The authors provide substantial evidence that the injured HO-2 null cornea, which experiences extensive oxidative stress, exaggerated inflammation, and impaired wound healing, can be rescued by the heme oxygenase product biliverdin, further supporting the notion that HO-2 is a critical cytoprotective system in the cornea.
Abstract
Purpose.
The heme oxygenase system (HO-1 and HO-2) represents an intrinsic cytoprotective and anti-inflammatory pathway based on its ability to modulate leukocyte migration and to inhibit the expression of inflammatory cytokines and proteins by its products biliverdin/bilirubin and carbon monoxide. Corneal injury in HO-2 null mice leads to impaired healing and chronic inflammatory complications, including ulceration and neovascularization. The authors examined whether topically administered biliverdin can counteract the effects of HO deficiency in a corneal epithelial injury model.
Methods.
HO-2 null mice were treated with biliverdin 1 hour before epithelial injury and twice a day thereafter. Reepithelialization and neovascularization were assessed by fluorescein staining and vital microscopy, respectively, and were quantified by image analysis. Inflammation was quantified by histology and Gr-1–specific immunofluorescence, and oxidative stress was assessed by DHE fluorescence.
Results.
Treatment with biliverdin accelerated wound closure, inhibited neovascularization and reduced epithelial defects. It also reduced inflammation, as evidenced by a reduction in the appearance of inflammatory cells and the expression levels of inflammatory and oxidant proteins, including KC and NOXs.
Conclusions.
The results clearly show that biliverdin, directly or through its metabolism to bilirubin by biliverdin reductase—the expression of which is increased after injury—rescues the aberrant inflammatory phenotype, further underscoring the importance of the HO system in the cornea for the execution of an ordered inflammatory and reparative response.
Tightly controlled inflammation contributes to normal wound healing. It facilitates and crucially drives tissue repair by stimulating resident cells to migrate and proliferate into the wound site, but, when prolonged, it severely disrupts wound healing. Thus, a self-resolving inflammatory-reparative process must include both proinflammatory and anti-inflammatory circuits that work in concert to initiate, mediate, and resolve inflammation in a tightly controlled manner, allowing for the repair process to proceed toward complete structural and functional restoration.
Heme oxygenase (HO) is the rate-limiting enzyme in heme catabolism. It cleaves heme into iron, carbon monoxide (CO), and biliverdin, which is subsequently converted by biliverdin reductase (BVR) to the potent endogenous antioxidant bilirubin.1 Two isoforms, HO-1 and HO-2, are expressed in most tissues. Whereas HO-2 displays, in general, a constitutive expression that is developmentally regulated2 and can be altered in human pathologic conditions,3,4 HO-1 expression is relatively low in most tissues during homeostasis but is readily induced in response to injury.1 HO-1 and HO-2 are similar in terms of mechanisms of heme oxidation, cofactor and substrate specificity, and susceptibility to inhibition by porphyrins.1,5
Although the mechanisms involved in cytoprotection are largely unknown, the elimination of excess cellular heme as well as the enzymatic products of the HO system, CO and bilirubin, have been shown to protect against tissue damage by exerting antioxidant and anti-inflammatory effects.6–9 The upregulation of HO activity and the administration of biliverdin/bilirubin or CO have been shown to downregulate the inflammatory response by attenuating the expression of adhesion molecules and, thus, inhibiting leukocyte recruitment,10,11 repressing the induction of cytokines and chemokines,12–19 or inhibiting proinflammatory hemoproteins such as cyclooxygenase-2 and cytochrome P450 4B1.20–23
We have previously documented the presence of HO expression and activity in human, mouse, and rabbit corneas and HO-1 inducibility after oxidative stress in vitro and hypoxic injury in vivo.20,22,24–27 In recent studies, we showed that HO-2 displays a prominent constitutive expression in the cornea and that deletion of the HO-2 gene markedly impairs the inflammatory and reparative response of the cornea to epithelial injury28 and in a model of suture-induced neovascularization.12 Hence, HO-2 deficiency led to unresolved corneal inflammation and chronic inflammatory complications, including ulceration, perforation, and neovascularization.
It is widely accepted that bilirubin, the product of biliverdin reductase, is the more powerful bioactive metabolite of the HO pathway. However, bilirubin is highly lipophilic, whereas biliverdin is water soluble and readily penetrates tissue. Studies have shown that the administration of biliverdin is as effective as the administration of bilirubin in conferring antioxidant and cytoprotective actions, in part because of its rapid conversion to bilirubin by biliverdin reductase. In the present study, we further characterized the response of the HO-2 null cornea to epithelial injury and examined whether supplementing HO activity by the administration of biliverdin is effective in reducing oxidative stress, inflammation, and neovascularization and in promoting healing of the HO-deficient cornea.
Materials and Methods
Animals and Epithelial Injury
All animal experiments were performed following an institutionally approved protocol in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The HO-2-null mice are direct descendants of the HO-2 mutants produced by Poss and colleagues.29 These well-characterized HO-2-null mice have a C57BL/6 × 129/Sv genetic background,30 which was used on age- and sex-matched controls (Jackson Laboratory, Bar Harbor, ME). Mice were anesthetized with ketamine (50 mg/kg) and xylazine (20 mg/kg) intramuscularly, and 1 drop of tetracaine-HCl 0.5% was applied to the eye to deliver local corneal anesthesia before the animals were subjected to injury. The corneal epithelium was removed up to the corneal/limbal border using a brush/burr with a 0.5-mm corneal rust ring remover (Algerbrush II; The Alger Company, Inc., Lago Vista, TX). Mice were treated with biliverdin (20 mg/kg in 100 μL administered intraperitoneally) once daily, starting 1 day before injury, and topical (10 μL of 100 μM) or its vehicle (Hanks balanced salt solution, pH 7.4) 1 hour before injury and two times daily thereafter. Corneal wound healing was measured by fluorescein using a Zeiss dissecting microscope coupled to a digital camera (Axiocam Hrc; Zeiss, Göttingen, Germany) and compatible software (Axiovision 4.5; Zeiss). Digital images were analyzed by image processing software (ImagePro; Media Cybernetics, Inc., Silver Spring, MD). Corneal vascularization was measured as the total length (millimeters) of vessels in the cornea. Mice were euthanatized at the indicated time, and the corneas were dissected and subjected to selected analyses.
