In this study, the authors characterize the expression of the microRNA-29 family in the trabecular meshwork and identify it as a critical node in the regulation of ECM gene networks under basal and TGF-β2 stimulatory conditions.
Abstract
Purpose.
The microRNA-29 (miR-29) family has emerged, in various tissues, as a key modulator of extracellular matrix (ECM) homeostasis. In this study, the authors investigate the role of the miR-29 family in the regulation of ECM synthesis in the trabecular meshwork (TM) under basal and TGF-β2 stimulatory conditions.
Methods.
Human TM cells were incubated with 2.5 ng/mL activated, recombinant human TGF-β2 for 24, 48, and 72 hours. A specific pharmacologic inhibitor was used to block SMAD3 function in the context of TGF-β2 stimulation. Changes in the expression of the miR-29 family were assessed by real-time PCR. The effect of miR-29 molecules and inhibitors on ECM levels was determined by immunoblot analysis.
Results.
All three members of the miR-29 family were expressed in cultured TM cells. Although the incubation of TM cells with TGF-β2 induced miR-29a and suppressed miR-29b levels, no significant effect was observed on miR-29c expression. Additional studies revealed that SMAD3 modulates miR-29b expression under basal and TGF-β2 conditions. Subsequent gain- and loss-of-function experiments demonstrated that the miR-29 family functions as a critical suppressor of various ECM proteins under basal and TGF-β2 stimulatory conditions.
Conclusions.
The findings derived from this study identify the miR-29 family as a critical regulator of ECM expression in the TM and suggest that its modulation by TGF-β2 may be important in controlling ECM synthesis. Together, these data provide further insight into the complex regulatory mechanisms mediating TGF-β2 signaling and ECM production in the TM.
Primary open-angle glaucoma (POAG) is a chronic, degenerative optic neuropathy that represents one of the leading causes of blindness worldwide.1,2 In the United States, glaucoma is the leading cause of preventable blindness in persons of Latino or African descent.3,4 Despite modern treatment, the estimated risk of blindness from glaucoma over a 15- to 20-year period is between 7% and 24%.5,6 Even in patients with impaired vision, but not blindness, vision loss can have profound consequences on daily functioning. For example, the extent of visual field loss from glaucoma correlates to both an increased risk of a motor vehicle accident and a fall with injury.7,8 Recent studies suggest that the number of Americans with open-angle glaucoma will increase by 50% to 4.2 million by the year 2020.9
The progressive optic nerve damage seen in glaucoma results from an elevation of intraocular pressure (IOP) beyond the structural and vascular capabilities of the optic nerve to withstand. IOP is determined by the balance between aqueous inflow and outflow. In POAG, elevated IOP results from impaired aqueous outflow.10 Normally, between 80% and 90% of aqueous outflow occurs through the trabecular meshwork (TM), with the remaining 10% to 20% occurring through the ciliary body face.11,12 The TM is the critical tissue for the regulation of aqueous outflow, and its dysregulation causes elevated IOP.13 The tissue that includes the inner wall of Schlemm's canal and the juxtacanalicular (JCT) region of the TM is the anatomic location of highest outflow resistance.14 The JCT region is an amorphous and dynamic layer composed of endothelial cells and extracellular matrix (ECM). Alterations in the balance between ECM synthesis and breakdown influence aqueous outflow.15–19 Hence, a rigorous understanding of the molecular mechanisms involved in the control of ECM homeostasis is pivotal for the development of new therapies for POAG.
The transforming growth factor-beta (TGF-β) family of cytokines functions as an important regulator of wound healing and ECM synthesis.20 One such isoform, TGF-β2, has been shown in several studies to be elevated in the aqueous humor of POAG patients.21–24 Tripathi et al.24 report total aqueous humor TGF-β2 levels (mean ± SD) to be 1.48 ± 0.68 ng/mL in healthy human subjects compared with 2.70 ± 0.76 ng/mL in age-matched POAG patients. In addition, the authors show that aqueous humor levels of intrinsically active TGF-β2 were elevated in POAG patients (0.45 ± 0.28 ng/mL) compared with age-matched healthy subjects (0.20 ± 0.24 ng/mL).24 Subsequent work has since demonstrated that TGF-β2 decreases aqueous humor outflow facility, which, in turn, elevates IOP.20,25,26 One possible mechanism for the decrease in outflow facility may involve the associated TGF-β2–mediated increase in ECM accumulation in the TM.20,25,26 Specifically, TGF-β2 has been reported to stimulate the expression of fibronectin, thrombospondin-1, laminin, collagen I, and collagen IV, key ECM components in the TM.26–29
MicroRNAs are small (∼22 nucleotides), single-stranded RNAs that modulate the posttranscriptional expression of genes.30 These short RNAs are predicted to regulate as many as 30% of human mRNA transcripts, with a given microRNA potentially binding up to 200 gene targets.31 Recent studies have suggested a critical involvement of microRNAs in the development of tissue fibrosis, with the microRNA-29 (miR-29) family serving as an important mediator of these effects.32–34 In nonocular human tissues, the miR-29 family regulates a number of ECM proteins, including SPARC (secreted protein, acidic, and rich in cysteine), laminin, collagen I, and collagen IV.32–37 Together, these data suggest that miR-29 may function as a node in the regulation of ECM homeostasis. The influence of microRNAs, and in particular the miR-29 family, in the development of elevated IOP remains unknown. To this end, we examined the importance of the miR-29 family in the TM, with a specific focus on its involvement in the regulation of ECM production under basal and TGF-β2 stimulatory conditions.
