In lacrimal gland cells, the activation of M3AChRs stimulates P2X7 receptors to increase [Ca2+]i and protein secretion.
Abstract
Purpose.
To determine the interaction of M3 muscarinic receptors (M3AChR) and P2X7 receptors to increase intracellular [Ca2+] ([Ca2+]i) and stimulate protein secretion in rat lacrimal gland cells.
Methods.
Exorbital lacrimal glands from male Sprague-Dawley rats were divided into pieces or digested with collagenase to form acinar clumps. [Ca2+]i was measured using an imaging system in acini incubated with fura-2/AM. Adenosine triphosphate (ATP) release was determined using the luciferin-luciferase reaction. Peroxidase secretion, our index for protein secretion, was measured spectrophotometrically. Acini were stimulated with the P2X7 receptor agonist, (benzoylbenzoyl)adenosine 5′ triphosphate (BzATP), cholinergic agonist carbachol, or the activator of conventional and novel PKC isoforms, phorbol 12-myristate 13-acetate (PMA).
Results.
The increase in [Ca2+]i caused by carbachol and BzATP used simultaneously was less than additive, but the increase in protein secretion was additive. The M3AChR antagonist atropine blocked the BzATP-stimulated increase in [Ca2+]i and peroxidase secretion. The P2X7 antagonist did not alter the carbachol-stimulated increase in [Ca2+]i or peroxidase. PMA- and BzATP-stimulated increases in [Ca2+]i were additive. Neither constitutively active PKCα, dominant-negative PKCα, nor PKCε altered BzATP-stimulated increases in [Ca2+]i. Carbachol increased ATP release from lacrimal gland pieces but not from acini.
Conclusions.
In lacrimal gland cells, the activation of M3AChRs stimulates P2X7 receptors to increase [Ca2+]i and protein secretion. The underlying mechanisms are unknown but could include the release of ATP or intracellular interactions not mediated by PKC isoforms. In addition, M3AChRs use signaling pathways that overlap with those used by P2X7 receptors to increase [Ca2+]i, but they also use signaling pathways not used by P2X7 receptors to stimulate protein secretion.
Lacrimal glands secrete protein, electrolytes, and water into the tear film that overspreads the cornea and conjunctiva.1 Lacrimal gland protein secretion is stimulated by multiple neurotransmitters, including the parasympathetic neurotransmitters acetylcholine (activates muscarinic type 3 acetylcholine receptors [M3AChR]) and vasoactive intestinal peptide (VIP) (stimulates VIPAC1 receptors) and the sympathetic neurotransmitter norepinephrine (interacts with α1D-adrenergic receptors [α1D-AR]).1 Each of these neurotransmitters activates a separate distinct signaling pathway. Thus lacrimal gland protein secretion can be induced by increasing the intracellular [Ca2+] ([Ca2+]i) and activating protein kinase C (PKC), raising the cellular level of cAMP, or elevating cellular cGMP levels.2–6
Cholinergic agonists stimulate lacrimal gland protein secretion by activating M3AChR coupled to Gαq G proteins, which activate phospholipase Cβ (PLCβ).7,8 PLCβ activation cleaves phosphatidylinositol 1,4-bisphosphate to produce the PKC activator diacylglycerol and inositol 1,4,5-trisphosphate (InsP3). InsP3 activates Ca2+-selective InsP3 receptors located in the endoplasmic reticulum of lacrimal gland acinar cells that increase the [Ca2+]i.2 Depletion of the endoplasmic reticulum Ca2+ pool triggers extracellular Ca2+ influx and a sustained elevation of [Ca2+]i.9 The increase in [Ca2+]i along with activation of the PKC isoforms PKCα, PKCδ, and PKCε stimulate the secretion of protein stored in preformed secretory granules.3 Protein secretion occurs across the apical membrane and along with isotonic electrolyte and water secretion, also induced by cholinergic agonists, forms lacrimal gland fluid.10 After modification by ductal cell secretion, lacrimal gland fluid is secreted onto the cornea and conjunctiva.
In addition to M3AChR, VIPAC1, and α1D-AR, lacrimal gland acinar cells express purinergic P2 receptors that are coupled to an increase in [Ca2+]i and stimulate protein secretion.11 The P2 receptor family consists of P2Y receptors that are G-protein–coupled (metabotropic) and P2X receptors that are ion channels (ionotropic).12 Both types of P2 receptors are activated by extracellular di- and tri-nucleotides. P2Y receptors cause an increase in [Ca2+]i by InsP3-induced Ca2+ mobilization from intracellular stores similar to muscarinic receptors, whereas P2X receptors act as ligand-gated, nonselective ion channels that allow the influx of extracellular Ca2+.12 P2X7 receptors are a major functional P2 receptor in the lacrimal gland.11 Activation of P2X7 receptors with (benzoylbenzoyl)adenosine 5′ triphosphate (BzATP) causes an increase in [Ca2+]i and the stimulation of lacrimal gland protein secretion.11 The BzATP-stimulated increase in [Ca2+]i in lacrimal acinar cells was increased in the absence of Mg2+ and was blocked by two P2X7 antagonists, brilliant blue G and A438979.11 Similarly, protein secretion induced by BzATP was prevented by brilliant blue G.11 Thus the activation of P2X7 receptors is a Ca2+-dependent stimulus of lacrimal gland protein secretion.
Neurotransmitters often work together, thus changing the secretory response. Simultaneous activation of two different receptors and their signaling pathways can cause three different outcomes: a less than additive response, an additive response, or potentiation of the response. A less than additive response can occur if two receptors activate the same or overlapping signaling pathways or if activation of one receptor inhibits the second receptor. In the lacrimal gland, a less than additive secretory response occurs when the cholinergic agonist M3AChR is activated with carbachol and PKC isoforms are activated by the phorbol ester 4β-phorbol 12,13 dibutyrate (PdBU) because M3AChR and PdBU both activate PKC isoforms.13 An additive response results if two receptors use different signaling pathways. No interaction of two separate signaling pathways occurs in the lacrimal gland in the presence of activation of the M3AChR (Ca2+- and PKC-dependent) and the α1D-AR (cGMP- and Ca2+-dependent), leading to additivity of secretion.13 Finally, potentiation is produced if the two pathways interact synergistically and cause a response that is greater than that of the two pathways activated together. In the lacrimal gland interaction of M3AChR (Ca2+- and PKC-dependent) and VIPAC1 (cAMP and Ca2+-dependent) receptors stimulated by cholinergic agonists and VIP, respectively, leads to potentiation.13,14
In the present study the interaction of M3AChR and P2X7 receptors is explored. Our results demonstrate that the activation of M3AChR stimulates P2X7 receptors to increase [Ca2+]i and induce protein secretion. M3AChR does not activate PKC to alter P2X7 receptors but can release adenosine triphosphate (ATP) from nonacinar cells. Activation of both receptors increases [Ca2+]i and stimulates lacrimal gland protein secretion because both receptors are at least partially Ca2+-dependent. Muscarinic agonists, however, activate an additional pathway, probably a PKC-dependent pathway, that causes protein secretion independently of P2X7 receptors because secretion stimulated by muscarinic and P2X7 agonists is additive.
