lymphoid organs and at inflammatory sites. Recent reports demonstrate that the response of immune cells to hyperosmotic stress is regulated by nuclear factor of activated T cells 5 (NFAT5) to induced expressions of hyperosmolarity-responsive genes and cytokine production.41 In these processes, serious signaling pathways, including p38 and JNK signaling pathways, were activated to protect cells from the damage of hyperosmotic pressure. In the present study, we provided first-hand information indicating that Plk3 plays important roles in the hyperosmotic stress-induced signaling pathways linked to c-Jun/AP-1 activation. It will be important in future studies to explore whether Plk3 is also associated with hyperosmotic stress-induced NFAT5 activation. The other interesting finding of the present study is that, unlike other solutes, hyperosmotic stress generated by high concentrations of extracellular NaCl inhibited the HCE cell survival index but did not induce apoptosis (Fig. 6). This may involve some sodium transport mechanisms in HCE cells and requires a further investigation in future studies.
Further evidence that Plk3 involves the regulation of hyperosmotic stress-induced c-Jun phosphorylation and apoptosis was provided by transfecting various constitutively active Plk3 mutants and by knocking down Plk3 in human corneal epithelial cells. After the transfection of constitutively active Plk3 to HCE cells, the phosphorylation level of c-Jun was significantly increased in both untreated and hyperosmotic stress-induced cells. In parallel to c-Jun activation, cell viability was significantly decreased. In contrast, the knockdown of Plk3 mRNA by the transfection of Plk3-specific siRNA resulted in decreased c-Jun phosphorylation in both untreated and hyperosmotic stress-induced cells. In addition, the knockdown of Plk3 activity in these cells partially protected them from hyperosmotic stress-induced apoptosis (Fig. 5E). As expected, alterations of Plk3 activity in human corneal epithelial cells could not totally suppress hyperosmotic stress-induced c-Jun phosphorylation and apoptotic responses because of the effect of endogenous JNK activity on the regulation of c-Jun, suggesting that Plk3 has no cross-talk with the JNK signaling pathway. This notion was verified by individually knocking down JNK and Plk3 activity through the transfection of cells with JNK- and Plk3-specific siRNA. Results demonstrate that the suppression of JNK and Plk3 activity in hyperosmotic stress-induced cells had no effect on Plk3 and JNK activity, respectively.
Footnotes
Supported by National Institutes of Health Grants EY 018343 (LL) and CA074229 (WD).
Disclosure: L. Wang, None; W. Dai, None; L. Lu, None
References
- 1. Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med (Maywood). 2001;226:653–664 [DOI] [PubMed] [Google Scholar]
- 2. Foulks GN. The correlation between the tear film lipid layer and dry eye disease. Surv Ophthalmol. 2007;52:369–374 [DOI] [PubMed] [Google Scholar]
- 3. Wu SG, Jeng FR, Wei SY, et al. Red blood cell osmotic fragility in chronically hemodialyzed patients. Nephron. 1998;78:28–32 [DOI] [PubMed] [Google Scholar]
- 4. Mitono H, Endoh H, Okazaki K, et al. Acute hypoosmolality attenuates the suppression of cutaneous vasodilation with increased exercise intensity. J Appl Physiol. 2005;99:902–908 [DOI] [PubMed] [Google Scholar]
- 5. Ito T, Itoh T, Hayano T, Yamauchi K, Takamata A. Plasma hyperosmolality augments peripheral vascular response to baroreceptor unloading during heat stress. Am J Physiol Regul Integr Comp Physiol. 2005;289:R432–R440 [DOI] [PubMed] [Google Scholar]
- 6. Corrales RM, Luo L, Chang EY, Pflugfelder SC. Effects of osmoprotectants on hyperosmolar stress in cultured human corneal epithelial cells. Cornea. 2008;27:574–579 [DOI] [PubMed] [Google Scholar]
- 7. Lang F, Busch GL, Ritter M, et al. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306 [DOI] [PubMed] [Google Scholar]
- 8. Yu SP, Choi DW. Ions, cell volume, and apoptosis. Proc Natl Acad Sci U S A. 2000;97:9360–9362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deutsch C, Slater L, Goldstein P. Volume regulation of human peripheral blood lymphocytes and stimulated proliferation of volume-adapted cells. Biochim Biophys Acta. 1982;721:262–267 [DOI] [PubMed] [Google Scholar]
- 10. Westfall PJ, Patterson JC, Chen RE, Thorner J. Stress resistance and signal fidelity independent of nuclear MAPK function. Proc Natl Acad Sci U S A. 2008;105:12212–12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev. 2007;87:1441–1474 [DOI] [PubMed] [Google Scholar]
- 12. Sheikh-Hamad D, Gustin MC. MAP kinases and the adaptive response to hypertonicity: functional preservation from yeast to mammals. Am J Physiol Renal Physiol. 2004;287:F1102–F1110 [DOI] [PubMed] [Google Scholar]
- 13. Lunn JA, Jacamo R, Rozengurt E. Preferential phosphorylation of focal adhesion kinase tyrosine 861 is critical for mediating an anti-apoptotic response to hyperosmotic stress. J Biol Chem. 2007;282:10370–10379 [DOI] [PubMed] [Google Scholar]