Materials and Methods
Trabecular Meshwork Cell Culture
Trabecular meshwork (TM) endothelial cells, in accordance with the Declaration of Helsinki, were isolated and cultured as previously described.38 Sixteen independent primary human TM cell lines were generated from 16 donors ranging in age from 9 to 74 years with no known history of ocular disease. Unless otherwise stated, cell cultures were maintained in Dulbecco's modified Eagle's medium (Invitrogen, Grand Island, NY) containing 20% fetal bovine serum, 1% l-glutamine (2 mM), and gentamicin (0.1 mg/mL) at 37°C in 10% CO2 atmosphere. All experiments used TM cells from passage 4 to 5 cultures. For each experimental paradigm, n refers to the number of independent experiments performed using different primary human TM cell lines.
RNA Isolation and Real-Time PCR
Total RNA was isolated as described by the manufacturer (TRIzol; Invitrogen, Carlsbad, CA). After pellet resuspension, RNA was treated with RQ1 RNase-free DNase I (Promega, Madison, WI) to remove DNA contaminants. RNA concentration and purity were assessed by spectrophotometry. Total RNA (10 ng) was reverse transcribed into cDNA using a microRNA reverse transcription kit (TaqMan; Ambion, Austin, TX) with specific, validated primers (TaqMan) for mature microRNA-29a/b/c and RNU6B (Applied Biosystems, Foster City, CA). Quantitative real-time PCR for mature microRNA-29a/b/c and internal control RNU6B was then performed according to the manufacturer's instructions for microRNA assays (TaqMan; Applied Biosystems). Levels of microRNA-29a/b/c were normalized to RNU6B using the formula 2-ΔCt. Relative fold changes in microRNA expression between control and experimental treatment groups was determined using the 2-ΔΔCt method.39
TGF-β2 Time Course Experiments
TM endothelial cells were plated and grown to 100% confluence. Two days after confluence, the cells were incubated for 24, 48, or 72 hours in serum-free media containing either 2.5 ng/mL activated, recombinant human TGF-β2 (R&D Systems, Minneapolis, MN) or 4 mM HCl solution containing 0.1% human serum albumin as vehicle. For the 48- and 72-hour time points, the media were changed every 24 hours.
SMAD3 Inhibition Experiments
Two days after confluence, TM cells were incubated for 2 hours with the SMAD3 inhibitor SIS3 (10 μM; Sigma-Aldrich, St. Louis, MO) or with vehicle (dimethyl sulfoxide [DMSO] 0.0907% vol/vol). Cells were then treated for 24 hours with 2.5 ng/mL activated, recombinant human TGF-β2 or 4 mM HCl solution containing 0.1% human serum albumin as vehicle.
MicroRNA Transfection Experiments
Transfection experiments were conducted as previously described,40 with minor modifications. For microRNA overexpression experiments, TM endothelial cells were transfected at 50% to 70% confluence with human pre-miR miRNA 29a/b/c precursors (50 nM; Ambion) or pre-miR miRNA negative control precursor molecules (50 nM; AM17110, Ambion) using oligofectamine (Invitrogen). Pre-miR miRNA 29a/b/c precursor molecules were used to mimic the respective endogenous mature microRNAs. Pre-miR miRNA negative control precursor molecules are double-stranded scrambled RNA oligonucleotides. At 24 hours after transfection, cells were washed twice with PBS and incubated in serum-free media for an additional 24 hours with either 2.5 ng/mL activated, recombinant human TGF-β2 or 4 mM HCl solution containing 0.1% human serum albumin as vehicle. Transfection efficiency for the microRNA overexpression experiments was 65% ± 6% (mean ± SD), as assessed using Cy3 dye-labeled pre-miR miRNA negative control precursor molecules (50 nM; AM17120; Ambion). For microRNA inhibitor experiments, TM cells were transfected at 35% to 55% confluence with human microRNA 29a/b/c hairpin inhibitors (miRIDIAN, 75 nM; Thermo Scientific, Lafayette, CO) or microRNA negative control hairpin inhibitors (miRIDIAN, 75 nM; IN-001005–01; Thermo Scientific) using oligofectamine. The microRNA (miRIDIAN; Thermo Scientific) 29a/b/c hairpin inhibitors suppress the function of their respective endogenous mature microRNAs by a proprietary design. The microRNA (miRIDIAN; Thermo Scientific) negative control hairpin inhibitor is based on the Caenorhabditis elegans miR-67 sequence and is validated to contain minimal sequence identity with known human, mouse, or rat microRNAs. At 24 hours after transfection, cells were washed twice with PBS and incubated in serum-free media for an additional 24 hours. Transfection efficiency for the microRNA inhibitor experiments was 44% ± 4% (mean ± SD), as assessed using Dy547-labeled microRNA negative control hairpin inhibitors (miRIDIAN, 75 nM; IP-004500–01; Thermo Scientific).