Materials and Methods
Materials
Fura-2 tetra-acetoxylmethyl ester (fura-2/AM) and reagent (Amplex Red) were purchased from Invitrogen Corporation (Carlsbad, CA). Collagenase CLSIII was from Worthington Biochemicals (Lakewood, NJ), and phorbol 12-myristate 13-acetate (PMA) was from LC Laboratories (Woburn, MA). All other chemicals were from Sigma-Aldrich (St. Louis, MO).
Animals
All experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Schepens Eye Research Institute Animal Care and Use Committee. Male Sprague-Dawley rats weighing 125 to 150 g were purchased from Taconic Farms (Germantown, NY). Rats were maintained in rooms at constant temperature with fixed light-dark intervals of 12 hours and were fed ad libitum. They were anesthetized for 1 minute in CO2 and then decapitated. Both exorbital lacrimal glands were removed immediately.
Preparation of Lacrimal Gland Acini
Collagenase digestion was used to prepare acini. Lacrimal glands were fragmented before incubation at 37°C with collagenase CLSIII (100 U/mL) in Krebs-Ringer bicarbonate buffer with HEPES (KRB-HEPES) (119 mM NaCl, 4.8 mM KCl, 1.0 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 MM NaHCO3, 10 mM HEPES, and 5.5 mM glucose, at pH 7.45) plus 0.5% BSA. After incubation, fragments were triturated, filtered through nylon mesh (150-μM pore size), and centrifuged at 50g for 2 minutes. The pellet was washed twice through KRB-HEPES containing 4% BSA. The dispersed acini recovered for 60 minutes at 37°C before use.
Measurement of [Ca2+]i
Acini were incubated for 30 minutes at room temperature in the dark with KRB-HEPES containing 0.5% BSA, 0.5 μM fura-2/AM, 8 μM pluronic acid F127, and 250 μM sulfinpyrazone followed by washing in KRB-HEPES containing sulfinpyrazone. Calcium measurements were made with a ratio imaging system (InCyt Im2; Intracellular Imaging, Cincinnati, OH) using excimer wavelengths of 340 and 380 nm and an emission wavelength of 505 nm. At least 10 clumps of acini were used for each condition, and experiments were repeated in at least three animals. Inhibitors or antagonists were added 20 minutes before agonists. After the addition of antagonists, inhibitors, and agonists, data were collected in real time. Data are presented as the actual [Ca2+]i with time or as the change in peak [Ca2+]i. Change in peak [Ca2+]I was calculated by subtracting the average of the basal value (no added agonist) from the peak [Ca2+]i. Although data are not shown, the plateau [Ca2+]i was affected similarly to the peak [Ca2+]i, except where indicated. All [Ca2+]i measurements in the presence of BzATP were performed in the absence of extracellular Mg2+ to increase the P2X7 receptor response.
Measurement of ATP Release
Lacrimal glands were removed and minced into small pieces, or acini were prepared by collagenase digestion. Pieces or acini were placed in cell strainers and preincubated in 0.4% BSA in KRB for 1 hour in a 12-well culture dish at 37°C. The strainers were moved to new wells containing fresh 0.4% BSA in KRB for an additional hour. The pieces or acini were then placed in fresh 0.4% BSA in KRB containing 10−4 M ARL-67516, α,β methylene ADP, and β,γ methylene ATP, inhibitors of ectonucleotide pyrophosphatase and ectoATPase. Agonists were added for 10 minutes, and the lacrimal gland pieces were removed. The ATP concentrations in the supernatants were determined with the chemiluminescent luciferin-luciferase reaction using an ATP assay kit (FLAA; Sigma-Aldrich, St. Louis, MO). The luciferin-luciferase working solution was 100 μL stock solution to 1 mL assay buffer, according to the manufacturer's instructions. Twenty-five microliters of samples were added to a 96-well plate and were placed in a luminometer (Synerg Mx microplate reader; BioTek Instruments, Winooski, VT). The luciferin-luciferase working solution (15 μL) was injected into each well using the internal injector system. The emitted light was recorded with an integration time of 100 ms/measurement every minute for 10 minutes. ATP levels were calculated by integration under the luminescence curve. Luminescence was converted to ATP concentration using an ATP standard curve. Data were expressed as fold increase above basal.
Expression of Constitutively Active and Dominant-Negative PKC Isoforms
Replication-defective adenovirus constructs expressing green fluorescent protein and dominant-negative PKCα or PKCε were kind gifts from George King (Joslin Diabetes Center, Boston, MA). Constitutively active PKCα (myrPKCα) was used as described previously.15 Acini were incubated overnight in the presence of 1 × 107 pfu of each adenovirus. Acini were allowed to recover for 1 hour before the addition of fura-2/AM for 1 hour.
Measurement of Peroxidase Secretion
Acini were incubated for 40 minutes in KRB-HEPES containing 4% BSA at 37°C in the presence of agonists. Inhibitors or antagonists were added 20 minutes before agonists. To terminate the incubation, acini were pelleted by centrifugation, and the supernatant was collected. The pellet was homogenized in 10 mM Tris-HCl (pH 7.5). Peroxidase activity, an index of protein secretion, was measured in duplicate in both the supernatant and the pellet. Peroxidase was measured using a reagent (Amplex Red; Invitrogen), which, when oxidized by peroxidase in the presence of hydrogen peroxide, produces a highly fluorescent molecule. The amount of fluorescence in the supernatant and pellet was quantified using a fluorescence microplate reader (model FL600; BioTek) with an excitation wavelength of 530 nm and an emission wavelength of 590 nm. Peroxidase was expressed as a percentage of peroxidase secreted into the media (supernatant) compared with total peroxidase present in the cells before stimulation (pellet plus supernatant). Data were expressed as fold increase × basal, which was set to 1.
Statistical Analysis
Results were expressed as mean ± SEM. Data were analyzed by Student's t-test. P < 0.05 was considered statistically significant.
Results
Cholinergic Agonists Increase [Ca2+]i in a Concentration-Dependent Manner
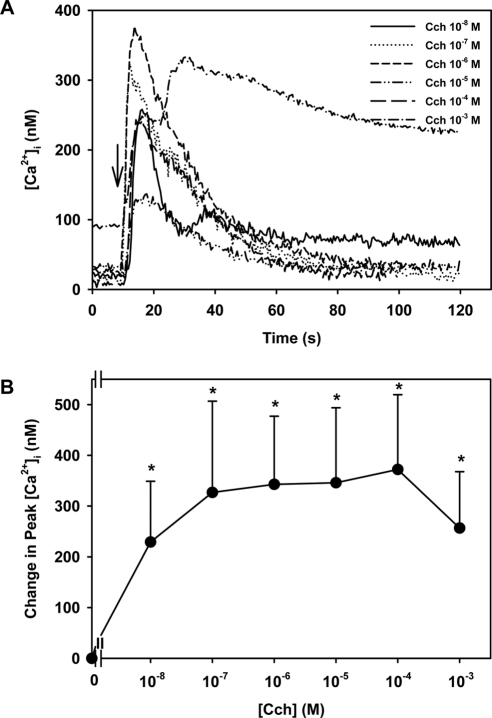
When the [Ca2+]i was determined in a large population of acinar cells using cells suspended in a cuvette, the cholinergic agonist carbachol increased [Ca2+]i in a concentration-dependent manner.2 A maximum increase of 153 nM was induced by carbachol at 10−3 M. In the present experiments using fura-2 in acini by fluorescence microscopy in which approximately 10 to 20 clumps of acini were measured, carbachol caused a concentration-dependent increase in [Ca2+]i (Fig 1A). A maximum increase in [Ca2+]i of 372.1 ± 127.7 nM occurred at 10−4 M carbachol (Fig 1). Using both measurement techniques, the change in [Ca2+]i caused by supramaximal concentrations of carbachol caused a decreased response.
