The authors have identified the purinergic receptors activated by ATP in the lacrimal gland that are responsible for stimulating protein secretion.
Abstract
Purpose.
To identify the type of purinergic receptors activated by adenosine triphosphate (ATP) in rat lacrimal gland and to determine their role in protein secretion.
Methods.
Purinergic receptors were identified by RT-PCR, Western blot analysis, and immunofluorescence techniques. Acini from rat lacrimal gland were isolated by collagenase digestion. Acini were incubated with the fluorescence indicator fura-2 tetra-acetoxylmethyl ester, and intracellular [Ca2+] ([Ca2+]i) was determined. Protein secretion was measured by fluorescence assay.
Results.
The authors previously showed that P2X7 receptors were functional in the lacrimal gland. In this study, they show that P2X1–4, and P2X6receptors were identified in the lacrimal gland by RT-PCR, Western blot, and immunofluorescence analyses. P2X5 receptors were not detected. ATP increased [Ca2+]i and protein secretion in a concentration-dependent manner. Removal of extracellular Ca2+ significantly reduced the ATP-stimulated increase in [Ca2+]i. Repeated applications of ATP caused desensitization of the [Ca2+]i response. Incubation with the P2X1 receptor inhibitor NF023 did not alter ATP-stimulated [Ca2+]i. Incubation with zinc, which potentiates P2X2 and P2X4 receptor responses, or lowering the pH to 6.8, which potentiates P2X2 receptor responses, did not alter the ATP-stimulated [Ca2+]i. P2X3 receptor inhibitors A-317491 and TNP-ATP significantly decreased ATP-stimulated [Ca2+]i and protein secretion, whereas the P2X3 receptor agonist α,β methylene ATP significantly increased them. The P2X7 receptor inhibitor A438079 had no effect on ATP-stimulated [Ca2+]i at 10−6 M but did have an effect at 10−4 M.
Conclusions.
Purinergic receptors P2X1–4 and P2X6 are present in the lacrimal gland. ATP uses P2X3 and P2X7 receptors to stimulate an increase in [Ca2+]i and protein secretion.
The components of tear film include the innermost mucous portion, the middle aqueous portion, and the outer lipid layer. The mucous layer is secreted by the conjunctival goblet cells and the corneal epithelial cells. The aqueous portion is secreted mainly by the main lacrimal gland, and the lipid portion is secreted by the meibomian glands.1 Functions of the tear film are varied and include providing the avascular cornea with nutrients, removing waste products from the ocular surface, and protecting the ocular surface from the external environment.2 Any changes in either the quantity or the quality of tears can have damaging effects on vision.
The main lacrimal gland is composed of three main cell types: acinar, myoepithelial, and ductal.1 Of these, acinar cells are in the majority, constituting approximately 80% of the gland. Acinar cells are arranged as pyramidal cells surrounding a central lumen separated by tight junctions at the apical membranes. Receptors for the neurotransmitters are located on the basolateral membranes.1 On activation, these receptors activate signal transduction pathways that lead to protein secretion across the apical membranes and into the lumen. Epithelial cells line the ducts and modify the primary fluid. The small ducts coalesce to form larger ducts and eventually the main excretory duct, which empties onto the ocular surface.1 Myoepithelial cells are large stellate cells that surround the acini and are believed to contract to help expel secretory products from the acinar cells, as occurs in the mammary gland.
Purinergic receptors bind purines and pyrimidines and are divided into P1 and P2 receptors. P1 receptors are G-protein–coupled receptors (GPCR) activated by adenosine. P2 receptors are activated by nucleotides and are further subdivided into P2X and P2Y categories. P2Y receptors are also GPCRs, whereas P2X receptors are adenosine triphosphate (ATP)–gated nonselective ion channels. Seven P2X receptors have been cloned to date and are identified as P2X1 to P2X7. Activation of these channels regulates cellular processes through Ca2+ influx or change in membrane potential.3
Although all P2X receptors have similar properties, there are some characteristics that are different and can be used to discriminate between these receptors. For example, P2X1, P2X3, and P2X4 receptors are known to become desensitized after repeated applications of ATP.4,5 P2X2 and P2X4 responses are potentiated in the presence of zinc and lower pH.6–8 P2X7 receptors are activated by high concentrations of ATP.5 In addition, specific agonists and inhibitors are available for P2X1, P2X3, and P2X7 receptors.
We previously determined that lacrimal gland acini contain P2X7 receptors and that they play a role in an increase in intracellular [Ca2+]([Ca2+]i), protein secretion, and extracellular regulated kinase 1/2 (ERK) activation.9 We did not investigate the other six P2X receptors. Therefore, the goal of this study was to determine whether the other P2X receptors are present in the lacrimal gland and are activated by ATP.
Materials and Methods
Materials
P2X1–6 rabbit polyclonal antibodies (catalog numbers APR-001, APR-003, APR-016, APR-002, APR-005, and APR-013, respectively) were purchased from Alomone Laboratories (Jerusalem, Israel). A second antibody to P2X5 (catalog number sc-15192) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit secondary antibody conjugated to horseradish peroxidase (HRP) was purchased from Millipore (Billerica, MA). Reagents (Amplex Red, TRIzol) and fura-2 tetra-acetoxylmethyl ester (fura-2 AM) were from Invitrogen (Carlsbad, CA). A reverse transcription system and reagents for PCR were purchased from Promega (Madison, WI). Collagenase (CLSIII) was purchased from Worthington Biochemicals (Lakewood, NJ). All other reagents were from Sigma Chemical Company (St. Louis, MO).
Methods
Animals.
All experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Schepens Eye Research Institute Animal Care and Use Committee. Male Sprague-Dawley rats (125–150 g) were purchased from Taconic Farms (Germantown, NY) and were maintained in constant-temperature rooms with fixed 12-hour light/12-hour dark intervals and were fed ad libitum. The rats were anesthetized for 1 minute in CO2 and then decapitated. Both exorbital lacrimal glands were removed.
RT-PCR.
The lacrimal gland was removed and homogenized in reagent (TRIzol; Invitrogen), and total RNA was isolated according to the manufacturer's instructions. RNA was also isolated from rat brain for use as a positive control because all P2X receptors are known to be present in this tissue. One microgram of purified total RNA was used for complementary DNA (cDNA) synthesis using the reverse transcription system. cDNA was amplified by polymerase chain reaction (PCR) using primers specific to receptors P2X1 to P2X6 (Table 1), which were derived from previously published sequences10 in a thermal cycler (PCR Sprint; Thermo Hybaid, Ashton, UK).
Table 1.
RT-PCR Primers
| Primer | Source | Sequence (5′–3′) | Predicted Length (bp) |
|---|---|---|---|
| P2X1 | X80447 | (S)-GAAGTGTGATCTGGACTGGCACGT | 452 |
| (AS)-GCGTCAAGTCCGGATCTCGACTAA | |||
| P2X2 | U14414 | (S)-GAATCAGAGTGCAACCCCAA | 357 |
| (AS)-TCACAGGCCATCTACTTGAG | |||
| P2X3 | X90651 | (S)-TGGCGTTCTGGGTATTAAGATCGG | 440 |
| (AS)-CAGTGGCCTGGTCACTGGCGA | |||
| P2X4 | X87763 | (S)-GAGGCATCATGGGTATCCAGATCAAG | 447 |
| (AS)-GAGCGGGGTGGAAATGTAACTTTAG | |||
| P2X5 | X92069 | (S)-AAAGACTGGTCAGTGTGTGGCGTTC | 418 |
| (AS)-TGCCTGCCCAGTGACAAGAATGTCAA | |||
| P2X6 | X92070 | (S)-GTGCCATTCTGACCAGGGTTGTATAAA | 520 |
| (AS)-GCCACCTCTGTAAAGTTCTCTCCGATT |
(S), sense; (AS), antisense.