Histology, Immunostaining, and Immunoblotting
Dissected corneas were washed twice with PBS and fixed in 4% paraformaldehyde-PBS for 1 hour at 4°C. Corneas were washed five times with PBS, placed in 30% sucrose for 24 hours, and embedded in OCT compound (Sakura Finetek, Torrance, CA). Cryostat sections were cut transversely into 5- to 7-μm–thick sections, stained with hematoxylin-eosin, and mounted on microscope slides in mounting medium (Cytoseal XYL; Richard-Allan Scientific, Kalamazoo, MI). For immunofluorescence studies, corneal sections (5 μm) were blocked in 5% goat serum in PBS-Triton X-100 (0.3%) overnight. Then sections were incubated with rabbit anti-mouse Gr-1 antibody (1:100; eBioscience, San Diego, CA) overnight at 4°C, washed, and further incubated with a Cy3-conjugated goat anti-rabbit antibody (1:500; Jackson ImmunoResearch, West Grove, PA) for 1 hour at room temperature. Sections were washed and counterstained for nuclei with 4′,6-diamidino-2-phenylindole (DAPI) for 5 minutes. Immunofluorescence was visualized using a fluorescence microscope (Axioplan-2; Zeiss). Images were captured and analyzed using multichannel image processing software (AxioVision 4.6; Zeiss). TUNEL assay was performed on cryostat sections using a kit for the detection and quantification of apoptosis (In Situ Cell Death Detection Kit, TMR red; Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions. Sections were counterstained with DAPI and visualized using a fluorescence microscope (Axioplan-2; Carl Zeiss, Inc. Oberkochen, Germany). Images were captured using the AxioVision 4.6 image processing software (Carl Zeiss, Inc.). Parallel image acquisitions of corneal sections from vehicle- and biliverdin-treated animals were performed with fixed parameters. To minimize any potential artifactual fluorescence caused by visible light, the entire procedure was performed under dark conditions.
For Western blot analysis, dissected corneas were homogenized in reagent (T-PER Tissue Protein Extraction Reagent; Thermo Fisher Scientific, Waltham, MA) containing a protease inhibitor cocktail (Complete Mini; Roche Diagnostics) and 1 mM phenylmethylsulfonyl fluoride. Proteins were separated by gel electrophoresis, and immunoblotting was performed using the following primary antibodies: anti-BVR (1:2500; Assay Designs, Ann Arbor, MI) and anti-B-actin antibody (1:5000; Sigma-Aldrich, St. Louis, MO). Secondary antibodies were either horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse, both from (Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
Detection of Superoxide by Fluorescence Microscopy
Dihydroethidium (DHE; Invitrogen) is widely used as a superoxide probe.31 Dissected corneas were washed twice with PBS and then embedded in OCT compound (Sakura Finetek). Cryostat sections were cut transversely into 5-μm thick sections, incubated with DHE (5 μM) in HBSS for 30 minutes, and washed with HBSS. DHE staining was visualized using a fluorescence microscope (Axioplan-2; Carl Zeiss, Inc). Image acquisition and analysis was performed as described.
Real-Time Polymerase Chain Reaction
Corneas were aseptically dissected from eyes and cleaned in sterile PBS (4°C) under a dissecting microscope to remove all noncorneal tissue. Total RNA was isolated (RNeasy Plus Micro Kit; Qiagen, Germantown, MD), and RNA was quantitated using a Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE). Reverse transcription reaction of total RNA was performed using a cDNA synthesis kit (qScript; Quanta BioSciences, Inc., Gaithersburg, MD) according to the manufacturer's instructions. Quantitative real-time PCR was performed using reaction mix (PerfeCTa SYBR Green FastMix Kit; Quanta BioSciences, Inc.) and the real-time PCR system (Mx3000; Stratagene, La Jolla, CA). Specific primers, purchased from Gene Link (Hawthorne, NY), were designed using Primerbank (http://pga.mgh.harvard.edu/primerbank) based on published sequences (GenBank) and were as follows: HO-1—sense, 5′-TCCAGACACCGCTCCTCCAG-3′, and antisense, 5′- GGATTTGGGGCTGCTGGTTTC-3′; 18S—sense, 5′-TGTCTCAAAGATTAAGCCATGCAT-3′, and antisense, 5′-AACCATAACTGATTTAATGAGCCATTC-3′; NOX-2—sense, 5′-TGAATGCCAGAGTCGGGATTT-3′, and antisense, 5′-CCCCCTTCAGGGTTCCTTGATTT-3′; NOX-4— sense, 5′-GAAGGGGTTAAACACCTCTGC-3′, and antisense, 5′-ATGCTCTGCTTAAACACAATCCT-3′; BVR—sense, 5′-GAAAGGGAGAGTCCTGCATGA-3′, and antisense, 5′-CTGGCTGTGAAGCGAAGAGAT-3′; MMP-2—sense, 5′-GACCTTGACCAGAACAACATC-3′, and antisense, 5′-CATCCACGGTTTCAGGGTCC-3′; MMP-9—sense, 5′-TGCCCATTTCGACGACGAC-3′, and antisense, 5′-GTGCAGGCCGAATAGGAGC-3′. Quantitative analysis was performed as previously described.12,32
Cytokine/Chemokine Analysis
Dissected corneas were homogenized in reagent (T-PER Tissue Protein Extraction Reagent; Thermo Fisher Scientific) containing a protease inhibitor cocktail (Complete Mini; Roche Diagnostics) and 1 mM phenylmethylsulfonyl fluoride. KC (IL-8 homolog), IL-1α, macrophage inflammatory protein-2 (MIP-2), and monocyte chemotactic protein-1 (MCP-1) were measured in corneal homogenates using a custom multiplex sandwich ELISA for protein analysis (SearchLight mouse microarray; Pierce Biotechnology, Woburn, MA).
Statistical Analysis
Student's t-test was used to evaluate the significance of differences between groups, and multiple comparisons were performed by regression analysis and one-way analysis of variance. P < 0.05 was considered significant. All data are presented as mean ± SE.