Figure 1.
Effect of [Ca2+]i in lacrimal gland acini. Time-dependent increase in [Ca2+]i induced by stimulation of lacrimal gland acini with increasing concentrations of the cholinergic agonist carbachol (Cch) added after 10 seconds of incubation. Trace is the mean from four experiments (A). Mean change in peak [Ca2+]i from four experiments (B). Values are mean ± SEM. Arrow: time of carbachol addition. *Significant difference from no additions (0).
Activated M3AChR and P2X7 Receptors Use Overlapping Cellular Pathways to Increase [Ca2+]i in Lacrimal Gland Acini
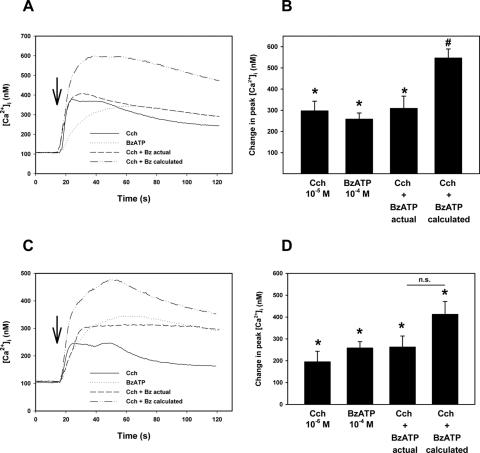
[Ca2+]i was measured in fura-2–loaded acini stimulated simultaneously with carbachol and BzATP at maximal and submaximal concentrations for increasing [Ca2+]i. Carbachol (10−4 M) and BzATP (10−4 M) at maximal concentrations each significantly increased [Ca2+]i to 297.3 ± 45.5 nM and 258.2 ± 29.1 nM, respectively (Figs. 2A, 2B). When the two were added at the same time, the increase in [Ca2+]i was significantly lower than the calculated additivity. Similar results were obtained when a submaximal concentration of carbachol (10−6 M) was used, although the difference in calculated and experimentally obtained increases in [Ca2+]i did not reach statistical significance (Figs. 2C, 2D).
Figure 2.
Effect of simultaneous addition of carbachol and BzATP on [Ca2+]i in lacrimal gland acini. Time-dependent increase in [Ca2+]i induced by the stimulation of lacrimal gland acini with cholinergic agonist carbachol (Cch), BzATP, or the two added simultaneously. The calculated [Ca2+]i response of the two agonists added simultaneously is also shown. Traces from seven experiments (A, C). Mean change in peak [Ca2+]i from seven experiments (B, D). Values are mean ± SEM. Arrows: time of agonist addition. *Significant difference from no additions (0). #Significant difference in experimental additivity from calculated additivity. n.s., no significant difference.
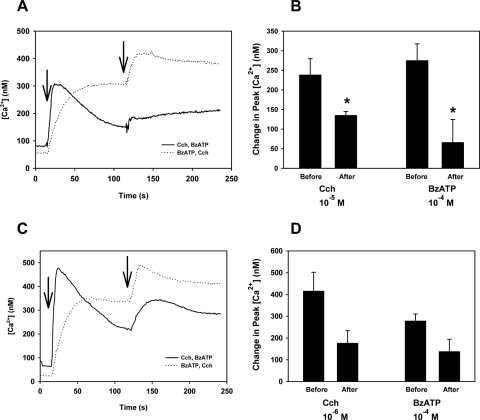
A second experimental paradigm was used to further explore the signaling pathways used by M3AChR and P2X7 receptors to increase [Ca2+]i. Instead of adding the two agonists simultaneously, they were added sequentially. If the two agonists used the same intracellular Ca2+ stores, the agonist added second should have had a reduced response from that added first. Conversely, if two agonists use different pools, the response of the second agonist should have been the same as that added first.16 When carbachol was used first, it significantly increased the [Ca2+]i by 235.7 ± 50.3 nM (Figs. 3A, 3B). When carbachol was used after BzATP, the response was significantly decreased to 142.3 ± 38.3 nM. Use of BzATP first increased the [Ca2+]i to 274.7 ± 43.0 nM. When BzATP was used after carbachol, the response was significantly decreased to 68.9 ± 46.7 nM. Similar results were obtained when a submaximal concentration of carbachol (10−6 M) was used, though the difference in the before and after results did not reach statistical significance (Figs. 3C, 3D). Maximal concentrations of M3AChR and P2X7 receptor agonists showed that these agonists used overlapping mechanisms to increase [Ca2+]i. When the M3AChR agonist concentration was decreased, less overlap of [Ca2+]i mechanisms was obtained.
Figure 3.
Effect of sequential addition of carbachol and BzATP on [Ca2+]i in lacrimal gland acini. Time-dependent increase in [Ca2+]i induced by stimulation of lacrimal gland acini with cholinergic agonist carbachol (Cch, 10−5 M; first arrow) followed by BzATP (10−4 M; second arrow) or the reverse. Traces from four experiments (A). Mean change in peak [Ca2+]i from four experiments (B). Values are mean ± SEM. Time-dependent increase in [Ca2+]i induced by the stimulation of lacrimal gland acini in a similar experimental paradigm but with a decreased concentration of Cch (10−6 M) and the same concentration of BzATP. Traces from three experiments (C). Mean change in peak [Ca2+]i from three experiments (D). Values are mean ± SEM. Arrows: time of addition of agonists. *Significant difference from agonist added before or after the second agonist.
Results from both types of experimental paradigms were consistent with activation of the M3AChR and the P2X7 receptors using overlapping signaling pathways.