The PCR reaction consisted of 0.5 μM sense and antisense primers, 200 μM each dNTP, 1.5 μM MgCl2, 1.25 U Taq polymerase, and 1 μL cDNA. Cycling conditions were 5 minutes hot start at 94°C, 40 cycles of denaturation for 1 minute at 94°C, annealing for 1 minute at 59°C, extension for 1 minute at 72°C, and a final extension at 72°C for 5 minutes. Samples with no cDNA served as the negative controls. After amplification, the products were separated by electrophoresis on a 1.5% agarose gel and were visualized by ethidium bromide staining.
Western Blot Analysis.
The presence of P2X receptors in lacrimal gland was determined by Western blot analysis. The gland was homogenized on ice with a tissue grinder in RIPA buffer containing 10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% deoxycholic acid, 1% Triton X-100, 0.1% SDS, and 1 mM EDTA containing protease inhibitors (100 μL/mL phenylmethylsulfonyl fluoride, 30 μL/mL aprotinin, and 100 nM sodium orthovanadate). Homogenized cells were sonicated and centrifuged at 2000g for 15 minutes at 4°C. Proteins in the supernatant were separated by SDS-PAGE on a 10% gel and were transferred onto nitrocellulose membranes. The nitrocellulose membranes were blocked overnight at 4°C in 5% nonfat dried milk in buffer containing 10 mM Tris-HCl (pH 8.0), 150 mM NaCl, and 0.05% Tween-20 and then were incubated with primary antibody at 1:500 dilution for 1 hour at room temperature or overnight at 4°C followed by incubation with HRP-conjugated secondary antibody. Immunoreactive bands were detected by the enhanced chemiluminescence method. Bands were scanned and analyzed using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html). Rat brain, processed according to the same method as the lacrimal gland, served as the positive control.
Immunohistochemistry.
Lacrimal glands were fixed in 4% formaldehyde diluted in phosphate-buffered saline (PBS; 145 mM NaCl, 7.3 mM Na2 HPO4, and 2.7 mM NaH2PO4 [pH 7.2]) for 4 hours at 4°C. The tissue was preserved in 30% sucrose in PBS at 4°C overnight and was then embedded in optimal cutting temperature embedding compound. Six-micrometer sections were cut, placed on slides, and air dried for 2 hours. The sections were rinsed for 5 minutes in PBS, and nonspecific sites were blocked by incubation with 10% normal goat serum, 1% bovine serum albumin, and 0.2% Triton X-100 in PBS for 45 minutes at room temperature. Sections were then incubated with the primary antibody for 2 hours at 1:50 dilution at room temperature in a humidified chamber. The secondary antibody conjugated to Cy3 was applied for 1 hour at room temperature. Coverslips were mounted with a medium consisting of glycerol and paraphenylenediamine. Sections incubated in the absence of primary antibody served as the negative control. The sections were viewed by microscope (Eclipse E80i; Nikon, Tokyo, Japan), and micrographs were taken with a digital camera (Spot; Diagnostic Instruments, Inc., Sterling Heights, MI).
Preparation of Lacrimal Gland Acini.
Acini were prepared by collagenase digestion. In brief, lacrimal glands were trimmed and fragmented before incubation with collagenase (CLSIII; 100 U/mL) in Krebs-Ringer bicarbonate (KRB) buffer (119 mM NaCl, 4.8 mM KCl, 1 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 10 mM HEPES, and 5.5 mM glucose [pH 7.45]) and 0.5% BSA at 37°C. Fragments were subjected to pipetting through tips of decreasing diameters, filtered through nylon mesh (150-μm pore size), and centrifuged briefly (50g, 2 minutes). The pellet was washed twice through a 4% BSA solution in KRB buffer. The dispersed acini were allowed to recover for 60 minutes at 37°C before use.
Measurement of [Ca2+]i.
Acini were allowed to attach to glass-bottom Petri dishes coated with cell and tissue adhesive (Cell-Tek; BD Biosciences, Franklin Lakes, NJ) for 30 minutes at 37°C. Acini were incubated for 1 hour at 37°C in the dark with fura-2 AM (0.5 μM) diluted in KRB-HEPES containing 0.5% BSA, 8 μM pluronic acid F127, and 250 μM sulfinpyrazone. The cells were washed in KRB buffer containing 250 μM sulfinpyrazone. Fluorescent images of cells were recorded and analyzed with a digital fluorescence imaging system (InCyt Im2; Intracellular Imaging, Cincinnati, OH) using excitation wavelengths of 340 and 380 nm. At least 10 acini clumps were selected for each condition. Acini were preincubated with inhibitors for 30 minutes before image collection. Agonists were added 15 seconds after the initiation of the experiment, and data were collected in real time for up to 2 minutes. All agonists and antagonists were dissolved in dH2O. [Ca2+]i was calculated using a standard curve. Change in peak [Ca2+]i was calculated by subtracting the average of the basal values (before the addition of agonist) from the peak [Ca2+]i.
Measurement of Peroxidase Secretion.
Acini were allowed to recover for 1 hour in KRB-HEPES buffer containing 0.5% BSA at 37°C. Acini were then incubated with the purinergic receptor agonist ATP or the P2X3 agonist α,β methylene ATP. Inhibitors were added 30 minutes before the addition of agonists. All agonists and antagonists were dissolved in dH2O. After 40 minutes, acini were centrifuged and the supernatant was collected. The pellet was sonicated on ice in 10 mM Tris-HCl, pH 7.5. Peroxidase activity, an index of protein secretion, was measured in duplicate in both the supernatant and the pellet. Peroxidase was measured using reagent (Amplex Red; Invitrogen), which, when oxidized by peroxidase in the presence of hydrogen peroxide, produces a highly fluorescent molecule. The amount of fluorescence in the supernatant and pellet was quantified on a fluorescent microplate reader (model FL600; BioTek, Winooski, VT) with an excitation wavelength of 530 nm and an emission wavelength of 590 nm. Peroxidase was expressed as a percentage of peroxidase secreted into the media (supernatant) compared with total peroxidase present in the cells (pellet + supernatant).
Statistical Analysis
All data are expressed as mean ± SEM. Data were analyzed using Student's t-test. P < 0.05 was considered statistically significant.