Results
Biliverdin Accelerates Wound Healing
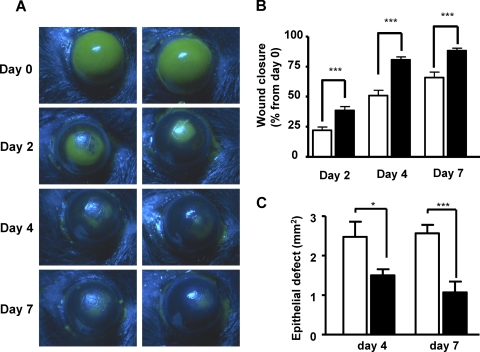
In vehicle-treated mice, the epithelial injury produced a consistent wound (8.06 ± 0.09 mm2, n = 30) that exhibited a linear rate of reepithelialization with 24.16% ± 4.6%, 34.92% ± 9.6%, and 68.95% ± 10.12% closure at days 2, 4, and 7 after injury, respectively. In contrast, reepithelialization of corneal wounds (7.76 ± 0.08 mm2, n = 30) in biliverdin-treated mice were significantly enhanced by 77%, 59%, and 33% compared with vehicle-treated mice at days, 2, 4, and 7, respectively (Figs. 1A, 1B), which confirms and extends our previous results obtained for day 2.28
Figure 1.
Effect of biliverdin treatment on corneal wound healing in HO-2-null mice. (A) Fluorescein-stained corneas after injury in vehicle- and biliverdin-treated HO-2-null (HO-2−/−) mice. (B) Wound closure as percentage change from day 0 (***P < 0.001 from vehicle-treated mice; n = 9–29). (C) Epithelial defects in vehicle- and biliverdin-treated HO-2−/− mice at days 4 and 7 after epithelial removal (*P < 0.05 and ***P < 0.001 from vehicle-treated mice; n = 8–10).
Previously we have shown that corneal epithelial wound healing in HO-2 knockout mice is frequently associated with ulceration, perforation, and epithelial defects, as often evidenced by the formation of a nonhealing crater around the corneal apex.28 In vehicle-treated mice, the size of the corneal epithelial defect was 2.48 ± 0.39 mm2 and 2.57 ± 0.22 mm2 at days 4 and 7, respectively. The size of the corneal epithelial defect in mice treated with biliverdin was evidently smaller (1.50 ± 0.16 mm2 and 1.07 ± 0.27 mm2 at days 4 and 7, respectively; Fig. 1C).
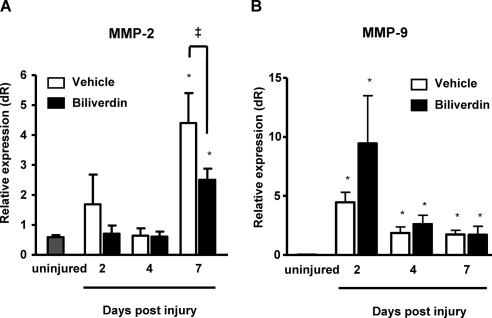
Matrix metalloproteinases (MMPs) have been shown to play an important role in epithelial repair and stromal remodeling after injury.33,34 In the cornea, MMP-9 plays an important role in epithelial cell migration, whereas MMP-2 participates in epithelial repair and stromal remodeling. We examined whether biliverdin affects the expression of these MMPs in response to injury and during wound healing. As seen in Figure 2, corneal MMP-2 and MMP-9 mRNA levels were elevated in response to injury, with significantly higher levels of MMP-2 at day 7 after injury and of MMP-9 at days 2, 4, and 7 after injury compared with uninjured corneas. Interestingly, the levels of MMP-2 mRNA were significantly lower at day 7 in the corneas of mice treated with biliverdin compared with vehicle-treated mice (Fig. 2A), whereas levels of MMP-9 were not significantly altered by biliverdin (Fig. 2B).
Figure 2.
Corneal MMP-2 and MMP-9 mRNA expression in HO-2-null mice. mRNA levels of (A) MMP-2 and (B) MMP-9 in uninjured corneas and in corneas 2, 4, and 7 days after epithelial debridement (*P < 0.05 from uninjured mice; ‡P < 0.05 from vehicle-treated mice; n = 3–8).
Amplified Neovascular Response in Corneas Is Ameliorated by Biliverdin
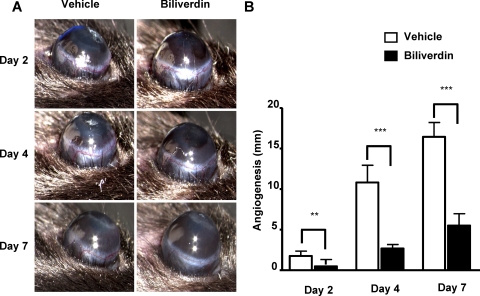
Epithelial debridement led to massive corneal neovascularization in the vehicle-treated mice, with significant corneal neovascularization seen as early as at day 2 after epithelial debridement. The total lengths of the blood vessels in the corneas of vehicle-treated mice were 1.85 ± 0.62 mm, 10.92 ± 2.13 mm, and 16.53 ± 1.78 mm at days 2, 4, and 7, respectively, whereas the total length of the corneal neovascularization in the mice treated with biliverdin was significantly reduced at all days—0.45 ± 0.11 mm, 2.79 ± 0.49 mm, and 5.60 ± 1.45 mm at days 2, 4, and 7 after epithelial debridement, respectively (Figs. 3A, 3B)—similar to results obtained in the suture-induced neovascularization model.12
Figure 3.
Effect of biliverdin treatment on injury-induced neovascularization in corneas of HO-2-null mice. (A) Photographs showing neovascularization in vehicle- and biliverdin-treated HO-2−/− mice at days 2, 4, and 7 after epithelial debridement. (B) Neovascularization expressed as total length of penetrating vessels (**P < 0.01 and ***P < 0.001 from vehicle-treated mice; n = 6–24).