Immunoblot Analysis
After completion of the respective experiments, conditioned media from TM cell cultures were removed and centrifuged at 2000g for 10 minutes at 4°C. The supernatant was collected, concentrated (Amicon Ultra-4 Filter Unit, 10 kDa; Millipore, Milford, MA), and protein content quantified (DC Protein Assay; Bio-Rad, Hercules, CA). Equal amounts of protein were mixed with 2× reducing buffer and boiled for 5 minutes, with the exception of samples analyzed for collagen types I and IV, which were mixed with 2× nonreducing buffer and were not boiled. Samples were then electrophoresed in 8% SDS-polyacrylamide gels, and proteins were transferred to nitrocellulose membranes (0.45-μm pore size; Invitrogen). The membranes were incubated for 1 hour at room temperature in a 1:1 mixture of 1× TBS-T (20 mM Tris-HCl [pH 7.6], 137 mM NaCl, 0.1% Tween-20) and blocking buffer (Rockland, Inc., Gilbertsville, PA), followed by overnight incubation at 4°C with the respective primary antibody. The dilutions used for the primary antibodies were 1:10,000 for SPARC (AON-5031; Hematologic Technologies Inc., Essex Junction, VT); 1:1000 for Collagen Type I (600–401-103; Rockland Inc.); 1:1000 for Collagen Type IV (600–401-106; Rockland Inc.); 1:1000 for thrombospondin-1 (AF3074; R&D Systems, Minneapolis, MN); 1:200 for laminin (recognizes laminin B2 chain; L8271; Sigma-Aldrich); and 1:2000 for fibronectin (F3648; Sigma-Aldrich). After the overnight primary antibody incubation, the membranes were washed three times with 1× TBS-T and incubated for 1 hour at room temperature with dye-conjugated affinity purified anti-mouse, anti-goat, or anti-rabbit IgG antibodies (IRDye 800; 1:10,000 dilution; Rockland Inc., Gilbertsville, PA). The membranes were then washed three times with 1× TBS-T and scanned, and band densities were quantified using an infrared imaging system (Odyssey; Li-Cor, Lincoln, NE).
Statistical Analysis
Data were analyzed using statistical software (GraphPad Prism 5; GraphPad, La Jolla, CA). For comparisons between two groups, a two-tailed Student's t-test was used. Comparisons between multiple groups were made using one-way ANOVA followed by Tukey's HSD post hoc test. Differences were considered statistically significant for P < 0.05.
Results
miR-29 Paralogs Are Expressed in the Trabecular Meshwork
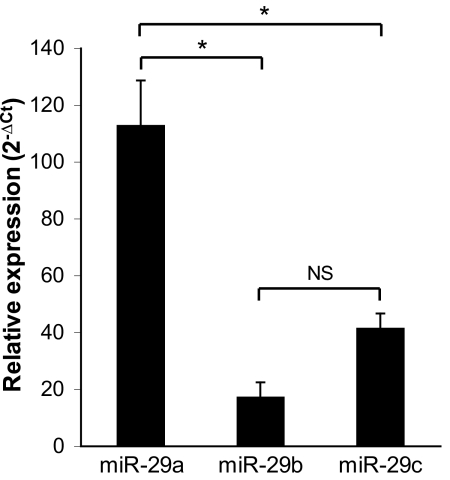
The human miR-29 family consists of three paralogs: miR-29a, miR-29b, and miR-29c. To determine the potential involvement of this family in the TM, we first characterized its expression in cultured TM cells. Real-time PCR analysis revealed the presence of all three members of the miR-29 family in the TM (Fig. 1). Among the family members, miR-29a exhibited the highest relative expression. Specifically, miR-29a levels were 6.7-fold and 2.7-fold greater than those of miR-29b and miR-29c, respectively. Although the average relative basal expression of miR-29c was 2.4-fold greater than that of miR-29b, this result was not statistically significant.
Figure 1.
Relative expression of the miR-29 family in the TM. Mature levels of human miR-29a, miR-29b, and miR-29c were measured in cultured human TM cells by real-time quantitative PCR and were normalized to internal control RNU6B using the formula 2-ΔCt. All data are expressed as the mean ± SEM (*P < 0.001; n = 10, where n refers to the number of independent experiments performed using n different primary human TM cell strains).
TGF-β2 Differentially Regulates miR-29a and miR-29b Expression
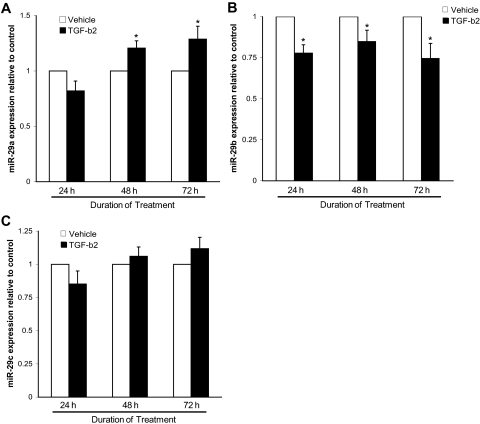
Recent studies have demonstrated a pivotal role for the miR-29 family in the modulation of ECM production.32–37,41 To determine whether the miR-29 family functions as an important regulator of TGF-β2–mediated ECM synthesis, miR-29 expression was analyzed after 24, 48, and 72 hours of incubation of TM cells with TGF-β2. Real-time quantitative PCR analysis of miR-29a levels revealed a statistically significant induction by TGF-β2 at 48 and 72 hours (Fig. 2A). In contrast, TGF-β2 treatment significantly reduced miR-29b expression at 24, 48, and 72 hours (Fig. 2B). Analysis of miR-29c levels showed no significant changes after TGF-β2 treatment (Fig. 2C).