Results
Identification and Localization of P2X1–6 Receptors in the Lacrimal Gland
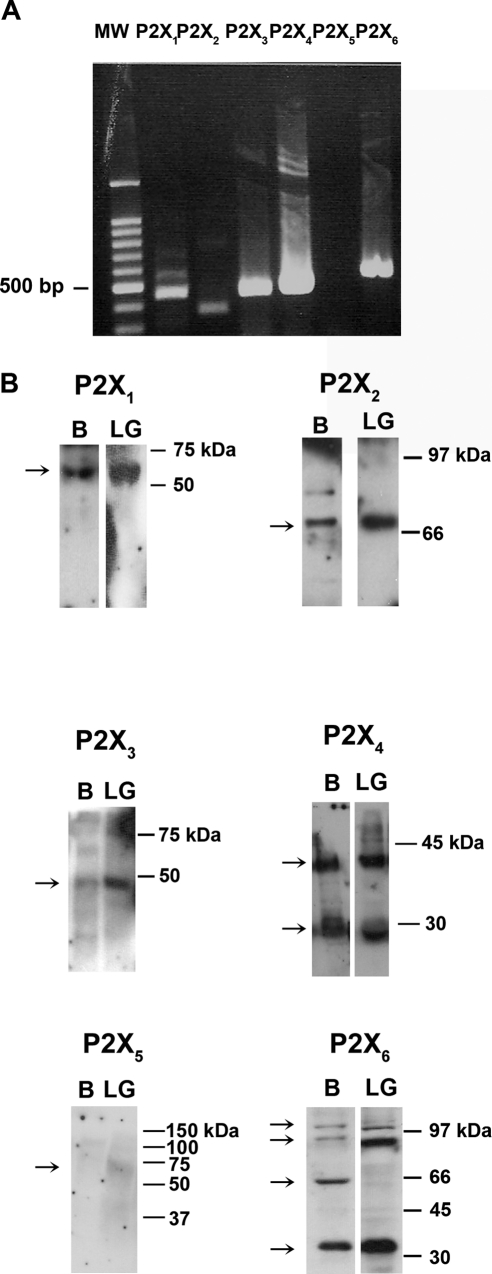
Rat lacrimal glands were homogenized, and RNA was isolated. RT-PCR was performed using primers specific for each P2X receptor (Table 1). As shown in Figure 1A, bands corresponding to the correct size in the lacrimal gland (Fig. 1A) and brain (not shown) were detected for all P2X receptors with the exception of P2X5.
Figure 1.
P2X receptors in the lacrimal gland. The presence of P2X1 to P2X6 receptors in the lacrimal gland was determined by RT-PCR (A) and Western blot analysis (B). Brain homogenate was used as a positive control. All blots are representative of three animals. Arrows: positions of bands. B, brain; LG, lacrimal gland.
Lacrimal glands were then homogenized, proteins were separated by SDS-PAGE, and Western blot analysis was performed using antibodies directed against P2X receptors. The predicted molecular weights of P2X receptors ranged from approximately 42 to 48 kDa. However, it is well established that P2X receptors can undergo significant glycosylation resulting in bands of different molecular weights, depending on the tissue.11 Single bands were obtained using antibodies against P2X1, P2X2, and P2X3 receptors in both rat brain (positive control) and lacrimal gland (Fig. 1B). Two bands were detected for P2X4 in both brain and lacrimal gland. As in other tissues, multiple bands were detected for P2X612,13 in brain and in lacrimal gland (Fig. 1B). A faint band was detected using the P2X5 antibody in both brain and lacrimal gland though the two bands were not at the same molecular weight (Fig. 1B). A second antibody against P2X5 receptor was also used, but no bands were detected in either lacrimal gland or brain samples (data not shown).
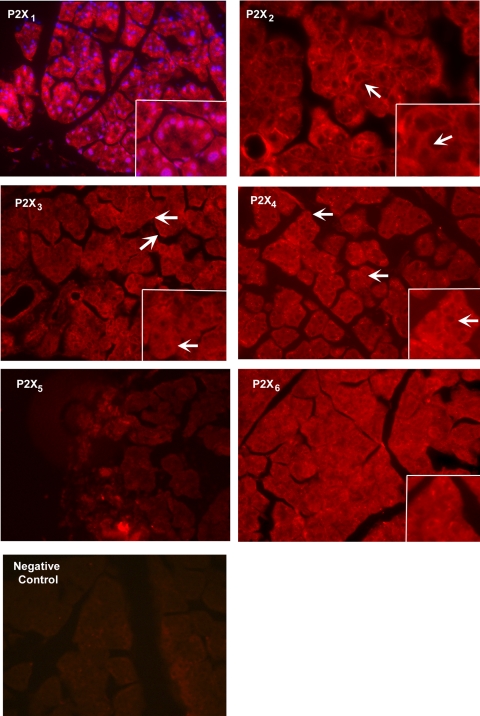
Using the same antibodies as in Western blot analysis, immunofluorescence experiments were performed in lacrimal gland sections. P2X1–4 and P2X6 immunoreactivity was detected in the lacrimal gland (Fig. 2). All five P2X receptors were present in the cytosol. In addition, P2X1 immunoreactivity was seen in the nuclei of acinar cells, whereas P2X2 receptors were detected in the basolateral membranes of acinar cells. The localization of P2X3 and P2X4 receptors was similar to that of P2X2 receptors. Again, we were unable to detect P2X5 immunoreactivity in the lacrimal gland despite using antibodies from two different sources. P2X6 receptors had a more cytosolic distribution than did the other P2X receptors. As we previously showed that P2X7 receptors were present, we can conclude that all the P2X receptors were present in the lacrimal gland with the exception of the P2X5 receptor.
Figure 2.
Localization of P2X receptors in the lacrimal gland. The localization of P2X1 to P2X6 receptors in the lacrimal gland was determined by immunofluorescence experiments. Arrows: basolateral membranes of acinar cells. Images are representative of three animals. Red: P2X receptor; blue: nuclei stained with DAPI in P2X1 receptor figure only. Magnification, ×200 (inset, ×400).
Effect of ATP on [Ca2+]i
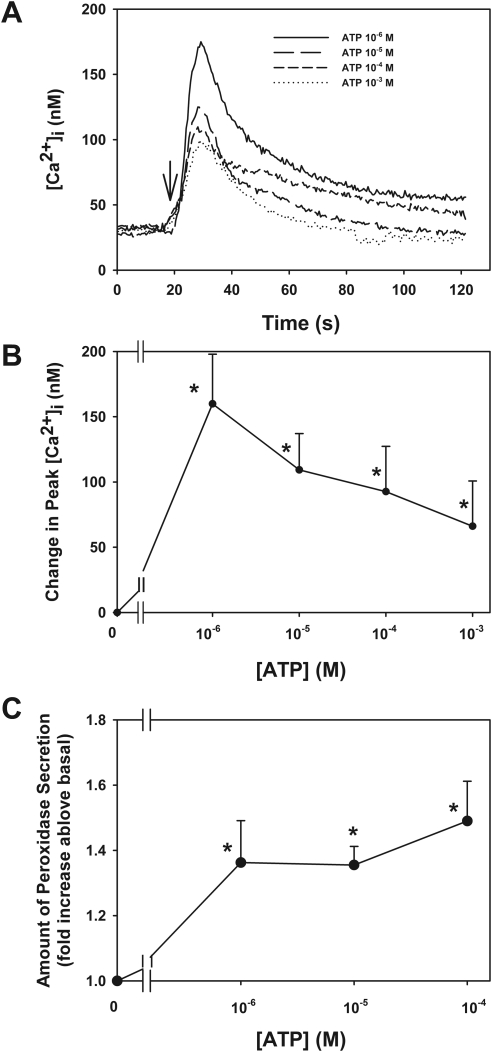
To determine which P2X receptors are functional in the lacrimal gland, acini containing fura-2 were stimulated with ATP and the [Ca2+]i was measured. ATP is known to bind to and activate all the P2X receptors with varying potencies and characteristics. As shown in Figure 3A, the application of ATP (10−6–10−3) increased [Ca2+]i in a concentration-dependent manner. When the change in peak [Ca2+]i was calculated, a maximum increase occurred at ATP 10−6 M that significantly increased [Ca2+]i by 160 ± 38 nM before declining to 109 ± 29, 93 ± 35, and 66 ± 35 nM at 10−5, 10−4, and 10−3 M ATP, respectively (Fig. 3B).
Figure 3.