Cholinergic Agonists Activate P2X7 Receptors to Increase [Ca2+]i in Lacrimal Gland Acinar Cells
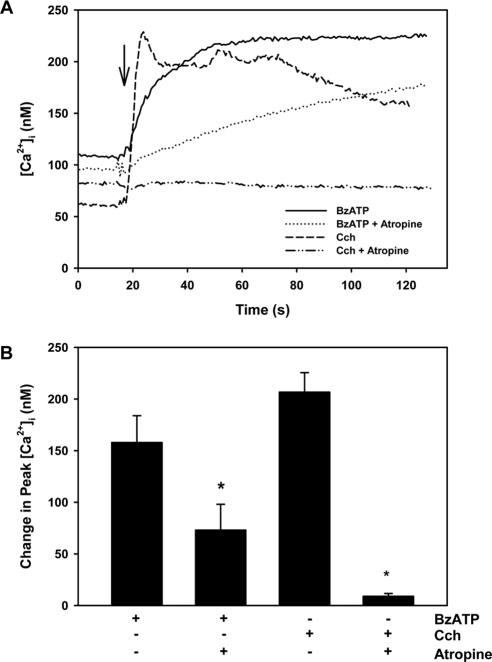
Activation of both cholinergic and P2X7 receptors increases the [Ca2+]i in lacrimal gland acinar cells. To determine whether these signaling pathways interact, we stimulated fura-2–loaded acinar cells with the P2X7 selective agonist BzATP after incubation with and without the cholinergic agonist atropine. BzATP at 10−4 M increased the [Ca2+]i by 157.8 ± 26.0 nM (Figs. 4A, 4B). Atropine (10−4 M) significantly inhibited the response to 73.0 ± 25.0 nM, suggesting that muscarinic receptor activation precedes P2X7 receptor stimulation. As a positive control, the cholinergic agonist carbachol (10−4 M) increased [Ca2+]i by 206.6 ± 18.9 nM, and atropine at 10−4 M completely blocked the carbachol response (Figs. 4A, 4B). These results suggest that most, but perhaps not the entire, BzATP-induced Ca2+ response was dependent on cholinergic agonist stimulation. Thus P2X7 receptor stimulation does not precede that of muscarinic receptors.
Figure 4.
Effect of cholinergic antagonist atropine on [Ca2+]i stimulated by BzATP or carbachol in lacrimal gland acini. Time-dependent increase in [Ca2+]i induced by stimulation of lacrimal gland acini with BzATP (10−4 M) or carbachol (Cch, 10−4 M) after a 30-minute preincubation with atropine (10−4 M). Traces from three to five experiments (A). Mean change in peak [Ca2+]i from three to five experiments (B). Values are mean ± SEM. Arrows: time of addition of agonists. *Significant difference between agonist alone and agonist plus antagonist.
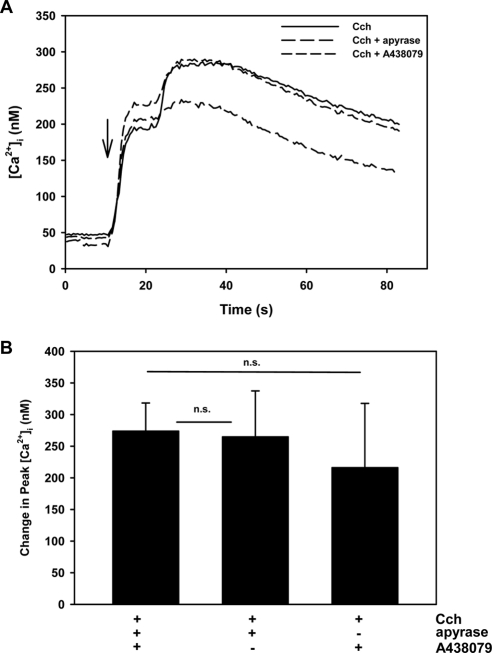
In separate experiments, cells were stimulated with the cholinergic agonist carbachol (10−5 M) with and without preincubation with the P2X7 antagonist A438079 (10−4 M). Carbachol increased the intracellular [Ca2+] by 273.7 ± 44.6 nM (Figs. 5A, 5B). A438079 did not alter the response. These data imply that the cholinergic agonist-induced [Ca2+]i response was not dependent on BzATP activation and that the muscarinic receptor activation preceded that of P2X7 receptors. These data also suggest that the activation of P2X7 receptors did not inhibit activation of the M3AChR-induced Ca2+ signaling pathway.
Figure 5.
Effect of P2X7 receptor antagonist A438079 and an inhibitor of ATPases, apyrase, on [Ca2+]i stimulated by carbachol in lacrimal gland acini. Time-dependent increase in [Ca2+]i induced by the stimulation of lacrimal gland acini with carbachol (Cch, 10−4 M) after a 30-minute preincubation with A438079 (10−4 M) or apyrase (20 U/mL). Traces from three to five experiments (A). Mean change in peak [Ca2+]i from three to five experiments (B). Values are mean ± SEM. Arrow: time of addition of agonist. *Significant difference between agonist alone and agonist plus antagonist or inhibitor. n.s., no significant difference.
Activation of PKC Isoforms Does Not Alter the P2X7 Receptor-Stimulated Increase in [Ca2+]i in Lacrimal Gland Acini
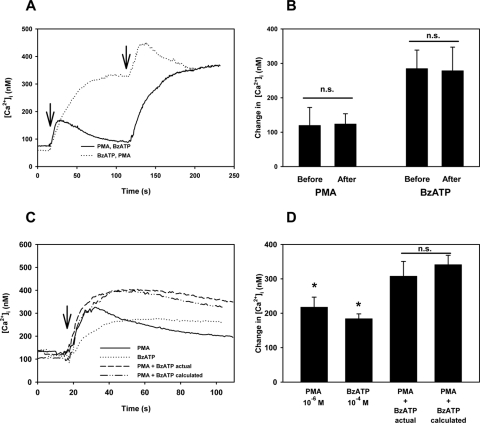
Given that the stimulation of M3AChR induces P2X7 receptor activity, we investigated the mechanism by which this occurs. Two possible mechanisms were the intracellular mediators produced by M3AChR stimulation activating the P2X7 receptor and the M3AChR-induced release of ATP into the extracellular space. We investigated one intracellular pathway stimulated by the activation of M3AChR—that is, the induction of PKC isoform activity. We first determined whether the activation of PKC isoforms alters the P2X7 receptor induced in [Ca2+]i by using PMA that activates conventional and novel, but not atypical, PKC isoforms. When acini were stimulated with PMA (10−6 M), [Ca2+]i was increased by 119.7 ± 52.1 nM (Figs. 6A, 6B). When BzATP (10−4 M) was added after PMA, the increase in [Ca2+]i was 278.7 ± 68.6 nM. When BzATP was added before PMA, the intracellular [Ca2+] was 284.9 ± 53.8 nM and was unchanged compared with the value obtained after the addition of PMA. Similarly, when PMA was added after BzATP, the response was unchanged compared with the value when PMA was added first. Another experimental paradigm with which to determine whether the activation of PKC isoforms altered the P2X7 receptor-induced Ca2+ response was to add PMA and BzATP simultaneously. PMA at 10−6 M increased [Ca2+]i by 217.9 ± 29.0 nM, and BzATP (10−4 M) raised the [Ca2+]i by 184.3 ± 13.8 nM (Figs. 6C, 6D). When PMA and BzATP were added simultaneously, the [Ca2+]i was increased to 307.9 ± 42.7 nM. The calculated activity was 341.4 ± 27.0 nM, a value not significantly different from the actual additive response.
Figure 6.