Figure 2.
TGF-β2 regulates miR-29 family expression. The levels of mature (A) miR-29a, (B) miR-29b, and (C) miR-29c were quantified by real-time PCR in human TM cells cultured for 24, 48, and 72 hours with either activated human recombinant TGF-β2 (2.5 ng/mL) or HCl vehicle. All data are expressed as the mean ± SEM (*P < 0.05 vs. its time-corresponding vehicle control; n = 9, where n refers to the number of independent experiments performed using n different primary human TM cell strains).
SMAD3 Is Necessary for mIR-29b Expression under TGF-β2 Stimulatory Conditions
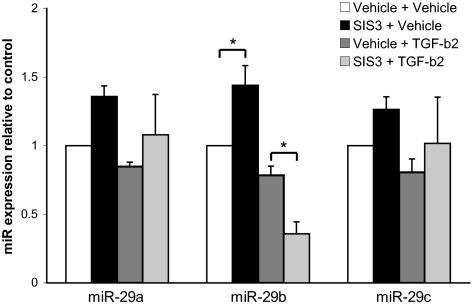
The SMAD3 transcription factor functions as a critical mediator of TGF-β signaling and tissue fibrosis.42–45 Our laboratory and others have reported the involvement of the TGF-β2/SMAD3 signaling pathway in the synthesis of ECM in the TM (Kang M, et al. IOVS 2010;51:ARVO E-Abstract 6100; Sethi A et al. IOVS 2010;51:ARVO E-Abstract 5836). Specifically, our laboratory has demonstrated that SMAD3 inhibition suppresses the induction of SPARC after 24 hours of TGF-β2 stimulation (Kang M, et al. IOVS 2010;51:ARVO E-Abstract 6100). Additional reports have further identified an important role for SMAD3 in facilitating microRNA maturation.46,47 In particular, SMAD3 signaling has been shown to regulate the expression of miR-29.48 To begin to examine the role of the miR-29 family in the TGF-β2/SMAD3 signaling pathway, TM cells were pretreated for 2 hours with the SMAD3 inhibitor SIS3, followed by incubation for 24 hours with TGF-β2. Neither miR-29a nor miR-29c levels were found to be significantly affected by SMAD3 inhibition under basal and 24-hour TGF-β2 stimulatory conditions (Fig. 3). In contrast, the inhibition of SMAD3 significantly induced miR-29b expression under basal conditions while significantly reducing its expression under TGF-β2 conditions (Fig. 3).
Figure 3.
Effect of SMAD3 inhibition on TGF-β2 modulation of miR-29 levels. Human TM cells were incubated for 2 hours with either 10 μM DMSO vehicle or the SMAD3 inhibitor SIS3. Cells were then stimulated for 24 hours with 2.5 ng/mL TGF-β2 or HCl vehicle, and the expression of the miR-29 family was measured by real-time quantitative PCR. All data are expressed as the mean ± SEM (*P < 0.05; n = 7, where n refers to the number of independent experiments performed using n different primary human TM cell strains).
Overexpression of the miR-29 Family Suppresses Extracellular Matrix Production under Basal and TGF-β2 Stimulatory Conditions
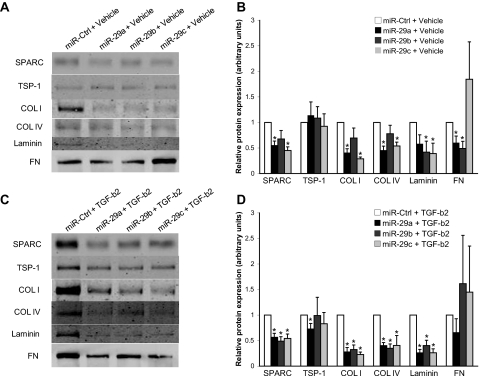
To assess the effect of the miR-29 family on ECM production in the TM, we overexpressed each paralog and examined, by immunoblot, its respective effect on ECM synthesis in TM-conditioned media. ECM proteins selected for analysis in this study focused largely on those previously shown in ocular and nonocular tissues32–37,41 to be regulated by the miR-29 family or predicted by computational algorithms, such as TargetScan version 5.149 and DIANA microT version 3.0,50 to be targets of the miR-29 family. After the transfection of miR-29a, a significant reduction in various ECM proteins, including SPARC, collagen I, collagen IV, and fibronectin, was observed in TM-conditioned media (Figs. 4A, 4B). In contrast, miR-29b overexpression significantly repressed laminin and fibronectin levels (Figs. 4A, 4B). Overexpression of miR-29c, meanwhile, was found to significantly decrease SPARC, collagen I, collagen IV, and laminin expression (Figs. 4A, 4B).