Effect of ATP on [Ca2+]i and protein secretion in lacrimal gland. Acini containing fura-2 were stimulated with ATP (10−6–10−4 M; A, B). Trace shown in (A) represents the mean of three animals. Arrow: addition of ATP. The peak [Ca2+]i was calculated from three animals (B). Acini from four animals were stimulated with ATP (10−6–10−4 M) for 40 minutes, and peroxidase secretion was measured (C). Data are mean ± SEM. *Significant difference from no addition.
Effect of ATP on Protein Secretion
To determine whether activation of P2X receptors by ATP plays a role in secretion, acini were stimulated with ATP (10−6–10−4 M) for 40 minutes and the amount of peroxidase secretion was determined (Fig. 3C). ATP increased peroxidase secretion in a concentration-dependent manner with an increase of 1.4 ± 0.1-, 1.4 ± 0.1-, and 1.5 ± 0.1-fold above basal at 10−6, 10−5, and 10−4 M, respectively. All concentrations were significantly increased above basal. These data indicate that ATP plays a functional role in the lacrimal gland acinar cells.
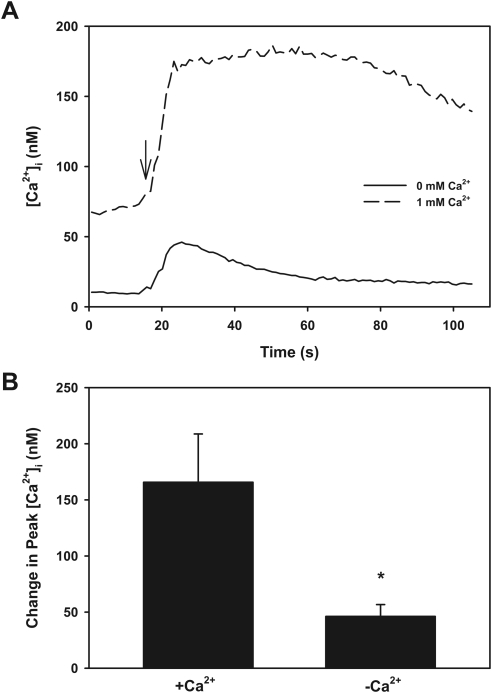
Effect of Removal of Extracellular [Ca2+]o on ATP-Stimulated [Ca2+]i
One way to differentiate P2X receptors from one another and from P2Y receptors is to determine the effect of removal of [Ca2+]o on the ATP-stimulated increase in [Ca2+]i. P2X2 receptors are known to be inhibited by [Ca2+]o; hence, the removal of [Ca2+]o should increase their activity.5 In contrast, P2X1 and P2X3 are dependent on [Ca2+]o for the increase in [Ca2+]i. P2Y receptors release Ca2+ from intracellular stores to generate an initial peak in [Ca2+]i and have a sustained increase in [Ca2+]i that is dependent on [Ca2+]o. In acini stimulated by ATP in buffer without Ca2+o, the ATP response was decreased compared with the ATP response in the presence of 1 mM Ca2+o (Fig. 4A). When the peak ATP response was analyzed, removal of [Ca2+]o significantly decreased the peak from 165 ± 43 nM in the presence of 1 mM Ca2+o to 46 ± 11 nM in the absence of [Ca2+]o (Fig. 4B). The plateau phase (after initial peak) of the Ca2+ response was reduced, but not significantly. The area under the curve in the presence of [Ca2+]o was 7532 ± 4122. This was decreased, but not significantly, to 1080 ± 33 in the absence of [Ca2+]o (data not shown). These results indicate that the ATP-stimulated increase in [Ca2+]i was predominately a P2X response, though a small portion might have been attributed to P2Y receptors. In addition, the fact that the response was not increased in the absence of [Ca2+]o implied that the response was not due to P2X2 receptors.
Figure 4.
Effect of removal of extracellular Ca2+ on ATP-stimulated [Ca2+]i. Acini containing fura-2 were washed in Ca2+-free buffer before stimulation with ATP (10−6 M). Trace shown in (A) represents the mean of three animals. Arrow: addition of ATP. Peak [Ca2+]i was calculated from each animal (B). Data are mean ± SEM. *Significant difference from ATP in the presence of extracellular Ca2+.
Effect of Repeated Applications of ATP on [Ca2+]i
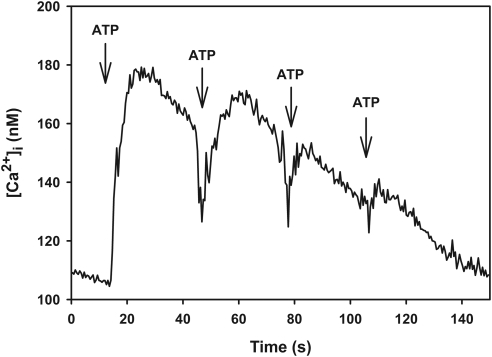
One way to differentiate the activities of P2X receptors from one another is to determine whether the receptors become desensitized with repeated applications of ATP. P2X1 and P2X3 become desensitized within milliseconds, P2X4 becomes desensitized within tens of seconds, P2X2 to P2X5 become desensitized within minutes, and P2X7 does not become desensitized.4,5 Therefore, lacrimal gland acini were subjected to four applications of ATP (10−6 M) every 30 seconds, and [Ca2+]i was measured. As shown in Figure 5, [Ca2+]i decreased with each application of ATP. The initial application of ATP increased [Ca2+]i by 83 ± 29 nM, whereas the fourth application of ATP increased [Ca2+]i by 33 ± 16 nM. These data indicate that ATP interacts predominantly with P2X1, P2X3, or P2X4 receptors.
Figure 5.
Effect of repeated applications of ATP on [Ca2+]i. Acini containing fura-2 were stimulated with ATP (10−6 M) every 30 seconds. Trace represents the mean of four animals. Arrows: addition of ATP.
Effect of Inhibition of P2X1 Receptors on ATP-Stimulated Peak [Ca2+]i
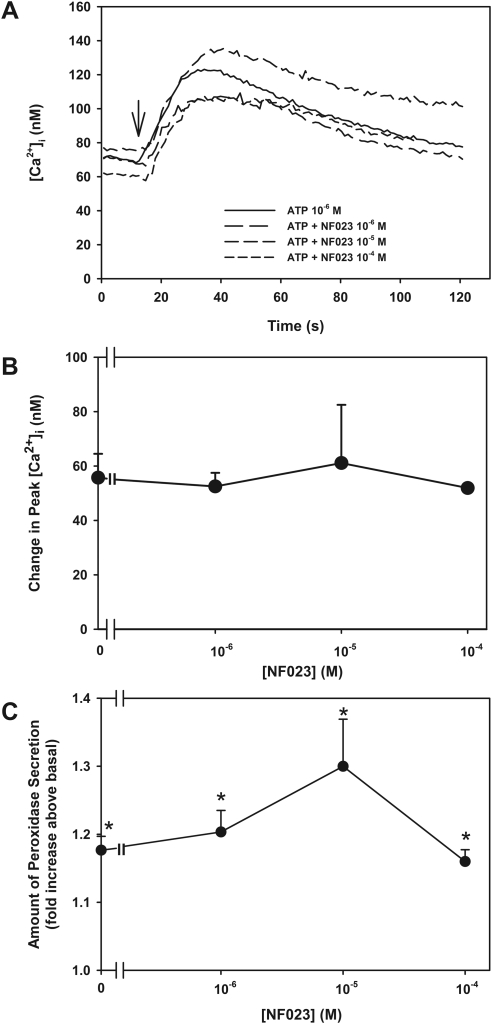
To determine whether ATP activates P2X1 receptors in the lacrimal gland, acini were preincubated for 30 minutes with the P2X1 receptor inhibitor NF023 (10−6–10−4 M).14,15 [Ca2+]i was then measured in response to the application of ATP (10−6 M; Fig. 6A). ATP increased peak [Ca2+]i by 56 ± 9 nM (Fig. 6B). ATP stimulation in acini preincubated with NF023 10−6, 10−5, and 10−4 M resulted in increases in [Ca2+]i of 53 ± 5, 61 ± 21, and 52 ± 1 nm, respectively. No concentration of NF023 had a significant effect on ATP-stimulated [Ca2+]i (Fig. 6B). Similarly, preincubation with NF023 (10−6–10−4 M) had no effect on ATP (10−6 M)–stimulated peroxidase secretion (Fig. 6C). Therefore, P2X1 receptors were not the predominant receptor activated by ATP in the lacrimal gland.