Effect of sequential and simultaneous addition of the PKC activator PMA and BzATP on [Ca2+]i in lacrimal gland acini. Time-dependent increase in [Ca2+]i induced by stimulation of lacrimal gland acini with PMA (10−6 M; first arrow) followed by BzATP (10−4 M; second arrow) or the reverse. Traces from four experiments (A). Mean change in peak [Ca2+]i from four experiments (B). Values are mean ± SEM. Time-dependent increase in [Ca2+]i induced by the stimulation of lacrimal gland acini with PMA (10−6 M), BzATP (10−4 M), or the two added simultaneously. Calculated [Ca2+]i response of the two agonists added simultaneously (C, D). Traces from three experiments (C). Mean change in peak [Ca2+]i from three experiments (D). Values are mean ± SEM. Arrow: time of carbachol addition. *Significant difference from no addition. n.s., no significant difference.
These data demonstrate that the activation of conventional and novel PKC isoforms does not alter the P2X7 receptor-induced Ca2+ response and that the activation of conventional and novel PKC isoforms and of P2X7 receptors use different signaling pathways to increase [Ca2+]i.
PKCα and PKCε Do Not Alter the P2X7 Receptor-Stimulated Increase in [Ca2+]i in Lacrimal Gland Acini
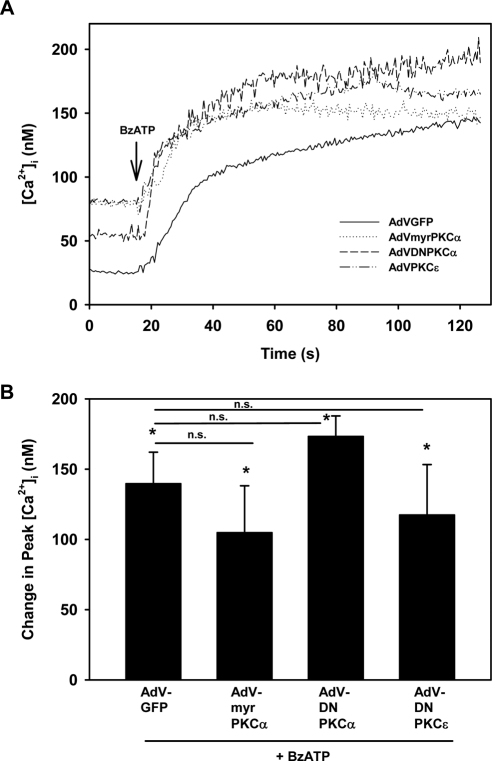
Multiple PKC isoforms are present in lacrimal gland acini, including conventional PKCα, novel PKCδ, and novel PKCε,17 and these PKC isoforms can have different, sometimes opposite, effects on function.3 Because PMA activates conventional and novel PKC isoforms, we sought to determine whether activation of the PKCα or inhibition of the PKCα or PKCε alters the P2X7 receptor effect on [Ca2+]i using adenoviruses that induced the expression of constitutively active PKCα (myrPKCα) or dominant-negative PKCα or PKCε. We previously showed that myrPKCα increased basal protein secretion from lacrimal gland acini,15 whereas dominant-negative PKCα and PKCε inhibited EGF-stimulated conjunctival goblet cell proliferation.18 None of the adenovirus constructs altered the basal [Ca2+]i (data not shown). The [Ca2+]i in nontransfected cells stimulated with BzATP (10−4 M) was 184.3 ± 13.8 nM (data not shown). In acinar cells transfected with a control adenovirus that expressed GFP and was stimulated with BzATP, the [Ca2+]i response was 139.6 ± 22.4 nM (Fig. 7). When compared with the response to the control adenovirus, neither the constitutive activation of PKCα nor the inhibition of PKCα altered the Ca2+ response to BzATP. Similarly, inhibiting PKCε did not alter the BzATP response. From these experiments, we concluded that neither the activation of PKCα nor the inhibition of PKCα or PKCε altered the BzATP-stimulated [Ca2+]i.
Figure 7.
Effect of constitutively active PKCα or dominant-negative PKCα or PKCε on [Ca2+]i stimulated by BzATP in lacrimal gland acini. Time-dependent increase in [Ca2+]i induced by stimulation of lacrimal gland acini with BzATP (10−4 M; arrow) after 24-hour preincubation with control adenovirus-producing GFP (AdV-GFP), adenovirus-producing constitutively active PKCα (AdV-myrPKCα), or adenovirus-producing dominant-negative PKCα or PKCε (AdV- DNPKCα and AdV-DNPKCε, respectively). Traces from three experiments (A). Mean change in peak [Ca2+]i from three experiments (B). Values are mean ± SEM. Arrow: time of carbachol addition. *Significant difference from no addition. n.s., no significant difference.
Results from three different experimental paradigms suggested that the activation of conventional and novel PKC isoforms did not affect the P2X7 receptor-induced increase in [Ca2+]i. Thus another mechanism was responsible for the M3AChR activation of P2X7 receptors.
Cholinergic Agonists Release ATP from Lacrimal Gland Pieces but Not from Acini
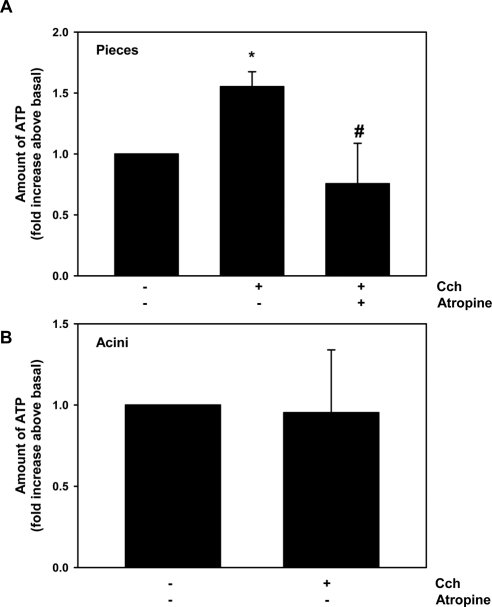
We sought to determine whether cholinergic agonists release ATP that could activate P2X7 receptors. Pieces of lacrimal gland were stimulated with no agonists (basal) or the cholinergic agonist carbachol (10−4 M). Basal ATP release was set to 1, and carbachol significantly increased the release 1.5 ± 0.1-fold (Fig. 8A). Preincubation with atropine at 10−4 M completely blocked carbachol-stimulated ATP release. To determine whether cholinergic agonists release ATP from acini, acini were stimulated with no agonists (basal) or carbachol at 10−4 M. Carbachol did not alter ATP release compared with basal, which was set to 1 (Fig. 8B). To confirm that cholinergic agonists did not stimulate ATP release from acini, acini were preincubated with apyrase, which breaks down extracellular ATP into AMP that can be converted to adenosine and should thus prevent cholinergic agonist activation of P2X7 receptors. Apyrase did not alter the carbachol-stimulated increase in [Ca2+]i from acinar cells (Figs. 5A, 5B). We conclude that cholinergic agonists release ATP from lacrimal gland pieces that contain nerve endings, acinar cells, myoepithelial cells, and duct cells but not from acini that are composed of acinar and myoepithelial cells. Thus M3AChR do not release ATP from acini to activate P2X7 receptors but do release it when other cell types are present. M3AChR could release ATP to activate P2X7 in vivo and thus have a physiological function. The M3AChR release of ATP, however, did not activate P2X7 receptors in the current experiments that used acini. Hence, another mechanism is responsible.