- 14. Galcheva-Gargova Z, Derijard B, Wu IH, Davis RJ. An osmosensing signal transduction pathway in mammalian cells. Science. 1994;265:806–808 [DOI] [PubMed] [Google Scholar]
- 15. Kyriakis JM, Banerjee P, Nikolakaki E, et al. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160 [DOI] [PubMed] [Google Scholar]
- 16. Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–1197 [DOI] [PubMed] [Google Scholar]
- 17. Ho SN. Intracellular water homeostasis and the mammalian cellular osmotic stress response. J Cell Physiol. 2006;206:9–15 [DOI] [PubMed] [Google Scholar]
- 18. Kultz D, Csonka L. What sets the TonE during osmotic stress? Proc Natl Acad Sci U S A. 1999;96:1814–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246 [DOI] [PubMed] [Google Scholar]
- 20. Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400 [DOI] [PubMed] [Google Scholar]
- 21. Lee JH, Kim M, Im YS, Choi W, Byeon SH, Lee HK. NFAT5 induction and its role in hyperosmolar stressed human limbal epithelial cells. Invest Ophthalmol Vis Sci. 2008;49:1827–1835 [DOI] [PubMed] [Google Scholar]
- 22. Lu L, Wang L, Shell B. UV-induced signaling pathways associated with corneal epithelial cell apoptosis. Invest Ophthalmol Vis Sci. 2003;44:5102–5109 [DOI] [PubMed] [Google Scholar]
- 23. Kimura K, Teranishi S, Yamauchi J, Nishida T. Role of JNK-dependent serine phosphorylation of paxillin in migration of corneal epithelial cells during wound closure. Invest Ophthalmol Vis Sci. 2008;49:125–132 [DOI] [PubMed] [Google Scholar]
- 24. Saika S, Okada Y, Miyamoto T, et al. Role of p38 MAP kinase in regulation of cell migration and proliferation in healing corneal epithelium. Invest Ophthalmol Vis Sci. 2004;45:100–109 [DOI] [PubMed] [Google Scholar]
- 25. Chen Z, Tong L, Li Z, et al. Hyperosmolarity-induced cornification of human corneal epithelial cells is regulated by JNK MAPK. Invest Ophthalmol Vis Sci. 2008;49:539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang L, Dai W, Lu L. Stress-induced c-Jun activation mediated by Polo-like kinase 3 in corneal epithelial cells. J Biol Chem. 2007;282:32121–32127 [DOI] [PubMed] [Google Scholar]
- 27. Wang L, Gao J, Dai W, Lu L. Activation of Polo-like kinase 3 by hypoxic stresses. J Biol Chem. 2008;283:25928–25935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dai W. Polo-like kinases, an introduction. Oncogene. 2005;24:214–216 [DOI] [PubMed] [Google Scholar]
- 29. Donohue PJ, Alberts GF, Guo Y, Winkles JA. Identification by targeted differential display of an immediate early gene encoding a putative serine/threonine kinase. J Biol Chem. 1995;270:10351–10357 [DOI] [PubMed] [Google Scholar]
- 30. Hamanaka R, Smith MR, O'Connor PM, et al. Polo-like kinase is a cell cycle-regulated kinase activated during mitosis. J Biol Chem. 1995;270:21086–21091 [DOI] [PubMed] [Google Scholar]
- 31. Li B, Ouyang B, Pan H, et al. Prk, a cytokine-inducible human protein serine/threonine kinase whose expression appears to be down-regulated in lung carcinomas. J Biol Chem. 1996;271:19402–19408 [DOI] [PubMed] [Google Scholar]
- 32. Conn CW, Hennigan RF, Dai W, Sanchez Y, Stambrook PJ. Incomplete cytokinesis and induction of apoptosis by overexpression of the mammalian polo-like kinase, Plk3. Cancer Res. 2000;60:6826–6831 [PubMed] [Google Scholar]
- 33. Wang Q, Xie S, Chen J, et al. Cell cycle arrest and apoptosis induced by human Polo-like kinase 3 is mediated through perturbation of microtubule integrity. Mol Cell Biol. 2002;22:3450–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ouyang B, Pan H, Lu L, et al. Human Prk is a conserved protein serine/threonine kinase involved in regulating M phase functions. J Biol Chem. 1997;272:28646–28651 [DOI] [PubMed] [Google Scholar]
- 35. Li T, Lu L. Epidermal growth factor-induced proliferation requires down-regulation of Pax6 in corneal epithelial cells. J Biol Chem. 2005;280:12988–91295 [DOI] [PubMed] [Google Scholar]
- 36. Wang L, Dai W, Lu L. Ultraviolet irradiation-induced K(+) channel activity involving p53 activation in corneal epithelial cells. Oncogene. 2005;24:3020–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aggeli IK, Gaitanaki C, Lazou A, Beis I. Activation of multiple MAPK pathways (ERKs, JNKs, p38-MAPK) by diverse stimuli in the amphibian heart. Mol Cell Biochem. 2001;221:63–69 [DOI] [PubMed] [Google Scholar]
- 38. Kultz D, Madhany S, Burg MB. Hyperosmolality causes growth arrest of murine kidney cells: induction of GADD45 and GADD153 by osmosensing via stress-activated protein kinase 2. J Biol Chem. 1998;273:13645–13651 [DOI] [PubMed] [Google Scholar]
- 39. Uhlik MT, Abell AN, Johnson NL, et al. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat Cell Biol. 2003;5:1104–1110 [DOI] [PubMed] [Google Scholar]
- 40. Lu J, Wang L, Dai W, Lu L. Effect of hypoxic stress-activated Polo-like Kinase 3 on corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2010;51:5034–5040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kino T, Takatori H, Manoli I, et al. Brx mediates the response of lymphocytes to osmotic stress through the activation of NFAT5. Sci Signal. 2009;2:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]