Figure 6.
Effect of inhibition of P2X1 receptors on ATP-stimulated [Ca2+]i. Acini containing fura-2 were preincubated with NF023 (10−6–10−4 M) before stimulation with ATP (10−6 M). Trace shown in (A) represents the mean of three animals. Peak [Ca2+]i was calculated from three animals (B). Acini were preincubated with NF023 (10−6–10−4 M) before stimulation with ATP (10−6 M). Peroxidase secretion was measured from three animals (C). Data are mean ± SEM. *Statistical significance from basal (0). Arrow: addition of ATP.
Effect of pH on ATP-Stimulated Peak [Ca2+]i
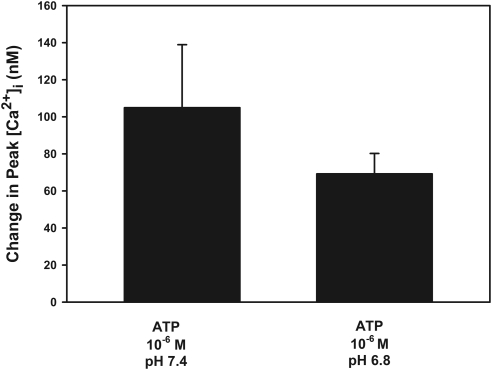
Unlike the other P2X receptors, lowering the pH has been shown to potentiate the response of P2X2 receptors to ATP.6,7 In normal KRB at pH 7.4, ATP (10−6 M) increased peak [Ca2+]i to 105 ± 34 nM. When the pH of the buffer was lowered to 6.8, [Ca2+]i was not potentiated but, rather, decreased slightly, though not significantly, to 69 ± 11 nM (Fig. 7). These results suggest that P2X2 receptors are not the predominant receptors activated by ATP in the lacrimal gland.
Figure 7.
Effect of pH on ATP-stimulated [Ca2+]i. Acini containing fura-2 were stimulated with ATP (10−6 M) in KRB-HEPES at either pH 7.4 (normal) or pH 6.8 (low). Peak [Ca2+]i was calculated from four animals. Data are mean ± SEM.
Effect of Zinc on ATP-Stimulated Peak [Ca2+]i
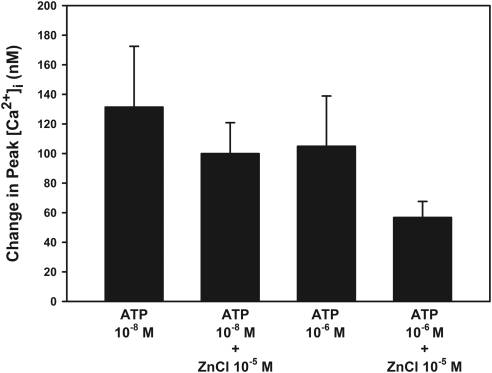
The addition of zinc has been demonstrated to potentiate the actions of P2X2 and, at a low concentration of ATP, P2X4.7,8 ATP 10−8 M alone increased [Ca2+]i by 131 ± 41 nM. The addition of ZnCl (10−5 M) simultaneously with ATP did not potentiate the ATP response and was 100 ± 21 nM (Fig. 8). ATP at 10−6 M increased [Ca2+]i by 105 ± 34 nM. When ZnCl was added, [Ca2+]i was again not potentiated by ZnCl but was decreased to 57 ± 11 nM (Fig. 8). This decrease was not significant. These results indicate that P2X2 and P2X4 do not play a significant role in the ATP-stimulated increase in [Ca2+]i.
Figure 8.
Effect of zinc on ATP-stimulated [Ca2+]i. Acini containing fura-2 were stimulated with ATP (10−8 and 10−6 M) in KRB-HEPES either with or without ZnCl (10−5 M). The peak [Ca2+]i was calculated from four animals. Data are mean ± SEM.
Effect of Inhibition of P2X3 on ATP-Stimulated Peak [Ca2+]i
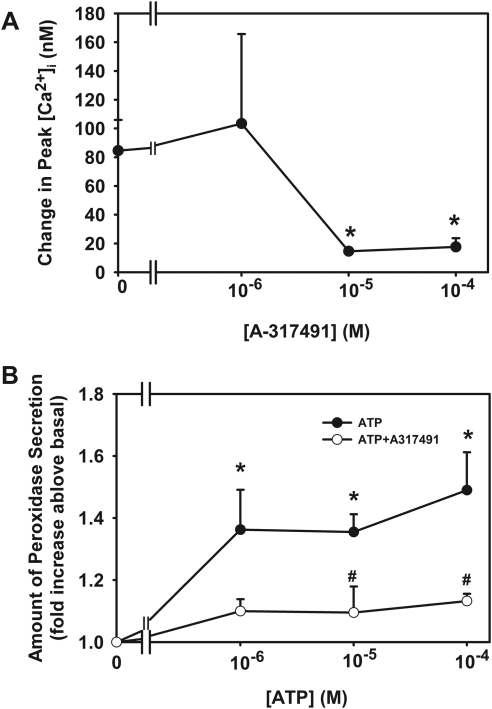
To determine whether ATP activates P2X3 receptors to increase [Ca2+]i, acini were incubated with the P2X3 receptor inhibitors A31749 (10−6–10−4 M) and TNP-ATP (10−8–10−6 M).4 ATP (10−6 M) increased peak [Ca2+]i to 85 ± 21 nM. Preincubation for 30 minutes with A31749 10−6 M did not alter the ATP response. However, incubation with A31749 at 10−5 and 10−4 M significantly decreased the ATP response to 15 ± 2 and 18 ± 6 nM (Fig. 9A).
Figure 9.
Effect of inhibition of P2X3 receptors on ATP-stimulated [Ca2+]i and protein secretion. Acini containing fura-2 were preincubated with either A317419 (10−6–10−4 M, A) for 30 minutes before stimulation with ATP (10−6 M). Peak [Ca2+]i was calculated from three animals. Data are mean ± SEM. Acini were also preincubated with A317491 (10−5 M) for 30 minutes and stimulated with ATP (10−6–10−4 M) for 40 minutes. Peroxidase secretion was measured (B). Data are mean ± SEM from four animals. ⧣Significant difference from ATP alone. *Significant difference from no additions. ATP alone data are repeated from Figure 3C because these two assays were performed at the same time.
In cells incubated with TNP-ATP 10−8, 10−7, and 10−6 M, peak [Ca2+]i was 41 ± 25, 16 ± 6, and 47 ± 40 nM. The decrease at 10−7 M was significantly different from that of ATP alone (data not shown).