Biliverdin Reduces the Number of Inflammatory Cells and the Levels of Proinflammatory Proteins
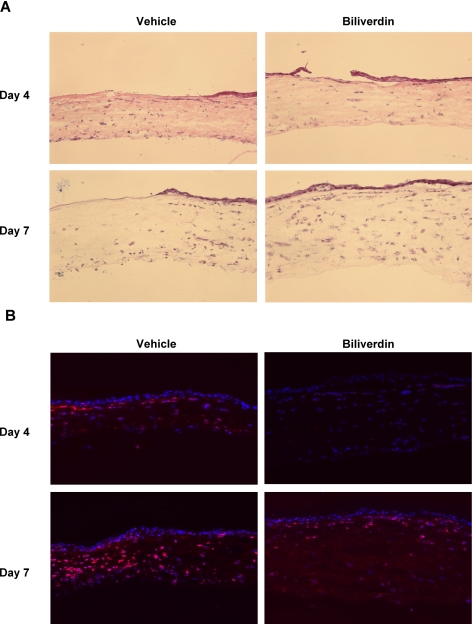
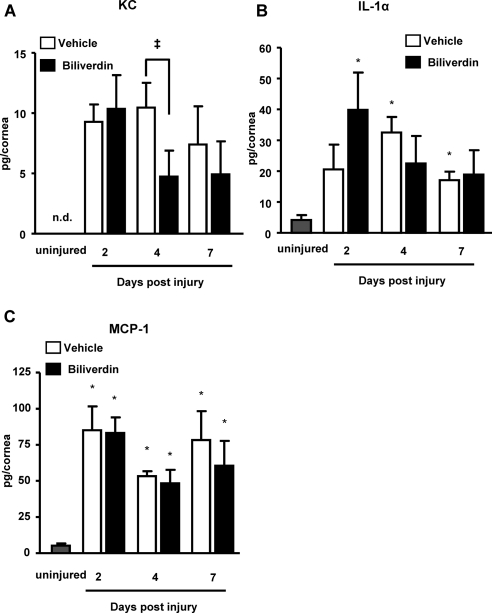
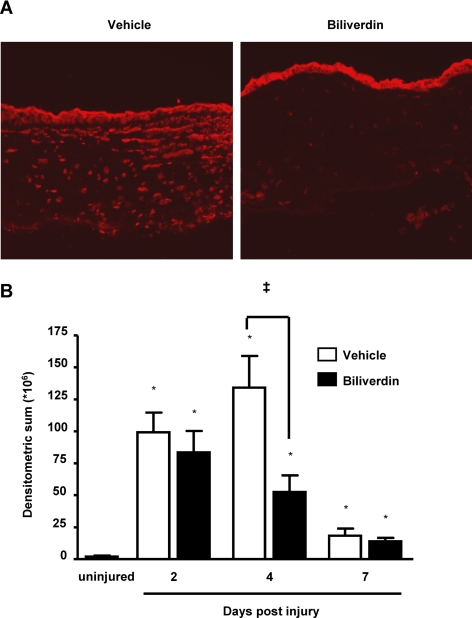
The neovascular response in vehicle-treated mice was accompanied by an exaggerated inflammatory response that was clearly seen by histologic staining and by the number of infiltrating neutrophils, as indicated by the immunofluorescence staining of neutrophils with an antibody against Gr-1 (Figs. 4A, 4B). Biliverdin treatment clearly reduced the number of inflammatory cells (Figs. 4A, 4B). Epithelial injury led to a release of proinflammatory cytokines, including KC, IL-1α, and MCP-1 (Fig. 5). Interestingly, only the level of neutrophil chemoattractant, KC, was affected by the supplementation of biliverdin, leading to a 50% reduction at day 4 after injury (Fig. 5A).
Figure 4.
Effect of biliverdin treatment on the injury-induced inflammatory response. (A) Representative histologic sections stained with hematoxylin and eosin of corneas from vehicle- and biliverdin-treated HO-2-null mice at days 4 and 7 after epithelial debridement. (B) Representative histologic sections stained with anti-Gr1 antibody (red) and counterstained with DAPI (blue) of corneas from vehicle- and biliverdin-treated HO-2-null mice at days 4 and 7 after epithelial debridement.
Figure 5.
Inflammatory cytokine profile in corneas of vehicle- and biliverdin-treated HO-2-null mice before, and 2, 4, and 7 days after epithelial debridement. (A) KC. (B) IL-1α. (C) MCP-1. (*P < 0.05 from uninjured mice; ‡P < 0.05 from vehicle-treated mice; n = 3–7).
Stromal Cell Death in Response to Epithelial Injury Is Reduced by Biliverdin Treatment
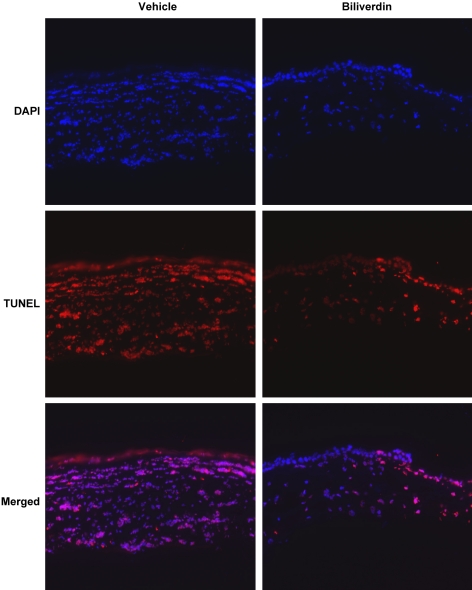
Injury of the corneal epithelium is followed by apoptosis of the keratocytes in the underlying stroma,35 a process that has been shown to be mediated by cytokines such as IL-1. As shown using the TUNEL assay (Fig. 6), corneas from HO-2 null mice at day 4 after injury show an abundance of cells throughout the stroma staining positive for DNA fragmentation. Further, biliverdin treatment significantly reduced the number of TUNEL+ cells (Fig. 6).
Figure 6.
Biliverdin treatment reduces the number of TUNEL+ cells. Representative images of TUNEL labeling of stromal cells in HO-2 null mouse corneas with and without treatment with biliverdin at day 4 after epithelial removal (n = 4–5).
Biliverdin Ameliorates Oxidative Stress
Injury is frequently associated with oxidative stress. Assessment of superoxide levels using DHE fluorescence indicated significantly lower levels in injured corneas treated with biliverdin compared with vehicle-treated injured corneas (Fig. 7A). Quantitative analysis of DHE fluorescence intensity indicated that corneas of HO-2 null mice treated with biliverdin experienced a 60% reduction in superoxide levels compared with corneas of vehicle treated HO-2 null mice (Fig. 7B).