Figure 4.
Overexpression of the miR-29 family suppresses ECM synthesis. (A) Representative immunoblot and (B) densitometric analyses of ECM proteins from conditioned media of TM cells transfected with miR control, miR-29a, miR-29b, or miR-29c molecules, followed by 24-hour incubation with HCl vehicle (*P < 0.05 vs. corresponding miR-Ctrl + Vehicle group). (C) Representative immunoblot and (D) densitometric analyses of ECM proteins from conditioned media of TM cells transfected with miR control, miR-29a, miR-29b, or miR-29c molecules, followed by 24-hour stimulation with 2.5 ng/mL activated, recombinant human TGF-β2 (*P < 0.05 vs. corresponding miR-Ctrl + TGF-β2 group). All data are expressed as the mean ± SEM (n = 5, where n refers to the number of independent experiments performed using n different primary human TM cell strains).
Given the important role of TGF-β2 in ECM deposition in the TM,20,25,26 we next examined whether overexpression of miR-29 could repress ECM synthesis under TGF-β2 stimulatory conditions. Immunoblot analysis showed that each paralog was able to suppress various ECM proteins after TGF-β2 treatment, including SPARC, collagen I, collagen IV, and laminin (Figs. 4C, 4D). Additionally, TSP-1 levels were found to be significantly reduced by miR-29a after TGF-β2 treatment (Figs. 4C, 4D). No significant effects on fibronectin expression were observed (Figs. 4C, 4D).
miR-29 Inhibition Upregulates the Synthesis of Extracellular Matrix Components
To investigate the endogenous role of the miR-29 family in ECM deposition, TM cells were transfected with specific inhibitors against each paralog. Immunoblot analysis revealed significant increases in SPARC, collagen I, and TSP-1 expression after the inhibition of each paralog (Figs. 5A, 5B). Additionally, miR-29a and miR-29b blockade resulted in significantly increase collagen IV expression (Figs. 5A, 5B). Although the inhibition of miR-29c also upregulated collagen IV levels, this finding was not statistically significant (P = 0.0544). Analysis of laminin and fibronectin expression after blockade of the miR-29 family did not reveal any significant increases (Figs. 5A, 5B).
Figure 5.
Inhibition of the miR-29 family induces ECM synthesis. (A) Representative immunoblot and (B) densitometric analyses of ECM proteins from conditioned media of TM cells transfected with miR control, miR-29a, miR-29b, or miR-29c inhibitors. All data are expressed as the mean ± SEM (*P < 0.05 vs. corresponding miR-Ctrl Inhibitor group; n = 5, where n refers to the number of independent experiments performed using n different primary human TM cell strains).
Discussion
MicroRNAs have emerged in recent years as critical modulators of posttranscriptional gene expression. Aberrant changes in microRNA profiles have been shown to have profound pathophysiological consequences, including most notably tissue fibrosis.33 Using a combination of gain- and loss-of-function experiments, we have identified the miR-29 family as an important regulator of ECM synthesis in the TM.
Analysis of miR-29 family expression in cultured TM cells revealed the presence of all three paralogs. Interestingly, the levels of miR-29a were found to be significantly greater than those of miR-29b and miR-29c, suggesting that endogenous miR-29a may mediate a more expansive role in global gene regulation than miR-29b and miR-29c. Loss-of-function studies using whole-genome microarrays will be necessary to evaluate the global importance of each miR-29 paralog.
Previous studies have shown that TGF-β2 promotes ECM synthesis in the TM.20,25,26 TM cells incubated with TGF-β2 for 24, 48, and 72 hours displayed a significant induction in miR-29a expression at 48 hours and 72 hours. The delayed induction of miR-29a may represent a negative feedback or homeostatic response by the TM to curtail TGF-β2–mediated ECM deposition. Several reports have provided evidence for the existence of an ECM homeostatic mechanism in the TM.51,52 In contrast to miR-29a, miR-29b expression was significantly reduced by TGF-β2 at 24, 48, and 72 hours, suggesting that its suppression may be important in facilitating ECM deposition by TGF-β2. Although no significant changes were observed in miR-29c expression after TGF-β2 treatment, the overexpression of miR-29c suppressed the synthesis of several ECM components by TGF-β2, suggesting an important role for miR-29c in the regulation of TGF-β2–mediated ECM production.