Effect of Inhibition of P2X3 on ATP-Stimulated Protein Secretion
To verify that ATP activates P2X3 receptors to increase peroxidase secretion, acini were preincubated with A31749 at 10−5 M, a concentration that inhibits the ATP-stimulated increase in [Ca2+]i. As shown in Figure 9B, A317491 significantly inhibited ATP-stimulated peroxidase secretion. These data indicate that ATP binds to and activates P2X3 receptors in the lacrimal gland.
Effect of Activation of P2X3 Receptors on Peak [Ca2+]i
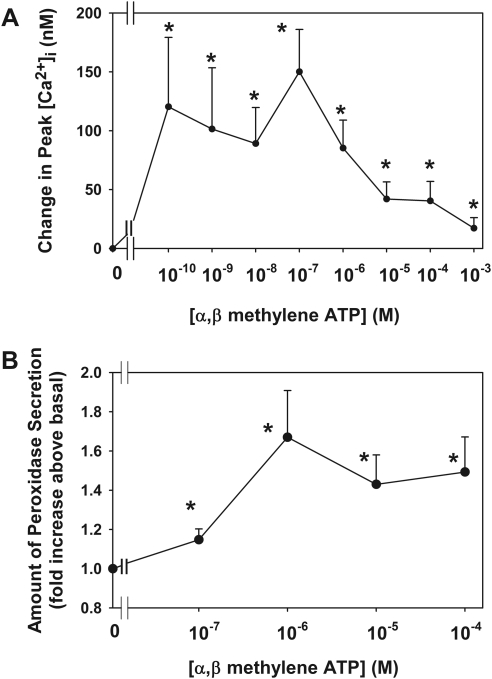
The ATP analog α,β methylene ATP (αβMeATP) shows specificity to P2X3 over the other P2X receptors.4,7 Figure 10A shows that αβMeATP increases peak [Ca2+]i in a concentration-dependent manner with a maximum concentration of 183 ± 59 nM at αβMeATP 10−7 M. All concentrations tested from 10−10 to 10−3 M significantly increased [Ca2+]i. These data indicate that P2X3 receptors are functional in the lacrimal gland acini.
Figure 10.
Effect of stimulation of P2X3 receptors on [Ca2+]i and protein secretion. Acini containing fura-2 were stimulated with α,β methylene ATP (10−10–10−3 M; A). Peak [Ca2+]i was calculated from three animals. Data are mean ± SEM. Acini were also stimulated with α,β methylene ATP (10−7–10−4 M) for 40 minutes. Peroxidase secretion was measured (B). Data are mean ± SEM of four animals. *Significant difference from basal (0).
Effect of Activation of P2X3 Receptors on Protein Secretion
To confirm that activation of the P2X3 receptors also stimulated secretion, acini were incubated with αβMeATP (10−7–10−4 M) for 40 minutes, and peroxidase secretion was measured (Fig. 10B). All concentrations of αβMeATP significantly increased peroxidase secretion above basal to 1.1 ± 0.1-, 1.7 ± 0.2-, 1.4 ± 0.3-, and 1.5 ± 0.2-fold at 10−7, 10−6, 10−5, and 10−4 M, respectively.
Effect of Inhibition of P2X7 on ATP-Stimulated [Ca2+]i
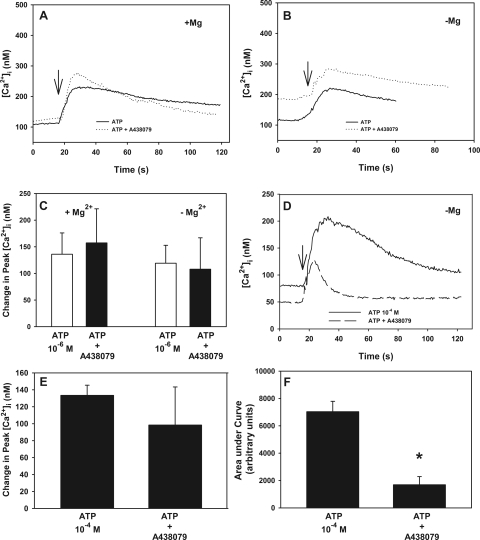
Using the P2X7 receptor agonist (benzoylbenzoyl)adenosine 5′ triphosphate (BzATP), we previously showed that P2X7 receptors are functional in the lacrimal gland and that their activation is linked to protein secretion and ERK activation.9 P2X7 receptors are unique among P2X receptors in that the response is potentiated in the absence of Mg2+ and high concentrations of ATP (10−4 M and greater) are necessary to activate the P2X7 receptors.5 To determine whether ATP activates P2X7, acini were stimulated with ATP (10−6 M) in the presence and absence of Mg2+. ATP increased [Ca2+]i in the presence (Fig. 11A) and absence (Fig. 11B) of Mg2+. Peak [Ca2+]i stimulated by ATP in the presence of Mg2+ was 136± 40 nM, which was not altered when measured in the absence of Mg2+ (Fig. 11C). Preincubation with the P2X7 receptor inhibitor A438079 (10−4 M) did not have an effect on the ATP response in either the presence or the absence of Mg2+ (Figs. 11A-C). The plateau phase (after initial peak) of the [Ca2+]i response was also unchanged in the presence or absence of either A438079 or Mg2+. When the area under each curve was integrated, the area in the presence of ATP alone was 7772.2 ± 2020.0 and was unchanged with ATP plus A438079 and was 16,527.5 ± 11,623.1 in the presence of Mg2+ (data not shown). In the absence of Mg2+, the area under the curve with ATP was 5877.3 and was unchanged in the presence of ATP and A438079 and was 7280.2 (data not shown).
Figure 11.
Effect of inhibition of P2X7 receptors on ATP-stimulated [Ca2+]i. Acini containing fura-2 were preincubated with A438079 (10−4 M) for 30 minutes in the presence or absence of Mg2+ before stimulation with ATP. Trace shown in (A) is mean of acini from three animals stimulated with ATP (10−6 M) in the presence of Mg2+. Trace shown in (B) is mean of acini from three animals stimulated with ATP (10−6 M) in the absence of Mg2+. Arrows: addition of ATP. Peak [Ca2+]i was calculated from three animals (C). Acini were preincubated with A438079 (10−4 M) for 30 minutes in the absence of Mg2+ before stimulation with ATP (10−4 M), and traces of three animals were averaged (D). Arrow: addition of ATP. Peak [Ca2+]i was calculated from three animals (E). Area under the curve from each animal was calculated, and the mean ± SEM is shown (F). *Significant difference from basal.
Increasing the concentration of ATP to 10−4 M increased [Ca2+]i in the absence of Mg2+ (Fig. 11D). The change in peak [Ca2+]i was 133± 12 (Fig. 11E). This was not significantly different from ATP 10−4 M obtained in the presence of Mg2+, which was 93 ± 35 nM (Fig. 1). Incubation with A438079 (10−4 M) did not alter the peak [Ca2+]i response stimulated by ATP 10−4 M in the absence of Mg2+, which was 98± 45 nM (Fig. 11E). However, the plateau phase of the [Ca2+]i response was reduced (Fig. 11D). When the area under each curve was integrated, the area in the presence of ATP alone was 7034.3 ± 758.8 (Fig. 11F). This was significantly decreased to 1687.6 ± 597.3 in the presence of ATP and A438079. These data indicate that at high concentrations, ATP activates P2X7 receptors to increase the sustained phase of the [Ca2+]i increase.