Figure 7.
Effect of biliverdin on injury induced superoxide levels. (A) Representative histologic sections of corneas from vehicle- and biliverdin-treated HO-2-null mice at day 4 after epithelial debridement stained for superoxide using DHE. (B) Quantitative analysis of DHE fluorescence intensity. Results are expressed as the total fluorescence intensity in the histologic section (*P < 0.05 from uninjured mice; ‡P < 0.05 from vehicle-treated mice; n = 3–6).
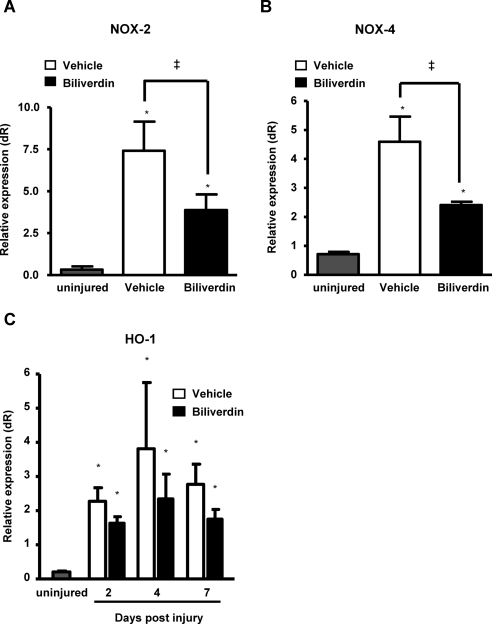
Bilirubin has been shown to inhibit the activation of NADP(H) oxidase,36 which is a major source of superoxide. Several NADP(H) oxidase homologues, including NOX-2 and NOX-4, are expressed in corneal tissues.37,38 Real-time PCR revealed that both NOX-2 and NOX-4 mRNA levels were significantly upregulated in response to injury and that at day 7 both were 47% lower in the corneas of biliverdin-treated animals compared with vehicle-treated animals (Figs. 8A, 8B).
Figure 8.
Effect of biliverdin on injury-induced expression of NADPH-oxidase and HO-1 mRNA. Corneal mRNA levels of (A) NOX-2 and (B) NOX-4 in uninjured corneas and at day 7 after injury and of (C) HO-1 in uninjured corneas at days 2, 4, and 7 after injury (*P < 0.05 from uninjured mice; ‡P < 0.05 from vehicle-treated mice; n = 3–10).
Oxidative stress is associated with the induction of HO-1, which in turn acts as an anti-oxidative system through the production of biliverdin.1 As seen in Figure 8C, HO-1 mRNA levels were significantly increased at days 2, 4, and 7 after injury in corneas of both vehicle- and biliverdin-treated mice. Further, biliverdin treatment did not affect HO-1 mRNA levels significantly in either direction (Fig. 8C) suggesting that the anti-oxidative effect of biliverdin is not due to increased HO-1 levels.
Biliverdin Reductase Is Expressed in the Cornea
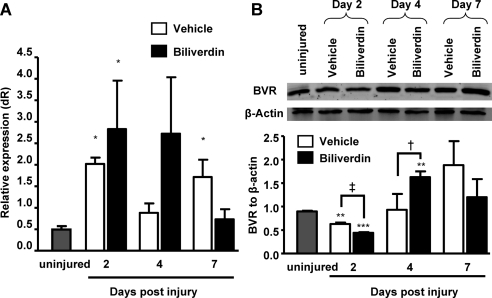
Biliverdin has to be reduced to bilirubin to exert its anti-oxidative properties. This reduction is catalyzed by the enzyme biliverdin IX reductase, which is constitutively expressed in most tissues. Quantitative analysis of biliverdin reductase mRNA in mouse corneas showed that biliverdin reductase was expressed and, further, that the levels were significantly upregulated at 2, 4, and 7 days after injury. The levels of biliverdin reductase mRNA appeared to increase by exogenous addition of its substrate, biliverdin, compared with those of vehicle-treated animals at days 2 and 4 after injury, although the differences did not reach statistical significance (Fig. 9A). The increased levels of biliverdin reductase mRNA were confirmed by Western blot analysis showing an increase in protein levels at day 4 after injury (Fig. 9B). Moreover, biliverdin supplementation brought about a significant increase in protein levels at day 4 compared with those of vehicle-treated mice (Fig. 9B).
Figure 9.
Biliverdin reductase mRNA and protein levels are increased in response to epithelial debridement. (A) Corneal biliverdin reductase mRNA levels before and at days 2, 4, and 7 after injury (*P < 0.05 from uninjured mice; n = 3–7). (B) Representative blot showing corneal biliverdin reductase protein levels and densitometric analysis at days 2, 4, and 7 after injury (**P < 0.01; ***P < 0.001 from uninjured mice; †P < 0.5; ‡P < 0.05 from vehicle-treated mice; n = 2–4).