TGF-β2 signaling promotes the deposition of various ECM components in the TM. Our laboratory and others have recently revealed that TGF-β2 induces SPARC expression in the TM (Kang M, et al. IOVS 2010;51:ARVO E-Abstract 6100; Bollinger KE, et al. IOVS 2010;51:ARVO E-Abstract 3207). Matricellular proteins, and in particular SPARC, are associated with increased fibrosis and aberrant tissue remodeling, processes implicated as major contributors to glaucoma pathogenesis. Mice bearing a homozygous deficiency in SPARC exhibit a 15% to 20% lower IOP than do control wild-type mice.53 Despite these findings, the regulatory mechanisms governing the TGF-β2–mediated synthesis of ECM in the TM remain incompletely understood. The SMAD3 transcription factor functions as an important downstream mediator of TGF-β2 signaling.54 Our data demonstrate that SMAD3 inhibition modulates miR-29b expression differently under basal and TGF-β2 stimulatory conditions. This effect may be attributed to differing gene expression environments, resulting in the altered recruitment of regulatory complexes. A working model for the role of miR-29 in TGF-β2–mediated signaling is shown in Figure 6. Although we have identified SMAD3 as an important modulator of miR-29b expression, recent studies have suggested the involvement of additional pathways in the control of miR-29b levels by TGF-β2. One such candidate is the p38 mitogen-activated protein kinase signaling pathway. p38 is activated by TGF-β2 and is important in its production of ECM proteins.54 A recent report55 has demonstrated the involvement of p38 in the regulation of miR-29b expression. Another possible factor involved in the regulation of miR-29b is SMAD7, an inhibitory SMAD that has been shown to antagonize TGF-β2–mediated signaling in the TM.56 Future studies will be necessary to assess the role of both p38 and SMAD7 in the regulation of miR-29b by TGF-β2 in the TM.
Figure 6.
Working model for the role of the miR-29 family in TGF-β2–mediated signaling. TGF-β2 stimulation activates SMAD3 signaling, which is necessary for both miR-29b expression and ECM induction by TGF-β2. Concurrently, TGF-β2 suppresses miR-29b levels by an unidentified molecular pathway dominant over that mediated by SMAD3 signaling. TGF-β2 stimulation also results in the induction of miR-29a expression. Together, the miR-29 family functions as an important modulator of ECM synthesis by TGF-β2.
The miR-29 family is transcribed from two bicistronic clusters: miR-29a and miR-29b-1 are coexpressed from one cluster, whereas miR-29c and miR-29b-2 are coexpressed from another.33 The exclusive effect of SMAD3 inhibition on miR-29b expression after treatment for 24 hours with TGF-β2 or its vehicle suggests that SMAD3 modulates miR-29b expression by a posttranscriptional mechanism. Noncanonical functions for SMAD3 have been identified.47 Specifically, SMAD3 has been reported to facilitate the posttranscriptional processing of microRNAs by its association with the DROSHA microprocessor complex.46,47
Dynamic modulation of ECM turnover is critical for IOP homeostasis. Combined data from our gain- and loss-of-function experiments reveal the importance of the miR-29 family in the regulation of ECM synthesis in the TM. In particular, the miR-29 family suppressed various ECM proteins in the context of both basal and TGF-β2 stimulation, including SPARC, collagen I, collagen IV, and laminin. Consistent with these observations, a recent study41 demonstrated, by microarray and real-time quantitative PCR analyses, that miR-29b overexpression suppresses basal levels of multiple ECM components in the TM, including SPARC, COL1A1, COL1A2, COL4A1, and LAMC1. Transgenic mice containing alterations in SPARC, collagen I, and collagen IV gene expression have been shown to develop significant changes in IOP.53,57,58 Future studies examining the consequence of alterations in miR-29 family expression on IOP in ex vivo and in vivo glaucoma models will be important.
Multiple reports have demonstrated the presence of elevated levels of TGF-β2 in the aqueous humor of POAG patients.21–24 The molecular mechanisms governing TGF-β2 signaling are complex and multifactorial.59 Although our data show that miR-29a levels are upregulated and miR-29b levels are downregulated by TGF-β2 stimulation, the relative contributions of these effects to the overall synthesis of the ECM remain unknown. As we have demonstrated, the relative expression of these paralogs varies significantly, making it unlikely that their effects would cancel. Additional studies using ex vivo and in vivo glaucoma models will be necessary to characterize the relative expression of the miR-29 paralogs in the TM and to assess their functional roles with regard to TGF-β2 signaling and ECM synthesis.
Collectively, the results presented here identify an important role for the miR-29 family in the coordinated regulation of ECM gene networks in the TM. Our findings lend further insight into the complex regulatory mechanisms governing ECM homeostasis and TGF-β2 signaling in the TM and may serve in the development of novel therapeutics for glaucoma.
Footnotes
Supported by a Fight for Sight Summer Student Fellowship (GV) and by National Eye Institute Grants EY 019654-01 (DJR) and EY 014104 (MEEI Vision-Core Grant).