Discussion
ATP is known to regulate many cellular functions.16 Virtually every cell type releases ATP, which can act in an autocrine (on the same cell) or a paracrine (on adjacent cells) manner. ATP is known to be released by mechanical stimulation through osmotic swelling or shrinking, host-pathogen interactions, or stretching forces.17–20 In addition, ATP is released with acetylcholine and norepinephrine.21 Therefore, in the lacrimal gland, the source of ATP could be parasympathetic and sympathetic nerves that innervate the gland, contraction of myoepithelial cells, or the acinar cells themselves.
We previously showed that P2X7 receptors stimulated by BzATP play an important role in lacrimal gland protein secretion and ERK activation. This study was undertaken to determine which P2X receptors play a role in the ATP-stimulated increase in [Ca2+]i in the lacrimal gland. P2X1 to P2X6 receptors have similar characteristics; however, there are subtle differences allowing for identification. Repeated applications of ATP to lacrimal gland acini decreased the [Ca2+]i response indicating that either P2X1, P2X3, and P2X4 are activated by ATP (Fig. 5). However, an inhibitor specific for P2X1 (Fig. 6) did not have any effect on ATP-stimulated increases in [Ca2+]i, arguing against a role for this receptor in this response.
It is well established that zinc potentiates the P2X4 response when activated by a submaximal concentration of ATP and the P2X2 response when activated by a maximal concentration of ATP. P2X2 and PX4 receptors have been shown to contain zinc binding sites, which increases the receptor's affinity for ATP.22,23 Given that zinc had no effect on the ATP-stimulated increase in [Ca2+]i at either a submaximal or a maximal concentration in the lacrimal gland, it is unlikely that either of these receptors plays a role in the ATP increase in [Ca2+]i (Fig. 8).
The evidence indicating that P2X3 receptors are activated in the lacrimal gland by ATP is strong. First, the ATP-induced increase in [Ca2+]i decreased with repeated applications of ATP (Fig. 5). Second, the ATP-stimulated increase in [Ca2+]i was inhibited by two P2X3 specific inhibitors, A31749 and TNP-ATP (Fig. 9). Third, a specific agonist for P2X3 receptors, α,β methylene ATP, elicited robust increases in [Ca2+]i and secretory responses over a large concentration range with a magnitude similar to that of ATP (Fig. 10).
We did not pursue the role of P2X5 receptors because we were unable to detect them either by RT-PCR, Western blot analysis, or immunofluorescence experiments.
P2X6 receptors were detected by immunofluorescence, Western blot analysis, and RT-PCR. However, it was difficult to assess the role they played in ATP-stimulated [Ca2+]i increases because P2X6 receptors do not form homomers.3 They do form heteromers with other P2X receptors, namely P2X2 and P2X4.5 The addition of zinc had no effect on the ATP-stimulated increase in [Ca2+]i (Fig. 8) and the [Ca2+]i response becomes desensitized relatively quickly (Fig. 5), suggesting that P2X2 and P2X4 receptors do not play a significant role in the lacrimal gland. Therefore, it seems unlikely that P2X6 forms heteromers with these two receptor subtypes to increase [Ca2+]i and stimulate lacrimal gland protein secretion.
ATP can also bind to and activate P2Y receptors. Thus a minor portion of the [Ca2+]i response elicited by ATP could be attributed to activation of the P2Y receptors. This is supported by the fact that the peak [Ca2+]i was not completely inhibited by the removal of [Ca2+]o. P2Y receptors differ from P2X receptors in that they are GPCRs instead of channels. When activated, P2Y receptors stimulate the production of inositol trisphosphate (IP3). IP3 releases Ca2+ from internal stores, causing a peak in [Ca2+]i, even in the absence of [Ca2+]o. Because the removal of [Ca2+]o does not completely abolish the peak increase in [Ca2+]i stimulated by ATP, it is possible that P2Y receptors play a small role in the lacrimal gland (Fig. 4). Of the eight P2Y receptors identified, only two, P2Y2 and P2Y11, preferentially bind ATP.16 We did not examine these two P2Y receptors in this study.
P2X3 receptors are known to dimerize with P2X2 receptors to form P2X2/P2X3 heterodimers.24–26 These heterodimers then have characteristics of each receptor such that they are activated by α,β methylene ATP and are inhibited by P2X3 receptor antagonists (P2X3 characteristics). However, their activity is potentiated by low pH, and they do not desensitize (P2X2 receptor characteristics). Because the [Ca2+]i response was not potentiated by low pH (Fig. 7) and the ATP-stimulated [Ca2+]i desensitized (Fig. 5), it was unlikely that P2X2/P2X3 heterodimers played a major role in the rat lacrimal gland.
P2X3 receptors usually exist as trimers and can collect to form large aggregates. They play a well-known role in visceral pain in pathologic conditions such as renal colic, dyspepsia, inflammatory bowel disease, angina, dysmenorrhoea, and interstitial cystitis. It is hypothesized that in visceral tubes and sacs, ATP is released from epithelial cells during distension, which activates P2X3 homomeric and P2X2/3 heteromeric receptors on subepithelial sensory nerves. Activation of these receptors initiates impulses in sensory pathways to pain centers in the central nervous system.27
P2X3 receptors have also been implicated in the exocytosis process. In the pancreas, P2X3 receptors are involved in a positive feedback loop to increase the secretion of insulin when glucose levels are high. Under these conditions, ATP is released with insulin from β cells. In humans, but not mice, this ATP activates P2X3 receptors, which increases [Ca2+]i and causes the release of insulin.28 It is likely that the activation of P2X3 receptors causes protein secretion in the lacrimal gland as well. An increase in [Ca2+]i is necessary for protein secretion from the rat lacrimal gland,29–32 and the activation of P2X3 receptors by ATP increases [Ca2+]i.
In the mouse parotid glands, P2X4 and P2X7 receptors have been identified.33 Casa-Pruneda et al.34 demonstrated that these two receptors form a functional unit to produce an ATP-activated current with unique characteristics, implying that these receptors form heterodimers. In addition, cAMP modulates P2X4 receptors to potentiate Ca2+ signaling in human parotid glands.35 Interestingly, cAMP has been shown to play an inhibitory role on P2X3 receptors in neurons.36 Given that cAMP also plays a major role in lacrimal gland function, it is possible that cAMP interacts with the P2X3 receptor to alter its activity.37–39
In conclusion, our data indicate that ATP activates P2X3 to increase [Ca2+]i and protein secretion from the rat lacrimal gland. In addition, at high concentrations, ATP also activates P2X7 receptors, which increases [Ca2+]i.
Acknowledgments
The authors thank Claire Mitchell and George Dubyak for their helpful discussions.
Footnotes
Supported by National Institutes of Health Grant EY06177.