Figure 8.
Effect of carbachol on ATP release from lacrimal gland pieces or acini. Release of ATP from lacrimal gland pieces (A) or acini (B) stimulated for 10 minutes with carbachol (Cch, 10−4 M) with or without a 30-minute preincubation with the cholinergic antagonist atropine (10−4 M). Values are the level of ATP release from three experiments. Values are mean ± SEM. *Significant difference from no additions. #Significant difference from Cch.
Cholinergic Agonists Activate P2X7 Receptors to Stimulate Protein Secretion from Lacrimal Gland Acinar Cells
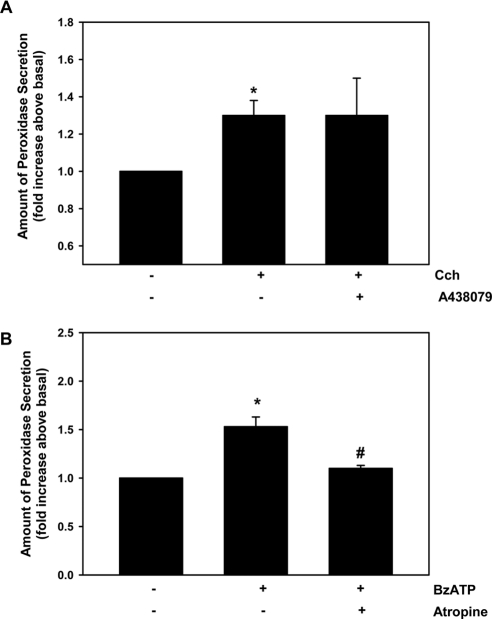
Given that cholinergic agonists activate P2X7 receptors to increase the [Ca2+]i, we sought to determine whether this effect was extended to the entire signaling pathway, resulting in protein secretion. Lacrimal gland acini were stimulated with the cholinergic agonist carbachol at 10−4 M, which increased secretion by 1.3 ± 0.1-fold (Fig. 9A). Preincubation with the P2X7 inhibitor A438079 (10−4 M) did not alter carbachol-stimulated secretion. Lacrimal gland acini were stimulated with BzATP at 10−4 M, and protein secretion was measured. BzATP significantly stimulated protein secretion by 1.5 ± 0.1-fold (Fig. 9B). When cells were preincubated with the cholinergic antagonist atropine (10−4 M), BzATP-stimulated secretion was almost completely blocked. As for the increase in [Ca2+]i, the activation of P2X7 receptors did not alter cholinergic agonist-induced secretion. In contrast, the activation of M3AChR induced P2X7 receptors to stimulate lacrimal gland protein secretion.
Figure 9.
Effect of cholinergic and P2X7 receptor antagonists on protein secretion stimulated by carbachol or BzATP from lacrimal gland acini. Peroxidase secretion, our index of protein secretion, was measured from lacrimal gland acini preincubated with the P2X7 receptor antagonist A438079 (10−4 M) for 30 minutes and then stimulated with carbachol (Cch) (10−4 M) for 40 minutes (A) or preincubated with the cholinergic antagonist atropine (10−4 M) for 30 minutes and then stimulated with BzATP (10−4 M) for 40 minutes (B). Values are mean ± SEM from four independent experiments. *Significant difference from no additions. #Significant difference from agonist.
Cholinergic and P2X7 Receptor Agonists Activate Different Cellular Pathways to Stimulate Protein Secretion from Lacrimal Gland Acini
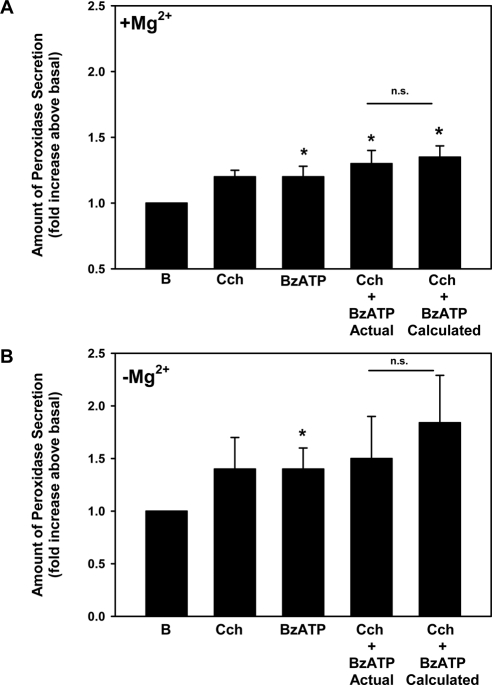
Protein secretion was measured from acini stimulated simultaneously with carbachol and BzATP at maximal concentrations for secretion. Carbachol (10−4 M) and BzATP (10−4 M) each increased protein secretion (Fig. 10A). When the two agonists were added simultaneously, the increase in protein secretion was not significantly different from the calculated additivity. Similar results were obtained when this experiment was performed in the absence of Mg2+ to increase the P2X7 receptor response (Fig. 10B). These results suggest that M3AChR and P2X7 receptors use different cellular pathways to stimulate protein secretion, unlike their effect on [Ca2+]i.
Figure 10.
Effect of simultaneous addition of carbachol and BzATP on protein secretion from lacrimal gland acini. Peroxidase secretion, our index of protein secretion, was measured from lacrimal gland acini stimulated for 40 minutes with carbachol (Cch, 10−4 M), BzATP (10−4 M), or the two agonists added simultaneously. The calculated amount of secretion from the two agonists added simultaneously is also plotted. Results obtained in the presence (A) and absence (B) of extracellular Mg2+. Values are mean ± SEM from four independent experiments. *Significant difference from no addition. n.s., no significant difference.
Discussion
In the present study, we demonstrated that one mechanism by which cholinergic agonists stimulate lacrimal gland protein secretion is to activate P2X7 receptors. In this tissue, cholinergic agonists activate P2X7 receptors to increase the [Ca2+]i and stimulate secretion. There are multiple mechanisms by which activated muscarinic agonists can stimulate P2X7 receptors. The mechanisms include the release of ATP, the production of intracellular signaling compounds, and the direct interaction of receptors. We found that cholinergic agonists did not release ATP from acini in which cholinergic antagonists decrease the activity of the P2X7 receptors. In exocrine glands, ATP, which stimulates P2X7 receptors, can come from multiple sources, including nerves, myoepithelial cells, acinar cells, and ductal cells. In the present study, the activation of muscarinic receptors caused the release of ATP from lacrimal gland pieces, but not from acini, implying that either nerves or duct cells are the source of ATP. The data indicating the lack of ATP release from acini are robust. Two different methods were used to investigate ATP release, and a positive control (phenylephrine, data not shown) induced ATP release. Our finding was in agreement with the results of Novak et al.,19 who found similar results from their preparation that included isolated acini and small ducts. However, the release of ATP did not account for cholinergic activation of P2X7 receptors when acini alone were used, as in the present study.