Disclosure: G. Villarreal, Jr, None; D.-J. Oh, None; M.H. Kang, None; D.J. Rhee, None
References
- 1. Allingham RR, Liu Y, Rhee DJ. The genetics of primary open-angle glaucoma: a review. Exp Eye Res. 2009;88:837–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360:1113–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hyman L, Wu SY, Connell AM, et al. Prevalence and causes of visual impairment in The Barbados Eye Study. Ophthalmology. 2001;108:1751–1756 [DOI] [PubMed] [Google Scholar]
- 4. Rodriguez J, Sanchez R, Munoz B, et al. Causes of blindness and visual impairment in a population-based sample of U.S. Hispanics. Ophthalmology. 2002;109:737–743 [DOI] [PubMed] [Google Scholar]
- 5. Coffey M, Reidy A, Wormald R, Xian WX, Wright L, Courtney P. Prevalence of glaucoma in the west of Ireland. Br J Ophthalmol. 1993;77:17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hattenhauer MG, Johnson DH, Ing HH, et al. The probability of blindness from open-angle glaucoma. Ophthalmology. 1998;105:2099–2104 [DOI] [PubMed] [Google Scholar]
- 7. Patino CM, McKean-Cowdin R, Azen SP, Allison JC, Choudhury F, Varma R. Central and peripheral visual impairment and the risk of falls and falls with injury. Ophthalmology. 2010;117:199–206 e191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Szlyk JP, Mahler CL, Seiple W, Edward DP, Wilensky JT. Driving performance of glaucoma patients correlates with peripheral visual field loss. J Glaucoma. 2005;14:145–150 [DOI] [PubMed] [Google Scholar]
- 9. Friedman DS, Wolfs RC, O'Colmain BJ, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122:532–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larsson LI, Rettig ES, Brubaker RF. Aqueous flow in open-angle glaucoma. Arch Ophthalmol. 1995;113:283–286 [DOI] [PubMed] [Google Scholar]
- 11. Bill A, Phillips CI. Uveoscleral drainage of aqueous humour in human eyes. Exp Eye Res. 1971;12:275–281 [DOI] [PubMed] [Google Scholar]
- 12. Pederson JE, Gaasterland DE, MacLellan HM. Uveoscleral aqueous outflow in the rhesus monkey: importance of uveal reabsorption. Invest Ophthalmol Vis Sci. 1977;16:1008–1007 [PubMed] [Google Scholar]
- 13. Rhee DJ, Haddadin RI, Kang MH, Oh DJ. Matricellular proteins in the trabecular meshwork. Exp Eye Res. 2009;88:694–703 [DOI] [PubMed] [Google Scholar]
- 14. Seiler T, Wollensak J. The resistance of the trabecular meshwork to aqueous humor outflow. Graefes Arch Clin Exp Ophthalmol. 1985;223:88–91 [DOI] [PubMed] [Google Scholar]
- 15. Barany EH, Scotchbrook S. Influence of testicular hyaluronidase on the resistance to flow through the angle of the anterior chamber. Acta Physiol Scand. 1954;30:240–248 [DOI] [PubMed] [Google Scholar]
- 16. Bradley JM, Vranka J, Colvis CM, et al. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998;39:2649–2658 [PubMed] [Google Scholar]
- 17. Ethier CR, Kamm RD, Palaszewski BA, Johnson MC, Richardson TM. Calculations of flow resistance in the juxtacanalicular meshwork. Invest Ophthalmol Vis Sci. 1986;27:1741–1750 [PubMed] [Google Scholar]
- 18. Gum GG, Samuelson DA, Gelatt KN. Effect of hyaluronidase on aqueous outflow resistance in normotensive and glaucomatous eyes of dogs. Am J Vet Res. 1992;53:767–770 [PubMed] [Google Scholar]
- 19. Keller KE, Bradley JM, Kelley MJ, Acott TS. Effects of modifiers of glycosaminoglycan biosynthesis on outflow facility in perfusion culture. Invest Ophthalmol Vis Sci. 2008;49:2495–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, Clark AF. TGFβ2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006;47:226–234 [DOI] [PubMed] [Google Scholar]
- 21. Ochiai Y, Ochiai H. Higher concentration of transforming growth factor-beta in aqueous humor of glaucomatous eyes and diabetic eyes. Jpn J Ophthalmol. 2002;46:249–253 [DOI] [PubMed] [Google Scholar]
- 22. Ozcan AA, Ozdemir N, Canataroglu A. The aqueous levels of TGF-β2 in patients with glaucoma. Int Ophthalmol. 2004;25:19–22 [DOI] [PubMed] [Google Scholar]
- 23. Picht G, Welge-Luessen U, Grehn F, Lutjen-Drecoll E. Transforming growth factor beta 2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefes Arch Clin Exp Ophthalmol. 2001;239:199–207 [DOI] [PubMed] [Google Scholar]
- 24. Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res. 1994;59:723–727 [DOI] [PubMed] [Google Scholar]
- 25. Gottanka J, Chan D, Eichhorn M, Lutjen-Drecoll E, Ethier CR. Effects of TGF-β2 in perfused human eyes. Invest Ophthalmol Vis Sci. 2004;45:153–158 [DOI] [PubMed] [Google Scholar]
- 26. Shepard AR, Millar JC, Pang IH, Jacobson N, Wang WH, Clark AF. Adenoviral gene transfer of active human transforming growth factor-β2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci. 2010;51:2067–2076 [DOI] [PubMed] [Google Scholar]
- 27. Fuchshofer R, Yu AH, Welge-Lussen U, Tamm ER. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-β2 in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007;48:715–726 [DOI] [PubMed] [Google Scholar]
- 28. Junglas B, Yu AH, Welge-Lussen U, Tamm ER, Fuchshofer R. Connective tissue growth factor induces extracellular matrix deposition in human trabecular meshwork cells. Exp Eye Res. 2009;88:1065–1075 [DOI] [PubMed] [Google Scholar]