Discussion
The eye is constantly subjected to oxidative stress through high metabolic activity, exposure to UV light, and high oxygen tension. Through evolutional selection pressure, the cornea has acquired several protective circuits to combat reactive oxygen species generated from environmental insult and pathologic conditions that would, if left uncontrolled, lead to loss of vision or impaired vision. The human cornea has been shown to be rich in SOD, catalase, and glutathione peroxidase.39,40 To this list, we added the HO system by demonstrating its presence in the rodent and human cornea12,25 and by documenting its function as a key cytoprotective system of the cornea; deficiency in its function, as in HO-2 null mice, leads to impaired inflammatory and repair response to injury.28
The present study provides further evidence of the critical role the HO system, in particular HO-2, and its metabolites play in the repair of corneal epithelial injury. In the absence of HO activity, wound repair is halted and inflammation is unresolved. Consequently, the HO-2 null cornea becomes vastly neovascularized and ulcerated. However, this phenotype can be rescued to a significant degree by pharmacologic supplementation with biliverdin, the product of HO activity; it accelerates corneal epithelial cell repair and reduces neovascularization and ulceration presumably through its reported anti-oxidative and anti-inflammatory properties.1
At physiological concentrations, bilirubin, and to lesser extent biliverdin, surpasses α-tocopherol as the most potent protector against lipid peroxidation6 and other reactive oxygen species.41 Administration of biliverdin/bilirubin reduces LPS-induced proinflammatory transcription factors (NF-κB), NADP(H) oxidases,36 and cytokines (IL-6, TNF-α)17 that might be mediated by its ROS-scavenging mechanisms because the expression of many proinflammatory cytokines is redox sensitive.42 In the present study, mRNA levels of NOX-2 and NOX-4 were upregulated at day 7 after injury, though superoxide levels, as measured by DHE fluorescence, were increased already at day 2 after injury. These results do not necessarily contradict each other. First, DHE staining represents total superoxide levels that are the balance between production and scavenging. Second, other enzymatic and nonenzymatic sources of superoxide exist, including cytochrome P450-dependent oxygenases, proteolytic conversion of xanthine dehydrogenase to xanthine oxidase, and the mitochondrial electron transport chain. NOXs have been shown as targets of antioxidants, including biliverdin,36 and are present in the cornea.12,39,40 Importantly, our results show that biliverdin reduces the total amount of superoxide and inhibits the expression of NOX-2 and NOX-4.
We have shown that HO induction after treatment with SnCl2 was associated with a significant decrease in the levels of IL-1α and MIP-2, inflammatory mediators with polymorphonuclear chemotactic and activating properties in the cornea.16 In the present study, supplementation with biliverdin did not alter HO-1 expression, but it significantly reduced the expression of the powerful neutrophil chemoattractant KC, a murine IL-8 homolog, and the number of infiltrated inflammatory cells into the cornea. Although cellular infiltrates are key components of the repair process,43 exaggerated responses are detrimental in that they delay wound healing. Thus, the reduced number of inflammatory cells infiltrating the corneas after biliverdin treatment may contribute to the significantly accelerated reepithelialization of the injured cornea and to decreased inflammation.
Epithelial injury was also associated with distinct changes in MMP expression. MMPs, including MMP-2 and MMP-9, are upregulated in response to injury and have been shown to play an important role in epithelial wound repair.34 MMP-2 is constitutively expressed in keratocytes and is upregulated in response to injury, whereas MMP-9 is normally not present during homeostasis, but, in response to injury, it is readily induced in epithelial cells and in infiltrating neutrophils.33,44 The role of MMP-9 in corneal remodeling is twofold. On one hand, its depletion has been shown to improve epithelial barrier function.45 On the other hand, it has been shown to be important for corneal epithelial migration and healing.33,44,46 In our study, the expression of MMP-2 and MMP-9 showed different patterns; though MMP-2 showed significantly elevated levels first at 7 days, MMP-9 displayed transient changes in its expression, with a fivefold increase at day 2 after injury. Biliverdin treatment caused a significant reduction in the levels of MMP-2 compared with vehicle-treated eyes at day 7 after injury, whereas the levels of MMP-9 were unaffected by supplementation with biliverdin.
Corneal injury, such as epithelial removal, has been shown to be associated with the immediate apoptotic activity of stromal keratocytes35 and the subsequent impairment of corneal healing. HO-1 induction and supplementation of CO or biliverdin have been shown to inhibit apoptosis in different cell types and tissues.47–50 In the present study, keratocyte apoptosis/necrosis seems to be more evident in the corneas of vehicle-treated mice than in the corneas of biliverdin-treated mice. Furthermore, the major apoptotic/necrotic activity seems to occur in the part of the corneas where the stroma is still not covered by epithelial cells, suggesting that the increased rate of reepithelialization seen in the corneas of mice treated with biliverdin prevents apoptosis of the keratocytes in the underlying stroma. At this stage it is unclear whether it is the potential antiapoptotic effect of biliverdin49 that leads to the increase in the rate of wound healing. Results acquired using human corneal epithelial cells in vitro have shown that biliverdin treatment increases wound closure, and this effect seems to be due to an increase in migratory rate.51
In this study, biliverdin supplementation led to a significant increase in BVR protein levels at day 4 after injury. BVR catalyzes the reduction of biliverdin into bilirubin; the latter displays anti-oxidant activity that is greater than its metabolic precursor.52 Studies by Snyder and colleagues53,54 suggested that the antioxidant action of bilirubin reflects an amplification cycle whereby bilirubin, acting as an antioxidant, is itself oxidized to biliverdin and then recycled by BVR back to bilirubin. These investigators further postulated that this redox cycle may constitute the principal physiologic function of bilirubin. The significance of the bilirubin-biliverdin recycling has been questioned recently.55,56 It is unclear what function, other than the reduction of biliverdin to bilirubin, BVR might have, but it has been suggested that in the presence of its substrate it favors the function of reductase over the function of a transcription factor capable of activating NF-κB.57 A recent study has shown that biliverdin treatment of macrophages leads to the translocation of BVR to the cell membrane and to phosphorylation by activation of the PI3K-Akt pathway, leading to the production of IL-10,58 which, in turn, has the potential to induce HO-1 expression.1 The increased expression of BVR in the biliverdin-treated corneas may contribute to an increased healing rate by producing the more potent anti-oxidant bilirubin and by promoting the anti-inflammatory program through the inhibition of NF-κB activation.
In summary, topical administration of biliverdin in mice deficient in HO activity accelerated the healing of epithelial defects. Biliverdin might be useful in the management of clinical conditions such as corneal abrasions. Its anti-inflammatory properties also suggest its use in the treatment of corneal surface inflammation. Moreover, nonhealing corneal ulcerations might benefit from topical biliverdin.
Footnotes
Supported by the National Institutes of Health Grant EY06513.