The second mechanism by which cholinergic agonists can activate or alter the activity of the P2X7 receptor is the production of second messengers that would directly interact with the intracellular domains of the P2X7 receptor or the P2X7-induced production of signaling intermediates. G-protein–coupled receptors often alter P2X7 activity by the activation of PKC or the release of Ca2+ from intracellular stores. All P2X receptor types have a consensus site for PKC phosphorylation on the intracellular C terminus.20 Brown and Yule21 found, however, that PKC catalytic subunits did not phosphorylate P2X3, P2X4, or P2X7 receptors in transfected cells. However, Faria et al.22 found that the inhibition of PKC as well as MAPK, PI3K, and cytoskeletal proteins blocked ATP induction of large conductance pores. Using multiple experimental paradigms, we found that the activation of PKC isoforms by phorbol esters, constitutively active PKCα, dominant-negative PKCα, or dominant-negative PKCε did not alter the P2X7 receptor–stimulated increase in [Ca2+]i in lacrimal gland acini. Thus it appears that modulation of the P2X7 receptors by PKC isoforms activated by cholinergic agonists does not lead to activation of the P2X7 receptors to increase [Ca2+]I in lacrimal gland acini. Alteration of the P2X7 receptor by other signaling compounds or by direct interaction with the M3AChRs (a third mechanism for activation) has yet to be tested.
We found that cholinergic- and P2X7 agonist-stimulated increases in [Ca2+]i were less than additive because these agonists use overlapping cellular pathways. Cholinergic agonists release Ca2+ from intracellular stores using InsP3.2 Depletion of this intracellular pool activates Ca2+ influx using Orai and STIM 1 to replenish the depleted Ca2+ pool.23 BzATP-induced activation of P2X7 receptors does not release intracellular Ca2+ stores, but it does activate Ca2+ influx because it is itself a cation selective ion channel. Even though M3AChR and P2X7 receptors activate Ca2+ influx by different mechanisms, these mechanisms appear to interact to account for the less than additive Ca2+ response. The M3AChR and P2X7 receptors could be physically close, but this was not tested in the present study. The less than additive Ca2+ response does not appear to be inhibition of the M3AChR by activated P2X7 receptors because a P2X7 receptor inhibitor does not block cholinergic agonist-induced increases in [Ca2+]i.
In contrast to their regulation of [Ca2+]i, cholinergic agonists increase protein secretion by an additional pathway not used by the P2X7 receptors, thereby accounting for secretion that is additive in the presence of both agonists. In addition to activating P2X7 receptors to increase [Ca2+]I and cause secretion, cholinergic agonists activate PLCβ to produce InsP3, which releases intracellular Ca2+ stores, and to produce diacylglycerol, which activates PKCα, PKCδ, and PKCε, all of which stimulate secretion.2,3 We found that P2X7 receptors do not use PKC isoforms to increase [Ca2+]i and that PKC isoforms do not alter the P2X7 receptor, suggesting that M3AChR activates PKC isoforms but that P2X7 receptors do not, and this could account for the additivity of secretion.
The interaction between P2X7 and muscarinic agonists has been studied in the three types of exocrine glands—lacrimal, pancreas, and salivary. In the only other study on the lacrimal gland, Novak et al.19 found that cholinergic stimulation leads to the release of ATP, which activates P2X7 receptors and downregulates the volume of tears produced. The volume of tears indicates electrolyte and water secretion and could come from sources other than the lacrimal gland, although most of the ACh-stimulated fluid secretion is likely from the lacrimal gland. Novak et al.19 also demonstrated that in the lacrimal gland, cholinergic agonist-stimulated increases in [Ca2+]i and fluid (tear) secretion were higher in P2X7−/− than in wild-type mice.19 The effect of P2X7 receptors was sex dependent because the effect occurred in male, but not in female, mice. Novak et al.19 concluded that cholinergic agonists release ATP from lacrimal gland acini and small ducts that activate P2X7 receptors and downregulate tear secretion.19 In the present study, which used male rat lacrimal glands, activation of the P2X7 receptors did not alter M3AChR-stimulated increases in [Ca2+]i or protein secretion because the P2X7 antagonist did not alter muscarinic agonist–stimulated functions and cholinergic agonists did not release ATP from acini. Similar to the Novak et al.19 study, in preparations that contained duct cells, our results showed that cholinergic agonists caused ATP release. However, in contrast to the Novak et al.19 study, we found that the activation of muscarinic receptors stimulated the P2X7 receptor to increase [Ca2+]i and to induce protein secretion. The difference between the results of the Novak et al.19 study and the present study could be species dependent (rat vs. mouse), function dependent (tear volume [electrolyte and water] vs. lacrimal gland protein secretion), or age dependent (8-week-old rats vs. 20- to 40-week-old mice).19 Given that P2X7 receptors exist as multiple splice variants, the mechanisms of interaction and activation of the P2X7 receptor could vary among tissues, species, and sexes.24
The results of the present study also differed from those of studies on the salivary glands in which activation of P2X7 receptors inhibited the muscarinic responses. Jorgensen et al.25 used ATP to activate P2z (now termed P2X7) receptors in rat parotid gland and found that ATP reduced the acetylcholine (ACh)-induced increase in [Ca2+]i and production of InsP3. In the Jorgensen 19 study, however, ATP increased InsP3 production and mobilized [Ca2+]i. Thus ATP was probably activating P2Y in addition to P2X7 receptors and not specifically activating P2X7 receptors. Fukushi,26 using rat parotid acinar cells, found that the activation of P2X7 receptors with BzATP blocked the ACh-induced Ca2+ response with increasing effect as the preincubation time with BzATP increased. A maximum effect occurred with a 60-second preincubation. The conclusion was that BzATP itself did not increase [Ca2+]i and that the activation of P2X7 receptors with BzATP induced phospholipase D to stimulate novel (Ca2+-independent) PKC isoforms that desensitized the muscarinic receptor.26 Nakamoto et al.27 found that ATP activation of P2X7 receptors stimulated fluid secretion from the mouse submandibular gland. In wild-type mice, activation of the P2X7 receptor with ATP partially inhibited muscarinic agonist-induced fluid secretion. The inhibition was prevented in P2X7−/− mice that lacked functioning P2X7 receptors. In contrast, Novak et al.19 using P2X7−/− mice, found that cholinergic stimulation led to the release of ATP to activate P2X7 receptors that upregulated salivary gland fluid secretion. Thus the results of the present study agree with those of Novak et al.19 on the salivary gland and pancreas but not on the lacrimal gland or the other studies on the salivary gland. Because ours is the only study measuring protein secretion, it is possible that the regulation of fluid and protein secretion by P2X7 receptors differs.
We conclude that in the male rat lacrimal gland, activation of the M3AChR stimulates P2X7 receptors to increase the [Ca2+]i and to induce protein secretion. The mechanisms of activation have yet to be clarified but could include ATP release when multiple cell types are present. In addition, M3AChR uses signaling pathways that overlap with those used by P2X7 receptors to increase [Ca2+]I, but M3AChR uses an additional signaling pathway to stimulate protein secretion.