- 29. Pattabiraman PP, Rao PV. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am J Physiol Cell Physiol. 2010;298:C749–C763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–234 [DOI] [PubMed] [Google Scholar]
- 31. Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269 [DOI] [PubMed] [Google Scholar]
- 32. Li Z, Hassan MQ, Jafferji M, et al. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem. 2009;284:15676–15684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Rooij E, Sutherland LB, Thatcher JE, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–13032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ogawa T, Iizuka M, Sekiya Y, Yoshizato K, Ikeda K, Kawada N. Suppression of type I collagen production by microRNA-29b in cultured human stellate cells. Biochem Biophys Res Commun. 2010;391:316–321 [DOI] [PubMed] [Google Scholar]
- 35. Du B, Ma LM, Huang MB, et al. High glucose down-regulates miR-29a to increase collagen IV production in HK-2 cells. FEBS Lett. 2010;584:811–816 [DOI] [PubMed] [Google Scholar]
- 36. Kapinas K, Kessler CB, Delany AM. miR-29 suppression of osteonectin in osteoblasts: regulation during differentiation and by canonical Wnt signaling. J Cell Biochem. 2009;108:216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sengupta S, den Boon JA, Chen IH, et al. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc Natl Acad Sci U S A. 2008;105:5874–5878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oh DJ, Martin JL, Williams AJ, Russell P, Birk DE, Rhee DJ. Effect of latanoprost on the expression of matrix metalloproteinases and their tissue inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006;47:3887–3895 [DOI] [PubMed] [Google Scholar]
- 39. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔC(T) method. Methods. 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 40. Parmar KM, Nambudiri V, Dai G, Larman HB, Gimbrone MA, Jr, Garcia-Cardena G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J Biol Chem. 2005;280:26714–26719 [DOI] [PubMed] [Google Scholar]
- 41. Luna C, Li G, Qiu J, Epstein DL, Gonzalez P. Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Mol Vis. 2009;15:2488–2497 [PMC free article] [PubMed] [Google Scholar]
- 42. Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471 [DOI] [PubMed] [Google Scholar]
- 43. Kobayashi K, Yokote K, Fujimoto M, et al. Targeted disruption of TGF-beta-Smad3 signaling leads to enhanced neointimal hyperplasia with diminished matrix deposition in response to vascular injury. Circ Res. 2005;96:904–912 [DOI] [PubMed] [Google Scholar]
- 44. Wang W, Huang XR, Canlas E, et al. Essential role of Smad3 in angiotensin II-induced vascular fibrosis. Circ Res. 2006;98:1032–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao J, Shi W, Wang YL, et al. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2002;282:L585–L593 [DOI] [PubMed] [Google Scholar]
- 46. Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hoover LL, Kubalak SW. Holding their own: the noncanonical roles of Smad proteins. Sci Signal. 2008;1:pe48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Divakaran V, Adrogue J, Ishiyama M, et al. Adaptive and maladaptive effects of SMAD3 signaling in the adult heart after hemodynamic pressure overloading. Circ Heart Fail. 2009;2:633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20 [DOI] [PubMed] [Google Scholar]
- 50. Maragkakis M, Reczko M, Simossis VA, et al. DIANA-microT web server: elucidating microRNA functions through target prediction. Nucleic Acids Res. 2009;37:W273–W276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bradley JM, Kelley MJ, Zhu X, Anderssohn AM, Alexander JP, Acott TS. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001;42:1505–1513 [PubMed] [Google Scholar]
- 52. Vittal V, Rose A, Gregory KE, Kelley MJ, Acott TS. Changes in gene expression by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol Vis Sci. 2005;46:2857–2868 [DOI] [PubMed] [Google Scholar]
- 53. Haddadin RI, Oh DJ, Kang MH, et al. SPARC-null mice exhibit lower intraocular pressures. Invest Ophthalmol Vis Sci. 2009;50:3771–3777 [DOI] [PubMed] [Google Scholar]
- 54. Saika S. TGFβ pathobiology in the eye. Lab Invest. 2006;86:106–115 [DOI] [PubMed] [Google Scholar]
- 55. Anastasiadou E, Boccellato F, Vincenti S, et al. Epstein-Barr virus encoded LMP1 downregulates TCL1 oncogene through miR-29b. Oncogene. 2010;29:1316–1328 [DOI] [PubMed] [Google Scholar]
- 56. Fuchshofer R, Stephan DA, Russell P, Tamm ER. Gene expression profiling of TGFβ2- and/or BMP7-treated trabecular meshwork cells: identification of Smad7 as a critical inhibitor of TGF-beta2 signaling. Exp Eye Res. 2009;88:1020–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dai Y, Lindsey JD, Duong-Polk X, Nguyen D, Hofer A, Weinreb RN. Outflow facility in mice with a targeted type I collagen mutation. Invest Ophthalmol Vis Sci. 2009;50:5749–5753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gould DB, Marchant JK, Savinova OV, Smith RS, John SW. Col4a1 mutation causes endoplasmic reticulum stress and genetically modifiable ocular dysgenesis. Hum Mol Genet. 2007;16:798–807 [DOI] [PubMed] [Google Scholar]
- 59. Pohlers D, Brenmoehl J, Loffler I, et al. TGF-beta and fibrosis in different organs—molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746–756 [DOI] [PubMed] [Google Scholar]