Disclosure: L. Bellner, None; J. Wolstein, None; K.A. Patil, None; M.W. Dunn, None; M. Laniado-Schwartzman, None
References
- 1. Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127 [DOI] [PubMed] [Google Scholar]
- 2. Liu N, Wang X, McCoubrey WK, Maines MD. Developmentally regulated expression of two transcripts for heme oxygenase-2 with a first exon unique to rat testis: control by corticosterone of the oxygenase protein expression. Gene. 2000;241:175–183 [DOI] [PubMed] [Google Scholar]
- 3. Zenclussen AC, Lim E, Knoeller S, et al. Heme oxygenases in pregnancy II: HO-2 is downregulated in human pathologic pregnancies. Am J Reprod Immunol. 2003;50:66–76 [DOI] [PubMed] [Google Scholar]
- 4. Atzori L, Caramori G, Lim S, et al. Effect of cigarette smoking on haem-oxygenase expression in alveolar macrophages. Respir Med. 2004;98:530–535 [DOI] [PubMed] [Google Scholar]
- 5. Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568 [PubMed] [Google Scholar]
- 6. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046 [DOI] [PubMed] [Google Scholar]
- 7. Otterbein LE. Carbon monoxide: innovative anti-inflammatory properties of an age-old gas molecule. Antioxid Redox Signal. 2002;4:309–319 [DOI] [PubMed] [Google Scholar]
- 8. Ryter SW, Otterbein LE. Carbon monoxide in biology and medicine. Bioessays. 2004;26:270–280 [DOI] [PubMed] [Google Scholar]
- 9. Bucolo C, Drago F. Focus on molecules: heme oxygenase-1. Exp Eye Res. 2009;89:822–823 [DOI] [PubMed] [Google Scholar]
- 10. Wagener FA, da Silva JL, Farley T, de Witte T, Kappas A, Abraham NG. Differential effects of heme oxygenase isoforms on heme mediation of endothelial intracellular adhesion molecule 1 expression. J Pharmacol Exp Ther. 1999;291:416–423 [PubMed] [Google Scholar]
- 11. Wagener FA, Volk HD, Willis D, et al. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol Rev. 2003;55:551–571 [DOI] [PubMed] [Google Scholar]
- 12. Bellner L, Vitto M, Patil KA, Dunn MW, Regan R, Laniado-Schwartzman M. Exacerbated corneal inflammation and neovascularization in the HO-2 null mice is ameliorated by biliverdin. Exp Eye Res. 2008;87:268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Minamino T, Christou H, Hsieh CM, et al. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci U S A. 2001;98:8798–8803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morse D, Pischke SE, Zhou Z, et al. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J Biol Chem. 2003;278:36993–36998 [DOI] [PubMed] [Google Scholar]
- 15. Otterbein LE, Bach FH, Alam J, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428 [DOI] [PubMed] [Google Scholar]
- 16. Patil K, Bellner L, Cullaro G, Gotlinger KH, Dunn MW, Schwartzman ML. Heme oxygenase-1 induction attenuates corneal inflammation and accelerates wound healing after epithelial injury. Invest Ophthalmol Vis Sci. 2008;49:3379–3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sarady-Andrews JK, Liu F, Gallo D, et al. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1131–L1137 [DOI] [PubMed] [Google Scholar]
- 18. Song R, Kubo M, Morse D, et al. Carbon monoxide induces cytoprotection in rat orthotopic lung transplantation via anti-inflammatory and anti-apoptotic effects. Am J Pathol. 2003;163:231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang J, Zhou HC, Pan P, Zhang N, Li WZ. Exogenous biliverdin improves the function of lung grafts from brain dead donors in rats. Transplant Proc. 2010;42:1602–1609 [DOI] [PubMed] [Google Scholar]
- 20. Conners MS, Stoltz RA, Davis KL, et al. A closed eye contact lens model of corneal inflammation, 2: inhibition of cytochrome P450 arachidonic acid metabolism alleviates inflammatory sequelae. Invest Ophthalmol Vis Sci. 1995;36:841–850 [PubMed] [Google Scholar]
- 21. Haider A, Olszanecki R, Gryglewski R, et al. Regulation of cyclooxygenase by the heme-heme oxygenase system in microvessel endothelial cells. J Pharmacol Exp Ther. 2002;300:188–194 [DOI] [PubMed] [Google Scholar]
- 22. Laniado-Schwartzman M, Abraham NG, Conners M, Dunn MW, Levere RD, Kappas A. Heme oxygenase induction with attenuation of experimentally induced corneal inflammation. Biochem Pharmacol. 1997;53:1069–1075 [DOI] [PubMed] [Google Scholar]
- 23. Li Volti G, Seta F, Schwartzman ML, Nasjletti A, Abraham NG. Heme oxygenase attenuates angiotensin II-mediated increase in cyclooxygenase-2 activity in human femoral endothelial cells. Hypertension. 2003;41:715–719 [DOI] [PubMed] [Google Scholar]
- 24. Abraham NG, Lavrovsky Y, Schwartzman ML, et al. Transfection of the human heme oxygenase gene into rabbit coronary microvessel endothelial cells: protective effect against heme and hemoglobin toxicity. Proc Natl Acad Sci U S A. 1995;92:6798–6802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abraham NG, Lin JH, Dunn MW, Schwartzman ML. Presence of heme oxygenase and NADPH cytochrome P-450(C) reductase in human corneal epithelium. Invest Ophthalmol Vis Sci. 1987;28:1464–1472 [PubMed] [Google Scholar]
- 26. Bonazzi A, Mastyugin V, Mieyal PA, Dunn MW, Laniado-Schwartzman M. Regulation of cyclooxygenase-2 by hypoxia and peroxisome proliferators in the corneal epithelium. J Biol Chem. 2000;275:2837–2844 [DOI] [PubMed] [Google Scholar]
- 27. Neil TK, Stoltz RA, Jiang S, et al. Modulation of corneal heme oxygenase expression by oxidative stress agents. J Ocul Pharmacol Ther. 1995;11:455–468 [DOI] [PubMed] [Google Scholar]
- 28. Seta F, Bellner L, Rezzani R, et al. Heme oxygenase-2 is a critical determinant for execution of an acute inflammatory and reparative response. Am J Pathol. 2006;169:1612–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poss KD, Thomas MJ, Ebralidze AK, O'Dell TJ, Tonegawa S. Hippocampal long-term potentiation is normal in heme oxygenase-2 mutant mice. Neuron. 1995;15:867–873 [DOI] [PubMed] [Google Scholar]