Footnotes
Supported by National Institutes of Health Grant EY06177.
Disclosure: D.A. Dartt, None; R.R. Hodges, None
References
- 1. Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28(3):155–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dartt DA, Dicker DM, Ronco LV, et al. Lacrimal gland inositol trisphosphate isomer and inositol tetrakisphosphate production. Am J Physiol. 1990;259(2 pt 1):G274–G281 [DOI] [PubMed] [Google Scholar]
- 3. Zoukhri D, Hodges RR, Sergheraert C, Toker A, Dartt DA. Lacrimal gland PKC isoforms are differentially involved in agonist-induced protein secretion. Am J Physiol. 1997;272(1 pt 1):C263–C269 [DOI] [PubMed] [Google Scholar]
- 4. Hodges RR, Shatos MA, Tarko RS, et al. Nitric oxide and cGMP mediate alpha1D-adrenergic receptor-stimulated protein secretion and p42/p44 MAPK activation in rat lacrimal gland. Invest Ophthalmol Vis Sci. 2005;46(8):2781–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hodges RR, Zoukhri D, Sergheraert C, Zieske JD, Dartt DA. Identification of vasoactive intestinal peptide receptor subtypes in the lacrimal gland and their signal-transducing components. Invest Ophthalmol Vis Sci. 1997;38(3):610–619 [PubMed] [Google Scholar]
- 6. Dartt DA, Baker AK, Vaillant C, Rose PE. Vasoactive intestinal polypeptide stimulation of protein secretion from rat lacrimal gland acini. Am J Physiol. 1984;247(5 pt 1):G502–G509 [DOI] [PubMed] [Google Scholar]
- 7. Meneray MA, Fields TY. Adrenergic stimulation of lacrimal protein secretion is mediated by G(q/11)alpha and G(s) alpha. Curr Eye Res. 2000;21(2):602–607 [PubMed] [Google Scholar]
- 8. Lemullois M, Rossignol B, Mauduit P. Immunolocalization of myoepithelial cells in isolated acini of rat exorbital lacrimal gland: cellular distribution of muscarinic receptors. Biol Cell. 1996;86(2–3):175–181 [DOI] [PubMed] [Google Scholar]
- 9. Smyth JT, Hwang SY, Tomita T, et al. Activation and regulation of store-operated calcium entry. J Cell Mol Med. 2010;14(10):2337–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gilbard JP, Dartt DA. Changes in rabbit lacrimal gland fluid osmolarity with flow rate. Invest Ophthalmol Vis Sci. 1982;23(6):804–806 [PubMed] [Google Scholar]
- 11. Hodges RR, Vrouvlianis J, Shatos MA, Dartt DA. Characterization of P2X7 purinergic receptors and their function in rat lacrimal gland. Invest Ophthalmol Vis Sci. 2009;50(12):5681–5689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burnstock G, Fredholm BB, North RA, Verkhratsky A. The birth and postnatal development of purinergic signalling. Acta Physiol (Oxf). 2010;199(2):93–147 [DOI] [PubMed] [Google Scholar]
- 13. Dartt DA, Ronco LV, Murphy SA, Unser MF. Effect of phorbol esters on rat lacrimal gland protein secretion. Invest Ophthalmol Vis Sci. 1988;29(11):1726–1731 [PubMed] [Google Scholar]
- 14. Funaki C, Hodges RR, Dartt DA. Role of cAMP inhibition of p44/p42 mitogen-activated protein kinase in potentiation of protein secretion in rat lacrimal gland. Am J Physiol Cell Physiol. 2007;293(5):C1551–C1560 [DOI] [PubMed] [Google Scholar]
- 15. Hodges RR, Raddassi I, Zoukhri D, et al. Effect of overexpression of constitutively active PKCalpha on rat lacrimal gland protein secretion. Invest Ophthalmol Vis Sci. 2004;45(11):3974–3981 [DOI] [PubMed] [Google Scholar]
- 16. Jorgensen NK, Petersen SF, Hoffmann EK. Thrombin-, bradykinin-, and arachidonic acid-induced Ca2+ signaling in Ehrlich ascites tumor cells. Am J Physiol. 1999;276(1 pt 1):C26–C37 [DOI] [PubMed] [Google Scholar]
- 17. Zoukhri D, Hodges RR, Willert S, Dartt DA. Immunolocalization of lacrimal gland PKC isoforms: effect of phorbol esters and cholinergic agonists on their cellular distribution. J Membr Biol. 1997;157(2):169–175 [DOI] [PubMed] [Google Scholar]
- 18. Shatos MA, Hodges RR, Oshi Y, et al. Role of cPKCalpha and nPKCepsilon in EGF-stimulated goblet cell proliferation. Invest Ophthalmol Vis Sci. 2009;50(2):614–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Novak I, Jans IM, Wohlfahrt L. Effect of P2X(7) receptor knockout on exocrine secretion of pancreas, salivary glands and lacrimal glands. J Physiol. 2010;588(pt 18):3615–3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roberts JA, Vial C, Digby HR, et al. Molecular properties of P2X receptors. Pflugers Arch. 2006;452(5):486–500 [DOI] [PubMed] [Google Scholar]
- 21. Brown DA, Yule DI. Protein kinase C regulation of P2X3 receptors is unlikely to involve direct receptor phosphorylation. Biochim Biophys Acta. 2007;1773(2):166–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Faria RX, Cascabulho CM, Reis RA, Alves LA. Large-conductance channel formation mediated by P2X7 receptor activation is regulated through distinct intracellular signaling pathways in peritoneal macrophages and 2BH4 cells. Naunyn Schmiedebergs Arch Pharmacol. 2010;382(1):73–87 [DOI] [PubMed] [Google Scholar]
- 23. Smyth JT, Dehaven WI, Bird GS, Putney JW., Jr Ca2+-store-dependent and -independent reversal of Stim1 localization and function. J Cell Sci. 2008;121(pt 6):762–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheewatrakoolpong B, Gilchrest H, Anthes JC, Greenfeder S. Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem Biophys Res Commun. 2005;332(1):17–27 [DOI] [PubMed] [Google Scholar]
- 25. Jorgensen TD, Gromada J, Tritsaris K, Nauntofte B, Dissing S. Activation of P2z purinoceptors diminishes the muscarinic cholinergic-induced release of inositol 1,4,5-trisphosphate and stored calcium in rat parotid acini: ATP as a co-transmitter in the stimulus-secretion coupling. Biochem J. 1995;312(pt 2):457–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fukushi Y. Heterologous desensitization of muscarinic receptors by P2Z purinoceptors in rat parotid acinar cells. Eur J Pharmacol. 1999;364(1):55–64 [DOI] [PubMed] [Google Scholar]
- 27. Nakamoto T, Brown DA, Catalan MA, et al. Purinergic P2X7 receptors mediate ATP-induced saliva secretion by the mouse submandibular gland. J Biol Chem. 2009;284(8):4815–4822 [DOI] [PMC free article] [PubMed] [Google Scholar]