Disclosure: R.R. Hodges, None; J. Vrouvlianis, None; R. Scott, None; D.A. Dartt, None
References
- 1. Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28:155–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hodges RR, Dartt DA. Regulatory pathways in lacrimal gland epithelium. Int Rev Cytol. 2003;231:129–196 [DOI] [PubMed] [Google Scholar]
- 3. Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gomes DA, Song Z, Stevens W, Sladek CD. Sustained stimulation of vasopressin and oxytocin release by ATP and phenylephrine requires recruitment of desensitization-resistant P2X purinergic receptors. Am J Physiol Regul Integr Comp Physiol. 2009;297:R940–R949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067 [DOI] [PubMed] [Google Scholar]
- 6. King BF, Wildman SS, Ziganshina LE, Pintor J, Burnstock G. Effects of extracellular pH on agonism and antagonism at a recombinant P2X2 receptor. Br J Pharmacol. 1997;121:1445–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ma B, Ruan HZ, Burnstock G, Dunn PM. Differential expression of P2X receptors on neurons from different parasympathetic ganglia. Neuropharmacology. 2005;48:766–777 [DOI] [PubMed] [Google Scholar]
- 8. Tittle RK, Hume RI. Opposite effects of zinc on human and rat P2X2 receptors. J Neurosci. 2008;28:11131–11140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hodges RR, Vrouvlianis J, Shatos MA, Dartt DA. Characterization of P2X7 purinergic receptors and their function in rat lacrimal gland. Invest Ophthalmol Vis Sci. 2009;50:5681–5689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shibuya I, Tanaka K, Hahori Y, et al. Evidence that multiple P2X purinoceptors are functionally expressed in rat supraoptic neurones. J Physiol. 1999;514(pt 2):351–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun B, Li J, Okahara K, Kambayashi J. P2X1 purinoceptor in human platelets: molecular cloning and functional characterization after heterologous expression. J Biol Chem. 1998;273:11544–11547 [DOI] [PubMed] [Google Scholar]
- 12. Suzuki-Kerr H, Vlajkovic S, Donaldson PJ, Lim J. Molecular identification and localization of P2X receptors in the rat lens. Exp Eye Res. 2008;86:844–855 [DOI] [PubMed] [Google Scholar]
- 13. Yunaev MA, Barden JA, Bennett MR. Changes in the distribution of different subtypes of P2X receptor clusters on smooth muscle cells in relation to nerve varicosities in the pregnant rat urinary bladder. J Neurocytol. 2000;29:99–108 [DOI] [PubMed] [Google Scholar]
- 14. Khaira SK, Pouton CW, Haynes JM. P2X2, P2X4 and P2Y1 receptors elevate intracellular Ca2+ in mouse embryonic stem cell-derived GABAergic neurons. Br J Pharmacol. 2009;158:1922–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Judkins CP, Sobey CG, Dang TT, et al. NADPH-induced contractions of mouse aorta do not involve NADPH oxidase: a role for P2X receptors. J Pharmacol Exp Ther. 2006;317:644–650 [DOI] [PubMed] [Google Scholar]
- 16. Corriden R, Insel PA. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal. 2010;3(104):re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Darby M, Kuzmiski JB, Panenka W, Feighan D, MacVicar BA. ATP released from astrocytes during swelling activates chloride channels. J Neurophysiol. 2003;89:1870–1877 [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci U S A. 1996;93:12020–12025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Piccini A, Carta S, Tassi S, et al. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A. 2008;105:8067–8072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sauer H, Hescheler J, Wartenberg M. Mechanical strain-induced Ca(2+) waves are propagated via ATP release and purinergic receptor activation. Am J Physiol Cell Physiol. 2000;279:C295–C307 [DOI] [PubMed] [Google Scholar]
- 21. Gourine AV, Wood JD, Burnstock G. Purinergic signalling in autonomic control. Trends Neurosci. 2009;32:241–248 [DOI] [PubMed] [Google Scholar]
- 22. Nagaya N, Tittle RK, Saar N, Dellal SS, Hume RI. An intersubunit zinc binding site in rat P2X2 receptors. J Biol Chem. 2005;280:25982–25993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Acuna-Castillo C, Morales B, Huidobro-Toro JP. Zinc and copper modulate differentially the P2X4 receptor. J Neurochem. 2000;74:1529–1537 [DOI] [PubMed] [Google Scholar]
- 24. Lewis C, Neidhart S, Holy C, et al. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435 [DOI] [PubMed] [Google Scholar]
- 25. Spelta V, Jiang LH, Surprenant A, North RA. Kinetics of antagonist actions at rat P2X2/3 heteromeric receptors. Br J Pharmacol. 2002;135:1524–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burgard EC, Correa-Medina M, Cabrero O, et al. Competitive antagonism of recombinant P2X(2/3) receptors by 2′, 3′-O-(2,4,6-trinitrophenyl) adenosine 5′-triphosphate (TNP-ATP). Mol Pharmacol. 2000;58:1502–1510 [DOI] [PubMed] [Google Scholar]
- 27. Burnstock G. Purinergic receptors and pain. Curr Pharm Des. 2009;15:1717–1735 [DOI] [PubMed] [Google Scholar]
- 28. Jacques-Silva MC, et al. ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic beta cell. Proc Natl Acad Sci U S A. 2010;107:6465–6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dartt DA, Rose PE, Joshi VM, Donowitz M, Sharp GW. Role of calcium in cholinergic stimulation of lacrimal gland protein secretion. Curr Eye Res. 1985;4:475–483 [DOI] [PubMed] [Google Scholar]
- 30. Hodges RR, Rios JD, Vrouvlianis J, et al. Roles of protein kinase C, Ca2+, Pyk2, and c-Src in agonist activation of rat lacrimal gland p42/p44 MAPK. Invest Ophthalmol Vis Sci. 2006;47:3352–3359 [DOI] [PubMed] [Google Scholar]
- 31. Zoukhri D, Hodges RR, Rawe IM, Dartt DA. Ca2+ signaling by cholinergic and α1-adrenergic agonists is up-regulated in lacrimal and submandibular glands in a murine model of Sjögren's syndrome. Clin Immunol Immunopathol. 1998;89:134–140 [DOI] [PubMed] [Google Scholar]
- 32. Zoukhri D, Hodges RR, Sergheraert C, Dartt DA. Cholinergic-induced Ca2+ elevation in rat lacrimal gland acini is negatively modulated by PKCdelta and PKCepsilon. Invest Ophthalmol Vis Sci. 2000;41:386–392 [PubMed] [Google Scholar]
- 33. Turner JT, Landon LA, Gibbons SJ, Talamo BR. Salivary gland P2 nucleotide receptors. Crit Rev Oral Biol Med. 1999;10:210–224 [DOI] [PubMed] [Google Scholar]
- 34. Casas-Pruneda G, Reyes JP, Perez-Flores G, Perez-Cornejo P, Arreola J. Functional interactions between P2X4 and P2X7 receptors from mouse salivary epithelia. J Physiol. 2009;587(pt 12):2887–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brown DA, Bruce JI, Straub SV, Yule DI. cAMP potentiates ATP-evoked calcium signaling in human parotid acinar cells. J Biol Chem. 2004;279:39485–39494 [DOI] [PubMed] [Google Scholar]
- 36. Mamenko MV, Chizhmakov IV, Volkova TM, Verkhratsky A, Krishtal OA. Extracellular cAMP inhibits P2X receptors in rat sensory neurones through G protein-mediated mechanism. Acta Physiol. 2010;199:199–204 [DOI] [PubMed] [Google Scholar]
- 37. Funaki C, Hodges RR, Dartt DA. Role of cAMP inhibition of p44/p42 mitogen-activated protein kinase in potentiation of protein secretion in rat lacrimal gland. Am J Physiol Cell Physiol. 2007;293:C1551–C1560 [DOI] [PubMed] [Google Scholar]
- 38. Dartt DA, Baker AK, Vaillant C, Rose PE. Vasoactive intestinal polypeptide stimulation of protein secretion from rat lacrimal gland acini. Am J Physiol. 1984;247(pt 1):G502–G509 [DOI] [PubMed] [Google Scholar]
- 39. Hodges RR, Zoukhri D, Sergheraert C, Zieske JD, Dartt DA. Identification of vasoactive intestinal peptide receptor subtypes in the lacrimal gland and their signal-transducing components. Invest Ophthalmol Vis Sci. 1997;38:610–619 [PubMed] [Google Scholar]