- 30. Rogers B, Yakopson V, Teng ZP, Guo Y, Regan RF. Heme oxygenase-2 knockout neurons are less vulnerable to hemoglobin toxicity. Free Radic Biol Med. 2003;35:872–881 [DOI] [PubMed] [Google Scholar]
- 31. Peshavariya HM, Dusting GJ, Selemidis S. Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free Radic Res. 2007;41:699–712 [DOI] [PubMed] [Google Scholar]
- 32. Bellner L, Martinelli L, Halilovic A, et al. Heme oxygenase-2 deletion causes endothelial cell activation marked by oxidative stress, inflammation, and angiogenesis. J Pharmacol Exp Ther. 2009;331:925–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsubara M, Girard MT, Kublin CL, Cintron C, Fini ME. Differential roles for two gelatinolytic enzymes of the matrix metalloproteinase family in the remodelling cornea. Dev Biol. 1991;147:425–439 [DOI] [PubMed] [Google Scholar]
- 34. Sivak JM, Fini ME. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Prog Retin Eye Res. 2002;21:1–14 [DOI] [PubMed] [Google Scholar]
- 35. Wilson SE, Mohan RR, Ambrosio R, Jr, Hong J, Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. 2001;20:625–637 [DOI] [PubMed] [Google Scholar]
- 36. Kwak JY, Takeshige K, Cheung BS, Minakami S. Bilirubin inhibits the activation of superoxide-producing NADPH oxidase in a neutrophil cell-free system. Biochim Biophys Acta. 1991;1076:369–373 [DOI] [PubMed] [Google Scholar]
- 37. O'Brien WJ, Krema C, Heimann T, Zhao H. Expression of NADPH oxidase in rabbit corneal epithelial and stromal cells in culture. Invest Ophthalmol Vis Sci. 2006;47:853–863 [DOI] [PubMed] [Google Scholar]
- 38. O'Brien WJ, Heimann T, Rizvi F. NADPH oxidase expression and production of superoxide by human corneal stromal cells. Mol Vis. 2009;15:2535–2543 [PMC free article] [PubMed] [Google Scholar]
- 39. Behndig A, Svensson B, Marklund SL, Karlsson K. Superoxide dismutase isoenzymes in the human eye. Invest Ophthalmol Vis Sci. 1998;39:471–475 [PubMed] [Google Scholar]
- 40. Cejkova J, Vejrazka M, Platenik J, Stipek S. Age-related changes in superoxide dismutase, glutathione peroxidase, catalase and xanthine oxidoreductase/xanthine oxidase activities in the rabbit cornea. Exp Gerontol. 2004;39:1537–1543 [DOI] [PubMed] [Google Scholar]
- 41. Dore S, Takahashi M, Ferris CD, et al. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci U S A. 1999;96:2445–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen XL, Kunsch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr Pharm Des. 2004;10:879–891 [DOI] [PubMed] [Google Scholar]
- 43. Li Z, Burns AR, Smith CW. Two waves of neutrophil emigration in response to corneal epithelial abrasion: distinct adhesion molecule requirements. Invest Ophthalmol Vis Sci. 2006;47:1947–1955 [DOI] [PubMed] [Google Scholar]
- 44. Ye HQ, Azar DT. Expression of gelatinases A and B, and TIMPs 1 and 2 during corneal wound healing. Invest Ophthalmol Vis Sci. 1998;39:913–921 [PubMed] [Google Scholar]
- 45. Pflugfelder SC, Farley W, Luo L, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005;166:61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gordon GM, Austin JS, Sklar AL, Feuer WJ, Lagier AJ, Fini ME. Comprehensive gene expression profiling and functional analysis of matrix metalloproteinases and TIMPs, and identification of ADAM-10 gene expression, in a corneal model of epithelial resurfacing. J Cell Physiol. 2011;226:1461–1470 [DOI] [PubMed] [Google Scholar]
- 47. Abraham NG, Kushida T, McClung J, et al. Heme oxygenase-1 attenuates glucose-mediated cell growth arrest and apoptosis in human microvessel endothelial cells. Circ Res. 2003;93:507–514 [DOI] [PubMed] [Google Scholar]
- 48. Brouard S, Otterbein LE, Anrather J, et al. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rodella L, Lamon BD, Rezzani R, et al. Carbon monoxide and biliverdin prevent endothelial cell sloughing in rats with type I diabetes. Free Radic Biol Med. 2006;40:2198–2205 [DOI] [PubMed] [Google Scholar]
- 50. Botros FT, Olszanecki R, Prieto-Carrasquero MC, Goodman AI, Navar LG, Abraham NG. Induction of heme oxygenase-1 in renovascular hypertension is associated with inhibition of apoptosis. Cell Mol Biol (Noisy-le-grand). 2007;53:51–60 [PubMed] [Google Scholar]
- 51. Halilovic A, Patil KA, Bellner L, et al. Knockdown of heme oxygenase-2 impairs corneal epithelial cell wound healing. J Cell Physiol. 2010. November 10 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stocker R, McDonagh AF, Glazer AN, Ames BN. Antioxidant activities of bile pigments: biliverdin and bilirubin. Methods Enzymol. 1990;186:301–309 [DOI] [PubMed] [Google Scholar]
- 53. Sedlak TW, Saleh M, Higginson DS, Paul BD, Juluri KR, Snyder SH. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci U S A. 2009;106:5171–5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci U S A. 2002;99:16093–16098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Maghzal GJ, Leck MC, Collinson E, Li C, Stocker R. Limited role for the bilirubin-biliverdin redox amplification cycle in the cellular antioxidant protection by biliverdin reductase. J Biol Chem. 2009;284:29251–29259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McDonagh AF. The biliverdin-bilirubin antioxidant cycle of cellular protection: Missing a wheel? Free Radic Biol Med. 2010;49:814–820 [DOI] [PubMed] [Google Scholar]
- 57. Gibbs PE, Maines MD. Biliverdin inhibits activation of NF-kappaB: reversal of inhibition by human biliverdin reductase. Int J Cancer. 2007;121:2567–2574 [DOI] [PubMed] [Google Scholar]
- 58. Wegiel B, Baty CJ, Gallo D, et al. Cell surface biliverdin reductase mediates biliverdin-induced anti-inflammatory effects via phosphatidylinositol 3-kinase and Akt. J Biol Chem. 2009;284:21369–21378 [DOI] [PMC free article] [PubMed] [Google Scholar]